Abstract
A process for extracting vanadium from ammonia slag is proposed in this work, taking advantage of the nature of V5+ vanadate ions condensing into a solid phase around a pH of 2. The slag is a mixture of oxides of Ca (36.0 mass %), Si (28.4 mass %), Al (9.3 mass %), Fe (7.1 mass %), V (6.9 mass %), S (3.9 mass %), Na (2.7 mass %), Ni (2.5 mass %), Mg (1.3 mass %), and other (K, P, and Ti) (1.9 mass %). The difficulty with extraction originates from the oxyanionic character of the vanadate ions, leading to the formation of vanadate salts with the concomitant cations. In contrast, a stepwise-pH-control process that is proposed in this work is effective for separating vanadium from the slag, as (1) the Si component in the slag is filtered off as the residue at the initial leaching step; (2) Fe, Al, and other cations are precipitated as the vanadate salts at pH 6, leaving Ca2+(aq) in the solution; (3) the vanadate component is transferred to the liquid phase by dissolving the precipitate in a NaOH(aq) solution of pH 13, leaving Fe, Ti, and Ni ions in the solid phase; and finally, (4) the pH of the solution is adjusted to 2. The vanadium component is solidified as sodium vanadate and V2O5. The maximum yield of vanadium from the slag is evaluated as 80.7%, obtaining for NaV6O15 and V2O5 with a purity of 97 mass %.
1. Introduction
Vanadium is an important element in the steel and chemical industries as it has been used to strengthen steels or as catalysts to produce sulfuric acid [1]. A high-strength alloy of aluminum, vanadium, and titanium is emerging in the aerospace field [1,2], and vanadium-redox-flow batteries have attracted much attention due to their potential applications for large-scale energy storages [3].
Since no high-vanadium-content ore is commercially available, vanadium has usually been produced from secondary sources and industrial wastes, such as titaniferrous magnetites, concentrates, slags, fly ashes, and petroleum residues [1,4,5]. The salt-roasting technique is a major process for extracting vanadium from those sources, by which a vanadium source is heated with a sodium salt to form sodium metavanadate. This material is typically converted into ammonium metavanadate in an aqueous solution by adding ammonium ions to collect it as the precipitate. This technique is quite effective for mass production of vanadium, especially in a large facility, but it is not suitable for small-to-middle-scale plants, because it requires kilns that can bear high-temperature treatment (e.g., 800–1230 °C) [2,6]. Energy consumption and toxic-gas emission during high-temperature treatments are also problematic [7,8].
Direct-leaching techniques have been investigated to collect vanadium under moderate conditions. The primary problem with direct leaching is the existence of concomitant cations, which makes the selective collection of vanadium difficult. The secondary sources and industrial wastes contain a range of metals such as Fe, Al, Ca, and Mg and they are usually extracted in the leachant, together with vanadium. The effectiveness of using organic extractants [9,10,11,12,13,14,15] and ion-exchanging resins [16,17,18,19,20,21] to collect vanadium from the extracted solutions has been examined.
A Japanese ammonia manufacture, for example, discharges a few thousand tons of ammonia slag every year. The slag typically contains up to 5–10 mass % vanadium as oxides, although it has not been used as a vanadium source because this content is relatively low. The concentration does not merit the costs that are involved in conventional vanadium-extraction processes, and therefore, the slag is wasted by solidifying it in cement.
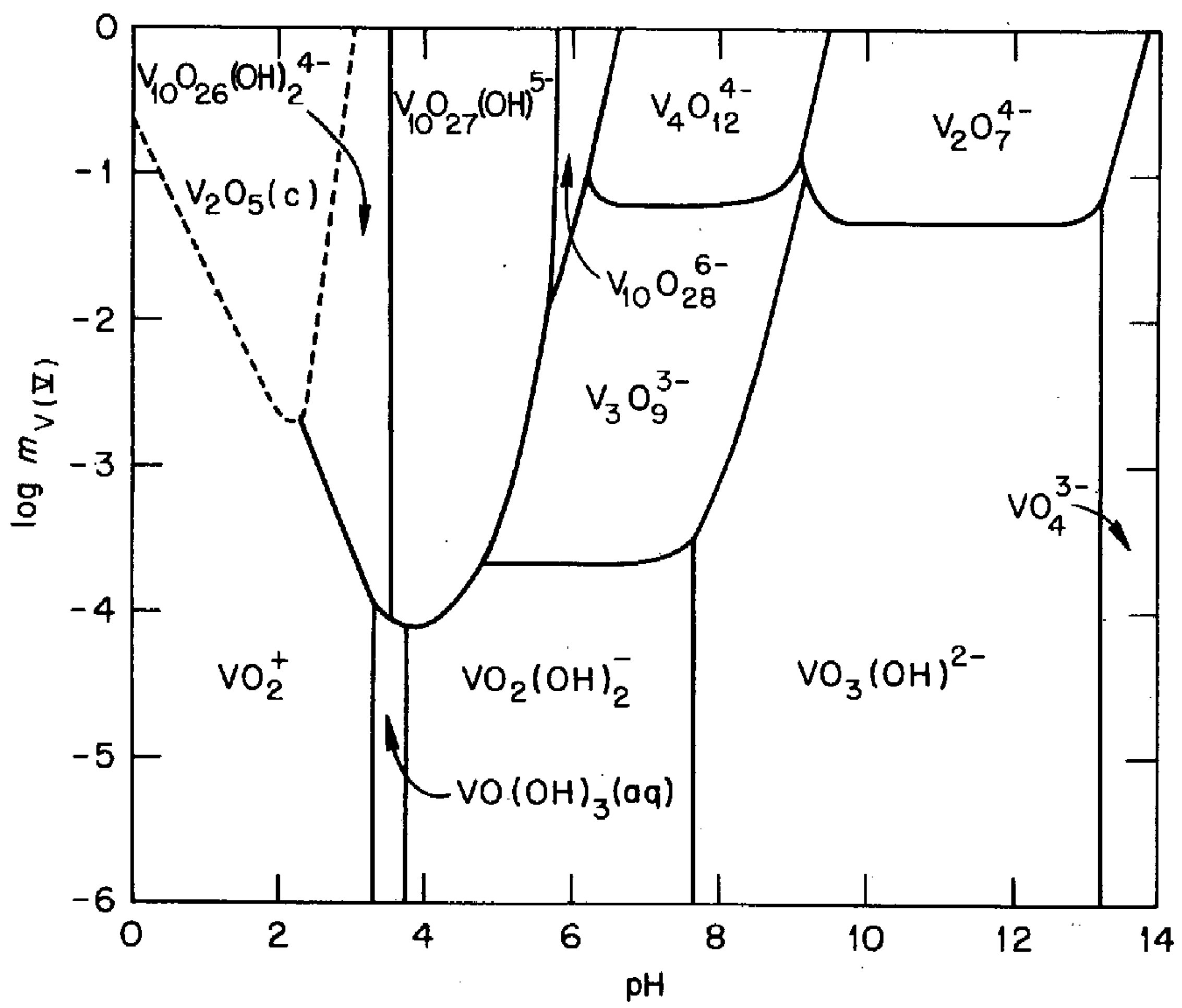
This work proposes a technique for collecting vanadium from ammonia slag. The process was based on an aqueous solution, which is beneficial from the environmental and economic points of view. The concept is explained with Figure 1, which shows an equilibrium diagram of V5+ vanadate ions in an aqueous solution [22]. The key points are as follows: (1) several types of vanadate ions are formed in an aqueous solution depending on the pH and the concentration; (2) they are stably soluble over a wide pH region; and, (3) solid-state V2O5 is condensed around pH 2 at relatively higher concentrations. The latter two points are contrasted with the nature of a typical cation being dissolved in an acidic solution and precipitating as hydroxide in the higher-pH region. One might suppose that the concomitant cations can be separated by raising the pH once to precipitate hydroxides and then lowering it to pH 2 to solidify the vanadate compounds simply lead to separation of vanadium from a solution containing several cations; however, in our previous paper [23], we reported that the oxyanionic character of the vanadate ions leads to the formation of vanadate salts, interfering with the selective collection of vanadium through a simple pH change. This paper builds upon [23] to establish a process for extracting vanadium from the slag by revealing the effects of the concomitant cations crucially influencing the formation behaviors of vanadate salts and the role of the each step in separating those cations.
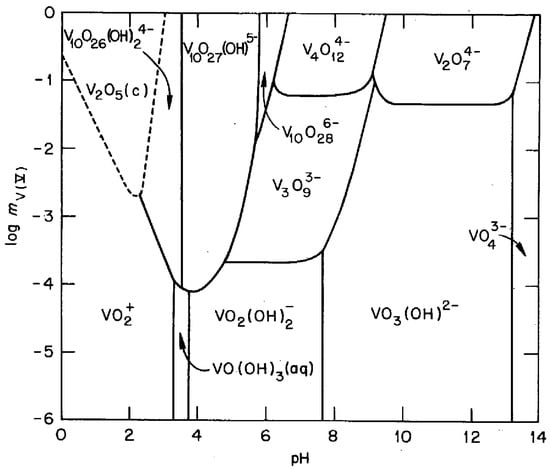
Figure 1.
Predominance diagram for V5+–OH− species at 25 °C [22]. The solid lines represent conditions under which the predominant species in adjacent regions contain equal amounts of V5+. The dashed lines represent the solubility of V2O5 in terms of the V5+ concentration. mV(V): moles of V5+ per kg of water. (Reprinted with permission from “The Hydrolysis of Cations”, Copyright (1976) John Wiley & Sons).
2. Experimental Section
2.1. Chemical Composition of the Ammonia Slag
The ammonia slag was offered from a Japanese ammonia manufacturer in Yamaguchi Prefecture. The slag is a mixture of oxides (and probably sulfides) of several metals. The chemical composition of the ash content excluding oxygen is shown in Table 1. The concentrations were evaluated by X-ray fluorescence spectroscopy (XRF) (EDXL 300, Rigaku, Tokyo, Japan), and they are shown as mass-percentages in elemental form. The examined slag contained Ca (36.0 mass %), Si (28.4 mass %), Al (9.3 mass %), Fe (7.1 mass %), V (6.9 mass %), S (3.9 mass %), Na (2.7 mass %), Ni (2.5 mass %), Mg (1.3 mass %), and other (K, P, and Ti) (1.9 mass %). Ca originated in CaCO3, which is an additive that acted as a melting-point depressant for coke ash. The other components were from the coke used to produce hydrogen for ammonia production via the water gas reaction. The composition of the slag was somewhat different from that used in our previous paper, and this difference was due to deviation of the usual nonuniformity of the composition of the industrial-waste mixture.

Table 1.
Chemical composition of the ash content of ammonia slag.
2.2. Extraction Procedure
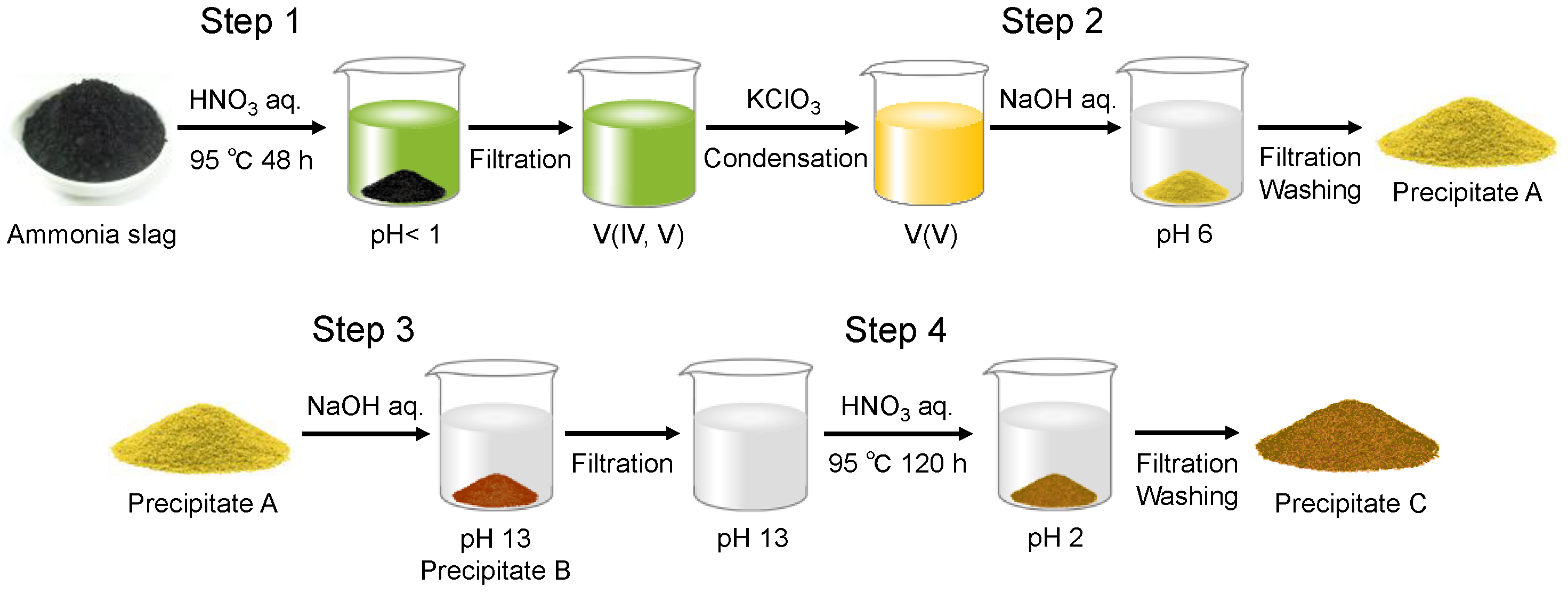
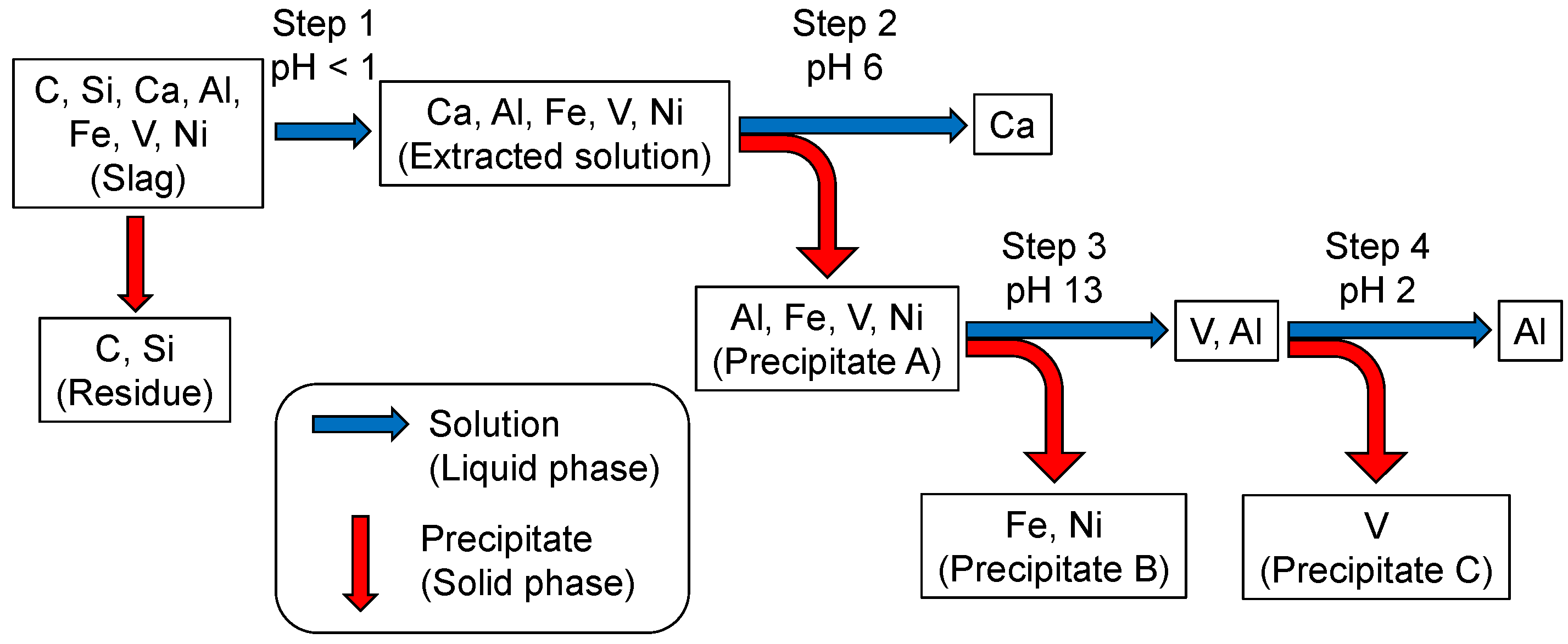
The optimized extraction process for collecting vanadium is shown in Scheme 1. The ammonia slag was dried at 95 °C on a hot plate and ground in an alumina mortar. A given amount of the slag was weighed for the extraction experiment. Typically, 2.0 g of the ammonia slag was treated in 50 mL HNO3(aq) (1.3 mol/L) in an autoclave at 95 °C for 48 h. After the insoluble residue was filtered off, 0.10 g of KClO3 was added to the filtrate to oxidize V4+ to V5+ [23], which led the solution to change the color from green to yellow. The solution was condensed on a hot plate at 95 °C to halve its volume. Then, the solution’s pH was adjusted to 6 using NaOH(aq) (15 mol/L) to form the precipitate (Precipitate A in Scheme 1). After being separated from the solution by filtration, Precipitate A was treated with 30 mL NaOH(aq) (1.5 mol/L). After stirring for 1 h, the insoluble residue (Precipitate B in Scheme 1) was filtered off from the solution. As discussed later in detail, the vanadium component was precipitated as Precipitate A at Step 2, and was transferred back to the liquid phase by treatment with NaOH(aq) at Step 3. Then, the pH of the filtrate was adjusted to 2 by adding HNO3(aq) (13 mol/L) to condense the vanadate ions into the solid phase (Precipitate C) in the solution during aging at 95 °C for 120 h.
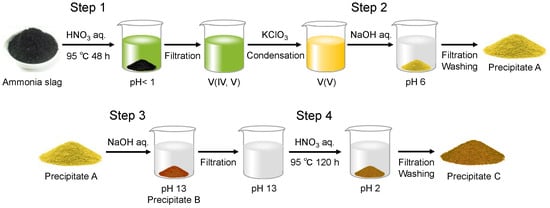
Scheme 1.
Procedure for extracting vanadium from ammonia slag by pH control.
The chemical reagents used in this work are shown in Table 2.

Table 2.
Chemical reagents used in this work.
The control experiments were carried out using the chemical reagents V2O5, Fe(NO3)3∙9H2O, Al(NO3)3∙9H2O, and CaCO3 (Table 2) to examine the individual effects of iron, aluminum, and calcium ions coexisting. Fe3+, Al3+, or Ca2+ were added to a 50 mL HNO3(aq) (mol/L) solution, dissolving 0.3 g of V2O5 at the molar ratio that was determined by XRF. The precipitating behaviors were investigated at several pHs, and the precipitates were heat-treated at 700 °C to improve the crystallinity to allow for the identification of the crystallographic phases by X-ray diffraction (XRD) (Rigaku, Tokyo, Japan).
2.3. Instruments
The concentrations of vanadate ions in the solution were determined using ultraviolet-visible-ray (UV-Vis) spectroscopy (PD-303, APEL, Saitama, Japan) with an absorbance curve calibrated with reference solutions prepared at several concentrations. (COOH)2∙2H2O was used as a reduction agent from V5+ to V4+ to use the absorption of VO2+(aq) at 760 nm to determine the concentration [23].
XRD (MiniFlex600, Rigaku, Tokyo, Japan) with Cu Kα radiation was used to identify the compounds with the samples being heat-treated at elevated temperatures to improve the crystallinity beforehand because they did not necessarily exhibit appreciable diffraction peaks in their as-prepared conditions. The compositions were analyzed with energy-dispersive X-ray spectroscopy (EDS) (Link ISIS, Oxford Instruments, Oxon, UK) attached to scanning electronic microscopy (SEM) (JSM-T330, JEOL, Tokyo, Japan) at an accelerating voltage of 20 kV with a working distance of 20 mm, for which the powder samples were dried at 300 °C in air and were pressed into compacts with a small amount of polyvinyl alcohol (PVA) (Kuraray Poval PVA-217) as a binder. The carbon coating was vacuum-evaporated onto the pellet samples before SEM-EDS and a Co foil was used as a calibration standard.
3. Results and Discussion
3.1. Extraction Process from the Slag (Step 1 in Scheme 1)
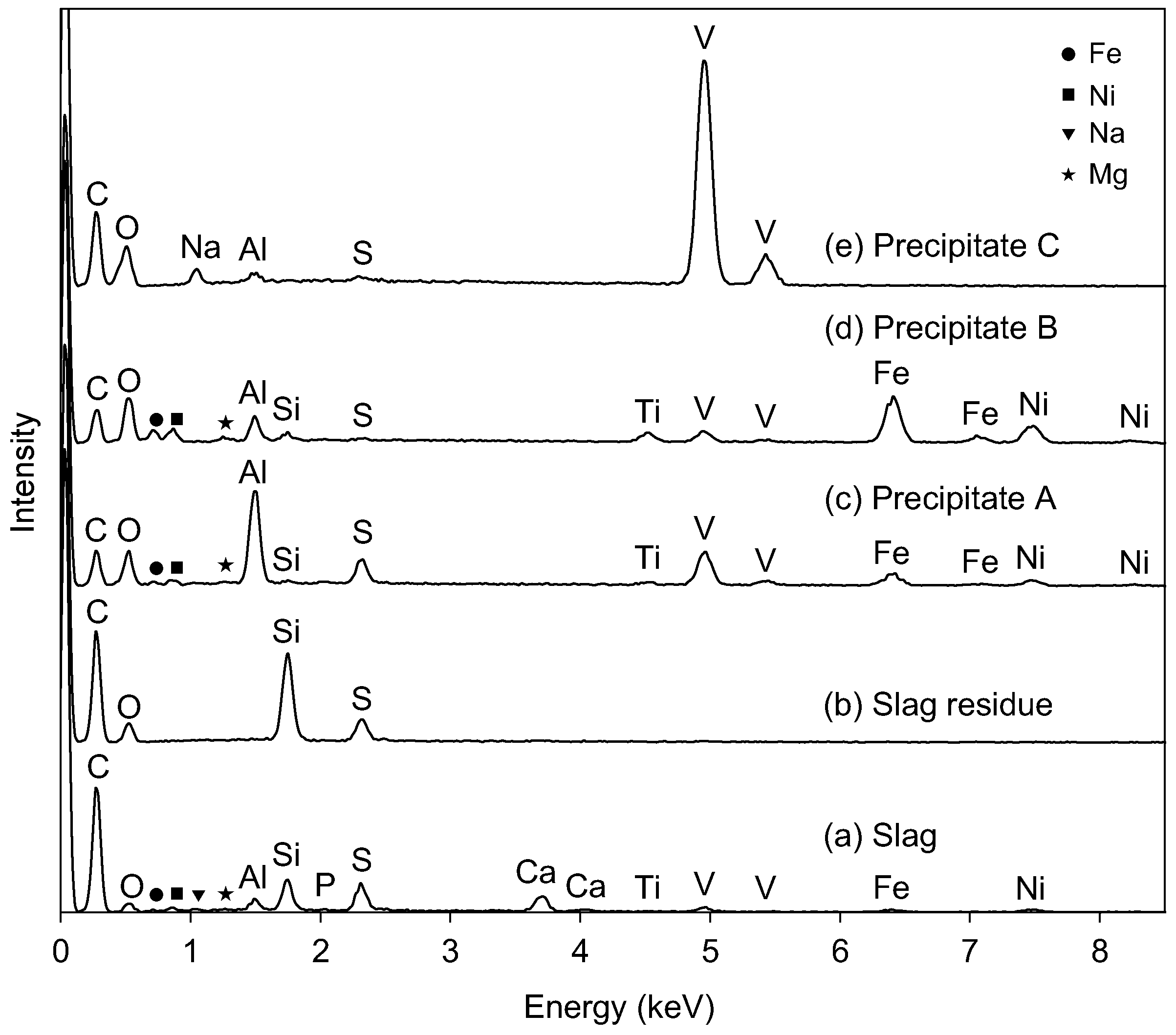
Figure 2 compares the EDS spectra of the starting slag and the precipitates that are formed at the subsequent extraction steps. EDS detected 12 elements in the slag (Figure 2a) other than vanadium, which was consistent with the elemental analysis using XRF (Table 1). The slag was mainly composed of Si, Ca, Al, V, and Fe. The carbon peak was attributed to the residual coke in the slag and to carbon coating for the SEM-EDS experiment.
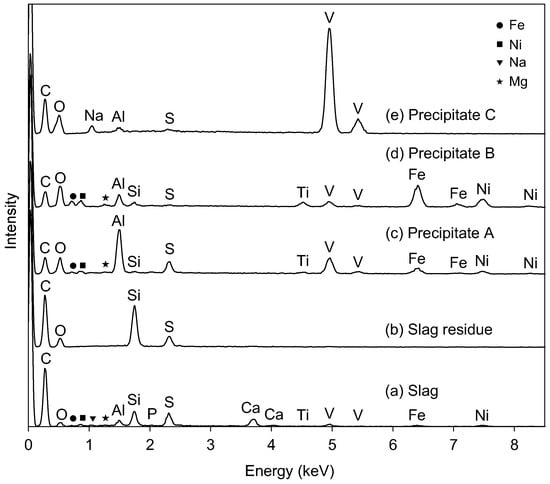
Figure 2b shows the EDS spectrum of the residue after the extraction by nitric acid at Step 1. The residue was composed of C, O, Si, and S. The peaks of Ca, V, and Fe were clearly weakened, indicating that they were effectively transferred into the filtrate from the slag.
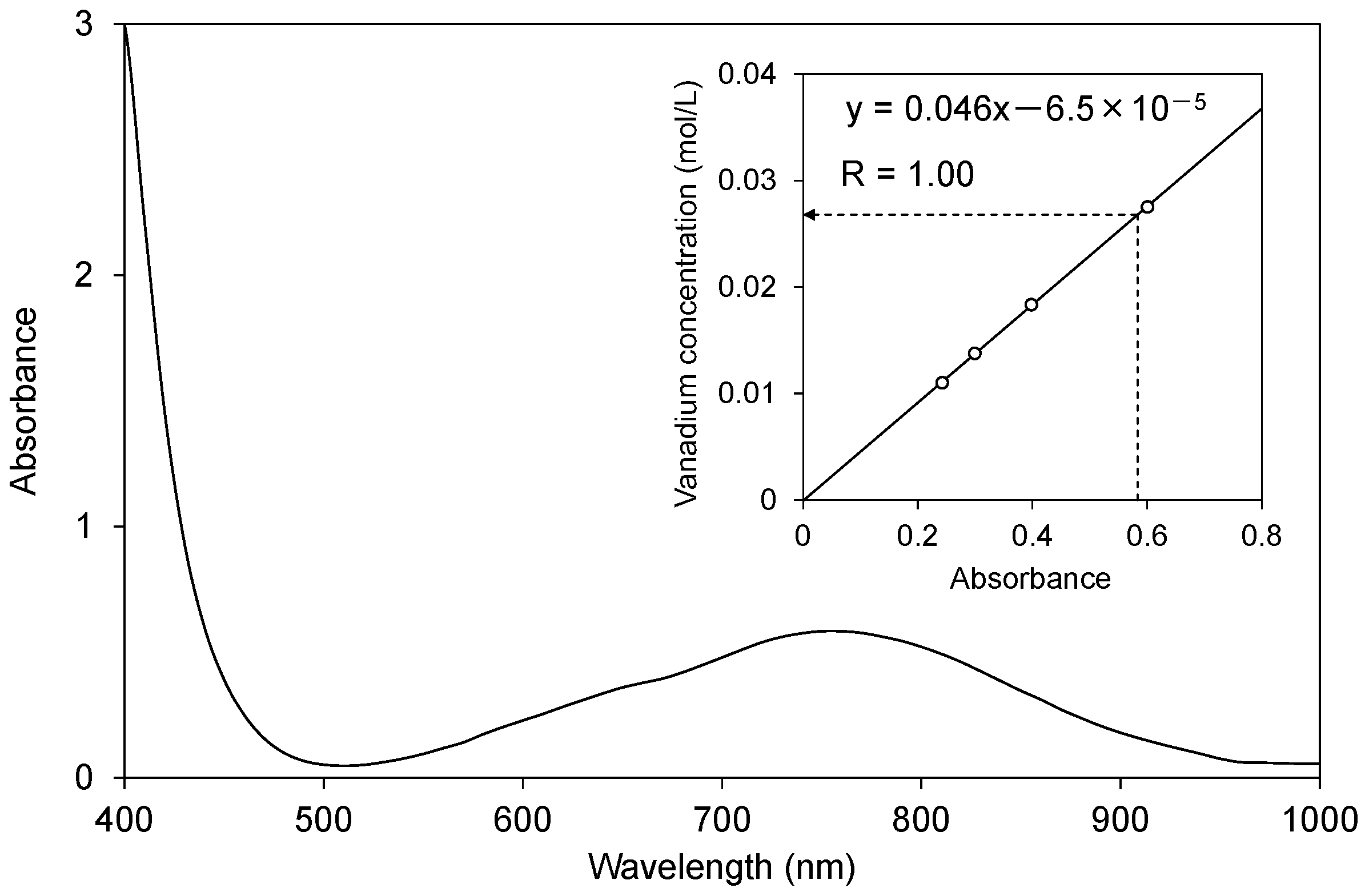
Figure 3 shows the absorption spectrum of the extracted solution at Step 1 after the addition of (COOH)2∙2H2O to reduce V5+ vanadate ions to VO2+(aq), which exhibits a broad absorption peak at around 760 nm. The inset shows the calibration curve, indicating an absorbance proportional to the VO2+(aq) concentration with a correlation coefficient of R = 1.00. The extraction yield from the slag to the solution at this step was estimated to be above 97%, based on the vanadium content in the slag and the VO2+(aq) concentration in the solution.
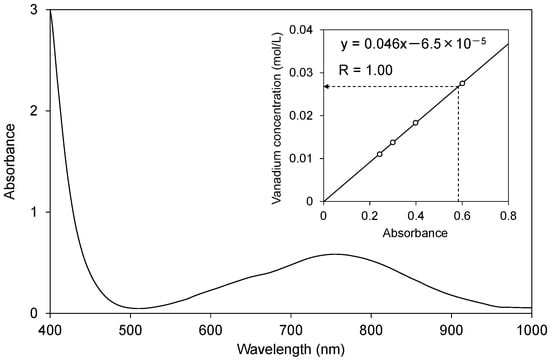
Figure 3.
Ultraviolet-visible-ray (UV-Vis) spectrum of the extracted solution. The vanadate species in the solution were reduced to VO2+ by (COOH)2∙2H2O so that the concentration of VO2+(aq) could be determined by the absorption at 760 nm. The inset shows the calibration curve drawn with the prepared solutions at several concentrations.
3.2. Precipitation of Aluminum Vanadate and Iron Vanadate (Step 2)
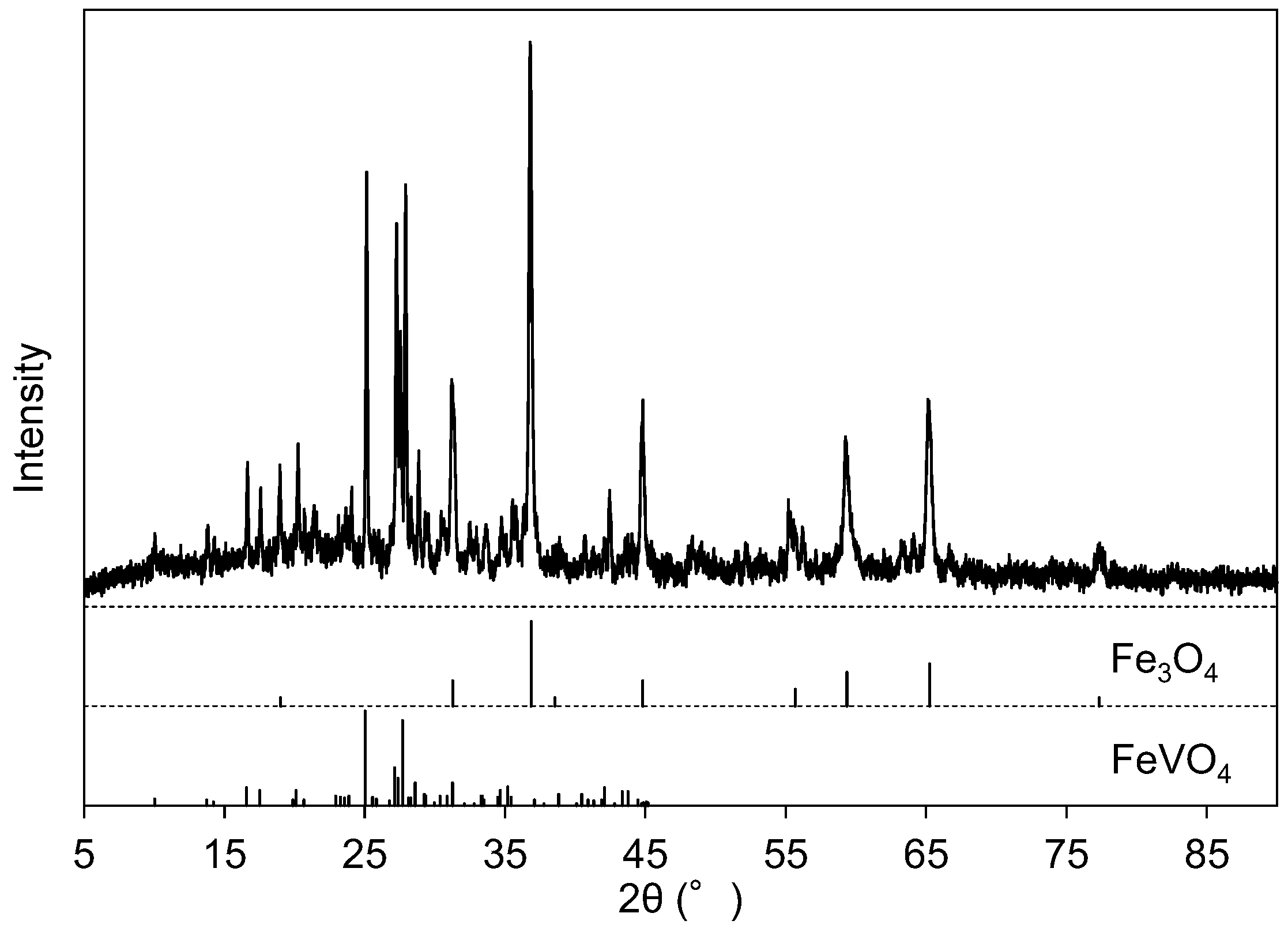
The EDS spectrum of Precipitate A is shown in Figure 2c, where Precipitate A was collected at Step 2 from the solution at pH 6. Figure 2c indicates that Precipitate A was mainly composed of aluminum, vanadium, and iron. The crystallographic phases of Precipitate A were identified as Fe3O4 (ICDD #26-1136) and FeVO4 (ICDD #38-1372) (Figure 4). Although vanadate ions are stably soluble in this pH region (Figure 1), the vanadium component precipitated as vanadate salts with Fe and Al (FeVO4 and AlVO4) at this step. Since an Al-related crystallographic phase was not observed in Figure 4, the Al component was considered to exist as amorphous or to be incorporated into FeVO4. Fe3O4 originated from the conversion of iron hydroxide into Fe3O4 during heat treatment for XRD. The absence of a Ca peak at 3.69 keV in EDS is characteristic of Precipitate A, indicating that Ca2+(aq) was left in the solution. The solution after the filtration was colorless.
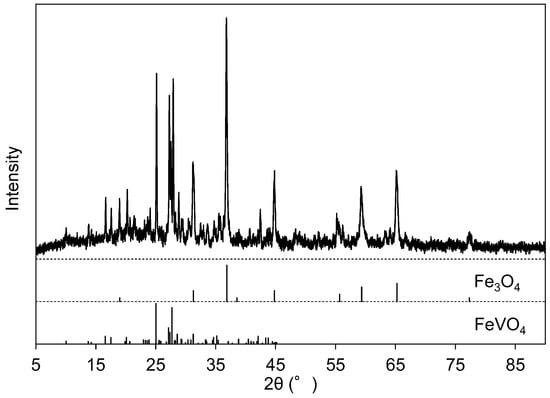
Figure 4.
X-ray diffraction (XRD) pattern of Precipitate A after the heat treatment at 700 °C. The line diagram below the pattern indicates the peak positions of Fe3O4 (ICDD #26-1136) and FeVO4 (ICDD #38-1372).
3.3. Separation of Vanadium from Fe and Al (Step 3)
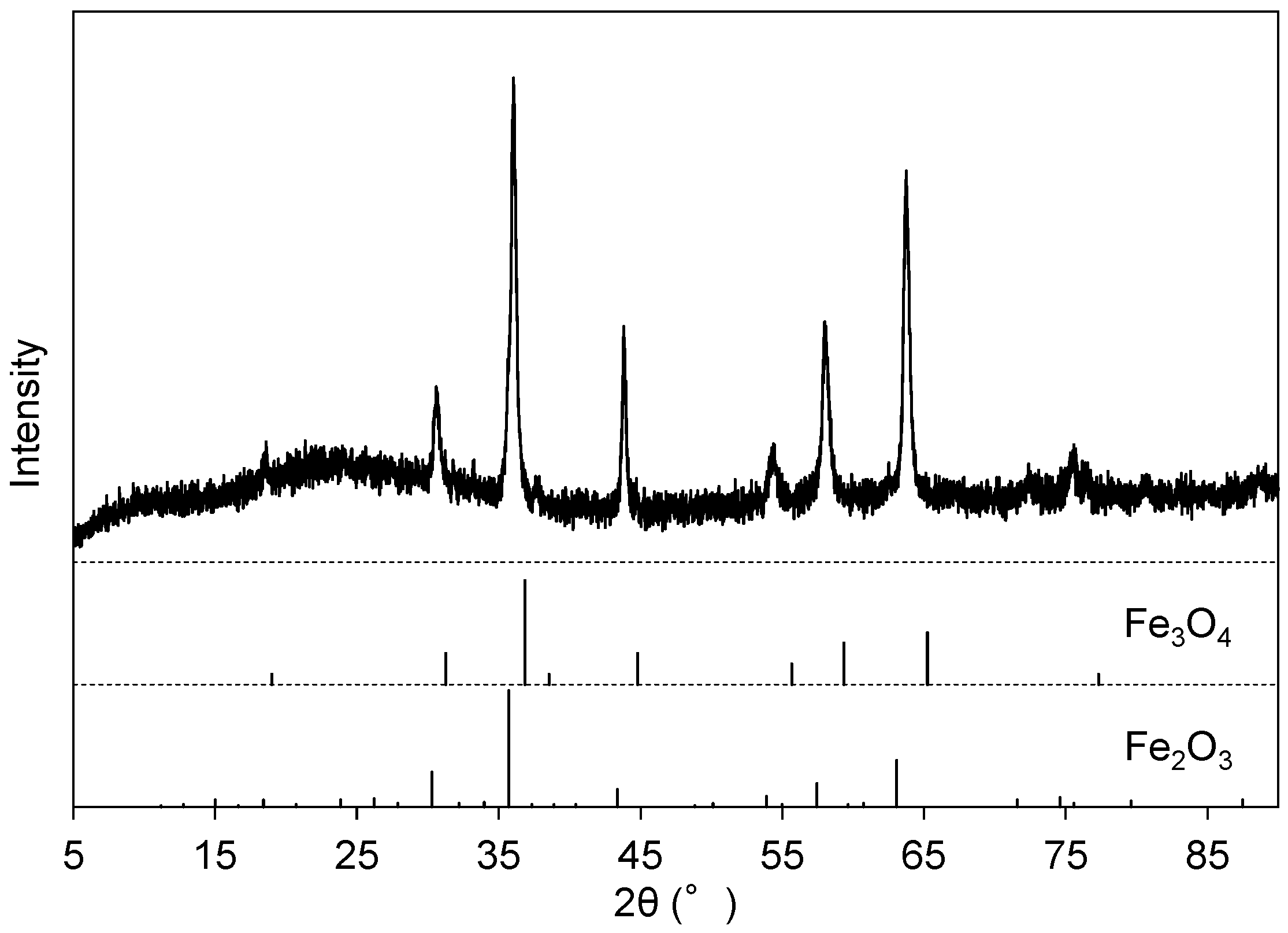
Figure 2d presents the EDS spectrum of Precipitate B, which was the residue at pH 13 after Precipitate A was dissolved in NaOH(aq). The peak intensities of Fe, Ni, and Ti increased after this step relative to Precipitate A (Figure 2d). The vanadate compound was preferentially dissolved into the solution from Precipitate A, leaving the Fe, Ni, and Ti components in Precipitate B. The XRD peaks of Precipitate B after heat treatment at 700 °C (Figure 5) were attributed to Fe2O3 (ICDD #33-664) and Fe3O4 (ICDD #26-1136), and the main component of the residue (Precipitate B) was assumed to be iron hydroxide with impurities of Ni and Ti. The Al component was not effectively separated from V at this step as the peak intensity of Al decreased from Figure 2c to Figure 2d. The peaks of V were still recognized in EDS (Figure 2d), and vanadium partly remained in Precipitate B.
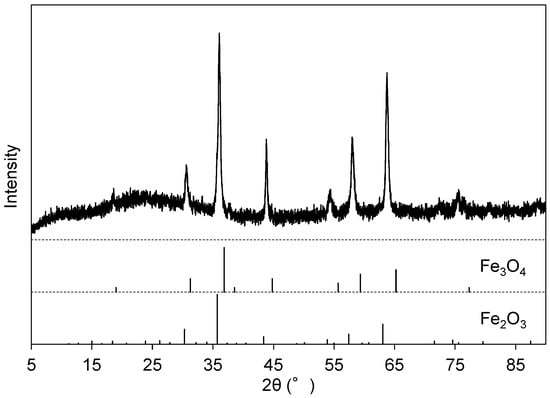
Figure 5.
XRD pattern of Precipitate B after heat treatment at 700 °C for 1 h.
3.4. Precipitation of Vanadate Compounds (Step 4)
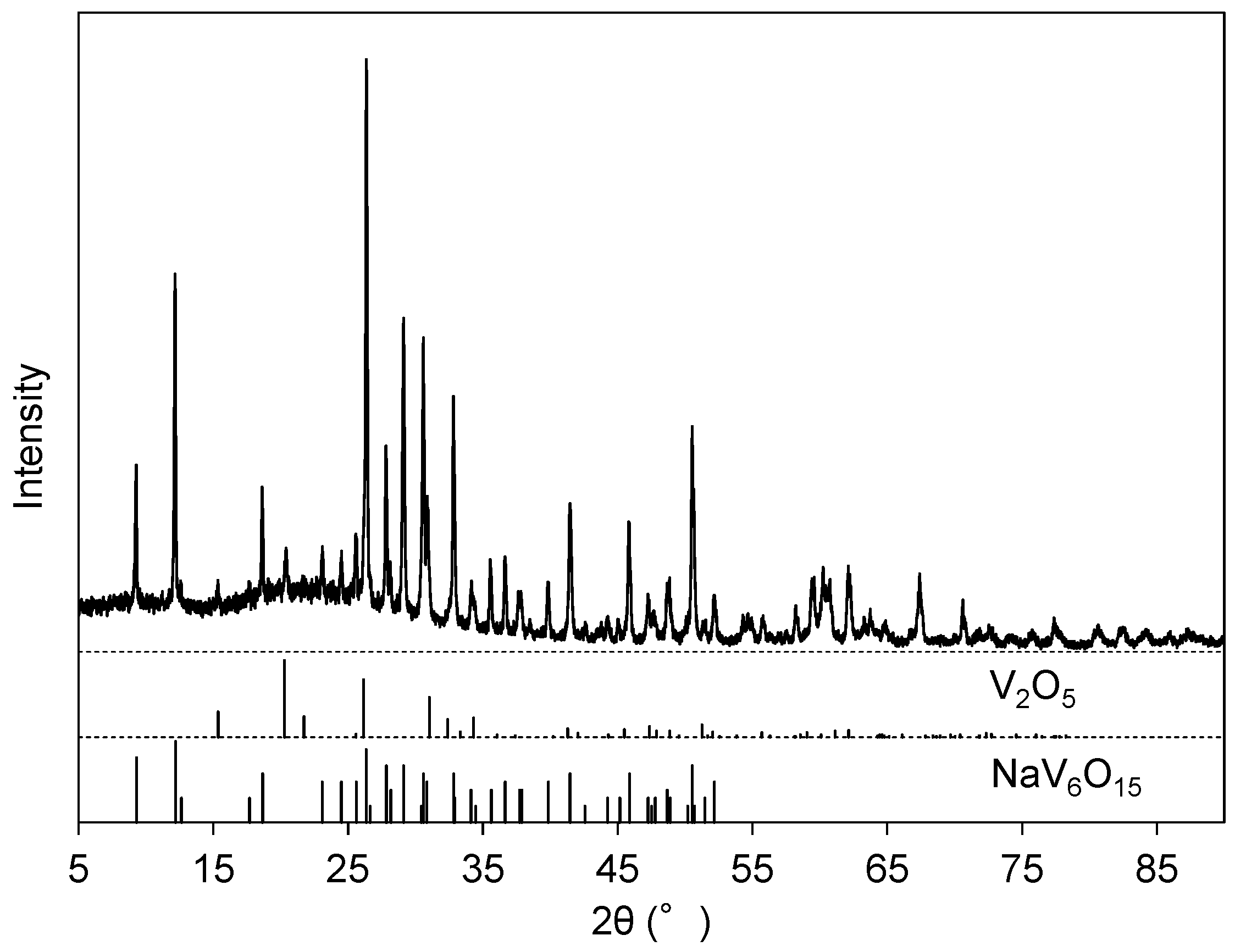
The pH of the filtrated solution at Step 3 was adjusted to 2 with HNO3(aq). A reddish-brown precipitate (Precipitate C) was obtained during aging at 95 °C. The EDS (Figure 2e) revealed that the main component of Precipitate C was vanadium with minor impurities of Na, Al, and S. Quantitative analysis by EDS gave a molar ratio of V:Na = 7.7:1, which is consistent with the observation of NaV6O15 (ICDD #24-1155) and V2O5 (ICDD #41-1426) in XRD for Precipitate C (Figure 6).
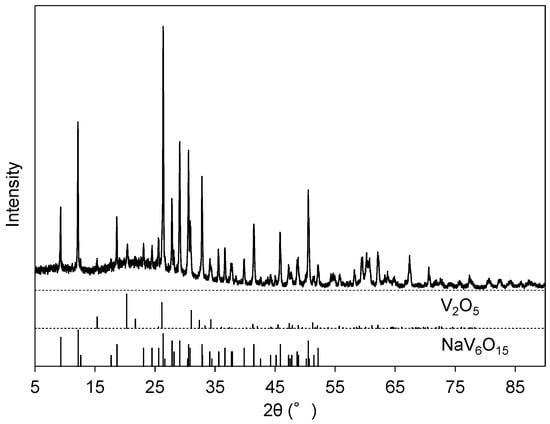
Figure 6.
XRD pattern of Precipitate C after heat treatment at 500 °C for 1 h.
Sodium vanadates are used together with vanadium oxides as raw materials in vanadium metallurgy [2], and therefore, Precipitate C can be regarded as a goal of our extraction process. The yield of vanadium from the slag was evaluated at 80.7% based on the vanadium content in the slag and the mass of obtained Precipitate C as a mixture of NaV6O15 and V2O5 at molar ratio V:Na = 7.7:1. The estimated content of the vanadate compounds (NaV6O15 and V2O5) was 97 mass % in Precipitate C.
The Al component that was dissolved in the NaOH(aq) solution together with V at Step 3 was separated at Step 4 by leaving it as Al3+(aq) in the solution at pH 2. Contamination by Al was recognized in Precipitate C in Figure 2e and lowering the pH below 2 could suppress it to some degree, but reduce the V yield instead.
3.5. Control Experiments with Chemical Reagents and Mechanisms for Vanadium Extraction
Several cationic species are contained in the slag, as shown in Table 1, and this makes it difficult to specify reactions that occur at Steps 2, 3, and 4. Then, the control experiments were carried out using chemical reagents for each pair of V and Fe, Al, or Ca (Table 3), where a chemical reagent of V2O5 was dissolved in HNO3(aq) and Fe(NO3)3∙9H2O, Al(NO3)3∙9H2O, or CaCO3 was added to the solution. The solution’s pH was initially lower than 1. This was then adjusted to 2, 6, or 13 with NaOH(aq) to collect the precipitate at each pH. The precipitates were heat-treated at 700 °C for 1 h in order to improve the crystallinity prior to XRD.

Table 3.
Crystallographic phases identified in the precipitates at pH 2, 6, and 13 with combinations of vanadate ions and (a) Fe3+, (b) Al3+, or (c) Ca2+.
As shown in Figure 1, vanadate ions themselves are stably soluble over a wide-pH region, except around pH 2. On the other hand, the coexistence of Fe3+(aq) led to the insolubility of iron vanadate, with FeVO4 being collected at pH 2. The precipitate at pH 6 was composed of FeVO4 and Fe2O3, indicating that Fe3+ precipitated as vanadate and hydroxide until pH 6. On the other hand, the main phase at pH 13 became Fe2O3, and therefore, the vanadium component was dissolved back into the liquid phase from FeVO4 in the higher-pH region.
Al3+(aq) did not form a vanadate salt at lower pH as the precipitate at pH 2 was V2O5 and NaV6O15, with sodium coming from NaOH to adjust the pH. The AlVO4 that was recognized in the product at pH 6 was dissolved again at higher pH with no precipitate at pH 13. Ca2+(aq) did not interact with the vanadate ions at a pH of 6 or lower. Ca10V6O25 and CaO were recognized in the precipitate at pH 13, where CaO resulted from Ca(OH)2 during the heat treatment for XRD.
The above results reveal rather complicated correlations between the vanadate ions and Fe3+, Na+, Al3+, and Ca2+, depending on the solution’s pH. FeVO4 is more favorably formed in the lower-pH region around 2, although Na+ also tends to form the vanadate salt NaV6O15. AlVO4 precipitates near the neutral-pH region when the solution’s pH is raised. These vanadates (FeVO4, NaV6O15, and AlVO4) are dissolved in the NaOH(aq) solution, whereas calcium vanadate stably exists in the higher-pH region even up to pH 13.
Scheme 2 summarizes the vanadium-condensation procedure from the slag through Scheme 1. Si is left in the residue during the extraction at Step 1. Precipitate A at Step 2 is composed of iron and aluminum vanadates with minor metals (Ni, Ti, etc.) and Ca2+ are separated at this step, being left in the solution. The vanadate compounds are dissolved in the solution by raising its pH, leaving Fe and other metals, such as Ni and Ti in the solid phase, as Precipitate B. The vanadate component is condensed from the solution into the solid phase (NaV6O15, V2O5, etc.) during aging at pH 2, leaving Al3+(aq) in the solution.
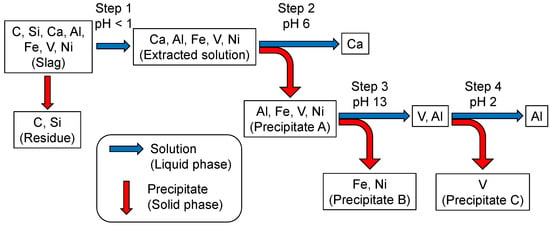
Scheme 2.
Major streams of elemental transfers of cations in the slag during the extraction process of this work. The contamination by impurities is ignored.
4. Conclusions
The extraction of vanadium from the ammonia slag was demonstrated by pH control based on the aqueous solution near ambient conditions (≤95 °C). The effects of the concomitant cations of Fe3+, Al3+, and Ca2+ were investigated on the precipitation behaviors of the vanadate salts were investigated. Fe3+ and Al3+ brought the vanadate salts up to a pH of 6. The vanadate components in these salts were dissolved in the NaOH(aq) solution until a pH of 13, which left Fe3+ in the insoluble residue. The vanadate component was separated from the Al component at pH 2 by forming solid-state NaV6O15 and V2O5 during aging. The maximum yield of vanadium was estimated to be 80.7%, obtaining for NaV6O15 and V2O5 with a purity of 97 mass %.
Author Contributions
Conceptualization, H.T. and Y.M.; Funding acquisition, H.T. and Y.M.; Investigation, H.T., W.F. and H.S.; Methodology, H.T. and Y.M.; Project administration, Y.M.; Supervision, Y.M.; Validation, H.T., H.S., T.Y. and Y.M.; Visualization, H.T.; Writing—original draft, H.T.; Writing—review & editing, H.T., T.Y. and Y.M.
Funding
This work is financially supported in part by Sasakawa Scientific Research Grant from The Japan Science Society (28-216).
Acknowledgments
The authors thank A. Sasaki and S. Mizunuma in Yamagata University for the XRF measurements. Poval PVA-217 used as a binder was offered by Kuraray co., ltd. The authors would like to thank Enago (www.enago.jp) for the English language review. The authors thank John Wiley & Sons Ltd. for permission to reuse Figure 1, which originally appears as Figure 10.7 in "The Hydrolysis of Cations (Charles Frederick Baes, Robert Eugene Messmer) (1976)".
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Moskalyk, R.R.; Alfantazi, A.M. Processing of vanadium: a review. Miner. Eng. 2003, 16, 793–805. [Google Scholar] [CrossRef]
- Ye, G. Recovery of Vanadium from LD slag, a State of the Art Report, Part 1—Facts and Metallurgy of Vanadium (JK Report 88031); Stalkretsoppet D816; Jernkontorets Forskning: Stockholm, Sweden, 2006. [Google Scholar]
- Parasuraman, A.; Lim, T.M.; Menictas, C.; Kazacos, M.S. Review of material research and development for vanadium redox flow battery applications. Electrochim. Acta 2013, 101, 27–40. [Google Scholar] [CrossRef]
- Shahnazi, A.; Rashchi, F.; Vahidi, E. A kinetics study on the hydrometallurgical recovery of vanadium from LD converter slag in alkaline media. In T.T. Chen Honorary Symposium on Hydrometallurgy, Electrometallurgy and Materials Characterization; Wang, S., Dutrizac, J.E., Free, M.L., Hwang, J.Y., Kim, D., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 707–715. [Google Scholar]
- USGS Minerals Information. Vanadium. Available online: https://minerals.usgs.gov/minerals/pubs/commodity/vanadium (accessed on 2 March 2018).
- Tavakoli, M.R.; Dreisinger, D.B. The kinetics of oxidative leaching of vanadium trioxide. Hydrometallurgy 2014, 147–148, 83–89. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Bao, S.-X.; Liu, T.; Chen, T.-J.; Huang, J. The technology of extracting vanadium from stone coal in China: History, current status and future prospects. Hydrometallurgy 2011, 109, 116–124. [Google Scholar] [CrossRef]
- Li, H.-Y.; Fang, H.-X.; Wang, K.; Zhou, W.; Yang, Z.; Yan, X.-M.; Ge, W.-S.; Li, Q.-W.; Xie, B. Asynchronous extraction of vanadium and chromium from vanadium slag by stepwise sodium roasting-water leaching. Hydrometallurgy 2015, 156, 124–135. [Google Scholar] [CrossRef]
- Zhu, Z.; Tulpatowicz, K.; Pranolo, Y.; Chen, C.Y. Solvent extraction of molybdenum and vanadium from sulphate solutions with Cyphos IL 101. Hydrometallurgy 2015, 154, 72–77. [Google Scholar] [CrossRef]
- Li, X.; Wei, C.; Deng, Z.; Li, M.; Li, C.; Fan, G. Selective solvent extraction of vanadium over iron from a stone coal/black shale acid leach solution by D2EHPA/TBP. Hydrometallurgy 2011, 105, 359–363. [Google Scholar] [CrossRef]
- Li, X.; Wei, C.; Wu, J.; Li, M.; Deng, Z.; Li, C.; Xu, H. Co-extraction and selective stripping of vanadium (IV) and molybdenum (IV) from sulphuric acid solution using 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester. Sep. Purif. Technol. 2012, 86, 64–69. [Google Scholar] [CrossRef]
- Zhang, P.; Inoue, K.; Yoshizuka, K.; Tsuyama, H. Extraction and selective stripping of molybdenum(IV) and vanadium(IV) from sulfuric acid solution containing aluminum(III), cobalt(II), nickel(II) and iron(III) by LIX 63 in Exxsol D80. Hydrometallurgy 1996, 41, 45–53. [Google Scholar] [CrossRef]
- Bal, Y.; Bal, K.-E.; Cote, G.; Lallam, A. Characterization of solid third phases that precipitate from the organic solutions of Aliquat® 336 after extraction of molybdenum(IV) and vanadium(V). Hydrometallurgy 2004, 75, 123–134. [Google Scholar] [CrossRef]
- Lozano, L.J.; Godinez, C. Comparative study of solvent extraction of vanadium from sulphate solutions by primine 81R and alamine 336. Miner. Eng. 2003, 16, 291–294. [Google Scholar] [CrossRef]
- Chagnes, A.; Rager, M.-N.; Courtaud, B.; Thiry, J.; Cote, G. Speciation of vanadium(V) extracted from acidic sulfate media by trioctylamine in n-dodecane modified with 1-tridecanol. Hydrometallurgy 2010, 104, 20–24. [Google Scholar] [CrossRef]
- Hu, J.; Wang, X.; Xiao, L.; Song, S.; Zhang, B. Removal of vanadium from molybdate solution by ion exchange. Hydrometallurgy 2009, 95, 203–206. [Google Scholar] [CrossRef]
- Li, Q.; Zeng, L.; Xiao, L.; Yang, Y.; Zhang, Q. Completely removing vanadium from ammonium molybdate solution using chelating ion exchange resins. Hydrometallurgy 2009, 98, 287–290. [Google Scholar] [CrossRef]
- Huang, J.; Su, P.; Wu, W.; Liao, S.; Qin, H.; Wu, X.; He, X.; Tao, L.; Fan, Y. Concentration and separation of vanadium from alkaline media by strong alkaline anion-exchange resin 717. Rare Met. 2010, 29, 439–443. [Google Scholar] [CrossRef]
- Wang, X.; Wang, M.; Shi, L.; Hu, J.; Qiao, P. Recovery of vanadium during ammonium molybdate production using ion exchange. Hydrometallurgy 2010, 104, 317–321. [Google Scholar] [CrossRef]
- Zeng, L.; Li, Q.; Xiao, L. Extraction of vanadium from the leach solution of stone coal using ion exchange resin. Hydrometallurgy 2009, 97, 194–197. [Google Scholar] [CrossRef]
- Zeng, L.; Li, Q.; Xiao, L.; Zhang, Q. A study of the vanadium species in an acid leach solution of stone coal using ion exchange resin. Hydrometallurgy 2010, 105, 176–178. [Google Scholar] [CrossRef]
- Baes, C.F.; Messmer, R.E. The Hydrolysis of Cations; John Wiley & Sons: New York, NY, USA, 1976; pp. 197–210. ISBN 0471039853. [Google Scholar]
- Takahashi, H.; Matsushima, Y. Extraction of vanadium from ammonia slags with environmentally friendly wet processes near the atmospheric conditions. Microsyst. Technol. 2016, 22, 73–76. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).