Abstract
The coupling relationships between dephosphorization and desulfurization abilities or potentials for CaO–FeO–Fe2O3–Al2O3–P2O5 slags over a large variation range of slag oxidization ability during the secondary refining process of molten steel have been proposed by the present authors as or in the reducing zone and as or in the oxidizing zone based on the ion and molecule coexistence theory (IMCT). In order to further verify the validation and feasibility of the proposed coupling relationships, the effects of chemical composition of the CaO-based slags are provided. The chemical composition of slags was described by three group parameters including reaction abilities of components represented by the mass action concentrations , two kinds of slag basicity as simplified complex basicity and optical basicity , and the comprehensive effect of iron oxides FetO and basic oxide CaO. Comparing with the strong effects of chemical composition of the CaO-based slags on dephosphorization and desulfurization abilities or potentials, the proposed coupling relationships have been confirmed to not only be independent of slag oxidization ability as expected but also irrelevant to the aforementioned three groups of parameters for representing the chemical composition of the CaO-based slags. Increasing temperature from 1811 to 1927 K (1538 to 1654 °C) can result in a decreasing tendency of the proposed coupling relationships. In terms of the proposed coupling relationships, chemical composition of slags or fluxes with assigned dephosphorization ability or potential can be theoretically designed or optimized from its desulfurization ability or potential, and vice versa. Considering the large difference of magnitude between phosphate capacity and sulfide capacity , the proposed coupling relationships between dephosphorization and desulfurization abilities for CaO-based slags are recommended to design or optimize chemical composition of slags.
1. Introduction
For the purpose of refining low or ultra-low phosphorus and sulfur steel products with high mechanical properties, simultaneous dephosphorization and desulfurization of iron-based melts has been widely applied as a routine sub-process during hot metal pretreatment operation and the secondary refining process of molten steel in most metallurgical companies. It is well known that the greater oxygen potential of slags or iron-based melts, higher content of basic oxides in slags, and lower temperature at dephosphorization zone are three preferred operation conditions for promoting dephosphorization reactions under a fixed mass ratio of slags to iron-based melts from the viewpoint of dephosphorization thermodynamics. However, the corresponding three preferred operation conditions for promoting desulfurization reactions can be summarized as smaller oxygen potential of slags or iron-based melts, higher content of basic oxides in slags, and higher temperature at the desulfurization zone. Evidently, conditions for promoting dephosphorization reactions are to some degree opposite to those for enhancing desulfurization reactions for an assigned slag system. Moreover, a larger amount of slags or fluxes is also beneficial for promoting dephosphorization as well as desulfurization from the viewpoint of kinetics. However, a reasonable mass ratio of slags to iron-based melts should be controlled in order to decrease production cost in industrial plants. It can be concluded that besides the easily controlled temperature and content of basic oxides in slags or fluxes, controlling the optimal range of slag oxidization ability is a challenging task to successfully maintain ideal dephosphorization ability and acceptable desulfurization ability during simultaneous dephosphorization and desulfurization processes of iron-based melts.
CaO–FetO–Al2O3 slag system was recommended by Ban–ya et al. [1] for simultaneous dephosphorization and desulfurization during the secondary refining process of molten steel. The recommended CaO–FeO–Fe2O3–Al2O3–P2O5 slags by Ban–ya et al. [1] exhibited a large variation range of slag oxidization ability with the mass percentage of FetO varying from 1.88 to 55.50. Nevertheless, no conclusions or results on the linkage between dephosphorization and desulfurization abilities or potentials of the slags were provided by Ban–ya et al. [1]. The coupling relationships between dephosphorization and desulfurization abilities or potentials for CaO–FeO–Fe2O3–Al2O3–P2O5 slags [1] over a large range of slag oxidization ability during the secondary refining process of molten steel have been recently proposed by Yang et al. [2] as or in the reducing zone and as or in the oxidizing zone through deleting or omitting the term of slag oxidization ability represented by the comprehensive mass action concentration of iron oxides FetO based on the ion and molecule coexistence theory (IMCT) [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18]. The proposed coupling relationships [2] for the CaO-based slags have been verified to be independent of slag oxidization ability as expected.
It should be specially mentioned that the linkage between phosphate capacity and sulfide capacity for slags was first correlated by Sano et al. [19] in 1990 as through deleting activity of oxygen ion O2– in slags. The defined constant term by Sano et al. [19] as can hold constant only under conditions that both ratios of and keep constants simultaneously, which is also derived in details by Yang et al. [2] However, Ban-ya et al. [20,21] clearly proved and argued that the ratio of the activity coefficient to the standard equilibrium constant, i.e., or , of dephosphorization and desulfurization products cannot hold constant in multi-components solutions like molten slags, fluxes and salts. Thus, the assumption of the term on the right-hand side of the relationship between and by Sano et al. [19] being constant is not a theoretically correct conclusion. Furthermore, the intrinsic relationship between the phosphorus distribution ratio and sulfur distribution ratio for slags with fixed chemical compositions has scarcely been investigated.
Under these circumstances, the proposed coupling relationships for the CaO-based slags should be further verified and evaluated from the viewpoint of whether or not they are also independent of slag chemical composition. The slag chemical composition was described in this contribution by three group parameters including the reaction abilities of components described by the mass action concentrations , activity relative to pure liquid or solid matters as standard state, slag basicity containing simplified complex basicity or optical basicity , and the comprehensive influence of iron oxides FetO and basic oxide CaO.
The ultimate objectives of this study can be summarized as (1) to further verify the linkage between dephosphorization and desulfurization abilities or potentials for a fixed flux or slags not only regardless of slag oxidization ability as expected but also independent of slag chemical composition; (2) to provide fundamental information for optimizing the chemical composition of slags or fluxes with the aim of enhancing simultaneous dephosphorization and desulfurization abilities or potentials of iron-based melts by a fixed flux or slags; (3) to enrich the foundations of the reaction mechanism during the simultaneous dephosphorization and desulfurization process of iron-based melts by a fixed flux or slags over a large variation range of slag oxidization ability; (4) moreover, to open new application fields of the IMCT [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18] for metallurgical slags.
2. Influence of Slag Chemical Composition on Proposed Coupling Relationships between Dephosphorization and Desulfurization Abilities and Potentials for CaO–based Slags
The chemical compositions of CaO–FeO–Fe2O3–Al2O3–P2O5 slags equilibrated with liquid iron by Ban-ya et al. [1] and three parameters for representing slag oxidization ability as the mass percentage of FetO through ; calculated [3] comprehensive mass action concentration of FetO and oxygen potential of the CaO-based slags over a temperature range from 1811 to 1927 K (1538 to 1654 °C) are summarized in Table 1. In addition, the determined [2] and calculated [2] coupling relationship terms as or in the reducing zone and as or in the oxidizing zone based on measured or measured by Ban-ya et al. [1], predicted [3] or [4] by the IMCT models, determined [3] or determined [5] after Ban–ya et al. [1], and predicted [3] or [5] by the IMCT models are also tabulated in Table 2 for comparison. Thus, the effects of chemical composition of slags described by three group parameters including the reaction abilities of components represented by the mass action concentrations , two kinds of slag basicity as simplified complex basicity and optical basicity , and the comprehensive effect of iron oxides FetO and basic oxide CaO on proposed coupling relationships [2] for the CaO-based slags are further evaluated in the next section.

Table 1.
Chemical compositions of CaO–FeO–Fe2O3–Al2O3–P2O5 slags over a large range of slag oxidization ability equilibrated with liquid iron after Ban-ya et al. [1] and three parameters for representing slag oxidization ability as the mass percentage of FetO, calculated [3] comprehensive mass action concentration of FetO, and oxygen potential [3] of the slags over a temperature range from 1811 to 1927 K (1538 to 1654 °C).

Table 2.
Comparison between determined and calculated coupling relationship terms between dephosphorization and desulfurization abilities or potentials for CaO–FeO–Fe2O3–Al2O3–P2O5 slags over a large range of slag oxidization ability equilibrated with liquid iron during the secondary refining process of molten steel based on measured or by Ban-ya et al. [1], predicted [3] or [4] by the IMCT models, determined [3] or determined [5] after Ban-ya et al. [1], and predicted [3] or [5] by the IMCT models over a temperature range from 1811 to 1927 K (1538 to 1654 °C).
2.1. Influence of Reaction Abilities of Components on Coupling Relationships between Dephosphorization and Desulfurization Abilities or Potentials for CaO-based Slags
It was verified [3] that good corresponding relationships between mass percentages of components and calculated [3] mass action concentrations of CaO, FeO, Fe2O3, and Al2O3 as components are established for the CaO-based slags. Thus, the calculated [3] mass action concentrations of components based on the IMCT [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18] can be applied to the representation of the chemical composition of the CaO-based slags, like the mass percentage (% i) of components. As a newly-formed structural unit , according to the IMCT [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18], the calculated [3,4,5] mass action concentration of can be used to describe the reaction ability of FeO·Fe2O3. In addition, the calculated [3,4,5] comprehensive mass action concentration of iron oxides FetO can also be applied to the description of reaction ability of FetO. Thus, the calculated [3,4,5] mass action concentrations of CaO, FeO, Fe2O3, Al2O3, FeO·Fe2O3, and FetO are used to represent reaction abilities of components in the CaO-based slags, like the traditional applied activities of components in the classical metallurgical physicochemistry.
2.1.1. Influences of Reaction Abilities of Components on Coupling Relationships between Dephosphorization and Desulfurization Abilities for CaO-based Slags
The relationships of calculated [3,4,5] mass action concentrations, , of six components CaO, FeO, Fe2O3, Al2O3, FeO·Fe2O3, and FetO against the calculated [3] phosphorus distribution ratio using the IMCT– model for the CaO-based slags equilibrated with liquid iron are shown in the first layers of Figure 1. Likewise, the relationships of the of six components against the calculated [4] sulfur distribution ratio using the IMCT– model for the CaO-based slags are also illustrated in the second layers of Figure 1. Meanwhile, the relationship of the aforementioned of the six components against determined or after original data from Ban-ya et al. [1], or calculated or based on results by Yang et al. [3,4] for the CaO-based slags is displayed in the first and second layers of Figure 2, respectively. The average values of term or after original data from Ban-ya et al. [1] or based on results from Yang et al. [3,4] over three sub-divided temperature ranges are also exhibited in Figure 2 by lines. The distinguishing lines between CaS and FeS for representing the reducing and oxidizing zones are added on the horizontal ordinates in the sub-figures of Figure 1 and other figures in the following text if necessary. To improve the display resolution, the horizontal ordinates in the sub-figures of Figure 1 and other figures in the following text, if necessary, are also split into two zones through adding break symbols for describing the reducing and oxidizing zones. It should be emphasized that the exponential growing tendency of the phosphorus distribution ratio against the slag oxidization ability expressed by of iron oxides in Figure 1(f1) as well as the backward tick-shaped or asymmetrical relationship of the sulfur distribution ratio against of iron oxides in Figure 1(f2) based on the normal scale of the horizontal ordinates cannot normally be displayed. However, the intrinsic relationships of the mass action concentrations of six components against or cannot be changed by adding break symbols on the horizontal ordinates in the sub-figures of Figure 1. It can be observed in Figure 1 and Figure 2 that the criterion for distinguishing the reducing and oxidizing zones corresponds to in 0.778, in 0.0626, in 1.00 × 10−4, in 1.50 × 10−3, in 4.10 × 10−5, and in 0.0637, respectively.
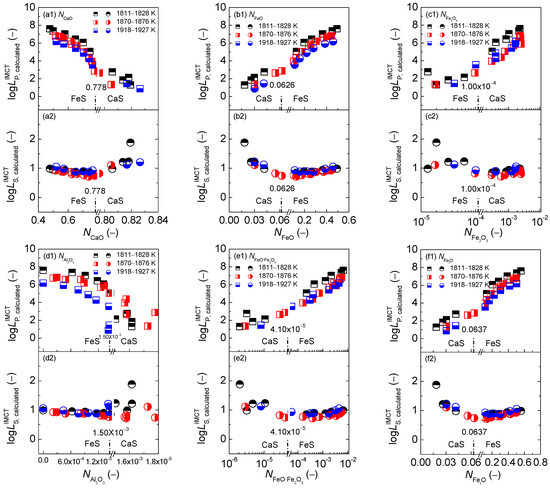
Figure 1.
Relationship of the calculated [3] mass action concentration of CaO (a), FeO (b), Fe2O3 (c), Al2O3 (d), FeO·Fe2O3 (e), and FetO (f) against the calculated [3] phosphorus distribution ratio by IMCT– model in the first layer or calculated [4] sulfur distribution ratio by IMCT– model in the second layer for CaO–FeO–Fe2O3–Al2O3–P2O5 slags equilibrated with liquid iron over a temperature range of 1811 to 1927 K (1538 to 1654 °C), respectively.
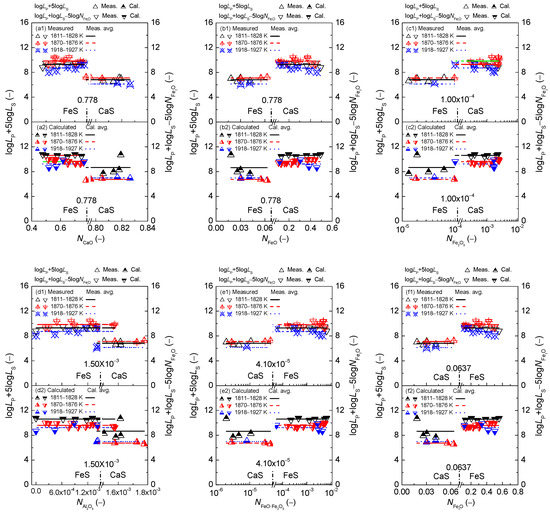
Figure 2.
Relationship of calculated [3] mass action concentration of CaO (a), FeO (b), Fe2O3 (c), Al2O3 (d), FeO·Fe2O3 (e), and FetO (f) against determined term or after Ban-ya et al. [1] in the first layer or calculated term or based on the results from Yang et al. [3,4] in the second layer for CaO–FeO–Fe2O3–Al2O3–P2O5 slags equilibrated with liquid iron over a temperature range of 1811 to 1927 K (1538 to 1654 °C), respectively.
With regard to the dephosphorization ability of the CaO-based slags, it can be observed in the first layers of Figure 1 that increasing or can result in a significantly decreasing tendency of as shown in Figure 1(a1,d1); however, increasing , or can lead to an increasing trend of as shown in Figure 1(b1,c1,e1). Certainly, the result in Figure 1(a1) is not consistent with the widely-accepted consensus that basic oxide CaO can promote dephosphorization reactions. It can be obtained from the relationship of calculated against or or or or for the CaO-basedslags as illustrated in Figure 3 that increasing or or can result in an increasing tendency of as shown in Figure 3(b,c,e); however, increasing can lead to a decreasing trend of or as illustrated in Figure 3(a,d). The promotive effect of increasing on can be counteracted by the decrease of for the CaO-based slags. There are some extreme proofs to support this finding as the CaO-based slags with high CaO but very low FetO, which are widely applied at reduction stage during electric arc furnace (EAF) steelmaking process or used during the blast furnace (BF) ironmaking process, can only extract sulphur, rather than phosphorus, from iron–based melts. Thus, not only the independent effect of iron oxides FetO and basic oxide CaO, but also the comprehensive effect of iron oxides FetO and basic oxide CaO plays a decisive role in between the CaO-based slags and liquid iron. This finding is in good agreement with the well-known conclusion that the CaO-based slags with middle FetO and high CaO, which are commonly used in a top–bottom combined oxygen blowing converter for the steelmaking process or dephosphorization pretreatment of hot metal, indicates a greater dephosphorization ability coupling with limited desulfurization ability for iron-based melts. This result can be applied to the explanation of reason that the promotive effect of increasing or or or on can be counteracted by a decrease of for the CaO-based slags as shown in Figure 1(a1).
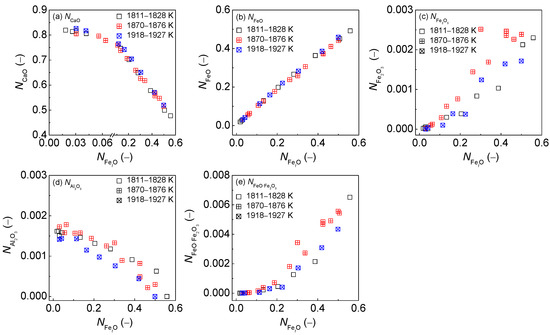
Figure 3.
Relationship of calculated [3] comprehensive mass action concentration of iron oxides against calculated [3] mass action concentration (a) or (b) or (c) or (d) or (e) for CaO–FeO–Fe2O3–Al2O3–P2O5 slags equilibrated with liquid iron over a temperature range of 1811 to 1927 K (1538 to 1654 °C), respectively.
With respect to the desulfurization ability of the CaO-based slags, it can be obtained from the second layers of Figure 1 that increasing or accompanied with a decrease in or or or can result in an obviously increasing tendency of the sulfur distribution ratio of the CaO-based slags in the reducing zone. This result can be reasonably explained by the IMCT– model [4] for the CaO-based slags in the reducing zone that basic oxide CaO expressed by shows a promoting effect on desulfurization ability, while iron oxides FetO expressed by exhibit a decaying influence on the desulfurization ability of the CaO-based slags in the reducing zone. However, increasing slag oxidization ability, i.e., increasing or or or , can lead to a slightly increasing trend of of the CaO-based slags in the oxidizing zone. This result can be explained by the IMCT– model [4] for the CaO-based slags in the oxidizing zone that only ferrous oxide FeO expressed by influences of the CaO-based slags in the oxidizing zone.
On the proposed coupling relationships [2] between and for the CaO–based slags, it can be observed in Figure 2 that the proposed terms in the reducing zone and in the oxidizing zone are doubtlessly independent of variation of or as well as or or or . Increasing temperature from 1811 to 1927 K (1538 to 1654 °C) can result in a slightly decreasing tendency of proposed coupling relationships between and for the CaO-based slags. Thus, the proposed coupling relationships between and are not only independent of the slag oxidization ability as shown in Figure 1(f1,f2) but is also irrelevant to the mass action concentrations of six components over a narrow temperature range.
2.1.2. Influences of Reaction Abilities of Components on Coupling Relationships between Dephosphorization and Desulfurization Potentials for CaO–based Slags
The relationship of calculated [3,4,5] mass action concentrations of six components as CaO, FeO, Fe2O3, Al2O3, FeO·Fe2O3, and FetO against the calculated [3] phosphate capacity using the IMCT– model for the CaO-based slags are shown in the first layers of Figure 4, respectively. Similarly, the relationship of of six components against the calculated [5] sulfide capacity using the IMCT– model for the CaO-based slags are also illustrated in the second layers of Figure 4, respectively. The relationship of of six components against determined or after original data from Ban-ya et al. [1], or calculated or based on results by Yang et al. [3,5] for the CaO-based slags are displayed in the first and second layers of Figure 5, respectively. In addition, the average values of term or after original data from Ban-ya et al. [1] or based on results from Yang et al. [3,5] over three sub-divided temperature ranges are also exhibited in Figure 5 by horizontal lines, respectively.
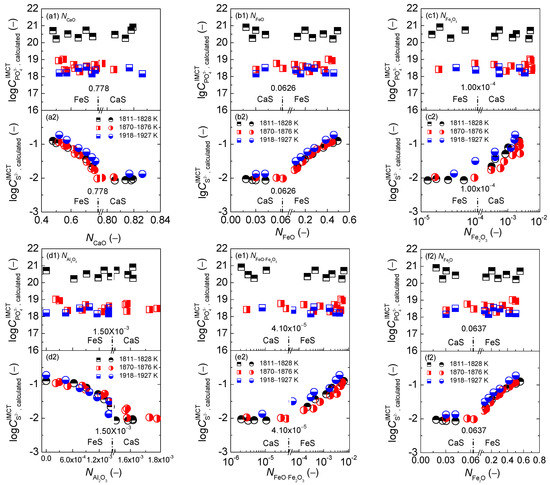
Figure 4.
Relationship of calculated [3] mass action concentration of CaO (a), FeO (b), Fe2O3 (c), Al2O3 (d), FeO·Fe2O3 (e), and FetO (f) against calculated [3] phosphate capacity by the IMCT– model in the first layer or calculated [5] sulfide capacity by the IMCT– model in the second layer for CaO–FeO–Fe2O3–Al2O3–P2O5 slags equilibrated with liquid iron over a temperature range of 1811 to 1927 K (1538 to 1654 °C), respectively.
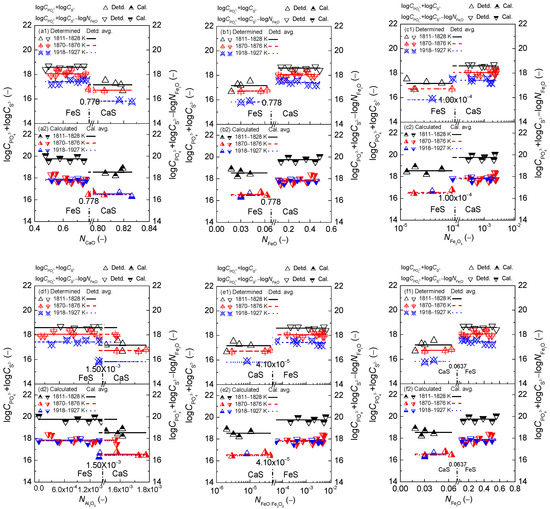
Figure 5.
Relationship of calculated [3] mass action concentration of CaO (a), FeO (b), Fe2O3 (c), Al2O3 (d), FeO·Fe2O3 (e), and FetO (f) against determined term or after Ban-ya et al. [1] in the first layer or calculated term or based on results from Yang et al. [3,5] in the second layer for CaO–FeO–Fe2O3–Al2O3–P2O5 slags equilibrated with liquid iron over a temperature range from 1811 to 1927 K (1538 to 1654 °C), respectively.
With respect to the dephosphorization potential of the CaO-based slags, it can be observed in the first layers of Figure 4 that phosphate capacity of the CaO-based slags is almost unchangeable with the increase of of six components over a narrow temperature range because the comprehensive effect of iron oxides FetO and basic oxide CaO plays the key role in dephosphorization potential of the CaO-based slags as discussed in Section 2.3.2 and elsewhere [3].
With regard to the desulfurization potential [5] of the CaO-based slags, it can be observed in the second layers of Figure 4 that sulfide capacity of the CaO-based slags in the reducing zone also keeps almost constant with the increase of of six components. However, sulfide capacity of the CaO-based slags in the oxidizing zone displays an obviously increasing tendency with the increase of or or or over a narrow temperature range, but exhibits a largely decreasing trend with the increase of or , which has been explained elsewhere [5]. Basic oxides CaO expressed by largely the affect desulfurization potential of the CaO-based slags in the reducing zone from the IMCT– model [5], while ferrous oxides expressed by significantly influence the desulfurization potential of the CaO-based slags in the oxidizing zone from the IMCT– model [5]. The very small decreasing tendency of in the reducing zone in Figure 3a cannot cause an obvious increasing tendency of desulfurization potential of the CaO-based slags in the reducing zone. Furthermore, sulfide capacity of the CaO-based slags in the reducing zone is also independent of or or or . The decreasing tendency of the sulfide capacity of the CaO-based slags in the oxidizing zone with the increase of or can be attributed to the largely decreasing trend of as shown in Figure 3. Increasing or or or can significantly promote the sulfide capacity of the CaO-based slags in oxidizing zone.
With regards to the proposed coupling relationships between and for the CaO-based slags, it can be observed in Figure 5 that the proposed terms [2] and are independent of of six components. Increasing temperature from 1811 to 1927 K (1538 to 1654 °C) can result in a decreasing tendency of proposed coupling relationships between and for the CaO-based slags. Thus, the proposed coupling relationships between and for the CaO-based slags are not only independent of slag oxidization ability as shown in Figure 5(f) but are also irrelevant to the mass action concentrations of six components over a narrow temperature range.
However, small discrepancies of proposed terms [2] and based on results by Yang et al. [3,5] and that based on determined ones after original data from Ban-ya et al. [1] for the CaO-based slags can be observed in each sub-figure of Figure 5. It is widely accepted that the phosphate capacity of slags can be determined or calculated from the corresponding phosphorus distribution ratio through the relationship [3,11] between and for slags, meanwhile, sulfide capacity of slags can also be determined or calculated from the sulfur distribution ratio through the relationship [5,7,9] between and for slags. It was verified by Yang et al. [4,5] that the calculated [4] results of by the IMCT– model are in good consistency with measured [1] ones by Ban-ya et al., meanwhile the calculated [5] results of by the IMCT– model are in good accord with determined [5] after the original data from Ban–ya et al. [1]. However, it was also verified by Yang et al. [3] that the calculated results of by the IMCT– model are not in good agreement with the measured by Ban–ya et al. [1], especially over the lower temperature range of 1811 to 1828 K (1538 to 1555 °C). Thus, the large deviation between calculated [3] by the IMCT– model and determined [3] results of after the original data from Ban–ya et al. [1], especially over the lower temperature range, is caused by the relationship [3,11] between and for slags. Evidently, the accuracy of the phosphorus distribution ratio is very important to obtain the precise phosphate capacity of slags through the relationship [3,11] between and for slags. This means that the experimental uncertainties for dephosphorization reactions by Ban-ya et al. [1] can be effectively relieved by the predicted results of by the IMCT– [3] and by the IMCT– model [3]. It can be deduced that the relationships of the mass action concentrations of six components against calculated coupling relationships between and based on results by Yang et al. [3,5] are more accurate than those against determined ones after original data from Ban-ya et al. [1] for the CaO–based slags.
2.2. Influence of Slag Basicity on Coupling Relationships between Dephosphorization and Desulfurization Abilities or Potentials for CaO-based Slags
For the purpose of investigating the influence of slag basicity on proposed coupling relationships, two kinds of slag basicity as simplified complex basicity and optical basicity are applied in this study. The commonly applied complex basicity [15,22,23,24] can be simplified as due to no SiO2 in the CaO-based slags.
Three group values of optical basicity for FeO and Fe2O3 have been recommended as (1) = 0.51 and = 0.48 from Pauling electronegativity [25]; (2) = 0.93 and = 0.69 from average electron density [26]; (3) = 1.0 and = 0.75 based on mathematical regression [27] from numerous experimental data. According to the evaluation results [3,4,5] of the aforementioned three group values of optical basicity for FeO and Fe2O3, the obtained = 1.0 and = 0.75 from mathematical regression [27] are recommended to represent optical basicity for FeO and Fe2O3, which are similar to those recommended = 1.0 and = 0.77 by Young et al. [28]
2.2.1. Influence of Slag Basicity on Coupling Relationships between Dephosphorization and Desulfurization Abilities for CaO-based Slags
The relationship between the simplified complex basicity or optical basicity and calculated [3] phosphorus distribution ratio for the CaO-based slags is shown in the first layers of Figure 6, respectively. Similarly, the relationship between the two kinds of slag basicity and the calculated [4] sulfur distribution ratio for the CaO-based slags is also illustrated in the second layers of Figure 6, respectively. The relationship between two kinds of slag basicity and determined term or after original data from Ban-ya et al. [1], or calculated or based on results by Yang et al. [3,4] for the CaO-based slags are displayed in the first and second layers of Figure 7, respectively. It can be obtained from Figure 6 and Figure 7 that the criterion for distinguishing, reducing and oxidizing zones corresponds to simplified complex basicity in 1.56 or optical basicity in 0.80, respectively. It should be pointed out that the obtained criterion of optical basicity as 0.80 for separating the reducing and oxidizing zones in this study is in good agreement with that by Young et al. [25] for developing the sulfide capacity prediction model of CaO–SiO2–MgO–FeO–MnO–Al2O3 slags.
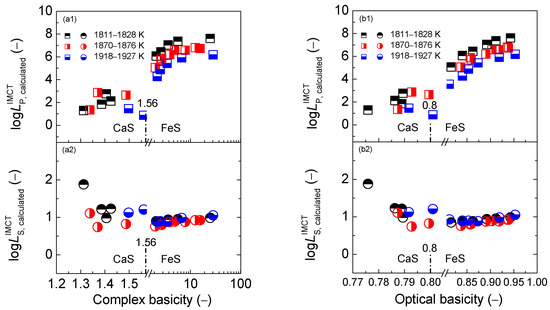
Figure 6.
Relationship of simplified complex basicity (a) or optical basicity (b) against the calculated [3] phosphorus distribution ratio by the IMCT– model in the first layer or calculated [4] sulfur distribution ratio by the IMCT– model in the second layer for CaO–FeO–Fe2O3–Al2O3–P2O5 slags equilibrated with liquid iron over a temperature range of 1811 to 1927 K (1538 to 1654 °C), respectively.
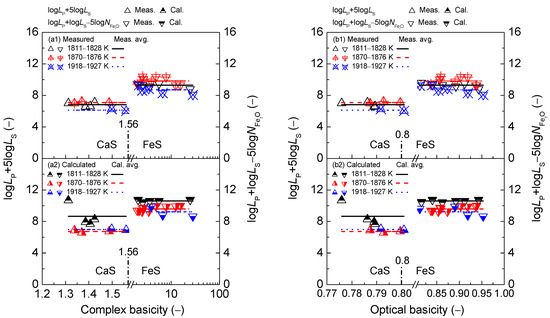
Figure 7.
Relationship of simplified complex basicity (a) or optical basicity (b) against the determined term or after Ban-ya et al. [1] in the first layer or calculated term or based on results from Yang et al. [3,4] in the second layer for CaO–FeO–Fe2O3–Al2O3–P2O5 slags equilibrated with liquid iron over a temperature range of 1811 to 1927 K (1538 to 1654 °C), respectively.
With respect to the dephosphorization ability of the CaO-based slags, it can be observed in the first layers of Figure 6 that increasing two kinds of slag basicity can result in an exponentially growing tendency of the phosphorus distribution ratio , which has been explained by Yang et al. elsewhere [3]. Regarding the desulfurization ability of the CaO–based slags, it can be obtained from the second layers of Figure 6 that increasing two kinds of slag basicity can lead to a backward tick-shaped variation trend of sulfur distribution ratio , which has been explained by Yang et al. elsewhere [4]. Certainly, adding break symbols on the horizontal ordinates in the sub-figures of Figure 6 can only destroy the apparent relationship of the exponentially growing tendency of against two kinds of slag basicity in the first layers of Figure 6 as well as the backward tick-shaped or asymmetrical relationship of against two kinds of slag basicity in the second layers of Figure 6. The intrinsic relationships of two kinds of slag basicity against or cannot be changed through adding break symbols on the horizontal ordinates in the sub-figures of Figure 6.
With respect to the proposed coupling relationships [2] between and for the CaO-based slags, it can be observed in Figure 7 that increasing two kinds of slag basicity cannot cause a visible variation of the proposed term or for the CaO-based slags over a narrow temperature range. Thus, the proposed coupling relationships [2] between and for the CaO-based slags are also independent of simplified complex basicity or optical basicity .
2.2.2. Influence of Slag Basicity on Coupling Relationship between Dephosphorization and Desulfurization Potentials for CaO-based Slags
The relationships of two kinds of slag basicity against calculated [3] phosphate capacity for the CaO-based slags are shown in the first layers of Figure 8, respectively. Likewise, the relationships of two kinds of slag basicity against calculated [5] sulfide capacity for the CaO-based slags are also illustrated in the second layers of Figure 8, respectively. The relationships of two kinds of slag basicity against determined term or after original data from Ban-ya et al. [1], or calculated term or based on results by Yang et al. [3,5] for the CaO-based slags are displayed in the first and second layers of Figure 9, respectively.
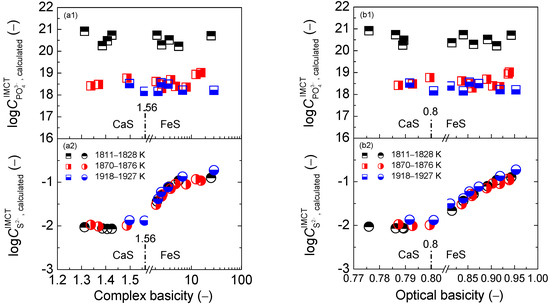
Figure 8.
Relationship of simplified complex basicity (a) or optical basicity (b) against the calculated [3] phosphate capacity by the IMCT– model in first layer or calculated [5] sulfide capacity by the IMCT– model in second layer for CaO–FeO–Fe2O3–Al2O3–P2O5 slags equilibrated with liquid iron over a temperature range of 1811 to 1927 K (1538 to 1654 °C), respectively.
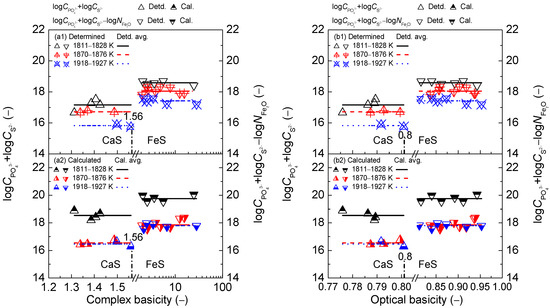
Figure 9.
Relationship of simplified complex basicity (a) or optical basicity (b) against determined term or after Ban-ya et al. [1] in the first layer or calculated term or based on results from Yang et al. [3,5] in the second layer for CaO–FeO–Fe2O3–Al2O3–P2O5 slags equilibrated with liquid iron over a temperature range of 1811 to 1927 K (1538 to 1654 °C), respectively.
With respect to the dephosphorization potential of the CaO-based slags, it can be observed in the first layers of Figure 8 that increasing two kinds of slag basicity cannot result in an obvious influence on dephosphorization potential over a narrow temperature range, which has been explained by Yang et al. elsewhere [3]. With regard to the desulfurization potential of the CaO-based slags, it can be observed in the second layers of Figure 8 that the desulfurization potential of the CaO-based slags in the reducing zone keeps almost constant with the increase of two kinds of slag basicity as illustrated in the left regions of Figure 8(a2,b2); however, sulfide capacity of the CaO-based slags in oxidizing zone displays an obviously increasing tendency with the increase of two kinds of slag basicity as illustrated in the right regions of Figure 8(a2,b2), which has been explained by Yang et al. elsewhere [5].
Regarding the proposed coupling relationships [2] between and for the CaO-based slags, it can be observed in Figure 9 that increasing two kinds of slag basicity cannot cause a visible variation of proposed coupling relationships between and for the CaO-based slags over a narrow temperature range. The relationships of two kinds of slag basicity against calculated [2] term or based on the results by Yang et al. [3,4] are more accurate than those against determined ones after original data from Ban-ya et al. [1] for the CaO-based slags in the reducing or oxidizing zone. Thus, the proposed coupling relationships [2] between and for the CaO-based slags are also independent of two kinds of slag basicity over a narrow temperature range.
2.3. Comprehensive Effect of FetO and CaO on Coupling Relationships between Dephosphorization and Desulfurization Abilities or Potentials for CaO-based Slags
It was verified by Yang et al. [3,4,5] that the mass percentage ratios or the mass action concentration ratios of various iron oxides to basic oxide CaO can be applied to the elucidation of the comprehensive effect of iron oxides FetO and basic oxide CaO on dephosphorization and desulfurization reactions of the CaO-based slags. It was also verified by Yang et al. [4] that the mass percentage ratio (% FeO)/(% CaO) or (% Fe2O3)/(% CaO) or (% FetO)/(% CaO) can correlate a good linear relationship with the mass action concentration ratio or or for the CaO-based slags, respectively. Thus, the aforementioned mass action concentration ratios of various iron oxides to basic oxide CaO can be reliably substituted by the corresponding mass percentage ratios. As the newly formed structural unit FeO·Fe2O3 in the CaO-based slags in terms of the IMCT [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18], the mass action concentration ratio is also applied to the evaluation of the comprehensive influence of FeO·Fe2O3 and basic oxide CaO on the proposed coupling relationships [2].
2.3.1. Comprehensive Effect of FetO and CaO on Coupling Relationships between Dephosphorization and Desulfurization Abilities for CaO-based Slags
The relationship between the mass action concentration ratios of various iron oxides to basic oxide CaO, i.e., or or or and calculated [3] phosphorus distribution ratio for the CaO-based slags is shown in the first layers of Figure 10, respectively. Similarly, the relationship between aforementioned four mass action concentration ratios and calculated [4] sulfur distribution ratio for the CaO-based slags is also illustrated in the second layers of Figure 10, respectively. The relationships between four mass action concentration ratios and determined term or after original data from Ban-ya et al. [1], or calculated term or based on results by Yang et al. [3,4] for the CaO-based slags are displayed in the first and second layers of Figure 11, respectively. It can be observed in Figure 10 and Figure 11 that the criterion for distinguishing the reducing and oxidizing zones corresponds to in 0.08, in 1.59 × 10−4, in 5.27 × 10−5, and in 0.082, respectively.
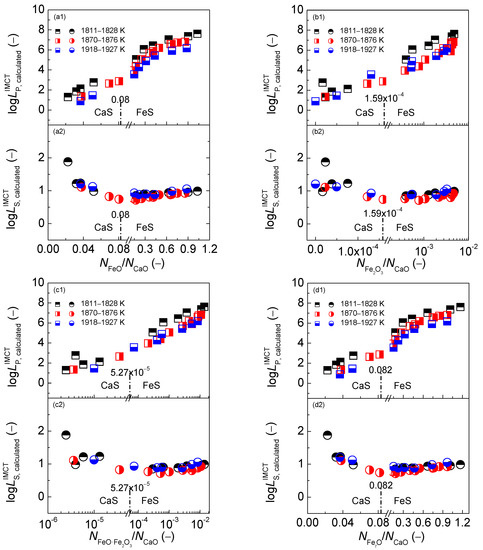
Figure 10.
Relationship of the mass action concentration ratio (a) or (b) or (c) or (d) against the calculated [3] phosphorus distribution ratio by the IMCT– model in first layer or the calculated [4] sulfur distribution ratio by IMCT– model in the second layer for CaO–FeO–Fe2O3–Al2O3–P2O5 slags equilibrated with liquid iron over a temperature range of 1811 to 1927 K (1538 to 1654 °C), respectively.
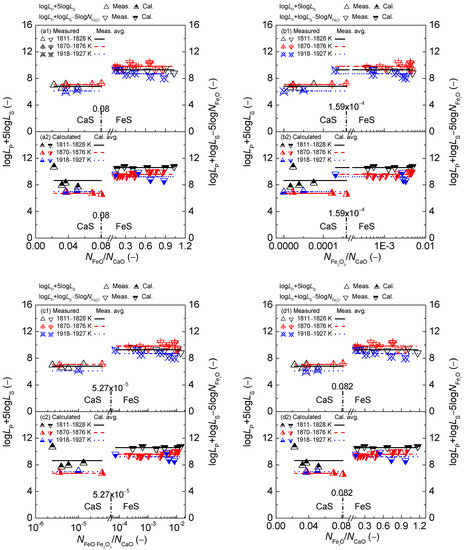
Figure 11.
Relationship of the mass action concentration ratio (a) or (b) or (c) or (d) against the determined term or after Ban-ya et al. [1] in the first layer or calculated term or based on the results from Yang et al. [3,4] in the second layer for CaO–FeO–Fe2O3–Al2O3–P2O5 slags equilibrated with liquid iron over a temperature range of1811 to 1927 K (1538 to 1654 °C), respectively.
With respect to the dephosphorization ability of the CaO-based slags, it can be observed in the first layers of Figure 10 that increasing four mass action concentration ratios can result in an exponentially growing tendency of the phosphorus distribution ratio , which has been discussed by Yang et al. elsewhere [3]. With regard to the desulfurization ability of the CaO-based slags, it can be obtained from the second layers of Figure 10 that increasing four mass action concentration ratios can lead to a backward tick-shaped variation trend of sulfur distribution ratio , which has been discussed by Yang et al. elsewhere [4].
On the proposed coupling relationships [2] between and for the CaO-based slags, it can be observed in Figure 11 that the proposed term or after original data from Ban-ya et al. [1] or based on results by Yang et al. [3,4] keeps almost constant with the increase of four mass action concentration ratios for the CaO-based slags in reducing and oxidizing zones over a narrow temperature range. Thus, the proposed coupling relationships [2] between and for the CaO-based slags are independent of the comprehensive influence of iron oxides FetO and basic oxides expressed by the mass action concentration ratio or or or , or the mass percentage ratio (% FeO)/(% CaO) or (% Fe2O3)/(% CaO) or (% FetO)/(% CaO).
2.3.2. Comprehensive Effect of FetO and CaO on Coupling Relationships between Dephosphorization and Desulfurization Potentials for CaO-based Slags
The relationship between the aforementioned four mass action concentration ratios of various iron oxides to basic oxide CaO and calculated [3] phosphate capacity for the CaO-based slags is shown in the first layers of Figure 12, respectively. Likewise, the relationship between four mass action concentration ratios and the calculated [4] sulfide capacity for the CaO-based slags is also illustrated in the second layers of Figure 12, respectively. The relationship between four mass action concentration ratios and the determined term or after the original data from Ban-ya et al. [1], or calculated term or based on results by Yang et al. [3,5] for the CaO-based slags is also illustrated in the first and second layers of Figure 13, respectively.
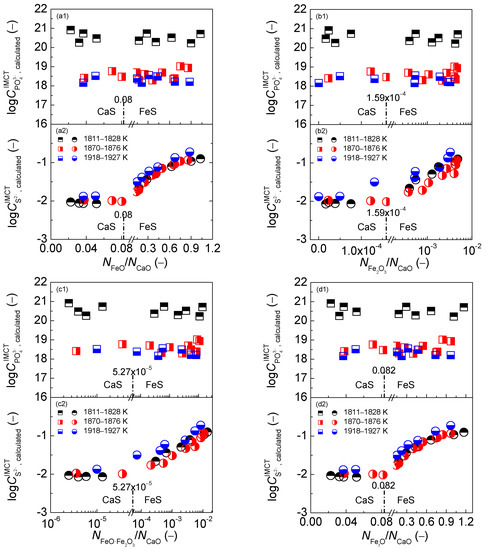
Figure 12.
Relationship of mass action concentration ratio (a) or (b) or (c) or (d) against the calculated [3] phosphate capacity by the IMCT– model in the first layer or calculated [5] sulfide capacity by the IMCT– model in the second layer for CaO–FeO–Fe2O3–Al2O3–P2O5 slags equilibrated with liquid iron over a temperature range of 1811 to 1927 K (1538 to 1654 °C), respectively.
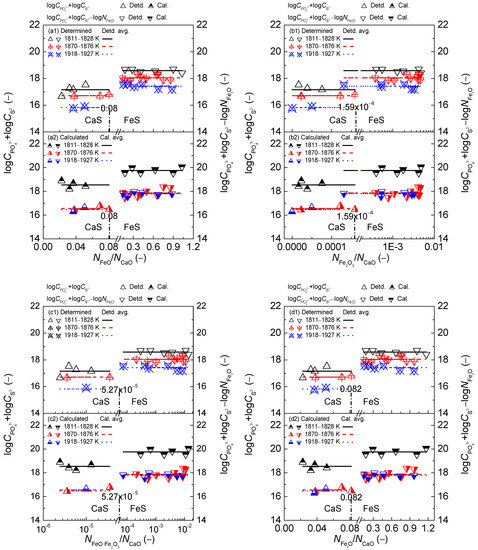
Figure 13.
Relationship of the mass action concentration ratio (a) or (b) or (c) or (d) against the determined term or after Ban-ya et al. [1] in the first layer or calculated term or based on results from Yang et al. [3,5] in the second layer for CaO–FeO–Fe2O3–Al2O3–P2O5 slags equilibrated with liquid iron over a temperature range of 1811 to 1927 K (1538 to 1654 °C), respectively.
With respect to the dephosphorization potential of the CaO-based slags, it can be observed in the first layers of Figure 12 that increasing the four mass action concentration ratios cannot result in an obvious variation of dephosphorization potential over a narrow temperature range. This result was explained by Yang et al. [3] as that greater values of calculated dephosphorization ability are the reason for larger ones of dephosphorization potential by the IMCT− model over the lower temperature range of 1811 to 1828 K (1538 to 1555 °C). With regard to the desulfurization potential of the CaO-based slags, it can be obtained from the second layers of Figure 12 that increasing four mass action concentration ratios can lead to the similar variation trend of sulfide capacity against slag oxidization ability expressed by of iron oxides in Figure 4(f2), which has been explained by Yang et al. elsewhere [5].
On the proposed coupling relationships [2] between and for the CaO-based slags, it can be observed in Figure 13 that the proposed term or after the original data from Ban-ya et al. [1] or based on results by Yang et al. [3,4] keeps almost constant with the increase of aforementioned four mass action concentration ratios for the CaO-based slags in the reducing and oxidizing zones over a narrow temperature range. The relationships of four mass action concentration ratios against calculated term or based on results by Yang et al. [3,4] are more accurate than those against determined terms after original data from Ban-ya et al. [1] for the CaO-based slags in the reducing and oxidizing zones. Thus, the proposed coupling relationships [2] between and for the CaO-based slags are certainly independent of the comprehensive influence of iron oxides FetO and basic oxides expressed by the mass action concentration ratio or or or , or the mass percentage ratio (% FeO)/(% CaO) or (% Fe2O3)/(% CaO) or (% FetO)/(% CaO).
3. Discussion on Proposed Coupling Relationships between Dephosphorization and Desulfurization Abilities or Potentials for CaO-based Slags
3.1. Magnitude of Proposed Coupling Relationships between Dephosphorization and Desulfurization Abilities for CaO-based Slags
Values of the phosphorus distribution ratio for the CaO-based slags in the reducing zone as shown in the first layers of Figure 1, Figure 6 and Figure 10 vary from 1.0 to 3.0, while data of the sulfur distribution ratio for the CaO-based slags in the reducing zone, as illustrated in the second layers of Figure 1, Figure 6 and Figure 10, change from 1.7 to 0.9. However, results of the proposed term for the CaO-based slags in the reducing zone, as displayed in Figure 2, Figure 7 and Figure 11, fluctuate from 6.7 to 8.5. Thus, the magnitude of proposed term for the CaO-based slags in the reducing zone is mainly decided by that of because the desulfurization ability indicates a five-time contribution to the proposed term compared with the one-time dephosphorization ability of . It is a well-known viewpoint that reducing slags exhibits good desulfurization ability with limited dephosphorization ability. This means that the magnitude of the proposed term for the CaO-based slags in the reducing zone is decided by desulfurization ability. Theoretically, higher temperature can promote the desulfurization reaction of the CaO-based slags. However, increasing the temperature from 1811 to 1927 K (1538 to 1654 °C) cannot cause an obvious increase of desulfurization ability for the CaO-based slags as shown in the second layers of Figure 1, Figure 6 and Figure 10. Furthermore, higher temperature can inhibit dephosphorization reactions of the CaO-based slags. Thus, increasing the temperature from 1811 to 1927 K (1538 to 1654 °C) can result in an effectively decreasing influence on dephosphorization ability of the CaO-based slags as illustrated in the first layers of Figure 1, Figure 6 and Figure 10. Evidently, increasing temperature from 1811 to 1927 K (1538 to 1654 °C) can lead to a slightly decreasing tendency of the proposed term for the CaO-based slags in the reducing zone in Figure 2, Figure 7 and Figure 11.
Values of the phosphorus distribution ratio for the CaO-based slags in the oxidizing zone, as shown in the first layers of Figure 1, Figure 6 and Figure 10 vary from 3.0 to 8.0, while the data of the sulfur distribution ratio for the CaO-based slags in the oxidizing zone as illustrated in the second layers of Figure 1, Figure 6 and Figure 10 change from 0.7 to 1.0. However, the results of the proposed term for the CaO-based slags in the oxidizing zone, as displayed in Figure 2, Figure 7 and Figure 11, fluctuate from 9.2 to 10.6. Thus, the magnitude of proposed term for the CaO-based slags in the oxidizing zone is mainly decided by that of . It is a widely-accepted viewpoint that oxidizing slags exhibit good dephosphorization ability with limited desulfurization ability. This indicates that the proposed term for the CaO-based slags in the oxidizing zone is controlled by the dephosphorization ability. This means that the temperature effect on the dephosphorization ability of the CaO-based slags can decide the influence of the increasing temperature from 1811 to 1927 K (1538 to 1654 °C) on the proposed term for the CaO-based slags in the oxidizing zone in Figure 2, Figure 7 and Figure 11.
3.2. Magnitude of Proposed Coupling Relationships between Dephosphorization and Desulfurization Potentials for CaO-based Slags
Values of phosphate capacity for the CaO-based slags in the reducing zone, as shown in the first layers of Figure 4, Figure 8 and Figure 10, vary from 18.0 to 20.0, while the data of sulfide capacity for the CaO-based slags in the reducing zone, as illustrated in the second layers of Figure 4, Figure 8 and Figure 10, keep almost constant at −2.0. However, the results of the proposed term for the CaO-based slags in the reducing zone, as shown Figure 5, Figure 9 and Figure 13 fluctuate from 16.5 to 18.5. Thus, the magnitude of the proposed term for the CaO-based slags in the reducing zone is mainly decided by that of because the dephosphorization potential of the CaO-based slags in the reducing zone with smaller oxygen partial potential can produce a large value of according to the defined phosphate capacity by Wagner [29]. Thus, using inaccurate values of phosphate capacity in the proposed term for the CaO-based slags in the reducing zone can cause some degree of risk for designing or optimizing slag chemical composition, as described in Section 3.3. As pointed out in Section 2.3.2 that the calculated [3] can relieve the experimental uncertainties. Therefore, the calculated [3] for the CaO-based slags, rather than the determined [3] after the original data from Ban-ya et al. [1], is applied in this study.
Values of phosphate capacity for the CaO-based slags in the oxidizing zone, as shown in the first layers of Figure 4, Figure 8 and Figure 10, also vary from 18.0 to 20, while the data of sulfide capacity for the CaO-based slags in the oxidizing zone, as illustrated in the second layers of Figure 4, Figure 8 and Figure 10 change from −2.0 to −0.75. However, the results of proposed term for the CaO-based slags in the oxidizing zone, as displayed in Figure 5, Figure 9 and Figure 13 fluctuate from 17.5 to 20.0. Thus, the magnitude of the proposed term for the CaO-based slags in the oxidizing zone is mainly decided by that of . Oxidizing slags have good dephosphorization ability with limited desulfurization ability. This means that the proposed term or for the CaO-based slags in reducing and oxidizing zones includes the key factor of dephosphorization potential. In addition, higher temperature can restrain the dephosphorization ability and potential of the CaO–based slags. Increasing the temperature from 1811 to 1927 K (1538 to 1654 °C) can effectively decrease the dephosphorization potential of the CaO-based slags as illustrated in the first layers of Figure 4, Figure 8 and Figure 12. Thus, increasing the temperature from 1811 to 1927 K (1538 to 1654 °C) can result in a slightly decreasing tendency of the proposed term or for the CaO-based slags in the reducing and oxidizing zones in Figure 5, Figure 9 and Figure 13.
Considering the large difference of magnitude between and , the proposed coupling relationships [2] as and , rather than and , are recommended to design or optimize the chemical composition of slags under the fixed experimental uncertainties as described in Section 3.3.
3.3. Prospect and application for Proposed Coupling Relationship between Dephosphorization and Desulfurization Abilities or Potentials for CaO–based Slags
The proposed coupling relationships [2] for CaO–FeO–Fe2O3–Al2O3–P2O5 slags are not only independent of slag oxidization ability as expected but are also irrelevant to slag chemical composition represented by the reaction abilities of components, two kinds of slag basicity as simplified complex basicity and optical basicity , and the comprehensive effect of iron oxides FetO and basic oxide CaO. Thus, the proposed coupling relationships [2] for the CaO-based slags remain almost constant over a narrow temperature range although changing slag chemical composition can significantly affect its dephosphorization and desulfurization abilities or potentials. This means that the maximum values of the sum of dephosphorization and desulfurization abilities or potentials for the assigned slags in reducing and oxidizing zones can be determined by the proposed coupling relationships [2]. Additionally, the counteraction characteristics between the dephosphorization and desulfurization abilities or potentials for reducing slags can be theoretically explained and quantitatively expressed as or . The promotive effect of slag oxidization ability described by the comprehensive mass action concentration of iron oxides on the maximum values of the sum of dephosphorization and desulfurization abilities or potentials for oxidizing slags can be reasonably explained and quantitatively described as or .
It has been verified by Yang et al. [3,4,5] that the IMCT– [3] or IMCT– [3] or IMCT– [4] or IMCT– [5] models can be accurately applied to the prediction of dephosphorization and desulfurization abilities or potentials of the assigned slags. Thus, the dephosphorization abilities or potentials of the assigned slags or fluxes can be theoretically predicted from its desulfurization abilities or potentials based on the proposed coupling relationships [2], and vice versa. This means that a new method of designing or optimizing chemical composition of slags or fluxes with the assigned dephosphorization abilities or potentials can be developed based on the proposed coupling relationships [2].
The proposed coupling relationships [2] between dephosphorization and desulfurization abilities as in the reducing zone and as in the oxidizing zone have been verified to be valid based on the reported equilibrium experiments in laboratory scale by Ban-ya et al. [1] Actually, reactions of dephosphorization and desulfurization at the final stage of many refining processes such as the dephosphorization pretreatment process of hot metal [15,16,17], the simultaneous dephosphorization and desulfurization operation of iron-based melts during secondary refining process [1,2,3,4,5], desulfurization reaction during the ladle furnace (LF) refining process [8,9], dephosphorization reaction at the blowing end-point during top–bottom combined blown converter steelmaking process [10,11], and so on can be considered to reach quasi-equilibrium at the interface between the slags and metal. It can be deduced that the proposed coupling relationships [2] are also suitable to industrial operations during the dephosphorization and desulfurization processes.
4. Conclusions
The proposed coupling relationships between the dephosphorization and desulfurization abilities or potentials for CaO–FeO–Fe2O3–Al2O3–P2O5 slags over a large variation range of slag oxidization ability during the secondary refining process of molten steel as or in the reducing zone and as or in the oxidizing zone have been further verified as valid and feasible through investigating the influence of slag chemical composition. The main summary remarks can be obtained as follows:
- (1)
- The proposed coupling relationships for the CaO-based slags in both the reducing and oxidizing zones are not only independent of slag oxidization ability described by the comprehensive mass action concentration of iron oxides but are also irrelevant to the reaction abilities of components expressed by the mass action concentrations over a narrow temperature range in comparison with significant influences of slag oxidization ability as well as reaction abilities of components on dephosphorization and desulfurization abilities or potentials.
- (2)
- The proposed coupling relationships for the CaO-based slags in both the reducing and oxidizing zones keep almost constant with the variation of two kinds of slag basicity as the simplified complex basicity and optical basicity over a narrow temperature range compared with the strong effects of two kinds of slags basicity on dephosphorization and desulfurization abilities or potentials.
- (3)
- The proposed coupling relationships for the CaO-based slags in both reducing and oxidizing zones are independent of the comprehensive effect of iron oxides FetO and basic oxide CaO described by the mass action concentration ratio or or or , or the mass percentage ratio (% FeO)/(% CaO) or (% Fe2O3)/(% CaO) or (% FetO)/(% CaO) in comparison with the large influences of the aforementioned comprehensive effect of iron oxides FetO and basic oxide CaO on dephosphorization and desulfurization abilities or potentials.
- (4)
- Increasing the temperature from 1811 to 1927 K (1538 to 1654 °C) can result in a slightly decreasing tendency of the proposed coupling relationships for the CaO-based slags in reducing and oxidizing zones.
- (5)
- Chemical composition of slags or fluxes with the assigned dephosphorization ability or potential can be theoretically designed or optimized by its desulfurization ability or potential, and vice versa, in terms of the obtained maximum values of dephosphorization and desulfurization abilities or potentials for the CaO-based slags in both reducing and oxidizing zones.
- (6)
- The proposed coupling relationships between and for the CaO-based slags as and in reducing and oxidizing zones are recommended to design or optimize the chemical composition of slags or fluxes due to a large difference of magnitude between phosphate capacity and sulfide capacity .
Author Contributions
X.M.Y. conceived and designed the study. J.Y.L. and M.Z. performed the simulations. X.M.Y. and J.Y.L. wrote the main draft of the manuscript. X.M.Y., J.Y.L. and F.J.Y. revised the manuscript. All authors contributed to the discussion of the results, and commented on the manuscript.
Funding
This research was funded by the Beijing Natural Science Foundation [Grant No. 2182069] and the National Natural Science Foundation of China [Grant No. 51174186].
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclatures
| Activity of components i in slags or element i in liquid iron relative to pure solid or liquid component i or element i as standard state with mole fraction as concentration unit and following Raoult’s law under the condition of taking ideal solution as reference state, i.e., , (–); | |
| Phosphate capacity of slags based on gas–slag equilibrium, (–); | |
| Sulfide capacity of slags based on gas–slag equilibrium, (–); | |
| Activity coefficient of element i in liquid iron related with activity , (–); | |
| Standard equilibrium constant of chemical reaction for forming component i or structural unit i, (–); | |
| Phosphorus distribution ratio between slags and liquid iron, defined as , (–); | |
| Sulphur distribution ratio between slags and liquid iron, defined as , (–); | |
| Mi | Relative atomic mass of element i or relative molecular mass of component i, (–); |
| Total mole number of all components in 100 g slags, (mol). | |
| Greek symbols | |
| Activity coefficient of component i in slags related with activity , (–); | |
| Optical basicity of slags, (–). | |
References
- Ban–ya, S.; Hino, M.; Sato, A.; Terayama, A.O. O, P and S Distribution Equilibria between Liquid Iron and CaO–Al2O3–FetO Slag Saturated with CaO. Tetsu–to–Hagané 1991, 77, 361–368. [Google Scholar]
- Yang, X.M.; Li, J.Y.; Zhang, M.; Chai, G.M.; Duan, D.P.; Zhang, J. Coupling Relationship between Dephosphorization and Desulfurization Abilities or Potentials for CaO–FeO–Fe2O3–Al2O3–P2O5 Slags over a Large Variation Range of Slag Oxidization Ability Based on the Ion and Molecule Coexistence Theory. Ironmak. Steelmak. 2018, 45, 25–43. [Google Scholar] [CrossRef]
- Yang, X.M.; Chai, G.M.; Zhang, M.; Li, J.Y.; Liang, Q.; Zhang, J. Thermodynamic Models for Predicting Dephosphorization Ability and Potential of CaO–FeO–Fe2O3–Al2O3–P2O5 Slags during Secondary Refining Process of Molten Steel Based on the Ion and Molecule Coexistence Theory. Ironmak. Steelmak. 2016, 43, 663–687. [Google Scholar] [CrossRef]
- Yang, X.M.; Li, J.Y.; Zhang, M.; Zhang, J. Prediction Model of Sulfur Distribution Ratio between CaO–FeO–Fe2O3–Al2O3–P2O5 Slags and Liquid Iron in a Large Variation Range of Oxygen Potential during Secondary Refining Process of Molten Steel Based on the Ion and Molecule Coexistence Theory. Ironmak. Steelmak. 2016, 43, 39–55. [Google Scholar] [CrossRef]
- Yang, X.M.; Li, J.Y.; Chai, G.M.; Zhang, M.; Zhang, J. Prediction Model of Sulfide Capacity for CaO–FeO–Fe2O3–Al2O3–P2O5 Slags in a Large Variation Range of Oxygen Potential Based on the Ion and Molecule Coexistence Theory. Metall. Mater. Trans. B 2014, 45, 2118–2137. [Google Scholar] [CrossRef]
- Yang, X.M.; Jiao, J.S.; Ding, R.C.; Shi, C.B.; Guo, H.J. A Thermodynamic Model for Calculating Sulphur Distribution Ratio between CaO–SiO2–MgO–Al2O3 Ironmaking Slags and Carbon Saturated Hot Metal Based on the Ion and Molecule Coexistence Theory. ISIJ Int. 2009, 49, 1828–1837. [Google Scholar] [CrossRef]
- Shi, C.B.; Yang, X.M.; Jiao, J.S.; Li, C.; Guo, H.J. A Sulphide Capacity Prediction Model of CaO–SiO2–MgO–Al2O3 Ironmaking Slags Based on the Ion and Molecule Coexistence Theory. ISIJ Int. 2010, 50, 1362–1372. [Google Scholar] [CrossRef]
- Yang, X.M.; Shi, C.B.; Zhang, M.; Chai, G.M.; Wang, F. A Thermodynamic Model of Sulfur Distribution Ratio between CaO–SiO2–MgO–FeO–MnO–Al2O3 Slags and Molten Steel during LF Refining Process Based on the Ion and Molecule Coexistence Theory. Metall. Mater. Trans. B 2011, 42, 1150–1180. [Google Scholar] [CrossRef]
- Yang, X.M.; Shi, C.B.; Zhang, M.; Chai, G.M.; Zhang, J. A Sulfide Capacity Prediction Model of CaO–SiO2–MgO–FeO–MnO–Al2O3 Slags during LF Refining Process Based on the Ion and Molecule Coexistence Theory. Metall. Mater. Trans. B 2012, 43, 241–266. [Google Scholar] [CrossRef]
- Yang, X.M.; Duan, J.P.; Shi, C.B.; Zhang, M.; Zhang, Y.L.; Wang, J.C. A Thermodynamic Model of Phosphorus Distribution Ratio between CaO–SiO2–MgO–FeO–Fe2O3–MnO–Al2O3–P2O5 Slags and Molten Steel during Top–Bottom Combined Blown Converter Steelmaking Process Based on the Ion and Molecule Coexistence Theory. Metall. Mater. Trans. B 2011, 42, 738–770. [Google Scholar] [CrossRef]
- Yang, X.M.; Shi, C.B.; Zhang, M.; Duan, J.P.; Zhang, J. A Thermodynamic Model of Phosphate Capacity for CaO–SiO2–MgO–FeO–Fe2O3–MnO–Al2O3–P2O5 Slags Equilibrated with Molten Steel during a Top–Bottom Combined Blown Converter Steelmaking Process Based on the Ion and Molecule Coexistence Theory. Metall. Mater. Trans. B 2011, 42, 951–976. [Google Scholar] [CrossRef]
- Yang, X.M.; Shi, C.B.; Zhang, M.; Zhang, J. A Thermodynamic Model for Prediction of Iron Oxide Activity in Some FeO–Containing Slag Systems. Steel Res. Int. 2012, 83, 244–258. [Google Scholar] [CrossRef]
- Yang, X.M.; Zhang, M.; Zhang, J.L.; Li, P.C.; Li, J.Y.; Zhang, J. Representation of Oxidation Ability for Metallurgical Slags Based on the Ion and Molecule Coexistence Theory. Steel Res. Int. 2014, 85, 347–375. [Google Scholar] [CrossRef]
- Li, J.Y.; Zhang, M.; Guo, M.; Yang, X.M. Enrichment Mechanism of Phosphate in CaO–SiO2–FeO–Fe2O3–P2O5 Steelmaking Slags. Metall. Mater. Trans. B 2014, 45, 1666–1682. [Google Scholar] [CrossRef]
- Yang, X.M.; Li, J.Y.; Chai, G.M.; Duan, D.P.; Zhang, J. A Thermodynamic Model for Predicting Phosphorus Partition between CaO–based Slags and Hot Metal during Hot Metal Dephosphorization Pretreatment Process Based on the Ion and Molecule Coexistence Theory. Metall. Mater. Trans. B 2016, 47, 2279–2301. [Google Scholar] [CrossRef]
- Yang, X.M.; Li, J.Y.; Chai, G.M.; Duan, D.P.; Zhang, J. Critical Evaluation of Prediction Models for Phosphorus Partition between CaO–based Slags and Iron–based Melts during Dephosphorization Processes. Metall. Mater. Trans. B 2016, 47, 2302–2329. [Google Scholar] [CrossRef]
- Yang, X.M.; Li, J.Y.; Chai, G.M.; Duan, D.P.; Zhang, J. A Thermodynamic Model for Predicting Phosphate Capacity of CaO−based Slags during Hot Metal Dephosphorization Pretreatment Process. Ironmak. Steelmak. 2017, 44, 437–454. [Google Scholar] [CrossRef]
- Zhang, J. Computational Thermodynamics of Metallurgical Melts and Solutions; Metallurgical Industry Press: Beijing, China, 2007. [Google Scholar]
- Tsukihashi, F.; Nakamura, M.; Orimoto, T.; Sano, N. Thermodynamics of Phosphorus for the CaO–BaO–CaF2–SiO2 and CaO–Al2O3 Systems. Tetsu–to–Hagané 1990, 76, 1664–1671. [Google Scholar] [CrossRef]
- Ban-ya, S.; Hobo, M.; Kaji, T.; Itoh, T.; Hino, M. Sulphide Capacity and Sulphur Solubility in CaO-Al2O3 and CaO-Al2O3-CaF2 Slags. ISIJ Int. 2004, 44, 1810–1816. [Google Scholar] [CrossRef]
- Ban-ya, S.; Hino, M. Comments on “Evaluation of Thermodynamic Activity of Metallic Oxide in a Ternary Slag from the Sulphide Capacity of the Slag”. ISIJ Int. 2005, 45, 1754–1756. [Google Scholar] [CrossRef]
- Wei, S.K. Thermodynamics of Metallurgical Processes; Science Press: Beijing, China, 2010. [Google Scholar]
- Zhang, J.Y. Metallurgical Physicochemistry; Metallurgical Industry Press: Beijing, China, 2004. [Google Scholar]
- Chen, J.X. Handbook of Common Figures, Tables and Data for Steelmaking, 2nd ed.; Metallurgical Industry Press: Beijing, China, 2010. [Google Scholar]
- Sosinsky, D.J.; Sommerville, I.D. The Composition and Temperature Dependence of the Sulfide Capacity of Metallurgical Slags. Metall. Trans. B 1986, 17, 331–337. [Google Scholar] [CrossRef]
- Nakamura, T.; Ueda, Y.; Toguri, J.M. A Critical Review of Optical Basicity on Metallurgical application. In Proceedings of the Third International Conference on Metallurgical Slags and Fluxes, University of Strathclyde, Glasgow, Scotland, 27–29 June 1988; The Institute of Metals: London, UK; pp. 146–149. [Google Scholar]
- Mills, K.C.; Sridhar, S. Viscosities of Ironmaking and Steelmaking Slags. Ironmak. Steelmak. 1999, 26, 262–268. [Google Scholar] [CrossRef]
- Young, R.W.; Duffy, J.A.; Hassall, G.J.; Xu, Z. Use of Optical Basicity Concept for Determining Phosphorus and Sulphur Slag–Metal Partitions. Ironmak. Steelmak. 1992, 19, 201–219. [Google Scholar]
- Wagner, C. The Concept of the Basicity of Slags. Metall. Trans. B 1975, 6, 405–409. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).