Abstract
More than one million cases of leptospirosis occur across the globe annually, resulting in about 59,000 deaths. Dogs are one of the most important reservoirs of Leptospira species and play an important role in transmitting the pathogen to humans. Many of these infections are controlled by routine vaccination that has reduced the possible reintroduction of leptospiral serovars into the human population. However, it is still not clear how a vaccinated dog can become infected with one or more Leptospira serovars contained in the vaccine formulation and thus against which it should be immunized. Here, we present the case of an asymptomatic dog who developed leptospiral infection despite being vaccinated. This unusual case emphasizes the substantial impact of immunization on mitigating the acute signs of the disease, even while providing limited protection against infection. Further studies will be required to better understand the role of dogs in the environmental circulation of leptospiral serovars in Sardinia. Asymptomatic leptospiral infection in vaccinated dogs should be considered to allow for better diagnosis and management of the infection. This will be essential for preventing Leptospira outbreaks in the future.
1. Introduction
Canine leptospirosis is a zoonotic disease caused by spirochetes of the genus Leptospira. Although serovars Canicola and Icterohaemorrhagiae have been historically associated with canine leptospirosis in Europe [1], other serovars, such as Grippotyphosa, Australis, Hardjo, and Bratislava, have been detected in European dogs [2,3,4]. Because leptospiral infection is frequently asymptomatic in dogs [5,6], through a combination of being undiagnosed or diagnosed retrospectively, the disease is probably underdiagnosed, likely leading to a significant underestimation of the true burden of the disease. Vaccination is the most effective measure to control the spread of the infection in dogs and to prevent the development of leptospiral infection in humans [7,8]. In Europe, canine Leptospira vaccines containing antigens from different serogroups have been available for more than 60 years [9]. Currently, the vaccine commercially available in Italy is Nobivac L4 (MSD Animal Health), which is composed of four Leptospira interrogans strains, representing the serogroups Canicola, Icterohaemorrhagiae, Australis, and Grippotyphosa (http://www.ema.europa.eu; accessed on 28 December 2021). This inactivated bacterial vaccine has been shown to work well in dogs and to play a significant role in the development of protective immunity against leptospirosis [10]. However, cases of vaccinated dogs infected by Leptospira serogroups included in the vaccine formulation have been previously reported [11], highlighting that vaccination protects animals from clinical disease but may not prevent the infection if the animal is exposed to high bacterial load [12]. Leptospirosis should be considered as a potential cause of granulomatous hepatitis, even in vaccinated dogs and dogs without evidence of renal dysfunction or seroconversion [13]. Previous results from a serological study conducted in Sardinia indicated the presence of pathogenic Leptospira serotypes, including L. interrogans serovar Canicola, in vaccinated dogs [14]. In the same study, Piredda et al. speculated on the role of dogs in the possible transmission of Leptospira serovars to humans and on dogs’ contribution to make this region a zoonotic hotspot of the disease.
In this report, we present a case of canine leptospirosis documented by complete clinical presentation, diagnostic findings, and isolation of the etiological agent from the urine of one vaccinated dog from Sardinia, Italy.
2. Case Report
A 7-year-old, sterilized female, Cirneco dell’Etna dog, weighing 18 kg, was presented to the Clinical Hospital of the University of Sassari for a regular routine check-up in April 2020. The dog, used in pet therapy, underwent clinical examination and blood analysis once a year to check overall health. According to the WSAVA guidelines [15], routine vaccinations (including Novibac L4 against canine leptospirosis) were up to date. Heartworm prophylactic treatment based on ivermectin (6 µg/kg) and pyrantel pamoate (5 mg/kg) was also given orally once a month.
The dog appeared well, and physical examination showed no abnormalities. Lymph nodes were normal in shape and of average size. Temperature (38.5 °C), respiratory rate (20 breaths per minute), heart rate (80 beats per minute), and capillary refill time (<2 s) were normal. Palpation of the abdomen did not reveal any abnormalities. The dog was well-hydrated, and membranes were pink and moist. Appetite was normal and weight stable. Stools were of normal consistency and volume.
Hematology and chemistry were performed at day 0 using an automated hematology system (Dimension RxL Max Integrated Chemistry System (Siemens Healthcare Diagnostics, Milan Italy)) and a chemistry analyzer (Dimension RXL, Siemens S.p.A., Milan, Italy), respectively. Electrophoresis of serum proteins was also performed using the GENIO S device (Interlab s.r.l., Rome, Italy). Biochemical analyses indicated a significant increase in liver enzymes with abnormal values of transaminases, consistent with liver dysfunction. Creatinine levels were also altered (Table 1), while no abnormalities were detected on complete blood count (CBC) and serum protein electrophoresis. The dog was then subjected to abdominal ultrasound, which was unremarkable. Based on these results, a specific supplement for liver problems (containing Silybum 160 mg, DL-methionine 60 mg, Cynara scolymus 50 mg, curcuma 50 mg, and phosphatidylcholine 30 mg) was given twice daily. A commercial dog food for liver disease was also utilized.

Table 1.
Metabolic results at day 0 indicating altered values of alkaline phosphatase, transaminase, and creatinine.
Seven days after the first sample, serum was collected from whole blood, and indirect immunofluorescent antibody (IFA) titers for A. phagocytophilum, Ehrlichia canis, Leishmania infantum, Toxoplasma gondii, Rickettsia spp., and Bartonella spp. were quantified. Microscopic agglutination test (MAT) for Leptospira was also performed at the seroimmunology laboratory (Istituto Zooprofilattico Sperimentale Sardegna, Sassari, Italy). The antibody titers were tested against eight different serogroups, as previously described [16]. All serological tests and the leptospirosis MAT were negative. At the same time, genomic DNA extraction and real-time PCR (qPCR) targeting the lipL32 gene specific for pathogenic Leptospira from urine samples were performed, as previously described [14]. A total of 1000 μL of urine was suspended in EMJH-fluorouracil semisolid medium at 28 °C and cultured for a period of three months, as previously reported [17]. Amplification of the gene target specified above was achieved from the dog urine, while we failed to isolate the Leptospira strain from urine culture.
A diagnosis of Leptospira was made based on the qPCR-positive result, and the dog was immediately treated with intramuscular penicillin (0.3 mg/10 kg once daily for 21 days). Hematology and chemistry were performed at days 7, 15, 30, 45, 60, and 90 following treatment initiation. Serum creatinine levels returned to normal at day 7 after starting the penicillin therapy, while glutamic pyruvic transaminase and alkaline phosphatase returned to within the reference interval by days 30 and 45 after initiation of antibiotics, respectively (Table 2).

Table 2.
Metabolic profile in dog monitored at day 7, 15, 30, 45, 60, and 90 after the start of penicillin therapy.
Twenty-one days after the penicillin treatment, further blood and urine samples were collected due to the presence of leptospiruria and leptospiremia in the infected dog as determined by qPCR, MAT analyses, and urine-culture. The qPCR and MAT were negative. After approximately 40 days, positive urine-culture was obtained, and treatment with doxycycline (5 mg/kg orally twice daily for 21 days) was given.
Partial sequencing of the rrs gene of strain isolated from the dog’s urine resulted in a 100% identity match with analogous sequences of Leptospira interrogans serovar Canicola present in GenBank. Multi-locus sequence-typing (MLST) analysis was applied to the leptospiral DNA that had been isolated following the seven-loci scheme proposed by Boonsilp et al. [18], revealing sequence type 37 (ST = 37) belonging to L. interrogans serovar Canicola.
For whole-genome sequencing of the Leptospira strain that had been isolated from the dog’s urine, an Illumina library was prepared with the Illumina Nextera XT kit, following the manufacturer’s instructions, then checked with BioAnalyzer (Agilent, Santa Clara, CA, USA) and quantified using Qubit fluorometer (Thermo Fisher, Bedford, MA, USA). The library was finally pooled with other samples, loaded onto the MiSeq system (Illumina, Inc., Ann Arbor, MI, USA) and sequenced following the V3-300PE strategy, producing 655,676 paired-end reads. Raw reads were processed using Cutadapt (v1.16) [19] in order to remove short (length < 150 bp) and low-quality (q < 30) reads and residual adapter sequences. Cleaned reads were assembled using SPAdes (v3.12.0) [20] with standard parameters and assembly metrics were calculated using QUAST (v4.6.3) [21]. The estimated genome size was 4,377,069 bp, with a mean coverage of 32X, L50 of 140, and N50 of 9044. The assembled sequences are available at the NCBI database under accession number JAJSDI000000000. Finally, quantitative evaluation of genome assembly was done using Busco (v5.1.2) against the spirochaetia_odb10 lineage. In parallel, the MetaPhlAn 3 [22] was used with standard parameters to characterize the sequenced isolate at species level and to evaluate the presence of possible contaminants. Lineage analysis revealed complete overlap with the Spirochetia class (239/239 BUSCO marker), and this finding was also supported by MetaPhlAn, which detected no contamination. In addition, PhyloPhlAn 3 [23] was applied in accurate mode with low diversity for the large-scale phylogenetic profiling of 14 Leptospira genomes, including isolate 769341 and eight Canicola serogroup isolates, available in the NCBI database (accessed: 4 November 2021). Thus, the isolate 769341 was included within the pathogenic isolates belonging to Leptospira interrogans serovar Canicola serogroup. The final refined tree was drawn with iTOL [24] (Figure 1).
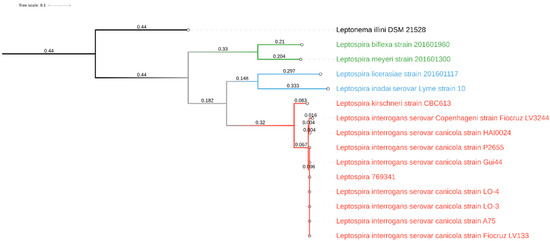
Figure 1.
Graphical representation of the phylogenetic tree drawn with iTOL based on 14 whole-genome sequences of Leptospira spp. The various clades were colored differently according to their major group: pathogens in red, intermediates or opportunists in blue, and nonpathogens in green. Leptonema illini was used as the outgroup.
3. Discussion
Dogs can act as carriers for pathogenic Leptospira strains, maintaining the bacteria in their renal tubules and eliminating them through urine into the environment for prolonged periods after infection [7,25]. Proper management of chronically infected dogs should be implemented to reduce environmental contamination; however, the identification of such individuals remains challenging [26]. In this report, the case of one asymptomatic dog infected with L. interrogans Canicola, a serovar included in the vaccine formulation, has been documented. The dog from this case report had received the vaccine four months prior to disease detection (December 2019). Although the vaccines represent a key point in the control of canine leptospirosis and in preventing clinical disease and renal carrier status in about 85% of immunized dogs [27], vaccination failure has been described in previous studies [11]. Results from this study corroborate the hypothesis that vaccination does not always guarantee complete protection against Leptospira serovars, and that vaccines are designed to prevent the disease, but not the infection [28]. The presence of leptospiral DNA in vaccinated dogs has previously been reported on the island [29], raising important questions regarding the role of dogs in the epidemiology of leptospirosis. In the dog under study, the absence of clinical signs consistent with leptospiral infection, along with culture isolation of Leptospira, suggested that the infection was asymptomatic, and that the microorganism may remain in the body after recovery from acute infection. Vaccinated dogs could represent a zoonotic risk, as described by several authors [8,26,30,31,32]. Although the serovar Canicola detected in this study has been described in humans [33,34], the dog owners from this study tested negative for leptospiral infection. Moreover, even if anemia is common in dogs with leptospirosis [35], in this study we did not find significant changes in CBC of the infected dog. Transaminase levels were elevated, with increases of alkaline phosphatase (321 U/L), transaminase (GOT 125 U/L and GPT 555 U/L), and creatinine. (1.55 mg/dl). These results were in accordance with other studies in which the increase of liver values has been associated with infected dogs. In particular, the increase of aspartate aminotransferase–alanine aminotransferase levels were indicative of leptospiral infection [36]. Liver function typically returns to normal values after treatment of leptospirosis with antibiotics, as observed in this case report.
In this study, leptospiral isolation from dog urine was reported, confirming that, although culture of leptospires is labor-intensive and isolation can take 8 to 12 weeks, isolation of a leptospiral strain can be achieved. Moreover, isolation of the bacterium in culture is essential to determine the phylogenetic behavior of the genus [37] and to specify the disease [38,39]. Further, our study showed that Leptospira was isolated from dog urine after penicillin treatment. Previous research has reported that penicillin is effective during the leptospiremic phase but was not always efficacious at eliminating leptospiral organisms from the renal tubules [40,41,42]. Since persistent leptospiruria occurs in dogs after penicillin treatment, a course of doxycycline is routinely used due to eliminate leptospiruria and prevent zoonotic transmission [43].
This case supports the fact that leptospirosis is a complex disease in terms of diagnosis, and clinicians should evaluate the possibility of persistent leptospiruria in dogs despite penicillin treatment. Moreover, vaccines should be regularly kept up to date for the presence of new serogroups and serovars in an area. In this study, MAT failed to detect the disease, but rt-PCR analysis and subsequent isolation from urine-culture confirmed its presence. This is in agreement with previous studies that have highlighted the need to use more than one diagnostic tool for the detection of Leptospira species in reservoir hosts [4,44].
4. Conclusions
Our results suggest that, although the advent of animal vaccines against specific serotypes has reduced the incidence of transmission to humans, clinicians should be alert to the risk of Leptospira infection in vaccinated dogs and to the possibility of transmitting infection to its owners. Future studies will focus on establishing the role of dogs in the zoonotic transmission of leptospirosis.
Author Contributions
Conceptualization, I.P.; software, L.B.; validation, I.P. and V.C.; formal analysis, I.P. and V.C.; investigation, I.P., B.P., S.S. and R.C.; data curation, I.P., S.S. and L.B.; writing—original draft preparation, I.P. and V.C.; writing—review and editing, I.P., V.C., S.S., R.C. and L.B.; resources, I.P., S.S., R.C. and L.B.; visualization, I.P. and V.C.; supervision, I.P. and V.C.; project administration, I.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study adhered to strict guidelines outlined by the ethical committee of the Istituto Zooprofilattico Sperimentale della Sardegna (IZS). In addition, permission was granted by the Italian Ministry of Health (Ministero della Salute) in accordance with Council Directive 2010/63/EEC of the European Union and the Italian D.Lgs 26/2014 (protocol 1248/2015-PR), whose representatives personally oversaw that animals were handled with respect according to the laws on experimental animal care.
Informed Consent Statement
Written informed consent was obtained from the patient owner to be included in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ellis, W.A. Control of canine leptospirosis in Europe: Time for a change? Vet. Rec. 2010, 167, 602–605. [Google Scholar] [CrossRef] [Green Version]
- Mayer-Scholl, A.; Luge, E.; Draeger, A.; Nöckler, K.; Kohn, B. Distribution of Leptospira serogroups in dogs from Berlin, Germany. Vector-Borne Zoonotic Dis. 2013, 13, 200–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renaud, C.; Andrews, S.; Djelouadji, Z.; Lecheval, S.; Corrao-Revol, N.; Buff, S.; Demont, P.; Kodjo, A. Prevalence of the Leptospira serovars bratislava, grippotyphosa, mozdok and pomona in French dogs. Vet. J. 2013, 196, 126–127. [Google Scholar] [CrossRef] [PubMed]
- López, M.C.; Vila, A.; Rodón, J.; Roura, X. Leptospira seroprevalence in owned dogs from Spain. Heliyon 2019, 5, e02373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Major, A.; Schweighauser, A.; Francey, T. Increasing incidence of canine leptospirosis in Switzerland. Int. J. Environ. Res. Public Health 2014, 11, 7242–7260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuller, S.; Arent, Z.J.; Gilmore, C.; Nally, J. Prevalence of antileptospiral serum antibodies in dogs in Ireland. Vet. Rec. 2015, 177, 126. [Google Scholar] [CrossRef] [PubMed]
- Rojas, P.; Monahan, A.M.; Schuller, S.; Miller, I.S.; Markey, B.K.; Nally, J.E. Detection and quantification of leptospires in urine of dogs: A maintenance host for the zoonotic disease leptospirosis. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, J.R.; Krupka-Dyachenko, I.; Rettinger, A.L.; Dyachenko, V.; Stamm, I.; Kopp, P.A.; Straubinger, R.K.; Hartmann, K. Urinary shedding of leptospires and presence of Leptospira antibodies in healthy dogs from Upper Bavaria. Berl. Und Munch. Tierarztl. Wochenschr. 2016, 129, 251–257. [Google Scholar]
- Jull, D.J.; Heath, K.R. The evaluation of a combined L. canicola and L. icterohaemorrhagiae vaccine on hamsters and dogs. J. Small Anim. Pract. 1961, 1, 245–258. [Google Scholar] [CrossRef]
- Azócar-Aedo, L.; Monti, G. Meta-Analyses of Factors Associated with Leptospirosis in Domestic Dogs. Zoonoses Public Health 2016, 63, 328–336. [Google Scholar] [CrossRef] [PubMed]
- André-Fontaine, G.; Branger, C.; Gray, A.W.; Klaasen, H.L. Comparison of the efficacy of three commercial bacterins in preventing canine leptospirosis. Vet. Rec. 2003, 153, 165–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertasio, C.; Boniotti, M.B.; Lucchese, L.; Ceglie, L.; Bellinati, L.; Mazzucato, M.; Furlanello, T.; D’Incau, M.; Natale, A. Detection of New Leptospira Genotypes Infecting Symptomatic Dogs: Is a New Vaccine Formulation Needed? Pathogens 2020, 9, 484. [Google Scholar] [CrossRef] [PubMed]
- McCallum, K.E.; Constantino-Casas, F.; Cullen, J.M.; Warland, J.H.; Swales, H.; Linghley, N.; Kortum, A.J.; Sterritt, A.J.; Cogan, T.; Watson, P.J. Hepatic leptospiral infections in dogs without obvious renal involvement. J. Vet. Intern. Med. 2019, 33, 141–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piredda, I.; Ponti, M.N.; Piras, A.; Palmas, B.; Pintore, P.; Pedditzi, A.; Chisu, V. New Insights on Leptospira Infections in a Canine Population from North Sardinia, Italy: A Sero-Epidemiological Study. Biology 2021, 10, 507. [Google Scholar] [CrossRef]
- Day, M.J.; Horzinek, M.C.; Schultz, R.D.; Squires, R.A.; Vaccination Guidelines Group (VGG) of the World Small Animal Veterinary Association (WSAVA). WSAVA Guidelines for the vaccination of dogs and cats. J. Small Anim. Pract. 2016, 57, E1–E45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piredda, I.; Ponti, M.N.; Palmas, B.; Noworol, M.; Pedditzi, A.; Rebechesu, L.; Chisu, V. Molecular Typing of Pathogenic Leptospira Species Isolated from Wild Mammal Reservoirs in Sardinia. Animals 2021, 11, 1109. [Google Scholar] [CrossRef] [PubMed]
- Gleiser, C.A. The Laboratory Diagnosis of Leptospirosis. Am. J. Trop. Med. Hyg. 1955, 4, 158–159. [Google Scholar] [CrossRef]
- Boonsilp, S.; Thaipadungpanit, J.; Amornchai, P.; Wuthiekanun, V.; Bailey, M.S.; Holden, M.T.G.; Zhang, C.; Jiang, X.; Koizumi, N.; Taylor, K.; et al. A Single Multilocus Sequence Typing (MLST) Scheme for Seven Pathogenic Leptospira Species. PLoS Negl. Trop. Dis. 2013, 7, e1954. [Google Scholar] [CrossRef] [Green Version]
- Kechin, A.; Boyarskikh, U.; Kel, A.; Filipenko, M. cutPrimers: A New Tool for Accurate Cutting of Primers from Reads of Targeted Next Generation Sequencing. J. Comput. Biol. 2017, 24, 1138–1143. [Google Scholar] [CrossRef]
- Prjibelski, A.; Antipov, D.; Meleshko, D.; Lapidus, A.; Korobeynikov, A. Using SPAdes De Novo Assembler. Curr. Protoc. Bioinf. 2020, 70, e102. [Google Scholar] [CrossRef]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Beghini, F.; McIver, L.J.; Blanco-Míguez, A.; Dubois, L.; Asnicar, F.; Maharjan, S.; Mailyan, A.; Manghi, P.; Scholz, M.; Thomas, A.M.; et al. Integrating taxonomic, functional, and strain-level profiling of diverse microbial communities with bioBakery 3. Elife 2021, 10, e65088. [Google Scholar] [CrossRef]
- Asnicar, F.; Thomas, A.M.; Beghini, F.; Mengoni, C.; Manara, S.; Manghi, P.; Zhu, Q.; Bolzan, M.; Cumbo, F.; May, U.; et al. Precise phylogenetic analysis of microbial isolates and genomes from metagenomes using PhyloPhlAn 3.0. Nat. Commun. 2020, 11, 2500. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Khedkar, S.; Bork, P. SMART: Recent updates, new developments and status in 2020. Nucleic Acids Res. 2021, 49, D458–D460. [Google Scholar] [CrossRef] [PubMed]
- Harkin, K.R.; Roshto, Y.M.; Sullivan, J.T.; Purvis, T.J.; Chengappa, M.M. Comparison of polymerase chain reaction assay, bacteriologic culture, and serologic testing in assessment of prevalence of urinary shedding of leptospires in dogs. J. Am. Vet. Med. Assoc. 2003, 222, 1230–1233. [Google Scholar] [CrossRef]
- Miotto, B.A.; Guilloux, A.G.A.; Tozzi, B.F.; Moreno, L.Z.; Da Hora, A.S.; Dias, R.A.; Heinemann, M.B.; Moreno, A.M.; de Souza Filho, A.F.; Lilenbaum, W.; et al. Prospective study of canine leptospirosis in shelter and stray dog populations: Identification of chronic carriers and different Leptospira species infecting dogs. PLoS ONE 2018, 13, e0200384. [Google Scholar] [CrossRef] [Green Version]
- Murcia, C.A.; Astudillo, M.; Romero, M.H. Prevalence of leptospirosis in vaccinated working dogs and humans with occupational risk. Prevalencia de leptospirosis en perros de trabajo vacunados y en población humana con riesgo ocupacional. Biomedica 2020, 40 (Suppl. S1), 62–75. [Google Scholar] [CrossRef] [PubMed]
- Arent, Z.J.; Andrews, S.; Adamama-Moraitou, K.; Gilmore, C.; Pardali, D.; Ellis, W.A. Emergence of novel Leptospira serovars: A need for adjusting vaccination policies for dogs? Epidemiol. Infect. 2013, 141, 1148–1153. [Google Scholar] [CrossRef] [PubMed]
- Piredda, I.; Bertoldi, L.; Benvenuto, G.; Palmas, B.; Pedditzi, A.; Pintore, P.; Chisu, V. First Isolation and Molecular Typing of Pathogenic and Intermediate Leptospira Species from Urine of Symptomatic Dogs. Vet. Sci. 2021, 8, 304. [Google Scholar] [CrossRef] [PubMed]
- Delaude, A.; Rodriguez-Campos, S.; Dreyfus, A.; Counotte, M.J.; Francey, T.; Schweighauser, A.; Lettry, S.; Schuller, S. Canine leptospirosis in Switzerland-A prospective cross-sectional study examining seroprevalence, risk factors and urinary shedding of pathogenic leptospires. Prev. Vet. Med. 2017, 141, 48–60. [Google Scholar] [CrossRef] [Green Version]
- Sant’Anna, R.; Vieira, A.S.; Grapiglia, J.; Lilenbaum, W. High number of asymptomatic dogs as leptospiral carriers in an endemic area indicates a serious public health concern. Epidemiol. Infect. 2017, 145, 1852–1854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khorami, N.; Malmasi, A.; Zakeri, S.; Zahraei Salehi, T.; Abdollahpour, G.; Nassiri, S.M.; Nejati, A. Screening urinalysis in dogs with urinary shedding of leptospires. Comp. Clin. Path. 2010, 19, 271–274. [Google Scholar] [CrossRef]
- Ellis, W.A. Animal leptospirosis. Curr. Top. Microbiol. Immunol. 2015, 387, 99–137. [Google Scholar] [CrossRef] [PubMed]
- Dreyfus, A.; Ruf, M.T.; Mayer-Scholl, A.; Zitzl, T.; Loosli, N.; Bier, N.S.; Hiereth, S.; Ulrich, S.; Poppert, S.; Straubinger, R.K.; et al. Exposure to Leptospira spp. and Associated Risk Factors in the Human, Cattle and Dog Populations in Bhutan. Pathogens 2021, 10, 308. [Google Scholar] [CrossRef]
- Beck, A.; Huber, D.; Antolić, M.; Anzulović, Ž.; Reil, I.; Polkinghorne, A.; Baneth, G.; Beck, R. Retrospective study of canine infectious haemolytic anaemia cases reveals the importance of molecular investigation in accurate postmortal diagnostic protocols. Comp. Immunol. Microbiol. Infect. Dis. 2019, 65, 81–87. [Google Scholar] [CrossRef]
- Birnbaum, N.; Barr, S.C.; Center, S.A.; Schermerhorn, T.; Randolph, J.F.; Simpson, K.W. Naturally acquired leptospirosis in 36 dogs: Serological and clinicopathological features. J. Small Anim. Pract. 1998, 39, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.T.; Schiettekatte, O.; Goarant, C.; Neela, V.K.; Bernet, E.; Thibeaux, R.; Ismail, N.; Khalid, M.K.N.M.; Amran, F.; Masuzawa, T.; et al. Revisiting the taxonomy and evolution of pathogenicity of the genus Leptospira through the prism of genomics. PLoS Negl. Trop. Dis. 2019, 13, e0007270. [Google Scholar] [CrossRef] [Green Version]
- Director, A.; Penna, B.; Hamond, C.; Loureiro, A.P.; Martins, G.; Medeiros, M.A.; Lilenbaum, W. Isolation of Leptospira interrogans Hardjoprajitno from vaginal fluid of a clinically healthy ewe suggests potential for venereal transmission. J. Med. Microbiol. 2014, 63, 1234–1236. [Google Scholar] [CrossRef] [Green Version]
- Pinto, P.S.; Libonati, H.; Lilenbaum, W. A systematic review of leptospirosis on dogs, pigs, and horses in Latin America. Trop. Anim. Health Prod. 2017, 49, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Hetrick, K.; Harkin, K.R.; Peddireddi, L.; Henningson, J. Evaluation by polymerase chain reaction assay of persistent shedding of pathogenic leptospires in the urine of dogs with leptospirosis. J. Vet. Intern. Med. 2022, 36, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Sykes, J.E.; Hartmann, K.; Lunn, K.F.; Moore, G.E.; Stoddard, R.A.; Goldstein, R.E. 2010 ACVIM small animal consensus statement on leptospirosis: Diagnosis, epidemiology, treatment, and prevention. J. Vet. Intern. Med. 2011, 25, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuller, S.; Francey, T.; Hartmann, K.; Hugonnard, M.; Kohn, B.; Nally, J.E.; Sykes, J. European consensus statement on leptospirosis in dogs and cats. J. Small Anim. Pract. 2015, 56, 159–179. [Google Scholar] [CrossRef] [PubMed]
- Truccolo, J.; Charavay, F.; Merien, F.; Perolat, P. Quantitative PCR assay to evaluate ampicillin, ofloxacin, and doxycycline for treatment of experimental leptospirosis. Antimicrob. Agents Chemother. 2002, 46, 848–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tagliabue, S.; Figarolli, B.M.; D’Incau, M.; Foschi, G.; Gennero, M.S.; Giordani, R.; Giordani, R.; Natale, A.; Papa, P.; Ponti, N.; et al. Serological surveillance of Leptospirosis in Italy: Two-year national data (2010–2011). Vet. Ital. 2016, 52, 129–138. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).