Abstract
A high epilepsy prevalence has been reported in onchocerciasis meso- and hyper-endemic regions in sub-Saharan Africa, including in the Democratic Republic of Congo (DRC). We investigated whether onchocerciasis-associated epilepsy can also be suspected in onchocerciasis hypo-endemic regions. Stored serum samples from 342 patients admitted with recent onset neurological symptoms admitted to Mosango general hospital, in the Kwilu province, DRC, between 2012 and 2015 were screened for onchocerciasis (OV16) antibodies by ELISA and Taenia solium antigen (using an in-house B158/B60 antigen test). Eighty-one (23.7%; 95% CI 19.5–28.5%) of these samples were positive for OV16 antibodies and 43/340 (12.6%; 95% CI 9.5–16.6%) were positive for T. solium antigen. Of the 58 persons clinically diagnosed with late onset epilepsy of unknown etiology, 19 (32.8%) were OV16 positive and nine (16%) T. solium antigen positive. In total, 16 persons with epilepsy were OV16 positive and T. solium negative, of whom 12 (75%) were between the ages seven to 31 years old, an age rage in which onchocerciasis-associated epilepsy is observed. Our study suggests that in onchocerciasis hypo-endemic areas, in T. solium antigen negative persons with epilepsy, onchocerciasis should be considered as a potential trigger of epilepsy.
1. Introduction
Neurological disorders in low and middle income countries have a variety of causes, including various infectious diseases [1,2]. Many of these diseases, such as cerebral malaria, bacterial meningitis, and human African trypanosomiasis (HAT) are treatable when the correct diagnosis is made in time [1,2]. The latter remains challenging in the rural settings where these diseases are most prevalent, especially in the early stages where most people have nonspecific symptoms and advanced diagnostic tools are absent. This leads to delay of diagnosis and consequently a larger disease burden in the affected communities.
A study was conducted between 2012 and 2015 to improve field diagnosis of common and severe neglected neurological diseases in Mosango general referral hospital in Kwilu province, Democratic Republic of Congo (DRC) [3]. In this study, patients above five years old who were admitted to the hospital with recent-onset neurological symptoms were recruited [3]. These patients all had a normal development without neurological symptoms before the age of five years. In 18% of the patients, the neurological symptoms could be attributed to common infections of the central nervous system, such as meningitis, HIV and related infections, and HAT [3]. However, many of them ended up with a syndromic diagnosis (without etiological agent), mainly epilepsy. In a post-hoc study using the stored samples of these neurological patients, we determined the presence of Taenia solium antigens and found 12.6% positivity in the whole neurological cohort and 16% positivity in the subset with clinical diagnosis of epilepsy [4].
High epilepsy prevalence has been reported in onchocerciasis meso- and hyper-endemic regions (i.e., regions where >20% of adults present onchocerciasis nodules [5,6,7,8]) including in the DRC [9,10,11,12,13,14]. In such regions, a form of epilepsy, called onchocerciasis-associated epilepsy, has been described. This form of epilepsy is characterized by the following criteria [15]: (1) the person has lived in an onchocerciasis endemic region for at least three years (2) onset of seizures occurred between 3–18 years of age; (3) there is a high prevalence of epilepsy in the village, and there are several families with more than one child with epilepsy in this village; (4) there is no obvious cause of epilepsy (for example perinatal trauma, recent head trauma, cerebral malaria, encephalitis, or neurocysticercosis); (5) prior to the onset of epilepsy, the psychomotor development of the child was normal; and (6) the person presents onchocerciasis antibodies and/or microfilariae in skin snips.
What we do not know is whether certain persons with epilepsy in onchocerciasis hypo-endemic regions may have onchocerciasis-associated epilepsy. We were interested to screen persons with neurological conditions including epilepsy in an onchocerciasis hypo-endemic area for the presence of Onchocerca volvulus antibodies. Initially, O. volvulus was not considered as a potential causative agent of neurological symptoms in the study in Mosango. We therefore set out to determine the prevalence of O. volvulus antibodies in the Mosango neurological study population in general, and in patients with epilepsy without evidence of T. solium infection in particular. We hypothesized that a limited number of persons with epilepsy would suffer from onchocerciasis-associated epilepsy.
2. Materials and Methods
2.1. Study Design
2.1.1. Study Population
This study is part of the project “Better Diagnosis for Infectious Diseases” (NIDIAG; www.nidiag.eu, accessed on 19 March 2021). Consecutive patients with recent-onset neurological disorders admitted to Mosango general referral hospital in Kwilu province, DRC, were recruited between 2012 and 2015. The study area is classified as hypo-endemic according to rapid epidemiological mapping of onocherciasis (REMO). Detailed description of the patient population and inclusion criteria are published elsewhere [3]. All eligible patients were older than five years, with at least one of the following symptoms: (1) altered state of consciousness, (2) changes in sleep pattern, (3) cognitive decline, (4) changes in personality/behavior, (5) recent epileptic seizure (within less than two weeks), (6) recent, severe and progressive headache, (7) meningism, (8) new onset cranial nerve lesion(s), (9) new onset sensory-motor focal deficits, and (10) new onset gait/walking disorders. These symptoms had to be either of recent onset or ongoing for a longer time but still present on admission. Patients younger than five years or with neurological symptoms due to recent trauma or a past neurological event were not eligible for the study [3].
2.1.2. Diagnosis of Epilepsy
Symptoms and diagnosis of epilepsy were registered in the following ways: (1) as epileptic seizure regardless the etiology as a motive for inclusion in the study or (2) as late-onset epilepsy of unknown etiology (using the 2014 ILAE definition of epilepsy [16] and after ruling out a set of infectious diseases). The conditions for which the study participants were systematically tested were: (1) second-stage HAT, (2) cerebral malaria, (3) bacterial meningitis and unspecified meningoencephalitis, (4) tuberculosis of the CNS, (5) neurosyphilis, and (6) HIV-related neurological disorders.
2.1.3. Laboratory Tests for Onchocerca volvulus and Taenia solium Infection
Cryopreserved serum collected during the clinical study was retrospectively screened for OV16 IgG4 antibodies by enzyme-linked immunosorbent assay (ELISA) with Horseradish peroxidase (HRP) as described earlier [17]. Briefly, plates were coated with OV16 antigen (Abcam, Cambridge, UK) overnight and washed three times with washing buffer (Phosphate buffered saline with 0.5% Tween 20). Plates were blocked with SuperBlock Blocking buffer (Invitrogen, Carlsbad, CA, USA) for 30 min and washed three times. Samples were diluted 1:200 and incubated at room temperature for 1 h, followed by five washing steps. HRP-conjugated anti-human IgG antibodies (Abcam) were used as detection antibody, diluted 1:10,000 and incubated for 1 h. After five washing steps, one-step Ultra TMB substrate solution (ThermoFisher Scientific, Waltham, MA, USA) was added, and the reaction was stopped by adding 1N HCl after 10 min. The absorbance was measured at an optical density of 450 nm. T. solium antigens were also measured on collected sera by an in-house B158/B60 antigen ELISA as described before [18]. This test has a sensitivity of 90% to 100% to detect current cysticercosis and a specificity of 83% to 98% [19].
2.2. Data Analysis
We first determined the frequency of OV16 antibody positivity among all patients with neurological disorders and compared demographic and clinical characteristics of OV16-seropositive and OV16-seronegative patients. We then described the age distribution in OV16-seropositive and T. solium antigen-positive patients. Next, we focused on the subgroup of patients clinically diagnosed with epilepsy and described the characteristics of epilepsy patients with positive versus negative tests for O. volvulus (OV16 antibody test) and T. solium (in-house B158/B60 antigen test). We used the chi-squared test with Yates’ continuity correction, the Fisher exact test, and the Wilcoxon rank sum test to assess statistical significance, taking an alpha level of 0.05. We used Microsoft Excel for data processing and R for statistical analyses.
3. Results
3.1. All Patients with Neurological Disorders
Serum samples from 342 out of 351 (97%) patients with neurological disorders enrolled in the NIDIAG study were available for OV16 testing, while 340 had been tested previously for T. solium testing. Eighty-one (23.7%; 95% CI 19.5–28.5%) of these samples were positive for the presence of OV16 antibodies and 43/340 (12.6%; 95% CI 9.3–16.7%) were positive for T. solium antigen. The median age of the OV16-seropositive patients was 40 years (interquartile range 20 to 50) and that of the OV16-seronegative patients 41 years (interquartile range 25 to 54; p = 0.2). There were three OV16-seropositive children under 10 years old (one child of six and two children of seven years old) and the prevalence of OV16 positivity among the 10–20 year old age group was 33% (Figure 1). The median age of the T. solium antigen-positive patients was 42 years (interquartile range 31 to 50). The prevalence of antigen positivity was 4/70 (5.7) % up to the age of 20 years.
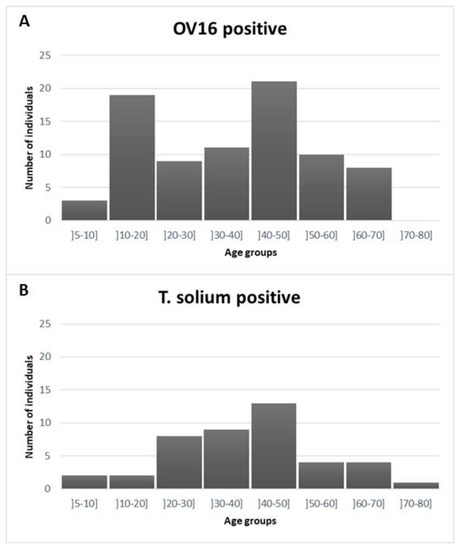
Figure 1.
Distribution of age in OV16-seropositive (panel (A)) and T. solium antigen-positive patients (panel (B)).
There were no statistically significant differences in demographic and clinical characteristics when we compared 81 OV16-seropositive with 261 OV16-seronegative patients (Table 1).

Table 1.
Number and proportion of patients with positive serology for Onchocerca stratified by demographic characteristics, clinical symptoms and signs, and final diagnosis.
Twenty-four percent of men and 23% of women were OV-16 seropositive (p = 0.9). Skin and soft tissue symptoms (n = 7) and itching (n = 2) were uncommon in the study population; all participants with these symptoms were OV16 seronegative. The majority of patients (n = 227; 66%) came from the Mosango health zone, and this proportion was similar among OV16-seropositive (65%) and OV16-seronegative (67%) patients.
3.2. Persons with Epilepsy
The study population included 58 patients with a final diagnosis of epilepsy. Nineteen of them (32.8%) were OV16 seropositive. In 57 of these 58 epilepsy patients, results of the antigen test for T. solium were also available, and nine out of 57 (16%) were positive. Detailed results of the two tests are given in Table 2.

Table 2.
Test results for Onchocerca volvulus (OV16 antibody test) and for Taenia solium (in-house B158/B60 antigen test) in 57 patients with a diagnosis of epilepsy, stratified by sex and age.
Taking all diagnostic test results together, there were 16 persons with epilepsy (28%) with a positive serology for onchocerciasis and a negative antigen test results for cysticercosis. Other infectious diseases (HIV, tuberculosis, malaria, second-stage human African trypanosomiasis, bacterial meningitis, and neurosyphilis) were ruled out in these patients. The characteristics of these 16 patients, for whom onchocerciasis could be the trigger of epilepsy, are summarized in Table 3. They all had a recent epileptic seizure (within less than two weeks) when they were enrolled. The median age of the 16 persons with epilepsy that were OV16 positive only was 22 years (interquartile range 18 to 34 years), and that of the six only T. solium antigen positive epilepsy patients was 33 years (interquartile range 27 to 35 years, p-value 0.18).

Table 3.
Characteristics of 16 patients with epilepsy, a positive test result for Onchocerca volvulus (OV16 antibody test) and a negative test result for Taenia solium antigen (in-house B158/B60 antigen test).
4. Discussion
This study among patients with neurological disorders enrolled at the Mosango hospital, a rural hospital located in an onchocerciasis hypo-endemic area of the DRC, revealed that 23.7% were OV16 ELISA test positive, with no other evidence of infectious etiologies including cysticercosis. As the OV16 ELISA test has a high specificity for the presence of O. volvulus antibodies [20], this means these individuals had been exposed to this infection. Moreover, the finding of three OV16-seropositive children under 10 years old suggests that there was relatively recent ongoing onchocerciasis transmission in the area where these children originated from (Mosango and Masimanimba). A slightly higher percentage (32.8%) of the people with epilepsy were positive for onchocerciasis, but this difference was not significant. Among the 57 persons with epilepsy, 16 (28%) presented O. volvulus antibodies only. No information was available about the age of onset of the epilepsy, but because 12 (75%) of the 16 persons with epilepsy were between the ages 7–31 years old, it is possible that some of them had onchocerciasis-associated epilepsy. The mean age of onset of onchocerciasis-associated epilepsy is between 10 and 12 years, and nearly all affected persons die before the age of 35 [7]. Severe progressive headache is generally not a symptom of onchocerciasis-associated epilepsy. Therefore, in patient 8 and 9 and in particular patient 9, who presented meningism, most likely the epilepsy was not triggered by onchocerciasis. Certainly, the epilepsy of the 60 year old person from Kinshasa was not related to onchocerciasis. It is interesting to note that the OV16 seropositivity started at an earlier age than T. solium antigen positivity. This may also explain that onchocerciasis associated epilepsy is generally observed at an earlier age than epilepsy caused by neurocysticercosis.
Our study has several limitations. First, no skin snips were taken to determine active O. volvulus infection and infection load. A positive OV16 antibody test is only an indication of exposure and does not provide information on current infection. Furthermore, no information on past ivermectin use was collected. Second, data on the area of residence and duration of residence in the area were unknown, as only the location from where the patient was admitted to the hospital was collected. Therefore, it is not certain which patients were residing or had in the past resided in regions meso- or hyper-endemic for onchocerciasis. Finally, no brain imaging was performed in the persons with epilepsy
Despite these limitations, our study suggests that there may be O volvulus transmission in the broader Mosango area and that some of the epilepsy was induced by onchocerciasis. It has been suggested that neurocysticercosis is the most important parasitic cause of epilepsy in sub-Saharan Africa. However, in Mosango, more persons with epilepsy tested positive for the presence of O. volvulus antibodies (28%) than T. solium antigen (11%) only. Our study suggest that in onchocerciasis hypo-endemic areas in T. solium antigen negative persons with epilepsy, onchocerciasis should be considered as a potential trigger of epilepsy. Moreover, if our OV16 prevalence data are confirmed in a community based study, the Congolese onchocerciasis elimination program should consider including the Mosango area for onchocerciasis elimination mapping [21].
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/pathogens10040389/s1. Table S1: In a subset of 290 patients with negative antigen test results for Taenia solium: number and proportion of patients with positive serology for Onchocerca, stratified by demographic characteristics, clinical symptoms and signs, and final diagnosis.
Author Contributions
Conceptualization: R.C., A.H., and K.V.; methodology: R.C., A.H., K.V., and E.B.; software: A.H.; validation: R.C. and A.H.; formal analysis: K.V.; Investigation: D.M., E.B., L.H., J.-R.L.-K., P.L., B.B. and A.H.; resources: R.C., J.J., and M.B.; data curation: K.V.; writing—original draft preparation: R.C., A.H., and K.V.; writing—review and editing: R.C., A.H., and K.V.; visualization: A.H.; supervision: R.C., J.J., and K.V.; project administration: R.C., J.J., and M.B.; funding acquisition: R.C., J.J., and M.B. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by a grant from the European Research Council (ERC 671055) and the European Union 7th Framework program for research, grant 260260. The study sponsor had no role in the design, execution, interpretation, or writing of the study.
Institutional Review Board Statement
The protocol of the original NIDIAG study was approved by the Institutional Review Board of the Institute of Tropical Medicine of Antwerp (ITM), and by the Ethics Committees of the University of Antwerp, Belgium, and the Public Health School of Kinshasa, DRC.
Informed Consent Statement
Written informed consent was obtained from each participant or from his/her legal representative for those <18 years or in case the neurological condition did not allow for adequate decision. For minors from 12 to 18 years, in addition to the parental consent, informed assent was also obtained. Additional ethical approval was obtained from the same Institutional Review Board and Ethics committees for OV16 testing on stored samples.
Data Availability Statement
The datasets generated during the current study are available from the corresponding authors on reasonable request.
Acknowledgments
We would like to thank all medical and nursing staff of the general referral hospital of Mosango and all study participants.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Winkler, A.S.; Mosser, P.; Schmutzhard, E. Neurological disorders in rural Africa: A systematic approach. Trop. Dr. 2009, 39, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Howlett, W.P. Neurology in Africa. Neurol. Afr. 2015, 83, 654–655. [Google Scholar] [CrossRef] [PubMed]
- Mukendi, D.; Kalo, J.-R.L.; Mpanya, A.; Minikulu, L.; Kayembe, T.; Lutumba, P.; Barbé, B.; Gillet, P.; Jacobs, J.; Van Loen, H.; et al. Clinical spectrum, etiology, and outcome of neurological disorders in the rural hospital of Mosango, the Democratic Republic of Congo. Am. J. Trop. Med. Hyg. 2017, 97, 1454–1460. [Google Scholar] [CrossRef] [PubMed]
- Mukendi, D.; Kalo, J.-R.L.; Lutumba, P.; Barbe, B.; Jacobs, J.; Yansouni, C.P.; Gabriel, S.; Dorny, P.; Chappuis, F.; Boelaert, M.; et al. High frequency of Taenia solium antigen positivity in patients admitted for neurological disorders in the Rural Hospital of Mosango, Democratic Republic of Congo. BMC Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Siewe, J.N.F.; Tatah, G.; Tabah, E.N.; Ngarka, L.; Nfor, L.N.; Chokote, S.E.; Mengnijo, M.K.; Dema, F.; Sitouok, A.T.; Nkoro, G.; et al. Epidemiology of onchocerciasis-associated epilepsy in the Mbam and Sanaga river valleys of Cameroon: Impact of more than 13 years of ivermectin. Infect. Dis. Poverty 2018, 7, 114. [Google Scholar] [CrossRef] [PubMed]
- Mmbando, B.P.; Suykerbuyk, P.; Mnacho, M.; Kakorozya, A.; Matuja, W.; Hendy, A.; Greter, H.; Makunde, W.H.; Colebunders, R. High Prevalence of Epilepsy and Onchocerciasis after 20 Years of Ivermectin Use in Four Villages of the Mahenge Are in Tanzania. In Proceedings of the 10th European Conference on Tropical Medicine and International Health, Antwerp, Belgium, 16–20 October 2017; p. 4. [Google Scholar]
- Colebunders, R.; Carter, J.Y.; Olore, P.C.; Puok, K.; Bhattacharyya, S.; Menon, S.; Abd-Elfarag, G.; Ojok, M.; Ensoy-Musoro, C.; Lako, R.; et al. High prevalence of onchocerciasis-associated epilepsy in villages in Maridi County, Republic of South Sudan: A community-based survey. Seizure 2018, 63, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Gumisiriza, N.; Mubiru, F.; Fodjo, J.N.S.; Kayitale, M.M.; Hotterbeekx, A.; Idro, R.; Makumbi, I.; Lakwo, T.; Opar, B.; Kaducu, J.; et al. Prevalence and incidence of nodding syndrome and other forms of epilepsy in onchocerciasis-endemic areas in northern Uganda after the implementation of onchocerciasis control measures. Infect. Dis. Poverty 2020, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Colebunders, R.; Mandro, M.; Mokili, J.L.; Mucinya, G.; Mambandu, G.; Pfarr, K.; Reiter-Owona, I.; Hoerauf, A.; Tepage, F.; Levick, B.; et al. Risk factors for epilepsy in Bas-Uélé Province, Democratic Republic of the Congo: A case–control study. Int. J. Infect. Dis. 2016, 49, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Levick, B.; Laudisoit, A.; Tepage, F.; Ensoy-Musoro, C.; Mandro, M.; Osoro, C.B.; Suykerbuyk, P.; Kashama, J.M.; Komba, M.; Tagoto, A.; et al. High prevalence of epilepsy in onchocerciasis endemic regions in the Democratic Republic of the Congo. PLoS Negl. Trop. Dis. 2017, 11, e0005732. [Google Scholar] [CrossRef] [PubMed]
- Lenaerts, E.; Mandro, M.; Mukendi, D.; Suykerbuyk, P.; Dolo, H.; Wonya’Rossi, D.; Ngave, F.; Ensoy-Musoro, C.; Laudisoit, A.; Hotterbeekx, A.; et al. High prevalence of epilepsy in onchocerciasis endemic health areas in Democratic Republic of the Congo. Infect. Dis. Poverty 2018, 7, 68. [Google Scholar] [CrossRef] [PubMed]
- Mandro, M.; Suykerbuyk, P.; Tepage, F.; Rossy, D.; Ngave, F.; Hasan, M.N.; Hotterbeekx, A.; Mambandu, G.; Kashama, J.M.; Laudisoit, A.; et al. Onchocerca volvulus as a risk factor for developing epilepsy in onchocerciasis endemic regions in the Democratic Republic of Congo: A case control study. Infect. Dis. Poverty 2018, 7, 79. [Google Scholar] [CrossRef]
- Mukendi, D.; Tepage, F.; Akonda, I.; Siewe, J.N.F.; Rotsaert, A.; Ndibmun, C.N.; Laudisoit, A.; Couvreur, S.; Kabutako, B.; Menon, S.; et al. High prevalence of epilepsy in an onchocerciasis endemic health zone in the Democratic Republic of the Congo, despite 14 years of community-directed treatment with ivermectin: A mixed-method assessment. Int. J. Infect. Dis. 2019, 79, 187–194. [Google Scholar] [CrossRef]
- Fodjo, J.N.S.; Mandro, M.; Mukendi, D.; Tepage, F.; Menon, S.; Nakato, S.; Nyisi, F.; Abhafule, G.; Wonya’Rossi, D.; Anyolito, A.; et al. Onchocerciasis-associated epilepsy in the Democratic Republic of Congo: Clinical description and relationship with microfilarial density. PLOS Negl. Trop. Dis. 2019, 13, e0007300. [Google Scholar] [CrossRef]
- Colebunders, R.; Siewe, F.N.; Hotterbeekx, A. Onchocerciasis-associated epilepsy, an additional reason for strengthening onchocerciasis elimination programs. Trends Parasitol. 2018, 34, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.S.; Acevedo, C.; Arzimanoglou, A.; Bogacz, A.; Cross, J.H.; Elger, C.E.; Engel, J.; Forsgren, L.; French, J.A.; Glynn, M.; et al. ILAE Official Report: A practical clinical definition of epilepsy. Epilepsia 2014, 55, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Golden, A.; Stevens, E.J.; Yokobe, L.; Faulx, D.; Kalnoky, M.; Peck, R.; Valdez, M.; Steel, C.; Karabou, P.K.; Banla, M.; et al. A recombinant positive control for serology diagnostic tests supporting elimination of Onchocerca volvulus. PLoS Negl. Trop. Dis. 2016, 10, e0004292. [Google Scholar] [CrossRef] [PubMed]
- Carabin, H.; Millogo, A.; Praet, N.; Hounton, S.; Tarnagda, Z.; Ganaba, R.; Dorny, P.; Nitiéma, P.; Cowan, L.D. Seroprevalence to the antigens of taenia solium cysticercosis among residents of three villages in Burkina Faso: A cross-sectional study. PLoS Negl. Trop. Dis. 2009, 3, e555. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gabriel, S.; Blocher, J.; Dorny, P.; Abatih, E.N.; Schmutzhard, E.; Ombay, M.; Mathias, B.; Winkler, A.S. Added Value of Antigen ELISA in the Diagnosis of Neurocysticercosis in Resource Poor Settings. PLoS Negl. Trop. Dis. 2012, 6, e1851. [Google Scholar] [CrossRef] [PubMed]
- Hotterbeekx, A.; Perneel, J.; Mandro, M.; Abhafule, G.; Fodjo, J.N.S.S.; Dusabimana, A.; Abrams, S.; Kumar-Singh, S.; Colebunders, R. Comparison of diagnostic tests for Onchocerca volvulus in the Democratic Republic of Congo. Pathogens 2020, 9, 435. [Google Scholar] [CrossRef] [PubMed]
- Lakwo, T.; Oguttu, D.; Ukety, T.; Post, R.; Bakajika, D. Onchocerciasis Elimination: Progress and Challenges. Res. Rep. Trop. Med. 2020, 11, 81–95. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).