Abstract
Toxoplasmosis is a globally distributed disease of warm-blooded animals. It is caused by the opportunistic parasite Toxoplasma gondii (T. gondii). One-third of the global human population is believed to be infected with T. gondii. Cats serve as final host of T. gondii and are the main source of contamination of soil and water. This study aimed to detect genotypes of T. gondii in cats. Fecal samples (n = 400) were collected from districts of South Punjab (Khanewal and Sahiwal), and were processed by polymerase chain reaction (PCR) followed by sequencing and phylogenetic analysis. The obtained oligonucleotide sequences (T. gondii) were submitted to the GenBank database, and the evolutionary tree was constructed using MEGA-X software. Seven fecal samples (3.5%) from cats were positive. Five out of thirteen fecal samples (38.46%) found to be positive for T. gondii with microscopy were confirmed by PCR. After phylogenetic analysis with 3 clonal types and atypical strains, isolates of T. gondii in current study were more closely linked to a typical strain (AF249696). Besides genotyping from cats, seroprevalence from humans and ruminants is still considered to be the best and easiest way to identify the Toxoplasma. Blood samples were collected from sheep and goats (n = 2000 each), and human blood samples (n = 400) were collected from the same vicinity. Seroprevalence was determined using a commercial enzyme-linked immunosorbent assay (ELISA) kit. In Khanewal, the blood samples of 292 goats (29.2%) and 265 sheep (26.5%), and 6 fecal samples from cats (3%) were positive. Out of 200 human blood samples, 52 were positive, with a seroprevalence of 26%. In the Sahiwal district, the blood samples from 49 humans, 235 sheep and 348 goats were positive, with seroprevalence of 24.5%, 23.5% and 34.8%, respectively. The present study revealed the current circulating genotype of T. gondii from cats in the districts Khanewal and Sahiwal and the seroprevalence of the organism in small ruminants and humans living in the same vicinity. Further genotype analyses of the organism from ruminants and humans are needed.
1. Introduction
Toxoplasma gondii is a common protozoan parasite of zoonotic significance that causes serious disease in small ruminants and mammals, including humans [1,2]. As one of the five parasitic diseases targeted for public health action and prevention, this malady has become a priority (Centers for Disease Control and Prevention (CDC), 2013). Toxoplasmosis is a cause of communal infection among caprine animals around the globe [3,4]. It is known to cause reproductive miscarriage [5]. This may result in huge economic losses due to abortion or the birth of weak offspring in food animals [6,7]. The infection rate varies widely from herd to herd due to inbreeding; an average rate of 30% has been recorded. The high prevalence of toxoplasmosis among domesticated animals such as cattle, sheep and goats can be an important cause of disease transmission to humans [8].
Toxoplasmosis is a highly zoonotic disease that is instigated by T. gondii, a food-borne pathogen [9,10]. Human toxoplasmosis is closely linked with the use of raw or undercooked food items [11]. Meat obtained from domestic animals containing T. gondii is considered an important source of infection in humans.
Efforts have been made to isolate the T. gondii tachyzoites from the saliva, urine, vaginal mucosa and nasal secretions of infected food animals [12]. Consumption of products, including milk, from diseased food animals stands as an imperative cause of human infection, which is of concern due to a rise in the consumption of sheep and goat’s milk among children with an allergy to cow’s milk [13]. Approximately 30% of the human population is chronically infected with T. gondii [14].
Seroprevalence at childbearing age in women has been shown to range from 4 to 100%. During pregnancy, infection rates vary (between 1 to 310 in 10,000 pregnancies) according to the locality of the pregnant population, for example, Europe and the USA [15]. Presently, there is no gold standard test for the diagnosis of toxoplasmosis. Different methods have been recommended to analyze infected populations based on the organs affected [16]. The best approach is indirect hemagglutination [17], which can be used to detect this disease in animals with the help of readily available kits.
Despite the economic and zoonotic significance, as well as the high seroprevalence of this pathogen, little or no research has been conducted in Pakistan. This project was undertaken to persuade assessment of the seroprevalence of toxoplasmosis in sheep, goats and humans in the populated districts of South Punjab, and the molecular characterization of T. gondii from cats. The proposed study provides solutions to address the dilemma between animal-friendly production and safety regarding T. gondii as a food pathogen with a high health impact.
2. Materials and Methods
2.1. Study Area and Sample Collection
The study comprised two districts of South Punjab (Khanewal and Sahiwal) (Figure 1). Samples were collected and processed between May and November 2020. A total of 2400 blood samples were taken: 1000 goats and sheep each; 200 from humans in different localities from each of the mentioned districts. Approximately 5 mL of blood was collected from the above-mentioned species for serum separation. Samples were collected rando mLy from these animals through appropriate methods and were transported to the immuno-parasitology laboratory, Department of Parasitology, UVAS, Lahore. In addition, 200 fecal samples from cats were also collected. The presence of cats and their accessibility to animal feed and water was observed within each zone.
Figure 1.
Map representing the sites where samples were collected.
2.2. Seroprevalence Determined Using ELISA
Blood samples were centrifuged at 2300× g for 10 minutes and the serum was separated [18]. Immunoglobulin G (IgG) antibodies were detected and separated using commercial indirect ELISA as described by [19]. The optical densities (ODs) were calculated through use of the Microplate Reader RT-6000 (Devrim limited, Romford, UK). The samples that revealed OD values >11 were considered to represent positive samples as per the manufacturer’s instructions for results interpretation.
2.3. Fecal Examination
A direct smear of feces was prepared and analyzed through use of an Olympus CX21 microscope. Oocysts were identified in cat fecal samples. Flotation/sedimentation techniques were used for the identification of oocysts. Collected un-sporulated oocysts were placed in 2.5% potassium dichromate (1:5) for sporulation. After five days, sporulated oocysts were observed under a compound microscope at 40×. Oocysts were counted using the McMaster technique. Washed oocysts were subjected to excystation using glass beads [20]. The sporozoites released from oocysts suspensions were subjected to DNA extraction.
2.4. Molecular Identification
DNA was extracted from the fecal samples using QIAamp Fast DNA Stool Mini Kit (Qiagen, Germany) for molecular detection as per the manufacturer’s instructions. PCR was performed to amplify the COX1 gene with the use of the universal primers, 400F (5′-GGDTCAGGTRTTGGTTGGAC-3′) and 1202R (5′-CCAAKRAYHGCACCAAGAGATA-3′). The PCRs were optimized by varying the concentrations of the DNA template [21]. Specific forward primers (5′-CCTGGTGTCTCTTCAAGCGT-3′) and reverse primers (5′-AAAGGAGAATGAGCGCACGA-3′) of the SAG2 gene with amplicon size 529 bp were used [22]. The PCRs were performed using the following conditions: initial denaturation at 95 °C for two minutes; followed by 35 cycles comprising of denaturation at 95 °C for 30 seconds; annealing at 59 °C for 30 seconds; extension at 72 °C for two minutes; and final extension at 72 °C for 10 minutes. Agarose gel (1.5%) was prepared and stained with ethidium bromide in order to analyze the PCR-generated amplicons. Gel electrophoresis was performed at 113 V, 230 mA for 35 minutes in order to visualize the bands in the gel documentation system (Bio-Rad Laboratories, Hercules, CA, USA).
2.5. Sequencing and Phylogenetic Analysis
T. gondii amplicons were arbitrarily selected for molecular analysis, purified with Gene JET Gel Extraction Kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions and subjected to Sanger’s sequencing method on an Applied BioSystems 3130 automated DNA sequencer. Phylogenetic analysis was performed using MEGA X software (Version: 11) through the neighbor-joining method with 1000 bootstraps to accomplish several sequence alignments. A phylogenetic tree of T. gondii SAG2 was constructed and compared with sequences that were already available in GenBank.
2.6. Statistical Analysis
Seroprevalence was assessed using the chi-square test. The p-values for results from both districts were found to be significant (p ≤ 0.05). Detection of T. gondii through the use of both the microscope and PCR was also found to be statistically significant.
3. Results
3.1. Seroprevalence of Toxoplasmosis in Small Ruminats and Humans
In Khanewal, 292 goats (29.2%), 265 sheep (26.5%) and 52 humans (26%) tested positive for T. gondii antibodies. In the Sahiwal district, 384 goats (38.4%), 235 sheep (23.5%) and 49 humans (24.5%) tested positive. The antibodies that were detected for seropositive samples by ELISA in small ruminants and human samples are presented in Table 1. The seroprevalence of toxoplasmosis in sheep, goats and humans in Sahiwal and Khanewal districts were found to be statistically significant (p ≤ 0.05) using the Chi-square test in SPSS.

Table 1.
Seroprevalence of toxoplasmosis in small ruminants and humans.
3.2. Examination of Fecal Samples
Through the use of microscopy, six (3%) and seven (3.5%) cat fecal samples were found to be positive in Khanewal and Sahiwal, respectively (Table 2). Unsporulated oocysts were spherical or somewhat round in shape. Their size ranged from 10 µm to 14 µm, measured by micrometry.

Table 2.
Prevalence of T. gondii in cat fecal samples (microscopy and PCR).
3.3. Molecular Detection by PCR
Microscopically positive fecal samples were confirmed by PCR. Five out of thirteen samples from both districts (38.46%) of T. gondii oocysts under the microscope were confirmed through PCR. Partial amplification of repetitive 800 bp and 529 bp sections are shown in Figure 2 and Figure 3, with universal and specific primers, respectively. The prevalence of T. gondii oocysts according to microscopy and PCR in the cats was analyzed by chi-square tests and was found to be statistically significant (p ≤ 0.05).
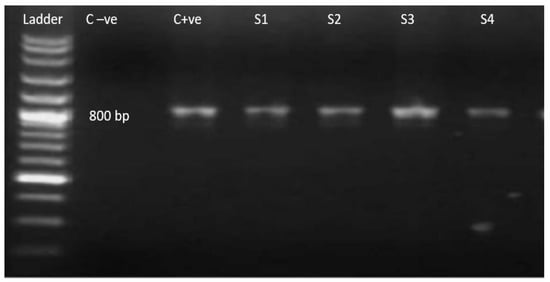
Figure 2.
Ethidium bromide-stained agarose gel with PCR amplification 800 bp (using universal primers). Ladder: 100 bp molecular weight marker; C−ve: negative control; C+ve: positive control; S1–S4 current study isolates ensuring amplified product size.
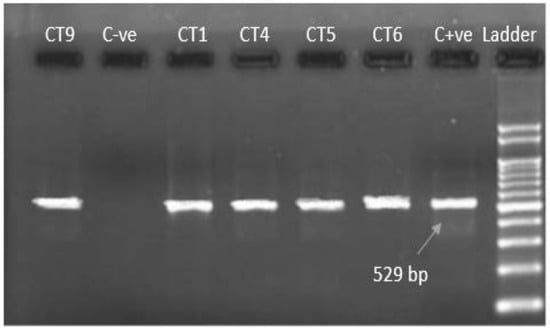
Figure 3.
Ethidium bromide-stained agarose gel with PCR amplification 529 bp (using specific primer). C−ve: negative control; CT9, CT1, CT4, CT5, CT6: current study isolates; C+ve: positive control; Ladder: 100 bp DNA marker.
3.4. Sequencing and Phylogenetic Analysis
The SAG2 gene of three current isolates was sequenced and compared with published sequences from all clonal types of T. gondii (Table 3). Clustal W analysis and bootstrapping were performed to enable the genotypic comparison of three isolates from cat fecal samples with published strains of Toxoplasma in GenBank. The current study isolates were found to be closer to the atypical strain AF249696 (Figure 4). Representative strains of each genotype from type I, II or III were taken after Clustal W analysis. Thus, it was found that our three isolates were near to atypical.

Table 3.
SAG2 sequences along with their accession numbers retrieved from NCBI with current isolates for phylogenetic analysis.
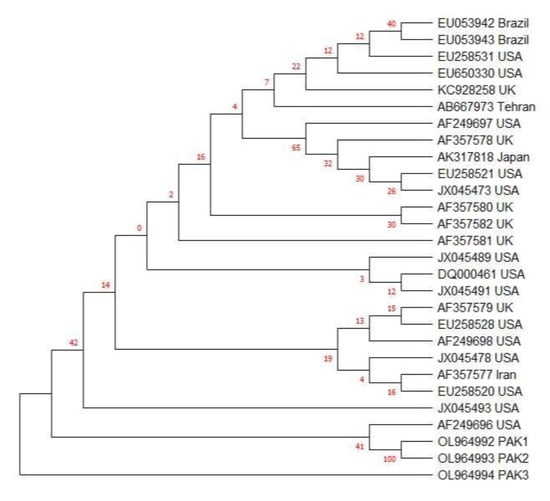
Figure 4.
SAG2 gene-based phylogenetic analysis of 3 current isolates from cat fecal samples using the neighbor-joining method with 1000 bootstrap replicates, with the help of Tamura Nei model (29) through MEGA-X software. PAK1, PAK2 and PAK3 are current study isolates from cat feces. AF249696 is the SAG2 sequence of the atypical T. gondii strain.
4. Discussion
In this study, ELISA was performed to estimate the seroprevalence of T. gondii in ruminants and humans. The findings of the present study provided evidence of toxoplasmosis in ruminants, humans and cats. The seroprevalence of T. gondii in humans from both districts was in agreement with the findings of [23], in upper Myanmar [24] and Iran [25]. Higher seroprevalence of T. gondii has been reported in Egypt [26], Iran [27], Northern Iraq [28], Egypt [29], Sudan [30], North East India [31] and Saudi Arabia [32] than in current study. However, lower seroprevalence in humans has been reported in Khyber Pakhtunkhwa, Pakistan [33], South-Eastern China [34], India [35], West Africa [36], Pothwar region, Northern Punjab, Pakistan [37], Ghana [38] and Algeria [39].
The present study revealed a higher seroprevalence of toxoplasmosis in both South Punjab districts (Khanewal and Sahiwal). These findings may be due to the warm and humid environment in Southern Punjab, which is favorable for the survival of T. gondii oocysts. Different rates of seroprevalence were recorded at different locations, which may be due to changes in the environment, sanitary conditions and management practices.
The prevalence of T. gondii oocysts in the present study was similar to the prevalence of T. gondii reported in Lahore, Pakistan (2.3%) [40]. Similar prevalence has also been reported in China (2.78%) [41] and Iran (2.56%) [42]. The prevalence was higher in Indonesia (6.81%) [22] and Kenya (7.8%) than in the present study [1]. Microscopy is not a reliable way to screen for oocyst shedding in cats because this method of oocyst identification lacks sensitivity and specificity. Infective oocysts can only be detected in cats infected with T. gondii. Antibodies and tissue cysts remain in the body for a long period of time, whereas oocysts persist for three weeks after infection [43].
In the current study, the prevalence of toxoplasmosis in cats was in line with the prevalence reported in Yogyakarta, Indonesia [22]. The prevalence reported in this study was higher than that reported in Izmir, Turkey (15.34%) [44]. Lower prevalence of T. gondii in domestic cats has been reported in Brazil (3.74%) [45] and Poland (2.4%) [46]. A higher prevalence of 47.2% has been reported in South Korea [47] and Southern Thailand (61.5%) [48]. The low prevalence of toxoplasmosis reported in domestic cats may be because these cats are mostly kept indoors [49]. Pet cats are usually prevented from eating raw or poorly cooked meat and have little chance of contact with wild animals.
Sequencing and phylogenetic results revealed that atypical SAG2 was the predominant genotype of T. gondii circulating in the study area. Limited research has been conducted in Pakistan to identify and characterize the molecules of T. gondii. Previous studies have indicated that the T. gondii SAG2 gene consists of three chief clonal lineages, nominated as types I, II and III, which are important in Europe and the USA [50], whereas the atypical genotype is dominant in South America and Asia [51,52]. In the current research, the phylogenetic approach revealed the SAG2 atypical strain through nucleotide sequence analysis. Although the lack of other genotypes in this study was consistent with previous findings, some studies have also established types II or III as the main genotypes [53]. In previous studies, the SAG2 atypical strain was also identified from Pakistan [54].
Atypical T. gondii genotypes were found to be associated with a number of severe cases of toxoplasmosis in immunocompetent individuals [55]. Atypical genotypes can develop when a cat ingests prey infected with T. gondii of more than one clonal type, followed by sexual recombination in the gut of the cat which can result in progeny representing a mixture of the two parental genotypes [56]. It is most likely that these atypical genotypes are the result of sexual recombination in cats [57]. Type 1 has been reported in China [58] and Iran [59]. These conflicting results are probably due to the presence of various genotypes of T. gondii in different geographical regions. It has been shown that Toxoplasma strains in animals and humans have different distributions in the same geographical regions.
5. Conclusions
The present study revealed the current circulating genotype of T. gondii from cats in the districts Khanewal and Sahiwal, and the seroprevalence of the organism in small ruminants and humans living in the same vicinity. Further studies are needed to assess the genotyping of T. gondii in intermediate hosts (humans and small ruminants) in Pakistan.
Author Contributions
Conceptualization, A.A., O.O.A., M.A.B.S., M.M.A. and U.C.; writing—original draft preparation, M.A.H., M.M. and S.F.W.; writing—review and editing, F.A., K.A., M.T.A., A.R.K., A.S., S.F.W. and A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Higher Education Commission Pakistan under the National Research Program for Universities with project no. 6941/Punjab/NRPU/R&D/HEC/2017 and the researchers supporting project number (RSP2022R494), King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
This study was performed under the regulation of the Ethical Review Committee (ERC) of the University of Veterinary and Animal Sciences (UVAS), Lahore, Pakistan, permit No. ERC-3355.
Data Availability Statement
The datasets generated during and/or analyzed during the current study can be find in the main text.
Acknowledgments
Researchers supporting project number6941/Punjab/NRPU/R&D/HEC/2017 and researchers supporting project number (RSP2022R494), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this article.
References
- Njuguna, A.N.; Kagira, J.M.; Karanja, S.M.; Ngotho, M.; Mutharia, L.; Maina, N.W. Prevalence of Toxoplasma gondii and other gastrointestinal parasites in domestic cats from households in Thika region, Kenya. BioMed Res. Int. 2017, 2017, 7615810. [Google Scholar]
- Filho, P.C.G.A.; Ribeiro-Andrade, M.; Santos, J.F.; Reis, A.C.; Valença, S.R.F.A.; Fernandes, E.F.T.S.; Junior, J.W.P.; Mota, R.A. Serological survey and risk factors for Toxoplasma gondii infection in cattle from Amazonas, Brazil. Prev. Vet. Med. 2020, 176, 104885. [Google Scholar] [CrossRef]
- Li, J.X.; He, J.J.; Elsheikha, H.M.; Chen, D.; Zhai, B.T.; Zhu, X.Q.; Yan, H.K. Toxoplasma gondii ROP17 inhibits the innate immune response of HEK293T cells to promote its survival. Parasitol. Res. 2019, 118, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Gazzonis, A.L.; Marino, A.M.F.; Garippa, G.; Rossi, L.; Mignone, W.; Dini, V.; Manfredi, M.T. Toxoplasma gondii seroprevalence in beef cattle raised in Italy: A multicenter study. Parasitol. Res. 2020, 119, 3893–3898. [Google Scholar] [CrossRef]
- Vilares, A. Genetic Study of Toxoplasma gondii Strains Isolated from Humans and Animals; Universidade de Lisboa, Faculdade de Ciências: Lisboa, Portugal, 2019. [Google Scholar]
- Stelzer, S.; Basso, W.; Silván, J.B.; Ortega-Mora, L.M.; Maksimov, P.; Gethmann, J.; Schares, G. Toxoplasma gondii infection and toxoplasmosis in farm animals: Risk factors and economic impact. Food Waterborne Parasitol. 2019, 15, e00037. [Google Scholar] [CrossRef] [PubMed]
- Nayeri, T.; Sarvi, S.; Moosazadeh, M.; Daryani, A. Global prevalence of Toxoplasma gondii infection in the aborted fetuses and ruminants that had an abortion: A systematic review and meta-analysis. Vet. Parasitol. 2021, 290, 109370. [Google Scholar] [CrossRef]
- Dubey, J.P.; Murata, F.H.A.; Cerqueira-Cézar, C.K.; Kwok, O.C.H.; Su, C. Economic and public health importance of Toxoplasma gondii infections in sheep: The last decade. Vet. Parasitol. 2020, 286, 100028. [Google Scholar] [CrossRef]
- Der Straße, U.A. Shadow Disease Chronic Active Toxoplasmosis: How It Deceives Medicine and Makes Us sick-and How to Diagnose and Treat It; BoD–Books on Demand: Nordstedt, Germany, 2019. [Google Scholar]
- Lam, A.P.; de Sordi, D.; Müller, H.H.; Lam, M.C.; Carl, A.; Kohse, K.P.; Philipsen, A. Aggravation of symptom severity in adult attention-deficit/hyperactivity disorder by latent Toxoplasma gondii infection: A case–control study. Sci. Rep. 2020, 10, 14382. [Google Scholar] [CrossRef]
- Ramakrishnan, C.; Maier, S.; Walker, R.A.; Rehrauer, H.; Joekel, D.E.; Winiger, R.R.; Smith, N.C. An experimental genetically attenuated live vaccine to prevent transmission of Toxoplasma gondii by cats. Sci. Rep. 2019, 9, 1474. [Google Scholar] [CrossRef]
- Atail, H.B.M. Sero-Prevalence of Toxoplasmosis in Sheep and Goats in El-Gadarif State. Ph.D. Thesis, Sudan University of Science & Technology, Kharsmu, Sudan, 2017. [Google Scholar]
- Rehman, F.; Shah, M.; Ali, A.; Ahmad, I.; Sarwar, M.T.; Rapisarda, A.M.C.; Cianci, A. Unpasteurised milk consumption as a potential risk factor for toxoplasmosis in females with recurrent pregnancy loss. J. Obstet. Gynaecol. 2020, 40, 1106–1110. [Google Scholar] [CrossRef]
- Laboudi, M. Review of toxoplasmosis in Morocco: Seroprevalence and risk factors for toxoplasma infection among pregnant women and HIV-infected patients. Pan Afr. Med. J. 2017, 27, 269. [Google Scholar] [CrossRef]
- Maldonado, Y.A.; Read, J.S. Committee on Infectious Diseases. Diagnosis, treatment, and prevention of congenital toxoplasmosis in the United States. Pediatrics 2017, 139, 2. [Google Scholar] [CrossRef] [Green Version]
- Al-Malki, E.S. Toxoplasmosis: Stages of the protozoan life cycle and risk assessment in humans and animals for an enhanced awareness and an improved socio-economic status. Saudi J. Biol. Sci. 2021, 28, 962. [Google Scholar] [CrossRef]
- Luo, H.; Li, K.; Zhang, H.; Gan, P.; Shahzad, M.; Wu, X.; Wang, J. Seroprevalence of Toxoplasma gondii infection in zoo and domestic animals in Jiangxi Province, China. Parasite 2017, 24, 7. [Google Scholar] [CrossRef] [Green Version]
- Xiong, S.; Qiu, L.; Su, Y.; Zheng, H.; Yi, C. Platelet-rich plasma and platelet-rich fibrin enhance the outcomes of fat grafting: A comparative study. Plast. Reconstr. Surg. 2019, 143, 1201e–1212e. [Google Scholar] [CrossRef]
- Fallahi, S.; Rostami, A.; Shiadeh, M.N.; Behniafar, H.; Paktinat, S. An updated literature review on maternal-fetal and reproductive disorders of Toxoplasma gondii infection. J. Gynecol. Obstet. Hum. 2018, 47, 133–140. [Google Scholar] [CrossRef]
- Tang, X.; Huang, G.; Liu, X.; El-Ashram, S.; Tao, G.; Lu, C.; Suo, X. An optimized DNA extraction method for molecular identification of coccidian species. Parasitol. Res. 2018, 117, 655–664. [Google Scholar] [CrossRef]
- El-Sherry, S.; Ogedengbe, M.E.; Hafeez, M.A.; Barta, J.R. Divergent nuclear 18S rDNA paralogs in a turkey coccidium, Eimeria meleagrimitis, complicate molecular systematics and identification. Int. J. Parasitol. 2013, 43, 679–685. [Google Scholar] [CrossRef]
- Hanafiah, M.; Prastowo, J.; Hartati, S.; Aliza, D.; Nurcahyo, R.W. Detection of Toxoplasma gondii copro-prevalence by polymerase chain reaction using repetitive 529 bp gene in feces of pet cats (Felis catus) in Yogyakarta, Indonesia. Vet. World 2018, 11, 1338. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, N.; Khan, I.A.; Iqbal, Z.; Naseem, A.A.; Kayani, A.R.; Afshan, K.; Qayyum, M. Seroepidemiology of Toxoplasmosis in Human Population with Reference to Its Zoonotic Potential in Sub-Tropical Areas of Pakistan. Pak. Vet. J. 2019, 39, 2. [Google Scholar]
- Thái, T.L.; Jun, H.; Park, S.H.; Lê, H.G.; Lee, J.; Ahn, S.K.; Nam, H.W. Seroprevalence of Toxoplasma gondii among School Children in Pyin Oo Lwin and Naung Cho, Upper Myanmar. Korean J. Parasitol. 2019, 57, 303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khademi, S.Z.; Ghaffarifar, F.; Dalimi, A.; Davoodian, P.; Abdoli, A. Prevalence and risk factors of Toxoplasma gondii infection among pregnant women in Hormozgan Province, South of Iran. Iran. J. Parasitol. 2019, 14, 167. [Google Scholar] [CrossRef] [PubMed]
- Tzanidakis, N.; Maksimov, P.; Conraths, F.J.; Kiossis, E.; Brozos, C.; Sotiraki, S.; Schares, G. Toxoplasma gondii in sheep and goats: Seroprevalence and potential risk factors under dairy husbandry practices. Vet. Parasitol. 2012, 190, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Izadyar, N.; Abd Nikfarjam, B.; Rastaghi, A.R.E.; Alizadeh, S.A.; Heydarian, P.; Saraei, M. A serologic study on Toxoplasma gondii infection in slaughtered sheep and goats in Qazvin Province, Iran. Trop. Anim. Health Prod. 2019, 51, 1289–1293. [Google Scholar] [CrossRef]
- Al Hamada, A.; Habib, I.; Barnes, A.; Robertson, I. Risk factors associated with seropositivity to Toxoplasma among sheep and goats in Northern Iraq. Vet. Parasitol. 2019, 15, 100264. [Google Scholar] [CrossRef]
- Mandour, A.; Mounib, M.; Eldeek, H.; Ahmad, A.; Abdel-Kader, A. Prevalence of congenital toxoplasmosis in pregnant women with complicated pregnancy outcomes in Assiut governorate. J. Egypt. Soc. 2017, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Taha, R.K.M.; Hamad, M.N.M.; Taha, K.M. Seroprevalence of toxoplasmosis between aborted ladies in Atbara district, Sudan. MOJWH 2019, 8, 86–87. [Google Scholar]
- Borkakoty, B.; Biswas, D.; Jakharia, A.; Mahanta, J. Seroprevalence of Toxoplasma gondii among pregnant women in Northeast India. J. Assoc. Physicians India 2016, 64, 24–28. [Google Scholar]
- Alghamdi, J.; Elamin, M.H.; Alhabib, S. Prevalence and genotyping of Toxoplasma gondii among Saudi pregnant women in Saudi Arabia. Saudi Pharm. J. 2016, 24, 645–651. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.N.; Khan, S.; Ayaz, S.; Jan, A.H.; Jehangir, S.; Attaullah, S.; Shams, S. Seroprevalance and risk factors of toxoplasmosis among pregnant women in District Kohat, Khyber Pakhtunkhwa, Pakistan. World Appl. Sci. J. 2011, 14, 1032–1036. [Google Scholar]
- Chen, X.; Chen, B.; Hou, X.; Zheng, C.; Yang, X.; Ke, J.; Tan, F. Association between Toxoplasma gondii infection and psychiatric disorders in Zhejiang, Southeastern China. Acta Trop. 2019, 192, 82–86. [Google Scholar] [CrossRef]
- Bachan, M.; Deb, A.R.; Maharana, B.R.; Sudhakar, N.R.; Sudan, V.; Saravanan, B.C.; Tewari, A.K. High seroprevalence of Toxoplasma gondii in goats in Jharkhand state of India. Vet. Parasitol. 2018, 12, 61–68. [Google Scholar] [CrossRef]
- Tonouhewa, A.B.N.; Akpo, Y.; Sherasiya, A.; Sessou, P.; Adinci, J.M.; Aplogan, G.L.; Farougou, S. A serological survey of Toxoplasma gondii infection in sheep and goat from Benin, West-Africa. J. Parasit. Dis. 2019, 43, 343–349. [Google Scholar] [CrossRef]
- Ahmad, N.; Iqbal, Z.; Mukhtar, M.; Mushtaq, M.; Khan, K.M.; Qayyum, M. Seroprevalence and associated risk factors of toxoplasmosis in sheep and goats in Pothwar region, Northern Punjab, Pakistan. Pak. J. Zool. 2015, 47, 161–167. [Google Scholar]
- Bentum, K.E.; Folitse, R.D.; Amemor, E.; Burimuah, V.; Opoku-Agyemang, T.; Emikpe, B.O. Seroprevalence of Toxoplasma gondii antibodies in sheep and goats slaughtered at the Kumasi Abattoir, Ghana. J. Immunoass. Immunochem. 2019, 40, 495–501. [Google Scholar] [CrossRef]
- Abdallah, M.C.; Kamel, M.; Karima, B.; Samir, A.; Djamel, K.; Rachid, K.; Khatima, A.O. Cross-sectional survey on Toxoplasma gondii infection in cattle, sheep, and goats in Algeria: Seroprevalence and risk factors. Vet. Sci. 2019, 6, 63. [Google Scholar] [CrossRef] [Green Version]
- Nabi, H.; Rashid, M.I.; Islam, S.; Bajwa, A.A.; Gul, R.; Shehzad, W.; Ashraf, K. Prevalence of Toxoplasma gondii oocysts through Copro-PCR in cats at Pet Center (UVAS), Lahore, Pakistan. J. Pak. Med. Assoc. 2018, 68, 115–118. [Google Scholar]
- Yang, Y.; Ying, Y.; Verma, S.K.; Cassinelli, A.B.M.; Kwok, O.C.H.; Liang, H.; Dubey, J.P. Isolation and genetic characterization of viable Toxoplasma gondii from tissues and feces of cats from the central region of China. Vet. Parasitol. 2015, 211, 283–288. [Google Scholar] [CrossRef]
- Khodaverdi, M.; Razmi, G. Prevalence and genotyping of Toxoplasma gondii in stray cats in Mashhad area, Iran. BMC Vet. Res. 2019, 15, 463. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, K.; Bahia-Oliveira, L.; Dixon, B.; Dumètre, A.; de Wit, L.A.; VanWormer, E.; Villena, I. Environmental transmission of Toxoplasma gondii: Oocysts in water, soil and food. Food Waterborne Parasitol. 2019, 15, e00049. [Google Scholar] [CrossRef]
- Karakavuk, M.; Can, H.; Selim, N.; Yeşilsiraz, B.; Atlı, E.; Şahar, E.A.; Döşkaya, M. Investigation of the role of stray cats for transmission of toxoplasmosis to humans and animals living in İzmir, Turkey. J. Infect. Dev. Ctries. 2021, 15, 155–162. [Google Scholar] [CrossRef]
- Bolais, P.F.; Vignoles, P.; Pereira, P.F.; Keim, R.; Aroussi, A.; Ismail, K.; Mercier, A. Toxoplasma gondii survey in cats from two environments of the city of Rio de Janeiro, Brazil by Modified Agglutination Test on sera and filter-paper. Parasit. Vectors 2017, 10, 88. [Google Scholar] [CrossRef] [Green Version]
- Sroka, J.; Bilska-Zając, E.; Wójcik-Fatla, A.; Zając, V.; Dutkiewicz, J.; Karamon, J.; Cencek, T. Detection and molecular characteristics of Toxoplasma gondii DNA in retail raw meat products in Poland. Foodborne Pathog. Dis. 2019, 16, 195–204. [Google Scholar] [CrossRef] [Green Version]
- Jung, B.K.; Lee, S.E.; Lim, H.; Cho, J.; Kim, D.G.; Song, H.; Chai, J.Y. Toxoplasma gondii B1 gene detection in feces of stray cats around Seoul, Korea and genotype analysis of two laboratory-passaged isolates. Korean J. Parasitol. 2015, 53, 259. [Google Scholar] [CrossRef]
- Chemoh, W.; Sawangjaroen, N.; Nissapatorn, V.; Sermwittayawong, N. Genotyping of Toxoplasma gondii isolated from cat feces in Songkhla, Southern Thailand. Vet. Parasitol. Reg. Stud. Rep. 2018, 13, 105–109. [Google Scholar] [CrossRef]
- Bizhga, B.; Selami, F.; Shehdula, D.; Lika, E. The evaluation of Toxoplasma gondii infection in cats at tiranacity. Ann. Univ. Craiova-Agric. Montanology Cadastre Ser. 2017, 46, 50–55. [Google Scholar]
- Darde, M.L.; Bouteille, B.; Pestre-Alexandre, M. Isoenzyme analysis of 35 Toxoplasma gondii isolates and the biological and epidemiological implications. J. Parasitol. 1992, 78, 786–794. [Google Scholar] [CrossRef]
- Dubey, J.P.; Velmurugan, G.V.; Ulrich, V.; Gill, J.; Carstensen, M.; Sundar, N.; Kwok, O.C.; Thulliez, P.; Majumdar, D.; Su, C. Transplacental toxoplasmosis in naturally-infected white-tailed deer: Isolation and genetic characterisation of Toxoplasma gondii from foetuses of different gestational ages. Int. J. Parasitol. 2008, 38, 1057–1063. [Google Scholar] [CrossRef]
- Lehmann, T.; Marcet, P.L.; Graham, D.H.; Dahl, E.R.; Dubey, J.P. Globalization and the population structure of Toxoplasma gondii. Proc. Natl. Acad. Sci. USA 2006, 103, 11423–11428. [Google Scholar] [CrossRef] [Green Version]
- Dubey, J.P.; Graham, D.H.; da Silva, D.S.; Lehmann, T.; Bahia-Oliveira, L.M. Toxoplasma gondii isolates of free-ranging chickens from Rio de Janeiro, Brazil: Mouse mortality, genotype, and oocyst shedding by cats. J. Parasitol. 2003, 89, 851–853. [Google Scholar] [CrossRef]
- Nabi, H.; Islam, S.; Bajwa, A.A.; Rashid, I.; Akbar, H.; Shehzad, W.; Durrani, A. Sequence Analysis of SAG2 of Feline Toxoplasma gondii Oocysts in Pakistan. Pak. J. Zool. 2017, 49, 6. [Google Scholar] [CrossRef]
- Carme, B.; Demar, M.; Ajzenberg, D.; Darde, M.L. Severe acquired toxoplasmosis caused by wild cycle of Toxoplasma gondii, French Guiana. Emerg. Infect. Dis. 2009, 15, 656–658. [Google Scholar] [CrossRef] [PubMed]
- Saeij, J.P.; Boyle, J.P.; Coller, S.; Taylor, S.; Sibley, L.D.; Brooke-Powell, E.T.; Ajioka, J.W.; Boothroyd, J.C. Polymorphic secreted kinases are key virulence factors in toxoplasmosis. Science 2006, 314, 1780–1783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrmann, D.C.; Pantchev, N.; Vrhovec, M.G.; Barutzki, D.; Wilking, H.; Frohlich, A.; Luder, C.G.; Conraths, F.J.; Schares, G. Atypical Toxoplasma gondii genotypes identified in oocysts shed by cats in Germany. Int. J. Parasitol. 2010, 40, 285–292. [Google Scholar] [CrossRef]
- Lass, A.; Ma, L.; Kontogeorgos, I.; Zhang, X.; Li, X.; Karanis, P. First molecular detection of Toxoplasma gondii in vegetable samples in China using qualitative, quantitative real-time PCR and multilocus genotyping. Sci. Rep. 2019, 9, 17581. [Google Scholar] [CrossRef]
- Armand, B.; Solhjoo, K.; Kordshooli, M.S.; Davami, M.H.; Pourahmad, M.; Orfaee, V. Toxoplasma gondii Type I, predominant genotype isolated from sheep in South of Iran. Vet. World 2017, 10, 386–392. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).