Abstract
Dolutegravir (DTG) is a potent anti-HIV drug that is used to treat HIV globally. There have been reports of mutations in the HIV-1 3′-polypurine tract (3′PPT) of the nef gene, contributing to DTG failure; however, there are limited ‘real-world’ data on this. In addition, there is a knowledge gap on the variability of 3′PPT residues in patients receiving combination antiretroviral therapy (cART) with and without viral load (VL) suppression. HIV-1 subtype C (HIV-1C) whole-genome sequences from cART naïve and experienced individuals were generated using next-generation sequencing. The nef gene sequences were trimmed from the generated whole-genome sequences using standard bioinformatics tools. In addition, we generated separate integrase and nef gene sequences by Sanger sequencing of plasma samples from individuals with virologic failure (VF) while on a DTG/raltegravir (RAL)-based cART. Analysis of 3′PPT residues was performed, and comparison of proportions computed using Pearson’s chi-square test with p-values < 0.05 was considered statistically significant. A total of 6009 HIV-1C full genome sequences were generated and had a median log10 HIV-1 VL (Q1, Q3) copies/mL of 1.60 (1.60, 2.60). A total of 12 matching integrase and nef gene sequences from therapy-experienced participants failing DTG/ RAL-based cART were generated. HIV-1C 3′PPT nef gene sequences from therapy-experienced patients failing DTG cART (n = 12), cART naïve individuals (n = 1263), and individuals on cART with and without virological suppression (n = 4696) all had a highly conserved 3′PPT motif with no statistically significant differences identified. Our study confirms the high conservation of the HIV-1 nef gene 3′PPT motif in ‘real-world’ patients and showed no differences in the motif according to VL suppression or INSTI-based cART failure. Future studies should explore other HIV-1 regions outside of the pol gene for associations with DTG failure.
1. Introduction
Dolutegravir (DTG) is a widely used second-generation integrase strand transfer inhibitor (INSTI) with a high genetic barrier to resistance [1,2,3,4,5]. It prevents the HIV integrase enzyme from incorporating viral DNA into the host cell genome [1]. However, resistance mutations within the integrase enzyme can cause reduced susceptibility to DTG [1,6]. Most major DTG resistance mutations amongst therapy-experienced individuals are usually located within the HIV-1 catalytic core domain of the integrase region [1,7]. However, a handful of studies have suggested associations between DTG failure and mutations outside the integrase region in the 3′-polypurine tract (3′PPT) of the HIV-1 nef gene [8,9].
The nef gene of HIV-1 is a small accessory protein of about 206 amino acids that contributes to HIV disease progression mainly by downregulating the expression of CD4 and major histocompatibility complex class I molecules, amongst other functions [10,11,12,13]. It has a highly conserved purine-dominated 15-nucleotide sequence (3′PPT) that is involved in the reverse transcription process, resulting in the production of double-stranded viral DNA, enabling the integration into the host cell genome [14,15,16].
Malet et al. unexpectedly cultured a virus that had mutations in the 3′PPT motif conferring resistance to DTG [8]. Similar mutations were identified in the guanine-tract (G-tract) motif at the 3′ end of 3′PPT of one patient with virologic failure (VF) while on a DTG monotherapy trial [9].
A subsequent study by Malet et al. with a larger number of individuals failing INSTI-based regimens revealed a highly conserved 3′PPT with no associations with DTG failure discernible [17]. Further in vitro work went on to confirm this [18]. A recent study from Cameroon amongst INSTI-naïve individuals also showed a highly conserved 3′PPT region [19]. Furthermore, analysis of publicly available HIV-1 nef gene sequences from the Los Alamos HIV-1 database reveals a highly conserved 3′PPT region across various subtypes [17].
Given these inconsistent study results and the fact that there are limited ‘real-world’ data on the contribution of 3′PPT to failure of DTG-based regimens, we conducted this study to address these knowledge gaps. In our study, we sought to determine the diversity of 3′PPT of the HIV-1 subtype C (HIV-1C) nef gene amongst cART-naïve and cART-treated individuals with and without VF. We also assessed whether HIV-1C mutations in 3′PPT contribute to VF amongst individuals failing INSTI-based cART regimens regardless of the presence of mutations in the integrase region.
2. Materials and Methods
2.1. Selection of Study Population and HIV-1 Genotyping
Participant samples were obtained from two studies conducted in Botswana. The first study consisted of sequences generated from residual plasma specimens obtained from therapy-experienced individuals experiencing VF while on DTG- or raltegravir (RAL)-based cART described elsewhere (BOSELE study; Figure 1) [7]. VF was defined as two or more consecutive plasma HIV-1 RNA levels (viral loads (VL)) > 400 copies/mL as per standard of care guidelines in Botswana. The HIV-1 integrase region was amplified using nested reverse transcription-polymerase chain reactions (RT-PCRs) where necessary and sequenced using a BigDye™ Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Carlsbad, CA, USA) on a 3130xl Genetic Analyser (Life Technologies Corporation, Applied Biosystems, Carlsbad, CA, USA) as previously described [7,20]. Sequencing of the nef gene was attempted from the same HIV-1 extracts that integrase sequences were successfully generated from. Briefly, products of about 620 base pairs were amplified using nested RT-PCRs where necessary using the following primers numbered relative to HIV-1 reference strain (HxB2) nucleotide positions (shown in brackets): NEF8683F_pan TAGCAGTAGCTGRGKGRACAGATAG (8683–8707), NEF9536R_pan TACAGGCAAAAAGCAGCTGCTTATATGYAG (9507–9536), NEF8746_SgrI_AscI_F AGAGCACCGGCGCGCCTCCACATACCTASAAGAATMAGACARG (8736–8772), and NEF9474_SacII_ClaI_R GCCTCCGCGGATCGATCAGGCCACRCCTCCCTGGAAASKCCC (9449–9491) [21]. Amplicons were bidirectionally Sanger-sequenced as described above. The Sequencher software, version 5.0 (Gene Codes Corporation, Ann Arbor, MI, USA), was used to manually edit our electropherograms and form contigs with further downstream analysis carried using the BioEdit software.
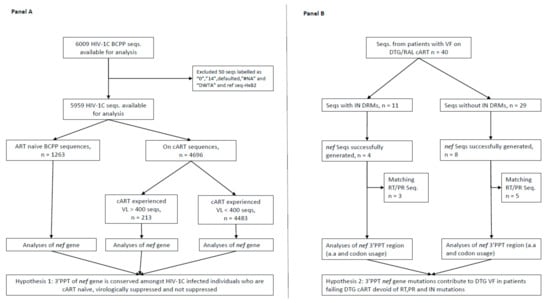
Figure 1.
Study schema depicting the selection of study sequences. (A), selection and analysis of 3′PPT nef gene sequences according to participant cART status and HIV-1 RNA levels; (B), selection and analysis of 3′PPT of nef gene amongst individuals with VF while on DTG/RAL-based cART. Seqs, sequences; PID, participant identification number; VL, viral load; VF, virologic failure; RAL, raltegravir; DTG, dolutegravir; cART, combination antiretroviral therapy; 3′PPT, 3′-polypurine tract; RT, reverse transcriptase HIV-1 gene; PR, protease HIV-1 gene; DRMs, drug resistance mutations; HIV-1C, HIV-1 subtype C.
The second group consisted of full-genome HIV-1C sequences obtained from participants enrolled in a large community-randomised HIV-1 prevention trial described elsewhere (BCPP study) [22,23]. The sequences were aligned to the HIV reference strain (HxB2) at the nucleotide level using virulign [24] and block-trimmed to the nef gene of HxB2 in the BioEdit, version 7.2.0, software [25]. We included two nucleotides before and one nucleotide after the 3′PPT nef gene sequence to have a complete amino acid coding for the 3′PPT tails. The sequences were assessed for hypermutations using the Hypermut tool at the Los Alamos National Laboratory HIV Database website (http://www.hiv.lanl.gov/ accessed on 8 March 2021). All sequences were exported to Microsoft® Excel® for Microsoft 365 MSO (16.0.13901.20148) 32-bit for further downstream analysis, and graphs were created. Additional statistical computations were performed using Stata version 14 (Stata Corporation, College Station, TX, USA) and R version 4.0.3; Pearson’s chi-square test was used to compare the proportion of 3′PPT of nef gene mutations per position by ART status and VL suppression. p-Values < 0.05 were considered statistically significant.
The Abbott RealTime HIV-1 assay, Cobas TaqMan/Cobas AmpliPrep HIV test (Roche Molecular Systems, Branchburg, NJ, USA), or Aptima HIV-1 Quant assay on Panther Systems (Hologic Inc., San Diego, CA, USA) was used to quantify HIV-1 RNA levels.
2.2. Ethical Statement
Both study protocols were approved by the health research and development division of the Botswana Ministry of Health and Wellness (Botswana’s IRB of authority). For the BOSELE study participants, a waiver of informed consent was obtained, and for the BCPP study, all the participants provided informed consent. The BCPP study was approved by the IRB at the U.S. Centers for Disease Control and Prevention and is registered at ClinicalTrials.gov (NCT01965470). All studies were conducted according to the principles stated in the Declaration of Helsinki.
3. Results
A total of 6021 HIV-1C nef gene sequences were available for analysis from both studies, and their basic demographics are shown in Table 1.

Table 1.
Basic demographics of 6009 HIV-1C diagnosed, cART-naïve, and cART-experienced individuals.
We included two nucleotides before (HxB2 nct position 9067 and 9068) and one nucleotide after 3′PPT (HxB2 nct position 9084) in our analysis of the 15-nucleotide 3′PPT region (5′ AAAAGAAAAGGGGGG 3′-HXB2 numbering 9069 to 9083) to complete the amino acid translation of the flanking regions of 3′PPT (Table 2, Figure 2).

Table 2.
HIV-1C nef 3′PPT variability amongst 12 therapy-experienced individuals experiencing VF while on DTG/RAL cART.
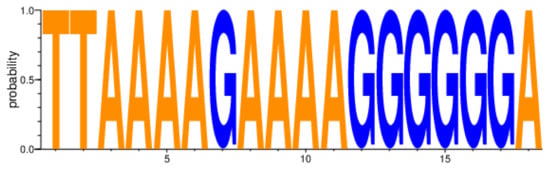
Figure 2.
HIV-1C nef gene 3′PPT variability amongst 6009 sequences from individuals on cART and not on cART. 3′PPT, 3′polypurine tract; cART, combination antiretroviral therapy; HIV-1C, HIV-1 subtype C.
All nucleotide positions of our HIV-1C nef gene 3′PPT sequences were highly conserved regardless of whether cART-naïve (n = 1263), on ART with VL < 400 (n = 4483) copies/mL, or on cART with VL ≥ 400 copies/mL (n = 213) groups (Figure 2). In addition, there was no statistically significant difference between ‘cART naïve’ and ‘on cART’ groups (p = 0.81), ‘on cART < 400′ and ‘on cART ≥ 400′ groups (p = 0.88), ‘ART naïve’ and ‘on cART < 400′ groups (p = 0.72), ‘cART naïve’ and ‘on cART ≥ 400′ groups (p = 0.99), ‘on cART and cART < 400′ groups (p = 0.86), ‘on cART and cART ≥ 400′ groups (p = 0.92), and ‘on ART’ and individuals with VF while on DTG/RAL cART group (p = 0.81). Analysis of sequences derived from buffy coat (n = 6009) adjusted for hypermutations (n = 2992) also revealed highly conserved nef 3′PPT residues with no statistically significant difference determined. The terminal six guanine stretch of 3′PPT also showed a high degree of conservation with all nucleotide residues having a mean rate of 99.47% (Figure 2, Supplementary Materials).
4. Discussion
We analysed 12 HIV-1C nef 3′PPT sequences (eight had paired integrase sequences without INSTI drug resistance mutations and four had paired IN sequences with INSTI drug resistance mutations) from patients with VF while on DTG/RAL-based cART to search for changes in 3′PPT sequence that could be linked to DTG VF as previously reported, and we did not find any. Amongst the eight patients who were failing an INSTI-based regimen but who did not have INSTI resistance mutations in the integrase region, all had a 100% conservation in their 3′PPT sequences (they had no mutations at the nucleotide or amino acid level). In addition, we analysed 1263 3′PPT sequences from patients who were cART naïve to investigate HIV-1C 3′PPT variability. We further analysed 4696 3′PPT sequences from patients on cART (but not on a DTG-based regimen) stratified according to virological suppression and found no 3′PPT region variability.
Malet et al. found some significant variability at position 9071 (25% and 10% in HIV-1 subtype B and CRF01, respectively) [17] and position 9075 (10% variability in HIV-1 subtype D). In our analysis, position 9071 revealed a variability of 0.29% (n = 6009) and 0% (n = 12) of sequences from patients not exposed to DTG cART and those failing DTG/RAL cART, respectively. Position 9075 was also conserved with a variability of 0.4% (n = 6009) and 0% (n = 12) amongst the two groups. Perhaps this difference in variability could be explained by the different HIV-1 subtypes—all our sequences were HIV-1C.
We observed a high conservation amongst the six nucleotides of the G-tract residues of 3′PPT (mean of 99.47%) similar to what others have found (99.95%) [8,17,18,19].
We did not explore the entire HIV-1C genome (5′ long terminal repeat (LTR), gag, protease, reverse transcriptase, vif, vpr, vpu, envelope, and 3′ LTR) for other mutations that could be linked to INSTI resistance. We did not measure plasma DTG or RAL levels to check whether issues of nonadherence could be contributing to VF.
In conclusion, we conducted one of the largest analyses of the HIV-1C 3′PPT region, showing great conservation of the region at the nucleotide and amino acid level. Although we did not detect any association of 3′PPT mutations with VF on INSTI-based cART, our data were limited on the number of 3′PPT sequences generated from patients failing an INSTI-containing regimen without INSTI mutations. However, our data add to a growing list of studies that have found no association of 3′PPT mutations with INSTI resistance [17,18]. Future studies should also investigate the role of other HIV-1 genes outside of Pol as this might enhance our understanding of mechanisms associated with INSTI resistance.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/pathogens10081027/s1, Table S1: HIV-1C nef 3′PPT variability amongst 12 therapy experienced individuals experiencing VF while on DTG/RAL ART. We included the two proximal and last distal nct positions to the 3′PPT to complete aminoacid picture of the region; 3′polypurine tract, 3′PPT; nct, nucleotide; VL, viral load; seqs., sequences; VF, virological failure; DTG, dolutegravir; RAL, raltegravir; cART, combination antiretroviral therapy, Adapted from Figure 2a of Malet I et al. [7]; Figure S1: HIV-1C nef 3′PPT variability amongst sequences from individuals on cART and not on ART, n = 5959. cART, combination antiretroviral therapy; 3′PPT, 3′polypurine tract; Figure S2: HIV-1C nef 3′PPT variability amongst cART naive individuals, n = 1263; Figure S3: HIV-1C nef 3′PPT variability amongst individuals on ART with VL ≤ 400 copies/mL, n = 4483; Figure S4: HIV-1C nef 3′PPT variability amongst individuals on ART with VL > 400 copies/mL, n = 213; Figure S5: HIV-1C nef 3′PPT variability amongst sequences from individuals on ART and not on ART, n = 2992 (hypermutations removed); Figure S6: HIV-1C nef 3′PPT variability amongst ART naive individuals, n = 762 (hypermutations removed); Figure S7: HIV-1C nef 3′PPT variability amongst individuals on ART with VL ≤ 400 copies/mL, n = 2074 (hypermutations removed); Figure S8: HIV-1C nef 3′PPT variability amongst individuals on ART with VL > 400 copies/mL, n = 119 (hypermutations removed).
Author Contributions
Writing–original draft preparation, K.K.S.; conceptualization, S.G., K.K.S.; investigation, K.K.S.; writing—reviewing and editing, K.K.S., S.G., I.K., S.L., S.M., O.M., V.N., D.M., W.T.C., data curation, K.K.S., S.G.; formal analysis, K.K.S., O.M., S.M., S.G.; project administration, S.G., S.L.; supervision, S.G., I.K; funding acquisition, S.G., K.K.S., S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This publication was made possible with help from the Harvard University Center for AIDS Research (CFAR), an NIH-funded program (P30 AI060354), which is supported by the following NIH cofunding and participating institutes and centres: National Institute of Allergy and Infectious Diseases; National Cancer Institute; Eunice Kennedy Shriver National Institute of Child Health and Human Development; National Heart, Lung, and Blood Institute; National Institute on Drug Abuse; National Institute of Mental Health; National Institute on Aging; National Institute of Diabetes and Digestive and Kidney Diseases; National Institute of General Medical Sciences; National Institute on Minority Health and Health Disparities; National Institute of Dental and Craniofacial Research; Office of AIDS Research; and Fogarty International Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was supported through the Sub-Saharan African Network for TB/HIV Research Excellence (SANTHE), a DELTAS Africa Initiative (grant # DEL-15-006). The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences’ (AAS) Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa’s Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust (grant # 107752/Z/15/Z) and the UK government. The views expressed in this publication are those of the author(s) and not necessarily those of AAS, NEPAD Agency, Wellcome Trust, or the UK government. ‘Research reported in this publication was supported by the Fogarty International Center and National Institute of Mental Health of the National Institutes of Health under Award Number D43 TW010543. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.’ S.L. was supported by the National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases K24 mentoring grant—NIH K24 AI131928. All funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The Botswana Combination Prevention Project Impact Evaluation was supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC) under the terms of cooperative agreements U01 GH000447 and U2G GH001911. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official positions of the funding agencies.
Institutional Review Board Statement
Both study protocols were approved by the health research and development division of the Botswana Ministry of Health and Wellness (Botswana’s IRB of authority). For the BOSELE study participants, a waiver of informed consent was obtained, and for the BCPP study, all the participants provided informed consent. The BCPP study was approved by the IRB at the U.S. Centers for Disease Control and Prevention and is registered at ClinicalTrials.gov (NCT01965470). All studies were conducted according to the principles stated in the Declaration of Helsinki.
Informed Consent Statement
For the BOSELE study participants, a waiver of informed consent was obtained, and for the BCPP study, all the participants provided informed consent.
Data Availability Statement
Sequences available at national centre for biotechnology information (NCBI) GenBank, accession numbers MW690052-MW690089, MG989443.1, MG989444.
Acknowledgments
We thank our patients and the staff of all infectious disease care clinics (PMH IDCC) that we visited throughout Botswana. We thank the Botswana Harvard HIV Reference Laboratory staff, Botswana Harvard Partnership, and Botswana Ministry of Health and Wellness for their collaboration. We thank the study participants from both study cohorts without whom this study would not have been possible.
Conflicts of Interest
All authors have no conflict of interest to declare.
References
- Anstett, K.; Brenner, B.; Mesplede, T.; Wainberg, M.A. HIV drug resistance against strand transfer integrase inhibitors. Retrovirology 2017, 14, 36. [Google Scholar] [CrossRef]
- DHHS; Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV; Department of Health and Human Services. 2021. Available online: https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv/whats-new-guidelines (accessed on 13 August 2021).
- MoHW. Handbook of the Botswana 2016 Integrated HIV Clinical Care Guidelines. Available online: https://www.moh.gov.bw/Publications/Handbook_HIV_treatment_guidelines.pdf (accessed on 15 April 2020).
- Raffi, F.; Rachlis, A.; Stellbrink, H.J.; Hardy, W.D.; Torti, C.; Orkin, C.; Bloch, M.; Podzamczer, D.; Pokrovsky, V.; Pulido, F.; et al. Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet 2013, 381, 735–743. [Google Scholar] [CrossRef]
- WHO. Update of Recommendations on First- and Second-Line Antiretroviral Regimens; (WHO/CDS/HIV/19.15); Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Oliveira, M.; Ibanescu, R.I.; Anstett, K.; Mesplede, T.; Routy, J.P.; Robbins, M.A.; Brenner, B.G.; The Montreal Primary HIV (PHI) Cohort Study Group. Selective resistance profiles emerging in patient-derived clinical isolates with cabotegravir, bictegravir, dolutegravir, and elvitegravir. Retrovirology 2018, 15, 56. [Google Scholar] [CrossRef]
- Seatla, K.K.; Maruapula, D.; Choga, W.T.; Ntsipe, T.; Mathiba, N.; Mogwele, M.; Kapanda, M.; Nkomo, B.; Ramaabya, D.; Makhema, J.; et al. HIV-1 Subtype C Drug Resistance Mutations in Heavily Treated Patients Failing Integrase Strand Transfer Inhibitor-Based Regimens in Botswana. Viruses 2021, 13, 594. [Google Scholar] [CrossRef]
- Malet, I.; Subra, F.; Charpentier, C.; Collin, G.; Descamps, D.; Calvez, V.; Marcelin, A.G.; Delelis, O. Mutations Located outside the Integrase Gene Can Confer Resistance to HIV-1 Integrase Strand Transfer Inhibitors. mBio 2017, 8, e00922-17. [Google Scholar] [CrossRef]
- Wijting, I.E.A.; Lungu, C.; Rijnders, B.J.A.; van der Ende, M.E.; Pham, H.T.; Mesplede, T.; Pas, S.D.; Voermans, J.J.C.; Schuurman, R.; van de Vijver, D.; et al. HIV-1 Resistance Dynamics in Patients With Virologic Failure to Dolutegravir Maintenance Monotherapy. J. Infect. Dis. 2018, 218, 688–697. [Google Scholar] [CrossRef]
- Anderson, S.J.; Lenburg, M.; Landau, N.R.; Garcia, J.V. The cytoplasmic domain of CD4 is sufficient for its down-regulation from the cell surface by human immunodeficiency virus type 1 Nef. J. Virol. 1994, 68, 3092–3101. [Google Scholar] [CrossRef]
- Garcia, J.V.; Miller, A.D. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature 1991, 350, 508–511. [Google Scholar] [CrossRef]
- Schwartz, O.; Maréchal, V.; Le Gall, S.; Lemonnier, F.; Heard, J.-M. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV–1 Nef protein. Nat. Med. 1996, 2, 338–342. [Google Scholar] [CrossRef]
- Watkins, R.L.; Zou, W.; Denton, P.W.; Krisko, J.F.; Foster, J.L.; Garcia, J.V. In vivo analysis of highly conserved Nef activities in HIV-1 replication and pathogenesis. Retrovirology 2013, 10, 125. [Google Scholar] [CrossRef]
- Das, A.T.; Berkhout, B.; Paraskevis, D. How Polypurine Tract Changes in the HIV-1 RNA Genome Can Cause Resistance against the Integrase Inhibitor Dolutegravir. mBio 2018, 9, e00006-18. [Google Scholar] [CrossRef]
- Julias, J.G.; McWilliams, M.J.; Sarafianos, S.G.; Alvord, W.G.; Arnold, E.; Hughes, S.H. Effects of Mutations in the G Tract of the Human Immunodeficiency Virus Type 1 Polypurine Tract on Virus Replication and RNase H Cleavage. J. Virol. 2004, 78, 13315–13324. [Google Scholar] [CrossRef]
- Jones, F.D.; Hughes, S.H. In vitro analysis of the effects of mutations in the G-tract of the human immunodeficiency virus type 1 polypurine tract on RNase H cleavage specificity. Virology 2007, 360, 341–349. [Google Scholar] [CrossRef][Green Version]
- Malet, I.; Delelis, O.; Nguyen, T.; Leducq, V.; Abdi, B.; Morand-Joubert, L.; Calvez, V.; Marcelin, A.G. Variability of the HIV-1 3′ polypurine tract (3′PPT) region and implication in integrase inhibitor resistance. J. Antimicrob. Chemother. 2019, 74, 3440–3444. [Google Scholar] [CrossRef]
- Wei, Y.; Sluis-Cremer, N. Mutations in the HIV-1 3′-Polypurine Tract and Integrase Strand-Transfer Inhibitor Resistance. Antimicrob. Agents Chemother. 2021, 65, e02432-20. [Google Scholar] [CrossRef]
- Acharya, A.; Tagny, C.T.; Mbanya, D.; Fonsah, J.Y.; Nchindap, E.; Kenmogne, L.; Jihyun, M.; Njamnshi, A.K.; Kanmogne, G.D. Variability in HIV-1 Integrase Gene and 3’-Polypurine Tract Sequences in Cameroon Clinical Isolates, and Implications for Integrase Inhibitors Efficacy. Int. J. Mol. Sci. 2020, 21, 1553. [Google Scholar] [CrossRef]
- Seatla, K.K.; Choga, W.T.; Mogwele, M.; Diphoko, T.; Maruapula, D.; Mupfumi, L.; Musonda, R.M.; Rowley, C.F.; Avalos, A.; Kasvosve, I.; et al. Comparison of an in-house ‘home-brew’ and commercial ViroSeq integrase genotyping assays on HIV-1 subtype C samples. PLoS ONE 2019, 14, e0224292. [Google Scholar] [CrossRef]
- Jones, B.R.; Miller, R.L.; Kinloch, N.N.; Tsai, O.; Rigsby, H.; Sudderuddin, H.; Shahid, A.; Ganase, B.; Brumme, C.J.; Harris, M.; et al. Genetic Diversity, Compartmentalization, and Age of HIV Proviruses Persisting in CD4(+) T Cell Subsets during Long-Term Combination Antiretroviral Therapy. J. Virol. 2020, 94, e01786-19. [Google Scholar] [CrossRef]
- Gaolathe, T.; Wirth, K.E.; Holme, M.P.; Makhema, J.; Moyo, S.; Chakalisa, U.; Yankinda, E.K.; Lei, Q.; Mmalane, M.; Novitsky, V.; et al. Botswana’s progress toward achieving the 2020 UNAIDS 90-90-90 antiretroviral therapy and virological suppression goals: A population-based survey. Lancet HIV 2016, 3, e221–e230. [Google Scholar] [CrossRef]
- Makhema, J.; Wirth, K.E.; Pretorius Holme, M.; Gaolathe, T.; Mmalane, M.; Kadima, E.; Chakalisa, U.; Bennett, K.; Leidner, J.; Manyake, K.; et al. Universal Testing, Expanded Treatment, and Incidence of HIV Infection in Botswana. N. Engl. J. Med. 2019, 381, 230–242. [Google Scholar] [CrossRef]
- Libin, P.J.K.; Deforche, K.; Abecasis, A.B.; Theys, K. VIRULIGN: Fast codon-correct alignment and annotation of viral genomes. Bioinformatics 2018, 35, 1763–1765. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Proc. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).