Application and Mechanism Study on Optimal Design of Cement-Based Building Materials Based on Polymer Binder
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Sample Preparation
2.2. Direct Mixed with CNC
3. Test Results and Discussions
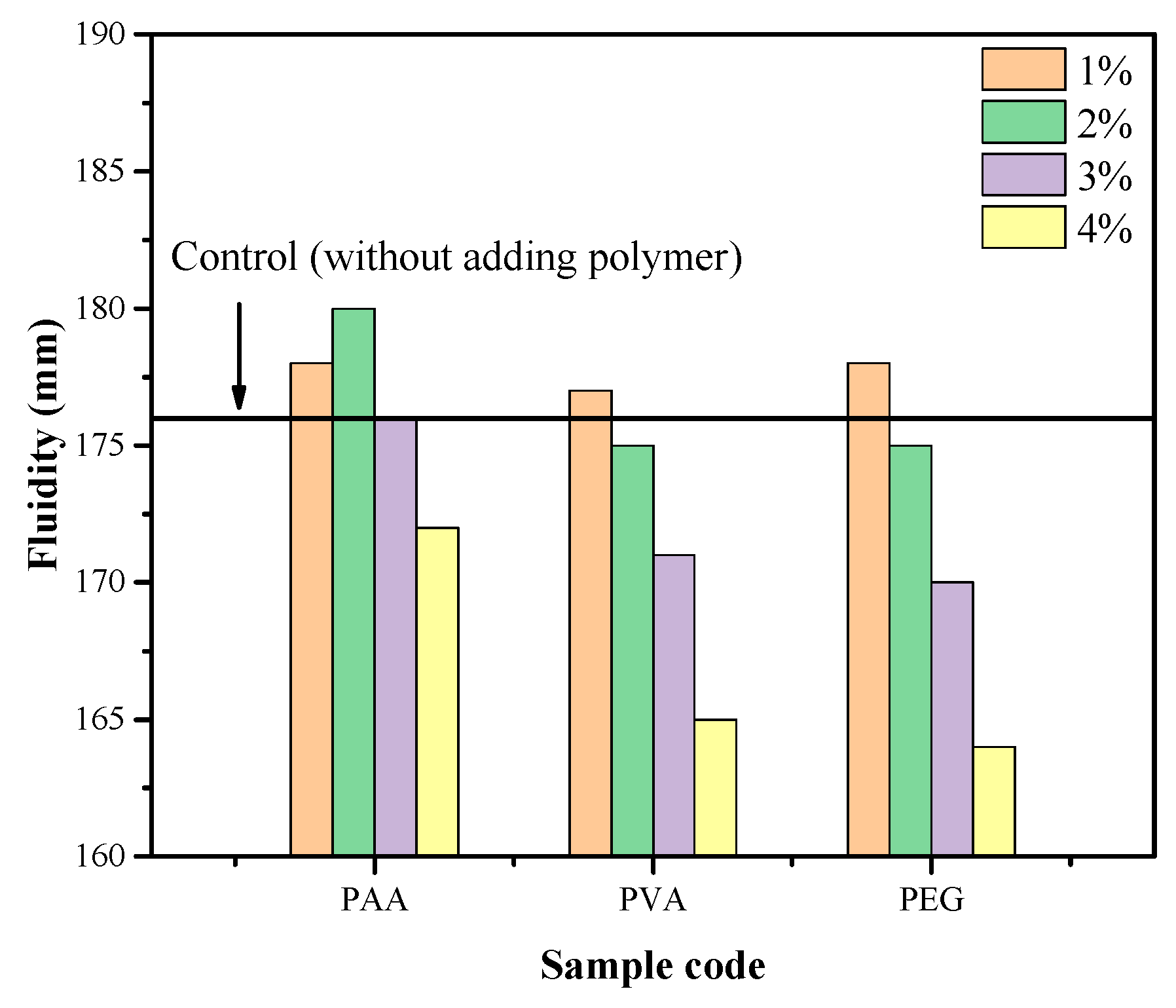
3.1. Fluidity
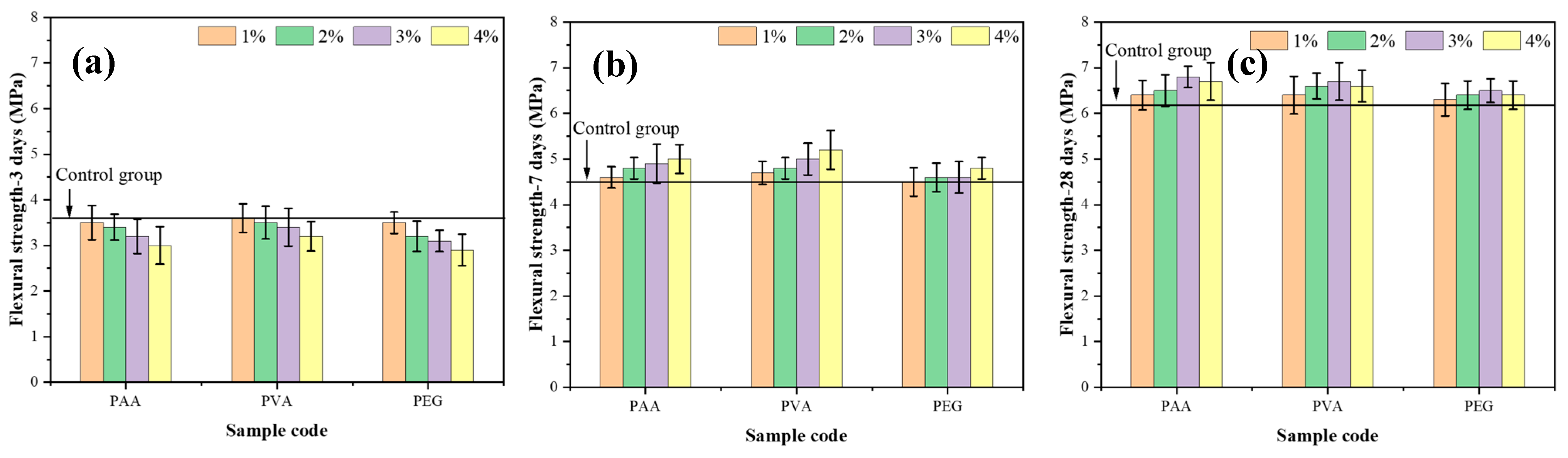
3.2. Effect of Different Curing Ages on Flexural Strength
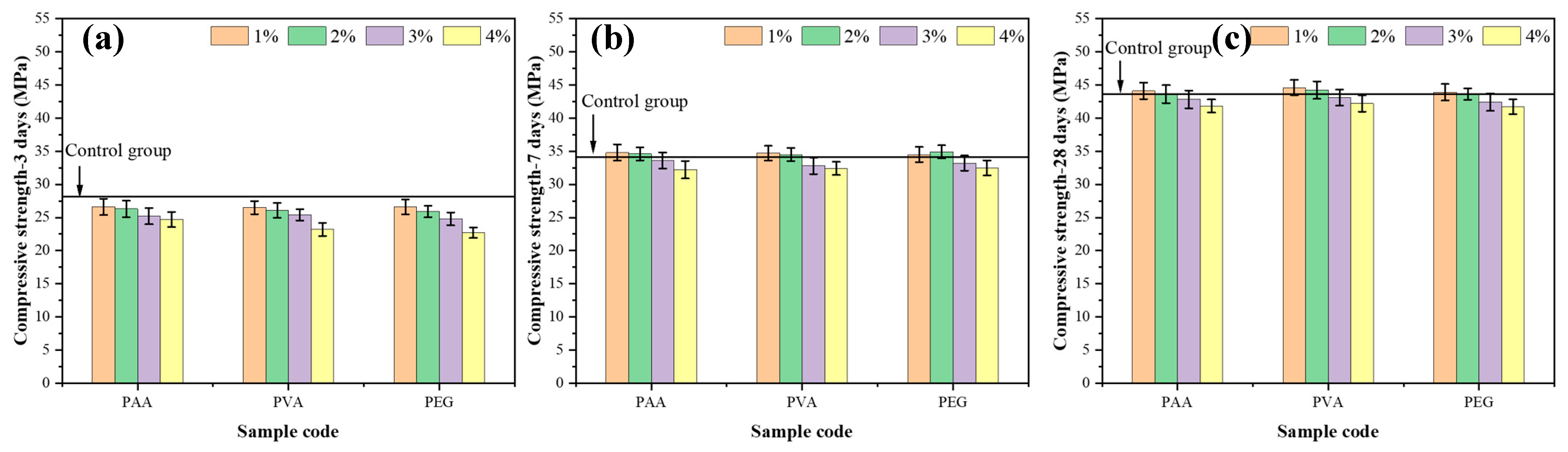
3.3. Effect of Different Curing Ages on Compressive Strength
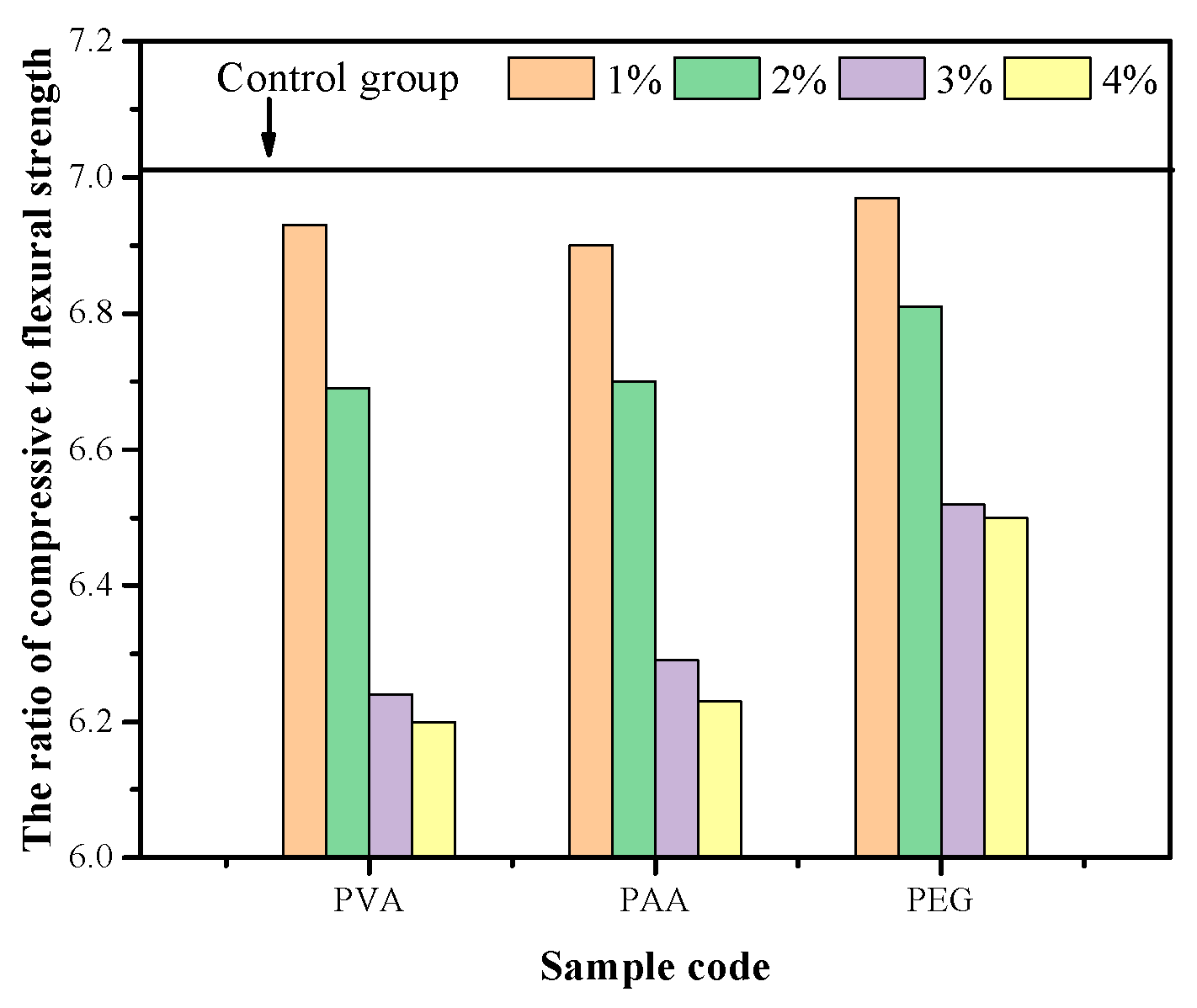
3.4. Compression and Flexural Ratio and Toughness Analysis
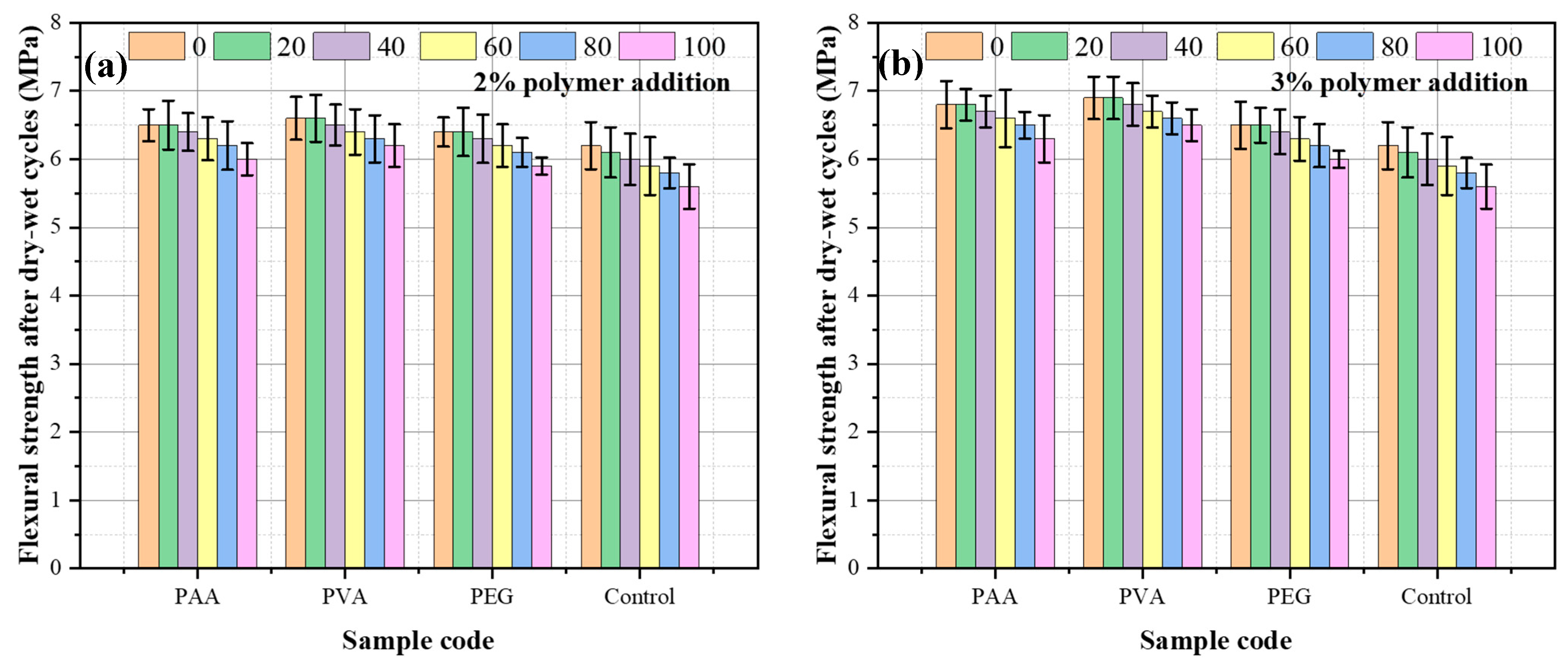
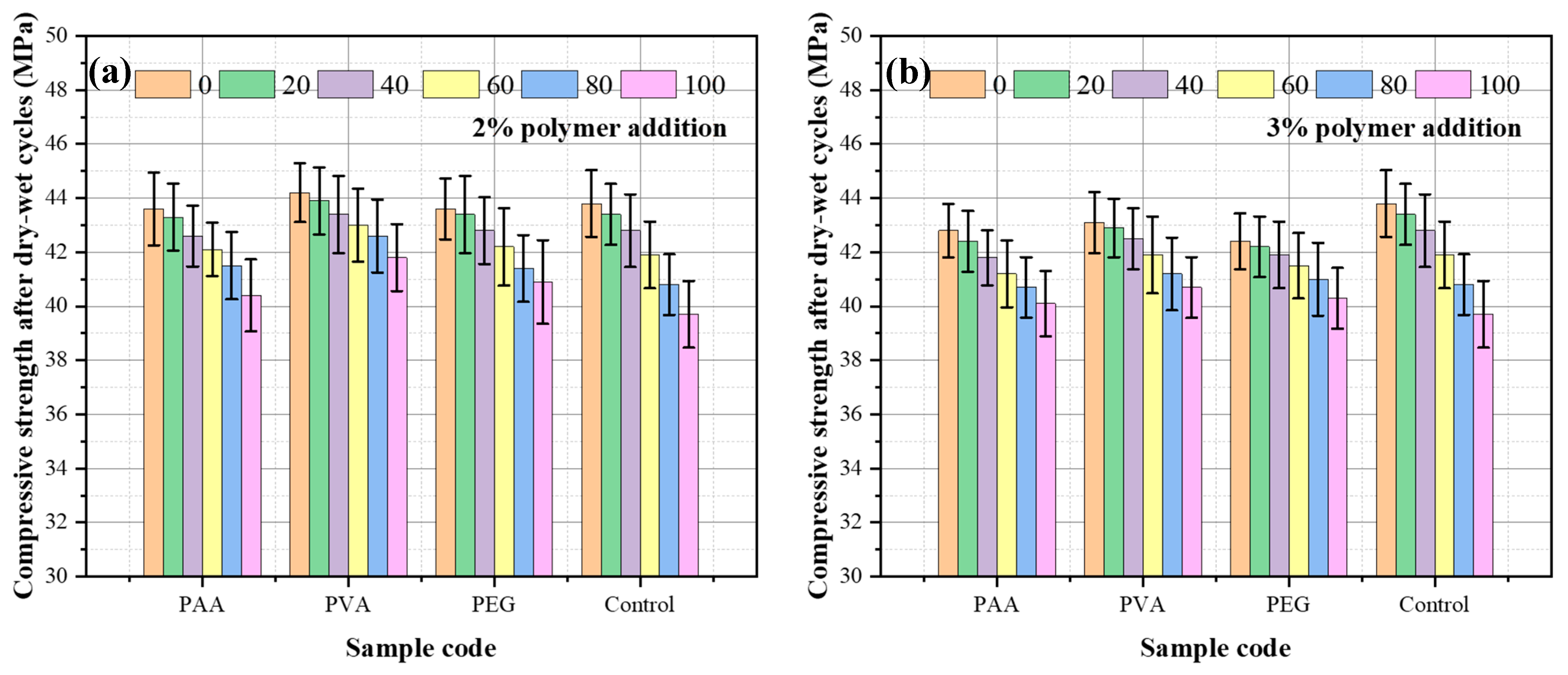
3.5. Dry–Wet Cycling Resistance
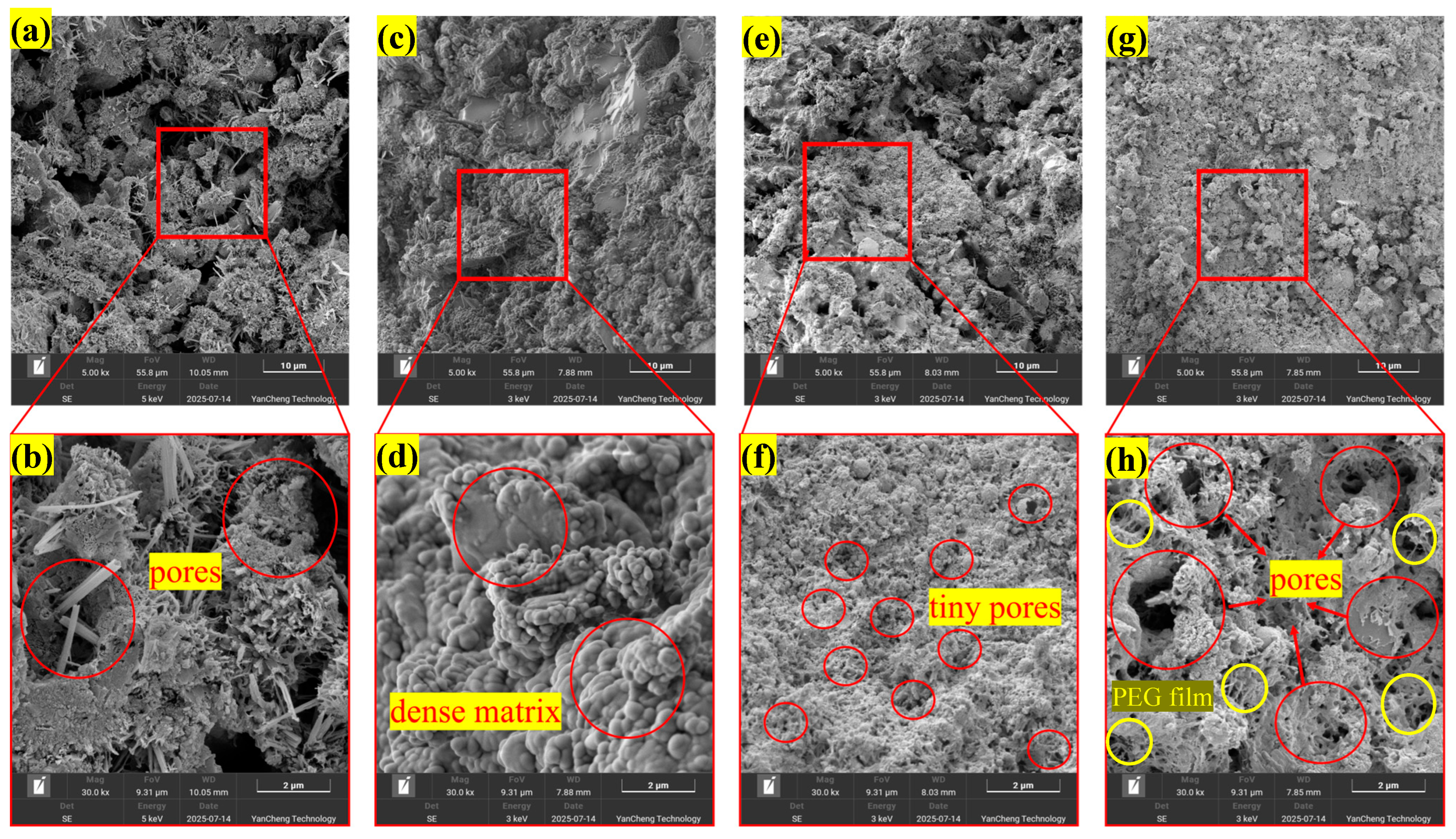
3.6. Microstructure of Hydration Products
4. Molecular Dynamics Simulations
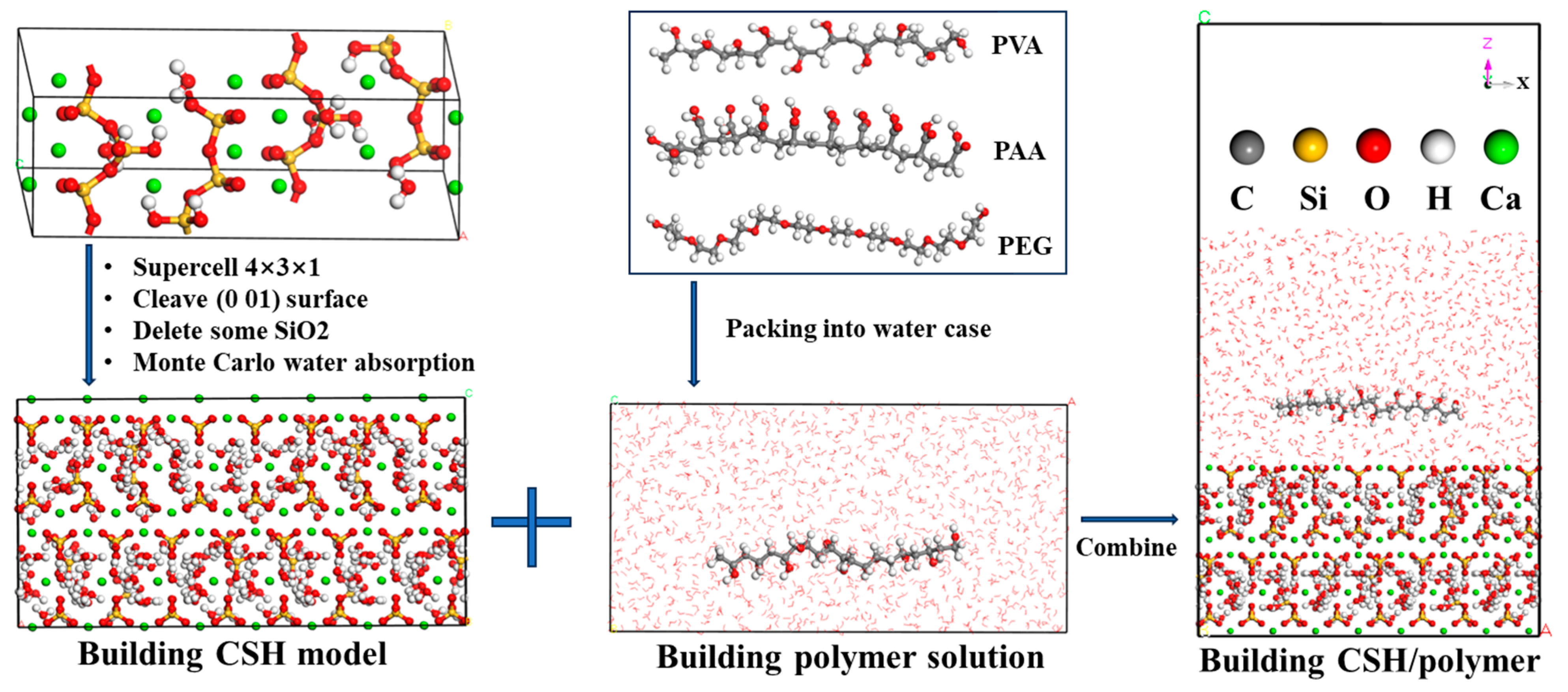
4.1. Simulation Details
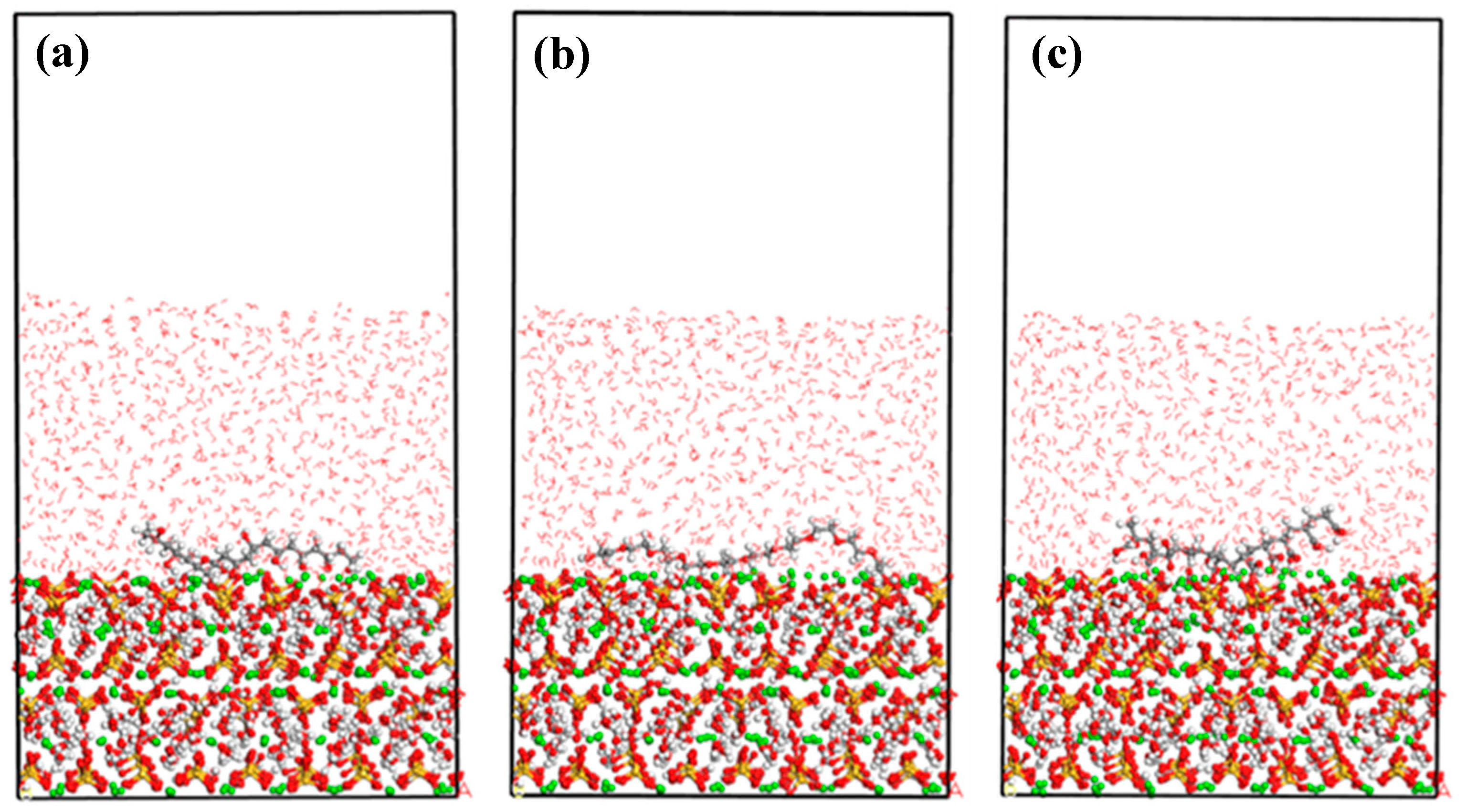
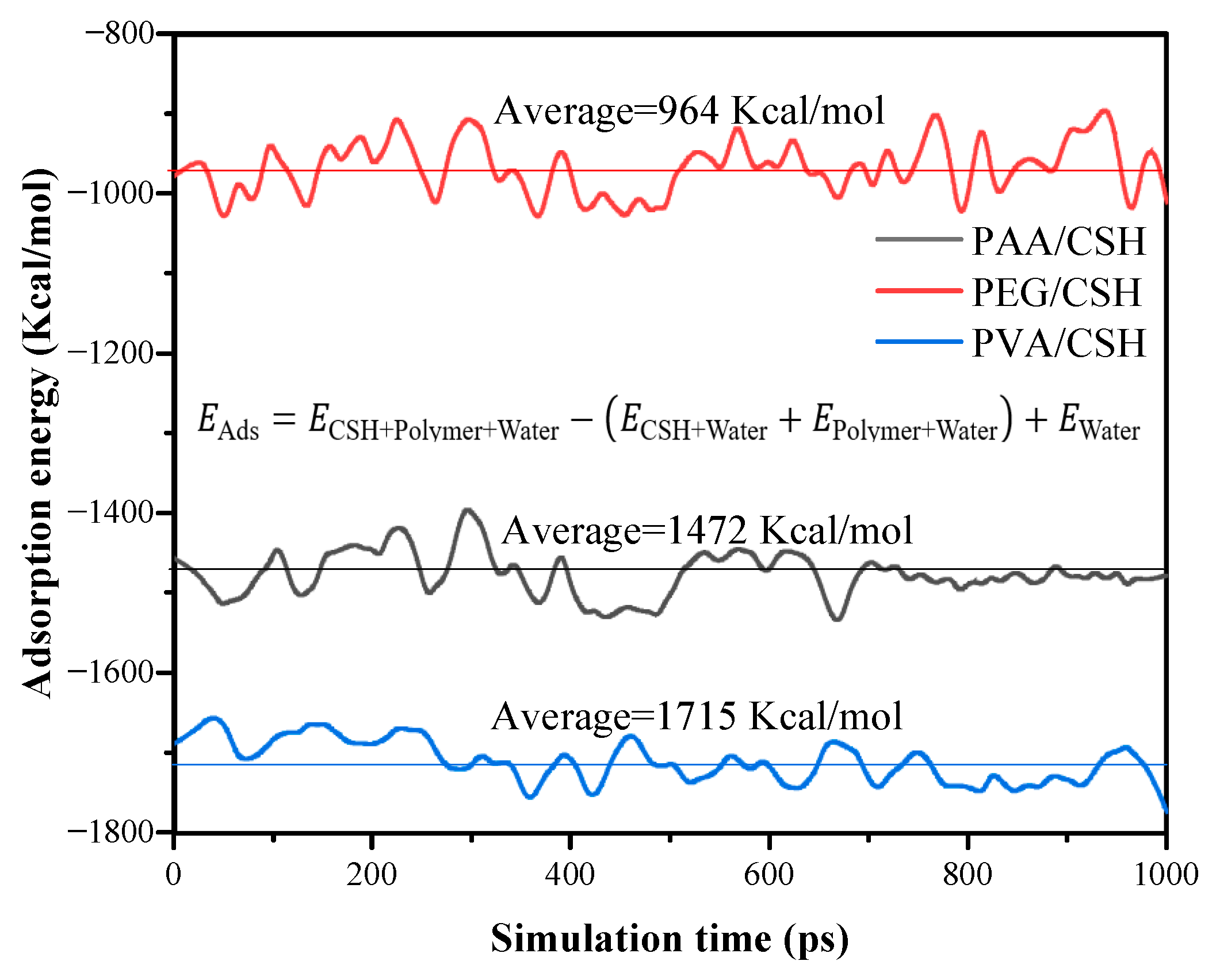
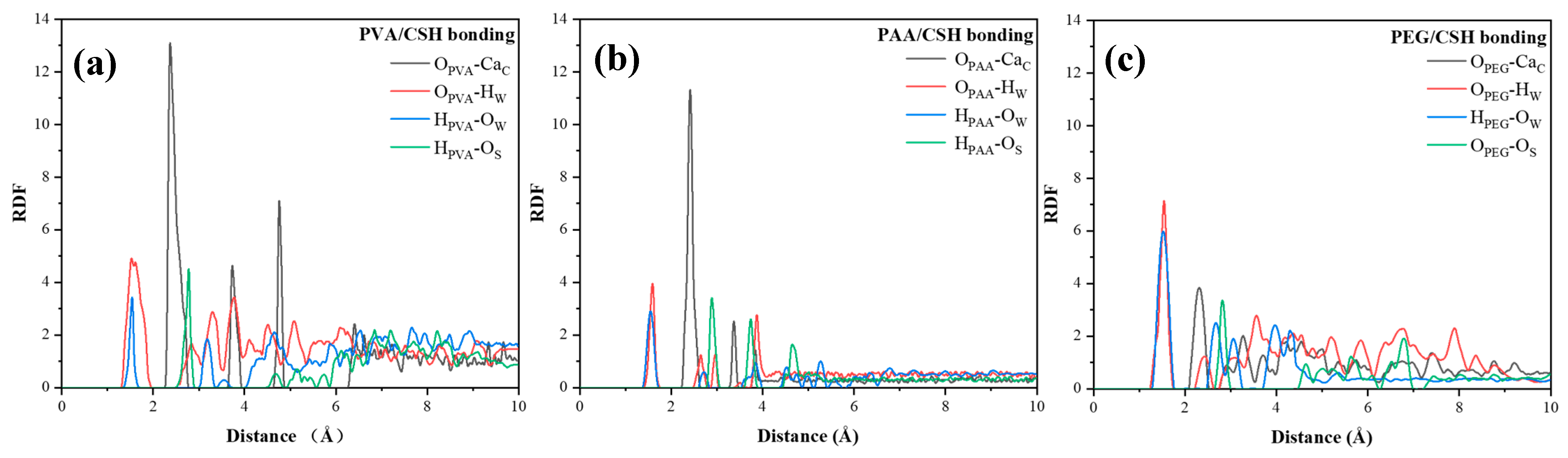
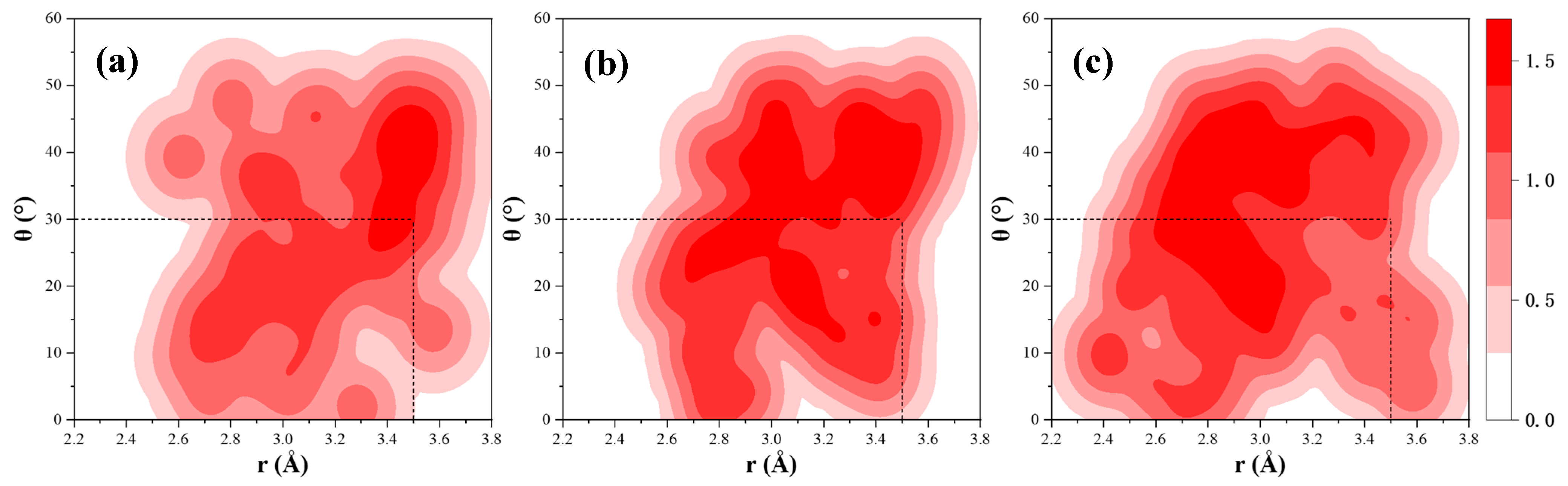
4.2. Interfacial Properties of CSH/Polymers
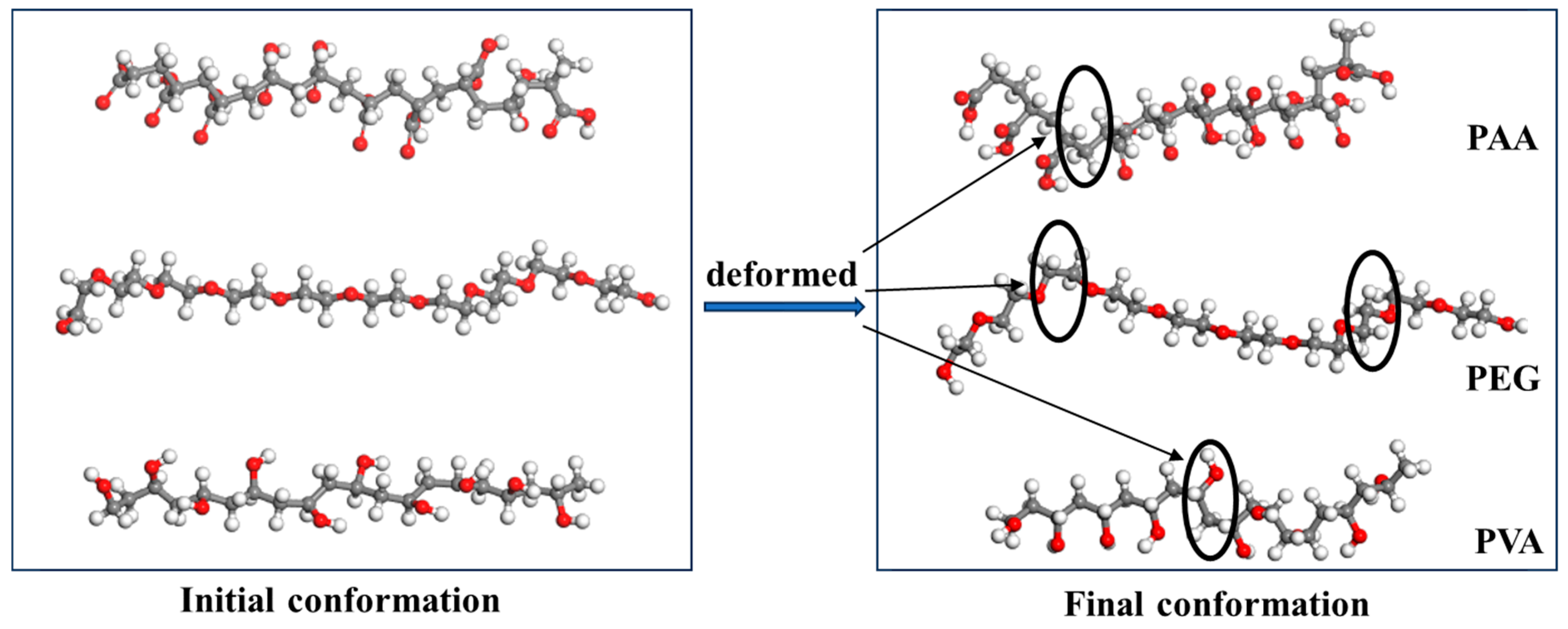
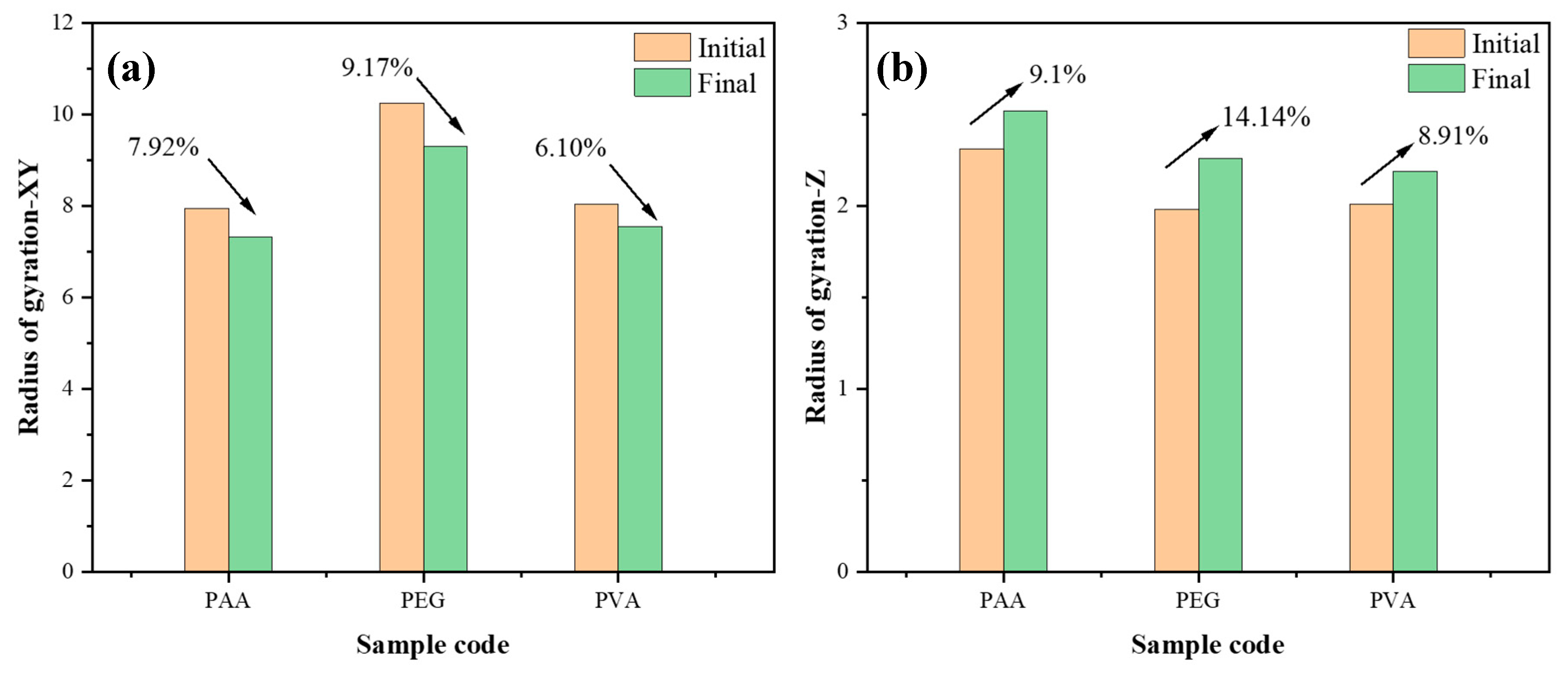
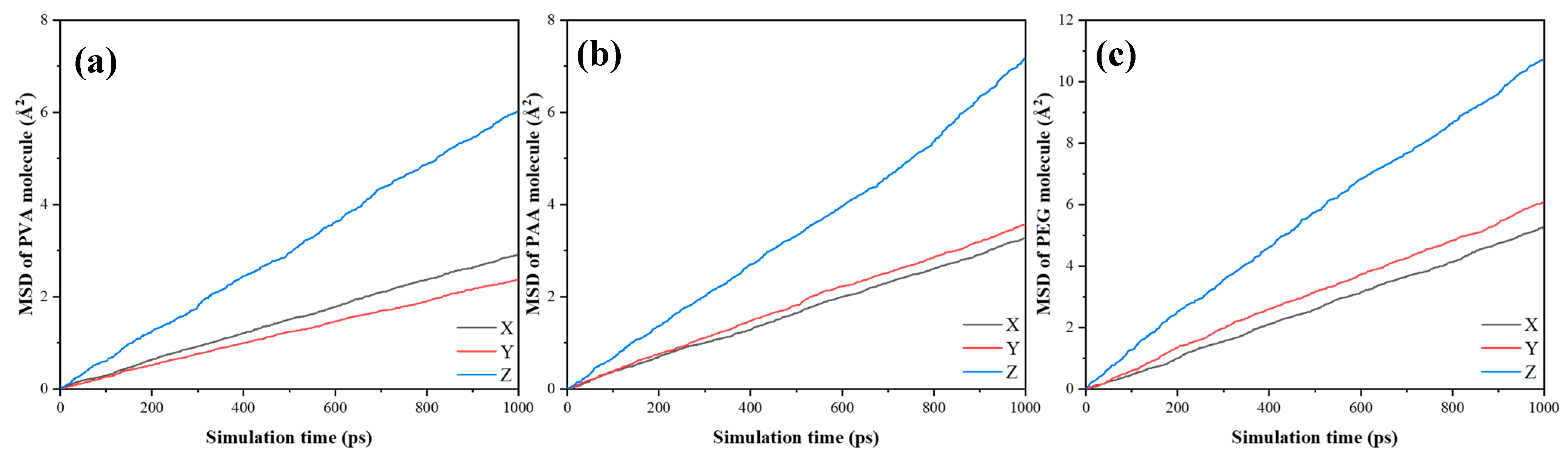
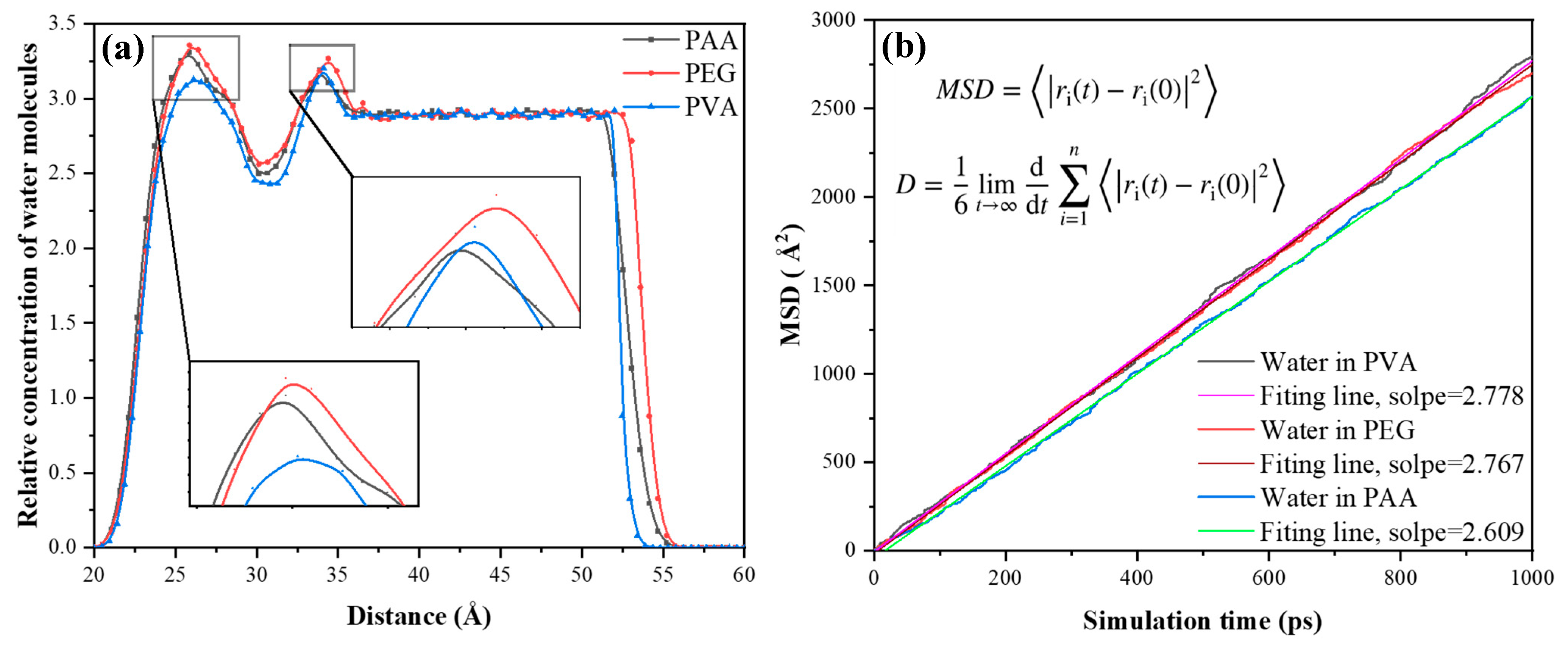
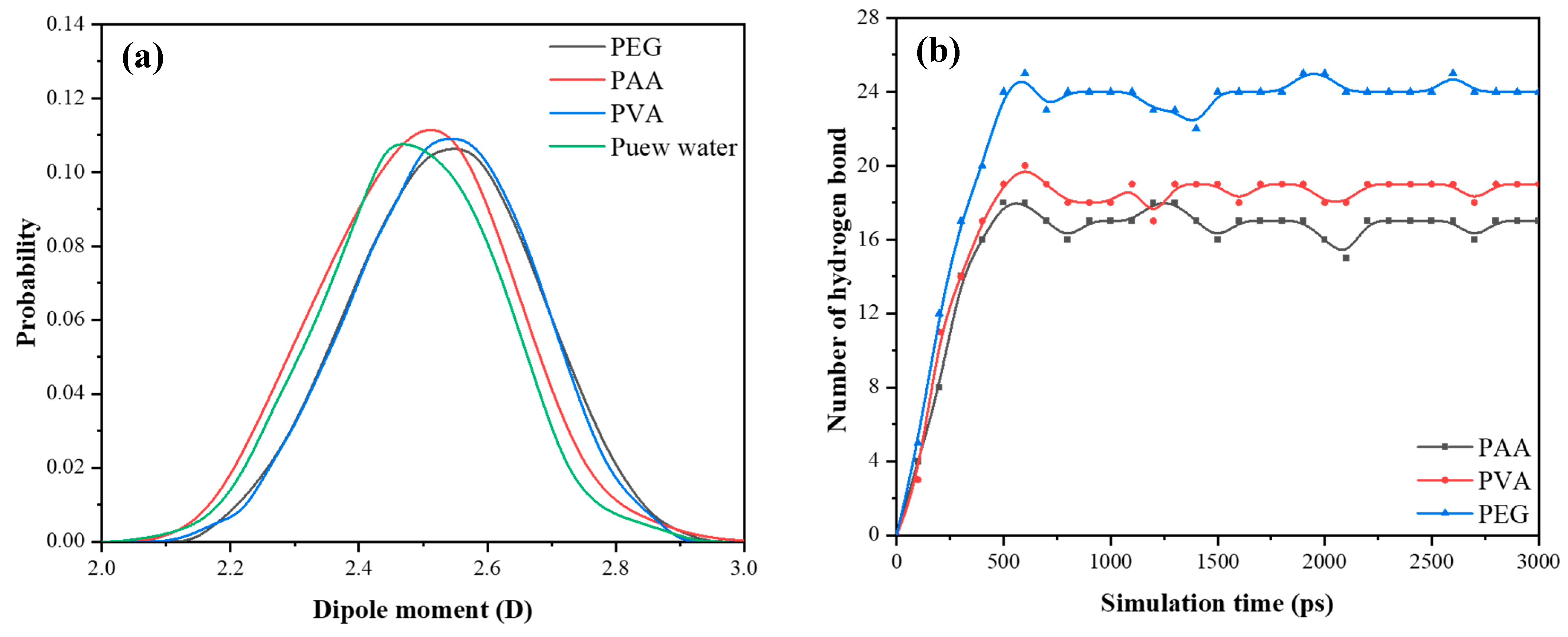
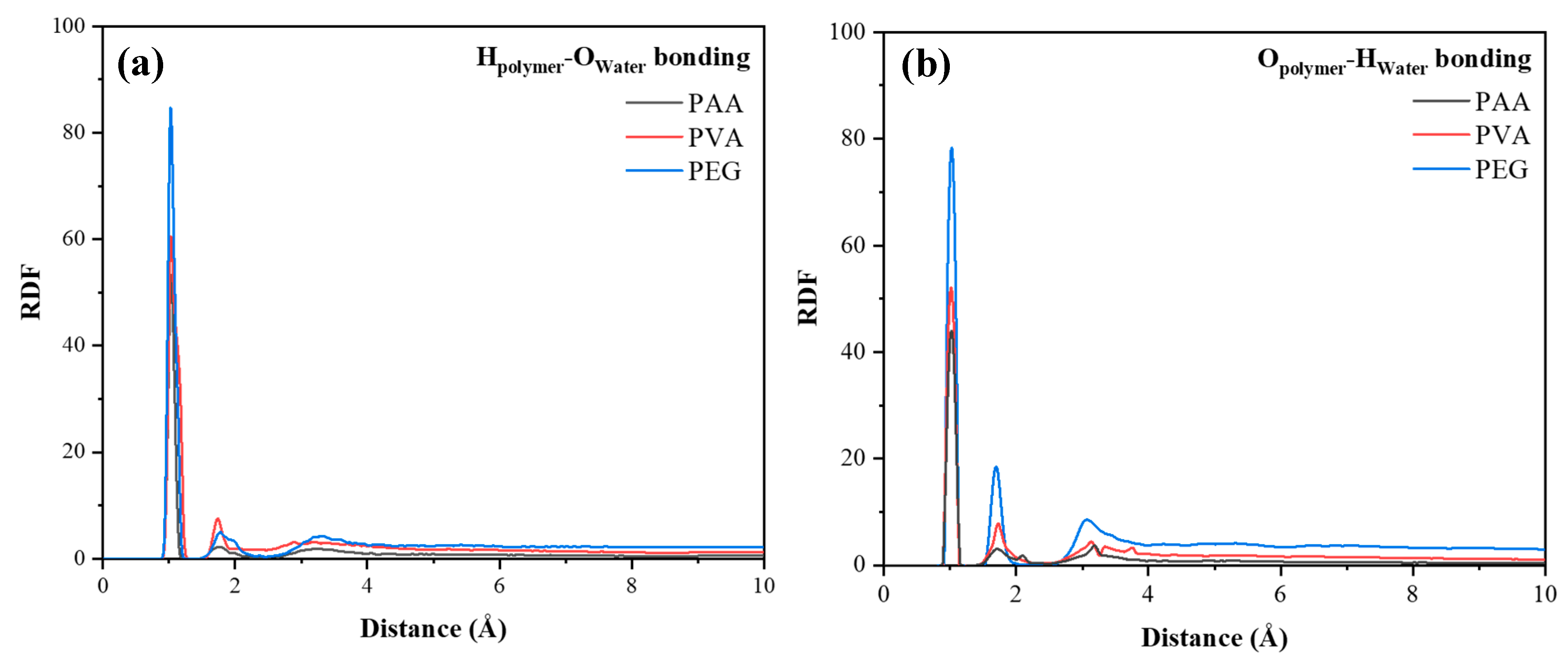
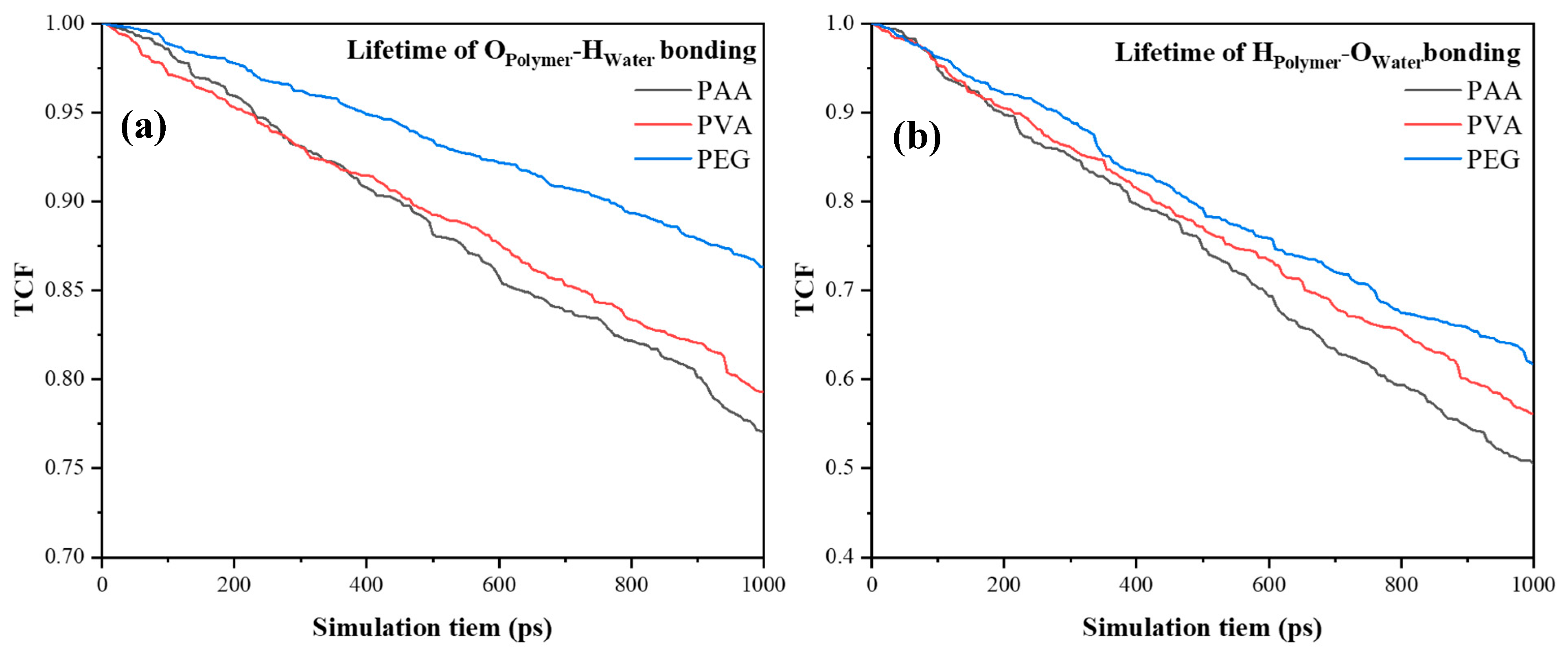
4.3. The Simulation of Polymers/Water
5. Conclusions
- (1)
- Polymers enhance the flexural strength of mortar by improving interfacial bonding, with PVA showing the greatest effect. Low dosages (1%) improve compressive strength, while higher dosages (2–3%) balance strength and hydration inhibition, identifying 2–3% as the optimal range.
- (2)
- Incorporation of polymers markedly improves resistance to wet–dry cycles, reducing strength loss through better cohesion and water-binding capacity. The effectiveness follows the order PVA > PEG > PAA.
- (3)
- MD simulations reveal that PVA and PAA strengthen the CSH interface via Ca-O coordination, while PEG interacts primarily with CSH through hydrogen bonding. PVA and PAA exhibit good adsorption energy, confirming superior reinforcement in mechanical performance.
- (4)
- PEG forms the most stable hydrogen bonding network with water molecules, effectively anchoring them and reducing ingress, which underpins its contribution to improved durability under cyclic exposure.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tang, S.W.; Yao, Y.; Andrade, C.; Li, Z.J. Recent durability studies on concrete structure. Cem. Concr. Res. 2025, 78, 143–154. [Google Scholar] [CrossRef]
- Salifu, N.; Bassuoni, M.T.; Guven, G. Performance Evaluation of Limestone-Blended Cement and Cellulose Nanomaterials in 3D Concrete Printing. Case Stud. Constr. Mater. 2025, 22, e04758. [Google Scholar] [CrossRef]
- Bai, J.; Li, M.; Yuan, Y.; Zhao, T.; Yang, Y.; Xu, Z.; Zhou, Y. Surface modified cellulose nanofibrils with γ-Aminopropyltriethoxysilane (KH550) for the performance enhancement of Portland cement systems. Constr. Build. Mater. 2025, 486, 141956. [Google Scholar] [CrossRef]
- Claramunt, J.; Ventura, H.; Toledo Filho, R.D.; Ardanuy, M. Effect of nanocelluloses on the microstructure and mechanical performance of CAC cementitious matrices. Cem. Concr. Res. 2019, 119, 64–76. [Google Scholar] [CrossRef]
- Du, X.; Li, Y.; Huangfu, B.; Si, Z.; Huang, L.; Wen, L.; Ke, M. Modification mechanism of combined nanomaterials on high performance concrete and optimization of nanomaterial content. J. Build. Eng. 2023, 64, 105648. [Google Scholar] [CrossRef]
- Monteiro, H.; Moura, B.; Soares, N. Advancements in nano-enabled cement and concrete: Innovative properties and environmental implications. J. Build. Eng 2022, 56, 104736. [Google Scholar] [CrossRef]
- Yue, Y.; Zhou, Y.; Xing, F.; Gong, G.; Hu, B.; Guo, M. An industrial applicable method to improve the properties of recycled aggregate concrete by incorporating nano-silica and micro-CaCO3. J. Clean. Prod. 2020, 259, 120920. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, S.; Huang, X.; Xi, B.; Huang, Z.; Guo, M. Performance enhancement of green high-ductility engineered cementitious composites by nano-silica incorporation. Constr. Build. Mater. 2021, 281, 122618. [Google Scholar] [CrossRef]
- Pezeshkian, M.; Delnavaz, A.; Delnavaz, M. Effect of natural zeolite on mechanical properties and autogenous shrinkage of ultrahigh-performance concrete. J. Mater. Civ. Eng. 2020, 32, 04020093. [Google Scholar] [CrossRef]
- Chin, K.M.; Sung Ting, S.; Ong, H.L.; Omar, M. Surface functionalized nanocellulose as a veritable inclusionary material in contemporary bioinspired applications: A review. J. Appl. Polym. Sci. 2018, 135, 46065. [Google Scholar] [CrossRef]
- Wang, L.; Rehman, N.U.; Curosu, I.; Zhu, Z.; Beigh, M.A.B.; Liebscher, M.; Mechtcherine, V. On the use of limestone calcined clay cement (LC3) in high-strength strain-hardening cement-based composites (HS-SHCC). Cem. Concr. Res. 2021, 144, 106421. [Google Scholar] [CrossRef]
- Lepech, M.D.; Li, V.C. Application of ECC for bridge deck link slabs. Mater. Struct. 2009, 42, 1185–1195. [Google Scholar] [CrossRef]
- Chun, B.; Lee, S.W.; Piao, R.; Kim, S.; Yoo, D.Y. Enhanced impact resistance of RC beams using various types of high-performance fiber-reinforced cementitious composites. Eng. Struct. 2024, 319, 118790. [Google Scholar] [CrossRef]
- Song, Z.; Chen, R.; Wang, T.; Wu, H. Synergistic effects of CO2 sequestration on mechanical, microstructural, and environmental performance in carbonated MgO-based ECC. J. CO2 Util. 2025, 91, 103010. [Google Scholar] [CrossRef]
- Wu, J.D.; Guo, L.P.; Qin, Y.Y. Preparation and characterization of ultra-high-strength and ultra-high-ductility cementitious composites incorporating waste clay brick powder. J. Clean. Prod. 2021, 312, 127813. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Z.; Yang, Y.; Yao, Y. Measurement and correlation of ductility and compressive strength for engineered cementitious composites (ECC) produced by binary and ternary systems of binder materials: Fly ash, slag, silica fume and cement. Constr. Build. Mater. 2014, 68, 192–198. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, J.; Qin, F.; Huang, Y.; Sun, F. Mechanical and self-healing properties of calcium-sulfoaluminate-cement-based engineered cementitious composites (ECC). J. Build. Eng. 2023, 77, 107512. [Google Scholar] [CrossRef]
- Mostafaei, H.; Bahmani, H. Sustainable high-performance concrete using zeolite powder: Mechanical and carbon footprint analyses. Buildings 2024, 14, 3660. [Google Scholar] [CrossRef]
- Fan, Q.; Zheng, Y.; Meng, D.; Guo, Q.; Liu, Y.; Wu, H. Study on improving the performance of engineered cement-based composites by modifying binder system and polyethylene fiber/matrix interface. Colloids Surf. A 2025, 707, 135862. [Google Scholar] [CrossRef]
- Fan, Q.; Wu, H.; Meng, D.; Zheng, Y.; Tan, T. Hydration, Microstructure, and Strength Properties of Eco-ECC Incorporating Agricultural Waste: Peanut Shell Ash. Colloids Surf. A 2025, 137396. [Google Scholar] [CrossRef]
- Heidarnezhad, F.; Jafari, K.; Ozbakkaloglu, T. Effect of polymer content and temperature on mechanical properties of lightweight polymer concrete. Constr. Build. Mater. 2020, 260, 119853. [Google Scholar] [CrossRef]
- Khan, R.M.A.; Shafighfard, T.; Ali, H.Q.; Mieloszyk, M.; Yildiz, M. Strength prediction and experimental damage investigations of plain woven CFRPs with interacting holes using multi-instrument measurements. Polym. Compos. 2023, 44, 3594–3609. [Google Scholar] [CrossRef]
- Knapen, E.; Van Gemert, D. Polymer film formation in cement mortars modified with water-soluble polymers. Cem. Concr. Comp. 2015, 58, 23–28. [Google Scholar] [CrossRef]
- Fan, J.; Li, G.; Deng, S.; Wang, Z. Mechanical properties and microstructure of polyvinyl alcohol (PVA) modified cement mortar. Appl. Sci. 2019, 9, 2178. [Google Scholar] [CrossRef]
- Bentz, D.P.; Snyder, K.A.; Cass, L.C.; Peltz, M.A. Doubling the service life of concrete structures. I: Reducing ion mobility using nanoscale viscosity modifiers. Cem. Concr. Comp. 2008, 30, 674–678. [Google Scholar] [CrossRef]
- Zhao, L.; Feng, P.; Shao, L.; Ye, S.; Liu, X. Using viscosity modifying admixture to reduce diffusion in cement-based materials: Effect of molecular mass. Constr. Build. Mater. 2021, 290, 123207. [Google Scholar] [CrossRef]
- Fan, Q.; Zheng, Y.; Liu, Y.; Meng, X.; Quan, G.; Guo, Q.; Meng, D. Effect of modified cellulose nanocrystals on the structure of calcium silicate hydrate studied by molecular dynamics simulation and experiment. Langmuir 2023, 39, 16244–16260. [Google Scholar] [CrossRef]
- Fan, Q.; Meng, X.; Li, Z.; Ma, G.; Wang, Z.; Zhang, K.; Meng, D. Experiment and molecular dynamics simulation of functionalized cellulose nanocrystals as reinforcement in cement composites. Constr. Build. Mater. 2022, 341, 127879. [Google Scholar] [CrossRef]
- Fan, Q.; Liu, Y.; Zheng, Y.; Meng, D.; Guo, Q.; Hu, Z.; Liao, W. The microscopic reinforcement mechanism of Zhuhai soft soil by cement-based stabilizer: From microscopic characterization to molecular dynamics simulation. Appl. Surf. Sci. 2025, 681, 161574. [Google Scholar] [CrossRef]
- Lu, Z.; Yu, J.; Yao, J.; Hou, D. Experimental and molecular modeling of polyethylene fiber/cement interface strengthened by graphene oxide. Cem. Concr. Comp. 2020, 112, 103676. [Google Scholar] [CrossRef]
- Lu, M.; Zheng, Y.Y.; Yin, Z.Y. From sedimentation to consolidation of kaolinite: A molecular dynamic study. Comput. Geotech. 2024, 170, 106285. [Google Scholar] [CrossRef]
- Lu, M.; Zheng, Y.Y.; Yin, Z.Y. A molecular dynamics study on the softening of kaolinite in water: Weakening of tensile property during stretching and disintegration of structure during soaking. Comput. Geotech. 2024, 173, 106562. [Google Scholar] [CrossRef]
- Wei, P.; Zhou, S.; Zheng, Y.Y.; Yin, Z.Y.; Xu, W. Nanoscale stick-slip behavior and hydration of hydrated illite clay. Comput. Geotech. 2024, 166, 105976. [Google Scholar] [CrossRef]
- Hou, D.; Yu, J.; Wang, P. Molecular dynamics modeling of the structure, dynamics, energetics and mechanical properties of cement-polymer nanocomposite. Compos. Part B-Eng. 2019, 162, 433–444. [Google Scholar] [CrossRef]
- GB/T 2419-2005; Test Method for Fluidity of Cement Mortar. Standardization Administration of the People’s Republic of China: Beijing, China, 2005.
- GB/T 17671-2021; Test Method of Cement Mortar Strength (ISO Method). Standardization Administration of the People’s Republic of China: Beijing, China, 2021.
- Wang, P.; Duan, Y.; Zheng, H.; Chen, Z.; Wang, M.; Wang, X.; Hou, D. Molecular structure and dynamics of water on the surface of cement hydration products: Wetting behavior at nanoscale. Appl. Surf. Sci. 2023, 611, 155713. [Google Scholar] [CrossRef]
- Lv, C.; Xue, Q.; Xia, D.; Ma, M. Effect of chemisorption structure on the interfacial bonding characteristics of graphene-polymer composites. Appl. Surf. Sci 2012, 258, 2077–2082. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Y.; Yang, Y.; Shu, X.; Yan, H.; Ran, Q. Effect of hydrophobic groups on the adsorption conformation of modified polycarboxylate superplasticizer investigated by molecular dynamics simulation. Appl. Surf. Sci. 2017, 407, 8–15. [Google Scholar] [CrossRef]
- Fan, Q.; Zheng, Y.; Yang, Y.; Liu, S.C.; Meng, D.; Guo, Q.; Liu, Y. Effect of interface properties between functionalized cellulose nanocrystals and tricalcium silicate on the early hydration mechanism of cement. Colloids Surf. A 2024, 698, 134552. [Google Scholar] [CrossRef]
- Sanchez, F.; Zhang, L. Molecular dynamics modeling of the interface between surface-functionalized graphitic structures and calcium-silicate-hydrate: Interaction energies, structure, and dynamics. Colloid Interface Sci. 2008, 323, 349–358. [Google Scholar] [CrossRef]
- Joos, M.; Kang, X.; Merkle, R.; Maier, J. Water uptake of solids and its impact on ion transport. Nat. Mater. 2025, 24, 821–834. [Google Scholar] [CrossRef]
- Liu, Y.M.; Zheng, Y.Y.; Lin, H.J.; Wei, P.C.; Fan, Q.C.; Huang, G.G.; Meng, D. Calculation of contact angle via Young-Dupré equation with molecular dynamic simulation: Kaolinite as an example. Colloids Surf. A 2024, 697, 134469. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Lin, H.; Fan, Q.; Tan, T. Effect of temperature on structure and mechanical properties of kaolinite via experiments and reactive molecular dynamics simulations. Appl. Clay Sci. 2025, 276, 107918. [Google Scholar] [CrossRef]
- Fan, Q.; Wang, Z.; Meng, X.; Zhang, K.; Ma, G.; Li, Z.; Meng, D. Multi-scale analysis of the strengthening mechanism of functionalized graphene as reinforcement in cement composites. Colloids Surf. A 2022, 651, 129729. [Google Scholar] [CrossRef]
| Composition | CaO | Al2O3 | Fe2O3 | SiO2 | SO3 | MgO | Loss |
|---|---|---|---|---|---|---|---|
| Ratio | 63.12% | 5.14% | 3.21% | 20.13% | 3.11% | 3.02% | 2.27% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, L.; Fan, Q.; Meng, D.; Meng, X.; Xu, B. Application and Mechanism Study on Optimal Design of Cement-Based Building Materials Based on Polymer Binder. Buildings 2025, 15, 3192. https://doi.org/10.3390/buildings15173192
Yu L, Fan Q, Meng D, Meng X, Xu B. Application and Mechanism Study on Optimal Design of Cement-Based Building Materials Based on Polymer Binder. Buildings. 2025; 15(17):3192. https://doi.org/10.3390/buildings15173192
Chicago/Turabian StyleYu, Lei, Qichang Fan, Dan Meng, Xue Meng, and Binghua Xu. 2025. "Application and Mechanism Study on Optimal Design of Cement-Based Building Materials Based on Polymer Binder" Buildings 15, no. 17: 3192. https://doi.org/10.3390/buildings15173192
APA StyleYu, L., Fan, Q., Meng, D., Meng, X., & Xu, B. (2025). Application and Mechanism Study on Optimal Design of Cement-Based Building Materials Based on Polymer Binder. Buildings, 15(17), 3192. https://doi.org/10.3390/buildings15173192




