Abstract
Bluetongue virus (BTV) produces an economically important disease in ruminants of compulsory notification to the OIE. BTV is typically transmitted by the bite of Culicoides spp., however, some BTV strains can be transmitted vertically, and this is associated with fetus malformations and abortions. The viral factors associated with the virus potency to cross the placental barrier are not well defined. The potency of vertical transmission is retained and sometimes even increased in live attenuated BTV vaccine strains. Because BTV possesses a segmented genome, the possibility of reassortment of vaccination strains with wild-type virus could even favor the transmission of this phenotype. In the present review, we will describe the non-vector-based BTV infection routes and discuss the experimental vaccination strategies that offer advantages over this drawback of some live attenuated BTV vaccines.
1. Introduction
Bluetongue (BT) is a disease of mandatory notification to the World Organization of Animal Health (OIE) that causes important economic losses globally estimated to be around three billion dollars per year [1]. Bluetongue virus (BTV) is the etiological agent responsible for BT, a disease that affects domestic and wild ruminants and that can be particularly severe in sheep [2,3]. BT disease clinical signs are characterized by the virus preferred tropism for endothelial cells [4]. As a consequence of endothelial cell damage, edema and hemorrhages can take place in BTV infections. Early clinical signs are pyrexia, depression, and loss of appetite [5,6]. In some cases, the disease progresses to conjunctivitis, congestion of the nasal and oral mucosa and edema of the face and lip. Sometimes hemorrhagic lesions occur which can progress to the cyanosis of the tongue that gave its name to the disease. In the most severe cases, respiratory distress and esophageal paresis can develop which can ultimately lead to the death of the infected animal. Although BTV infection is not always fatal, it typically leads to reduced productivity in ruminants (e.g., reduced milk yield, weakness of the animal, abortion or stillbirth)[5,6]. BTV therefore produces a debilitating disease that affects the livestock industry.
BTV circulation was once restricted to the subtropical regions with occasional incursion in more temperate areas of the globe. However, it has now become apparent that the disease has become endemic in the European part of the Mediterranean basin [7,8]. Vaccination can control outbreaks; however, at least 28 different BTV serotypes with little cross-reactivity have been identified so far [9,10,11,12]. This complicates disease control as multiple vaccines are required for protection in regions where multiple serotypes are circulating. BTV serotypes have been classified as “classical” (serotypes 1–24) or “atypical” for some recent isolates that predominantly affect small ruminants with little to no clinical signs [13,14,15,16]. Only “classical” BTV serotypes (1–24) are notifiable to the OIE [17].
2. BTV Viral Particle
BTV belongs to the Reoviridae family and is prototypical of the Orbivirus genus. BTV is a double stranded RNA (dsRNA) virus, its genetic material consists of 10 segments (Figure 1) [18], encoding for 7 structural proteins (VP1 to VP7) and at least 4 non-structural proteins (NS1 to NS4). A putative fifth non-structural protein (NS5) has also been reported [19]. The viral particle consists of a two-layer core that encapsulates the RNA polymerase and the segmented genome. The outer core is composed of the highly variable VP2 protein and the VP5 protein that acts as the main anchor of this layer to the inner core. This outer core is responsible for the interaction with the host cellular components that allow virus cell entry. Most neutralizing antibodies are also directed against the proteins in this layer and mostly against VP2. The high variability of VP2 confers the virus with a means to evade neutralizing antibodies, which, as a result, generates the 28 serotypes with little cross-reactivity.
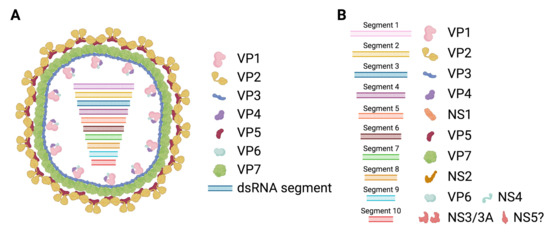
Figure 1.
Schematic representation of bluetongue virus (BTV). (A) The bluetongue viral particle is composed of an outer capsid that consists of the VP2 and VP5 proteins, and an inner core formed by the VP7 and VP3 proteins. VP3 anchors the RNA polymerase VP1 to the capsid. The RNA capping and methyl transferase VP4 and the helicase VP6 are associated with VP1. Enclosed within the inner core, the BTV genome consisting of 10 segments of dsRNA is found. (B) The segmented genome of BTV encodes for 7 structural proteins (VP1 to VP7) and at least 4 non-structural proteins (NS1 to NS4). Segment 1 encodes for the RNA polymerase VP1. Segment 2 encodes for the highly variable VP2. Segment 3 encodes for the inner core protein VP3. Segment 4 encodes for the methyl transferase and RNA capping enzyme VP4. Segment 5 encodes for NS1, a non-structural protein that forms cytoplasmic tubules. Segment 6 encodes for the outer capsid protein VP5. Segment 7 encodes for the inner core protein VP7. Segment 8 encodes for NS2, an RNA binding non-structural protein expressed in viral inclusion bodies. Segment 9 encodes for the helicase VP6 and for NS4, a non-structural protein involved in immune evasion. Segment 10 encodes for NS3 and its isoform NS3a, which are polyfunctional non-structural proteins involved in viral particle exit from the cell as well as in interference with the mammalian IFN system. Segment 10 also putatively encodes for a fifth non-structural protein (NS5), which could be implicated in cellular shutdown. (Created with Biorender.com).
Once internalized, the outer core is destabilized by low pH, which allows VP5-mediated liberation of the highly stable inner core into the cytoplasm [20,21]. The inner core is composed of the VP7 and VP3 proteins and serves as a protective shell for the viral replication machinery. VP3 also anchors the RNA polymerase VP1 to the inner core [22]. Core-like particle assembly experiments have also indicated that the RNA capping enzyme and methyl transferase VP4 is associated with the VP3-VP1 complex [23]. The spatial distribution within the core of the RNA helicase VP6 is less well characterized, but its presence is important for the correct packaging of the dsRNA genome [24]. The inner core also contains the segmented RNA genome.
Non-structural proteins are involved in promoting viral replication in the host cells and in interfering with immunity. NS1 forms cytoplasmic tubules that promote viral protein expression [25]. NS1 enhancement of viral mRNA translation relies on two zinc finger-like motifs present in the protein and on the transition from the inactive tubular state to an active non-tubular form [26]. NS2 is the most abundant protein in viral inclusion bodies (VIB). VIB formation is dependent on NS2 phosphorylation, which is enhanced by calcium ions [27]. NS2 is an RNA binding protein that facilitates the assembly of new viral inner cores [28]. NS3, and its shorter isoform NS3a which lacks the first 13 N-terminal amino-acid residues, is involved in virion egress [29,30,31]. NS3 can act as a viroporin, thus easing the release of new viral particles [32]. NS3 also contributes to the maturation of the viral particle, possibly through its binding to VP2 [33] which promotes the release of two-layered mature viral particles. VP3, NS3, NS4 and the putative NS5 are involved in countering the antiviral cell response. VP3, NS3 and NS4 can act as IFN antagonists (reviewed in [34]). VP3 can impair IFN induction [35], while NS3 and NS4 can counter IFN induction as well as type I and type II IFN signaling [36,37,38,39,40]. Finally, the putative NS5 has been shown to promote cellular shut-off in transfection experiments [19].
3. BTV Is Mainly an Arbovirus, but It Can Be Transmitted through Other Routes
BTV is principally an arthropod-borne virus (arbovirus) that is transmitted by the bite of Culicoides spp. to ruminants [41] (Figure 2A). However, BTV can also be transmitted through other routes (Figure 2B–E). There is evidence that large African carnivores can become infected probably through feeding on BTV-infected ruminants [42]. Similarly, BTV-8 could be transmitted to Eurasian lynx through this oral route [43]. The significance of these findings in the wider context of BTV transmission is unclear, but it is unlikely to have a high epidemiological impact.
Horizontal transmission in ruminants of BTV-1, BTV-2 and BTV-8 has been documented under experimental conditions [44,45,46]. Naïve animals housed with infected counterparts can, in some cases, become infected. This transmission route is probably the result of animals being in close proximity and/or sharing food and water troughs. Oral transmission in ruminants is also suspected in the field as a result of ingestion of contaminated placenta or colostrum [47,48]. The direct contact route appears to be particularly important in the transmission of some “atypical” BTV serotypes that specifically infect small ruminants [49,50]. From an epidemiological perspective, horizontal transmission is unlikely to be a major component of epizootic episodes, although it could have an impact on BTV morbidity in farms with densely housed livestock.
Since BTV possesses an affinity for erythrocytes [51], it is plausible that it can be transmitted through mechanical means. Indeed, there are instances in which this transmission route has been demonstrated. Transmission through sharing infected needles is documented, even in the absence of visible blood contamination (subcutaneous inoculation) [52], thus indicating that sharing needles for inoculations between ruminants poses a risk of BTV transmission. Tick transmission has also been documented [53]. Indeed, several species of ticks can become infected by BTV-8, and the virus can be found in salivary glands and thus could potentially be transmitted to ruminant hosts [54]. In the same study, BTV was shown to pass transstadial stages in hard ticks (nymph to adult) and to infect eggs in soft ticks [54]. These phenomena could contribute to BTV overwintering mechanisms, although this has yet to be confirmed. Tick transmission is nonetheless unlikely to be a major route of disease spreading.
BTV is also known to target ram and bull semen quality and can be isolated from this fluid in infected animals [55,56]. Recently, BTV transmission to heifers through insemination with semen from naturally infected bulls has been demonstrated [57]. Previous reports had already established that BTV could be transmitted through this route using semen from experimentally infected ruminants [58,59]. This transmission route has implications in disease control, as it is now suspected that the re-emergence of BTV-8 in France in 2015 could be the result of insemination with frozen semen obtained from a 2008 infected animal [60].
Vertical transmission from the pregnant female to the fetus is the alternative BTV transmission route with the most epidemiological significance. Indeed, venereal BTV transmission can also result in vertical transmission of the virus to the fetus, which often leads to abortions [57]. Vertical BTV transmission was first suspected in the 1950s as a result of vaccination with a live attenuated virus that increased stillbirth and weak lambs in vaccinated flocks [61]. Transplacental transmission was subsequently confirmed in sheep, cattle, goat and elk [62,63,64,65,66]. For a while, transplacental transmission was associated with live attenuated vaccine strains that had been passaged in embryonated chicken eggs (expertly reviewed in [63]). However, this feature has now also been associated with some BTV field strains [44,46,64,67,68,69,70,71] such as the BTV-8 responsible for the 2006 European outbreak, and thus, vector infected ruminants can transmit the virus to their offspring. It should be noted that BTV vertical transmission depends greatly on isolates. The factors that govern BTV vertical transmission are unknown but appear to be intrinsic to the virus as the rescued reverse genetic virus of a BTV-2 strain known to cross the placental barrier maintained this phenotype [44]. Curiously, BTV effects on reproduction are not limited to the ruminant hosts of the disease. There is evidence that BTV can produce abortions in dogs and even cause mortality in pregnant bitches [72,73,74,75]. This further indicates that BTV possesses intrinsic mechanisms that allow it to cross the placental barrier. From an epidemiological perspective, vertical transmission could be involved in overwintering, as newborn calves/lambs can be BTV positive, and thus could potentially start a new cycle of infection by passing the virus to the arthropod vector. Thus, vertical transmission is an aspect of BT disease that needs close attention.
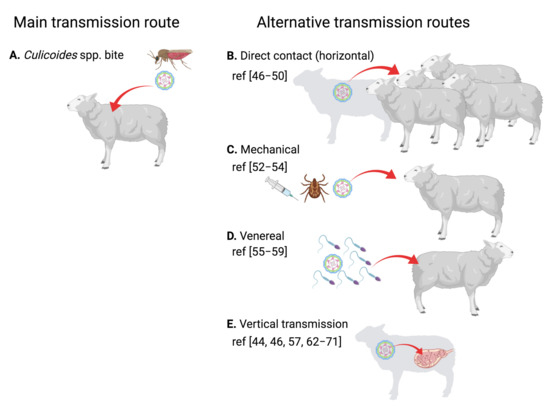
Figure 2.
Transmission routes of BTV in ruminants. (A) Typically, BTV is transmitted to the mammalian host through the bite of infected Culicoides spp. (B–E) Other transmission routes have nonetheless been documented. (B) BTV can be transmitted by direct contact in some rare cases, probably through sharing of water and food trough or consumption of infected placenta or colostrum. (C) BTV affinity for erythrocytes makes mechanical transmission possible. Infection by sharing contaminated needles and transmission by tick bites has been documented. (D) Venereal transmission through the semen of infected ruminants has also been demonstrated. (E) Finally, vertical transmission from the mother to the fetus is associated with some BTV strains. This often leads to abortions, stillbirths or lambs/calves with neurological issues. (Created with Biorender.com).
4. Impact of BTV Vertical Transmission
As previously stated, BTV infection in pregnant cows and ewes can lead to abortion or weak offspring. This represents an important economic setback for livestock farming. Moreover, transplacental transmission could contribute to BTV overwintering mechanisms. This aspect of BTV infection is often underestimated, and indeed a study found a 56% probability of vertical transmission events for BTV-8 which indicates that this transmission route could be more frequent than previously thought for some BTV isolates [76].
Early studies established BTV tropism for brain tissue in infected fetuses that resulted in congenital brain malformation [77,78,79]. The structural protein VP5 has been associated with viral neural tropism in newborn mice [80]. The teratogenic effects on fetuses of BTV during gestation depend greatly on the time of infection (reviewed in [81]). Effects on the fetuses are more severe at the early stage of gestation, and they appear to decrease as fetus immunocompetence develops from days 60–70 in sheep and days 120–130 in cattle [81,82,83]. Nonetheless, brain affectations, such as encephalitis, can still be detected in animals apparently born healthy but that were exposed to the virus [84]. Vertical transmission appears to be more likely when infection occurs in early to mid-gestation [85,86,87].
Fetus exposure to BTV in early pregnancy leads to cavitating white matter brain lesions [77] that are the results of the destruction by the virus of stem cells from the central nervous system [81]. Once pregnancy advances and the BTV-susceptible glial and neuronal precursor cells migrate to the white matter, the teratogenic effects of BTV infection in fetuses are diminished [81]. Infections in late pregnancy typically produce mild encephalitis and premature births [84,88,89]. Newborn calves/lambs exposed to BTV in utero can be born PCR positive. This has been proposed as a mechanism for virus overwintering in climates in which vector activity is greatly reduced in winter [62]. Indeed, the virus can be isolated in some instances from newborn calves [67] and newborn calves can remain PCR positive for up to five months [70], which supports the idea that transplacental transmission can lead to BTV overwintering.
In most cases, newborns that became infected in utero develop antibodies and are seropositive at birth. In some cases, PCR positive but seronegative calves have been reported [67]. This could be indicative of a tolerance to BTV, which could lead to chronic infection in these animals. Given the differences between the infant and adult immune system, viral infection in early life can have very different outcomes to infection in adulthood [90]. For instance, perinatal infection with hepatitis B virus results in persistent infection in approximately 90% of cases, whereas infection in adults only results in 5% of cases becoming persistent [90]. In the case of BTV, a study has found that infected newborn calves become PCR negative by 6 months [70]. BTV is therefore unlikely to produce chronic infections in young animals, but rather, as in the case of infection in adults [91], prolonged viremia is observed. This feature of BTV infection is thought to facilitate the transfer of the virus back to the vector and could possibly contribute to the re-emergence of the virus in spring.
The effects of BTV infection in early life on the repertoire of cells that respond to BTV are unknown. Infection could lead to an immunocompromised repertoire of T and B cells that respond to the virus. Further longitudinal studies will be required to assess the effects of BTV infection on adaptive immunity at different timepoints in animals’ lives. A compromised adaptive response to BTV due to an early life encounter with the virus could contribute to the characteristic prolonged viremia as adaptive immune cells fail to be optimally activated upon subsequent encounters. Indeed, we have shown in sheep that BTV limits humoral responses by targeting follicular dendritic cells, and this delays antibody response and potentially reduces IgG affinity for BTV antigens [92]. Furthermore, BTV infection is known to produce leukopenia [93] and, in some cases, limits the response to T cell mitogens [94]. These immunosuppressive phenomena could prolong virus circulation. Further work will be required to fully elucidate the effects of BTV infection on the immune system of young ruminants and determine whether infection in early life leads to deleterious effects on viral recognition in later life.
It is important to note that vertical transmission appears to be a feature of BTV infections limited to some strains. As previously mentioned, vertical transmission was initially thought to result from virus adaption to tissue culture conditions that favored the transmission through the transplacental barrier [63]. The overwhelming evidence that the field BTV-8 strain responsible for the 2006 European outbreak can be transmitted vertically has nonetheless challenged this view [44,45,46,67,68,69,70]. Since BTV genetic material is segmented, host co-infection with several BTV serotypes can result in reassorted viral progeny (i.e., a viral progeny in which segments that originate from the different serotypes are mixed) [95]. Sequence analysis indicated that the BTV-8 strain responsible for the outbreak in Northern Europe in 2006 did not originate directly from the BTV-8 live attenuated vaccine strain, but that it was a reassortant carrying segments from different serotypes [96]. This could have led to the introduction of the genetic determinants responsible for transplacental transmission in this strain. In the absence of studies that characterize the viral factors responsible for vertical transmission, it is difficult to discuss whether this feature was always present in the field strains or was introduced as a result of reassortment of field strains with live attenuated vaccine strains. Evidence that a reverse genetic BTV-2 strain was still capable of vertical transmission indicates that this characteristic is part of the virus make-up [44]. In any case, it is now clear that vertical transmission can be a feature of some BTV outbreaks and should therefore be monitored given its impact on reproduction.
5. Vaccination as a Strategy to Prevent BTV Vertical Transmission
Vaccination remains one of the most effective methods to combat infectious disease. This prophylaxis is probably the most cost-effective control method to prevent disease spreading: it protects animals, limits or stops disease transmission, and saves on resources that would have to be destined for disease treatment. Vaccination is an essential tool in animal health and in the fight against poverty [97].
Vaccination that would prevent BTV vertical transmission has several benefits for ruminant production. It would limit the abortions, stillbirths and weak offspring that result from in utero BTV infection, thus increasing productivity. It would also limit the possibility of disease overwintering in temperate climates, as newborns would not carry infective BTV, and thus could not trigger a new cycle of infection in the spring. Maternal vaccination could also provide passive immunity to the offspring through antibody transfer by colostrum intake after birth [98]. In the case of BTV, protection through colostrum intake could prevent newborns from becoming a reservoir for BTV transmission.
Vaccines are still being developed for arboviruses such as Chikungunya, dengue and Zika viruses, which can be transmitted vertically in humans [99]. An important consideration for these vaccines is their capacity to block vertical transmission, as these infections can have severe implications for the fetus [99]. The current vaccine for dengue virus is not recommended during pregnancy as insufficient data is available on its benefit [100], while preclinical studies have demonstrated some promising results for Zika virus candidate vaccines in preventing vertical transmission [101,102]. Evaluation of vaccine efficacy in terms of protection from vertical transmission in clinical trials can be difficult in these diseases owing to the unpredictable nature of arbovirus outbreaks. This implies that robust preclinical models are necessary to evaluate the effects of vaccination on vertical transmission.
Models to study BTV vertical transmission have been described in ruminants and mice [103,104]. Infection of pregnant ruminants in the most susceptible gestation period (typically between 1/3rd and 2/3rd of the gestation period) has been used to study the frequency of vertical transmission and BTV teratogenic effects [103]. A murine model in which the type I IFN receptor activity was blocked by antibody injection has also been described to study BTV transplacental transmission [104]. The classic IFNAR(-/-) murine model for screening BTV vaccines [105] is, however, unlikely to be useful to study vaccine effectivity against vertical transmission as infected mice typically succumb to the disease within 5–10 days. Thus, the assessment of vaccination efficacy against transplacental transmission will require the use of BTV vertical transmission models.
The identification of BTV strains that are consistently capable of vertical transmission is also a requisite to study not only the pathogenesis of the infection but also the putative capacity of vaccines to prevent transmission through this route. Indeed, there is evidence that vaccination with inactivated BTV vaccines can limit vertical transmission of BTV-8 [71]. Santman-Berends et al. showed that none of the 256 calves born from BTV-8 vaccinated dams were positive by PCR for BTV [71]. Moreover, 13 dams that were seropositive before pregnancy did not give birth to BTV positive calves, indicating that exposure to the same BTV strain prior to pregnancy may also limit vertical transmission events [71]. There is a report of a calf born with hemorrhagic artery lesions (a hallmark of BTV infection) from a vaccinated dam, although the calf was negative by PCR at the time of assessment [106]. Overall, it appears that vaccination with inactivated vaccines could limit BTV vertical transmission, although further work will be required to confirm this. In the next section we will provide a brief overview of the vaccination strategies being developed for BTV and whether they could protect from vertical transmission.
6. BTV Vaccines: Live Attenuated, Inactivated or Recombinant Vaccines?
The pros and cons of BTV vaccine strategies are summarized in Table 1. As previously discussed, the main problem of BTV live attenuated vaccines is the possibility that, in spite of their attenuation, they acquire a phenotype capable of crossing the placental barrier that leads to abortions and teratogenesis in the fetus [61,77,78,79]. Moreover, live attenuated vaccines can be contaminated with exogenous viruses that can be pathogenic in some cases [72,107,108]. These drawbacks led to the development of inactivated BTV vaccines, which are effective and safe, but typically protect against only one serotype. The reduction in incidence of BTV-8 vertical transmission in vaccinated dams indicates that classical inactivated BTV vaccines can also offer protection to the fetus [71]. This is probably the result of the protection provided to the mother by the vaccine, which limits infection, and of antibody transfer from the mother to the newborn, which protects the newborn in early life. Overall, it appears that immunity to BTV can limit vertical transmission, but little is known on the mechanisms that afford this protection.
Another issue of “classical” vaccines is that they cannot differentiate infected from vaccinated animals (the so-called DIVA approach). A DIVA vaccine simplifies serological surveillance of vaccinated populations; this is therefore highly recommendable for disease control in disease-free regions that are at risk of outbreaks. DIVA vaccines are also ideal for eradication programs as they allow surveillance once vaccination campaigns are finished and animal trade is ready to resume. Typically, “classical” vaccines” only offer protection against re-infection with a virus from the same serotypes, which implies that multiple BTV vaccines need to be administered in regions where several serotypes are circulating. Thus, one of the ultimate goals in BTV vaccinology is to develop vaccine formulations that provide protection against multiple serotypes. Advances in molecular biology and recombinant protein technology have promoted the development of vaccine alternatives to BTV live attenuated and inactivated vaccines that aim to overcome these drawbacks of “classical” vaccines.
Broadly speaking, alternative BTV vaccines can be divided into three categories: (1) recombinant BTV protein vaccines; (2) live reverse genetics BTV vaccines; and (3) viral vector vaccines expressing BTV proteins [109]. The capacity of these vaccines to prevent vertical transmission has not been tested so far, but it is likely that if they confer good BTV immunity they will also limit all transmission routes. It should be noted that the description of a murine model of vertical transmission [104] could now allow testing of these alternative vaccine formulations in a preclinical model, thus facilitating the screening of candidate vaccines that could prevent vertical transmission.
Recombinant BTV protein vaccines include BTV subunit proteins expressed by different systems (insect cells [110,111], plant [112], yeast [113]); or bluetongue virus-like particles [114] that consist of the BTV capsid proteins expressed without the virus genetic material. Recombinant BTV protein vaccines can elicit immune responses in ruminants and even provide protection [115,116,117]. These approaches are deemed extremely safe, as these formulations are unable to replicate and therefore cause disease. They are also DIVA, as serological tests can easily differentiate animals vaccinated with vaccine subunits, as opposed to infected animals, which will also present antibodies to BTV proteins that are not present in the vaccine formulation. In spite of their safety, these approaches remain nonetheless quite expensive for veterinary medicine, and inactivated whole virus vaccines, which are cheaper to manufacture, are preferred. The advent of plant-based expression systems for these BTV constructs [112,115] could, however, change this in the long-term.
Reverse genetics technology for BTV [118] has opened new doors for the development of live vaccines in the field. This has allowed, for instance, for the introduction of alternative serotype-defining outer core proteins (VP2 and VP5) on the backbone of a live attenuated virus [119]. Disabled infectious single cycle (DISC) BTV vaccines have been developed by packaging a segment 9 that contains large deletions into the viral particle [120]. Since segment 9 encodes for the helicase VP6 that is critical for new viral particle assembly [24], this DISC virus could infect and express BTV RNA (except for VP6) but could not package new viral particles and thus spread in the host. Disabled infectious single animal (DISA) vaccines have also been described [121,122,123]. This was achieved by a deletion in segment 10 that encodes for NS3/NS3a. NS3/NS3a is not required for replication in mammalian cells but it is critical for virus release from Culicoides spp. [124]. Thus, the DISA vaccine can replicate in the ruminant host but cannot in the vector [124]. These live BTV vaccines designed by reverse genetics have been shown to protect ruminants from virulent virus challenge [119,120,121,122]. Because they mimic a natural infection, they have the potential to be effective as a single dose vaccine. Diagnostic tests can also be designed so that they can be considered DIVA vaccines. The risk of reversion to virulence is nonetheless still present, and reassortment during concomitant infection with wild-type BTV remains a possibility. Moreover, because attenuation has been associated with vertical transmission for some vaccine strains [61,63,65], the safety assessment of these live reverse genetics attenuated vaccines should probably include their capacity to cross the placental barrier.
Viral vector vaccines are based on the premise of activating innate immunity to provide sufficient adjuvancy so that an adaptive immune response is mounted to the antigen expressed by the vector [125,126,127]. Several platforms have been employed to induce immunity to BTV in the natural host. These include, among others, poxviruses [128,129,130], adenoviruses [130,131,132], Rift Valley fever virus (RVFV) [133,134], or herpesviruses [135,136]. These recombinant constructs were able to induce immunity to BTV in murine models and/or in the natural host, and protection was also demonstrated in some studies in the natural host [129,130,131,133,134]. It should be noted that most protection studies with viral vectors in ruminants only detected partial protection, as in most cases, in spite of the absence of clinical signs, some level of viral replication could be detected by PCR. Vaccines based on viral vectors nonetheless offer multiple advantages over “classical” vaccines. They are typically thermotolerant formulations, which facilitate transportation to remote areas with little infrastructures. They are non-pathogenic as they are often based on replication-defective viruses. They can offer protection over multiple BTV serotypes with the same formulation [128,132]. Recombinant vector vaccines based on attenuated vaccine strains, such as RVFV, can even induce bivalent protection in ruminants against RVFV and BTV [133]. They are also DIVA vaccines as only a fraction of BTV antigens are expressed in the recombinant vector, and thus a DIVA diagnostic test can be designed around these formulations. The correct cocktail of BTV antigens that provides a broad spectrum of protection is still the subject of active research in the field. It remains to be determined whether the immunity to BTV that viral vector vaccines produce is sufficient to limit vertical transmission.

Table 1.
Pros and cons of BTV vaccine strategies.
Table 1.
Pros and cons of BTV vaccine strategies.
| Vaccine Type | Protection | Risk of BTV Vertical Transmission | DIVA 1 | |
|---|---|---|---|---|
| Classical | Live attenuated | Yes (serotype specific) | Possible | No |
| Inactivated | Yes (serotype specific) | No | No | |
| Alternative | Recombinant protein | |||
| BTV proteins [110,111,112,113] | Yes | No | Yes | |
| BTV VLP 2 [114] | Yes | No | Yes | |
| Live reverse genetics | ||||
| DISC 3 [120] | Yes | Unlikely; Needs to be tested | Yes 5 | |
| DISA 4 [121,123] | Yes | Needs to be tested | Yes 5 | |
| Viral recombinant vectors | ||||
| Poxvirus [128,129,130] | Yes 6 (potential for multiserotype) | No | Yes | |
| Adenovirus [130,131,132] | Yes 6 (potential for multiserotype) | No | Yes | |
| Rift Valley Fever Virus [133,134] | Yes 6 (bivalent BTV and RVFV) | No | Yes | |
| Herpesvirus [135,136] | Yes 6 (not tested in natural host) | No | Yes | |
1 DIVA: differentiation between infected and vaccinated animals. 2 VLP: virus-like particle. 3 DISC: disabled infectious single cycle. 4 DISA: disabled infectious single animal. 5: DIVA test needs to be designed around segment product deficiency. 6: Protection is often partial.
As previously stated, none of these experimental vaccines have been tested for their potency in inhibiting BTV vertical transmission. Data from vaccinated cattle with inactivated vaccines indicates that inducing good immunity to BTV is probably sufficient to greatly limit vertical transmission and therefore prevent abortions and newborn malformations [71]. It would therefore be interesting to evaluate whether these experimental vaccines can limit the transmission of BTV strains prone to cross the placental barrier.
7. Conclusions
Even though vertical transmission has long been associated with live attenuated BTV vaccine strains, the 2006 BTV-8 outbreak in Europe demonstrated that vertical transmission could be a feature of some BTV field strains. In utero infection can lead to abortions and/or congenital malformations that limit ruminant productivity. Moreover, vertical transmission can also contribute to the disease overwintering in temperate climates in which vector activity is reduced in colder months. As such, this transmission route and its consequences on reproduction should be monitored during BTV outbreaks. The factors involved in the crossing of the placental barrier by the virus remain elusive, and thus further work will be necessary to pinpoint these. As often seen, vaccination appears to be an effective tool to limit disease spreading and to impair the teratogenic effects of BTV. Establishing adequate models of BTV vertical transmission will also help in the development of strategies to counter this transmission route. Since classic models for screening BTV vaccine candidates are unlikely to be useful in protection studies against vertical transmission, establishing robust models of vertical transmission for BTV will be a necessity. This includes the characterization of BTV strains prone to transmission through this route as well as precisely defining the experimental conditions that favor transplacental barrier crossing. These issues are critical to adequately assess vaccine efficacy against vertical transmission. Much work remains to be done to fully understand BTV capacity to be transmitted vertically and produce harm to the developing fetus.
Author Contributions
Conceptualization, J.M.R., V.M. and N.S.; writing—original draft preparation, J.M.R.; writing—review and editing, J.M.R., V.M. and N.S.; funding acquisition, V.M. and N.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by grants RTI2018-094616-B-100 from the Ministerio de Ciencia e Innovación (Spain) and S2018/BAA-4370-PLATESA2 from Comunidad de Madrid (Fondo Europeo de Desarrollo Regional, FEDER).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors wish to thank all the members of the New Strategies for the Control of Relevant Pathogens in Animal Health laboratory for helpful discussions.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Rushton, J.; Lyons, N. Economic impact of Bluetongue: A review of the effects on production. Vet. Ital. 2015, 51, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Elbers, A.R.; Backx, A.; Meroc, E.; Gerbier, G.; Staubach, C.; Hendrickx, G.; van der Spek, A.; Mintiens, K. Field observations during the bluetongue serotype 8 epidemic in 2006. I. Detection of first outbreaks and clinical signs in sheep and cattle in Belgium, France and the Netherlands. Prev. Vet. Med. 2008, 87, 21–30. [Google Scholar] [CrossRef]
- Katsoulos, P.D.; Giadinis, N.D.; Chaintoutis, S.C.; Dovas, C.I.; Kiossis, E.; Tsousis, G.; Psychas, V.; Vlemmas, I.; Papadopoulos, T.; Papadopoulos, O.; et al. Epidemiological characteristics and clinicopathological features of bluetongue in sheep and cattle, during the 2014 BTV serotype 4 incursion in Greece. Trop. Animal Health Prod. 2016, 48, 469–477. [Google Scholar] [CrossRef]
- Howerth, E.W. Cytokine release and endothelial dysfunction: A perfect storm in orbivirus pathogenesis. Vet Ital. 2015, 51, 275–281. [Google Scholar]
- Williamson, S.; Woodger, N.; Darpel, K. Differential diagnosis of bluetongue in cattle and sheep. In Pract. 2008, 30, 242–251. [Google Scholar] [CrossRef]
- Rojas, J.M.; Rodríguez-Martín, D.; Martín, V.; Sevilla, N. Diagnosing bluetongue virus in domestic ruminants: Current perspectives. Vet. Med. 2019, 10, 17–27. [Google Scholar] [CrossRef]
- Zientara, S.; Sanchez-Vizcaino, J.M. Control of bluetongue in Europe. Vet. Microbiol. 2013, 165, 33–37. [Google Scholar] [CrossRef]
- Alkhamis, M.A.; Aguilar-Vega, C.; Fountain-Jones, N.M.; Lin, K.; Perez, A.M.; Sánchez-Vizcaíno, J.M. Global emergence and evolutionary dynamics of bluetongue virus. Sci. Rep. 2020, 10, 21677. [Google Scholar] [CrossRef]
- Bumbarov, V.; Golender, N.; Jenckel, M.; Wernike, K.; Beer, M.; Khinich, E.; Zalesky, O.; Erster, O. Characterization of bluetongue virus serotype 28. Transbound. Emerg. Dis. 2020, 67, 171–182. [Google Scholar] [CrossRef]
- Sun, E.C.; Huang, L.P.; Xu, Q.Y.; Wang, H.X.; Xue, X.M.; Lu, P.; Li, W.J.; Liu, W.; Bu, Z.G.; Wu, D.L. Emergence of a Novel Bluetongue Virus Serotype, China 2014. Transbound. Emerg. Dis. 2016, 63, 585–589. [Google Scholar] [CrossRef]
- Schulz, C.; Breard, E.; Sailleau, C.; Jenckel, M.; Viarouge, C.; Vitour, D.; Palmarini, M.; Gallois, M.; Hoper, D.; Hoffmann, B.; et al. Bluetongue virus serotype 27: Detection and characterization of two novel variants in Corsica, France. J. Gen. Virol. 2016, 97, 2073–2083. [Google Scholar] [CrossRef]
- Fay, P.C.; Mohd Jaafar, F.; Batten, C.; Attoui, H.; Saunders, K.; Lomonossoff, G.P.; Reid, E.; Horton, D.; Maan, S.; Haig, D.; et al. Serological Cross-Reactions between Expressed VP2 Proteins from Different Bluetongue Virus Serotypes. Viruses 2021, 13, 1455. [Google Scholar] [CrossRef]
- Chaignat, V.; Worwa, G.; Scherrer, N.; Hilbe, M.; Ehrensperger, F.; Batten, C.; Cortyen, M.; Hofmann, M.; Thuer, B. Toggenburg Orbivirus, a new bluetongue virus: Initial detection, first observations in field and experimental infection of goats and sheep. Vet. Microbiol. 2009, 138, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Maan, S.; Maan, N.S.; Nomikou, K.; Batten, C.; Antony, F.; Belaganahalli, M.N.; Samy, A.M.; Reda, A.A.; Al-Rashid, S.A.; El Batel, M.; et al. Novel bluetongue virus serotype from Kuwait. Emerg. Infect. Dis. 2011, 17, 886–889. [Google Scholar] [CrossRef]
- Zientara, S.; Sailleau, C.; Viarouge, C.; Höper, D.; Beer, M.; Jenckel, M.; Hoffmann, B.; Romey, A.; Bakkali-Kassimi, L.; Fablet, A.; et al. Novel bluetongue virus in goats, Corsica, France, 2014. Emerg. Infect. Dis. 2014, 20, 2123–2125. [Google Scholar] [CrossRef] [PubMed]
- Ries, C.; Domes, U.; Janowetz, B.; Böttcher, J.; Burkhardt, K.; Miller, T.; Beer, M.; Hoffmann, B. Isolation and Cultivation of a New Isolate of BTV-25 and Presumptive Evidence for a Potential Persistent Infection in Healthy Goats. Viruses 2020, 12, 983. [Google Scholar] [CrossRef]
- OIE. Bluetongue (Infection with Bluetongue Virus). In OIE Terrestrial Manual; OIE: Paris, France, 2014. [Google Scholar]
- Roy, P. Bluetongue virus structure and assembly. Curr. Opin. Virol. 2017, 24, 115–123. [Google Scholar] [CrossRef]
- Stewart, M.; Hardy, A.; Barry, G.; Pinto, R.M.; Caporale, M.; Melzi, E.; Hughes, J.; Taggart, A.; Janowicz, A.; Varela, M.; et al. Characterization of a second open reading frame in genome segment 10 of bluetongue virus. J. Gen. Virol. 2015, 96, 3280–3293. [Google Scholar] [CrossRef]
- Zhang, X.; Patel, A.; Celma, C.C.; Yu, X.; Roy, P.; Zhou, Z.H. Atomic model of a nonenveloped virus reveals pH sensors for a coordinated process of cell entry. Nat. Struct. Mol. Biol. 2016, 23, 74–80. [Google Scholar] [CrossRef]
- Wu, W.; Celma, C.C.; Kerviel, A.; Roy, P. Mapping the pH Sensors Critical for Host Cell Entry by a Complex Nonenveloped Virus. J. Virol. 2019, 93, e01897–e18. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Shivakoti, S.; Ding, K.; Cui, Y.; Roy, P.; Zhou, Z.H. In situ structures of RNA-dependent RNA polymerase inside bluetongue virus before and after uncoating. Proc. Natl. Acad. Sci. USA 2019, 116, 16535–16540. [Google Scholar] [CrossRef] [PubMed]
- Nason, E.L.; Rothagel, R.; Mukherjee, S.K.; Kar, A.K.; Forzan, M.; Prasad, B.V.V.; Roy, P. Interactions between the inner and outer capsids of bluetongue virus. J. Virol. 2004, 78, 8059–8067. [Google Scholar] [CrossRef]
- Sung, P.Y.; Vaughan, R.; Rahman, S.K.; Yi, G.; Kerviel, A.; Kao, C.C.; Roy, P. The Interaction of Bluetongue Virus VP6 and Genomic RNA Is Essential for Genome Packaging. J. Virol. 2019, 93, e02023–e18. [Google Scholar] [CrossRef] [PubMed]
- Boyce, M.; Celma, C.P.; Roy, P. Bluetongue virus non-structural protein 1 is a positive regulator of viral protein synthesis. Virol. J. 2012, 9, 178. [Google Scholar] [CrossRef] [PubMed]
- Kerviel, A.; Ge, P.; Lai, M.; Jih, J.; Boyce, M.; Zhang, X.; Zhou, Z.H.; Roy, P. Atomic structure of the translation regulatory protein NS1 of bluetongue virus. Nat. Microbiol. 2019, 4, 837–845. [Google Scholar] [CrossRef]
- Rahman, S.K.; Kerviel, A.; Mohl, B.-P.; He, Y.; Zhou, Z.H.; Roy, P. A Calcium Sensor Discovered in Bluetongue Virus Nonstructural Protein 2 Is Critical for Virus Replication. J. Virol. 2020, 94, e01099–e20. [Google Scholar] [CrossRef]
- Kar, A.K.; Bhattacharya, B.; Roy, P. Bluetongue virus RNA binding protein NS2 is a modulator of viral replication and assembly. BMC Mol. Biol. 2007, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Wirblich, C.; Bhattacharya, B.; Roy, P. Nonstructural protein 3 of bluetongue virus assists virus release by recruiting ESCRT-I protein Tsg101. J. Virol. 2006, 80, 460–473. [Google Scholar] [CrossRef]
- Celma, C.C.; Roy, P. A viral nonstructural protein regulates bluetongue virus trafficking and release. J. Virol. 2009, 83, 6806–6816. [Google Scholar] [CrossRef]
- Labadie, T.; Jegouic, S.; Roy, P. Bluetongue Virus Nonstructural Protein 3 Orchestrates Virus Maturation and Drives Non-Lytic Egress via Two Polybasic Motifs. Viruses 2019, 11, 1107. [Google Scholar] [CrossRef]
- Han, Z.; Harty, R.N. The NS3 protein of bluetongue virus exhibits viroporin-like properties. J. Biol. Chem. 2004, 279, 43092–43097. [Google Scholar] [CrossRef]
- Beaton, A.R.; Rodriguez, J.; Reddy, Y.K.; Roy, P. The membrane trafficking protein calpactin forms a complex with bluetongue virus protein NS3 and mediates virus release. Proc. Natl. Acad. Sci. USA 2002, 99, 13154–13159. [Google Scholar] [CrossRef]
- Rojas, J.M.; Avia, M.; Martín, V.; Sevilla, N. Inhibition of the IFN Response by Bluetongue Virus: The Story So Far. Front. Microbiol. 2021, 12, 692069. [Google Scholar] [CrossRef]
- Pourcelot, M.; Amaral Moraes, R.; Fablet, A.; Bréard, E.; Sailleau, C.; Viarouge, C.; Postic, L.; Zientara, S.; Caignard, G.; Vitour, D. The VP3 Protein of Bluetongue Virus Associates with the MAVS Complex and Interferes with the RIG-I-Signaling Pathway. Viruses 2021, 13, 230. [Google Scholar] [CrossRef]
- Avia, M.; Rojas, J.M.; Miorin, L.; Pascual, E.; Van Rijn, P.A.; Martín, V.; García-Sastre, A.; Sevilla, N. Virus-induced autophagic degradation of STAT2 as a mechanism for interferon signaling blockade. EMBO Rep. 2019, 20, e48766. [Google Scholar] [CrossRef] [PubMed]
- Ratinier, M.; Shaw, A.E.; Barry, G.; Gu, Q.; Di Gialleonardo, L.; Janowicz, A.; Varela, M.; Randall, R.E.; Caporale, M.; Palmarini, M. Bluetongue Virus NS4 Protein Is an Interferon Antagonist and a Determinant of Virus Virulence. J. Virol. 2016, 90, 5427–5439. [Google Scholar] [CrossRef] [PubMed]
- Chauveau, E.; Doceul, V.; Lara, E.; Breard, E.; Sailleau, C.; Vidalain, P.O.; Meurs, E.F.; Dabo, S.; Schwartz-Cornil, I.; Zientara, S.; et al. NS3 of bluetongue virus interferes with the induction of type I interferon. J. Virol. 2013, 87, 8241–8246. [Google Scholar] [CrossRef]
- Li, Z.; Lu, D.; Yang, H.; Li, Z.; Zhu, P.; Xie, J.; Liao, D.; Zheng, Y.; Li, H. Bluetongue virus non-structural protein 3 (NS3) and NS4 coordinatively antagonize type Ⅰ interferon signaling by targeting STAT1. Vet. Microbiol. 2021, 254, 108986. [Google Scholar] [CrossRef] [PubMed]
- Pourcelot, M.; Zemirli, N.; Da Costa, L.S.; Loyant, R.; Garcin, D.; Vitour, D.; Munitic, I.; Vazquez, A.; Arnoult, D. The Golgi apparatus acts as a platform for TBK1 activation after viral RNA sensing. BMC Biol. 2016, 14, 69. [Google Scholar] [CrossRef] [PubMed]
- Baylis, M.; O’Connell, L.; Mellor, P.S. Rates of bluetongue virus transmission between Culicoides sonorensis and sheep. Med. Vet. Entomol. 2008, 22, 228–237. [Google Scholar] [CrossRef]
- Alexander, K.A.; MacLachlan, N.J.; Kat, P.W.; House, C.; O’Brien, S.J.; Lerche, N.W.; Sawyer, M.; Frank, L.G.; Holekamp, K.; Smale, L.; et al. Evidence of natural bluetongue virus infection among African carnivores. Am. J. Trop. Med. Hyg. 1994, 51, 568–576. [Google Scholar] [CrossRef]
- Jauniaux, T.P.; De Clercq, K.E.; Cassart, D.E.; Kennedy, S.; Vandenbussche, F.E.; Vandemeulebroucke, E.L.; Vanbinst, T.M.; Verheyden, B.I.; Goris, N.E.; Coignoul, F.L. Bluetongue in Eurasian lynx. Emerg. Infect. Dis. 2008, 14, 1496–1498. [Google Scholar] [CrossRef]
- Rasmussen, L.D.; Savini, G.; Lorusso, A.; Bellacicco, A.; Palmarini, M.; Caporale, M.; Rasmussen, T.B.; Belsham, G.J.; Bøtner, A. Transplacental transmission of field and rescued strains of BTV-2 and BTV-8 in experimentally infected sheep. Vet. Res. 2013, 44, 75. [Google Scholar] [CrossRef]
- van der Sluijs, M.; Timmermans, M.; Moulin, V.; Noordegraaf, C.V.; Vrijenhoek, M.; Debyser, I.; de Smit, A.J.; Moormann, R. Transplacental transmission of Bluetongue virus serotype 8 in ewes in early and mid gestation. Vet. Microbiol. 2011, 149, 113–125. [Google Scholar] [CrossRef]
- van der Sluijs, M.T.; Schroer-Joosten, D.P.; Fid-Fourkour, A.; Vrijenhoek, M.P.; Debyser, I.; Moulin, V.; Moormann, R.J.; de Smit, A.J. Transplacental transmission of Bluetongue virus serotype 1 and serotype 8 in sheep: Virological and pathological findings. PLoS ONE 2013, 8, e81429. [Google Scholar] [CrossRef]
- Mayo, C.E.; Crossley, B.M.; Hietala, S.K.; Gardner, I.A.; Breitmeyer, R.E.; Maclachlan, N.J. Colostral transmission of bluetongue virus nucleic acid among newborn dairy calves in California. Transbound. Emerg. Dis. 2010, 57, 277–281. [Google Scholar] [CrossRef]
- Menzies, F.D.; McCullough, S.J.; McKeown, I.M.; Forster, J.L.; Jess, S.; Batten, C.; Murchie, A.K.; Gloster, J.; Fallows, J.G.; Pelgrim, W.; et al. Evidence for transplacental and contact transmission of bluetongue virus in cattle. Vet. Rec. 2008, 163, 203–209. [Google Scholar] [CrossRef]
- Batten, C.A.; Henstock, M.R.; Steedman, H.M.; Waddington, S.; Edwards, L.; Oura, C.A. Bluetongue virus serotype 26: Infection kinetics, pathogenesis and possible contact transmission in goats. Vet. Microbiol. 2013, 162, 62–67. [Google Scholar] [CrossRef]
- Bréard, E.; Schulz, C.; Sailleau, C.; Bernelin-Cottet, C.; Viarouge, C.; Vitour, D.; Guillaume, B.; Caignard, G.; Gorlier, A.; Attoui, H.; et al. Bluetongue virus serotype 27: Experimental infection of goats, sheep and cattle with three BTV-27 variants reveal atypical characteristics and likely direct contact transmission BTV-27 between goats. Transbound. Emerg. Dis. 2018, 65, e251–e263. [Google Scholar] [CrossRef]
- Barratt-Boyes, S.M.; MacLachlan, N.J. Dynamics of viral spread in bluetongue virus infected calves. Vet. Microbiol. 1994, 40, 361–371. [Google Scholar] [CrossRef]
- Darpel, K.E.; Barber, J.; Hope, A.; Wilson, A.J.; Gubbins, S.; Henstock, M.; Frost, L.; Batten, C.; Veronesi, E.; Moffat, K.; et al. Using shared needles for subcutaneous inoculation can transmit bluetongue virus mechanically between ruminant hosts. Sci. Rep. 2016, 6, 20627. [Google Scholar] [CrossRef]
- Stott, J.L.; Osburn, B.I.; Alexander, L. Ornithodoros coriaceus (pajaroello tick) as a vector of bluetongue virus. Am. J. Vet. Res. 1985, 46, 1197–1199. [Google Scholar]
- Bouwknegt, C.; van Rijn, P.A.; Schipper, J.J.; Hölzel, D.; Boonstra, J.; Nijhof, A.M.; van Rooij, E.M.; Jongejan, F. Potential role of ticks as vectors of bluetongue virus. Exp. Appl. Acarol. 2010, 52, 183–192. [Google Scholar] [CrossRef]
- Vanbinst, T.; Vandenbussche, F.; Dernelle, E.; De Clercq, K. A duplex real-time RT-PCR for the detection of bluetongue virus in bovine semen. J. Virol. Methods 2010, 169, 162–168. [Google Scholar] [CrossRef]
- Leemans, J.; Raes, M.; Vanbinst, T.; De Clercq, K.; Saegerman, C.; Kirschvink, N. Viral RNA load in semen from bluetongue serotype 8-infected rams: Relationship with sperm quality. Vet. J. 2012, 192, 304–310. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, K.; Vandaele, L.; Vanbinst, T.; Riou, M.; Deblauwe, I.; Wesselingh, W.; Pinard, A.; Van Eetvelde, M.; Boulesteix, O.; Leemans, B.; et al. Transmission of Bluetongue Virus Serotype 8 by Artificial Insemination with Frozen-Thawed Semen from Naturally Infected Bulls. Viruses 2021, 13, 652. [Google Scholar] [CrossRef]
- Bowen, R.A.; Howard, T.H.; Pickett, B.W. Seminal shedding of bluetongue virus in experimentally infected bulls. Prog. Clin. Biol. Res. 1985, 178, 91–96. [Google Scholar]
- Thomas, F.C.; Singh, E.L.; Hare, W.C. Embryo transfer as a means of controlling viral infections. VI. Bluetongue virus-free calves from infectious semen. Theriogenology 1985, 24, 345–350. [Google Scholar] [CrossRef]
- Pascall, D.J.; Nomikou, K.; Bréard, E.; Zientara, S.; Filipe, A.D.S.; Hoffmann, B.; Jacquot, M.; Singer, J.B.; De Clercq, K.; Bøtner, A.; et al. “Frozen evolution” of an RNA virus suggests accidental release as a potential cause of arbovirus re-emergence. PLoS Biol. 2020, 18, e3000673. [Google Scholar] [CrossRef]
- Schultz, G.; Delay, P.D. Losses in newborn lambs associated with bluetongue vaccination of pregnancy ewes. J. Am. Vet. Med. Assoc. 1955, 127, 224–226. [Google Scholar]
- Gibbs, E.P.; Lawman, M.J.; Herniman, K.O.A. Preliminary observations on transplacental infection of bluetongue virus in sheep-a possible overwintering mechanism. Res. Vet. Sci. 1979, 27, 118–120. [Google Scholar] [CrossRef]
- van der Sluijs, M.T.; de Smit, A.J.; Moormann, R.J. Vector independent transmission of the vector-borne bluetongue virus. Crit. Rev. Microbiol. 2016, 42, 57–64. [Google Scholar] [CrossRef]
- Richardson, C.; Taylor, W.P.; Terlecki, S.; Gibbs, E.P. Observations on transplacental infection with bluetongue virus in sheep. Am. J. Vet. Res. 1985, 46, 1912–1922. [Google Scholar]
- Savini, G.; Lorusso, A.; Paladini, C.; Migliaccio, P.; Di Gennaro, A.; Di Provvido, A.; Scacchia, M.; Monaco, F. Bluetongue serotype 2 and 9 modified live vaccine viruses as causative agents of abortion in livestock: A retrospective analysis in Italy. Transbound. Emerg. Dis. 2014, 61, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Stott, J.L.; Lauerman, L.H.; Luedke, A.J. Bluetongue virus in pregnant elk and their calves. Am. J. Vet. Res. 1982, 43, 423–428. [Google Scholar] [PubMed]
- De Clercq, K.; De Leeuw, I.; Verheyden, B.; Vandemeulebroucke, E.; Vanbinst, T.; Herr, C.; Méroc, E.; Bertels, G.; Steurbaut, N.; Miry, C.; et al. Transplacental infection and apparently immunotolerance induced by a wild-type bluetongue virus serotype 8 natural infection. Transbound. Emerg. Dis. 2008, 55, 352–359. [Google Scholar] [CrossRef]
- Darpel, K.E.; Batten, C.A.; Veronesi, E.; Williamson, S.; Anderson, P.; Dennison, M.; Clifford, S.; Smith, C.; Philips, L.; Bidewell, C.; et al. Transplacental transmission of bluetongue virus 8 in cattle, UK. Emerg. Infect. Dis. 2009, 15, 2025–2028. [Google Scholar] [CrossRef] [PubMed]
- Vercauteren, G.; Miry, C.; Vandenbussche, F.; Ducatelle, R.; Van der Heyden, S.; Vandemeulebroucke, E.; De Leeuw, I.; Deprez, P.; Chiers, K.; De Clercq, K. Bluetongue virus serotype 8-associated congenital hydranencephaly in calves. Transbound. Emerg. Dis. 2008, 55, 293–298. [Google Scholar] [CrossRef]
- Santman-Berends, I.M.; van Wuijckhuise, L.; Vellema, P.; van Rijn, P.A. Vertical transmission of bluetongue virus serotype 8 virus in Dutch dairy herds in 2007. Vet. Microbiol. 2010, 141, 31–35. [Google Scholar] [CrossRef][Green Version]
- Santman-Berends, I.M.G.A.; Hage, J.J.; van Rijn, P.A.; Stegeman, J.A.; van Schaik, G. Bluetongue virus serotype 8 (BTV-8) infection reduces fertility of Dutch dairy cattle and is vertically transmitted to offspring. Theriogenology 2010, 74, 1377–1384. [Google Scholar] [CrossRef]
- Akita, G.Y.; Ianconescu, M.; MacLachlan, N.J.; Osburn, B.I. Bluetongue disease in dogs associated with contaminated vaccine. Vet. Rec. 1994, 134, 283–284. [Google Scholar] [CrossRef]
- Brown, C.C.; Rhyan, J.C.; Grubman, M.J.; Wilbur, L.A. Distribution of bluetongue virus in tissues of experimentally infected pregnant dogs as determined by in situ hybridization. Vet. Pathol. 1996, 33, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Dubovi, E.J.; Hawkins, M.; Griffin, R.A., Jr.; Johnson, D.J.; Ostlund, E.N. Isolation of Bluetongue virus from canine abortions. J. Vet. Diagn. Investig. 2013, 25, 490–492. [Google Scholar] [CrossRef]
- Evermann, J.F. Letter to the Editor, regarding Bluetongue virus and canine abortions. J. Vet. Diagn. Investig. 2013, 25, 670. [Google Scholar] [CrossRef]
- Courtejoie, N.; Bournez, L.; Zanella, G.; Durand, B. Quantifying bluetongue vertical transmission in French cattle from surveillance data. Vet. Res. 2019, 50, 34. [Google Scholar] [CrossRef]
- Barnard, B.J.; Pienaar, J.G. Bluetongue virus as a cause of hydranencephaly in cattle. Onderstepoort J. Vet. Res. 1976, 43, 155–157. [Google Scholar]
- MacLachlan, N.J.; Osburn, B.I. Bluetongue virus-induced hydranencephaly in cattle. Vet. Pathol. 1983, 20, 563–573. [Google Scholar] [CrossRef]
- MacLachlan, N.J.; Osburn, B.I.; Ghalib, H.W.; Stott, J.L. Bluetongue virus-induced encephalopathy in fetal cattle. Vet. Pathol. 1985, 22, 415–417. [Google Scholar] [CrossRef]
- Carr, M.A.; De Mattos, C.C.; De Mattos, C.A.; Osburn, B.I. Association of bluetongue virus gene segment 5 with neuroinvasiveness. J. Virol. 1994, 68, 1255–1257. [Google Scholar] [CrossRef] [PubMed]
- Maclachlan, N.J.; Osburn, B.I. Teratogenic bluetongue and related orbivirus infections in pregnant ruminant livestock: Timing and pathogen genetics are critical. Curr. Opin. Virol. 2017, 27, 31–35. [Google Scholar] [CrossRef]
- Silverstein, A.M.; Uhr, J.W.; Kraner, K.L.; Lukes, R.J. Fetal response to antigenic stimulus. II. Antibody production by the fetal lamb. J. Exp. Med. 1963, 117, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Schultz, R.D.; Dunne, H.W.; Heist, C.E. Ontogeny of the bovine immune response. Infect. Immun. 1973, 7, 981–991. [Google Scholar] [CrossRef]
- Waldvogel, A.S.; Anderson, G.A.; Phillips, D.L.; Osburn, B.I. Association of virulent and avirulent strains of bluetongue virus serotype 11 with premature births of late-term bovine fetuses. J. Comp. Pathol. 1992, 106, 333–340. [Google Scholar] [CrossRef]
- Kirkland, P.D.; Hawkes, R.A. A comparison of laboratory and ‘wild’ strains of bluetongue virus-is there any difference and does it matter? Vet. Ital. 2004, 40, 448–455. [Google Scholar]
- Osburn, B.I.; Johnson, R.T.; Silverstein, A.M.; Prendergast, R.A.; Jochim, M.M.; Levy, S.E. Experimental viral-induced congenital encephalopathies. II. The pathogenesis of bluetongue vaccine virus infection in fetal lambs. Lab. Investig. J. Tech. Methods Pathol. 1971, 25, 206–210. [Google Scholar]
- Osburn, B.I.; Silverstein, A.M.; Prendergast, R.A.; Johnson, R.T.; Parshall, C.J., Jr. Experimental viral-induced congenital encephalopathies. I. Pathology of hydranencephaly and porencephaly caused by bluetongue vaccine virus. Lab. Investig. J. Tech. Methods Pathol. 1971, 25, 197–205. [Google Scholar]
- Nusinovici, S.; Madouasse, A.; Fourichon, C. Quantification of the increase in the frequency of early calving associated with late exposure to bluetongue virus serotype 8 in dairy cows: Implications for syndromic surveillance. Vet. Res. 2016, 47, 18. [Google Scholar] [CrossRef] [PubMed]
- Marceau, A.; Madouasse, A.; Lehébel, A.; van Schaik, G.; Veldhuis, A.; Van der Stede, Y.; Fourichon, C. Can routinely recorded reproductive events be used as indicators of disease emergence in dairy cattle? An evaluation of 5 indicators during the emergence of bluetongue virus in France in 2007 and 2008. J. Dairy Sci. 2014, 97, 6135–6150. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, A.J.; Klenerman, P.; Goulder, P.J. The impact of differential antiviral immunity in children and adults. Nat. Rev. Immunol. 2012, 12, 636–648. [Google Scholar] [CrossRef]
- Bonneau, K.R.; DeMaula, C.D.; Mullens, B.A.; MacLachlan, N.J. Duration of viraemia infectious to Culicoides sonorensis in bluetongue virus-infected cattle and sheep. Vet. Microbiol. 2002, 88, 115–125. [Google Scholar] [CrossRef]
- Melzi, E.; Caporale, M.; Rocchi, M.; Martin, V.; Gamino, V.; di Provvido, A.; Marruchella, G.; Entrican, G.; Sevilla, N.; Palmarini, M. Follicular dendritic cell disruption as a novel mechanism of virus-induced immunosuppression. Proc. Natl. Acad. Sci. USA 2016, 113, E6238–E6247. [Google Scholar] [CrossRef]
- Rodríguez-Martín, D.; Louloudes-Lázaro, A.; Avia, M.; Martín, V.; Rojas, J.M.; Sevilla, N. The Interplay between Bluetongue Virus Infections and Adaptive Immunity. Viruses 2021, 13, 1511. [Google Scholar] [CrossRef]
- Ellis, J.A.; Luedke, A.J.; Davis, W.C.; Wechsler, S.J.; Mecham, J.O.; Pratt, D.L.; Elliott, J.D. T Lymphocyte Subset Alterations Following Bluetongue Virus Infection in Sheep and Cattle. Vet. Immunol. Immunopathol. 1990, 24, 49–67. [Google Scholar] [CrossRef]
- Nomikou, K.; Hughes, J.; Wash, R.; Kellam, P.; Breard, E.; Zientara, S.; Palmarini, M.; Biek, R.; Mertens, P. Widespread Reassortment Shapes the Evolution and Epidemiology of Bluetongue Virus following European Invasion. PLoS Pathog. 2015, 11, e1005056. [Google Scholar] [CrossRef]
- Maan, S.; Maan, N.S.; Ross-smith, N.; Batten, C.A.; Shaw, A.E.; Anthony, S.J.; Samuel, A.R.; Darpel, K.E.; Veronesi, E.; Oura, C.A.L.; et al. Sequence analysis of bluetongue virus serotype 8 from the Netherlands 2006 and comparison to other European strains. Virology 2008, 377, 308–318. [Google Scholar] [CrossRef]
- MacLennan, C.A.; Saul, A. Vaccines against poverty. Proc. Natl. Acad. Sci. USA 2014, 111, 12307–12312. [Google Scholar] [CrossRef]
- Roopenian, D.C.; Akilesh, S. FcRn: The neonatal Fc receptor comes of age. Nat. Rev. Immunol. 2007, 7, 715–725. [Google Scholar] [CrossRef]
- Evans-Gilbert, T. Vertically transmitted chikungunya, Zika and dengue virus infections: The pathogenesis from mother to fetus and the implications of co-infections and vaccine development. Int. J. Pediatrics Adolesc. Med. 2020, 7, 107–111. [Google Scholar] [CrossRef]
- World Health Organization. Dengue vaccine: WHO position paper, September 2018–Recommendations. Vaccine 2019, 37, 4848–4849. [Google Scholar] [CrossRef]
- Shan, C.; Xie, X.; Luo, H.; Muruato, A.E.; Liu, Y.; Wakamiya, M.; La, J.H.; Chung, J.M.; Weaver, S.C.; Wang, T.; et al. Maternal vaccination and protective immunity against Zika virus vertical transmission. Nat. Commun. 2019, 10, 5677. [Google Scholar] [CrossRef]
- Barrett, A.D.T. Current status of Zika vaccine development: Zika vaccines advance into clinical evaluation. NPJ Vaccines 2018, 3, 24. [Google Scholar] [CrossRef]
- Martinelle, L.; Dal Pozzo, F.; Thiry, E.; De Clercq, K.; Saegerman, C. Reliable and Standardized Animal Models to Study the Pathogenesis of Bluetongue and Schmallenberg Viruses in Ruminant Natural Host Species with Special Emphasis on Placental Crossing. Viruses 2019, 11, 753. [Google Scholar] [CrossRef]
- Saminathan, M.; Singh, K.P.; Vineetha, S.; Maity, M.; Biswas, S.K.; Manjunathareddy, G.B.; Chauhan, H.C.; Milton, A.A.P.; Ramakrishnan, M.A.; Maan, S.; et al. Virological, immunological and pathological findings of transplacentally transmitted bluetongue virus serotype 1 in IFNAR1-blocked mice during early and mid gestation. Sci. Rep. 2020, 10, 2164. [Google Scholar] [CrossRef]
- Calvo-Pinilla, E.; Rodriguez-Calvo, T.; Anguita, J.; Sevilla, N.; Ortego, J. Establishment of a bluetongue virus infection model in mice that are deficient in the alpha/beta interferon receptor. PLoS ONE 2009, 4, e5171. [Google Scholar] [CrossRef]
- Martinelle, L.; Dal Pozzo, F.; Sarradin, P.; De Leeuw, I.; De Clercq, K.; Thys, C.; Thiry, E.; Saegerman, C. Pulmonary artery haemorrhage in newborn calves following bluetongue virus serotype 8 experimental infections of pregnant heifers. Vet. Microbiol. 2013, 167, 250–259. [Google Scholar] [CrossRef]
- Miyazawa, T.; Yoshikawa, R.; Golder, M.; Okada, M.; Stewart, H.; Palmarini, M. Isolation of an infectious endogenous retrovirus in a proportion of live attenuated vaccines for pets. J. Virol. 2010, 84, 3690–3694. [Google Scholar] [CrossRef]
- Su, Q.; Li, Y.; Zhang, Y.; Zhang, Z.; Meng, F.; Cui, Z.; Chang, S.; Zhao, P. Newcastle disease virus-attenuated vaccine LaSota played a key role in the pathogenicity of contaminated exogenous virus. Vet. Res. 2018, 49, 80. [Google Scholar] [CrossRef]
- van Rijn, P.A. Prospects of Next-Generation Vaccines for Bluetongue. Front. Vet. Sci. 2019, 6, 407. [Google Scholar] [CrossRef]
- Urakawa, T.; French, T.J.; Adachi, Y.; Fukusho, A.; LeBlois, H.; Flamand, M.; Mertens, P.; Roy, P. Synthesis of recombinant baculoviruses expressing the outer capsid protein VP2 of five BTV serotypes and the induction of neutralizing antibodies to homologous and heterologous BTV serotypes. Virus Res. 1994, 31, 149–161. [Google Scholar] [CrossRef]
- Inumaru, S.; Roy, P. Production and characterization of the neutralization antigen VP2 of bluetongue virus serotype 10 using a baculovirus expression vector. Virology 1987, 157, 472–479. [Google Scholar] [CrossRef]
- Fay, P.C.; Attoui, H.; Batten, C.; Mohd Jaafar, F.; Lomonossoff, G.P.; Daly, J.M.; Mertens, P.P.C. Bluetongue virus outer-capsid protein VP2 expressed in Nicotiana benthamiana raises neutralising antibodies and a protective immune response in IFNAR −/− mice. Vaccine: X 2019, 2, 100026. [Google Scholar] [CrossRef] [PubMed]
- Athmaram, T.N.; Bali, G.; Kahng, G.G.; Dwarakanath, S. Heterologous expression of Bluetongue VP2 viral protein fragment in Pichia pastoris. Virus Genes 2007, 35, 265–271. [Google Scholar] [CrossRef] [PubMed]
- French, T.J.; Marshall, J.J.; Roy, P. Assembly of double-shelled, viruslike particles of bluetongue virus by the simultaneous expression of four structural proteins. J. Virol. 1990, 64, 5695–5700. [Google Scholar] [CrossRef]
- Thuenemann, E.C.; Meyers, A.E.; Verwey, J.; Rybicki, E.P.; Lomonossoff, G.P. A method for rapid production of heteromultimeric protein complexes in plants: Assembly of protective bluetongue virus-like particles. Plant. Biotechnol. J. 2013, 11, 839–846. [Google Scholar] [CrossRef]
- Roy, P. Genetically engineered structure-based vaccine for bluetongue disease. Vet. Ital. 2004, 40, 594–600. [Google Scholar] [PubMed]
- Anderson, J.; Hägglund, S.; Bréard, E.; Riou, M.; Zohari, S.; Comtet, L.; Olofson, A.S.; Gélineau, R.; Martin, G.; Elvander, M.; et al. Strong protection induced by an experimental DIVA subunit vaccine against bluetongue virus serotype 8 in cattle. Vaccine 2014, 32, 6614–6621. [Google Scholar] [CrossRef] [PubMed]
- Boyce, M.; Celma, C.C.P.; Roy, P. Development of Reverse Genetics Systems for Bluetongue Virus: Recovery of Infectious Virus from Synthetic RNA Transcripts. J. Virol. 2008, 82, 8339–8348. [Google Scholar] [CrossRef]
- van Gennip, R.G.; van de Water, S.G.; Maris-Veldhuis, M.; van Rijn, P.A. Bluetongue viruses based on modified-live vaccine serotype 6 with exchanged outer shell proteins confer full protection in sheep against virulent BTV8. PLoS ONE 2012, 7, e44619. [Google Scholar] [CrossRef][Green Version]
- Matsuo, E.; Celma, C.C.; Boyce, M.; Viarouge, C.; Sailleau, C.; Dubois, E.; Bréard, E.; Thiéry, R.; Zientara, S.; Roy, P. Generation of replication-defective virus-based vaccines that confer full protection in sheep against virulent bluetongue virus challenge. J. Virol. 2011, 85, 10213–10221. [Google Scholar] [CrossRef]
- Feenstra, F.; van Gennip, R.G.P.; Maris-Veldhuis, M.; Verheij, E.; van Rijn, P.A. Bluetongue virus without NS3/NS3a expression is not virulent and protects against virulent bluetongue virus challenge. J. Gen. Virol. 2014, 95, 2019–2029. [Google Scholar] [CrossRef]
- van Rijn, P.A.; Maris-Veldhuis, M.A.; van Gennip, R.G.P. The Bluetongue Disabled Infectious Single Animal (DISA) Vaccine Platform Based on Deletion NS3/NS3a Protein Is Safe and Protective in Cattle and Enables DIVA. Viruses 2021, 13, 857. [Google Scholar] [CrossRef]
- van Rijn, P.A.; Daus, F.J.; Maris-Veldhuis, M.A.; Feenstra, F.; van Gennip, R.G.P. Bluetongue Disabled Infectious Single Animal (DISA) vaccine: Studies on the optimal route and dose in sheep. Vaccine. 2017, 35, 231–237. [Google Scholar] [CrossRef]
- Feenstra, F.; Drolet, B.S.; Boonstra, J.; van Rijn, P.A. Non-structural protein NS3/NS3a is required for propagation of bluetongue virus in Culicoides sonorensis. Parasites Vectors 2015, 8, 476. [Google Scholar] [CrossRef]
- Rojas, J.M.; Sevilla, N.; Martín, V. A New Look at Vaccine Strategies Against PPRV Focused on Adenoviral Candidates. Front. Vet. Sci. 2021, 8, 1005. [Google Scholar] [CrossRef]
- García-Arriaza, J.; Esteban, M. Enhancing poxvirus vectors vaccine immunogenicity. Hum. Vaccines Immunother. 2014, 10, 2235–2244. [Google Scholar] [CrossRef]
- Jiménez-Cabello, L.; Utrilla-Trigo, S.; Calvo-Pinilla, E.; Moreno, S.; Nogales, A.; Ortego, J.; Marín-López, A. Viral Vector Vaccines against Bluetongue Virus. Microorganisms 2020, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Pinilla, E.; Navasa, N.; Anguita, J.; Ortego, J. Multiserotype protection elicited by a combinatorial prime-boost vaccination strategy against bluetongue virus. PLoS ONE 2012, 7, e34735. [Google Scholar] [CrossRef]
- Lobato, Z.I.P.; Coupar, B.E.H.; Gray, C.P.; Lunt, R.; Andrew, M.E. Antibody responses and protective immunity to recombinant vaccinia virus-expressed bluetongue virus antigens. Vet. Immunol. Immunopathol. 1997, 59, 293–309. [Google Scholar] [CrossRef]
- Utrilla-Trigo, S.; Jiménez-Cabello, L.; Alonso-Ravelo, R.; Calvo-Pinilla, E.; Marín-López, A.; Moreno, S.; Lorenzo, G.; Benavides, J.; Gilbert, S.; Nogales, A.; et al. Heterologous Combination of ChAdOx1 and MVA Vectors Expressing Protein NS1 as Vaccination Strategy to Induce Durable and Cross-Protective CD8+ T Cell Immunity to Bluetongue Virus. Vaccines 2020, 8, 346. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.; Pascual, E.; Avia, M.; Pena, L.; Valcarcel, F.; Sevilla, N. Protective Efficacy in Sheep of Adenovirus-Vectored Vaccines against Bluetongue Virus Is Associated with Specific T Cell Responses. PLoS ONE 2015, 10, e0143273. [Google Scholar] [CrossRef]
- Rojas, J.M.; Barba-Moreno, D.; Avia, M.; Sevilla, N.; Martín, V. Vaccination With Recombinant Adenoviruses Expressing the Bluetongue Virus Subunits VP7 and VP2 Provides Protection Against Heterologous Virus Challenge. Front. Vet. Sci. 2021, 8, 158. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Pinilla, E.; Marín-López, A.; Moreno, S.; Lorenzo, G.; Utrilla-Trigo, S.; Jiménez-Cabello, L.; Benavides, J.; Nogales, A.; Blasco, R.; Brun, A.; et al. A protective bivalent vaccine against Rift Valley fever and bluetongue. NPJ Vaccines 2020, 5, 70. [Google Scholar] [CrossRef]
- Moreno, S.; Calvo-Pinilla, E.; Devignot, S.; Weber, F.; Ortego, J.; Brun, A. Recombinant Rift Valley fever viruses encoding bluetongue virus (BTV) antigens: Immunity and efficacy studies upon a BTV-4 challenge. PLoS Negl. Trop. Dis. 2020, 14, e0008942. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, V.; Capocefalo, A.; Calvo-Pinilla, E.; Redaelli, M.; Mucignat-Caretta, C.; Mertens, P.; Ortego, J.; Donofrio, G. Immunization of knock-out α/β interferon receptor mice against lethal bluetongue infection with a BoHV-4-based vector expressing BTV-8 VP2 antigen. Vaccine 2011, 29, 3074–3082. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Eschbaumer, M.; Said, A.; Hoffmann, B.; Beer, M.; Osterrieder, N. An equine herpesvirus type 1 (EHV-1) expressing VP2 and VP5 of serotype 8 bluetongue virus (BTV-8) induces protection in a murine infection model. PLoS ONE 2012, 7, e34425. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).