Abstract
Porphyromonas gingivalis (P. gingivalis) is a unique pathogen implicated in severe forms of periodontitis (PD), a disease that affects around 50% of the US population. P. gingivalis is equipped with a plethora of virulence factors that it uses to exploit its environment and survive. These include distinct fimbrial adhesins that enable it to bind to other microbes, colonize inflamed tissues, acquire nutrients, and invade cells of the stroma and immune system. Most notable for this review is its ability to invade dendritic cells (DCs), which bridge the innate and adaptive immune systems. This invasion process is tightly linked to the bridging functions of resultant DCs, in that it can disable (or stimulate) the maturation function of DCs and cytokines that are secreted. Maturation molecules (e.g., MHCII, CD80/CD86, CD40) and inflammatory cytokines (e.g., IL-1b, TNFa, IL-6) are essential signals for antigen presentation and for proliferation of effector T-cells such as Th17 cells. In this regard, the ability of P. gingivalis to coordinately regulate its expression of major (fimA) and minor (mfa-1) fimbriae under different environmental influences becomes highly relevant. This review will, therefore, focus on the immunoregulatory role of P. gingivalis fimbriae in the invasion of DCs, intracellular signaling, and functional outcomes such as alveolar bone loss and immune senescence.
1. Introduction
1.1. Periodontitis
Periodontitis (PD) is a chronic inflammatory disease resulting from a microbial dysbiosis. Unresolved inflammation in the soft and hard tissues surrounding the teeth results in the loss of supporting tissue and eventually loss of the teeth. While bacterial biofilm is a causative factor, smoking and diabetes remain the strongest risk factors for the severity and progression of periodontal disease in a susceptible host. Periodontitis affects almost half of the US population and, as the population ages, the prevalence of periodontal disease will also increase. Eke et al. reported on the prevalence of periodontitis from the NHANES (2009–2010 and 2011–2012). In 2009 to 2012, 46% of US adults, representing 64.7 million people, had PD, with 8.9% having severe PD. The prevalence of PD was higher among males and was positively associated with increasing age through as yet unclear mechanisms, though immune senescence has been proposed [1,2]. PD prevalence was highest in Hispanics (63.5%) and non-Hispanic blacks (59.1%), followed by non-Hispanic Asian Americans (50.0%), and lowest in non-Hispanic whites (40.8%). Prevalence varied two-fold between the lowest and highest levels of socioeconomic status, whether defined by poverty or education [3]. Not only has smoking been shown to affect the humoral and cellular immune responses but also the vasculature, cell signaling mechanisms, tissue homeostasis, and even the composition and quantity of the subgingival microflora [4,5,6,7,8,9,10,11]. Data derived from the NHANES III study suggested that up to 42% of PD cases in the US can be attributed to current smoking and 11% to former smoking. In longitudinal studies, adjusted for co-variates, smoking has been found to confer a statistically significant increased risk for PD progression [12,13,14,15,16,17,18]. Smoking also has a negative effect on the outcome of both non-surgical and surgical periodontal therapy: Current smokers exhibit poorer responses than former or never smokers [19,20,21,22,23,24,25,26,27,28,29,30]. Smoking cessation results in beneficial effects on periodontal status, and former smokers have the potential to experience periodontal stability similar to non-smokers [31,32,33,34,35]. The effects of diabetes mellitus (DM) span multiple organs throughout the entire body. The oral cavity and, more precisely, the periodontal apparatus are also affected [36,37,38,39]. Chávarry et al. confirmed a strong association between type 2 DM and PD, but concluded that the evidence for type 1 DM was weaker in a recent meta-analysis [40]. Poor metabolic control and extended duration of DM increase the adverse effects on a patient’s periodontal status [41,42,43,44]. Furthermore, previous studies have reported the relationship between poor metabolic control and the severity as well as the progression of PD [45,46,47,48,49,50]. Similarly to smoking, patients with poorly controlled DM display a poorer treatment outcome of periodontal treatment [51], whereas well-controlled diabetic patients and non-diabetic subjects have similar responses [52,53,54]. The interaction between DM and PD seems to be a “two-way street”: Just as an increased severity of periodontal tissue destruction is observed in subjects with DM, studies indicate a higher incidence of DM complications and poorer metabolic control of diabetes in PD patients [50]. Therefore, it is of importance that the diabetic control be established to increase control of the periodontal condition as well as the periodontal condition be addressed in conjunction with medications, diet, and exercise to aid in maintaining diabetic control. The consensus report of the 1996 World Workshop in Periodontics identified three species, Actinobacillus actinomycetemcomitans (now Aggregatibacter actinomycetemcomitans) [55], P. gingivalis, and Bacteroides forsythus (now Tannerella forsythia) [56,57], as causative factors for PD. As such, much research has been devoted to these three species but they should not be considered to be the only causative pathogens because only approximately 50% of the bacteria of the oral cavity are currently recognized [58]. Two decades of research have contributed to our understanding of the role of specific periodontal bacteria as risk factors for PD and have clarified that (1) the clinical presentation of a patient is determined by the burden of the exposure to the specific micro-organisms rather than its mere presence, (2) within a microbial species, there may be clonal types that are more virulent and can cause more or less disease at a faster or slower rate. and (3) reducing said pathogens to undetectable levels from the subgingival microbiome results in improved periodontal clinical markers [13,14,59,60,61,62,63,64,65,66,67]. Based on systematic reviews, it is clear that an antimicrobial approach consisting of the removal of subgingival plaque with or without adjunctive local or systemic antibiotics followed by adequate maintenance care is currently the single most successful and consistent strategy in the treatment of PD and maintenance of long-term stability [68,69,70].
1.2. P. gingivalis Fimbriae and the Prevalence of Periodontal Disease
P. gingivalis is a Gram-negative, black-pigmented, anaerobic coccobacillus. P. gingivalis is considered a keystone pathogen in the development of PD [71]. In addition, it is part of the anaerobic bacterial complex, known as the red complex, consisting of Treponema denticola (T. denticola) and Tannerella forsythia (T. forsythia), which has been implicated in severe forms of PD [72]. The progression from gingivitis to PD is associated with a dramatic shift from a symbiotic, aerobic microbial community bacteria to a dysbiotic, anaerobic polymicrobial complex that elicits a pro-inflammatory immune response [73]. P. gingivalis is equipped with an arsenal of virulence factors, including fimbriae, cysteine proteinases, hemagglutinins, and LPS, which together strongly support its pathogenicity. This occurs though a combination of binding to host cells and to other microbes, using blood hemin for growth and cellular invasion. This review focused on the role of P. gingivalis fimbria.
Fimbriae are appendages present on the outer surface that are involved in the P. gingivalis cell membrane and greatly contribute to its virulence [74]. P. gingivalis fimbriae play a crucial role in nearly all interactions between the bacterium and the host, as well as with other bacteria. More importantly, P. gingivalis fimbriae have been identified as a key factor in its adhesion, invasion, and colonization of the oral mucosa [75,76]. P. gingivalis has two distinct types of fimbriae: long and short fimbriae [77,78]. The long fimbriae (FimA) are also known as major fimbriae while the short fimbriae (Mfa1) are known as minor fimbriae. Both P. gingivalis fimbriae are involved in the initial attachment and organization of the biofilm and the attachment to other bacteria [79]. Most notably, the fimbriae of P. gingivalis are required for invasion of DCs. The minor fimbriae, comprised of a 67-kDa glycoprotein that is encoded by the mfa1 gene [80], targets the C-type lectin DC-SIGN on DCs for entry [78] and survival within [81]. The major fimbriae are composed of a 41-kDa protein called fimbrillin and encoded by the fimA gene [82]. The fimA gene has been classified into six types (I, Ib, II, III, IV, V) [83,84,85], based on nucleotide sequence variation. The difference between fimA genotypes in the context of pathogenicity and ability to adhere and invade human cells has been studied before. Studies using recombinant FimA protein corresponding to fimA genotype II have reported that genotype II has a greater ability to adhere to and invade human epithelial cells than FimA corresponding to the protein from other genotypes. Furthermore, it was shown that fimA genotypes II, Ib, and IV cause stronger infectious symptoms and inflammatory response in animal models, relative to fimA genotypes I and III [86,87,88].
Results of various clinical studies also support findings that nucleotide variation of the fimA gene is contributing to the virulence of P. gingivalis strains. Isolates with fimA genotypes II, IV, and Ib have been shown to be significantly more prevalent than isolates with other genotypes [84,85,89,90,91,92,93,94]. More recently, a meta-analysis by Wang et al. showed that the fimA II and fimA IV genotypes of P. gingivalis are highly prevalent in patients with PD [95], suggesting that these two genotypes may be related to the pathogenesis and progression of PD.
1.3. Regulation of P. gingivalis Fimbriae
P. gingivalis fimbriae expression is regulated by the microenvironment below the gumline and controlled by a variety of endogenous and exogenous factors. The biogenesis of fimA fimbria is controlled by the activation of the FimS-FimR two-component signal transduction system. FimR does not bind directly to the fimA promoter, but rather binds to the promoter region of the first gene (PG2130) in the fimA cluster. PG2130, in turn, regulates the expression of other genes in the fimA cluster, including PG2131 and PG2132, the fimA gene. In addition, using microarray experiments, it was reported that fimR mutant strains showed significant reduction in the expression levels of PG2133 and PG2134, postulating that FimA regulates the expression of PG2133 and PG2134 through an as yet unknown mechanism (Figure 1) [96]. Unlike fimA, mfa1 gene regulation is accomplished by FimR directly binding to the promoter region of mfa1 [97]. Previous studies showed that low temperature (34 °C) is necessary for the transcription of the fimA gene [98,99]. Additionally, the presence of the gingipains (RgpA and Kgp) is essential for the transcription of fimA, where fimA mRNA expression levels were significantly decreased in rgp and kgp mutant P. gingivalis strains [100]. Furthermore, the interaction of P. gingivalis with other periodontal pathogens influences the expression level of its fimbriae. It was shown that the development of P. gingivalis communities with Streptococcus gordonii, Streptococcus sanguinis, and Streptococcus mitis leads to downregulation of its minor fimbria [101], whereas the development of P. gingivalis communities with Streptococcus cristatus leads to the downregulation of the major fimbria [102].
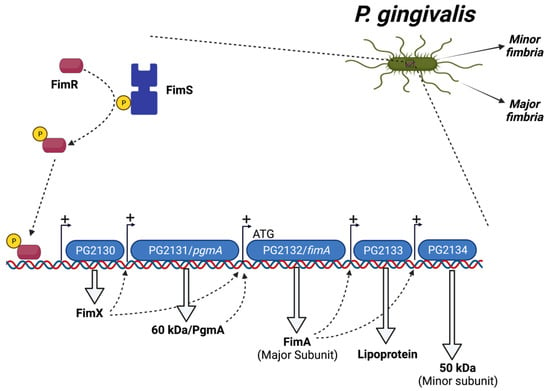
Figure 1.
Regulation of P. gingivalis FimA fimbria by a FimS-FimR system.
1.4. Dendritic Cells: Revisiting the Dogma of DC Subsets and Differentiation
Dendritic cells (DCs) are antigen-capturing and -presenting cells (APCs) that play an important role in the innate immune system by capturing antigens and in the adaptive immune response by presenting the antigens to T-cells [103]. DCs have the ability to infiltrate several mucosal sites, including oral mucosa. Previous studies reported that DCs actively mobilize in and out of oral mucosal sites at different stages of periodontal health and disease [104,105,106,107], distinctly increasing in the lamina propria of PD tissues. DCs infiltrate the peripheral tissues in the immature state, where immature DCs capture and/or respond to a wide variety of microbes in the peripheral tissues via a set of extracellular as well as intracellular pattern recognition receptors (PRRs). PRRs identify pathogens via recognition of antigens such as pathogen-associated molecular patterns (PAMPs), including lipopolysaccharide (LPS), fimbriae, or flagellin [108]. Upon encountering a pathogen, PRRs activate a large number of complex intracellular signaling pathways, resulting in activation of gene expression and production of pro- and anti-inflammatory cytokines, chemokines, cell adhesion molecules, and immunoreceptors, orchestrating the early host response to invading pathogens and activation of the adaptive immune response [108]. Upon pathogen recognition and capture, immature DCs undergo maturation and must process the antigens and activate CD4+ T-cells and CD8+ T-cells via presenting antigens on their surface MHC-I and MHC-II molecules, respectively. The DC maturation process involves upregulation of co-stimulatory molecules (CD80, CD86), maturation markers (CD83), and antigen-presenting molecules (MHC classes I and II). Mature DCs then acquire a highly migratory profile through upregulation of chemokine receptors (e.g., CCR7) and secretion of cytokines (e.g., IL-12p70) [109]. Under optimum conditions, DCs migrate to secondary lymphoid organs and present the captured antigens to T-cells and prime naïve T-cells. As DCs migrate to lymphoid organs, blood DCs and monocytes migrate into the tissues and differentiate into DCs to replace migrating DCs and maintain DCs’ proper homeostasis, a cycle that is highly regulated by a variety of cytokines.
DCs develop in the bone marrow from hematopoietic progenitors, expressing the transcription factor IRF8 [110] and the cytokine receptor FLT3 [111], which complete their differentiation in the periphery. DCs have evolved into multiple subsets throughout the body [112]. Conventional DCs (cDCs) are potent APCs and are classified into two major subsets. The cDC1 subset is specialized in presenting cell-associated antigens through cross presentation to CD8+ T-cells in addition to activating a type I immune response. On the other hand, the cDC2 subset (also called myeloid DC (mDC)), the focus of most of our work, is known to be more involved in activating CD4+ T-cells’ responses via MHC-II. Contrary to cDCs, the plasmacytoid DCs’ (pDCs) subset consists of relatively poor APCs and, instead, are more specialized in the rapid production of type I interferons (IFN-I); hence, they play an important role in responding to viral infection. In humans, DCs are generally divided into two major subpopulations, the pDC (CD11c−CD123+) and mDC (CD11c+CD123−) lineages. The mDCs can be further subdivided into CD141 (BDCA-3)+, CD16+ DC, and CD1c (BDCA-1)+ DC subsets [113,114,115]. The mDCs are further subdivided into CD141 (BDCA-3)+, CD16+ DC, and CD1c (BDCA-1)+ DC subsets [113,114,115]. In addition, DCs are subdivided into Langerhans cells and monocyte-derived DCs (MO-DC). Langerhans cells are present in the epidermis and oral mucosa and have a role in both tolerance and immune priming in that compartment. Mo-DCs differentiate from monocytes recruited in the event of tissue inflammatory responses and, in turn, instruct the differentiation of CD4+ T-cells into Th1, Th2, or Th17 cells [116,117,118].
1.5. Modulation of DC–T Cell Interaction by P. gingivalis
CD1c+(BDCA-1) CD209+ blood myeloid DCs have been shown to be expanded in subjects with PD, relative to healthy controls. More interestingly, it was also shown that this expansion further increases 24 h after mechanical debridement, which has been attributed to bacteremia [81]. In addition, myeloid DCs have been reported to be increased in PD patients with existing coronary artery disease. The increase in DC populations in the systemic circulation of a PD subject with coronary artery disease is associated with microbial carriage state of the DCs, most notably P. gingivalis. Postmortem analysis of coronary artery samples of coronary artery-diseased patients with PD shows co-localization of myeloid DCs’ marker, CD209 (DC-SIGN), with P. gingivalis minor fimbria protein (mfa-1) in the atherosclerotic plaques. Epidemiologic studies have reported that PD is associated with cardiovascular diseases, but the mechanism remains unclear. Recent studies implicated DCs in the microbial dissemination of periodontal pathogens. It was hypothesized that this was due to the manipulation of intracellular signaling in DCs by P. gingivalis minor fimbria via targeting the C-type lectin receptor DC-SIGN. Recently, it was shown that targeting DC-SIGN on DCs by P. gingivalis minor fimbria extends the survival of P. gingivalis-loaded DCs through the inhibition of apoptosis and autophagy [119]. Autophagy is a process whereby the cell disposes its intracellular damaged proteins and organelles by sequestering and directing cargo to the lysosome for degradation. Autophagy is crucial for the cell not only to maintain proper cellular homeostasis but also to defend against invading pathogens [120,121]. By trafficking intracellular bacteria to lysosomes, autophagy comprises an important element of the first line of defense, the innate immune response. Autophagy is involved in many immune functions such as clearance of intracellular pathogens [122,123,124], secretion of inflammatory cytokines [125], antigen presentation [126,127], and development of lymphocytes [128]. In addition, autophagy is regulated by a variety of immunological signals in response to the exposure of PRRs, such as TLRs and NLRs, to ligands or to cytokines. Moreover, TLR ligand-coated particles stimulate phagocytes and LC3-PE conjugation, a process called LC3-associated phagocytosis (LAP) [129]. Several in vitro studies reported the influence of P. gingivalis fimbria on autophagy in DCs. P. gingivalis has evolved an immune escape tactic whereby it evades intracellular killing in DCs by targeting DC-SIGN with its minor fimbria [122]. The same study reported that the intracellular killing of P. gingivalis inside DCs decreases while intracellular content increases via a DC-SIGN-dependent uptake of P. gingivalis by DCs. Furthermore, by blocking DC-SIGN by HIV glycoprotein 120, P. gingivalis survival inside DCs is reduced, but the mechanism of this phenomenon was unclear [122]. A more recent study showed that inhibition of autophagy in DCs by P. gingivalis involves targeting a crucial regulator of autophagy, the Akt-mTOR pathway [119]. P. gingivalis infection increases the expression of important elements in mTOR-dependent autophagy inhibition such as p-Akt Ser473, p-mTOR Ser2448, p-Raptor Ser792, and p-ULK1 Ser757 [119]. Other studies indicated that the hyperactivation of AKT [130] or of mTOR [131,132,133] plays influential roles in cellular senescence, with the mTOR inhibitor rapamycin obviating alveolar bone loss in mice [131]. Our work established a significant role for immune senescence induction by P. gingivalis in disabling the immune functions of DCs, including the ability of DCs to mature and induce antigen-specific T-cell proliferation [2]. Not yet clear is the role of major and minor fimbriae in promoting immune senescence (Figure 2).
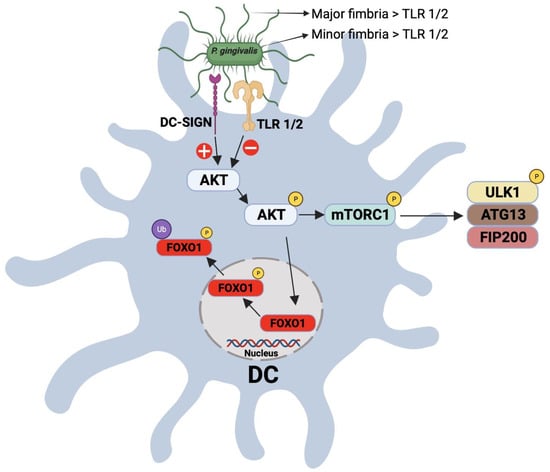
Figure 2.
Schematic representation for the interaction between P. gingivalis fimbria and DC-SIGN and TLR receptors on DCs. Targeting of the DC-SIGN receptor on DCs results in the activation/phosphrylation of AKT, which, in turn, posphorylates and inactivates FOXO1, leading to the inhibition of apoptosis. Phophorylated AKT also phosphorylates mTORC1, resulting in its activation and inhibition of autophagy.
1.6. Significance of DC–T Cells’ Clusters in Periodontitis: Oral Lymphoid Foci
When DCs were discovered in the early 1970s [134,135], they were thought to be predominantly immune stimulatory. It was decades later, in the 1990s, that the immune-regulatory functions of certain DC subtypes were recognized, including tolerogenic DCs [136,137]. Immature DCs monitor the periphery for antigens, which they phagocytose and process for antigen presentation to T-cells in the context of MHC-II molecules. DCs exposed to pro-inflammatory signals during antigen acquisition undergo maturation by upregulating their expression of MHC-II, co-stimulatory (e.g., CD40, CD80, CD86, CD83) lymph node-homing migratory chemokine receptors, and of inflammatory cytokines such as IL-12, while downregulating phagocytic mediators such as C-type lectins. DCs then migrate to regional lymph nodes, where they present their processed antigen peptides to T-cells in an immunostimulatory context, activating effector T-cell-type (e.g., Th1, Th17) responses [138]. We have reported in human studies the infiltration of oral lamina propria in PD with CD83+ matured DCs [139]. These mature DCs form immune complexes in situ with CD4+ T-cells [139]. These clusters in PD, called “oral lymphoid foci” [107], are analogous to ectopic lymphoid follicles found in many chronic inflammatory diseases [140] and are thought to result from continuous exposure to oral microbes and repeated damage to the oral mucosal epithelium [104,141,142]. In the experimental PD model in mice, a destructive role for in situ, matured DCs in promoting Th17-mediated alveolar bone loss was documented [143]. The exposure of DCs to innocuous antigens (e.g., apoptotic cells) in the absence of proinflammatory stimulants maintains their immature profile. In this scenario, very low levels of MHC-II-bound antigen peptides, co-stimulatory molecules, and secreted cytokines are expressed by DCs, inducing T-cell anergy [144]. TGF-β1 and IL-10 inhibit DC maturation and promote regulatory T-cell (Tregs) responses, while inhibiting Th17 effectors. This combination of cytokines loaded into DC-derived exosomes was shown to inhibit experimental PD in mice (Figure 3) [143].
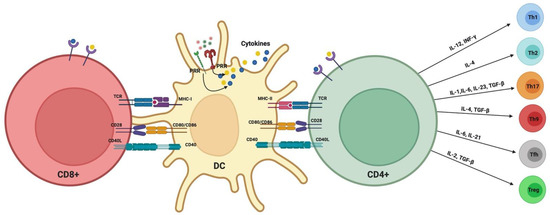
Figure 3.
Classical DC–T-cell activation signals and polarization of T-cells.
2. Conclusions
Dendritic cells play a very active role in clearing infecting microbes, and other antigens when immature, and have been observed infiltrating oral mucosa at all stages of health and disease. Matured DCs are a unique feature of the established periodontitis lesion, forming immune conjugates with T-cells that are evocative of lymphoid tissues. These matured DCs promote Th17-mediated alveolar bone degeneration. The ability of P. gingivalis to coordinately regulate its fimbrial types, promoting or disabling DC maturation and Th17-type responses, should have a profound effect on the promotion or resolution of alveolar bone loss, most notably involving the process of immune senescence, though this will require further cause-and-effect studies in mice.
Author Contributions
Conceptualization, M.M.M. and C.W.C.; Original draft preparation M.M.M., M.G. and C.W.C.; Review and editing, M.M.M. and C.W.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by This project was supported by a grant from the Carlos and Marguerite Mason Trust and by NIH/NIDCR R01DE02946801 (to CWC).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ebersole, J.L.; Dawson, D.A., III; Huja, P.E.; Pandruvada, S.; Basu, A.; Nguyen, L.; Zhang, Y.; Gonzalez, O.A. Age and Periodontal Health—Immunological View. Curr. Oral. Health Rep. 2018, 5, 229–241. [Google Scholar] [CrossRef]
- Elsayad, R.; Elashiry, M.; Lui, Y.; El-Awady, A.; Hamrick, M.; Cutler, C.W. Porphyromonas gingivalis provokes exosome secretion and paracrine immune senescence in bystander dendritic cells. Front. Cell. Infect. Microbiol. 2021, 11, 471. [Google Scholar] [CrossRef] [PubMed]
- Eke, P.I.; Dye, B.A.; Wei, L.; Slade, G.D.; Thornton-Evans, G.O.; Borgnakke, W.S.; Taylor, G.W.; Page, R.C.; Beck, J.D.; Genco, R.J. Update on Prevalence of Periodontitis in Adults in the United States: NHANES 2009 to 2012. J. Periodontol. 2015, 86, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Bagaitkar, J.; Daep, C.A.; Patel, C.K.; Renaud, D.E.; De Muth, N.R.; Scott, D.A. Tobacco Smoke Augments Porphyromonas gingivalis—Streptococcus gordonii Biofilm Formation. PLoS ONE 2011, 6, e27386. [Google Scholar] [CrossRef] [PubMed]
- Brook, I. The Impact of Smoking on Oral and Nasopharyngeal Bacterial Flora. J. Dent. Res. 2011, 90, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Haffajee, A.D.; Socransky, S.S. Relationship of cigarette smoking to the subgingival microbiota. J. Clin. Periodontol. 2001, 28, 377–388. [Google Scholar] [CrossRef]
- Kinane, D.F.; Chestnutt, I.G. Smoking and periodontal disease. Crit. Rev. Oral. Biol. Med. 2000, 11, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Kubota, M.; Tanno-Nakanishi, M.; Yamada, S.; Okuda, K.; Ishihara, K. Effect of smoking on subgingival microflora of patients with periodontitis in Japan. BMC Oral Health 2011, 11, 1. [Google Scholar] [CrossRef]
- Kumar, P.S.; Matthews, C.R.; Joshi, V.; de Jager, M.; Aspiras, M. Tobacco Smoking Affects Bacterial Acquisition and Colonization in Oral Biofilms. Infect. Immun. 2011, 79, 4730–4738. [Google Scholar] [CrossRef]
- Palmer, R.M.; Wilson, R.F.; Hasan, A.S.; Scott, D.A. Mechanisms of action of environmental factors—tobacco smoking. J. Clin. Periodontol. 2005, 32, 180–195. [Google Scholar] [CrossRef]
- Shchipkova, A.; Nagaraja, H.; Kumar, P. Subgingival Microbial Profiles of Smokers with Periodontitis. J. Dent. Res. 2010, 89, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.D.; Koch, G.G.; Offenbacher, S. Incidence of attachment loss over 3 years in older adults—new and progressing lesions. Community Dent. Oral Epidemiol. 1995, 23, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.D.; Cusmano, L.; Green-Helms, W.; Koch, G.G.; Offenbacher, S. A 5-year study of attachment loss in community-dwelling older adults: Incidence density. J. Periodontal Res. 1997, 32, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Machtei, E.E.; Hausmann, E.; Dunford, R.; Grossi, S.; Ho, A.; Davis, G.; Chandler, J.; Zambon, J.; Genco, R.J. Longitudinal study of predictive factors for periodontal disease and tooth loss. J. Clin. Periodontol. 1999, 26, 374–380. [Google Scholar] [CrossRef]
- Norderyd, O.; Hugoson, A.; Grusovin, G. Risk of severe periodontal disease in a Swedish adult population. J. Clin. Periodontol. 1999, 26, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wolff, L.; Aeppli, D.; Guo, Z.; Luan, W.-M.; Baelum, V.; Fejeskov, O. Cigarette smoking, salivary/gingival crevicular fluid cotinine and periodontal status A 10-year longitudinal study. J. Clin. Periodontol. 2001, 28, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, H.; Yoshihara, A.; Hirotomi, T.; Ando, Y.; Miyazaki, H. Risk factors for periodontal disease progression among elderly people. J. Clin. Periodontol. 2002, 29, 592–597. [Google Scholar] [CrossRef]
- Paulander, J.; Wennström, J.L.; Axelsson, P.; Lindhe, J. Some risk factors for periodontal bone loss in 50-year-old individuals. A 10-year cohort study. J. Clin. Periodontol. 2004, 31, 489–496. [Google Scholar] [CrossRef]
- Ah, M.K.B.; Johnson, G.K.; Kaldahl, W.B.; Patil, K.D.; Kalkwart, K.L. The effect of smoking on the response to periodontal therapy. J. Clin. Periodontol. 1994, 21, 91–97. [Google Scholar] [CrossRef]
- Garcia, R.I. Smokers have less reductions in probing depth than non-smokers following nonsurgical periodontal therapy. Evid.-Based Dent. 2005, 6, 37–38. [Google Scholar] [CrossRef][Green Version]
- Grossi, S.G.; Zambon, J.; Machtei, E.E.; Schifferle, R.; Andreana, S.; Genco, R.J.; Cummins, D.; Harrap, G. Effects of Smoking and Smoking Cessation on Healing after Mechanical Periodontal Therapy. J. Am. Dent. Assoc. 1997, 128, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Kaldahl, W.B.; Johnson, G.K.; Patil, K.D.; Kalkwarf, K.L. Levels of Cigarette Consumption and Response to Periodontal Therapy. J. Periodontol. 1996, 67, 675–681. [Google Scholar] [CrossRef]
- Labriola, A.; Needleman, I.; Moles, D. Systematic review of the effect of smoking on nonsurgical periodontal therapy. Periodontology 2000 2005, 37, 124–137. [Google Scholar] [CrossRef]
- Patel, R.A.; Wilson, R.F.; Palmer, R.M. The Effect of Smoking on Periodontal Bone Regeneration: A Systematic Review and Meta-Analysis. J. Periodontol. 2012, 83, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Paulander, J.; Axelsson, P.; Lindhe, J.; Wennström, J.L. Intra-oral pattern of tooth and periodontal bone loss between the age of 50 and 60 years. A longitudinal prospective study. Acta Odontol. Scand. 2004, 62, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Rieder, C.; Joss, A.; Lang, N.P. Influence of compliance and smoking habits on the outcomes of supportive periodontal therapy (SPT) in a private practice. Oral Health Prev. Dent. 2004, 2, 89–94. [Google Scholar] [PubMed]
- Sculean, A.; Stavropoulos, A.; Berakdar, M.; Windisch, P.; Karring, T.; Brecx, M. Formation of human cementum following different modalities of regenerative therapy. Clin. Oral Investig. 2005, 9, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulos, A.; Mardas, N.; Herrero, F.; Karring, T. Smoking affects the outcome of guided tissue regeneration with bioresorbable membranes: A retrospective analysis of intrabony defects. J. Clin. Periodontol. 2004, 31, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S. Cigarette Smoking and Periodontal Diseases: Etiology and Management of Disease. Ann. Periodontol. 1998, 3, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.P.; Leung, W.K.; Wong, M.C.M.; Wong, R.M.S.; Wan, P.; Lo, E.C.M.; Corbet, E.F. Effects of smoking on healing response to non-surgical periodontal therapy: A multilevel modelling analysis. J. Clin. Periodontol. 2009, 36, 229–239. [Google Scholar] [CrossRef]
- Bolin, A.; Eklund, G.; Frithiof, L.; Lavstedt, S. The effect of changed smoking habits on marginal alveolar bone loss. A longitudinal study. Swed. Dent. J. 1993, 17, 211–216. [Google Scholar] [PubMed]
- Krall, E.; Dawson-Hughes, B.; Garvey, A.; Garcia, R. Smoking, smoking cessation, and tooth loss. J. Dent. Res. 1997, 76, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Bergström, J.; Eliasson, S.; Dock, J. A 10-Year Prospective Study of Tobacco Smoking and Periodontal Health. J. Periodontol. 2000, 71, 1338–1347. [Google Scholar] [CrossRef] [PubMed]
- Rosa, E.F.; Corraini, P.; De Carvalho, V.F.; Inoue, G.; Gomes, E.F.; Lotufo, J.P.B.; De Micheli, G.; Pannuti, C.M. A prospective 12-month study of the effect of smoking cessation on periodontal clinical parameters. J. Clin. Periodontol. 2011, 38, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Duarte, P.M.; Nogueira, C.F.P.; Silva, S.M.; Pannuti, C.M.; Schey, K.C.; Miranda, T.S. Impact of Smoking Cessation on Periodontal Tissues. Int. Dent. J. 2021, 72, 31–36. [Google Scholar] [CrossRef]
- Lalla, E.; Lamster, I.B.; Drury, S.; Fu, C.; Schmidt, A.M. Hyperglycemia, glycoxidation and receptor for advanced glycation endproducts: Potential mechanisms underlying diabetic complications, including diabetes-associated periodontitis. Periodontology 2000 2000, 23, 50–62. [Google Scholar] [CrossRef]
- Lalla, E.; Papapanou, P.N. Diabetes mellitus and periodontitis: A tale of two common interrelated diseases. Nat. Rev. Endocrinol. 2011, 7, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Mealey, B.L.; Oates, T.W. American Academy of Periodontology. Diabetes Mellitus and Periodontal Diseases. J. Periodontol. 2006, 77, 1289–1303. [Google Scholar] [CrossRef] [PubMed]
- Graves, D.T.; Ding, Z.; Yang, Y. The impact of diabetes on periodontal diseases. Periodontology 2000 2020, 82, 214–224. [Google Scholar] [CrossRef]
- Chávarry, N.G.M.; Vettore, M.V.; Sansone, C.; Sheiham, A. The relationship between diabetes mellitus and destructive periodontal disease: A meta-analysis. Oral Health Prev. Dent. 2009, 7, 107–127. [Google Scholar]
- Grossi, S.G.; Genco, R.J. Periodontal Disease and Diabetes Mellitus: A Two-Way Relationship. Ann. Periodontol. 1998, 3, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Lalla, E.; Park, D.B.; Papapanou, P.N.; Lamster, I.B. Oral Disease Burden in Northern Manhattan Patients with Diabetes Mellitus. Am. J. Public Health 2004, 94, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.W.; Burt, B.A.; Becker, M.P.; Genco, R.J.; Shlossman, M. Glycemic Control and Alveolar Bone Loss Progression in Type 2 Diabetes. Ann. Periodontol. 1998, 3, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.W.; Burt, B.A.; Becker, M.P.; Genco, R.J.; Shlossman, M.; Knowler, W.C.; Pettitt, D.J. Severe Periodontitis and Risk for Poor Glycemic Control in Patients with Non-Insulin-Dependent Diabetes Mellitus. J. Periodontol. 1996, 67, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, D.; Marlow, N.M.; Fernandes, J.K.; Leite, R.S. Periodontal disease progression and glycaemic control among Gullah African Americans with type-2 diabetes. J. Clin. Periodontol. 2010, 37, 501–509. [Google Scholar] [CrossRef]
- Guzman, S.; Karima, M.; Wang, H.-Y.; Van Dyke, T.E. Association Between Interleukin-1 Genotype and Periodontal Disease in a Diabetic Population. J. Periodontol. 2003, 74, 1183–1190. [Google Scholar] [CrossRef]
- Seppala, B.; Ainamo, J. A longitudinal study on insulin-dependent diabetes mellitus and periodontal disease. J. Clin. Periodontol. 1993, 20, 161–165. [Google Scholar] [CrossRef]
- Tervonen, T.; Karjalainen, K. Periodontal disease related to diabetic status. A pilot study of the response to periodontal therapy in type 1 diabetes. J. Clin. Periodontol. 1997, 24, 505–510. [Google Scholar] [CrossRef]
- Tervonen, T.; Oliver, R.C. Long-term control of diabetes mellitus and periodontitis. J. Clin. Periodontol. 1993, 20, 431–435. [Google Scholar] [CrossRef]
- Holmlund, A.; Lind, L. Periodontal disease and a poor response to periodontal treatment were associated with an increased risk of incident diabetes: A longitudinal cohort study in Sweden. J. Clin. Periodontol. 2021, 48, 1605–1612. [Google Scholar] [CrossRef]
- Costa, F.O.; Cortelli, J.R.; Cortelli, S.C.; Costa, A.A.; Lima, R.P.E.; Costa, A.M.; Pereira, G.H.M.; Cota, L.O.M. The loss of molars in supportive periodontal care: A 10-year follow-up for tooth- and patient-related factors. J. Clin. Periodontol. 2022, 49, 292–300. [Google Scholar] [CrossRef]
- Westfelt, E.; Rylander, H.; Biohme, G.; Jonasson, P.; Lindhe, J. The effect of periodontal therapy in diabetics. Results after 5 years. J. Clin. Periodontol. 1996, 23, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Christgau, M.; Palitzsch, K.-D.; Schmalz, G.; Kreiner, U.; Frenzel, S. Healing response to non-surgical periodontal therapy in patients with diabetes mellitus: Clinical, microbiological, and immunologic results. J. Clin. Periodontol. 1998, 25, 112–124. [Google Scholar] [CrossRef]
- Faria-Almeida, R.; Navarro, A.; Bascones, A. Clinical and Metabolic Changes After Conventional Treatment of Type 2 Diabetic Patients with Chronic Periodontitis. J. Periodontol. 2006, 77, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Nørskov-Lauritsen, N.; Kilian, M. Reclassification of Actinobacillus actinomycetemcomitans, Haemophilus aphrophilus, Haemophilus paraphrophilus and Haemophilus segnis as Aggregatibacter actinomycetemcomitans gen. nov., comb. nov., Aggregatibacter aphrophilus comb. nov. and Aggregatibacter segnis comb. nov., and emended description of Aggregatibacter aphrophilus to include V factor-dependent and V factor-independent isolates. Int. J. Syst. Evol. Microbiol. 2006, 56, 2135–2146. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, M.; Suzuki, M.; Umeda, M.; Ishikawa, I.; Benno, Y. Reclassification of Bacteroides forsythus (Tanner et al., 1986) as Tannerella forsythensis corrig., gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 2002, 52, 841–849. [Google Scholar]
- Maiden, M.F.; Cohee, P.; Tanner, A.C. Proposal to conserve the adjectival form of the specific epithet in the reclassification of Bacteroides forsythus Tanner et al., 1986 to the genus Tannerella Sakamoto et al. 2002 as Tannerella forsythia corrig., gen. nov., comb. nov. Request for an Opinion. Int. J. Syst. Evol. Microbiol. 2003, 53, 2111–2112. [Google Scholar] [CrossRef] [PubMed]
- Paster, B.J.; Boches, S.K.; Galvin, J.L.; Ericson, R.E.; Lau, C.N.; Levanos, V.A.; Sahasrabudhe, A.; Dewhirst, F.E. Bacterial Diversity in Human Subgingival Plaque. J. Bacteriol. 2001, 183, 3770–3783. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.D.; Koch, G.G.; Rozier, R.G.; Tudor, G.E. Prevalence and Risk Indicators for Periodontal Attachment Loss in a Population of Older Community-Dwelling Blacks and Whites. J. Periodontol. 1990, 61, 521–528. [Google Scholar] [CrossRef]
- Haffajee, A.D.; Socransky, S.S.; Smith, C.; Dibart, S. Relation of baseline microbial parameters to future periodontal attachment loss. J. Clin. Periodontol. 1991, 18, 744–750. [Google Scholar] [CrossRef]
- Grossi, S.G.; Zambon, J.J.; Ho, A.W.; Koch, G.; Dunford, R.G.; Machtei, E.E.; Norderyd, O.M.; Genco, R.J. Assessment of Risk for Periodontal Disease. I. Risk Indicators for Attachment Loss. J. Periodontol. 1994, 65, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Grossi, S.; Genco, R.; Machtet, E.; Ho, A.; Koch, G.; Dunford, R.; Zambon, J.; Hausmann, E. Assessment of Risk for Periodontal Disease. II. Risk Indicators for Alveolar Bone Loss. J. Periodontol. 1995, 66, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N.; Baelum, V.; Luan, W.-M.; Madianos, P.N.; Chen, X.; Fejerskov, O.; Dahlén, G. Subgingival Microbiota in Adult Chinese: Prevalence and Relation to Periodontal Disease Progression. J. Periodontol. 1997, 68, 651–666. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, M.F.; van der Weijden, G.A.; Abbas, F.; Arief, E.M.; Armand, S.; Winkel, E.G.; van Winkelhoff, A.J.; van der Velden, U. Untreated periodontal disease in Indonesian adolescents. Longitudinal clinical data and prospective clinical and microbiological risk assessment. J. Clin. Periodontol. 2000, 27, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N.; Teanpaisan, R.; Obiechina, N.S.; Pithpornchaiyakul, W.; Pongpaisal, S.; Pisuithanakan, S.; Baelum, V.; Fejerskov, O.; Dahlén, G. Periodontal microbiota and clinical periodontal status in a rural sample in southern Thailand. Eur. J. Oral Sci. 2002, 110, 345–352. [Google Scholar] [CrossRef]
- Fine, D.H.; Markowitz, K.; Furgang, D.; Fairlie, K.; Ferrandiz, J.; Nasri, C.; Mc Kiernan, M.; Gunsolley, J. Aggregatibacter actinomycetemcomitans and Its Relationship to Initiation of Localized Aggressive Periodontitis: Longitudinal Cohort Study of Initially Healthy Adolescents. J. Clin. Microbiol. 2007, 45, 3859–3869. [Google Scholar] [CrossRef]
- Haubek, D.; Ennibi, O.-K.; Poulsen, K.; Væth, M.; Poulsen, S.; Kilian, M. Risk of aggressive periodontitis in adolescent carriers of the JP2 clone of Aggregatibacter (Actinobacillus) actinomycetemcomitans in Morocco: A prospective longitudinal cohort study. Lancet 2008, 371, 237–242. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Chapple, I.L.C. Biological approaches to the development of novel periodontal therapies—Consensus of the Seventh European Workshop on Periodontology. J. Clin. Periodontol. 2011, 38, 114–118. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J.A.; Trombelli, L.; Heitz, F.; Needleman, I.; Moles, D. A systematic review of the effect of surgical debridement vs. non-surgical debridement for the treatment of chronic periodontitis. J. Clin. Periodontol. 2002, 29, 92–102. [Google Scholar] [CrossRef]
- Herrera, D.; Sanz, M.; Jepsen, S.; Needleman, I.; Roldán, S. A systematic review on the effect of systemic antimicrobials as an adjunct to scaling and root planing in periodontitis patients. J. Clin. Periodontol. 2002, 29, 136–159. [Google Scholar] [CrossRef]
- How, K.Y.; Song, K.P.; Chan, K.G. Porphyromonas gingivalis: An Overview of Periodontopathic Pathogen below the Gum Line. Front. Microbiol. 2016, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D. Dental biofilms: Difficult therapeutic targets. Periodontology 2000 2002, 28, 12–55. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Samaranayake, L.P. Essential Microbiology for Dentistry, 2nd ed.; Churchil Livingstone Elsevier: London, UK, 2002. [Google Scholar]
- Cutler, C.; Kalmar, J.; Genco, C. Pathogenic strategies of the oral anaerobe, Porphyromonas gingivalis. Trends Microbiol. 1995, 3, 45–51. [Google Scholar] [CrossRef]
- Zeituni, A.E.; Mc Caig, W.; Scisci, E.; Thanassi, D.G.; Cutler, C.W. The Native 67-Kilodalton Minor Fimbria of Porphyromonas gingivalis Is a Novel Glycoprotein with DC-SIGN-Targeting Motifs. J. Bacteriol. 2010, 192, 4103–4110. [Google Scholar] [CrossRef] [PubMed]
- Ezzo, P.J.; Cutler, C.W. Microorganisms as risk indicators for periodontal disease. Periodontology 2000 2003, 32, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Zeituni, A.E.; Jotwani, R.; Carrion, J.; Cutler, C.W. Targeting of DC-SIGN on Human Dendritic Cells by Minor Fimbriated Porphyromonas gingivalis Strains Elicits a Distinct Effector T Cell Response. J. Immunol. 2009, 183, 5694–5704. [Google Scholar] [CrossRef]
- Holt, S.C.; Kesavalu, L.; Walker, S.; Genco, C. Virulence factors of Porphyromonas gingivalis. Periodontology 2000 1999, 20, 168–238. [Google Scholar] [CrossRef]
- Hamada, N.; Sojar, H.T.; Cho, M.I.; Genco, R.J. Isolation and characterization of a minor fimbria from Porphyromonas gingivalis. Infect. Immun. 1996, 64, 4788–4794. [Google Scholar] [CrossRef]
- Carrion, J.; Scisci, E.; Miles, B.; Sabino, G.J.; Zeituni, A.E.; Gu, Y.; Bear, A.; Genco, C.A.; Brown, D.L.; Cutler, C.W. Microbial Carriage State of Peripheral Blood Dendritic Cells (DCs) in Chronic Periodontitis Influences DC Differentiation, Atherogenic Potential. J. Immunol. 2012, 189, 3178–3187. [Google Scholar] [CrossRef]
- Xie, H.; Lamont, R.J. Promoter architecture of the Porphyromonas gingivalis fimbrillin gene. Infect. Immun. 1999, 67, 3227–3235. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Morishima, S.; Takahashi, I.; Hamada, S. Molecular Cloning and Sequencing of the Fimbrilin Gene of Porphyromonas gingivalis Strains and Characterization of Recombinant Proteins. Biochem. Biophys. Res. Commun. 1993, 197, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, I.; Amano, A.; Kimura, R.K.; Nakamura, T.; Kawabata, S.; Hamada, S. Distribution and molecular characterization of Porphyromonas gingivalis carrying a new type of fimA gene. J. Clin. Microbiol. 2000, 38, 1909–1914. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, I.; Amano, A.; Ohara-Nemoto, Y.; Endoh, N.; Morisaki, I.; Kimura, S.; Kawabata, S.; Hamada, S. Identification of a new variant of fimA gene of Porphyromonas gingivalis and its distribution in adults and disabled populations with periodontitis. J. Periodontal Res. 2002, 37, 425–432. [Google Scholar] [CrossRef]
- Genco, C.A.; Van Dyke, T.; Amar, S. Animal models for Porphyromonas gingivalis-mediated periodontal disease. Trends Microbiol. 1998, 6, 444–449. [Google Scholar] [CrossRef]
- Nakano, K.; Kuboniwa, M.; Nakagawa, I.; Yamamura, T.; Nomura, R.; Okahashi, N.; Ooshima, T.; Amano, A. Comparison of inflammatory changes caused by Porphyromonas gingivalis with distinct fimA genotypes in a mouse abscess model. Oral Microbiol. Immunol. 2004, 19, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Neiders, M.E.; Chen, P.B.; Suido, H.; Reynolds, H.S.; Zambon, J.J.; Shlossman, M.; Genco, R.J. Heterogeneity of virulence among strains of Bacteroides gingivalis. J. Periodontal Res. 1989, 24, 192–198. [Google Scholar] [CrossRef]
- Amano, A.; Kuboniwa, A.; Nakagawa, I.; Akiyama, S.; Morisaki, I.; Hamada, S. Prevalence of Specific Genotypes of Porphyromonas gingivalis fimA and Periodontal Health Status. J. Dent. Res. 2000, 79, 1664–1668. [Google Scholar] [CrossRef] [PubMed]
- Amano, A.; Nakagawa, I.; Kataoka, K.; Morisaki, I.; Hamada, S. Distribution of Porphyromonas gingivalis strains with fimA genotypes in periodontitis patients. J. Clin. Microbiol. 1999, 37, 1426–1430. [Google Scholar] [CrossRef]
- Amano, A.; Nakagawa, I.; Okahashi, N.; Hamada, N. Variations of Porphyromonas gingivalis fimbriae in relation to microbial pathogenesis. J. Periodontal Res. 2004, 39, 136–142. [Google Scholar] [CrossRef]
- Beikler, T.; Peters, U.; Prajaneh, S.; Prior, K.; Ehmke, B.; Flemmig, T.F. Prevalence of Porphyromonas gingivalis fimA genotypes in Caucasians. Eur. J. Oral Sci. 2003, 111, 390–394. [Google Scholar] [CrossRef]
- Enersen, M.; Olsen, I.; Kvalheim, Ø.; Caugant, D.A. fimA Genotypes and Multilocus Sequence Types of Porphyromonas gingivalis from Patients with Periodontitis. J. Clin. Microbiol. 2008, 46, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Missailidis, C.G.; Umeda, J.E.; Ota-Tsuzuki, C.; Anzai, D.; Mayer, M.P.A. Distribution of fimA genotypes of Porphyromonas gingivalis in subjects with various periodontal conditions. Oral Microbiol. Immunol. 2004, 19, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, W.; Wang, W.; Zhang, L. The prevalence of fimA genotypes of Porphyromonas gingivalis in patients with chronic periodontitis: A meta-analysis. PLoS ONE 2020, 15, e0240251. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, K.; Yoshimura, F.; Duncan, M.J. A regulation cascade controls expression of Porphyromonas gingivalis fimbriae via the FimR response regulator. Mol. Microbiol. 2004, 54, 546–560. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lin, X.; Xie, H. Porphyromonas gingivalis short fimbriae are regulated by a FimS/FimR two-component system. FEMS Microbiol. Lett. 2007, 271, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Amano, A.; Sharma, A.; Sojar, H.T.; Kuramitsu, H.K.; Genco, R.J. Effects of temperature stress on expression of fimbriae and superoxide dismutase by Porphyromonas gingivalis. Infect. Immun. 1994, 62, 4682–4685. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Cai, S.; Lamont, R.J. Environmental regulation of fimbrial gene expression in Porphyromonas gingivalis. Infect. Immun. 1997, 65, 2265–2271. [Google Scholar] [CrossRef]
- Xie, H.; Chung, W.O.; Park, Y.; Lamont, R.J. Regulation of the Porphyromonas gingivalis fimA (Fimbrillin) Gene. Infect. Immun. 2000, 68, 6574–6579. [Google Scholar] [CrossRef][Green Version]
- Park, Y.; James, C.E.; Yoshimura, F.; Lamont, R.J. Expression of the short fimbriae of Porphyromonas gingivalis is regulated in oral bacterial consortia. FEMS Microbiol. Lett. 2006, 262, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.-Y.; Wu, J.; Lamont, R.J.; Lin, X.; Xie, H. Negative Correlation of Distributions of Streptococcus cristatus and Porphyromonas gingivalis in Subgingival Plaque. J. Clin. Microbiol. 2009, 47, 3902–3906. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M. Dendritic cells and the control of immunity: Enhancing the efficiency of antigen presentation. Mt. Sinai J. Med. 2001, 68, 160–166. [Google Scholar]
- Cutler, C.; Jotwani, R. Dendritic Cells at the Oral Mucosal Interface. J. Dent. Res. 2006, 85, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Jotwani, R.; Cutler, C.W. Multiple Dendritic Cell (DC) Subpopulations in Human Gingiva and Association of Mature DCs with CD4+ T-cells in situ. J. Dent. Res. 2003, 82, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Jotwani, R.; Muthukuru, M.; Cutler, C. Increase in HIV Receptors/Co-receptors/α-defensins in Inflamed Human Gingiva. J. Dent. Res. 2004, 83, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Jotwani, R.; Palucka, A.K.; Al-Quotub, M.; Nouri-Shirazi, M.; Kim, J.; Bell, D.; Banchereau, J.; Cutler, C.W. Mature Dendritic Cells Infiltrate the T Cell-Rich Region of Oral Mucosa in Chronic Periodontitis: In Situ, In Vivo, and In Vitro Studies. J. Immunol. 2001, 167, 4693–4700. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Zhang, Y.; Chen, L.; Zhou, T.; Huang, W.; Zhou, X.; Shao, L. The role of Toll-like receptors in periodontitis. Oral Dis. 2016, 23, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Miles, B.; Zakhary, I.; El-Awady, A.; Scisci, E.; Carrion, J.; O’Neill, J.C.; Rawlings, A.; Stern, J.K.; Susin, C.; Cutler, C.W. Secondary Lymphoid Organ Homing Phenotype of Human Myeloid Dendritic Cells Disrupted by an Intracellular Oral Pathogen. Infect. Immun. 2014, 82, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Kurotaki, D.; Kawase, W.; Sasaki, H.; Nakabayashi, J.; Nishiyama, A.; Morse, H.C.; Ozato, K.; Suzuki, Y.; Tamura, T. Epigenetic control of early dendritic cell lineage specification by the transcription factor IRF8 in mice. Blood 2019, 133, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, A.; Wu, L. The Early Progenitors of Mouse Dendritic Cells and Plasmacytoid Predendritic Cells Are within the Bone Marrow Hemopoietic Precursors Expressing Flt3. J. Exp. Med. 2003, 198, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Guilliams, M.; Ginhoux, F.; Jakubzick, C.; Naik, S.H.; Onai, N.; Schraml, B.U.; Segura, E.; Tussiwand, R.; Yona, S. Dendritic cells, monocytes and macrophages: A unified nomenclature based on ontogeny. Nat. Rev. Immunol. 2014, 14, 571–578. [Google Scholar] [CrossRef]
- Dzionek, A.; Fuchs, A.; Schmidt, P.; Cremer, S.; Zysk, M.; Miltenyi, S.; Buck, D.W.; Schmitz, J. BDCA-2, BDCA-3, and BDCA-4: Three Markers for Distinct Subsets of Dendritic Cells in Human Peripheral Blood. J. Immunol. 2000, 165, 6037–6046. [Google Scholar] [CrossRef] [PubMed]
- Lindstedt, M.; Lundberg, K.; Borrebaeck, C.A.K. Gene Family Clustering Identifies Functionally Associated Subsets of Human In Vivo Blood and Tonsillar Dendritic Cells. J. Immunol. 2005, 175, 4839–4846. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, K.; Munster, D.J.; Clark, G.J.; Dzionek, A.; Schmitz, J.; Hart, D.N.J. Characterization of human blood dendritic cell subsets. Blood 2002, 100, 4512–4520. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.R. TGFbeta1 and TNFalpha secreted by mast cells stimulated via the FcepsilonRI activate fibroblasts for high-level production of monocyte chemoattractant protein-1 (MCP-1). Cell Immunol. 2000, 201, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Waskow, C.; Liu, X.; Yao, K.; Hoh, J.; Nussenzweig, M. Origin of dendritic cells in peripheral lymphoid organs of mice. Nat. Immunol. 2007, 8, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Fucikova, J.; Palova-Jelinkova, L.; Bartunkova, J.; Spisek, R. Induction of Tolerance and Immunity by Dendritic Cells: Mechanisms and Clinical Applications. Front. Immunol. 2019, 10, 2393. [Google Scholar] [CrossRef]
- Meghil, M.; Tawfik, O.K.; Elashiry, M.; Rajendran, M.; Arce, R.M.; Fulton, D.J.; Schoenlein, P.V.; Cutler, C.W. Disruption of Immune Homeostasis in Human Dendritic Cells via Regulation of Autophagy and Apoptosis by Porphyromonas gingivalis. Front. Immunol. 2019, 10, 2286. [Google Scholar] [CrossRef]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of Cells and Tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef]
- Lamb, C.; Yoshimori, T.; Tooze, S. The autophagosome: Origins unknown, biogenesis complex. Nat. Rev. Mol. Cell Biol. 2013, 14, 759–774. [Google Scholar] [CrossRef]
- El-Awady, A.R.; Miles, B.; Scisci, E.; Kurago, Z.B.; Palani, C.D.; Arce, R.M.; Waller, J.L.; Genco, C.A.; Slocum, C.; Manning, M.; et al. Porphyromonas gingivalis Evasion of Autophagy and Intracellular Killing by Human Myeloid Dendritic Cells Involves DC-SIGN-TLR2 Crosstalk. PLOS Pathog. 2015, 11, e1004647. [Google Scholar] [CrossRef] [PubMed]
- Thurston, T.L.; Wandel, M.P.; von Muhlinen, N.; Foeglein, A.; Randow, F. Galectin 8 Targets Damaged Vesicles for Autophagy to Defend Cells against Bacterial Invasion. Nature 2012, 482, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Wild, P.; Farhan, H.; McEwan, D.G.; Wagner, S.; Rogov, V.V.; Brady, N.R.; Richter, B.; Korac, J.; Waidmann, O.; Choudhary, C.; et al. Phosphorylation of the Autophagy Receptor Optineurin Restricts Salmonella Growth. Science 2011, 333, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, T.; Fujita, N.; Jang, M.H.; Uematsu, S.; Yang, B.; Satoh, T.; Omori, H.; Noda, T.; Yamamoto, N.; Komatsu, M.; et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature 2008, 456, 264–268. [Google Scholar] [CrossRef]
- Paludan, C.; Schmid, D.; Landthaler, M.; Vockerodt, M.; Kube, D.; Tuschl, T.; Münz, C. Endogenous MHC Class II Processing of a Viral Nuclear Antigen After Autophagy. Science 2005, 307, 593–596. [Google Scholar] [CrossRef]
- Loi, M.; Müller, A.; Steinbach, K.; Niven, J.; da Silva, R.B.; Paul, P.; Ligeon, L.-A.; Caruso, A.; Albrecht, R.A.; Becker, A.C.; et al. Macroautophagy Proteins Control MHC Class I Levels on Dendritic Cells and Shape Anti-viral CD8 + T Cell Responses. Cell Rep. 2016, 15, 1076–1087. [Google Scholar] [CrossRef]
- Wei, J.; Long, L.; Yang, K.; Guy, C.; Shrestha, S.; Chen, Z.; Wu, C.; Vogel, P.; Neale, G.; Green, D.R.; et al. Autophagy enforces functional integrity of regulatory T cells by coupling environmental cues and metabolic homeostasis. Nat. Immunol. 2016, 17, 277–285. [Google Scholar] [CrossRef]
- Sanjuan, M.A.; Dillon, C.P.; Tait, S.W.G.; Moshiach, S.; Dorsey, F.; Connell, S.; Komatsu, M.; Tanaka, K.; Cleveland, J.L.; Withoff, S.; et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature 2007, 450, 1253–1257. [Google Scholar] [CrossRef]
- Nogueira, V.; Park, Y.; Chen, C.-C.; Xu, P.-Z.; Chen, M.-L.; Tonic, I.; Unterman, T.; Hay, N. Akt Determines Replicative Senescence and Oxidative or Oncogenic Premature Senescence and Sensitizes Cells to Oxidative Apoptosis. Cancer Cell 2008, 14, 458–470. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Quarles, E.; Mekvanich, S.; Kang, A.; Liu, A.; Santos, D.; Miller, R.A.; Rabinovitch, P.S.; Cox, T.C.; Kaeberlein, M. Rapamycin treatment attenuates age-associated periodontitis in mice. GeroScience 2017, 39, 457–463. [Google Scholar] [CrossRef]
- Herranz, N.; Gallage, S.; Mellone, M.; Wuestefeld, T.; Klotz, S.; Hanley, C.J.; Raguz, S.; Acosta, J.C.; Innes, A.J.; Banito, A.; et al. mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat. Cell Biol. 2015, 17, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Kucheryavenko, O.; Nelson, G.; Von Zglinicki, T.; Korolchuk, V.I.; Carroll, B. The mTORC1-autophagy pathway is a target for senescent cell elimination. Biogerontology 2019, 20, 331–335. [Google Scholar] [CrossRef]
- Steinman, R.M. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 1991, 9, 271–296. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M.; Cohn, Z.A. Identification of a novel cell type in peripheral lymphoid organs of mice: I. morphology, quantitation, tissue distribution. J. Exp. Med. 1973, 137, 1142–1162. [Google Scholar] [CrossRef] [PubMed]
- Enk, A.H.; Angeloni, V.L.; Udey, M.C.; Katz, S.I. Inhibition of Langerhans cell antigen-presenting function by IL-10. A role for IL-10 in induction of tolerance. J. Immunol. 1993, 151, 2390–2398. [Google Scholar] [PubMed]
- Simon, J.; Hara, H.; Denfeld, R.; Martin, S. UVB-Irradiated Dendritic Cells Induce Nonproliferating, Regulatory Type T Cells. Ski. Pharmacol. Physiol. 2002, 15, 330–334. [Google Scholar] [CrossRef]
- Inaba, K.; Inaba, M.; Romani, N.; Aya, H.; Deguchi, M.; Ikehara, S.; Muramatsu, S.; Steinman, R.M. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 1992, 176, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Heitbrock, L.; Ancuta, P.; Crowe, S.; Dalod, M.; Grau, V.; Hart, D.N.; Leenen, P.; Liu, Y.-J.; Mac Pherson, G.; Randolph, G.J.; et al. Nomenclature of monocytes and dendritic cells in blood. Blood 2010, 116, e74–e80. [Google Scholar] [CrossRef]
- Christensen, J.R.; Börnsen, L.; Ratzer, R.; Piehl, F.; Khademi, M.; Olsson, T.; Sørensen, P.S.; Sellebjerg, F. Systemic inflammation in progressive multiple sclerosis involves follicular T-helper, Th17- and activated B-cells and correlates with progression. PLoS ONE 2013, 8, e57820. [Google Scholar] [CrossRef]
- Cutler, C.W.; Jotwani, R.; Palucka, K.A.; Davoust, J.; Bell, D.; Banchereau, J. Evidence and a novel hypothesis for the role of dendritic cells and Porphyromonas gingivalis in adult periodontitis. J. Periodontal Res. 1999, 34, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Cutler, C.W.; Teng, Y.-T.A. Oral mucosal dendritic cells and periodontitis: Many sides of the same coin with new twists. Periodontology 2000 2007, 45, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Elashiry, M.; Elashiry, M.M.; Elsayed, R.; Rajendran, M.; Auersvald, C.; Zeitoun, R.; Rashid, M.H.; Ara, R.; Meghil, M.M.; Liu, Y.; et al. Dendritic cell derived exosomes loaded with immunoregulatory cargo reprogram local immune responses and inhibit degenerative bone disease in vivo. J. Extracell. Vesicles 2020, 9, 1795362. [Google Scholar] [CrossRef]
- Steinbrink, K.; Mahnke, K.; Grabbe, S.; Enk, A.H.; Jonuleit, H. Myeloid dendritic cell: From sentinel of immunity to key player of peripheral tolerance? Hum. Immunol. 2009, 70, 289–293. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).