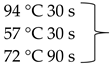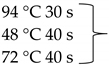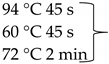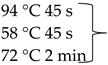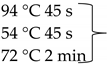Abstract
The genus Chaetomium is a frequently occurring fungal taxon world-wide. Chaetomium and Chaetomium-like species occur in indoor environments, where they can degrade cellulose-based building materials, thereby causing structural damage. Furthermore, several species of this genus may also cause adverse effects on human health. The aims of this research were to identify Chaetomium and Chaetomium-like strains isolated from indoor environments in Hungary and Finland, two geographically distant regions of Europe with drier and wetter continental climates, respectively, and to study their morphological and physiological properties, as well as their extracellular enzyme activities, thereby comparing the Chaetomium and Chaetomium-like species isolated from these two different regions of Europe and their properties. Chaetomium and Chaetomium-like strains were isolated from flats and offices in Hungary, as well as from schools, flats, and offices in Finland. Fragments of the translation elongation factor 1α (tef1α), the second largest subunit of RNA polymerase II (rpb2) and β-tubulin (tub2) genes, as well as the internal transcribed spacer (ITS) region of the ribosomal RNA gene cluster were sequenced, and phylogenetic analysis of the sequences performed. Morphological examinations were performed by stereomicroscopy and scanning electron microscopy. Thirty-one Chaetomium sp. strains (15 from Hungary and 16 from Finland) were examined during the study. The most abundant species was Ch. globosum in both countries. In Hungary, 13 strains were identified as Ch. globosum, 1 as Ch. cochliodes, and 1 as Ch. interruptum. In Finland, 10 strains were Ch. globosum, 2 strains were Ch. cochliodes, 2 were Ch. rectangulare, and 2 isolates (SZMC 26527, SZMC 26529) proved to be representatives of a yet undescribed phylogenetic species from the closely related genus Dichotomopilus, which we formally describe here as the new species Dichotomopilus finlandicus. Growth of the isolates was examined at different temperatures (4, 15, 20, 25, 30, 37, 35, 40, and 45 °C), while their extracellular enzyme production was determined spectrophotometrically.
1. Introduction
Chaetomium Kunze (Ascomycota, Sordariales) is the largest genus of the family Chaetomiaceae, present in various substrates and geographical regions [1]. More than 400 Chaetomium species have been described. The type species is Ch. globosum Kunze [2]. As cellulose-degrading fungi they possess the ability to degrade wet cellulosic building materials such as wood and plywood and synthetic building materials such as plastics and drywall [3]. Wet building materials are dominated by colonization and forming a dense mycelium covering most of the building materials, thereby damaging the building structure [4,5]. In addition to colonizing building materials, members of the genus Chaetomium are also known to produce more than 500 bioactive metabolites [6]. Chaetomium globosum, producing various mycotoxins—such as chaetoglobosin, chaetomin, chaetomugilin, and chaetoviridine—is the most common species of the genus in indoor environments [5,7].
Several Chaetomium species have been described to cause onychomycosis [8,9,10,11,12], skin surface infections [13], and cerebral mycosis [14,15,16]. Among them, some are able to opportunistically cause systemic infections and trigger severe allergic reactions that increase the risk of developing asthma [7]. On the other hand, some species of the genus Chaetomium are plant endophytes [17,18,19,20], producing high levels of various enzymes [21,22,23,24], antioxidants [25,26], as well as antifungal [25,27,28,29,30,31], antibacterial [28,32,33,34,35,36,37], and nematicidal metabolites [38,39], possessing anticancer [26,28,30,40,41,42,43,44,45,46,47,48,49,50,51] and anti-inflammatory activities [39,52], and being able to biosynthesize several types of nanoparticles [44,53,54]. The genus includes psychrotolerant [55], mesophilic, thermotolerant, and thermophilic species [5], which are widely used in the medicinal and industrial field (e.g., food, textile, and fermentation industries), agriculture (as biocontrol agents and in agricultural waste degradation), and waste processing (composting) [56].
The genus Chaetomium is generally characterized by rounded, ovoid, or obovate ostiolate ascomata covered with characteristic hairs. The walls of the ascomata are usually textura intricata (tissue of interwoven irregularly disposed hyphae with distinct interhyphal spaces, the walls not united), but they occur in textura angularis (tissue of short polyhedral cells without intercellular spaces). Ascomatal hairs can be straight (seta-like), flexible, curved, wavy, circulating, spirally curled, or otherwise branched in various morphologies. Asci are clavate or fusiform with 8 biseriate or irregularly arranged ascospores, evanescent. Ascospores are limoniform to globose, or irregular in a few species, bilaterally flattened, usually more than 7 μm in length. Asexual morphs, if present, are Acremonium-like [7].
Chaetomium is an intensively studied fungal genus worldwide, as it causes one of the biggest problems in indoor environments with damaging effects both to buildings and human health [3,4,7,54,57,58,59,60,61]. The closely related genus Dichotomopilus was first described by Wang et al. [7]; until then, members of the genus Dichotomopilus belonged to the genus Chaetomium. The genus name Dichotomopilus refers to the shape of terminal hairs of the ascomata, which are usually dichotomously branched. So far, this genus has included 12 species.
To the best of our knowledge, the diversity of this group of fungi has not yet been studied in Hungary. This study aimed to identify Chaetomium and Chaetomium-like strains isolated from Hungary and Finland, two geographically distant regions of Europe with drier and wetter continental climates, respectively, to study their morphology, determine their physiological properties, measure their extracellular enzyme activities, and compare the properties of Chaetomium and Chaetomium-like strains isolated from different sites of the two regions.
2. Results
Based on tef1α sequences, the most abundant species in this study was Ch. globosum in both countries (Table 1, Figure 1). In Hungary, 13 strains were identified as Ch. globosum, 1 as Ch. cochliodes, and 1 as Ch. interruptum, while in Finland, 10 strains were Ch. globosum, 2 strains were Ch. cochliodes, 2 were Ch. rectangulare, and 2 isolates (SZMC 26527, SZMC 26529) proved to be representatives of a yet undescribed phylogenetic species from the closely related genus Dichotomopilus (Figure 1).

Table 1.
Chaetomium and Chaetomium-like isolates, reference strains and their sequences involved in the study.
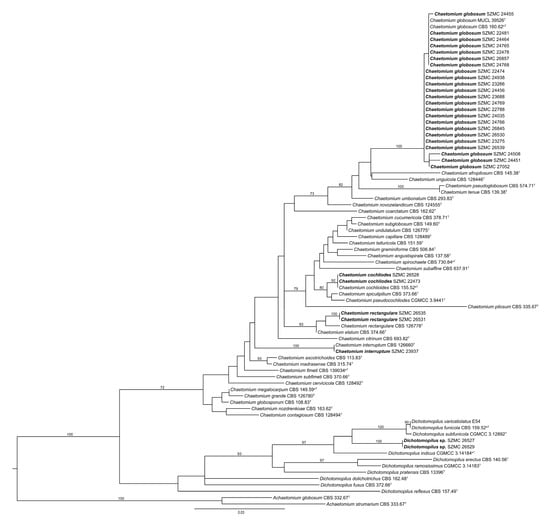
Figure 1.
Maximum likelihood phylogeny of the examined isolates (set in bold) based on translation elongation factor 1α. T = ex-type, nT = ex-neotype, eT = ex-epitype. Numbers above branches are bootstrap values. Only values greater than 70% are shown.
2.1. Morphological Features of the Isolated Chaetomium Strains
Among the previously described Chaetomium species, Ch. cochliodes colonies grew rapidly on MEA, OA, and PDA (Figure 2A) reaching 65–70 mm in diameter after 7 days at 25 °C. Hyphae were light beige on MEA, while brownish on OA and PDA, with powdery surface, undulate colony edges and without colored exudates. The strains were unable to produce ascospore-containing ascomata on MEA, while strong dark green ascospore formation was observed after 7 days on OA and PDA. Ch. interruptum (Figure 2B) formed white mycelium on all media, brownish exudates diffusing into the media, and did not produce spores during 7 days of culturing at 25 °C. On MEA and OA, it formed regular circular colonies, while on PDA the edges of the colonies grew irregularly. Colony diameters after 7 days were 40–45, 50–60, and 30–40 mm on MEA, OA, and PDA, respectively. Ch. globosum (Figure 2C) colonies overgrew both MEA and OA media in 7 days at 25 °C. On PDA the strains grew slowly, with colony diameters of 30–40 mm after 7 days and a lobate edge. No ascospores were produced on MEA medium, but greenish ascospores were produced on OA and PDA. Colonies ranged from beige (MEA, OA) to brown (PDA) in color, the surface texture was floccose or velvety, and a brownish exudate was produced on all media. Ch. rectangulare (Figure 2D) completely overgrew all media in 7 days at 25 °C with white, cottony mycelium and without colored exudates. No ascospores were produced under any of the conditions tested.
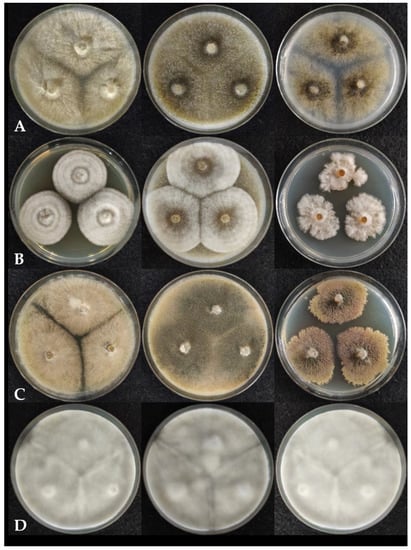
Figure 2.
Colony morphology of Chaetomium strains on different agar media left to right: MEA, OA and PDA after seven days of incubation. (A). Ch. cochliodes SZMC 22473, (B). Ch. interruptum SZMC 23937, (C). Ch. globosum SZMC 23266, (D). Ch. rectangulare SZMC 26535.
Ascomata of Ch. cochliodes (Figure 3(A1–A5)) were ostiolate, ovoid, greenish olivaceous, with brown wall, textura intricata. Terminal hairs were usually around the ostiolum, light brown or brown, spirally coiled, lateral hairs undulate or loosely coiled, tapering towards the tip. Mature ascospores were brown, limoniform, usually biapiculate at both ends, bilaterally flattened. Ascomata of Ch. globosum (Figure 3(B1–B5)) were ostiolate, greenish olivaceous, with brown wall, textura intricata. Terminal hairs were light brown or brown, undulate to loosely coiled, lateral hairs brown, flexuous, tapering towards the tips. Mature ascospores were greenish or brown, subglobose or limoniform, bilaterally flattened. Ascomata of Ch. interruptum (Figure 3(C1–C5)) were ostiolate, brown, with brown wall, textura epidermoidea (tissue of closely interwoven irregularly disposed hyphae without interhyphal spaces, the walls united, usually forming a membranous or epidermis-like tissue). Terminal hairs were brown undulate, lateral hairs brown, flexuous, tapering towards the tips. Mature ascospores were greenish or brown, subglobose, or limoniform, bilaterally flattened.

Figure 3.
Ascomata of Chaetomium strains. (A1–A5): Chaetomium cochliodes SZMC 22473, (B1–B5): Chaetomium globosum SZMC 23266, (C1–C5): Chaetomium interruptum SZMC 23937. 1—ascomata side view; 2—ascomata top view; 3—ascomatal hairs; 4—ascospores; 5—ascomatal wall.
2.2. Phylogeny and Taxonomy
The tef1α, ITS, rpb2, and tub2 dataset consisted of 935, 639, 525, and 571 characters, respectively. The indel-based binary dataset was 100 characters long. Isolates SZMC 26527 and SZMC 26529 resolved as members of a new species with high confidence values on the phylograms obtained from both tef1α (Figure 1) and the other three loci (data not shown). For the final inference the four loci were concatenated and partitioned. Based on the maximum likelihood phylogenetic tree inferred from the concatenated sequences (Figure 4), isolates SZMC 26527 and SZMC 26529 formed a well-supported distinct branch inside the genus Dichotomopilus with the closest relatives being D. funicola, D. pseudofunicola, D. subfunicola, and D. variostiolatus. This new species is described below as Dichotomopilus finlandicus sp. nov.
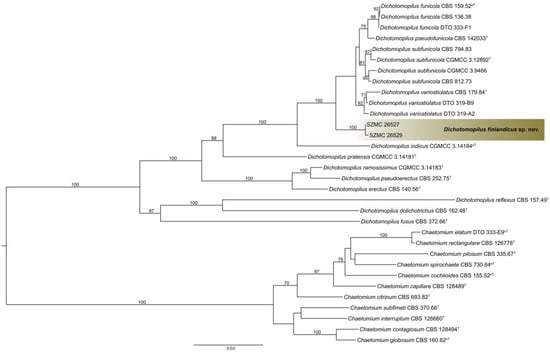
Figure 4.
Maximum likelihood phylogeny of the genus Dichotomopilus inferred from the concatenated translation elongation factor 1α (tef1α), internal transcribed spacer (ITS), second largest subunit of RNA polymerase II (rpb2), and β-tubulin (tub2) sequences. T = ex-type, nT = ex-neotype, eT = ex-epitype. Numbers above branches are bootstrap values. Only values greater than 70% are shown.
Dichotomopilus finlandicus O. Kedves, S. Kocsubé, and L. Kredics sp. nov. MycoBank accession number: 840621. Etymology: Refers to the country of origin. Colonies on PDA (Figure 5(A1)) rapidly growing, about 51–54 mm in diameter after 7 days at 25 °C, with a slightly undulate edge, usually with a floccose, white to cream mycelium, irregular concentric rings, without colored exudates, and producing grey or black ascomata in 7 days. Colonies on MEA (Figure 5(A2)) rapidly growing, approximately 55–60 mm in diameter after 7 days at 25 °C with lobate edge, not forming ascospores under seven days. Colony color yellowish-white; the surface texture folded velvety to floccose. Colonies slowly growing on OA (Figure 5(A3)) at 25 °C for 7 days, about 35–38 mm in diameter with a lobate edge; with velvety surface texture. Culture color white, producing grey or black ascomata in seven days. Colonies slowly growing on vegetable juice agar media (Figure 5(A4)), about 31–33 mm in diameter over seven days at 25 °C, with a slightly undulate edge, usually with a floccose, white to cream mycelium, without colored exudates, not producing ascomata in seven days. On DG18 agar media (Figure 5(A5)) regular circular colonies showing weak growth, 12–13 mm in diameter. Colony color orange and white, producing orange exudates. Ascomata (Figure 5, B1–C3) superficial, ostiolate, subglobose to ovoid, dark brown, 150–180 µm high and 110–130 µm wide ascocarp. Ascomatal wall (Figure 5(D1)) comprising of brown, elongated, or irregular cells (textura intricata). Terminal hairs (Figure 5(C1–C3,D1)) usually around the ostiolum, light brown, or olivaceous brown, dichotomously branched 4–6 times, up to 250–320 µm long, 3–4.5 µm in diameter at the base, at wide angles and starting primarily from the upper half part, verrucose, regularly septate. Lateral hairs unbranched, seta-like, tapering towards the tip. Asci (Figure 5(D3)) fasciculate, clavate and long stipitate, stalked, 8 irregularly-arranged ascospores, spore-bearing portion 18–19 × 7–8 μm, stalks 6–11 μm long, evanescent. Ascospores: brown, broadly ellipsoid or almond-shaped 5.12–6.42 (5.88) × 4.06–4.82 (4.42) × 2.31–3.58 (2.82) µm (length × width × thickness). Growth temperature: optimum 25–30 °C, minimum 15 °C, and maximum 38 °C. Specimens examined: A piece of inlet air filter (2 × 2 cm), public building, Espoo, Finland; Holotype: freeze dried culture specimen in the Szeged Microbiological Collection (SZMC) at the Department of Microbiology, Faculty of Science and Informatics, University of Szeged, Hungary, SZMC 26529; Non-sporulating strain: SZMC 26527 from a school building, Vantaa, Finland.
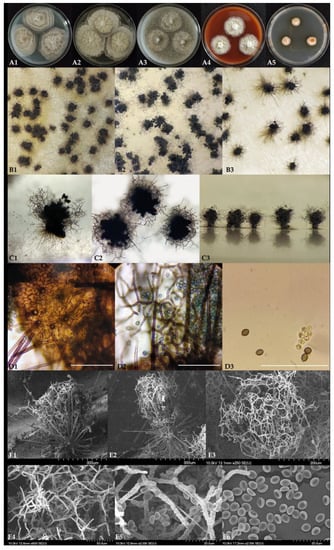
Figure 5.
Dichotomopilus finlandicus SZMC 26529. (A1–A5): colony morphology of seven-day-old cultures on different agar media (A1—PDA, A2—MEA, A3—OA, A4—vegetable juice agar, A5—DG18). (B1–B3): morphology of ascomata on different agar media (left to right OA, MEA, and PDA). (C1–C3): Ascomata C1, C3—side view, C2—top view. (D1): ascomatal wall. (D2): ascomatal hairs and ascospores. (D3): Asci and ascospores (Bars: D1–D3 50 µm). (E1–E6): Scanning electron-microscopic images of: E1,E2: ascomata; E3: terminal ascomatal hairs; E4,E5: ascomatal hairs and ascospores; E6: ascospores.
2.3. Physiological Characterization of the Isolated Chaetomium and Chaetomium-like Strains
The optimal growth temperature of all isolates was between 25–30 °C (Figure 6). The Ch. globosum strains grew at temperatures between 15–40 °C, they were unable to grow at 4 °C, and only three Hungarian Ch. globosum isolates (SZMC 22788, SZMC 24508, and SZMC 24938) grew at 40 °C (Figure 6A,B). In addition, four Hungarian isolates of Ch. globosum (SZMC 23266, SZMC 24938, SZMC 26845, and SZMC 26857) showed more intensive growth at 30 °C than at 25 °C (Figure 6B). Ch. cochliodes SZMC 22473 and SZMC 26528, Ch. interruptum SZMC 23937 and Ch. rectangulare SZMC 26535 grew at 4 °C but among these strains Ch. cochliodes SZMC 26528, Ch. interruptum SZMC 23937 and Ch. rectangulare SZMC 26535 showed no growth at 37 °C, nor Ch. cochliodes SZMC 26528 at 35 °C (Figure 6C).
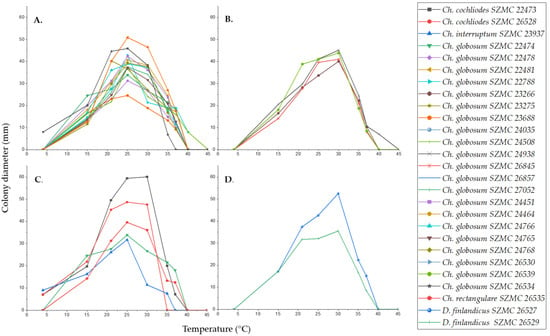
Figure 6.
Temperature effect of growth rate: Colony diameters (cm) of the examined Chaetomium and Chaetomium-like isolates on PDA after four days measured at various temperatures ranging from 4 °C to 45 °C. (A): C. globosum strains with a temperature optimum at 25 °C. (B): C. globosum strains with a temperature optimum at 30 °C. (C): strains of other Chaetomium species. (D): D. finlandicus strains.
Strains of the new species D. finlandicus (SZMC 26527 and SZMC 26529) showed similar growth at all temperatures tested (Figure 6D). Their colony diameters were the same at 15 °C (17 mm); furthermore, at higher temperatures, strain SZMC 26527 showed a slightly higher growth. However, the optimum growth temperature for both strains was around 30 °C, and both could also grow at 37 °C.
As Chaetomium and Chaetomium-like species are known as cellulolytic fungi, the polysaccharide (cellulose, hemicellulose, and chitin) degrading ability of the isolated strains was determined. The examined Chaetomium and Dichotomopilus strains showed various enzyme activities (Figure 7). Ch. globosum strains produced the highest amounts of extracellular enzymes, ranging from 20.64 to 71.67 U/mL of cellobiohydrolase, 7.57 to 18.99 U/mL of β-glucosidase, 8.54 to 41.57 U/mL of β-xylosidase, and 16.38 to 45.22 U/mL of β-1,4-N-acetyl-glucosaminidase activity. Two strains of Ch. globosum (SZMC 27052 and SZMC 26539) showed the highest cellobiohydrolase enzyme activities, which were almost identical in amount (71.13 ± 2.7 and 71.67 ± 2.3 U/mL, respectively). The smallest amount of extracellular enzymes was produced by Ch. interruptum SZMC 23937 (7.88 U/mL cellobiohydrolase, 5.29 U/mL β-glucosidase, 2.28 U/mL β-xylosidase, 6.98 U/mL β-1,4-N-acetyl-glucosaminidase) and by the two Ch. cochliodes strains SZMC 22473 and SZMC 26528 (11.31 and 8.83 U/mL cellobiohydrolase, 7.76 and 5.83 U/mL β-glucosidase, 20.88 and 20.43 U/mL β-xylosidase, 12.41 and 18.31 U/mL β-1,4-N-acetyl-glucosaminidase, respectively). The Ch. rectangulare strain SZMC 26535 also produced low amounts of polysaccharide-degrading enzymes (18.57 U/mL cellobiohydrolase, 3.24 U/mL β-glucosidase, 4.58 U/mL β-xylosidase), but the β-1,4-N-acetyl-glucosaminidase enzyme activity (39.21 U/mL) was prominent. The strains of the new species D. finlandicus (SZMC 26527 and SZMC 26529) had lower cellobiohydrolase enzyme production (32.14 and 24.91 U/mL) than the Ch. globosum strains, but in the case of the other enzymes we found similar enzyme activities (21.27 and 7.06 U/mL β-glucosidase, 29.01 and 36.08 U/mL β-xylosidase, 18.29 and 26.89 U/mL β-1,4-N-acetyl-glucosaminidase) (Figure 7).
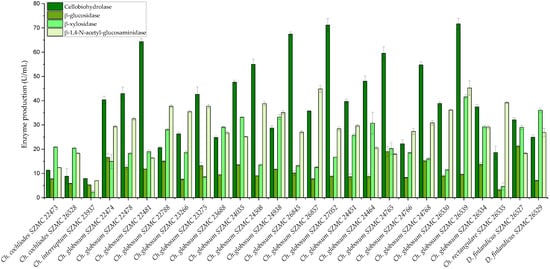
Figure 7.
Extracellular enzyme activities of the examined Chaetomium and Chaetomium-like strains. Error bars show standard deviation of three replicates.
3. Discussion
The dominant species in this study was Ch. globosum in both countries in indoor environments, as also determined in several previous studies [5,7,62]. In both countries, the species Ch. cochliodes was found to be also common in indoor environments. Ch. interruptum was isolated only from Hungary, while Ch. rectangulare and D. finlandicus only from Finland. Due to the tendency of application of cellulose-based materials (e.g., wallpapers and drywalls) in modern buildings, cellulose-degrading fungi, such as Chaetomiaceae have an increasing relevance. Most indoor strains were isolated from house dust or surface samples, while isolates from air samples were relatively rare. Similar observations were made by Fogle et al. [63] based on the analysis of samplings performed in 794 buildings in Dallas. Although several theories have emerged to explain this phenomenon, further experiments are needed to clarify the dispersal strategy of these fungi indoors.
In a previous study, Salo et al. [5] tested 42 toxin-producing Chaetomium isolates from Finland. In addition to the most common Ch. globosum, three other species, Ch. cochliodes, Ch. rectangulare, and a Chaetomium-like species were described for the first time from Finnish buildings. In a study by Vornanen-Winquist et al. [61], unknown indoor Chaetomium-like strains were designated as Dichotomophilus sp. The molecular results presented here revealed that the Chaetomium-like isolate Ch1/tu (SZMC 26529) in Salo et al. [5] and the Dichotomopilus sp. isolate C5/LM (SZMC 26527) from Vornanen-Winquist et al. [61] belong to the same new, previously undescribed species of the genus Dichotomopilus. Strain Ch1/tu was isolated from an inlet air filter and suggested to originate from the outdoor air [5], while strain C5/LM was isolated from an exhaust air filter. This may indicate that C5/LM had a possible indoor source. On the other hand, the fact that this new species was detected in both inlet and outlet air filters may also suggest that the strains were already incorporated into the filter material during production. Contamination of gypsum wall board with Chaetomium strains during production has been described by Andersen et al. [64].
The species D. finlandicus described in the recent study could be morphologically and molecularly differentiated from related species, the results of the phylogenetic analyses of the combined dataset of ITS, tef1α, rpb2, and tub2 (Figure 4) was 100% bootstrap support. In addition, the phylogenetically closest relative species D. funicola, D. pseudofunicola, D. subfunicola, D. variostiolatus, and D. indicus are morphologically different from the strain we studied. Based on the morphological properties of these species studied by Wang et al. [7], ascomata, terminal hairs, and the asci were different while the shape and the size of ascospores were similar to D. finlandicus, which we describe here as a new species.
The enzymatic activity of the Chaetomium and Chaetomium-like strains proved to be diverse, and no correlation was found with either the isolation site or the growing substrate. These results are consistent with the findings of Abdel Azeem et al. [22], that enzyme production is isolate-dependent. The authors concluded that enzyme production has no detectable association with ecology, however, although this may be true in the case of plant host specificity, we suggest the ability to produce cellulolytic enzymes as a clear ecological advantage in the case of fungal growth on cellulose-based building materials.
In the rapid screening assays described by Salo et al. [5] and Vornanen-Winquist et al. [65], Dichotomopilus strains gave weaker responses than the Ch. globosum, Ch. cochliodes and Ch. rectangulare strains. However, strain Ch1/tu (SZMC 26529), which was designated here as the type strain of the newly described species D. finlandicus, inhibited boar sperm motility after 3 d of exposure, indicating that the strain produced a bioactive agent possibly affecting mitochondrial functions, or ion homeostasis [66]. Purification and identification of this substance and characterization of its biological activities will be the subject of further research.
4. Materials and Methods
4.1. Sample Collection and Isolation
Chaetomium and Chaetomium-like strains were collected and isolated from schools, flats, and offices in Finland as described previously by Salo et al. [5], as well as from houses, flats, and offices in Hungary (Table 1). To collect fungi from walls, visible colonies, or wet surfaces detected by moisture meter (Greisinger GMI 15) were sampled with sterile swabs. House dust samples were also collected with swabs. Samples were spread directly onto malt extract agar (MEA) supplemented with 2% chloramphenicol, Dichloran - Rose Bengal Agar, or Casitone Agar on site. To collect airborne fungi, air samples of 100 L were collected at 150 cm a.g.l. with 400-hole one-stage Andersen samplers [67] (MAS 100, EMD Millipore, Merck, Darmstadt, Germany; SAS IAQ, International PBI SpA, Milan, Italy; Samp l’Air MK2, AES Chemunex, Bruz, France), at a flow of 100 L/min onto MEA. Between samplings, the devices were sterilized with ethanol (abs.). Incubation of the samples was performed for 5 to 7 days at room temperature. The isolated pure cultures were deposited in the Szeged Microbiology Collection (SZMC, http://szmc.hu), Szeged, Hungary.
4.2. Morphological Characterization
The morphology and colony characteristics of the isolates were examined on three different media: 2% (w/v) MEA (VWR, Debrecen, Hungary), 3% (w/v) oatmeal agar (OA, Merck, Darmstadt, Germany), and potato dextrose agar (PDA, VWR, Debrecen, Hungary), and incubated for seven days in the dark at 25 °C [2,7]. Microscopic studies were performed using light—(Zeiss Primostar, Carl Zeiss, Suzhou, China), stereo—(Zeiss Stemi 305, Carl Zeiss, Suzhou, China), and scanning electron microscopes. SEM samples were prepared by stabilization in 0.1 M phosphate buffer (pH 7.3) containing 2.5% glutaraldehyde (12 h, 4 °C). The samples were then dehydrated with ethanol-water, gradually increasing the volume ratio of ethanol (50% v/v, 60% v/v, 70% v/v, 80% v/v, 90% v/v, 95% v/v, 100% v/v). Finally, the samples were dried (3 h, 30 °C) and coated with gold for microscopic examination. Electron microscopy images were taken with a 10 kV accelerating voltage Hitachi S-4700 Type II FE-SEM microscope, observing secondary electrons with magnitudes of 150×, 250×, 600×, and 2000× [68].
4.3. DNA Extraction, Identification, and Phylogenetic Analysis
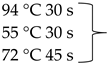
Pure cultures of fungi were grown in 2% (w/v) MEA for 7 days at room temperature. Fungal genomic DNA was then extracted using the E.Z.N.A.®Fungal DNA Mini Kit (Omega Biotek, Norcross, GA, USA). The extracted genomic DNA was amplified by PCR with the primers listed in Table 2. The PCR mixture (20 μL) contained 2 μL 10× DreamTaq Buffer with 20 mM MgCl2, 2 μL of 2 mM dNTP mix, 4 μL of each primer (100 μM), 7 μL bidistilled water, 0.1 μL of 5 U/μL DreamTaq DNA Polymerase (Thermo Fischer Scientific, Vilnius, Lithuania) and 1 μL genomic DNA. Amplifications were performed in a Doppio Gradient 2 × 48-well thermal cycler (VWR International, Debrecen, Hungary) according to the amplification cycles shown in Table 2. PCR products were purified using NucleoSpin™ Gel and PCR Clean-up Kit (Macherey-Nagel, Düren, Germany). Sequencing was performed on the sequencing platform of Eurofins Genomics (http://www.eurofinsgenomics.com, accessed on 2 September 2021). The resulting sequences were submitted to the GenBank Nucleotide database (ncbi.nlm.nih.gov) under the accession numbers listed in Table 1. In addition to the sequences generated in this study, sequences of reference strains were obtained from the GenBank Nucleotide database (Table 1).

Table 2.
List of the amplified genes, used primers, and PCR conditions.
Sequences of the two Dichotomopilus isolates were aligned with publicly available sequences of 12 and 11 previously described Dichotomopilus and Chaetomium species, respectively. Phylogenetic analyses were conducted using four loci (ITS, tef1α, rpb2, and tub2).
Sequences were aligned with Prank v170427 [69]. Alignments of the four loci were concatenated and partitioned. Tef1α and rpb2 sequences were defined as two single partitions, while the tub2 dataset was partitioned to exons and introns. The ITS dataset was divided to rDNA and ITS1-ITS2 regions. Alignments of tub2 and ITS datasets contained relative high number of indels, therefore gaps were coded as absence/presence characters by 2matrix v1.0 [70] using the simple indel coding algorithm [71]. The two indel matrices were concatenated and added as a single partition to the dataset. Best fitting model for the phylogenetic inference was selected by using ModelTest-NG v0.1.4 [72], based on the Bayesian information criterion [73], with discrete gamma rate categories. Best fit models for each partition are shown in Table 3. Maximum likelihood analysis was performed using RAxML-NG v0.9.0 [74]. Statistical support of the best ML tree was obtained with 1000 bootstrap replicates.

Table 3.
Best-fit models for each partition proposed by ModelTest-NG based on Bayesian information criterion.
4.4. Enzyme Production
For enzyme activity measurements, fungal strains were grown in cellulose-containing broth (20 g/L mannitol, 10 g/L KH2PO4, 5 g/L NaNO3, 2 g/L MgSO4·7H2O, 20 g/L cellulose powder) for seven days at 25 °C with shaking (150 rpm) on a MaxQ 8000 Incubated Stackable Shaker (Thermo Fisher Scientific, Waltham, USA). Enzyme activity measurements were performed from culture supernatants with the chromogenic substrates 4-nitrophenyl-β-D-glucopyranoside (β-glucosidase), 4-nitrophenyl-β-D-cellobiose (cellobiohydrolase), 4-nitrophenyl-β-D-xylopyranoside (β-xylosidase), 4-nitrophenyl-N-acetyl-β-D-glucosamine (β-1,4-N-acetyl-glucosaminidase) (Sigma Aldrich, St. Louis, MO, USA). 100 µL of the culture supernatants were pipetted into the wells of a microtiter plate and 100 µL of 3 mM 4-nitrophenyl substrate was added. The mixtures were incubated at 37 °C for 1 hour; thereafter the reactions were stopped by the addition of 100 μL Na2CO3 solution (0.1 M). The released 4-nitrophenol was measured on a Spectrostar Nano microtiter plate reader (BMG Labtech, Ortenberg, Germany) at 405 nm. The optical density values obtained were converted to units: 1 unit of enzyme activity was defined as the amount of enzyme required to release 1 µmol of p-nitrophenol per min under the determined reaction conditions. Calibration curve was prepared using standard solutions of 4-nitrophenol of known concentration.
4.5. Temperature Profiling
Optimal growth temperature ranges were determined for all Chaetomium and Chaetomium-like isolates. PDA plates were inoculated with 7 mm agar plates taken from the edge of seven-day-old colonies. The plates were incubated at 4, 15, 21, 25, 30, 35, 37, 40, and 45 °C, with six replicates each. Colony diameters were measured after four days.
Author Contributions
Conceptualization, O.K. and L.K.; Methodology, O.K., T.B., M.A.A., J.M.S., R.M., H.S., A.K. and Z.K.; Software, S.K. and A.S.; Validation, J.M.S., S.K., A.S., Z.K., C.V. and D.M.; Formal analysis, O.K., S.K. and A.S.; Investigation, O.K., T.B., M.A.A., R.M. and A.K.; Resources, H.S., Z.K., C.V. and D.M.; Data curation, O.K., M.A.A., D.M., S.K. and L.K.; Writing—original draft, O.K., S.K., M.A.A., A.S., A.K., D.M. and L.K.; Writing—review and editing, O.K., M.A.A., J.M.S., H.S., R.M., C.V., Z.K., D.M. and L.K.; Visualization, O.K., S.K. and A.K..; Supervision, O.K., Z.K. and L.K.; Project administration, H.S., D.M. and L.K.; Funding acquisition, O.K., M.A.A., H.S. and R.M. All authors have read and agreed to the published version of the manuscript.
Funding
O.K. was supported by the ÚNKP-20-3—New National Excellence Program of the Ministry for Innovation and Technology from the source of the National Research, Development and Innovation Fund. M.A.A., H.S. and R.M. are grateful to the Academy of Finland (CleanSchool-project, grant no. 330150) for financial support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Nucleotide sequences were deposited in the GenBank Nucleotide Database (https://www.ncbi.nlm.nih.gov), accession numbers are provided in Table 1.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rodríguez, K.; Stchigel, A.; Guarro, J. Three new species of Chaetomium from soil. Mycologia 2002, 94, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.W.; Wang, X.L.; Liu, F.J.; Zhao, X.M.; Li, J.; Cai, L. Phylogenetic assessment of Chaetomium indicum and allied species, with the introduction of three new species and epitypification of C. funicola and C. indicum. Mycol. Prog. 2014, 13, 719–732. [Google Scholar] [CrossRef]
- Došen, I.; Nielsen, K.F.; Clausen, G.; Andersen, B. Potentially harmful secondary metabolites produced by indoor Chaetomium species on artificially and naturally contaminated building materials. Indoor Air 2017, 27, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Andersen, B.; Frisvad, J.C.; Søndergaard, I.; Rasmussen, I.S.; Larsen, L.S. Associations between fungal species and water-damaged building materials. Appl. Environ. Microbiol. 2011, 77, 4180–4188. [Google Scholar] [CrossRef]
- Salo, J.M.; Kedves, O.; Mikkola, R.; Kredics, L.; Andersson, M.A.; Kurnitski, J.; Salonen, H. Detection of Chaetomium globosum, Ch. cochliodes and Ch. rectangulare during the diversity tracking of mycotoxin-producing Chaetomium-like isolates obtained in buildings in Finland. Toxins 2020, 12, 443. [Google Scholar] [CrossRef]
- Castagnoli, E.; Mikkola, R.; Vornanen-Winquist, C.; Andersson, M.; Kredics, L.; Marik, T.; Kurnitski, J.; Salonen, H. Indoor Chaetomium-like isolates; resistance to chemicals, fluorescence and mycotoxin production. Sisäilmastoseminaari 2017, 35, 227–232. [Google Scholar]
- Wang, X.W.; Houbraken, J.; Groenewald, J.Z.; Meijer, M.; Andersen, B.; Nielsen, K.F.; Crous, P.W.; Samson, R.A. Diversity and taxonomy of Chaetomium and Chaetomium-like fungi from indoor environments. Stud. Mycol. 2016, 84, 145–224. [Google Scholar] [CrossRef]
- Stiller, M.J.; Rosenthal, S.; Summerbell, R.C.; Pollack, J.; Chan, A. Onychomycosis of the toenails caused by Chaetomium globosum. J. Am. Acad. Dermatol. 1992, 26, 775–776. [Google Scholar] [CrossRef]
- Aspiroz, C.; Gené, J.; Rezusta, A.; Charlez, L.; Summerbell, R.C. First Spanish case of onychomycosis caused by Chaetomium globosum. Med. Mycol. 2007, 45, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.M.; Suh, M.K.; Ha, G.Y. Onychomycosis due to nondermatophytic molds. Ann. Dermatol. 2012, 24, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.M.; Lee, M.H.; Suh, M.K.; Ha, G.Y.; Kim, H.; Choi, J.S. Onychomycosis caused by Chaetomium globosum. Ann. Dermatol. 2013, 25, 232–236. [Google Scholar] [CrossRef]
- Shi, D.; Lu, G.; Mei, H.; de Hoog, G.S.; Zheng, H.; Liang, G.; Shen, Y.; Li, T.; Liu, W. Onychomycosis due to Chaetomium globosum with yellowish black discoloration and periungual inflammation. Med. Mycol. Case Rep. 2016, 13, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Hubka, V.; Mencl, K.; Skorepova, M.; Lyskova, P.; Zalabska, E. Phaeohyphomycosis and onychomycosis due to Chaetomium spp., including the first report of Chaetomium brasiliense infection. Med. Mycol. 2011, 49, 724–733. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abbott, S.P.; Sigler, L.; McAleer, R.; McGough, D.A.; Rinaldi, M.G.; Mizell, G. Fatal cerebral mycoses caused by the ascomycete Chaetomium strumarium. J. Clin. Microbiol. 1995, 33, 2692–2698. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Mileusnic, D.; Carey, R.B.; Kampert, M.; Anderson, D. Fatal Chaetomium cerebritis in a bone marrow transplant patient. Hum. Pathol. 1999, 874–879. [Google Scholar] [CrossRef]
- Barron, M.A.; Sutton, D.A.; Veve, R.; Guarro, J.; Rinaldi, M.; Thompson, E.; Cagnoni, P.J.; Moultney, K.; Madinger, N.E. Invasive mycotic infections caused by Chaetomium perlucidum, a new agent of cerebral phaeohyphomycosis. J. Clin. Microbiol. 2003, 41, 5302–5307. [Google Scholar] [CrossRef][Green Version]
- Gao, K.; Liu, X.; Kang, Z.; Mendgen, K. Mycoparasitism of Rhizoctonia solani by endophytic Chaetomium spirale ND35: Ultrastructure and cytochemistry of the interaction. J. Phytopathol. 2005, 153, 280–290. [Google Scholar] [CrossRef]
- Ding, G.; Song, Y.C.; Chen, J.R.; Xu, C.; Ge, H.M.; Wang, X.T.; Tan, R.X. Chaetoglobosin U, a cytochalasan alkaloid from endophytic Chaetomium globosum IFB-E019. J. Nat. Prod. 2006, 69, 302–304. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Kulkarni, G.; Sonawane, M.S.; Shouche, Y.S. A new endophytic species of Chaetomium from Jatropha podagrica. Mycotaxon 2013, 124, 117–126. [Google Scholar] [CrossRef]
- Kamat, S.; Kumari, M.; Sajna, K.V.; Jayabaskaran, C. Endophytic fungus, Chaetomium globosum, associated with marine green alga, a new source of Chrysin. Sci. Rep. 2020, 10, 18726. [Google Scholar] [CrossRef]
- Lakshmikant; Kamal; Mathur, S.N. Cellulolytic activities of Chaetomium globosum on different cellulosic substrates. World J. Microbiol. Biotechnol. 1990, 6, 23–26. [Google Scholar] [CrossRef]
- Abdel-Azeem, A.M.; Gherbawy, Y.A.; Sabry, A.M. Enzyme profiles and genotyping of Chaetomium globosum isolates from various substrates. Plant Biosyst. 2016, 150, 420–428. [Google Scholar] [CrossRef]
- Wanmolee, W.; Sornlake, W.; Rattanaphan, N.; Suwannarangsee, S.; Laosiripojana, N.; Champreda, V. Biochemical characterization and synergism of cellulolytic enzyme system from Chaetomium globosum on rice straw saccharification. BMC Biotechnol. 2016, 16, 82. [Google Scholar] [CrossRef]
- Zhou, Q.; Jia, J.; Ji, P.; Han, C. A novel application potential of GH6 cellobiohydrolase ctcel6 from thermophilic Chaetomium thermophilum for gene cloning, heterologous expression and biological characterization. Int. J. Agric. Biol. 2017, 19, 355–362. [Google Scholar] [CrossRef]
- Li, H.; Liao, Z.B.; Tang, D.; Han, W.B.; Zhang, Q.; Gao, J.M. Polyketides from two Chaetomium species and their biological functions. J. Antibiot. 2018, 71, 677–681. [Google Scholar] [CrossRef]
- Wang, Z.; Jia, S.; Cui, J.; Qu, J.; Yue, Y.; Sun, Q.; Zhang, H. Antioxidant activity of a polysaccharide produced by Chaetomium globosum CGMCC 6882. Int. J. Biol. Macromol. 2019, 141, 955–960. [Google Scholar] [CrossRef]
- Serena, C.; Ortoneda, M.; Capilla, J.; Pastor, F.J.; Sutton, D.A.; Rinaldi, M.G.; Guarro, J. In vitro activities of new antifungal agents against Chaetomium spp. and inoculum standardization. Antimicrob. Agents Chemother. 2003, 47, 3161–3164. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.; Feng, Y.; Blunt, J.W.; Cole, A.L.J.; Munro, M.H.G. Chaetoglobosins Q, R, and T, three further new metabolites from Chaetomium globosum. J. Nat. Prod. 2004, 67, 1722–1725. [Google Scholar] [CrossRef]
- Park, J.H.; Gyung, J.C.; Kyoung, S.J.; He, K.L.; Heung, T.K.; Kwang, Y.C.; Kim, J.C. Antifungal activity against plant pathogenic fungi of chaetoviridins isolated from Chaetomium globosum. FEMS Microbiol. Lett. 2005, 252, 309–313. [Google Scholar] [CrossRef]
- Huang, S.; Chen, H.; Li, W.; Zhu, X.; Ding, W.; Li, C. Bioactive chaetoglobosins from the mangrove endophytic fungus Penicillium chrysogenum. Mar. Drugs 2016, 14, 172. [Google Scholar] [CrossRef]
- Yan, W.; Cao, L.L.; Zhang, Y.Y.; Zhao, R.; Zhao, S.S.; Khan, B.; Ye, Y.H. New metabolites from endophytic fungus Chaetomium globosum CDW7. Molecules 2018, 23, 2873. [Google Scholar] [CrossRef]
- Chovanová, K.; Zámocký, M. Detection of the antibacterial effect of Chaetomium cochliodes Palliser CCM F-232 based on agar plugs and unprocessed fungal substances from cultivation media. Biologia 2016, 71, 1204–1211. [Google Scholar] [CrossRef]
- Dissanayake, R.K.; Ratnaweera, P.B.; Williams, D.E.; Wijayarathne, C.D.; Wijesundera, R.L.C.; Andersen, R.J.; de Silva, E.D. Antimicrobial activities of endophytic fungi of the Sri Lankan aquatic plant Nymphaea nouchali and chaetoglobosin A and C, produced by the endophytic fungus Chaetomium globosum. Mycology 2016, 7, 1–8. [Google Scholar] [CrossRef]
- Gao, W.; He, Y.; Li, F.; Chai, C.; Zhang, J.; Guo, J.; Chen, C.; Wang, J.; Zhu, H.; Hu, Z.; et al. Antibacterial activity against drug-resistant microbial pathogens of cytochalasan alkaloids from the arthropod-associated fungus Chaetomium globosum TW1-1. Bioorg. Chem. 2019, 83, 98–104. [Google Scholar] [CrossRef]
- Wang, Z.; Xue, R.; Cui, J.; Wang, J.; Fan, W.; Zhang, H.; Zhan, X. Antibacterial activity of a polysaccharide produced from Chaetomium globosum CGMCC 6882. Int. J. Biol. Macromol. 2019, 125, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Wani, N.; Khanday, W.; Tirumale, S. Evaluation of In vitro antioxidant potential of active metabolite constituents of different extracts of Chaetomium cupreum-SS02 by spectrophotometric method. Matrix Sci. Pharma 2020, 4, 50. [Google Scholar] [CrossRef]
- Attia, E.; Dashora, K.; Abdel-azeem, A. A potential antimicrobial, extracellular enzymes, and antioxidants resource: Endophytic fungi associated with medicinal plants. Int. J. Biosci. 2020, 17, 119–132. [Google Scholar] [CrossRef]
- Khan, B.; Yan, W.; Wei, S.; Wang, Z.; Zhao, S.; Cao, L.; Rajput, N.A.; Ye, Y. Nematicidal metabolites from endophytic fungus Chaetomium globosum YSC5. FEMS Microbiol. Lett. 2019, 366, fnz169. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, W.; Zhang, P.; Ruan, W.; Zhu, X. Nematicidal activity of chaetoglobosin A poduced by Chaetomium globosum NK102 against Meloidogyne incognita. J. Agric. Food Chem. 2013, 61, 41–46. [Google Scholar] [CrossRef]
- Chen, C.; Tong, Q.; Zhu, H.; Tan, D.; Zhang, J.; Xue, Y.; Yao, G.; Luo, Z.; Wang, J.; Wang, Y.; et al. Nine new cytochalasan alkaloids from Chaetomium globosum TW1-1 (Ascomycota, Sordariales). Sci. Rep. 2016, 6, 18711. [Google Scholar] [CrossRef]
- Ruan, B.H.; Yu, Z.F.; Yang, X.Q.; Yang, Y.B.; Hu, M.; Zhang, Z.X.; Zhou, Q.Y.; Zhou, H.; Ding, Z.T. New bioactive compounds from aquatic endophyte Chaetomium globosum. Nat. Prod. Res. 2018, 32, 1050–1055. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, P.; Tao, N.; Zhang, H.; Li, R.; Zhan, X.; Wang, F.; Shen, Y. Anticancer activity of polysaccharides produced from glycerol and crude glycerol by an endophytic fungus Chaetomium globosum CGMCC 6882 on human lung cancer A549 cells. Biomolecules 2018, 8, 171. [Google Scholar] [CrossRef] [PubMed]
- Senthil Kumar, V.; Kumaresan, S.; Tamizh, M.M.; Hairul Islam, M.I.; Thirugnanasambantham, K. Anticancer potential of NF-κB targeting apoptotic molecule “flavipin” isolated from endophytic Chaetomium globosum. Phytomedicine 2019, 61, 152830. [Google Scholar] [CrossRef]
- Wani, N.; Khanday, W.; Tirumale, S. Biosynthesis of iron oxide nanoparticles using ethyl acetate extract of Chaetomium cupreum and their anticancer activity. Matrix Sci. Pharma 2020, 4, 23. [Google Scholar] [CrossRef]
- Wijeratne, E.M.K.; Turbyville, T.J.; Fritz, A.; Whitesell, L.; Gunatilaka, A.A.L. A new dihydroxanthenone from a plant-associated strain of the fungus Chaetomium globosum demonstrates anticancer activity. Bioorg. Med. Chem. 2006, 14, 7917–7923. [Google Scholar] [CrossRef]
- Ming Ge, H.; Yun Zhang, W.; Ding, G.; Saparpakorn, P.; Chun Song, Y.; Hannongbua, S.; Xiang Tan, R. Chaetoglobins A and B, two unusual alkaloids from endophytic Chaetomium globosum culture. Chem. Commun. 2008, 45, 5978–5980. [Google Scholar] [CrossRef] [PubMed]
- Thohinung, S.; Kanokmedhakul, S.; Kanokmedhakul, K.; Kukongviriyapan, V.; Tusskorn, O.; Soytong, K. Cytotoxic 10-(indol-3-yl)-[13]cytochalasans from the fungus Chaetomium elatum ChE01. Arch. Pharm. Res. 2010, 33, 1135–1141. [Google Scholar] [CrossRef]
- Li, H.; Xiao, J.; Gao, Y.Q.; Tang, J.J.; Zhang, A.L.; Gao, J.M. Chaetoglobosins from Chaetomium globosum, an endophytic fungus in ginkgo biloba, and their phytotoxic and cytotoxic activities. J. Agric. Food Chem. 2014, 62, 3734–3741. [Google Scholar] [CrossRef]
- Zheng, Q.C.; Kong, M.Z.; Zhao, Q.; Chen, G.D.; Tian, H.Y.; Li, X.X.; Guo, L.D.; Li, J.; Zheng, Y.Z.; Gao, H. Chaetoglobosin Y, a new cytochalasan from Chaetomium globosum. Fitoterapia 2014, 93, 126–131. [Google Scholar] [CrossRef]
- Hamed Eman, M.H. Anticancer compounds from Chaetomium globosum. Biochem. Anal. Biochem. 2015, 4, 174. [Google Scholar] [CrossRef]
- Chen, C.; Shao, Y.; Tao, Y.; Wen, H. Optimization of dynamic microwave-assisted extraction of Armillaria polysaccharides using RSM, and their biological activity. LWT-Food Sci. Technol. 2015, 64, 1263–1269. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X.; Bao, Y.; Wang, X.; Zhai, J.; Zhan, X.; Zhang, H. Characterization and anti-inflammation of a polysaccharide produced by Chaetomium globosum CGMCC 6882 on LPS-induced RAW 264.7 cells. Carbohydr. Polym. 2021, 251, 117129. [Google Scholar] [CrossRef] [PubMed]
- Madbouly, A.K.; Abdel-Aziz, M.S.; Abdel-Wahhab, M.A. Biosynthesis of nanosilver using Chaetomium globosum and its application to control Fusarium wilt of tomato in the greenhouse. IET Nanobiotechnol. 2017, 11, 702–708. [Google Scholar] [CrossRef]
- El-Mohamedy, R.S.R.; El-Gamal, N.G.; El-Shamy, A.R.; Atalla, S.M.M. Biosynthesis of zinc nanoparticles and its effect on enzymes production by Bacillus subtilis and Pseudomonas fluorescens using different agricultural wastes. Int. J. Agric. Technol. 2018, 14, 833–844. [Google Scholar]
- Wang, X.W.; Zheng, R.Y. Chaetomium acropullum sp. nov. (Chaetomiaceae, Ascomycota), a new psychrotolerant mesophilic species from China. Nova Hedwigia 2005, 80, 413–417. [Google Scholar] [CrossRef]
- Abdel-Azeem, A.M.; Abu-Elsaoud, A.M.; Abo Nahas, H.H.; Abdel-Azeem, M.A.; Balbool, B.A.; Mousa, M.K.; Ali, N.H.; Darwish, A.M.G. Biodiversity and industrial applications of genus Chaetomium. In Industrially Important Fungi for Sustainable Development; Abdel-Azeem, A.M., Yadav, A.N., Yadav, N., Usmani, Z., Eds.; Springer: Cham, Switzerland, 2021; Volume 1, pp. 147–206. [Google Scholar]
- Gatumbi, R.W.; Kung’u, J.N. Notes on species of the genus Chaetomium from Kenya. East. Afr. Agric. For. J. 1994, 60, 79–89. [Google Scholar] [CrossRef]
- Udagawa, S.I. A new species of Chaetomium from house dust. Mycoscience 1997, 38, 399–402. [Google Scholar] [CrossRef]
- Andersen, B.; Nissen, A.T. Evaluation of media for detection of Stachybotrys and Chaetomium species associated with water-damaged buildings. Int. Biodeterior. Biodegrad. 2000, 46, 111–116. [Google Scholar] [CrossRef]
- Piecková, E. In vitro toxicity of indoor Chaetomium kunze ex fr. Ann. Agric. Environ. Med. 2003, 10, 9–14. [Google Scholar]
- Vornanen-Winqvist, C.; Järvi, K.; Andersson, M.A.; Duchaine, C.; Létourneau, V.; Kedves, O.; Kredics, L.; Mikkola, R.; Kurnitski, J.; Salonen, H. Exposure to indoor air contaminants in school buildings with and without reported indoor air quality problems. Environ. Int. 2020, 141, 105781. [Google Scholar] [CrossRef] [PubMed]
- McMullin, D.R.; Sumarah, M.W.; Miller, J.D. Chaetoglobosins and azaphilones produced by Canadian strains of Chaetomium globosum isolated from the indoor environment. Mycotoxin Res. 2013, 29, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Fogle, M.R.; Douglas, D.R.; Jumper, C.A.; Straus, D.C. Growth and mycotoxin production by Chaetomium globosum. Mycopathologia 2007, 164, 49–56. [Google Scholar] [CrossRef]
- Andersen, B.; Dosen, I.; Lewinska, A.M.; Nielsen, K.F. Pre-contamination of new gypsum wallboard with potentially harmful fungal species. Indoor Air 2017, 27, 6–12. [Google Scholar] [CrossRef]
- Vornanen-Winqvist, C.; Järvi, K.; Toomla, S.; Ahmed, K.; Andersson, M.A.; Mikkola, R.; Marik, T.; Kredics, L.; Salonen, H.; Kurnitski, J. Ventilation positive pressure intervention effect on indoor air quality in a school building with moisture problems. Int. J. Environ. Res. Public Health 2018, 15, 230. [Google Scholar] [CrossRef]
- Castagnoli, E.; Salo, J.; Toivonen, M.S.; Marik, T.; Mikkola, R.; Kredics, L.; Vicente-Carrillo, A.; Nagy, S.; Andersson, M.T.; Andersson, M.A.; et al. An evaluation of boar spermatozoa as a biosensor for the detection of sublethal and lethal toxicity. Toxins 2018, 10, 463. [Google Scholar] [CrossRef]
- Andersen, A.A. New sampler for the collection, sizing, and enumeration of viable airborne particles. J. Bacteriol. 1958, 76, 471–484. [Google Scholar] [CrossRef]
- Kedves, A.; Rónavári, A.; Kónya, Z. Long-term effect of graphene oxide on the aerobic granular sludge wastewater treatment process. J. Environ. Chem. Eng. 2021, 9, 104853. [Google Scholar] [CrossRef]
- Löytynoja, A. Phylogeny-aware alignment with PRANK. Meth. Mol. Biol. 2014, 1079, 155–170. [Google Scholar] [CrossRef]
- Salinas, N.R.; Little, D.P. 2matrix: A utility for indel coding and phylogenetic matrix concatenation. Appl. Plant Sci. 2014, 2, 1300083. [Google Scholar] [CrossRef] [PubMed]
- Simmons, M.P.; Ochoterena, H. Gaps as characters in sequence-based phylogenetic analysis. Syst. Biol. 2000, 49, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Posada, D.; Kozlov, A.M.; Stamatakis, A.; Morel, B.; Flouri, T. ModelTest-NG: A new and scalable tool for the selection of DNA and protein evolutionary models. Mol. Biol. Evol. 2020, 37, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, G. Estimating the dimension of a model. Ann. Stat. 1978, 6, 461–464. [Google Scholar] [CrossRef]
- Kozlov, A.M.; Darriba, D.; Flouri, T.; Morel, B.; Stamatakis, A. RAxML-NG: A fast, scalable, and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 2019, 35, 4453–4455. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).