Abstract
The coal washing and processing industry generates substantial quantities of coal gangue, which exerts significant impacts on soil and groundwater environments. Activating the reactivity of inert coal gangue to achieve comprehensive utilization in the field of cementitious materials holds considerable importance. This study investigates a method that synergistically utilizes thermal activation and mechanical activation to enhance the reactivity of coal gangue. The approach aims to reduce the temperature required for thermal activation while effectively stimulating the reactive properties. Furthermore, the mechanisms underlying the thermal–mechanical synergistic activation and its hydration characteristics are thoroughly examined. Experimental results demonstrate that thermo-mechanical synergistic activation, in comparison to sole thermal activation at 950 °C, enhances reaction activity by 28.3%, improves mechanical properties by 27.4%, reduces setting time by 65 min, and significantly optimizes flow performance. The XRD, FT-IR, and TG-DTG analyses demonstrate that the interlayer hydrogen bonds of kaolinite are disrupted during the thermal activation stage, resulting in the formation of amorphous and highly reactive metakaolinite. Subsequent mechanical activation after thermal treatment significantly reduces particle size, further breaks the interlayer hydrogen bonds of kaolinite, and leads to the complete disintegration of the lattice framework. This process markedly enhances the degree of amorphization and thoroughly disrupts the long-range ordered crystalline structure of the kaolinite mineral phase in coal gangue. Concurrently, the d002 interplanar spacing of kaolinite expands by 0.155 Å, leading to an increase in reactivity. SEM-EDS analysis reveals that C-S-H gel is embedded within the mortar matrix, with a reduction in calcium hydroxide content and Ca/Si ratio, and an increase in Al/Si ratio in coal gangue mortar. This confirms that the thermo-mechanical synergistic activation introduces highly reactive Ca2+ and Al3+ from coal gangue into the secondary hydration reaction, resulting in the formation of a gel structure characterized by high stability and enhanced durability.
1. Introduction
Coal gangue, a primary byproduct of coal mining and coal washing operations, constitutes approximately 15–20% of raw coal production annually [1]. Over the past decade (2015–2024), China’s coal output has risen from 3.75 billion tons to 4.85 billion tons, paralleled by a corresponding increase in coal gangue generation. Projections indicate that annual coal gangue emissions may reach 900 million tons by 2025, with historically accumulated stockpiles conservatively estimated at around 8 billion tons [2,3]. In major coal-producing regions—including Xinjiang, Inner Mongolia, and Shanxi Province—the extensive accumulation of coal gangue has not only resulted in significant land occupation but also poses dual environmental and public health risks due to spontaneous combustion, which releases toxic gases, and the leaching of heavy metals into surrounding ecosystems. These challenges hinder the realization of green and high-quality development goals in these regions [4,5,6]. In light of the current climate emergency, promoting the application of coal gangue activation for cementitious material production can significantly reduce raw material exploitation in the cement industry. Simultaneously, it enables source control of greenhouse gas and pollutant emissions, thereby profoundly mitigating climate impacts at the systemic level [7].
Chemically, coal gangue is predominantly composed of SiO2, Al2O3, and CaO at relatively high concentrations, exhibiting compositional similarities to pozzolanic materials such as fly ash, which are extensively employed in the production of supplementary cementitious materials and concrete admixtures, thereby providing substantial economic and environmental advantages [8,9,10]. Mineralogically, coal gangue contains significant quantities of clay minerals, including quartz and kaolinite. Through processes of dehydration, decomposition, and recrystallization, these minerals can transform into new silicon- and aluminum-rich phases that exhibit potential hydraulic or pozzolanic reactivity [11,12,13]. Therefore, the effective activation of coal gangue’s reactivity constitutes a research area of considerable significance.
Untreated coal gangs typically exhibit minimal reactivity. Numerous researchers have employed specific activation techniques to enhance the reactivity of coal gangue [14,15,16]. The primary activation methods include thermal activation and mechanical activation. Thermal activation disrupts the interlayer hydrogen bonds of kaolinite, inducing a comprehensive reconstruction of the crystal lattice framework. This process completely destroys the long-range ordered structure of the lattice, exposing a significant number of unsaturated Si-O- and Al-O- bonds and generating initial active sites. Consequently, the lattice transitions into a disordered state, ultimately yielding metakaolin characterized by both an amorphous structure and high pozzolanic activity [17,18,19,20,21]. The essence of mechanical activation lies in utilizing mechanical shearing and impact as energy input vectors. By applying mechanical force to the interlayer hydrogen bond regions of kaolinite, it significantly ruptures these interlayer hydrogen bonds. The continuous application of mechanical force results in the complete disintegration of the lattice framework, thereby markedly enhancing the degree of amorphization. Simultaneously, the shear forces and frictional effects generated by mechanical activation disrupt particle agglomerates, significantly reducing particle size and increasing specific surface area. This process enhances the number of active sites, thereby achieving the objective of improving reaction activity [22,23,24,25,26]. The application of a singular activation method exhibits limitations in enhancing the pozzolanic activity of coal gangue. Li et al. [27] conducted thermal activation of coal gangue at temperatures ranging from 700 to 1000 °C, revealing that single thermal activation is insufficient to fully activate the reactivity of clay minerals within the coal gangue. Manosa et al. [28] conducted thermal activation of kaolinite and concluded that single thermal activation can only achieve partial amorphization of the kaolinite matrix while being prone to agglomeration phenomena. Li et al. [29] conducted mechanical activation on molybdenum tailings and discovered that single mechanical activation demonstrated insufficient targeting capability for different minerals, resulting in a significant upper limit in activity enhancement. Hu et al. [30] conducted mechanical activation on coal gangue and discovered that single mechanical activation cannot effectively remove the internal and external hydroxyl groups in kaolinite, thus failing to achieve complete mineral phase reconstruction. Currently, a significant number of researchers continue to rely on elevating thermal activation temperatures or extending mechanical activation durations to enhance singular activation effects, which inevitably leads to a substantial surge in energy consumption. Han et al. [31] employed a thermo-mechanical composite activation approach to treat coal gangue, revealing that the composite activation method effectively addresses the issue of single activation sites being encapsulated by inert layers. Cheng et al. [32] conducted single activation and thermo-mechanical composite activation treatments on coal gangue, revealing that the thermo-mechanical composite activation enhances the expression of activity by promoting the consumption of more hydration products during the hydration process. Therefore, investigating the thermo-mechanical synergistic activation approach not only serves as an effective strategy for surpassing the upper limit of reaction activity but also constitutes a pivotal element in achieving low-carbon and environmentally sustainable transformation.
In summary, this study systematically evaluates the pozzolanic activity of coal gangue under both single and synergistic activation conditions through compressive strength ratio analysis. Furthermore, the mechanisms associated with thermal–mechanical synergistic activation and its influence on hydration processes were elucidated using a combination of advanced characterization techniques, including X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), scanning electron microscopy (SEM), energy dispersive spectroscopy (EDS), and thermogravimetric analysis (TG-DTG). This integrated approach not only constitutes a critical strategy for source-level solid waste reduction but also establishes a scientific basis for promoting sustainable development and facilitating the green transformation of the coal industry. This not only serves as a pivotal approach to reducing solid waste at the source, but also provides a scientific foundation for the sustainable development and green transformation of the coal industry.
2. Materials and Methods
2.1. Materials
The coal gangue utilized in this experiment was sourced from the Hengda Coal Mine in Fuxin City, Liaoning Province, China. Proximate analysis indicated a moisture content of 1.5%, ash content of 83.62%, volatile matter of 14.68%, fixed carbon content of 12%, and a calorific value of 261 MJ·kg−1. As shown in Table 1, XRF (S8 TIGER Series 2, Karlsruhe, Germany) analysis revealed that the primary chemical constituents of the coal gangue are silicon dioxide and aluminum oxide. The combined proportion of SiO2, Al2O3, and Fe2O3 exceeds 80%, meeting the ASTM C618 standard requirements for supplementary cementitious materials [33]. The structural characteristics and crystalline phases of the coal gangue were determined by X-ray diffraction (XRD) under conditions of 40 kV accelerating voltage and 40 mA current. The XRD pattern, presented in Figure 1, demonstrates that the main mineral phases in the coal gangue are quartz, kaolinite, pyrite, domolite, illite and albite, indicating its nature as an inert material with potential pozzolanic activity.

Table 1.
Chemical composition of cement, coal gangue (% by weight).
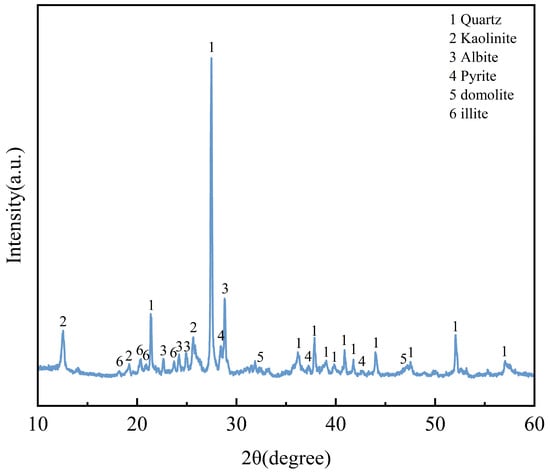
Figure 1.
XRD patten of coal gangue.
2.2. Methods
2.2.1. Activation of Coal Gangue
The coal gangue is crushed and pre-screened to obtain samples smaller than 2 mm, ensuring the uniformity and efficiency of thermo-mechanical synergistic activation. The samples are then placed in a drying oven at 105 °C for 1 h until the moisture content is less than 1%, thereby eliminating interference from moisture in the subsequent thermal activation process. The particle size distribution is illustrated in Figure 2. The specific activation process is as follows: A 100 g sample of pre-dried coal gangue at 105 °C is placed in a muffle furnace (SGM·M30/10). The temperature is increased from room temperature (20 °C) to the designated temperatures (500 °C and 950 °C) at a heating rate of 10 °C/min, followed by calcination for 60 min. After cooling to ambient temperature, the calcined sample is placed in a 250 mL ball mill jar and subjected to mechanical activation in a planetary ball mill, with a ball-to-material filling ratio of 60%. The activation process of coal gangue is divided into three distinct stages, as delineated in Table 2. The schematic diagram of the activated coal gangue technology is illustrated in Figure 3. Based on preliminary experiments and related research findings [28,29], the thermal activation temperatures of 500 °C and 950 °C were selected to target distinct transformation stages of the kaolinite component in coal gangue: 500 °C represents the primary temperature range for the dehydroxylation process leading to metakaolin formation, while 950 °C signifies the definitive temperature for complete kaolinite-to-metakaolin transformation with initial recrystallization. Simultaneously, mechanical activation durations of 30 min and 120 min were chosen to represent moderate and intensive grinding conditions, respectively, facilitating a systematic investigation into the evolution mechanism of mineral crystal structures from initial defects to profound structural degradation under varying mechanical energy inputs [31,32].
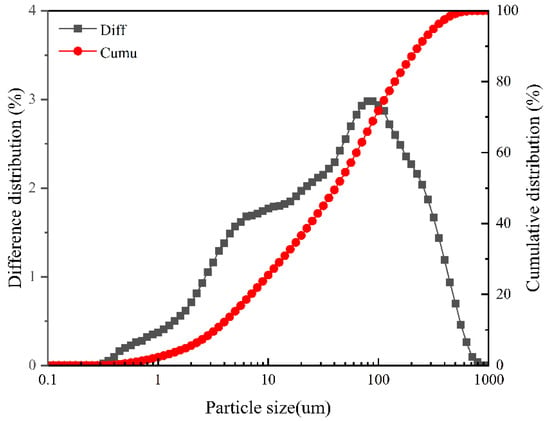
Figure 2.
Particle size distribution of raw coal gangue.

Table 2.
Coal gangue activation treatment.
Figure 3.
Experimental flowchart.
2.2.2. Mixing Ratio
In accordance with the standard “Methods of Testing Cement—Determination of Strength (ISO Method)” (GB/T 17671-2021) [34], a reference mortar mixture, designated as C0, was prepared by thoroughly mixing ordinary Portland cement (450 g) and standard sand (1350 g) at a water-to-cement ratio of 0.5 using a cement mortar mixer (model NJ-160, Wuxi Maofang Instrument Equipment Co., Ltd., Wuxi, China). Additional mortar mixtures, labeled C1 through C5, were prepared by replacing 20% of the Portland cement content with equivalent amounts of CG1, CG2, CG3, CG4, and CG5, respectively; the detailed mix proportions are provided in Table 3. The resulting mortar mixtures were cast into standard prismatic molds of dimensions 40 mm × 40 mm × 160 mm and compacted using a mortar vibrating table. After 24 h of curing in a standard conditioning chamber, the specimens were demolded and transferred to standard curing conditions (temperature: 20 ± 2 °C, relative humidity ≥ 95%) until reaching the specified testing ages.

Table 3.
Mixing proportion of samples.
2.2.3. Power Activity Index Test
In accordance with the “Methods of testing cement—Determination of strength (ISO method)” (GB/T 17671-2021), compressive strength tests were conducted on coal gangue mortar specimens [35]. The compressive strength ratio is calculated using Equation (1):
where SAI denotes the compressive strength ratio, expressed in percentage.
R1 represents the 28-day compressive strength of coal gangue mortar specimens, measured in MPa.
R2 indicates the 28-day compressive strength of cement mortar specimens, measured in MPa.
2.2.4. Mechanics Performance Testing
After curing the thermally–mechanically activated coal gangue concrete specimens to the designated ages in a standard curing chamber, performance tests were conducted in accordance with the following standards: Standard for Test Methods of Concrete Physical and Mechanical Properties (GB/T 50081-2019) [36]. Test Methods for Water Requirement of Normal Consistency, Setting Time and Soundness of Cement (GB/T 1346-2024) [37], and Method for Determining Fluidity of Cement Mortar (GB/T 2419-2005) [38].
2.2.5. Characterization Methods for Activation Mechanisms
X-ray diffraction analysis was conducted using a Bruker D8 instrument (Burker, Karlsruhe, Germany) to characterize the mineral composition of coal gangue under various activation methods. The scanning rate was set at 2°/min with a 2θ range of 5° to 40°, employing a copper target (λ = 0.15415 nm). To correct for potential instrumental shifts, a high-purity quartz standard (SiO2) was employed as an internal standard and thoroughly mixed with each coal gangue sample at a weight ratio of approximately 1:9. The position of the designated primary quartz (101) peak (at approximately 26.64° 2θ) was used as a reference to calibrate the 2θ scale for all measurements. The interplanar spacing of kaolinite in coal gangue was calculated based on integral breadth and Bragg’s Equation (2) [39].
dhkl denotes the interplanar spacing, Å. θ denotes the position of the diffraction peak, °. λ denotes the wavelength of the diffraction line, nm.
Fourier Transform Infrared (FT-IR) spectroscopy was employed to analyze structural changes in mineral phases via the potassium bromide pellet method using a spectrometer (Bruker Tensor II, Bruker Corporation, Karlsruhe, Germany), with measurements recorded in absorbance. The obtained FTIR spectra were subjected to baseline correction to ensure accurate data analysis. The powdered samples were required to be thoroughly dried and anhydrous, with a particle fineness exceeding 200 mesh. Particle size distribution of coal gangue under various activation conditions was determined using a laser particle size analyzer (BT-2003, Dandong Bettersize Instruments Ltd., Dandong, China). Morphological evolution of coal gangue during the activation process was examined by scanning electron microscopy (ZEISS EVO 10, Carl Zeiss AG, Oberkochen, Germany). Distinct and non-overlapping particles from samples subjected to different activation conditions were selected as subjects for analysis. Projected areas were quantified through threshold segmentation [40], and the average sphericity was calculated using Equation (3). This methodology relies on a single two-dimensional projection to reconstruct three-dimensional geometric parameters, which inherently possesses intrinsic limitations.
where Vp denotes the particle volume in mm3, and Sp represents the particle surface area in mm2.
Thermogravimetric analysis was conducted using a TGA instrument (Q50, TA Instruments, Newcastle, DE, USA) to determine the TGA-DTG curves of activated coal gangue samples within the temperature range of 30–900 °C. The heating rate was set at 10 °C/min under a nitrogen atmosphere with a flow rate of 20 mL/min. While the cement-based hardened paste specimens are being heated, various hydrates may decompose or dehydrate at different temperatures, so we can work out the contents of such hydrates through measuring their mass loss at specific phases of temperature. Because the loss in weight of Ca(OH)2 is just the mass of water (ΔG1) decomposed from Ca(OH)2, as indicated on the TG curve corresponding to the DTA curve between 450 and 750 °C (from the initial temperature to the end temperature of the endothermic peak), the content of Ca(OH)2 was calculated using Equations (4) and (5) [41].
ΔG denotes the mass at 900 °C, in mg; ΔG1 represents the mass loss between 450 °C and 750 °C, mg; ΔG2 corresponds to the mass of calcium hydroxide, mg; CH indicates the calcium hydroxide content, %.
2.2.6. Analysis of Hydration Properties
To investigate the hydration properties of thermally–mechanically activated coal gangue, the C1, C2, C3, C4, and C5 mortar specimens at specified ages were fractured at the midpoint, cured in anhydrous ethanol for 24 h, and subsequently dried in an oven at 60 °C for 24 h. The samples were then encapsulated in epoxy resin to fully expose the cross-sections, and ground into thin slices with a thickness of less than 0.5 mm and dimensions of less than 5 mm in length and width. These slices were utilized for microstructural morphology analysis (ZEISS EVO 10, Carl Zeiss AG, Oberkochen, Germany) and elemental analysis (X-Max 100, Oxford Instruments, Abingdon, UK), with the following analytical parameters: an acceleration voltage of 15 kV, a working distance of 10 mm, and a large beam spot size to ensure high beam current. The remaining samples were ground and subjected to TG-DTG (Q50 TA Instruments, Newcastle, DE, USA) and FT-IR (Bruker Tensor II, Bruker Corporation, Karlsruhe, Germany) analysis.
3. Results and Discussion
3.1. Impact of Activation Mechanisms on Reactivity and Performance
3.1.1. Power Activity Index
Figure 4 presents the 28-day compressive strength and strength activity index of coal gangue mortar specimens under various activation conditions. The activity indices of specimens C1 through C5 all exceed 65%, satisfying the requirement specified in GB/T 17671-2021 (equivalent to the ISO method for cement strength determination), which stipulates that the 28-day compressive strength ratio shall not be less than 65%. Therefore, these materials qualify as supplementary cementitious materials. A comparative analysis of C1, C3, and C5 reveals a notable increase in the strength activity index, indicating that the synergistic effect of thermal and mechanical activation can surpass the maximum reactivity enhancement achievable by single activation methods. For C2, C3, and C4, the activity index demonstrates a linear growth trend, reaching its peak at C4 with an SAI of 95.9%. This indicates that the introduction of mechanical activation in synergistic activation can overcome the inherent limitations of single thermal activation in terms of reactivity. This phenomenon occurs because the appropriate duration of thermal activation fully transforms kaolinite into metakaolin with higher reactivity, making it easier for mechanical activation to disrupt its crystal structure and generate more active sites. Additionally, the finely ground coal gangue powder contributes to dense packing in mortar specimens and serves as nucleation sites for C-S-H gel formation. Comparative analysis of C3, C4, and C5 reveals that the enhancement in activity index of C4 relative to C3 exhibits a diminishing trend, indicating that further prolongation of mechanical activation duration may result in diminishing returns in terms of reaction activity and performance improvement.
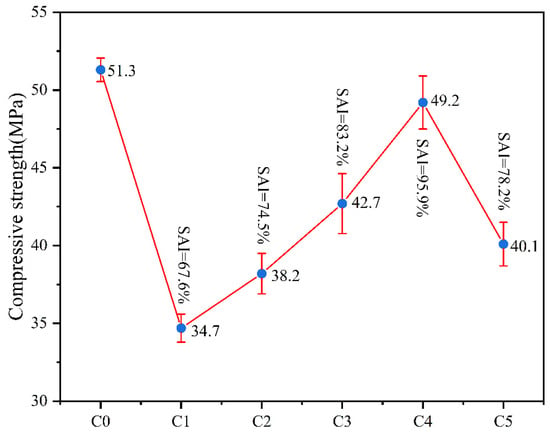
Figure 4.
Compressive Strength Ratio of Mortar Specimens Mixed with Coal Gangue under Various Activation Conditions.
3.1.2. Performance Characteristics of Activated Coal Gangue Mortar
The mechanical properties of coal gangue blended mortar specimens at 1, 3, and 7 days are presented in Figure 5a,b. With progressive optimization of activation conditions and increasing curing age, the compressive strength, flexural strength, and strength differential of the specimens exhibit a steady increase. This trend can be attributed to the combined effects of the micro-aggregate filling effect and the pozzolanic reactivity of activated coal gangue [42]. Compared with specimen C2, samples C3 and C4 showed improvements in compressive and flexural strength ranging from 7.9% to 37.8% at 1, 3, and 7 days, indicating that thermal–mechanical synergistic activation positively enhances the early-age strength development of coal gangue-based mortars. When compared to C1, specimens C5 and C3 demonstrated the most notable gains in compressive strength at 7 days, with increases of 5.9% and 9.8%, respectively, suggesting that thermal activation plays a more significant role in strength enhancement during the mid-curing stage. Based on previous strength activity index results, it can be inferred that activated coal gangue primarily contributes through a micro-aggregate densification effect in the initial hydration phase, while its pozzolanic reactivity becomes increasingly dominant in the later stages of hydration.
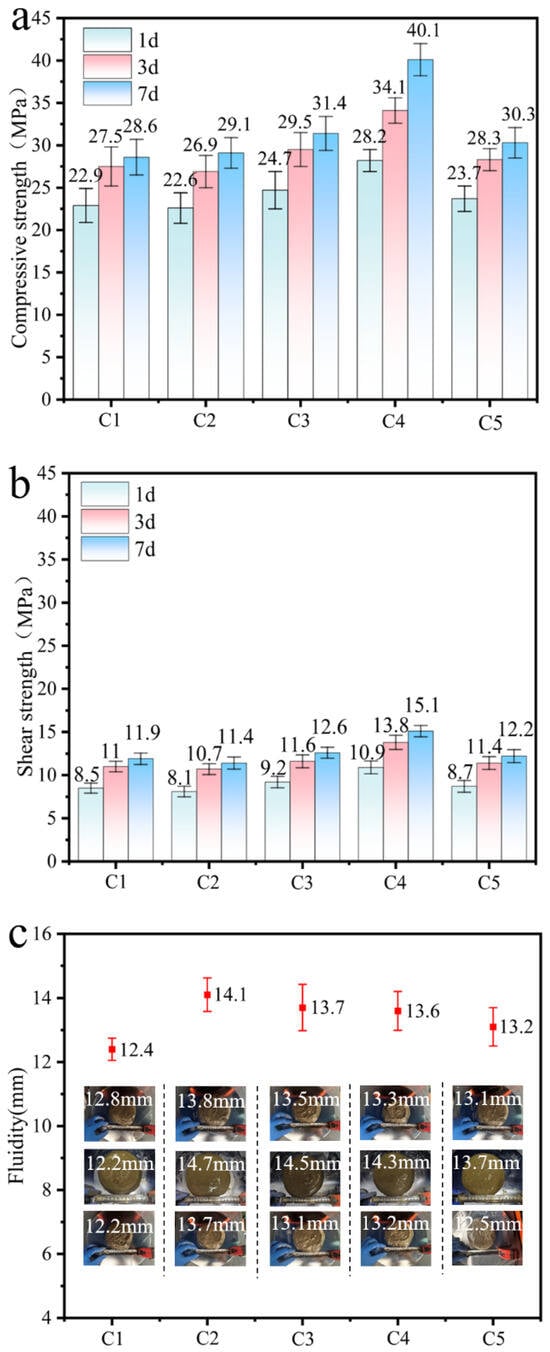
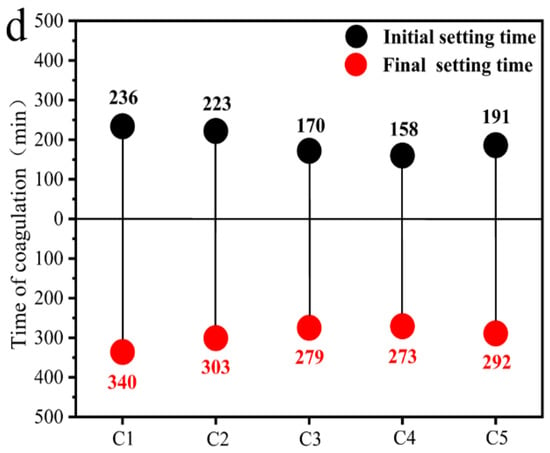
Figure 5.
Performance test results of activated coal gangue mortar specimens: (a) compressive strength, (b) flexural strength, (c) fluidity, (d) time of coagulation.
Figure 5c presents the fluidity test results of activated coal gangue mortar specimens under different activation conditions. A comparative analysis of groups C2, C3, and C4 reveals that extended mechanical activation duration significantly improves fluidity, a trend consistent with findings reported by Luo et al. [43]. Comparative analysis of C1, C3, and C5 demonstrates that the elevation of thermal activation temperature exerts a positive influence on fluidity. This phenomenon can be attributed to the disruption of lattice ordering through singular thermal activation, which consequently reduces interparticle sliding resistance, thereby enhancing fluidity. Concurrently, the generation of substantial amorphous phases facilitates the transition from extensive rigid contact to localized flexible interaction among particles, further diminishing sliding resistance and augmenting flow properties.
Figure 5d shows the setting time measurements for activated coal gangue mortar specimens under varying activation conditions. The initial and final setting times for C1 and C2 are 234 min, 338 min and 220 min, 301 min, respectively, indicating that individual activation methods have minimal impact on setting behavior. Comparative analysis of C3, C4 versus C2, and C5, C3 versus C1 demonstrates a reduction in final setting time by 29 min, 35 min, 50 min, and 66 min, respectively. C4 exhibits the optimal setting performance, indicating that under thermo-mechanical synergistic activation conditions, the upper limit of setting time influenced by single activation methods can be surpassed. In this process, the impact of thermal activation on setting time is twice that of mechanical activation.
3.2. Mechanism and Activation Pathway Analysis
3.2.1. Analysis of Phase Constituents
Figure 6 illustrates the changes in major mineral phases of coal gangue under different activation methods. Compared with CG1, the XRD diffraction peaks corresponding to the primary mineral phases in CG3 and CG5 exhibit significant alterations. Under thermal activation between 500 °C and 950 °C, the characteristic peaks of kaolinite gradually diminish, while weak signatures of metakaolin appear, which can be attributed to the breakdown of the long-range ordered crystal structure of kaolinite and its transformation into an amorphous phase. In the case of mechanical activation, the quartz diffraction peak in CG1 shows a marked reduction, indicating partial amorphization of the crystalline structure [44]. Concurrently, the baseline broadening observed in CG3, CG4, and CG5 within the 2θ range of 15–35° further confirms the formation of amorphous components as a result of thermo-mechanical synergistic activation [45]. Coal gangue particles undergo plastic deformation due to shear and friction forces generated during grinding with milling media [46]. The pronounced decrease in diffraction intensity of the main mineral phases in CG2 reflects extensive crystal lattice disruption and a higher degree of amorphization.
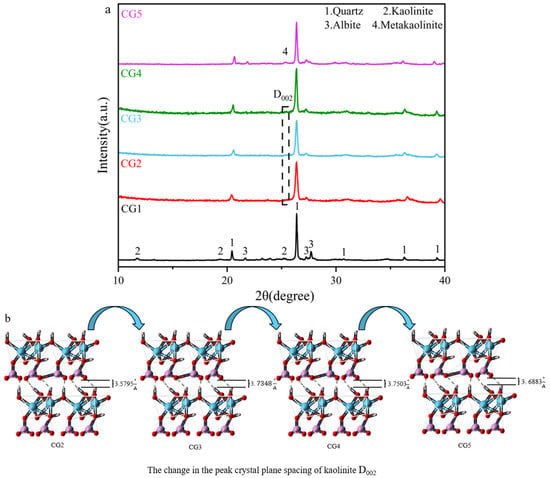
Figure 6.
Coal gangue under different activation methods: (a) X-ray diffraction analysis, (b) interplanar spacing variations.
The interplanar spacing of the kaolinite (002) plane in CG2, CG3, CG4, and CG5 was determined using Bragg’s law [47,48]. The calculated d-spacing values are 3.5795 Å, 3.7348 Å, 3.7503 Å, and 3.6883 Å, respectively. A comparison between CG3 and CG4 reveals that mechanical activation introduces additional crystal defects and lattice distortions within coal gangue, thereby generating more reactive sites and enhancing overall reactivity [49]. The comparison between CG3 and CG5 demonstrates that thermo-mechanical synergistic activation effectively alleviates issues such as particle consolidation associated with single-mode activation, leading to further improvement in pozzolanic reactivity.
3.2.2. FT-IR Analysis
Figure 7 presents the FT-IR spectra of coal gangue subjected to different activation methods. The spectra exhibit characteristic absorption peaks associated with O-H stretching vibrations at 3685 cm−1, Si-O-Si asymmetric stretching vibrations in the range of 950–1200 cm−1, and bending vibrations of Si-O-Al and Si-O-Si at 800 cm−1, 600–700 cm−1, and 550 cm−1 [50,51]. A comparative analysis of samples CG2, CG3, and CG4 reveals a progressive broadening and eventual disappearance of the characteristic absorption peaks corresponding to Si-O and Si-O-Al bending vibrations at 786 cm−1, 695 cm−1, 669 cm−1, and 534 cm−1 as the duration of mechanical activation increases. This trend indicates that thermo-mechanical synergistic activation effectively disrupts the long-range ordered crystalline structure of kaolinite within coal gangue, thereby generating a significant number of active sites [52]. Furthermore, the asymmetric stretching vibration peaks of Si-O-Si at 1068 cm−1 and 1020 cm−1 become increasingly diffuse, suggesting that appropriate thermo-mechanical conditions not only disrupt structural order but also promote amorphization [53]. The disappearance of the O–H stretching vibration band at 3685 cm−1 in samples CG2, CG3, and CG4, in contrast to its distinct presence in Raw, indicates that thermal activation progressively disrupts the kaolinite crystal structure, leading to the complete removal of structural hydroxyl groups [12].
Figure 7.
FT-IR analysis of coal gangue under various activation methods.
3.2.3. Morphological Analysis
Figure 8 presents the particle size distribution curves and sphericity analysis of morphological characteristics of coal gangue under different activation methods. A comparison between Figure 8c,g reveals that mechanical activation significantly reduces the particle size of C4 while increasing surface defects, indicating that the process disrupts the internal structure of coal gangue particles and fractures their surface chemical bonds, thereby expanding the reactive surface area available for hydration reactions [54]. As shown in Figure 8a, the particle size of C1 is predominantly distributed around 5 um, with a sphericity of 0.771, and exhibits sharp edges and irregular morphology. Following thermal activation under varying conditions, the particle size of C5 decreases and is primarily concentrated near 10 μm, accompanied by a slight increase in sphericity of 0.005. In contrast, C3 exhibits a marked reduction in particle size, with a uniform distribution in the range of 10–300 μm, and a significant improvement in sphericity by 0.011. The particles display reduced angularity, a more porous microstructure, and a relatively rounded, ellipsoidal shape. These observations suggest that thermo-mechanical synergistic activation enhances the breakdown of surface chemical bonds, increases the number of active sites, reduces particle size, and promotes more effective dense packing.
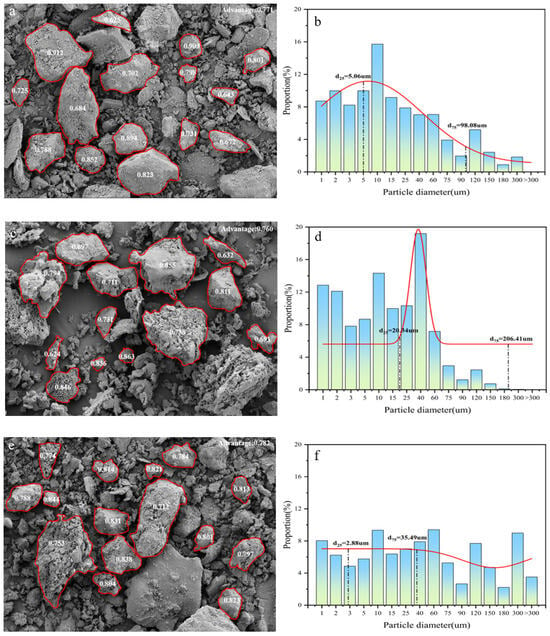
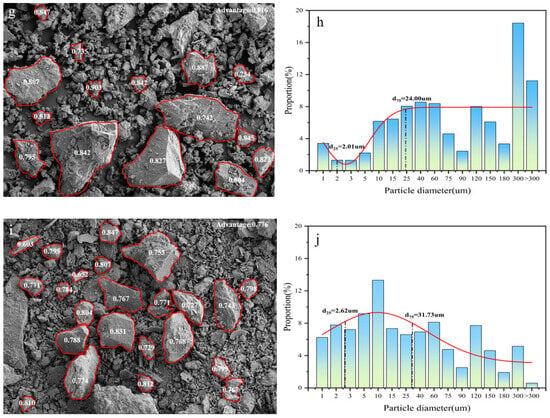
Figure 8.
Morphology sphericity of coal gangue samples C1 (a), C2 (c), C3 (e), C4 (g), and C5 (i) and particle size distribution curves of C1 (b), C2 (d), C3 (f), C4 (h), and C5 (j) under different activation methods.
3.2.4. Analysis of Ca(OH)2 Content
Figure 9 presents the TG-DTG curves of coal gangue mortar specimens C0, C1, C3, C4, and C5 after 28 days of curing. As shown in Table 4, with progressive optimization of the thermo-mechanical co-activation conditions, the calcium hydroxide content in the mortar specimens decreases, whereas the contents of C-S-H gel and ettringite increase. These results demonstrate that thermo-mechanical co-activation enhances the pozzolanic reactivity of coal gangue. The active Ca2+ and Al3+ species released from coal gangue react with calcium hydroxide generated during cement hydration, thereby promoting secondary hydration reactions and facilitating the formation of a substantial amount of low-calcium-to-silicon-ratio C-S-H gel. This contributes to a significant improvement in the performance of the mortar specimens, which is consistent with the mechanical strength results obtained from physical testing.
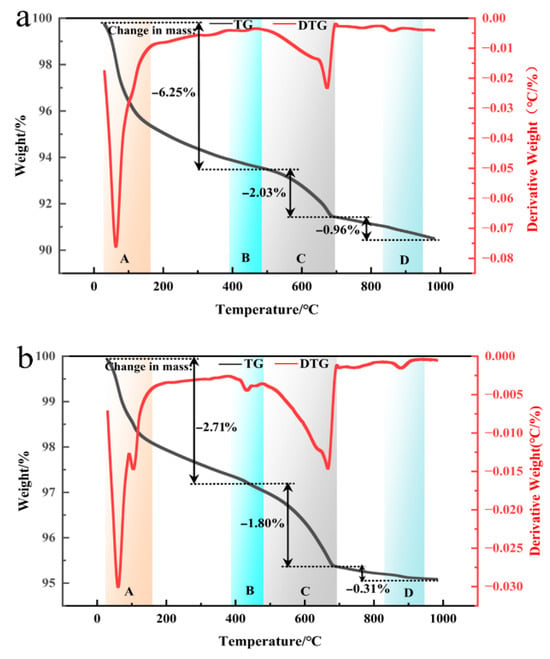
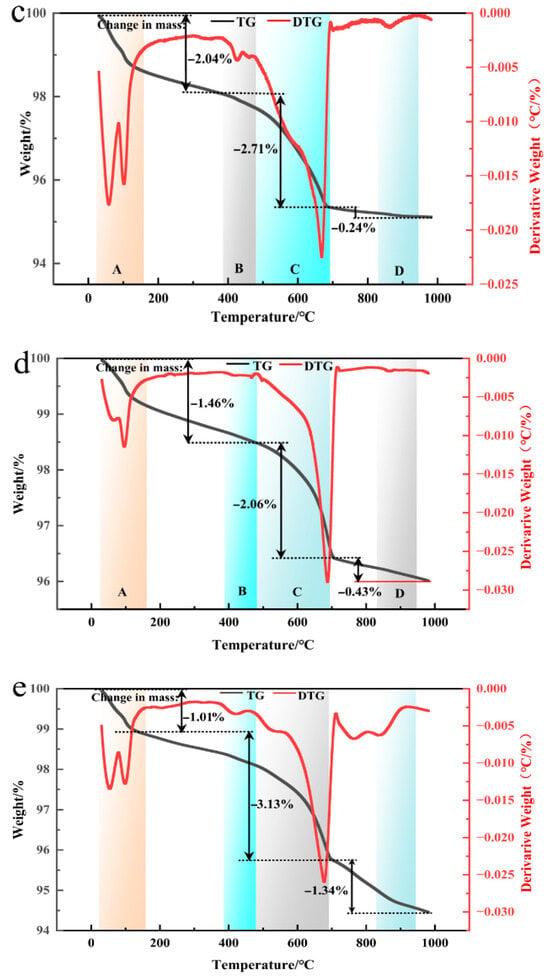
Figure 9.
DG-DTG curves of coal gangue mortar specimens C0 (a), C1 (b), C3 (c), C4 (d), and C5 (e) after 28 days of hardening.

Table 4.
The content of Ca(OH)2 in the hardened cement coal gangue slurry.
3.2.5. Microstructural Morphology and Elemental Analysis
Figure 10 illustrates the micro-morphology of coal gangue mortar after 28 days of curing. As shown in Figure 10e,g,i, fibrous C-S-H gels are embedded within the mortar matrix, effectively integrating the components into a cohesive bulk structure and contributing to a denser and more homogeneous microstructure in specimens C3, C4, and C5. To assess the progression of hydration reactions in coal gangue mortar under different activation conditions, energy-dispersive spectroscopy (EDS) analysis was performed on the C-S-H gel phases in regions A, B, C, D, and E. The atomic ratios of Ca/Si and Al/Si were calculated and are presented in Figure 10b,d,f,h,j. In comparison with C3 and C5, sample C4 exhibits a continuous decrease in the Ca/Si ratio along with an increase in the Al/Si ratio. This observation indicates that optimized thermo-mechanical synergistic activation promotes the participation of reactive silica and alumina from coal gangue in secondary hydration reactions, facilitating the formation of a more stable and durable C-S-H gel structure, thereby enhancing the mechanical and durability performance of coal gangue mortar [55,56,57].
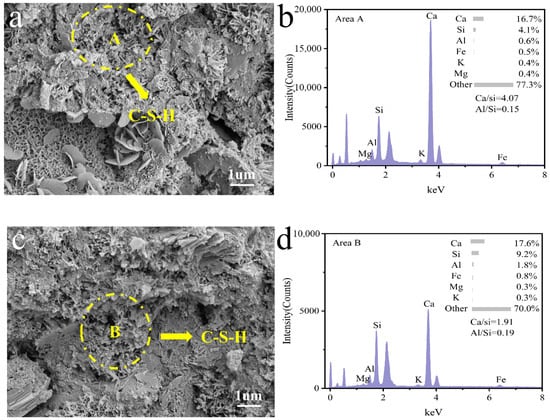
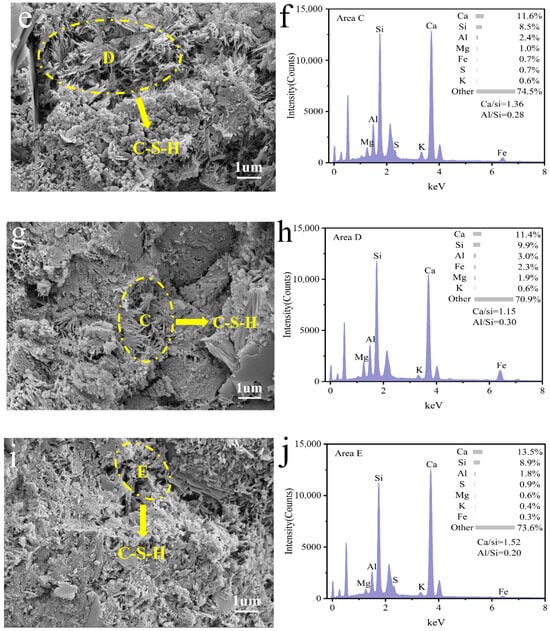
Figure 10.
Microstructural morphology of coal gangue mortar specimens C0 (a), C1 (c), C3 (e), C4 (g), and C5 (i) after 28 days of hardening and elemental analysis of C0 (b), C1 (d), C3 (f), C4 (h), and C5 (j).
3.3. Analysis of Thermomechanical Synergistic Activation Mechanisms
As illustrated in Figure 11, the reaction mechanism of thermal–mechanical synergistic activation of coal gangue can be regarded as a physicochemical process in which thermal energy and mechanical force collaboratively induce the destruction of crystal structures. Initially, thermal energy is employed to cleave the Al-OH bonds within the aluminum-oxygen octahedra, thereby disrupting the integrity of the layered structure through dehydroxylation. Under optimal conditions, thermal activation yields a maximized quantity of amorphous, metastable metakaolin, which aligns with the findings reported by Hu et al. [58,59,60,61]. Subsequently, under the influence of mechanical energy, the Si-O-Si and Si-O-Al bonds within the metakaolin are distorted through the cutting action of mechanical force, leading to the disintegration of its crystalline structure and its transformation into an amorphous structure characterized by high surface energy and a multitude of defects. The essence of this process lies in enhancing the efficiency of structural distortion and defect generation, thereby significantly increasing the reactivity. This constitutes the fundamental rationale for the significant enhancement of coal gangue reactivity through the thermo-mechanical synergistic activation process.
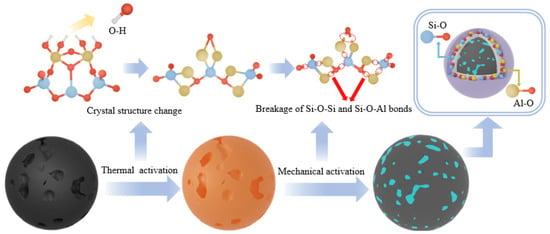
Figure 11.
Schematic illustration of the thermo-mechanical synergistic activation reaction mechanism.
4. Conclusions
This study investigates a novel approach that synergistically utilizes thermal activation and mechanical activation to enhance the reactivity of coal gangue. The proposed method not only reduces the thermal activation temperature of coal gangue but also significantly improves its reaction activity. The compressive strength, flexural strength, fluidity, and setting time of coal gangue mortar prepared under single activation method and thermo-mechanical synergistic activation were compared. The synergistic mechanism of thermo-mechanical activation and the hydration characteristics of activated coal gangue were investigated through XRD, FT-IR, SEM, EDS, TG-DTG, and sphericity analysis, leading to the following conclusions:
- (1)
- Thermo-mechanical synergistic activation enhances the reactivity of coal gangue by 21.4% compared to single thermal activation. The prepared coal gangue mortar test blocks exhibit a 27.4% improvement in mechanical properties, a 65-min reduction in setting time, and the thermo-mechanical synergistic activation can surpass the upper limit of reactivity constrained by single activation conditions.
- (2)
- During the thermo-mechanical synergistic activation process, the O-H and Al-OH bonds in coal gangue undergo cleavage at a thermal activation temperature of 950 °C, resulting in the formation of metastable, amorphous, and highly reactive metakaolin. Under mechanical activation, the generated shear forces forcibly disrupt the highly distorted Si-O-Si and Si-O-Al bonds within the metakaolin, leading to the disintegration of its crystalline structure and consequently enhancing the reactivity of the coal gangue.
- (3)
- Through particle morphology and sphericity analysis, it was observed that the thermo-mechanical synergistic activation significantly reduced the particle size of coal gangue, predominantly within the range of 75–300 μm. The sphericity of the particles increased by 0.045, transforming the surface voids into loosely structured ellipsoidal particles, thereby enhancing the densification of the gel structure during the secondary hydration reaction.
- (4)
- Thermo-mechanical synergistic activation facilitates the formation of highly stable and durable C-S-H gel structures through the hydration process of Ca2+ and Al3+ in coal gangue mortar specimens, thereby ensuring reliable long-term performance.
Author Contributions
Conceptualization, J.C. and Q.S.; methodology, J.C. and Q.S.; software, X.T.; validation, K.D., M.L. and Y.S.; formal analysis, J.C.; investigation, Q.S. and Y.S.; resources, Q.S. and M.L.; data curation, J.C. and Q.S.; writing—original draft preparation, J.C.; writing—review and editing, J.C. and Q.S.; visualization, J.C. and X.T.; supervision, K.D. and M.L.; project administration, Q.S. and M.L.; funding acquisition, Q.S. and M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (Grant No. 52004140), the Liaoning Provincial Science and Technology Plan Project (Grant No. 2025BS0916).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Luo, Y.; Zhao, X.; Zhang, K. Coal gangue in asphalt pavement: A review of applications and performance influence. Case Stud. Constr. Mater. 2024, 20, e03282. [Google Scholar] [CrossRef]
- Shen, Y.; Li, Q.; Yi, M.; Lu, L.; Shu, Q. Research on the expansion properties of concrete with coal gangue aggregate during a high temperature process. Constr. Build. Mater. 2025, 479, 141466. [Google Scholar] [CrossRef]
- National Bureau of Statistics of China. China Statistical Yearbook; China Statistics Press: Beijing, China, 2024.
- Shi, L.-Q.; Peng, J.-F.; Xu, D.-J.; Tian, J.-J.; Liu, T.-H.; Jiang, B.-B.; Zhang, F.-C. Leaching characteristics and pollution risk assessment of potentially harmful elements from coal gangue exposed to weathering for different periods of time. Environ. Sci. Pollut. Res. 2023, 30, 63200–63214. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, W.; Yang, Z. Numerical simulation of the dynamic change law of spontaneous combustion of coal gangue mountains. ACS Omega 2022, 7, 37201–37211. [Google Scholar] [CrossRef]
- Gao, L.; Liu, Y.; Xu, K.; Bai, L.; Guo, N.; Li, S. A short review of the sustainable utilization of coal gangue in environmental applications. RSC Adv. 2024, 14, 39285–39296. [Google Scholar] [CrossRef]
- Pierrehumbert, R. There is no Plan B for dealing with the climate crisis. Bull. At. Sci. 2019, 75, 215–221. [Google Scholar] [CrossRef]
- Jia, X.; Li, W.; Dong, X.; Liu, B.; Chen, J.; Li, J.; Ni, G. Mechanical and Durability Properties of Concrete Prepared with Coal Gangue: A Review. Buildings 2025, 15, 3048. [Google Scholar] [CrossRef]
- Kassem, M.; Soliman, A.; El Naggar, H. Sustainable approach for recycling treated oil sand waste in concrete: Engineering properties and potential applications. J. Clean. Prod. 2018, 204, 50–59. [Google Scholar] [CrossRef]
- Gunasekera, C.; Setunge, S.; Law, D.W. Correlations between mechanical properties of low-calcium fly ash geopolymer concretes. J. Mater. Civ. Eng. 2017, 29, 04017111. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, C.; Li, K.; Qu, F.; Yan, C.; Wu, Z. Toward understanding the activation and hydration mechanisms of composite activated coal gangue geopolymer. Constr. Build. Mater. 2022, 318, 125999. [Google Scholar] [CrossRef]
- Su, Z.; Li, X.; Zhang, Q. Influence of thermally activated coal gangue powder on the structure of the interfacial transition zone in concrete. J. Clean. Prod. 2022, 363, 132408. [Google Scholar] [CrossRef]
- Duan, D.-Y.; Wang, C.-Q.; Bai, D.-S.; Huang, D.-M. Representative coal gangue in China: Physical and chemical properties, heavy metal coupling mechanism and risk assessment. Sustain. Chem. Pharm. 2024, 37, 101402. [Google Scholar] [CrossRef]
- Miao, H.; Wang, Z.; Wang, Z.; Sun, H.; Li, X.; Liu, Z.; Dong, L.; Zhao, J.; Huang, J.; Fang, Y. Effects of Na2CO3/Na2SO4 on catalytic gasification reactivity and mineral structure of coal gangue. Energy 2022, 255, 124498. [Google Scholar] [CrossRef]
- Lu, S.H.; Pan, J.; Zhu, D.Q.; Guo, Z.Q.; Li, S.W.; Shi, Y.; Zhang, W.J. Investigation on activation technology of self-heating decarbonization of coal gangue by a sintering process. J. Cent. South Univ. 2023, 30, 1158–1167. [Google Scholar] [CrossRef]
- Han, T.; Shan, R.; Jing, G.; Zhao, W.; Xu, Z.; Qiao, D.; Wu, H. Interface structure between coal gangue ceramsite and cement matrix. Case Stud. Constr. Mater. 2025, 22, e04691. [Google Scholar] [CrossRef]
- Yuan, X.; Wu, H.; Wang, P.; Xu, F.; Ding, S. Thermal activation of coal gangue with low Al/Si ratio as supplementary cementitious materials. Molecules 2022, 27, 7268. [Google Scholar] [CrossRef]
- Zhou, J.; Hao, S.; Chen, Y.; Zhang, S.; Xu, W. Multiscale investigation of thermally activated coal gangue aggregate concrete interfacial transition zone evolution and failure mechanisms. Sci. Rep. 2025, 15, 28663. [Google Scholar] [CrossRef]
- Zhang, M.; Li, L.; Yang, F.; Zhang, S.; Zhang, H.; Zhu, Y.; An, J. Thermal activation of high-alumina coal gangue auxiliary cementitious admixture: Thermal transformation, calcining product formation and mechanical properties. Materials 2024, 17, 415. [Google Scholar] [CrossRef]
- Shao, S.; Ma, B.; Wang, C.; Chen, Y. Extraction of valuable components from coal gangue through thermal activation and HNO3 leaching. J. Ind. Eng. Chem. 2022, 113, 564–574. [Google Scholar] [CrossRef]
- Wang, A.; Liu, P.; Mo, L.; Liu, K.; Ma, R.; Guan, Y.; Sun, D. Mechanism of thermal activation on granular coal gangue and its impact on the performance of cement mortars. J. Build. Eng. 2022, 45, 103616. [Google Scholar] [CrossRef]
- Guo, Y.; Yan, K.; Cui, L.; Cheng, F. Improved extraction of alumina from coal gangue by surface mechanically grinding modification. Powder Technol. 2016, 302, 33–41. [Google Scholar] [CrossRef]
- Zhao, J.; Hu, Y.; Wang, H.; Xue, S.; Cheng, Y. Study on the Activation Effect of Mechanical Force in the Process of Ultrafine Grinding of Coal Gangue. Min. Met. Explor. 2025, 42, 1141–1148. [Google Scholar] [CrossRef]
- Guo, H.; Wang, H.; Li, H.; Xue, H.; Wei, L.; Li, Y.; Chen, Y.; Li, Q.; Dong, H. Comparison of performance, hydration behavior, and environmental benefits of coal gangue cementitious materials prepared by wet and dry grinding methods. Constr. Build. Mater. 2025, 471, 140665. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, D.; Marani, A.; Nehdi, M.L.; Wang, L.; Yang, G.; Zhang, J. Toward Sustainable Construction: Comprehensive Utilization of Coal Gangue in Building Materials. Case Stud. Constr. Mater. 2025, 23, e04930. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, Z.; Zhang, D.; Yang, Q. Properties of gangue powder modified fly ash-based geopolymer. Materials 2023, 16, 5719. [Google Scholar] [CrossRef]
- Li, Y.; Yao, Y.; Liu, X.; Sun, H.; Ni, W. Improvement on pozzolanic reactivity of coal gangue by integrated thermal and chemical activation. Fuel 2013, 109, 527–533. [Google Scholar] [CrossRef]
- Mañosa, J.; Torres-Carrasco, M.; Córdoba, J.C.; Maldonado-Alameda, A.; Chimenos, J.M. In-situ characterisation of early hydration of low-carbon cements containing thermally and mechanically activated kaolin. Constr. Build. Mater. 2024, 457, 139469. [Google Scholar] [CrossRef]
- Li, Y.; Dang, F.; Zhou, M.; Zhang, Y. Activity characteristics and activation mechanism of molybdenum tailings under mechanical, mechano-thermal, mechano-chemical, and mechano-thermal-chemical activation. Constr. Build. Mater. 2025, 463, 140109. [Google Scholar] [CrossRef]
- Hu, Y.; Han, X.; Sun, Z.; Jin, P.; Li, K.; Wang, F.; Gong, J. Study on the reactivity activation of coal gangue for efficient utilization. Materials 2023, 16, 6321. [Google Scholar] [CrossRef]
- Han, R.; Guo, X.; Guan, J.; Yao, X.; Hao, Y. Activation mechanism of coal gangue and its impact on the properties of geopolymers: A review. Polymers 2022, 14, 3861. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, H.; Feng, C.; Liu, Q.; Zhang, D.; Fan, Z.; Chen, K.; Zhao, C. Pozzolanic Evaluation Of Mechanical-Thermal Composite Activation Of Low-Quality Coal Gangue and its Impacts on the Hydration Properties of Ordinary Portland Cement. J. Build. Eng. 2025, 113, 114237. [Google Scholar] [CrossRef]
- ASTM C618-22; Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete. ASTM: West Conshohocken, PA, USA, 2012.
- GB/T 17671-2021(ISO 679:2009); Test method of cement mortar strength (ISO method). China Building Materials Academy: Beijing, China, 2021.
- GB/T 17671; (SBQTS) State Bureau of Quality Technical Supervision. Method of Testing Cements—Determination of Strength. Standard Press of China: Beijing, China, 2021.
- GB/T 50081-2019; Standard for test methods of concrete physical and mechanical properties. China Architecture & Building Press: Beijing, China, 2019.
- GB/T 1346-2024; Test methods for water requirement of standard consistency, setting time and soundness of the portland cement. National Standardization Administration: Beijing, China, 2024.
- GB/T 2419-2005; Test method for fluidity of cement mortar. Standards Press of China: Beijing, China, 2005.
- Li, Y.; Zhang, J.; Yan, C.; Bold, T.; Wang, J.; Cui, K. Effect of activated coal gangue on the hydration and hardening of Portland cement. Constr. Build. Mater. 2024, 422, 135740. [Google Scholar] [CrossRef]
- Wadell, H. Volume, shape, and roundness of rock particles. J. Geol. 1932, 40, 443–451. [Google Scholar] [CrossRef]
- Cheng, Y.; Huang, F.; Li, W.; Liu, R.; Li, G.; Wei, J. Test research on the effects of mechanochemically activated iron tailings on the compressive strength of concrete. Constr. Build. Mater. 2016, 118, 164–170. [Google Scholar] [CrossRef]
- Li, J.; Sheng, N.; Jin, H.; Chen, M.; Yang, Z. Efficient utilization of coal gangue, calcium carbide slag and flue-gas desulfurization gypsum waste for low-carbon all-solid-waste binders to develop eco-friendly grouting concrete. Chem. Eng. J. 2025, 523, 168282. [Google Scholar] [CrossRef]
- Ting, L.; Qiang, W.; Shiyu, Z. Effects of ultra-fine ground granulated blast-furnace slag on initial setting time, fluidity and rheological properties of cement pastes. Powder Technol. 2019, 345, 54–63. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, J.; Wu, P.; Sun, X.; Zhang, S.; Guo, Z. Calcined oil shale residue as a supplementary cementitious material for ordinary Portland cement. Constr. Build. Mater. 2021, 306, 124849. [Google Scholar] [CrossRef]
- Chen, B.; Pang, L.; Zhou, Z.; Chang, Q.; Fu, P. Study on the activation mechanism and hydration properties of gold tailings activated by mechanical-chemical-thermal coupling. J. Build. Eng. 2022, 48, 104014. [Google Scholar] [CrossRef]
- Li, J.; Hitch, M. Structural and chemical changes in mine waste mechanically-activated in various milling environments. Powder Technol. 2017, 308, 13–19. [Google Scholar] [CrossRef]
- Lim, D.J.; Marks, N.A.; Rowles, M.R. Universal Scherrer equation for graphene fragments. Carbon 2020, 162, 475–480. [Google Scholar] [CrossRef]
- Chen, R.; Li, Y.; Zhao, Y.; Li, S.; Xiang, R.; Xu, N.; Liu, F. Effect of inorganic acid on the phase transformation of alumina. J. Alloys Compd. 2017, 699, 170–175. [Google Scholar] [CrossRef]
- Li, G.; Wang, Y.; Chen, X.; Jiao, H.; Zhu, G.; Fan, Z.; Gao, M.; Xu, W.; Dong, F.; Yao, L. Research on the Mechanical Properties and Micro-Evolution Characteristics of Coal Gangue-Based Composite Cementitious Materials. Buildings 2025, 15, 3406. [Google Scholar] [CrossRef]
- Li, F.; Liu, L.; Liu, K.; Zheng, A.; Liu, J. Investigation on waterproof mechanism and micro-structure of cement mortar incorporated with silicane. Constr. Build. Mater. 2020, 239, 117865. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Xu, C.; Guan, X.; Zou, D.; Jing, G. Synergy effect of synthetic ettringite modified by citric acid on the properties of ultrafine sulfoaluminat cement-based materials. Cem. Concr. Compos. 2022, 125, 104312. [Google Scholar] [CrossRef]
- Zhou, H.; Ma, D.; Dai, L.; Wuri, L.; Wang, Y.; Liu, X.; Shu, X. Exploring solid-phase reaction mechanism to elucidate the formation of effective silica through thermal–chemical coupled activation of siliceous minerals in coal gangue. J. Alloys Compd. 2025, 1022, 180070. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Qian, L.; Ba, T.; Dong, W.; Yuan, F.; Sun, Z. Mechanical assisted thermal and chemical composite activation of coal gangue and its hydration characteristics. J. Build. Eng. 2025, 112, 113687. [Google Scholar] [CrossRef]
- Bakil, S.; Tóth, M.; Ibrahim, J.-E.F.; Mucsi, G. Influence of mechanical activation of coal gangue on the strength and microstructure of geopolymer. Constr. Build. Mater. 2025, 486, 141977. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, N.; Yao, Y.; Sun, H.; Feng, H. Micro-structural characterization of the hydration products of bauxite-calcination-method red mud-coal gangue based cementitious materials. J. Hazard. Mater. 2013, 262, 428–438. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, L.; Pan, J.; He, X.; Shi, S.; Long, X.; Yang, Y.; Zhao, X.; Zhou, C. Study on modes of occurrence and selective leaching of lithium in coal gangue via grinding-thermal activation. Chem. Eng. J. 2024, 482, 148941. [Google Scholar] [CrossRef]
- Wang, J.; Xu, H.; Cao, Y.; Fan, G.; Deng, J.; Huang, X.; Fu, B. Selective extraction of lithium from coal gangue through calcination and mechanochemical activation. J. Environ. Manag. 2025, 387, 125926. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, C.; Mao, L.; Han, Z.; Chen, L.; Zou, H.; Li, L.; Yu, P.; Dong, J.; Zhang, Y. Study on the effect of activated coal gangue on the mechanical and hydration properties of cement. Front. Mater. 2023, 10, 1186055. [Google Scholar] [CrossRef]
- Shao, S.; Ma, B.; Wang, C.; Chen, Y. Thermal behavior and chemical reactivity of coal gangue during pyrolysis and combustion. Fuel 2023, 331, 125927. [Google Scholar] [CrossRef]
- Fei, E.; Zhang, X.; Su, L.; Liu, B.; Li, B.; Li, W. Analysis of calcination activation modified coal gangue and its acid activation mechanism. J. Build. Eng. 2024, 95, 109916. [Google Scholar] [CrossRef]
- Guan, X.; Chen, J.; Zhu, M.; Gao, J. Performance of microwave-activated coal gangue powder as auxiliary cementitious material. J. Mater. Res. Technol. 2021, 14, 2799–2811. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.