Abstract
Background: Leishmaniasis is an infectious disease caused by Leishmania parasites. A Th1 immune response is necessary in the acute phase to control the pathogen. The triggering receptor expressed on myeloid cells (TREM)-1 is a potent amplifier of inflammation. Our aim is to identify whether the TREM1 variant rs2234237 A/T (Thr25Ser) is associated with the disease development of cutaneous leishmaniasis (CL) in Leishmania guyanensis-infected individuals. The effects of the rs2234237 genotypes on plasma cytokines IL-1β, IL-6, IL-8, IL-10, MCP-1 and TNF-α are also investigated. Methods: 838 patients with CL and 818 healthy controls (HCs) living in the same endemic areas were genotyped by Polymerase Chain Reaction-Restriction Fragment Length Polymorphism. Plasma cytokines were assayed in 400 patients with CL and 400 HCs using the BioPlex assay. Results: The genotypes’ and alleles’ frequencies were similar in both patients with CL (AA = 618, 74%; AT = 202, 24%; TT = 18, 2%) and in HCs (AA = 580, 71%; AT = 220, 27%; TT = 18, 2%). Rs2234237 showed a modest effect on plasma IL-10 that disappeared when correction of the p-value was applied. Plasma IL-10 by rs2234237 genotypes were (mean ± SEM; AA = 2.91 pg/mL ± 0.14; AT = 2.35 pg/mL ± 0.12; TT = 3.14 pg/mL ± 0.56; p = 0.05). Conclusion: The TREM1 rs2234237 (Thr25Ser) seems to have no influence on the susceptibility or resistance to L. guyanensis infections.
1. Introduction
Leishmania infections cause a broad spectrum of clinical manifestations. The clinical outcome depends on the Leishmania spp. and may range from asymptomatic, self-healing or non-healing skin lesions, classified as cutaneous leishmaniasis (CLs), to severe destructive mucosal lesions (MLs) or visceral leishmaniasis (VLs) that may even be fatal [1]. According to the World Health Organization (WHO), 97 countries were considered endemic for leishmaniasis in 2018, with 88 countries for CL, 78 for VL and 69 for both CL and VL [2]. In 2018, 253,435 new CL cases were reported worldwide [2]. In Brazil, 16,432 new cases of American Tegumentary Leishmaniasis (ATL) were reported in 2018 [3]. In the state of Amazonas, 1684 new cases of ATL were noted in 2018 [3].
The clinical outcome of Leishmania infections depends on a fine regulation of the immune response coordinated by T helper cells [4]. Th1 cells, producing high levels of IFN-γ, activate infected macrophages to induce superoxide production to contain the Leishmania parasite’s replication [4]. Alternatively, a Th2 response, producing interleukin-4 (IL-4), IL-5 and IL-13 favors the survival and replication of the Leishmania parasites [5].
Receptors expressed in innate immune cells play key roles in Th1/Th2 differentiation. A triggering receptor expressed on myeloid cells (TREM-1) is a receptor with a molecular mass of 30 kDa that belongs to the immunoglobulin-like superfamily. TREM-1 is highly expressed on activated monocytes/macrophages and neutrophils [6]. TREM-1 has a short intracellular tail without an immunoreceptor tyrosine-based activation motif (ITAM) domain. To activate its signaling pathway, TREM-1 forms a complex with the protein adapter DNAX-activating protein 12 (DAP12) for signal transduction [6,7]. The TREM-1/DAP12 complex signaling pathway activates phospholipase C γ (PLCγ), extracellular signal-regulated kinase (ERK) and phosphatidylinositol 3-kinase (PIK3) to trigger calcium mobilization, cell survival, neutrophil degranulation and increases in the expression of pro-inflammatory cytokines, resulting in hyper-inflammatory conditions [6,8].
Emerging evidence has shown the importance of TREM-1 in the modulation of an excessive pro-inflammatory response against Escherichia coli [7], Lipopolysaccharide-induced septic shock [7], Pseudomonas aeruginosa [9], Aspergillus fumigatus [10], Lymphocytic Choriomeningitis Virus (LCMV) [11], human immunodeficiency virus (HIV) [12] and Mycobacterium tuberculosis [13]. TREM-1 may be a promising therapeutic target in infectious diseases with exacerbation of inflammation.
Knockout mice of TREM1 (TREM1−/−), upon infection with L. major, showed smaller lesions with reduced neutrophil infiltration after 14 days when compared to wild-type mice, despite both presenting similar parasitic load [14]. In addition, TREM1+/+ neutrophils showed low Caspase3/7 activity compared to TREM1−/− neutrophils [14]. CCL7, IL8, IFI44L and IL1B genes were upregulated in L. braziliensis-stimulated peripheral blood mononuclear cells (PBMCs) from healthy high IFN-γ producing individuals [15]. The upregulation was cited to be related to the IL-17 and TREM-1 pathways, as determined by Ingenuity Pathway Analysis [15]. In L. braziliensis-infected patients with CL, miR-193b, miR-671 and TREM-1 were shown to be negatively correlated with good responses to treatment and faster wound healing [16]. TREM-1 also performed cross-talk with toll-like receptors 2 (TLR2) and TLR4 pathways to potentiate the intracellular TLR signal and upregulate cytokines’ expression [17,18,19,20].
Variants present in the TREM1 gene are associated with susceptibility to inflammatory bowel diseases [21], sepsis prognosis [22], pneumonia [23], coronary artery disease [24], septic shock [25], severe malaria [26] and atherosclerosis [27]. The variant rs2234237 A/T is a non-synonymous variant present in Exon 2 of the TREM1 gene. This variant results from the substitution of threonine with serine at the 25th amino acid position in the extracellular immunoglobulin-like domain, responsible for binding with TREM-1 ligands. Furthermore, this variant is correlated with an increased level of soluble TREM-1 [25].
Taking into account the importance of TREM-1 in inflammatory diseases, this study investigates whether rs2234237 A/T is a risk factor in the development of CL in patients infected by L. guyanensis. We also analyze rs2234237 A/T’s effects on circulating cytokines’ plasma levels.
2. Results
2.1. Characteristics of the Population
The characteristics of the study population are described elsewhere [28]. Briefly, in this study, the population was composed of 838 patients with CL and 818 HCs. The main characteristics of the participants of the study are shown in Table 1. Females were more frequent among the control subjects (p-value = 0.002). The controls were older than the patients with CL (p-value < 0.001). All patients with CL included in the study had fewer or equal to (≤) six skin lesions. Patients with HIV and pregnant women were excluded. The diameters of the lesions were not recorded as all patients sought treatment only after the lesions were well developed. Besides this, most patients applied local traditional treatment before going to the hospital.

Table 1.
Basic characteristics of the study population.
2.2. Genotypes and Allele Frequencies of the TREM1 Variant rs2234237 A/T’s Polymorphism
The genotype distributions did not present any HWE deviation in either patients with CL or HCs. The genotypes’ and alleles’ frequencies of rs2234237 were similar in both patients with CL (AA: 74%, n = 618; AT: 24%, n = 202; TT: 2%, n = 18) and the healthy controls (AA: 71%, n = 580; AT: 27%, n = 220; TT: 2%, n = 18), as shown in Table 2.

Table 2.
Genotypes’ and alleles’ frequencies for rs2234237 (Thr25Ser) polymorphism in patients with cutaneous leishmaniasis and healthy controls, and statistical comparisons adjusted by sex and age.
Genotypes’ and alleles’ comparisons did not show any statistical differences between the patients with CL and the HCs despite the frequency of T alleles being slightly higher (16%) among the HCs when compared with the patients with CL (14%). These results show that the polymorphism of the variant rs2234237 A/T (Thr25Ser) in the TREM1 gene is neither associated with the development of CL in patients infected by L. guyanensis nor with resistance.
2.3. Effects of TREM1 rs2234237’s Polymorphism on Circulating Plasma Cytokine Levels
The TREM-1 signaling pathway’s activation induces the expression of the pro-inflammatory cytokines IL-1β, IL-6, IL-8, MCP-1 and TNF-α and downregulates IL-10 expression [6,14]. The effect of the variant rs2234237 (Thr25Ser) on plasma cytokines was analyzed in 800 subjects (400 patients with CL and 400 HCs). The means and standard of the means in pg/mL (mean ± SEM pg/mL) of plasma cytokines, adjusted by sex and age, are shown for patients with CL, for HCs and for the total individuals (patients with CL + HCs) in Supplementary Figure S1A,B. The means ± the SEM pg/mL of each cytokine for the rs2234237 genotypes and inheritance models (codominant, dominant, recessive and over-dominant) are shown in Supplementary Table S1. Of all of the cytokines analyzed, only IL-10 seems to be influenced by the variant rs2234237 and the effect is modest. The distribution of the circulating plasma levels of IL-10, as shown in Figure 1, is different among the genotypes in the combined samples (patients with CL + HCs) (p = 0.05). Among carriers of the AA genotype, the mean circulating levels of IL10 are different (2.91 ± 0.14 pg/mL, n = 492) compared to the AT (2.35 ± 0.12 pg/mL, n = 174) and TT genotypes (3.14 ± 0.56 pg/mL, n = 15). In the dominant model, individuals bearing a T allele (AT+TT genotypes, n = 189) had a mean level of circulating plasma IL-10 of 2.41 ± 0.12 pg/mL compared to 2.91 ± 0.14 pg/mL for individuals homozygous for the A allele (AA genotype, n = 492; p = 0.03). However, in a recessive model, individuals homozygous for the T allele seemed to have higher levels of IL-10 (3.14 ± 0.56 pg/mL, n = 15) compared to individuals carrying the A allele (AA+AT: 2.76 ± 0.10 pg/mL, n = 666, p = 0.59). Interestingly, in the over-dominant model, heterozygous individuals seemed to produce lower levels of circulating plasma IL-10 (AT: 2.35 ± 0.12 pg/mL, n = 174) when compared with both sets of homozygous individuals (AA+TT: 2.91 ± 0.13 pg/mL, n = 507, p = 0.02). Furthermore, we did not observe a difference between the two sets of homozygous individuals (AA: 2.91 ± 0.14 pg/mL, n = 492; TT: 3.14 ± 0.56, n = 15; p = 0.76). Of note, the statistical significance disappeared when the Benjamini and Hochberg correction was applied, assuming five tests, as depicted by the p-value.
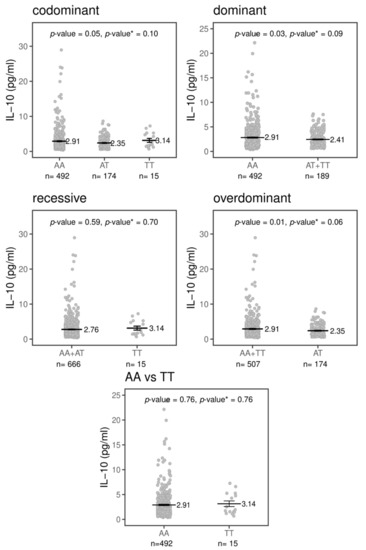
Figure 1.
TREM1 rs2234237 A/T (Thr25Ser) influence on circulating IL-10 plasma levels in total subjects in four inheritance models (codominant, dominant, recessive and over-dominant) and between homozygous genotypes adjusted by sex and age. The black bars represent the means of concentration in picogram per milliliter (pg/mL), whereas error bars represent the standard error of mean. p-value <0.05 is considered significant. p-value*: Benjamini and Hochberg’s correction assuming five tests.
3. Discussion
In the area of endemicity for Leishmania species, some individuals develop clinical symptoms while others sharing the same environment and exposure to the parasite do not show any symptoms or remain asymptomatic. The clinical spectrum of leishmaniasis also suggests that hosts’ genetic factors are involved in the disease outcome. This points to possible host genetic contributions to the development of leishmaniasis [28,29,30]. Understanding the role of host genetics in Leishmania infections may decipher the developmental mechanism of the disease and contribute to elaborating a novel treatment for leishmaniasis, especially in the field of immunotherapy.
In this study, our data showed that the variant rs2234237 (Thr25Ser) within the TREM1 gene in Exon 2 was not associated with the development of CL or resistance to L. guyanensis infection. In contrast, the variant may have influenced the expression of IL-10, as the T allele correlated with lower levels of circulating plasma IL-10, although the statistical significance disappeared when corrections were applied for multiple comparisons.
A few studies have shown that the rs2234237 T allele is associated with several diseases. The T-allele has been suggested to be a risk factor for the development of severe malaria in African children [26] and Behcet’s disease in the Korean population [21]. The T-allele has also been reported to be associated with sepsis in Chinese patients [22] and high risk of developing pneumonia caused by mechanic ventilation [23].
Conversely, other studies have reported a lack of association of rs2234237 with septic shock [25], severe sepsis [31], or a clinical course of sepsis [32]. One study showed that individuals homozygous for the rs2234237 A allele were at a high risk of developing coronary artery disease (CAD) among the Russian population [24].
The TREM-1 signaling pathway acts synergistically with TLR2 and TLR4. This leads to NF-kB activation and expressions of proinflammatory cytokines [17,18,19,20]. Plasmodium berghei-infected inbred mice treated with recombinant mouse TREM-1/Fc and mouse TREM-1/antibody to inhibit TREM-1 activity exhibited a sharp decrease in TNF-α, IL-6, IFN-γ and IL-10 levels and less tissue damage of affected organs, compared with untreated mice [33]. Whole blood from patients with simple sepsis showed a higher expression of TREM-1 and higher levels of IL-6, IL-8, IL-10 and TNF-α upon stimulation with LPS when compared with whole blood from patients with severe sepsis [20]. Pathway-specific microarray analysis revealed that the silencing of TREM-1 induced a reduction in the expression of myeloid differentiation protein (MyD88) (a protein adapter of TLRs), IL-10 and IL-1β [34]. In contrast, other studies have shown that TREM-1 can inhibit IL-10 expression [10,35]. Human peripheral monocytes from L. braziliensis-infected patients with CL exhibited higher expression of TLR2, TLR4, TNF-α and IL-10 upon infection in vitro with L. braziliensis [36].
In this study, the effects of rs2234237 on the levels of plasma circulating IL-1B, IL-6, IL-8, IL-10, TNF-α and MCP-1 were analyzed. Only plasma IL-10 seems to be influenced by the rs2234237 genotypes, as shown in Supplementary Figure S1A,B. The AA homozygotes were correlated with higher circulating IL-10 compared with the T genotypes (AT + TT). Of note, as the frequency of the T allele was low in our population, only 15 individuals had the TT genotypes out of the 666 individuals assayed for IL-10. When compared, the levels of circulating plasma IL-10 between the AA (2.91 ± 0.14 pg/mL) and TT (3.14 ± 0.56 pg/mL) homozygotes revealed that TT genotypes correlated with high levels of IL-10. However, the standard error of the mean among the TT genotypes was higher (0.56) when compared to 0.14 for the AA genotypes. The T allele may, in fact, correlate with low levels of IL-10.
Comparisons of genotypes’ frequencies did not reveal any statistical differences between the patients with CL and the HCs, but pointed out that carriers of the T allele may have an 18% risk of developing CL in a dominant model (OR = 1.18 (95% CI [0.95–1.47])). In a study of falciparum malaria with 293 and 87 African children with uncomplicated and severe malaria, respectively, individuals with the T allele had an increased risk of developing severe malaria (dominant model; OR = 2.4 (95%CI [1.2–4.5]) [26]). Likewise, under the dominant model, the T allele was associated with Behcet‘s disease (OR = 1.69 (95%CI [1.1–2.6])) [21]. Although no statistical significance has been observed in our analysis, we can hypothesize that individuals with the T allele may not properly control the inflammation and thus progress to the development of a lesion. The rs2234237 T allele was cited to correlate with an increased level of soluble TREM-1 in human whole blood stimulated with LPS [25]. Soluble TREM-1 (sTREM-1) may act as a decoy receptor for TREM-1 ligands and lead to a decrease of circulating IL-10. Interestingly, TREM-1 silencing lowered the expression of IL-10 [34]. In contrast, sTREM-1 was suggested as a possible modulator of the anti-inflammatory pathway [37].
This study has several limitations, especially with the correlation of plasma IL-10 by the rs223437 genotypes. The frequency of the T allele (16%) is low when compared with the A allele (84%). Of 1656 individuals’ genotypes, only 36 were homozygous for the T allele. Of these, only 15 were assayed for cytokines. To show that the T allele may be correlated with low plasma IL-10, we had to pool healthy controls and patients with CL together to have statistical power. The pooling was performed as we were looking at whether the allele had an influence on expression. Age and gender did not influence plasma IL-10, as shown by linear regression correlation analysis (Supplementary Figure S2). Future studies are needed to confirm this correlation as it is very modest and it might be a spurious association. It would be interesting in future studies to select only 15 individuals from each genotype and perform stimulation studies with whole blood in vitro to avoid confounding factors.
4. Materials and Methods
4.1. Population Studied
In this study, individuals were recruited from the perirural regions of Manaus, the capital city of the Amazonas state, considered endemic for L. guyanensis. The recruitment of patients with CL and healthy controls (HCs) (individuals with no history of CL or any scars and different lesions) from the endemic areas was initiated in January 2009 and ended in March 2016. Patients with CL attended the Fundação de Medicina Tropical Doutor Heitor Vieira Dourado (FMT-HVD), a referral hospital, for the treatment of tropical diseases including leishmaniasis. The majority of the participants in the study are agricultural or farmworkers. The population studied has a mixture of Native American (50 to 60%), European (40 to 50%), and African (around 10%) ancestries [38].
4.2. Ethics Statement
This study was conducted in accordance with the international Declaration of Helsinki and was approved by the Research Ethics Committee of the Fundação de Medicina Tropical Dr. Heitor Vieira Dourado (FMT-HVD), granted under the file number CAAE:09995212.0.0000.0005 on 31 May 2013. All of the participants (or their responsible party for individuals less than 18 years old) provided written informed consent for the collection of biological samples and their subsequent analysis.
4.3. Collection of Biological Samples
Five mL of peripheral blood from each participant in the study was collected using venipuncture and transferred into an EDTA-containing vacutainer tube (Becton Dickinson) for DNA extraction and plasma separation for cytokines’ assay. A skin biopsy sample around the lesions from all patients with CL was collected for the identification of Leishmania species.
4.4. Leishmania Genotyping
The presence of Leishmania in the skin lesions of patients with CL was confirmed by direct microscopic examination of Giemsa-stained scarifications of the lesions. DNA was extracted from biopsy lesions of all patients with CL. The discrimination of L. Viannia from the L. Leishmania subgenus was performed by polymerase chain reaction (PCR) as described elsewhere [39,40]. The identification of Leishmania species was obtained by direct nucleotide sequencing of heat-shock protein 70 (HSP70) and miniexons genes as described previously [29,30].
4.5. Cytokines’ Assay
At the time of enrolment in the study, all participants provided 5 mL of peripheral blood. All patients with CL were treatment naïve prior to collection of the blood. Plasma was separated from the peripheral blood of both patients with CL and the HCs, and all samples were kept frozen at −80 °C for the cytokines’ analyses. Concentrations of circulating cytokines IL-1β, IL-6, IL-8, IL-10, monocyte chemoattractant protein-1 (MCP-1) and tumor necrosis factor-α (TNF-α) were detected from the plasma using the multiplex cytokine commercial kit Bio-PlexProTM Human Cytokine Grp I Panel 27-Plex (Bio-Rad, Hercules, CA, USA), according to the manufacturer’s instructions, in the Bio-plex 200 Protein Array System (Luminex Corporation, Austin, TX, USA).
4.6. TREM1 Polymorphism Genotyping
DNA was extracted using proteinase K and the salting-out method. Allele identification was performed by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP). The following pair of primers, TREM1 Forward: 5′ TCT TTC CCT GCT TAT AGA ACT CCG 3′ and TREM1 Reverse: 5′ GGT CTT GGG CAT CTC TCC GTC CTT 3′, was designed to flank the polymorphism region to generate a fragment of 166 base pairs (bp) by PCR in a final volume of 25 µL containing 50 ng of genomic DNA, 0.2 pmol/L of each primer, 40µmol/L of each deoxynucleotide triphosphate (dNTP), 1 U of Taq polymerase, 2.0 mmol/L of MgCl2 in a buffer containing 500 mmol/L KCl and 100 mol/L of Tris-HCL (pH 8.3). The PCR reactions were optimized in the Mastercycler ep S (Eppendorf) under the following cycling conditions: initial denaturation step at 95 °C for 5 min; 45 cycles of 95 °C for 15 s, 56 °C for 15 s and 72 °C for 30 s; and a final step of 72 °C for 7 min. A volume of 10 µL of the PCR product was digested using two units of MseI enzyme restriction (New England Biolabs). The DNA fragments were size separated by electrophoresis in a 3% agarose gel stained with 1 µL of 10 mg/mL ethidium bromide per 100 mL of gel. In the presence of the A allele, two fragments of 128bp and 38bp were generated. When the T allele was present, the fragment of 166pb remained uncleaved.
4.7. Statistical Analyses
The Mann-Whitney and χ2 tests were used to compare the ages and sexes in both the CL and control groups. The allelic and genotypic frequencies were determined by direct counting. Deviations in the Hardy–Weinberg Equilibrium (HWE) were tested comparing the observed genotype frequencies to the expected genotype frequencies. Association analyses were performed by logistic regression using the statistical language R (http://www.r-project.org/ accessed on the 20 September 2020). Single nucleotide polymorphism (SNP) association to disease outcome and SNP influence on circulating plasma cytokines were tested using the SNPassoc library version 2.0-0 [41]. Multiple testing corrections were realized according to Benjamini and Hochberg’s method [42] to control for false discovery rates using the R stats library. To control for false-positives relating to the influence of the SNP on circulating cytokines, the tail area-based false discovery rate values (FDR q-values) using the R package fdrtool were applied [43]. Cytokines plots were designed with the ggplot2 package version 3.3.6 [44].
5. Conclusions
Altogether, our data showed that the variant rs2234237 of the TREM1 gene is not associated with the development of CL in patients infected with L. guyanensis. Further studies including other variants present in TREM1 gene are necessary to better understand the role of this receptor in the immune system and modulation of cytokine expression in response to Leishmania-infections.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/pathogens10040498/s1: Supplementary Figure S1A,B, TREM1 rs2234237 A/T (Ser25Thr) influence on circulating cytokines plasma levels; Supplementary Figure S2. Linear regression correlation analysis to show whether gender and age influence plasma IL-10 levels in patients with CL, healthy controls, and totals.; Supplementary Table S1. Circulating plasma cytokine levels in patients with CL and healthy controls adjusted by sex and age.
Author Contributions
Conceptualization, M.V.d.F.G. and R.R.; methodology, J.d.E.S.J., T.G.R.d.M., L.D.O.d.S., F.J.d.A., J.L.d.S., T.C.d.L., L.S.d.S., C.M.d.S.J., K.L.G.D.Q., D.M.d.S., C.C.d.S., H.D.G.S., M.T.H. and M.L.G.d.S.; formal analysis, J.d.E.S.J., T.G.R.d.M., L.D.O.d.S. and R.R.; data curation, J.d.E.S.J., T.G.R.d.M., L.D.O.d.S., F.J.d.A., J.L.d.S., T.C.d.L., L.S.d.S., C.M.d.S.J., K.L.G.D.Q., D.M.d.S., C.C.d.S., H.D.G.S., M.T.H. and M.L.G.d.S.; writing—original draft prepara-tion, J.d.E.S.J., T.G.R.d.M. and R.R.; writing—review and editing, M.V.d.F.G. and R.R.; supervision, R.R.; funding acquisition, R.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Brazilian Council for Scientific and Technological Development (CNPq), grant number 404181/2012-0, and Fundação de Amparo e Pesquisa do Estado Amazonas, grant number 06201954/2015.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Fundação de Medicina Tropical Dr. Heitor Vieira Dourado (FMT-HVD) Research Ethics Committee (protocol code CAAE:09995212.0.0000.0005 and date of approval 31 May 2013).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Acknowledgments
We thank all participants, both patients with CL and healthy controls, for participating in this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Burza, S.; Croft, S.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://www.who.int/publications/i/item/who-wer9525 (accessed on 17 September 2020).
- Ministério da Saúde. Available online: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z-1/l/leishmaniose-tegumentar-lt (accessed on 17 September 2020).
- Von Stebut, E.; Udey, M.C. Requirements for Th1-dependent immunity against infection with Leishmania major. Microbes Infect. 2004, 6, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Sacks, D.; Noben-Trauth, N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat. Rev. Immunol. 2002, 2, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Bouchon, A.; Dietrich, J.; Colonna, M. Cutting edge: Inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J. Immunol. 2000, 164, 4991–4995. [Google Scholar] [CrossRef]
- Bouchon, A.; Facchetti, F.; Weigand, M.A.; Colonna, M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature 2001, 410, 1103–1107. [Google Scholar] [CrossRef] [PubMed]
- Tessarz, A.S.; Cerwenka, A. The TREM-1/DAP12 pathway. Immunol. Lett. 2008, 116, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Peng, A.; Sun, M.; Deng, Q.; Hazlett, L.D.; Yuan, J.; Liu, X.; Gao, Q.; Feng, L.; He, J.; et al. TREM-1 amplifies corneal inflammation after Pseudomonas aeruginosa infection by modulating Toll-like receptor signaling and Th1/Th2-type immune responses. Infect. Immun. 2011, 79, 2709–2716. [Google Scholar] [CrossRef]
- Zhong, J.; Huang, W.; Deng, Q.; Wu, M.; Jiang, H.; Lin, X.; Sun, Y.; Huang, X.; Yuan, J. Inhibition of TREM-1 and Dectin-1 Alleviates the Severity of Fungal Keratitis by Modulating Innate Immune Responses. PLoS ONE 2016, 11, e0150114. [Google Scholar] [CrossRef] [PubMed]
- Kozik, J.H.; Trautmann, T.; Carambia, A.; Preti, M.; Lütgehetmann, M.; Krech, T.; Wiegard, C.; Heeren, J.; Herkel, J. Attenuated viral hepatitis in Trem1-/- mice is associated with reduced inflammatory activity of neutrophils. Sci. Rep. 2016, 6, 28556. [Google Scholar] [CrossRef]
- Yuan, Z.; Fan, X.; Staitieh, B.; Bedi, C.; Spearman, P.; Guidot, D.M.; Sadikot, R.T. HIV-related proteins prolong macrophage survival through induction of Triggering receptor expressed on myeloid cells-1. Sci. Rep. 2017, 7, 42028. [Google Scholar] [CrossRef]
- Wu, Y.; Fang, Y.M.; Ding, L.; Liu, X.; Francisco, N.M.; Wen, J.; Liao, C.; Ma, Z.; Li, Z.; Li, M.; et al. Activation and Regulation of Blood Vδ2 T Cells Are Amplified by TREM-1+ during Active Pulmonary Tuberculosis. J. Immunol. 2018, 200, 1627–1638. [Google Scholar] [CrossRef] [PubMed]
- Weber, B.; Schuster, S.; Zysset, D.; Rihs, S.; Dickgreber, N.; Schürch, C.; Riether, C.; Siegrist, M.; Schneider, C.; Pawelski, H.; et al. TREM-1 deficiency can attenuate disease severity without affecting pathogen clearance. PLoS Pathog. 2014, 10, e1003900. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, M.W.; Fukutani, K.F.; Andrade, B.B.; Curvelo, R.P.; Cristal, J.R.; Carvalho, A.M.; Barral, A.; Van Weyenbergh, J.; Barral-Netto, M.; de Oliveira, C.I. Gene Expression Profile of High IFN-γ Producers Stimulated with Leishmania braziliensis Identifies Genes Associated with Cutaneous Leishmaniasis. PLoS Negl. Trop. Dis. 2016, 10, e0005116. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.; Silva, I.B.; Ampuero, M.R.; de Noronha, A.L.L.; de Souza, L.C.L.; Correia, T.C.; Khouri, R.; Boaventura, V.S.; Barral, A.; Ramos, P.I.P.; et al. Integrated Analysis Reveals That miR-193b, miR-671, and TREM-1 Correlate with a Good Response to Treatment of Human Localized Cutaneous Leishmaniasis Caused by Leishmania braziliensis. Front Immunol. 2018, 9, 640. [Google Scholar] [CrossRef] [PubMed]
- Fortin, C.F.; Lesur, O.; Fulop, T., Jr. Effects of TREM-1 activation in human neutrophils: Activation of signaling pathways, recruitment into lipid rafts and association with TLR4. Int. Immunol. 2007, 19, 41–50. [Google Scholar] [CrossRef]
- Buckland, K.F.; Ramaprakash, H.; Murray, L.A.; Carpenter, K.J.; Choi, E.S.; Kunkel, S.L.; Lukacs, N.W.; Xing, Z.; Aoki, N.; Hartl, D.; et al. Triggering receptor expressed on myeloid cells-1 (TREM-1) modulates immune responses to Aspergillus fumigatus during fungal asthma in mice. Immunol. Investig. 2011, 40, 692–722. [Google Scholar] [CrossRef]
- Golovkin, A.S.; Matveeva, V.G.; Kudryavtsev, I.V.; Chernova, M.N.; Bayrakova, Y.V.; Shukevich, D.L.; Grigoriev, E.V. Perioperative Dynamics of TLR2, TLR4, and TREM-1 Expression in Monocyte Subpopulations in the Setting of On-Pump Coronary Artery Bypass Surgery. ISRN Inflamm. 2013, 2013, 817901. [Google Scholar] [CrossRef]
- Van Bremen, T.; Drömann, D.; Luitjens, K.; Dodt, C.; Dalhoff, K.; Goldmann, T.; Schaaf, B. Triggering receptor expressed on myeloid cells-1 (Trem-1) on blood neutrophils is associated with cytokine inducibility in human E. coli sepsis. Diagn. Pathol. 2013, 8, 24. [Google Scholar] [CrossRef]
- Jung, E.S.; Kim, S.W.; Moon, C.M.; Shin, D.J.; Son, N.H.; Kim, E.S.; Lee, H.J.; Hong, S.P.; Kim, T.I.; Kim, W.H.; et al. Relationships between genetic polymorphisms of triggering receptor expressed on myeloid cells-1 and inflammatory bowel diseases in the Korean population. Life Sci. 2011, 89, 289–294. [Google Scholar] [CrossRef]
- Su, L.; Liu, C.; Li, C.; Jiang, Z.; Xiao, K.; Zhang, X.; Li, M.; Yan, P.; Feng, D.; Xie, L. Dynamic changes in serum soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) and its gene polymorphisms are associated with sepsis prognosis. Inflammation 2012, 35, 1833–1843. [Google Scholar] [CrossRef]
- Rivera-Chávez, F.A.; Huebinger, R.M.; Burris, A.; Liu, M.M.; Minei, J.P.; Hunt, J.L.; Arnoldo, B.D.; Barber, R.C. A TREM-1 Polymorphism A/T within the Exon 2 Is Associated with Pneumonia in Burn-Injured Patients. ISRN Inflamm. 2013, 2013, 431739. [Google Scholar] [CrossRef] [PubMed]
- Golovkin, A.S.; Ponasenko, A.V.; Khutornaya, M.V.; Kutikhin, A.G.; Salakhov, R.R.; Yuzhalin, A.E.; Zhidkova, I.I.; Barbarash, O.L.; Barbarash, L.S. Association of TLR and TREM-1 gene polymorphisms with risk of coronary artery disease in a Russian population. Gene 2014, 550, 101–109. [Google Scholar] [CrossRef]
- Peng, L.S.; Li, J.; Zhou, G.S.; Deng, L.H.; Yao, H.G. Relationships between genetic polymorphisms of triggering receptor expressed on myeloid cells-1 and septic shock in a Chinese Han population. World J. Emerg. Med. 2015, 6, 123–130. [Google Scholar] [CrossRef]
- Adukpo, S.; Gyan, B.A.; Ofori, M.F.; Dodoo, D.; Velavan, T.P.; Meyer, C.G. Triggering receptor expressed on myeloid cells 1 (TREM-1) and cytokine gene variants in complicated and uncomplicated malaria. Trop. Med. Int. Health 2016, 21, 1592–1601. [Google Scholar] [CrossRef] [PubMed]
- Kutikhin, A.G.; Ponasenko, A.V.; Khutornaya, M.V.; Yuzhalin, A.E.; Zhidkova, I.I.; Salakhov, R.R.; Golovkin, A.S.; Barbarash, O.L.; Barbarash, L.S. Association of TLR and TREM-1 gene polymorphisms with atherosclerosis severity in a Russian population. Meta Gene 2016, 9, 76–89. [Google Scholar] [CrossRef]
- De Araújo Santos, F.J.; da Silva, L.S.; Júnior, J.D.E.S.; Ramos de Mesquita, T.G.; de Souza, M.L.G.; de Andrade Júnior, M.C.; Talhari, S.; Ramasawmy, R. Single nucleotide polymorphisms of the genes IL-2, IL-2RB, and JAK3 in patients with cutaneous leishmaniasis caused by Leishmania (V.) guyanensis in Manaus, Amazonas, Brazil. PLoS ONE 2019, 14, e0220572. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, G.A.V.; de Mesquita, T.G.R.; de Souza Encarnação, H.V.; do Espírito Santo Junior, J.; da Costa Sabino, K.; de Aguiar Neres, I.; de Almeida, S.A.; de Souza, M.L.G.; Talhari, S.; Ramasawmy, R. A polymorphism in the IL1B gene (rs16944 T/C) is associated with cutaneous leishmaniasis caused by Leishmania guyanensis and plasma cytokine interleukin receptor antagonist. Cytokine 2019, 123, 154788. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, G.A.V.; Mesquita, T.G.; Souza, V.C.; Junior, J.D.E.S.; Gomes de Souza, M.L.; Talhari, A.C.; Talhari, S.; Naveca, F.G.; Ramasawmy, R. A Single Haplotype of IFNG Correlating with Low Circulating Levels of Interferon-γ Is Associated With Susceptibility to Cutaneous Leishmaniasis Caused by Leishmania guyanensis. Clin. Infect. Dis. 2020, 71, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhou, H.; Wu, S.; Wang, H.; Lv, C.; Cheng, B.; Xie, G.; Fang, X. Lack of association between TREM-1 gene polymorphisms and severe sepsis in a Chinese Han population. Hum. Immunol. 2008, 69, 220–226. [Google Scholar] [CrossRef]
- Runzheimer, J.; Mewes, C.; Büttner, B.; Hinz, J.; Popov, A.F.; Ghadimi, M.; Kristof, K.; Beissbarth, T.; Schamroth, J.; Tzvetkov, M.; et al. Lack of an Association between the Functional Polymorphism TREM-1 rs2234237 and the Clinical Course of Sepsis among Critically Ill Caucasian Patients-A Monocentric Prospective Genetic Association Study. J. Clin. Med. 2019, 8, 301. [Google Scholar] [CrossRef]
- Chin, V.K.; Asyran, A.M.Y.; Zakaria, Z.A.; Abdullah, W.O.; Chong, P.P.; Nordin, N.; Ibraheem, Z.O.; Majid, R.A.; Basir, R. TREM-1 modulation produces positive outcome on the histopathology and cytokines release profile of Plasmodium berghei-infected mice. J. Parasit. Dis. 2019, 43, 139–153. [Google Scholar] [CrossRef]
- Ornatowska, M.; Azim, A.C.; Wang, X.; Christman, J.W.; Xiao, L.; Joo, M.; Sadikot, R.T. Functional genomics of silencing TREM-1 on TLR4 signaling in macrophages. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 293, 1377–1384. [Google Scholar] [CrossRef]
- Bleharski, J.R.; Kiessler, V.; Buonsanti, C.; Sieling, P.A.; Stenger, S.; Colonna, M.; Modlin, R.L. A role for triggering receptor expressed on myeloid cells-1 in host defense during the early-induced and adaptive phases of the immune response. J. Immunol. 2003, 170, 3812–3818. [Google Scholar] [CrossRef]
- Polari, L.P.; Carneiro, P.P.; Macedo, M.; Machado, P.R.L.; Scott, P.; Carvalho, E.M.; Bacellar, O. Leishmania braziliensis Infection Enhances Toll-Like Receptors 2 and 4 Expression and Triggers TNF-α and IL-10 Production in Human Cutaneous Leishmaniasis. Front Cell Infect. Microbiol. 2019, 9, 120. [Google Scholar] [CrossRef]
- Koussoulas, V.; Tzivras, M.; Giamarellos-Bourboulis, E.J.; Demonakou, M.; Vassilliou, S.; Pelekanou, A.; Papadopoulos, A.; Giamarellou, H.; Barbatzas, C. Can soluble triggering receptor expressed on myeloid cells (sTREM-1) be considered an anti-inflammatory mediator in the pathogenesis of peptic ulcer disease? Dig. Dis. Sci. 2007, 52, 2166–2169. [Google Scholar] [CrossRef]
- Ruiz-Linares, A.; Adhikari, K.; Acuña-Alonzo, V.; Quinto-Sanchez, M.; Jaramillo, C.; Arias, W.; Fuentes, M.; Pizarro, M.; Everardo, P.; de Avila, F.; et al. Admixture in Latin America: Geographic Structure, Phenotypic Diversity and Self-Perception of Ancestry Based on 7,342 Individuals. PLoS Genet 2014, 10, e1004572. [Google Scholar] [CrossRef] [PubMed]
- Garcia, L.; Kindt, A.; Bermudez, H.; Llanos-Cuentas, A.; De Doncker, S.; Arevalo, J.; Wilber Quispe Tintaya, K.; Dujardin, J.C. Culture-independent species typing of neotropical Leishmania for clinical validation of a PCR-based assay targeting heat shock protein 70 genes. J. Clin. Microbiol. 2004, 42, 2294–2297. [Google Scholar] [CrossRef]
- Marfurt, J.; Nasereddin, A.; Niederwieser, I.; Jaffe, C.L.; Beck, H.P.; Felger, I. Identification and differentiation of Leishmania species in clinical samples by PCR amplification of the miniexon sequence and subsequent restriction fragment length polymorphism analysis. J. Clin. Microbiol. 2003, 41, 3147–3153. [Google Scholar] [CrossRef] [PubMed]
- González, J.R.; Armengol, L.; Solé, X.; Guinó, E.; Mercader, J.M.; Estivill, X.; Moreno, V. SNPassoc: An R package to perform whole genome association studies. Bioinformatics 2007, 23, 644–645. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Strimmer, K. fdrtool: A versatile R package for estimating local and tail area-based false discovery rates. Bioinformatics 2008, 24, 1461–1462. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 1st ed.; Springer: New York, NY, USA, 2009; pp. 41–64. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).