Abstract
Diarrhea remains a public health problem in Mozambique, even with control strategies being implemented. This analysis aimed to determine the proportion and factors associated with intestinal parasitic infection (IPI) in children up to 14 years old with diarrheal disease, in the southern, central and northern regions of Mozambique. A single diarrheal sample of 1424 children was collected in hospitals and examined using the formol-ether concentration and modified Ziehl–Neelsen techniques to identify intestinal parasites using optical microscopy. Sociodemographic characteristics were obtained by questionnaires. Descriptive statistics and cross-tabulation were performed, and p-values <0.05 were considered statistically significant. A single IPI was detected in 19.2% (273/1424) of the children. Cryptosporidium spp. was the most common parasite (8.1%; 115/1424). Polyparasitism was seen in 26.0% (71/273), with the co-infection of Ascaris lumbricoides and Trichuris trichiura (26.8%; 19/71) being the most common. Age and province were related to IPI (p-value < 0.05). The highest occurrence of IPI was observed in the wet period (October to March), with 21.9% (140/640), compared to the dry period (April to September), with 16.9% (131/776) (p-value = 0.017). Cryptosporidium spp. and the combination of A. lumbricoides/T. trichiura were the main intestinal parasites observed in children hospitalized with diarrhea in Mozambique.
1. Introduction
In 2019, approximately half a million of the 5,050,000 total child deaths were due to diarrheal diseases and 53% of those deaths occurred in Sub-Saharan Africa, with a contribution of 7573 from Mozambique [1].
In Mozambique, diarrhea remains a public health problem even though strategies have been implemented since 1990, including the introduction of vaccination against rotavirus, national health week where children are vaccinated, dewormed and supplemented with vitamin A, and the improvement of water, sanitation and hygiene [2]. According to the United Nations Children’s Fund (UNICEF), Mozambique has about 320 daily deaths in children under five with malaria, respiratory infections or diarrhea [3]. Children aged between 6 and 23 months (19%) are more likely to have diarrhea compared to children under 6 months (5%) and children between 48 and 59 months (6%) in Mozambique [4].
Infectious diarrhea can be caused by multiple agents including viruses, bacteria, fungi and parasites. Among the parasites the main reported ones are Entamoeba histolytica, Giardia duodenalis, Cryptosporidium spp., Ascaris lumbricoides, Ancylostoma/Necator spp. and Trichuris trichiura [5]. Despite their worldwide distribution, Enterobius vermicularis [6] and Dientamoeba fragilis [7] have largely remained sub-diagnosed because of the limitations of the stool parasite screening methods commonly used. The symptoms and consequences of intestinal parasitic infections (IPI) include abdominal pain, intestinal obstruction, anaemia, poor appetite, worsening of nutritional status, impaired physical development and diarrhea [8].
Studies carried out in Mozambique in children up to 14 years old using microscopy between 2000 and 2013 revealed a wide range of parasites [9,10,11,12,13]. A national study conducted in school-age children (August 2005–June 2007) identified a great frequency of A. lumbricoides, T. trichiura and Entamoeba coli. Enterobius vermicularis was the fourth most common helminth found [9]. At a rural hospital in southern Mozambique, A. lumbricoides, G. duodenalis and Strongyloides stercoralis were the most frequent parasites [11]. In the south, Fonseca et al. [10] identified higher frequencies of G. duodenalis, T. trichiura and A. lumbricoides in the Hospital Central de Maputo (February and March 2009). In Sofala, one of the central provinces in Mozambique (June and August 2007), Meurs et al. [12] reported T. trichiura, A. lumbricoides and S. stercoralis as the most common parasites, while in the northern region of the country, in Nampula (2012 and 2013), Ferreira et al. [13] found G. duodenalis, S. stercoralis and Cryptosporidium spp. to be the most common; however, for G. duodenalis diagnosis, an Immunochromatographic assay was also applied in malnourished and HIV-positive children.
Among the protozoans, Dientamoeba fragilis was reported in Nampula Province in the northern region of the country in school-age children [14].
Except for a study developed nationwide in 2009, all other studies were conducted mainly in the southern region of the country. Other studies have been restricted to certain age groups (under five years old) or limited to children with some co-morbidities (HIV, malnutrition), and in some cases were carried out in children without suggestive symptoms. In this perspective, we used the data from the National Surveillance of Diarrhea (ViNaDia), implemented in 2014, with the aim of determining the burden and aetiology in children admitted with diarrhea. In this analysis, we aimed to describe the epidemiology of parasites in children up to 14 years of age in four of the eleven provinces of Mozambique, using a simple and inexpensive method—optical microscopy.
In addition, our data provide information regarding the variation in the distribution of intestinal parasites between 2014 and 2019.
2. Results
2.1. Characteristics of the Participants
Of the 2420 recruited children, 1424 provided a sufficient faecal sample for the screening techniques. Males accounted for 58.3% (830/1424) of these children, with 47.9% (682/1424) being below 12 months of age and 52.3% (745/1424) from Maputo (Table 1).

Table 1.
Demographic characteristics of children hospitalized with diarrhea in Mozambique, May 2014 to December 2019.
2.2. Intestinal Parasite Infections
Intestinal parasites were identified in 19.2% (273/1424; 95% CI: 17.2–21.3) and multiple intestinal parasite infections were detected in 26.0% (71/273; 95% CI: 21.1–31.4) of the children participating in the study.
The protozoan Cryptosporidium spp. was the most common parasite (8.1%; 115/1424) observed, followed by the helminth T. trichiura (3.8%; 54/1424) (Figure 1). Up to 39 combinations of multiple parasitic infections were recorded, ranging from two to four parasites (Figure 2). The most common parasitic combination was between the helminths A. lumbricoides and T. trichiura (26.8%; 19/71).
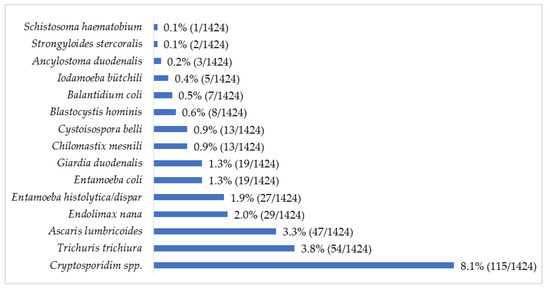
Figure 1.
Relative frequency (%) of intestinal parasites in children with diarrhea in Mozambique, May 2014 to December 2019.
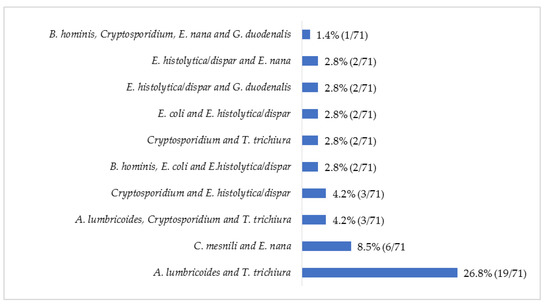
Figure 2.
Relative frequency (%) of the most common multiple parasitic infections in children with diarrhea, May 2014 to December 2019.
2.3. Factors Associated with Intestinal Parasite Infections
Age and province were related to IPI (p-value < 0.05), which was more common in children older than five years (30.8%; 20/65) and in children from Maputo (23.9%; 178/745) (Table 2). Multiple parasitic infections were more common in the provinces of Maputo (33.7%; 60/178) and Zambézia (28.6%; 6/21), followed by Nampula (3.6%; 2/56) and Sofala (16.7%; 3/18) (p-value < 0.001; Table 2).

Table 2.
Cross-tabulation between sociodemographic factors and intestinal parasitic infections in children with diarrhea in Mozambique, May 2014 to December 2019.
2.4. Parasitic Distribution by Provinces
The province of Sofala had the highest frequencies of the three most common parasites observed in the study: Cryptosporidium spp. 10.3% (8/78), T. trichiura 9.0% (7/78) and A. lumbricoides 5.1% (4/78). Despite this finding, all other parasites, with the exception of G. duodenalis and Cystoisospora belli, were not observed in this province, contrary to Maputo, which presented the highest parasite distribution (Figure 3). In Nampula Province, no G. duodenalis infections were diagnosed, but it was the only province where S. stercoralis was diagnosed (Figure 3).
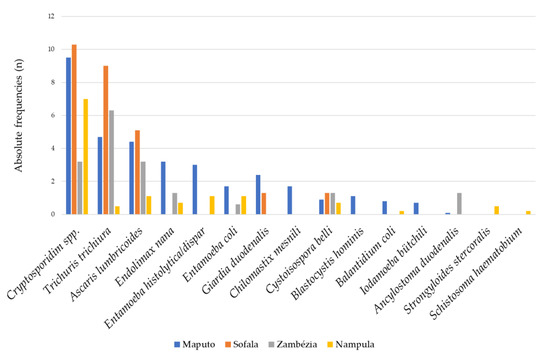
Figure 3.
Relative frequency (%) of individual intestinal parasitic infections by province in children with diarrhea in Mozambique, May 2014 to December 2019.
2.5. Evaluation of the Distribution of Intestinal Parasites during the Wet and Dry Periods
The overall frequency of parasites observed during the rainy period was 21.9% (140/640; 95% CI: 18.8–25.2) and during the dry period it was 16.9% (131/776; 95% CI: 14.4–19.6) (p-value = 0.017).
The peaks of infection by Cryptosporidium spp. were mostly observed in the wet period during the study by 10.0% (64/640; 95% CI: 7.9–12.5), compared to 6.6% in the dry periods (51/776; 95% CI: 5.0–8.5; p-value = 0.019).
G. duodenalis was more common in the dry period, with 1.9% (15/776; 95% CI: 1.1–3.1), and was less observed in the wet period, at 0.6% (4/640; 95% CI: 0.2–1.5); p-value = 0.033). Regarding T. trichiura, there were no significant differences in infection between the wet and dry periods (p-value = 0.657; Figure 4).
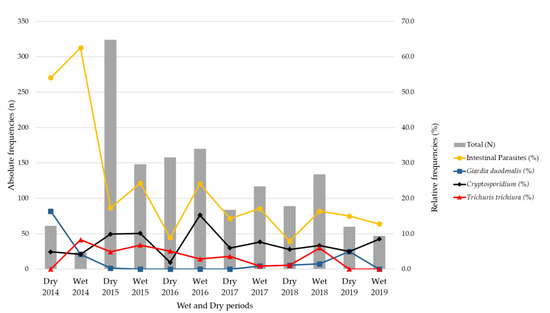
Figure 4.
Relative frequency (%) of intestinal parasitic infections and the most frequent parasites (Cryptosporidium spp., Trichuris trichiura and Giardia duodenalis) by wet and dry period in children with diarrhea in Mozambique, May 2014 to December 2019.
3. Discussion
One in five children with diarrhea was infected with at least one intestinal parasite. This finding was lower than that found in Nampula, which was 31.6% [13], and higher than that found in Hospital Central de Maputo, at 16.1% [10]. These differences can be explained by the different testing methods applied, causes of hospitalization and health unit level, inclusion criteria and possibly the local epidemiological conditions. A significant increase in infection was observed in children from Maputo and in those older than 59 months. An increase in exposure to the environment (soil, water, animals) associated with inadequate hygiene practices can help explain this result in this age group compared to the younger ones. It has also been proven that the youngest (breastfeeding children) are protected by natural immunological mechanisms decreasing the incidence as well as the duration of diarrhea [15]. Maputo was also the province with the greatest parasitic variability observed. This outcome may be due to the fact that this province had more hospitals involved in the study compared to the other provinces, along with a greater number of children tested.
In Mozambique, school starts at age six and the current strategy to control parasitic infections is focused on the school-age population. The Ministry of Health has been following WHO guidelines for preventive chemotherapy in schools since 2009 [16]. According to a geospatial analysis of the prevalence and intensity of soil-transmitted helminth infections in children, Sub-Saharan Africa has levels above the 2% threshold of the estimated prevalence of moderate-to-heavy infection. From this perspective, it is necessary to reinforce intervention to better target preventive chemotherapy to children at high risk of morbidity in order to monitor progress towards the soil-transmitted helminth targets for 2020 and 2030 [17].
One study carried out recently in Maputo markets reported that 29.3% of the horticultural products sampled presented at least one parasite (pathogenic and non-pathogenic) [18]. Therefore, it is also necessary to reinforce the improvement of sanitation and encourage healthy behaviours to avoid reinfections.
Protozoa were the most common group of parasites found, with an emphasis on Cryptosporidium spp., G. duodenalis, E. histolytica/dispar, Cystoisospora belli and Balantidium coli for being pathogenic. Even at low frequencies, pathogenic helminth infections (T. trichiura, A. lumbricoides, A. duodenalis and S. stercoralis) were recorded; these have been documented as predictors of growth retardation and impaired cognitive development, anaemia and vitamin A deficiency [19].
Cryptosporidium spp. was the most common parasite detected. This protozoon is known to cause diarrhea and malnutrition in young children in developing countries, and is associated with diarrhea outbreaks. In the study carried out by the Global Enteric Multicentre Study (GEMS) in the south of Mozambique, Cryptosporidium spp. was found to be the second most important cause of diarrhea [20]. In two hospitals in Maputo city, by microscopy, Cryptosporidium spp. was observed in 11% of the children sampled with diarrhea [21]. Its high prevalence can be justified by its opportunistic nature mainly in a country where the rates of HIV infection and malnutrition are higher [22]. G. duodenalis was less frequent than reported by other studies [10,13,23,24]. One of the possible reasons is the methods used, especially the immunological ones, which detect cyst epitopes, as opposed to the parasitological methods used by us, which only identify intact cysts and trophozoites. Additionally, the biology of the vegetative forms of G. duodenalis, the trophozoites, with the consistency of the stool samples collected may also have influenced the result. Trophozoites are considered to be the most common forms in watery and newly emitted stool; they are therefore not very resistant to the external environment [25]. The stool samples from our study were collected from children with diarrhea, and with the exception of Maputo, samples from the remaining provinces were sent to Maputo once a week and frozen, which probably destroyed the trophozoites and may have resulted in false negatives.
In this analysis we also observed that polyparasitism was prevalent, with the combination of A. lumbricoides and T. trichiura being the most common. A. lumbricoides was also reported as the most prevalent agent in a study conducted in South Africa, a country neighbouring Mozambique [26]. The occurrence of polyparasitism in this analysis may be due to the environmental contamination (poor sanitation, water supply provided), bad hygiene practices (poor hand washing, inadequate disposal of waste) as well as poverty. Individuals harbouring co-infections are usually at higher risk of significant morbidity than those infected with only one parasite species [27].
Mozambique has a tropical climate, with two periods: a cold-dry/winter period and a wet/summer period [28]. The wet periods are characterized by continuous heavy rains with flooding of residential and peri-urban areas. Our data, in general, indicate that parasitic infections were more common in wet periods. Among the analysed parasites, Cryptosporidium showed a significant seasonal pattern. These results are in agreement with studies in Kenya [29] and Nampula [30]. Cryptosporidium infection cases may be associated with floods and accidental ingestion of infectious forms, contamination of drinking water, unsafe treatment of sewage water or even the recycling of sewage for agriculture—situations that occur more often during the wet periods. Giardia duodenalis was common in dry periods, which contrasts with the study conducted in Nampula [30]. In Ghana, no significant association was found [31]. Although there was an association, between the dry period and giardiasis in this study, their interpretation must be performed carefully, given the smaller positive sample size found in the current study.
Our study had limitations, such as the lack of knowledge of the deworming status of the recruited children and the collection of one stool sample, which may have underestimated the real rate of infection by parasite once intestinal protozoa are irregularly shed [13,32]. Another limitation is related with the fact that samples from Sofala, Zambézia and Nampula were frozen before being sent to Maputo, where the analyses were carried out, a process which could have destroyed the diagnostic forms of the parasites. It is also important to mention the non-use of a cellulose tape slide test, a specific collection technique for Enterobius vermicularis, and the non-use of trichrome staining or modified iron-haematoxylin for Dientamoeba fragilis, which contributed significantly to its non-diagnosis and report in this study.
4. Materials and Methods
4.1. Design and Study Area
A cross-sectional hospital-based surveillance in the three regions of Mozambique was conducted in six health facilities from the National Diarrhea Surveillance (ViNaDia): in the southern region, Hospital Central de Maputo, Hospital Geral José Macamo and Hospital Geral de Mavalane in Maputo; in the centre, the Hospital Central da Beira in Sofala and the Hospital Geral de Quelimane in Zambézia and in the northern region, the Hospital Central de Nampula in Nampula. The sampling was carried out from May 2014 to December 2019, taking into account the cold/dry periods that occur from April to September and the wet season from October to March [28].
4.2. Population and Laboratory Procedures
The study population included 2420 children aged up to 14 years with diarrhea whose guardians had provided informed consent. Of these children, 1424 provided a sufficient faecal sample and were included in the present analysis. Children with nosocomial diarrhea were not eligible. Diarrhea was defined as the passage of three or more loose or liquid stools per day [33]. Sociodemographic data were obtained through a questionnaire administered by the health technicians to the caregivers or legal guardians of the participants.
A single stool sample was collected from each participant in a screw-capped container properly identified. The samples collected in Maputo were kept at 2 °C to 8 °C and sent on the same day to the laboratory of parasitology of the National Institute of Health/Instituto Nacional de Saúde–Moçambique (INS). The samples from the central and northern regions were kept at −20 °C and sent to the INS once a week. After delivery to the INS, the samples were subjected to the formalin-ether concentration technique for identification of intestinal parasites in general and to the modified Ziehl–Neelsen technique specifically for the identification of coccidian by optical microscopy [34]
Briefly, the presence of trophozoites, eggs and larvae of the parasites was identified by the formol-ether technique as described by the World Health Organization (WHO). The formol-ether technique consists in using 1 g of stool suspended in 10% formalin and ether and centrifuging it at 2500 rotation per minute. After centrifugation, the bottom was later used for observation in an optic microscopy after adding Lugol’s iodine solution. Parasites were observed in objective 10× and then changed to 40×.
The diagnosis of coccidian oocysts in stools was performed using the modified Ziehl–Neelsen stain method as described by the WHO [34]. This technique was performed by adding two smears in the slide, one from the fresh stool and the second from the formol-ether sediment. The smears were fixed with methanol and stained by adding a fuchsin solution, followed by an alcohol–acid solution. Finally, malachite green solution was added, and the preparation examined under the optical microscope at objective 100×.
4.3. Data Analysis
The data were analysed using the Statistical Package for the Social Sciences, Armok, NY, USA: IBM Corp, 2011, version 26.0 (IBM SPSS). All data collected were entered twice by two independent data entry typists and compared to evaluate consistency, prior analyses. Descriptive statistics were used to summarize population characteristics. Proportions and corresponding Jeffrey’s 95% confidence intervals (CI) were estimated for each infection and multiple infections. Bivariate analyses between dependent variables (infection by an intestinal parasite or infection by multiple parasites) and independent variables (sex, age and province) were conducted. Pearson’s Chi-square test or, if the assumptions were not met, the alternative Fisher’s exact test was used to identify factors related to the dependent variables, and the Mann–Whitney U test was used when the independent variable was quantitative. p-values < 0.05 were considered statistically significant.
5. Conclusions
In the present study, one in five children was infected with at least a parasite, and of these, one in four presented co-infection with more than one parasite. Pathogenic intestinal parasites were found to be circulating in the Mozambican paediatric population, with Cryptosporidium spp. and T. trichiura being the most common. Age was associated with IPI, and children in Maputo had the highest prevalence and the greatest parasite diversity. In evaluating some studies carried out in the country, it can be concluded that, in spite the effort of the governmental authorities, the frequency of IPI in Mozambique has remained unchanged and continues to be an indicator of poor hygiene and sanitation.
From this perspective, we recommend improving basic sanitation and runoff from rainwater. Awareness and education about individual and community hygiene practices with a special focus on kindergartens and schools will help to create a healthier society.
Author Contributions
Conceptualization, I.C.-M. and N.d.D.; methodology, O.L.N., I.C.-M. and A.F.L.B.; validation, O.L.N., I.C.-M., M.L.L. and O.M.; formal analysis, A.F.L.B. and A.C.; investigation, I.C.-M., A.F.L.B., A.C. and N.d.D.; resources, N.d.D.; data curation, A.F.L.B. and A.C.; writing—original draft preparation, O.L.N. and A.F.L.B.; writing—review and editing, O.L.N., I.C.-M., A.F.L.B., A.C., M.L.L., O.M. and N.d.D.; visualization, I.C.-M., A.F.L.B., A.C. and N.d.D.; supervision, N.d.D.; project administration, N.d.D.; funding acquisition, N.d.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by funds from the European Foundation Initiative for African Research into Neglected Tropical Diseases (EFINTD, grant number 98539), the World Health Organization, Deutsche Forschungsgemeinschaft (DFG, grant number JO369/5-1) and The Global Vaccine Alliance Initiative through Health System Strengthening. O.N., PhD, is supported by Camões—Instituto da Cooperação e da Língua.
Institutional Review Board Statement
The protocol was approved by the Mozambique National Bioethics Committee for Health (IRB00002657, reference Nr. 348/CNBS/13).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors wish to thank the caretakers for allowing their children to enrol in the surveillance. For their efforts in the study procedures the authors thank all the health personnel involved.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Paulson, K.R.; Kamath, A.M.; Alam, T.; Bienhoff, K.; Abady, G.G.; Abbas, J.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abd-Elsalam, S.M.; et al. Global, regional, and national progress towards Sustainable Development Goal 3.2 for neonatal and child health: All-cause and cause-specific mortality findings from the Global Burden of Disease Study 2019. Lancet 2021, 398, 870–905. [Google Scholar] [CrossRef]
- Chissaque, A.; de Deus, N.; Vubil, D.; Mandomando, I. The Epidemiology of Diarrhea in Children Under 5 Years of Age in Mozambique. Curr. Trop. Med. Rep. 2018, 5, 115–124. [Google Scholar] [CrossRef]
- Unicef The Children in Mozambique. Situation of the Children in Mozambique. Available online: https://www.unicef.org/mozambique/en/children-mozambique (accessed on 15 January 2021).
- Ministerio da Saude (MISAU); Instituto Nacional de Estatística (INE); ICF International (ICFI). Moçambique Inquérito Demográfico e de Saúde 2011; MISAU, INE e ICFI; Maputo: Calverton, MD, USA, 2013. [Google Scholar]
- Harhay, M.O.; Horton, J.; Olliaro, P.L. Epidemiology and control of human gastrointestinal parasites in children. Expert Rev. Anti. Infect. Ther. 2010, 8, 219–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burkhart, C.N.; Burkhart, C.G. Assessment of frequency, transmission, and genitourinary complications of enterobiasis (pinworms). Int. J. Dermatol. 2005, 44, 837–840. [Google Scholar] [CrossRef] [PubMed]
- Pietilä, J.-P.; Meri, T.; Siikamäki, H.; Tyyni, E.; Kerttula, A.-M.; Pakarinen, L.; Jokiranta, T.S.; Kantele, A. Dientamoeba fragilis—the most common intestinal protozoan in the Helsinki Metropolitan Area, Finland, 2007 to 2017. Eurosurveillance 2019, 24, 1800546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Soil-Transmitted Helminth Infections. Available online: https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections (accessed on 7 January 2021).
- Augusto, G.; Nalá, R.; Mapaco, L.; Casmo, V.; Sabonete, A.; Mapaco, L.; Monteiro, J. Geographic Distribution and Prevalence of Schistosomiasis and Soil-Transmitted Helminths among Schoolchildren in Mozambique. Am. J. Trop. Med. Hyg. 2009, 81, 799–803. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, A.M.; Fernandes, N.; Ferreira, F.S.; Gomes, J.; Centeno-Lima, S. Intestinal parasites in children hospitalized at the Central Hospital in Maputo, Mozambique. J. Infect. Dev. Ctries. 2014, 8, 786–789. [Google Scholar] [CrossRef]
- Mandomando, I.M.; Macete, E.V.; Ruiz, J.; Sanz, S.; Abacassamo, F.; Vallès, X.; Sacarlal, J.; Navia, M.M.; Vila, J.; Alonso, P.I.; et al. Etiology of diarrhea in children younger than 5 years of age admitted in a rural hospital of southern Mozambique. Am. J. Trop. Med. Hyg. 2007, 76, 522–527. [Google Scholar] [CrossRef] [Green Version]
- Meurs, L.; Polderman, A.M.; Vinkeles Melchers, N.V.S.; Brienen, E.A.T.; Verweij, J.J.; Groosjohan, B.; Mendes, F.; Mechendura, M.; Hepp, D.H.; Langenberg, M.C.C.; et al. Diagnosing Polyparasitism in a High-Prevalence Setting in Beira, Mozambique: Detection of Intestinal Parasites in Fecal Samples by Microscopy and Real-Time PCR. PLoS Negl. Trop. Dis. 2017, 11, e0005310. [Google Scholar] [CrossRef]
- Ferreira, F.S.; Pereira, F. da L.M.; Martins, M. do R.O. Intestinal parasitic infections in children under five in the Central Hospital of Nampula, Northern Mozambique. J. Infect. Dev. Ctries. 2020, 14, 532–539. [Google Scholar] [CrossRef]
- Guidetti, C.; Ricci, L.; Vecchia, L. Aetiology of intestinal parasites in a sample of students from Mozambique. Le Infez. Med. 2011, 19, 157–165. [Google Scholar]
- Lamberti, L.M.; Walker, C.L.F.; Noiman, A.; Victora, C.; Black, R.E. Breastfeeding and the risk for diarrhea morbidity and mortality. BMC Public Health 2011, 11, 515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Soil-Transmitted Helminthiases: Eliminating Soil-Transmitted Helminthiases as a Public Health Problem in Children: Progress Report 2001–2010 and Strategic Plan 2011–2020; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Sartorius, B.; Cano, J.; Simpson, H.; Tusting, L.S.; Marczak, L.B.; Miller-Petrie, M.K.; Kinvi, B.; Zoure, H.; Mwinzi, P.; Hay, S.I.; et al. Prevalence and intensity of soil-transmitted helminth infections of children in sub-Saharan Africa, 2000–18: A geospatial analysis. Lancet Glob. Health 2021, 9, e52–e60. [Google Scholar] [CrossRef]
- Salamandane, C.; Lobo, M.L.; Afonso, S.; Miambo, R.; Matos, O. Occurrence of Intestinal Parasites of Public Health Significance in Fresh Horticultural Products Sold in Maputo Markets and Supermarkets, Mozambique. Microorganisms 2021, 9, 1806. [Google Scholar] [CrossRef] [PubMed]
- Crompton, D.W.T.; Nesheim, M.C. Nutritional impact of intestinal helminthiasis during the human life cycle. Annu. Rev. Nutr. 2002, 22, 35–59. [Google Scholar] [CrossRef] [PubMed]
- Kotloff, K.; Nataro, J.; Blackwelder, W.; Nasrin, D.; Farag, T.; Panchalingam, S.; Wu, Y.; Sow, S.; Sur, D.; Breiman, R.; et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet 2013, 382. [Google Scholar] [CrossRef]
- Cossa-Moiane, I.; Cossa, H.; Bauhofer, A.F.L.; Chilaúle, J.; Guimarães, E.L.; Bero, D.M.; Cassocera, M.; Bambo, M.; Anapakala, E.; Chissaque, A.; et al. High Frequency of Cryptosporidium hominis Infecting Infants Points to A Potential Anthroponotic Transmission in Maputo, Mozambique. Pathogens 2021, 10, 293. [Google Scholar] [CrossRef]
- Unicef Situação das Crianças em Moçambique. Juntos Pelas Crianças. Available online: https://www.unicef.org/mozambique/media/571/file/Situação das Crianças em Moçambique 2014.pdf (accessed on 25 June 2021).
- Bauhofer, A.F.L.; Cossa-Moiane, I.; Marques, S.; Guimarães, E.L.; Munlela, B.; Anapakala, E.; Chilaúle, J.J.; Cassocera, M.; Langa, J.S.; Chissaque, A.; et al. Intestinal protozoan infections among children 0–168 months with diarrhea in Mozambique: June 2014–January 2018. PLoS Negl. Trop. Dis. 2020, 14, e0008195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nhampossa, T.; Mandomando, I.; Acacio, S.; Quintó, L.; Vubil, D.; Ruiz, J.; Nhalungo, D.; Sacoor, C.; Nhabanga, A.; Nhacolo, A.; et al. Diarrheal Disease in Rural Mozambique: Burden, Risk Factors and Etiology of Diarrheal Disease among Children Aged 0–59 Months Seeking Care at Health Facilities. PLoS ONE 2015, 10, e0119824. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention Laboratory Identification of Parasites of Public Health Concern. Available online: https://www.cdc.gov/dpdx/index.html (accessed on 7 February 2022).
- Nxasana, N.; Baba, K.; Bhat, V.; Vasaikar, S. Prevalence of intestinal parasites in primary school children of mthatha, Eastern Cape Province, South Africa. Ann. Med. Health Sci. Res. 2013, 3, 511–516. [Google Scholar] [CrossRef] [Green Version]
- Booth, M.; Bundy, D.A.P.; Alboico, M.; Chwaya, H.M.; Alawi, K.S.; Savioli, L. Associations among multiple geohelminth species infections in schoolchildren from Pemba Island. Parasitology 1998, 116, 85–93. [Google Scholar] [CrossRef] [PubMed]
- USAID Mozambique. Climate Vulnerability Profile. Available online: https://www.climatelinks.org/sites/default/files/asset/document/mozambique_climate_vulnerability_profile_jan2013.pdf (accessed on 23 June 2021).
- Muchiri, J.M.; Ascolillo, L.; Mugambi, M.; Mutwiri, T.; Ward, H.D.; Naumova, E.N.; Egorov, A.I.; Cohen, S.; Else, J.G.; Griffiths, J.K. Seasonality of Cryptosporidium oocyst detection in surface waters of Meru, Kenya as determined by two isolation methods followed by PCR. J. Water Health 2009, 7, 67–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, F.S. Estudo do Perfil Epidemiológico Molecular de Giardia Duodenalis em Crianças dos 0 aos 59 Meses de Idade no Hospital Central de Nampula e Sua Associação com o Estado Nutricional, Diarreia e VIH. Ph.D. Thesis, Universidade Nova em Lisboa, Lisboa, Portugal, 2017. [Google Scholar]
- Anim-Baidoo, I.; Narh, C.A.; Oddei, D.; Brown, C.A.; Enweronu-Laryea, C.; Bandoh, B.; Sampane-Donkor, E.; Armah, G.; Adjei, A.A.; Adjei, D.N.; et al. Giardia lamblia infections in children in Ghana. Pan. Afr. Med. J. 2016, 24. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control and Prevention. CDC-DPDx. Diagnostic Procedures-Other Specimens. Available online: https://www.cdc.gov/dpdx/diagnosticprocedures/index.html (accessed on 17 February 2022).
- World Health Organization Diarrhoeal disease. Available online: https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease (accessed on 12 January 2021).
- World Health Organization. Manual of BasicTecnhiques for Health Laboratory, 2nd ed.; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).