Abstract
The most common processes used for the recovery of gold and silver from cyanide leachates are Merril-Crowe, activated carbon in pulp, and ion exchange resins; the process of electrocoagulation (EC) also is a promising new technique. EC is an electrochemical process whose mechanisms include oxidation, reduction, decomposition, deposition, coagulation, absorption, flotation, and precipitation. It has been used for the treatment of water and wastewater with different degrees of success. This study aimed to determine the kinetics of the reaction and the energy consumption at constant voltage, and at constant current using aluminum electrodes with two different distances between electrodes. EC was run in three stages for the removal of gold and silver from aqueous cyanide solutions from samples supplied by a Mexican mining company. Characterization of the sample showed initial concentrations of 49.48 and 383 mg/L of gold and silver, respectively. Results showed the effectiveness of the process by achieving removals up to 98.59% of gold and 99.43% of silver. Additionally, it was determined that the kinetics of the reaction is of zero order and that the lowest energy consumption can be achieved when working at constant voltage and with a separation of 0.8 cm between electrodes.
1. Introduction
The two conventional processes for the recovery of gold and silver from cyanide leachates are the activated carbon adsorption process and the Merril-Crowe process of cementation on zinc powder. In the carbon adsorption process, precious metals are adsorbed within the activated carbon granules; after they are adsorbed, gold is separated using a solution of caustic cyanide. This solution is taken to an electrolytic cell in which gold and silver are deposited on steel fiber cathodes. In the Merril-Crowe process, which requires basic condition of deoxygenation using a vacuum tower, the product is a filtered zinc precipitated powder. The cathode of the carbon adsorption process, also known as the precipitate of the Merril-Crowe process, is melted in combination with fluxes such as borax, nitrates, and silica. The product resulting from the melting process is the dore (bullion) of precious metals, which typically contain more than 97% of the targeted precious metals [1,2,3]. This work is important because electrocoagulation might provide a simple and economic alternative for the removal of silver and gold from cyanide solutions. The goals of this research are: (1) To determine the reaction order because EC is an electrochemical process that depends on thermodynamics and kinetics, and (2) To determine the values of the operational parameters that yield the lowest energy consumption because costs are a determinant for the evaluation of industrial processes.
1.1. Electrocoagulation (EC)
Electrochemical studies have demonstrate the relationship in the change of chemical and electric energy. EC can be considered as an accelerated corrosion process and has been used for both water and wastewater treatment. It is a complex process that involves many chemical and physical mechanisms operating synergistically to remove pollutants from water. EC generates coagulants in situ and it works through the interactions among electrochemistry, coagulation, and flotation [2,4,5].
EC induces an electric current either in the water or in the solution to be treated using metallic parallel plates of different materials as electrodes; the most commonly used electrodes are iron and aluminum [6]. EC occurs when the metallic ions Al3+ or Fe3+, which are formed from the anode oxidation, react with the OH- produced on the cathode from the reduction of H2O to H2. The formed hydroxides act as flocculants agents and adsorbents of the contaminant [7]. The hydrolyzed aluminum ions can form very long chains of Al-O-Al-OH, which chemically might adsorb a significant amount of contaminants. Some of the reactions for EC using aluminum electrodes are [5]:
When using iron electrodes, the Fe(OH)3 forms a red brown colloid; depending on pH and the availability of ferrous ions, it might react with the dissolved oxygen to form Fe3O4. Iron electrodes also form green rust, which are unstable compounds that contain a mix of ferrous and ferric hydroxides [8,9]. The formed hydroxides remove contaminants present in water by complexation or electrostatic attraction.
EC removal mechanisms include oxidation, reduction, coagulation, absorption, adsorption, precipitation, and flotation. The treated solution separates in a floating layer as well as a sediment rich in metals that can be separated using conventional methods [10]. Parameters that affect the EC process include applied voltage, current density, size, material, and the distance of separation among electrodes, reactor design, temperature (T), electrical conductivity (ec), residence time (tR), and flow type [7,8,9,10,11].
An earlier study used an aqueous cyanide solution with 200 mg/L of free CN, 1400 mg/L of total CN, 13.25 mg/L of gold, 1357 mg/L of silver, 1 gr/L of sodium chloride to increase its conductivity, and conditioned to a pH of 8. It was treated using EC with iron electrodes. After five minutes of treatment, gold and silver were reduced to 0.1 mg/L and 0.9 mg/L, respectively. Another project used a solution with 3400 mg/L of free CN and 7000 mg/L of total CN, with 63.5 mg/L of gold and 336.35 mg/L of silver. EC was conducted in three stages. A 23 experimental design was used with three factors: pH of solution, a type of acid for pH conditioning, and electrodes material; their respective levels (low–high) were: for pH; 7–8, for acid; sulfuric and hydrochloric, and for electrodes iron-aluminum, and the dependent variables were the removal of gold and silver. The highest values for removal were obtained with a pH of 8, using hydrochloric acid as pH conditioner in addition to aluminum electrodes. As a result of the high concentrations of cyanide, formation of Prussian Blue or iron (III) ferrocyanide (C18Fe7N18) occurred when using iron electrodes. Removal of gold and silver was achieved up to 98%; accordingly, it was decided to use the same parameters to conduct this study [10,11].
1.2. Kinetics and Reaction Rates
EC is an electrochemical process; it depends on thermodynamics and kinetics. Reaction rates of the chemical reactions are the field of study of chemical kinetics. Reaction rates are a function of temperature, pressure, and concentrations of the different species of the reaction; they might also depend on the concentration of other species such as catalysts and inhibitors that might appear or not in the global reaction. The reaction rate in EC depends on the dissolution of iron or aluminum and on the elimination of [H+] through H2 evolution. This reaction is fast for low pH values (strong acids); for a weak acid, the rate will depend on the , where is the acid dissociation constant.
The constant of the reaction rate can be determined from measurements of the concentration of the species [A] and time t. These two values are adjusted to the functional form of the integrated equation; in each case, if the dependent variable is chosen appropriately, the problem is reduced to a linear adjustment. The integrated rate reactions are: zero order, first order, and second order. The integrated rate equations are given by:
where is the concentration of reactant A at time t, is the initial concentration of reactant A, is the number of moles of A, and is the velocity constant [4,12,13].
Zero order reactions commonly occur when an adsorption process involved; such reactions occur most often when the reaction rate is determined by the available surface area. When a chemical reaction is of zero order in the concentration of a particular reactant, in this case gold or silver, it does not mean that the reactant is not participating in the reaction. It suggests that the initial concentration of that particular reactant does not contribute to how fast the reaction proceeds. Another factor controls how fast the reaction proceeds. This other factor might be another rate-limiting step in a complex reaction or a rate-limiting condition in an elementary reaction [14].
2. Experimental
2.1. Materials and Instruments
The following equipment, materials, and reagents were used. A Kaselco EC equipment with a maximum capacity of 50 VDC (Shinner, TX, USA), two Hanna Instruments Model HI 255 pH and conductivity meters (Carrollton, TX, USA), an Orion 420+ pH meter (Bellefonte, PA, USA), a Thermoline Cimarec 2 magnetic stirrer (Dubuque, IA, USA), a Novatech HS60-A10 oven, a Novatech CE-120BAE extraction hood (Kingwood, TX, USA), an A&D Company Limited HR-250A analytical balance (Yeongdeungpo-gu, Seoul, Korea), an SEM JEOL model JSM-6610LV (Akishima, Tokyo, Japan), an acquisition data system controlled by LabView 5 (Austin, TX, USA), tension and current Hall effect sensors, two aluminum electrodes 3 cm × 6 cm × 0.2 cm, two mercury thermometers Brannan, No. 40 Whatman filter papers of 10 cm, reagent grade hydrochloric acid, sodium hydroxide and buffers of pH 4, 7 and 10 (J.T. Baker). The experimental setup is shown in Figure 1.
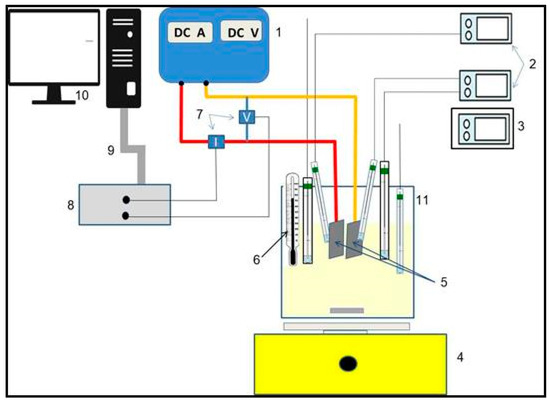
Figure 1.
Diagram of the experimental setup. 1. Kaselco EC Equipment 2. pH and conductivity meters, 3. pH meter 4. Magnetic stirrer 5. Al Electrodes 6. Thermometer 7. Hall effect sensors 8. Signal conditioning 9. Cable for data acquisition 10. Data acquisition system 11. Benchmark reactor.
2.2. Methods
Prior to the EC process, the cyanide solution of the industrial sample from a mining company was characterized to determine the initial concentrations of Au and Ag. The initial parameters were 49.48 and 383 mg/L for gold and silver, respectively, electrical conductivity of 58 mS, a temperature of 26 °C, and a pH of 11.81, which was conditioned to a pH of 8 using hydrochloric acid.
Four tests were run, denominated as P, Q, R, and T. Table 1 shows the operational parameters. Tests P and Q were run at constant current while tests R and T were held at constant voltage.

Table 1.
Operational parameters and conditions for the EC tests.
The tests were run in three EC stages of 10 min each using Al electrodes and reversing the polarity every minute to prevent polarization of the electrodes. In each stage, readings of pH were taken every minute in the vicinity of the anode and cathode, in the bulk solution. Temperature (T) and electrical conductivity (ec) were taken and recorded and a 10 mL sample was extracted. Samples were filtered to separate liquid and solid phases; both were sent to external laboratories to determine Au, Ag, free CN, and total CN concentrations. The first stage was initiated with 400 mL. At the end of the first step, liquid and solid phases were separated. Samples of both phases were analyzed. The second stage used the remaining 300 mL, and, correspondingly, 200 mL was used for the third stage. All of the tests were performed in the extraction hood and the produced gas was bubbled in a 10% NaOH solution to neutralize the hydrogen cyanide (HCN) produced.
Additionally, an Acquisition Data System was installed. It was programed to take 100 readings per second, to calculate the average behavior per minute of V and I, as well as to calculate the power and the energy consumption per liter. Using the average readings, electric power load was calculated in the three stages according to Equation (8).
where is the electric power load per volume in kW/m3, is the electric tension in volts, is the intensity of current in amperes, and is the volume of the solution in m3.
Energy consumption was calculated every minute for each stage of the EC process, and it is expressed as Equation (9).
where is the electric energy consumed per volume in kWh/m3, and are the final and initial time taken each minute.
3. Results and Discussion
The minute by minute gold and silver concentration results of the analyzed samples were graphed versus time for each stage. The rates of reaction for gold and silver were of zero order for each stage as seen in Figure 2 and Figure 3, indicating that it is an adsorption reaction and that the rate is constant. The slopes represent the constant (k) from Equation (5). Each stage shows a different velocity; however, if they are considered to be factors of an experimental design, there is no difference, suggesting that they are statistically equivalent. The same can be observed with silver.
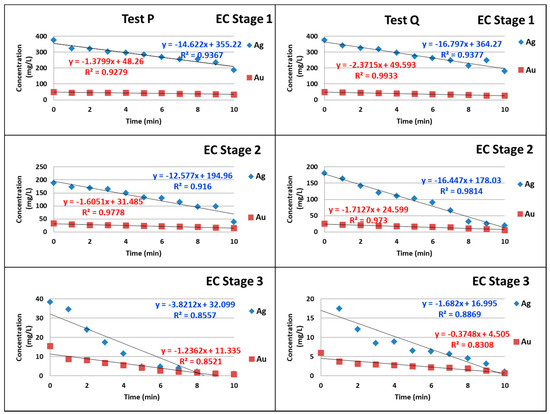
Figure 2.
Concentration versus time, equations, and R2 for tests P and Q, according to stage.
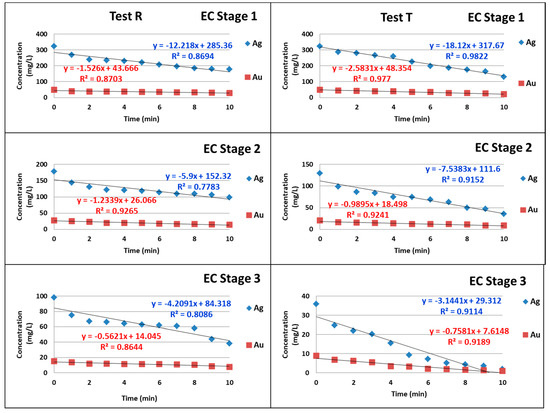
Figure 3.
Concentration versus time, equations, and R2 for tests R and T, according to stage.
The correlation coefficients (R2) for the rate of reaction (concentration versus time) have values between 0.8 and 0.99, with an average of 0.91.
Once the samples were characterized before and after EC, percentages of recovery of gold and silver were determined. Results obtained from the analyses and measurements are shown in Table 2, and in Figure 4 and Figure 5.

Table 2.
Parameters and results of tests P, Q, R, and T.
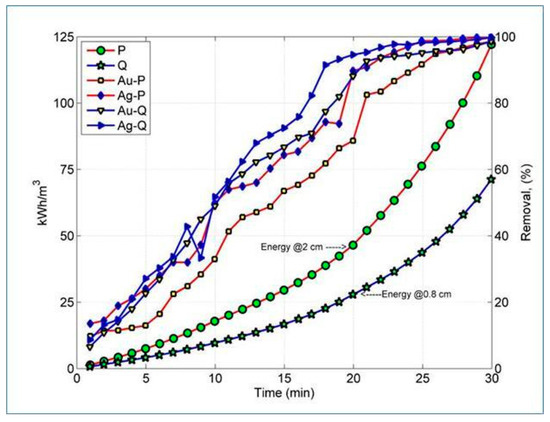
Figure 4.
Energy consumption and percent removal of metals versus tr, obtained in the tests at constant current.
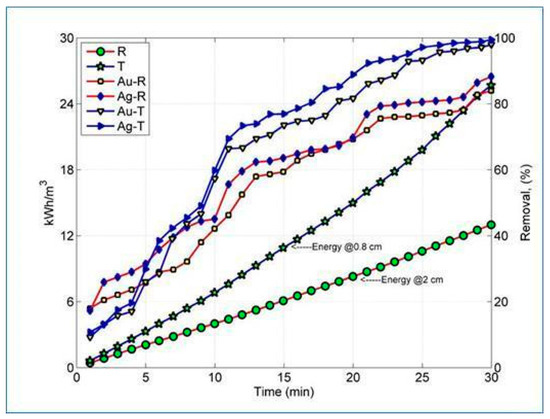
Figure 5.
Energy consumption and percent removal of metals versus tr obtained in the tests at constant voltage.
Au: The values for the recovery of gold were up to 99.43%, with 84% the lowest value achieved for test R. Test R exhibited less energy consumption in comparison with tests P, Q, and T which consumed seven, four, and two times more energy than test R, respectively. It would be beneficial to optimize parameters and conditions of test R in the future.
Ag: Similar to the recovery of gold, the maximum recovery value was of 99.73%. Test R exhibited a recovery value of 88.27% under the same considerations for the energy consumption as Au.
In the four tests, P, Q, R, and T, a decrease in conductivity was observed at an average rate of 36 mS due to the evolution of H2, neutralization of HCN, and precipitation of ions.
The weight loss in electrodes was significant but less in both test P and Q (constant V). The test that exhibited the least weight loss in electrodes was test R.
Parameters with a significant difference are:
Temperature: The average of the temperature increase by stages was 24.70, 15.37, 5.8 and 7.47 °C for tests P, Q, R, and T. The biggest increase was for test P (constant current and 2 cm of separation between electrodes), followed by test Q (constant current and 0.8 cm of separation). The smaller increments correspond to tests T and R, which were performed with a constant voltage. Test R exhibited the lowest temperature increase.
Energy consumption: The results are equivalent to the patterns exhibited in the temperature increase; this was expected because higher energy consumption leads to an increase in temperature. Energy consumption is higher when a constant current is used because Power = I2xR.
Test T gave the best results in recovery of gold and silver. Figure 6 shows the graph of test T, concentration of gold, silver, as well as free and total CN versus time. The four concentrations exhibit a similar pattern throughout the three stages of EC. One explanation as to why they are so related is that the CN also presents a zero order reaction.
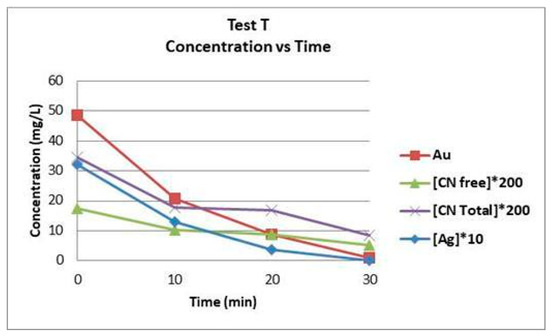
Figure 6.
Graph of concentration versus t, in test T.
The analyses of solid samples were carried out in a scanning electron microscope (SEM) to corroborate the concentration of gold and silver. Scanning Electron Microscope (SEM) images of test T, of gold and silver particles adsorbed in aluminum species are shown in Figure 7 and Figure 8. The spectrogram showed that the surface of the particles of the hydroxides of aluminum are covered by a layer of gold and silver, with concentrations in mass percentage of 14.6 for Au and 70.8 for Ag.
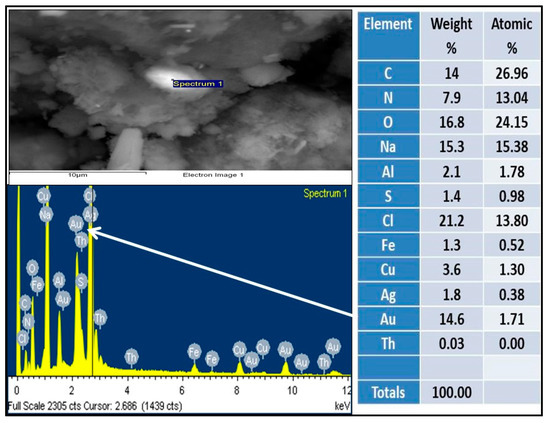
Figure 7.
Chemical composition of the solid product according to SEM, and presence of gold on the surface of aluminum hydroxide.
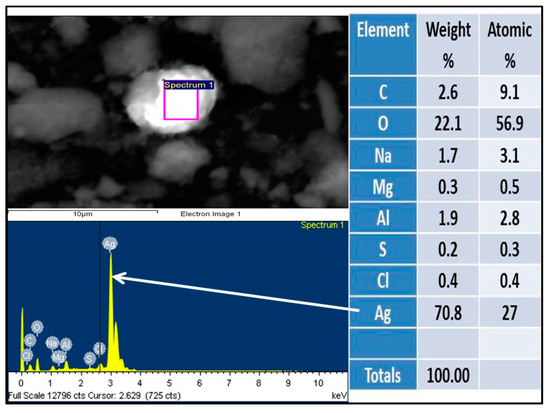
Figure 8.
Chemical composition of the solid product according to SEM, and presence of silver on the surface of aluminum hydroxide.
4. Conclusions
The rates of reaction of gold and silver removal by EC are of zero order.
The removal of gold and silver in cyanide solutions with EC in three stages using aluminum electrodes was determined to be very promising for solutions with high concentrations of cyanide.
The tests with constant current, P and Q, had elevated recoveries, 99.73% for silver and 98.59% for gold. However, they exhibited a high-energy consumption, largely attributable to the increase in resistance of the solution; they also presented a proportional increase in temperature.
The tests with constant voltage exhibit lower energy consumption, with removal rates of more than 98% in test T, with smaller distance between electrodes. Test R had percentages of removal between 84% and 88% and exhibited lower energy consumption, close to 50% when compared with test T. Future studies could optimize these conditions for test R, which could aid in determining if it is possible to increase the removal of gold and silver with a similar energy consumption.
Author Contributions
Conceptualization, C.G.-C., H.M.-C. and J.P.-T.; methodology, C.G.-C., H.M.-C., J.P.-T., and F.S.S.-S.; software, F.S.S.-S.; formal analysis, C.G.-C., H.M.-C. and F.S.S.-S.; investigation, C.G.-C., H.M.-C., J.P.-T., and F.S.S.-S.; writing—original draft preparation, C.G.-C. and H.M.-C.; writing—review and editing, C.G.-C. and H.M.-C.; supervision, C.G.-C., H.M.-C. and J.P.-T.
Funding
This research received no external funding.
Acknowledgments
To the Program of Enhancement of the Professorship (PRODEP) and to the TNM (Tecnológico Nacional de México), Technological Institute of Saltillo and Technological Institute of La Laguna for their support in scholarship, and for the use of facilities, laboratories, and equipment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Parga, J.R.; Cocke, D.L.; Valenzuela, J.L.; Gomes, J.A.; Kesmez, M.; Irwin, G.; Moreno, H.; Weir, M. Arsenic Removal Via EC from Heavy Metal Contaminated Groundwater in La Comarca Lagunera Mexico. J. Hazard. Mater. 2005, 124, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Parga, J.R.; Rodríguez, M.; Vazquez, V.; Valenzuela, J.L.; Moreno, H. Recovery of Silver and Gold from Cyanide Solution by Magnetic Species Formed in the Electrocoagulation Process. Miner. Procesing Extr. Metall. Rev. 2012, 33, 363–373. [Google Scholar] [CrossRef]
- Adams, M.D. Advances in Gold Ore Processing; Elsevier, B.V.: Amsterdam, The Netherlands, 2016; pp. 102–104. [Google Scholar]
- Logan, S.R. Fundamentos De Cinética Química; Addiso Wesley Iberoamericana: Boston, MA, USA, 2000; pp. 1–14. [Google Scholar]
- Moreno, C.H.A.; Cocke, D.L.; Gomes, J.A.G.; Morkovsky, P.; Parga, J.R.; Peterson, E.; Garcia, C. Electrochemical Reactions for Electrocoagulation Using Aluminum Electrodes; The Minerals, Metals & Materials Society: Warrendale, PA, USA, 2007; pp. 23–35. [Google Scholar]
- Arango Ruiz, A. La electrocoagulación: Una alternativa para el tratamiento de aguas residuales. In Revista Lasallista De Investigación; Corporación Universitaria Lasallista: Antioquia, Colombia, 2005; Volume 2, pp. 49–56. [Google Scholar]
- Cuicas, J.R.P.; Cuadra, R.L.A. Evaluación De La Electrocoagulación En El Tratamiento De Agua Potable; Química Viva Universidad de Buenos Aires: Buenos Aires, Argentina, 2017; Volume 16, pp. 56–69. [Google Scholar]
- Piña Soberanis, M.; Martín Domínguez, A.; González Ramírez, C.A.; Prieto García, F.; Guevara Lara, A.; García Espinoza, J.E. Revisión de Variables de Diseño y Condiciones de Operación en la Electrocoagulación. In Revista Mexicana de ingeniería química; Universidad Autónoma Metropolitana, Unidad Iztapalapa: Mexico City, Mexico, 2011; Volume 10, pp. 257–271. [Google Scholar]
- Moreno Casillas, H. Mechanisms, Chemical Pathways, and Optimization of Electrochemical Water Treatment by Electrocoagulation for Sustainable Water Utilization. Thesis, Lamar University, Beaumont, TX, USA, 2007. [Google Scholar]
- García Carrillo, M.C.; Parga Torres, J.R.; Moreno Casillas, H.A.; Paredes Soto, J. Determinación de los factores que influyen en la recuperación de oro y plata de soluciones del proceso de cianuración mediante electrocoagulación, aplicando diseño de experimentos 23. In Revista de Energía Química y Física; Ecorfan: Bolivia, South America, 2016; Volume 3, pp. 40–49. [Google Scholar]
- Parga Torres, J.R.; García Carrillo, M.C.; Valenzuela García, J.L.; Sánchez Valdéz, E.; Moreno Casillas, H.A. Adsorption of Gold and Silver on Magnetic Species Formed during the Electrocoagulation Process. In Revista Internacional de Investigación e Innovación Tecnológica; Centro Kappa de Conocimiento: Saltillo, Coah, Mexico, 2018; Volume 5, pp. 1–12. [Google Scholar]
- Smith, J.M. Ingeniería de la Cinética Química; Décimo tercera reimpresión; Compañía Editorial Continental: México, North America, 2001; pp. 26–28. [Google Scholar]
- Castellan Gilbert, W. Fisicoquímica, 2nd ed.; Addison-Weslwy Iberoamericana: Boston, MA, USA, 1987; pp. 841–855. [Google Scholar]
- Ray, M. How Can a Zero Order Reaction be Possible? How Can a Reaction Not Depend on Its Reactant? Available online: https://www.quora.com/How-can-a-zero-order-reaction-be-possible-How-can-a-reaction-not-depend-on-its-reactant (accessed on 24 June 2019).
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).