The ESTPHAD Concept: An Optimised Set of Simplified Equations to Estimate the Equilibrium Liquidus and Solidus Temperatures, Partition Ratios, and Liquidus Slopes for Quick Access to Equilibrium Data in Solidification Software Part II: Ternary Isomorphous Equilibrium Phase Diagram
Abstract
1. Introduction
- (i)
- (ii)
2. The Thermodynamic Basis of the ESTPHAD Formalism in the Case of Ternary Alloys
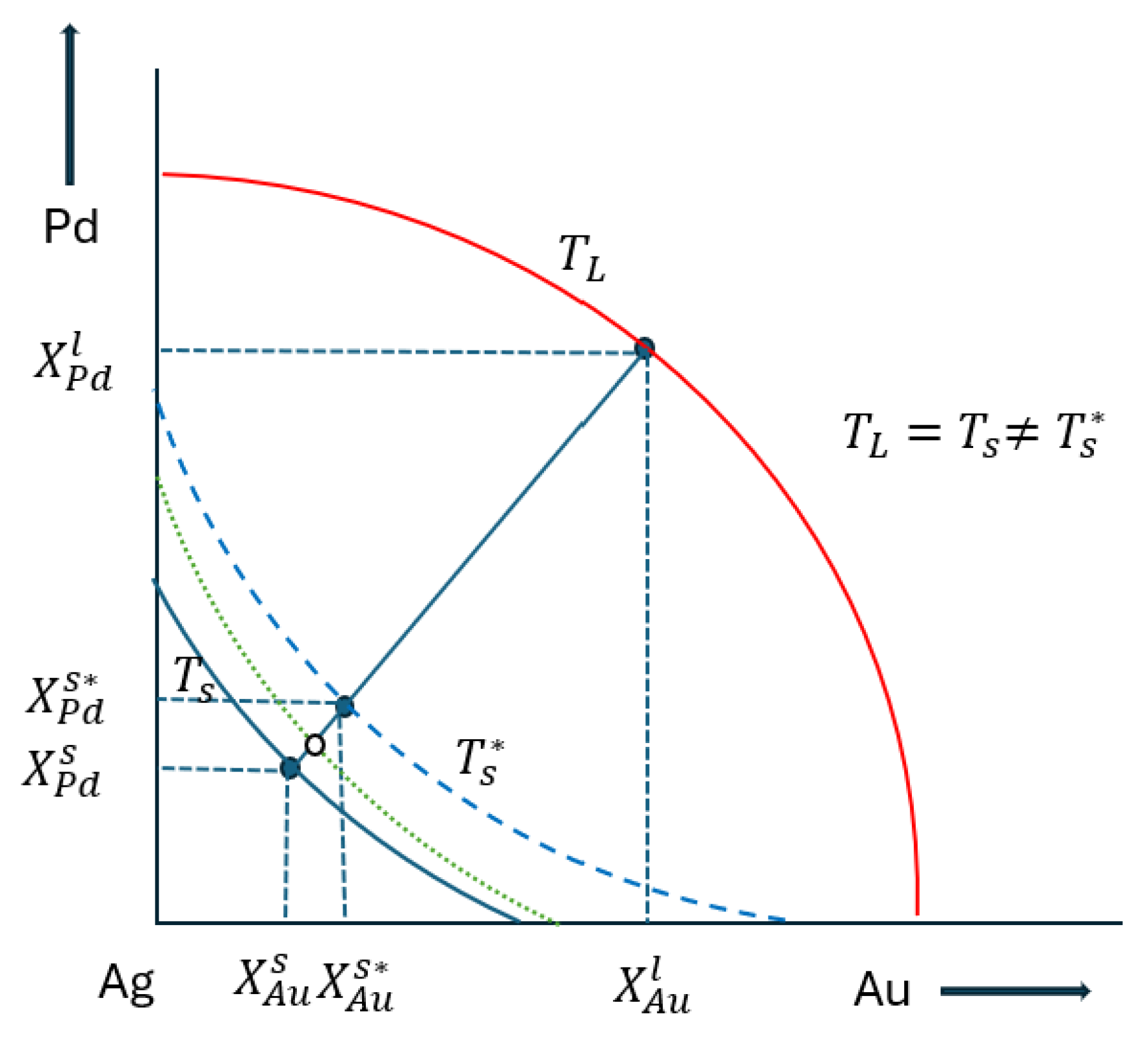
3. Determination of the Liquidus (TL) and Solidus (Ts) Temperature
3.1. Liquidus Temperature
3.2. Solidus Temperature
4. Determination of the Partition Ratios
4.1. as a Function of Liquidus Concentrations
4.2. as a Function of Solidus Concentrations
4.3. as a Function of Liquidus Concentration
4.4. as a Function of Solidus Concentration
5. Determination of the Constants of Liquidus Slopes
6. Calculation Methods of the Constants
6.1. Calculation of the Constants of Liquidus and Solidus Functions
6.1.1. First Estimation (Only the A-B and A-C BEPD Are Known)
6.1.2. Second Estimation (The A-B, A-C and B-C BEPD Are Known)
6.1.3. Third Estimation (The A-B and A-C BEPD and the Data of the Liquidus and Solidus Surfaces of the TEPD Are Known, the B-C BEPD Is Unknown)
6.1.4. Fourth Estimation (The A-B, A-C, B-C BEPD and the Data of the Liquidus and Solidus Surface of the TEPD Are Known)
6.1.5. Determination of the Liquidus and Solidus Isotherms Using an Iteration Method
6.2. Calculation of the Constants of the Partition Ratio
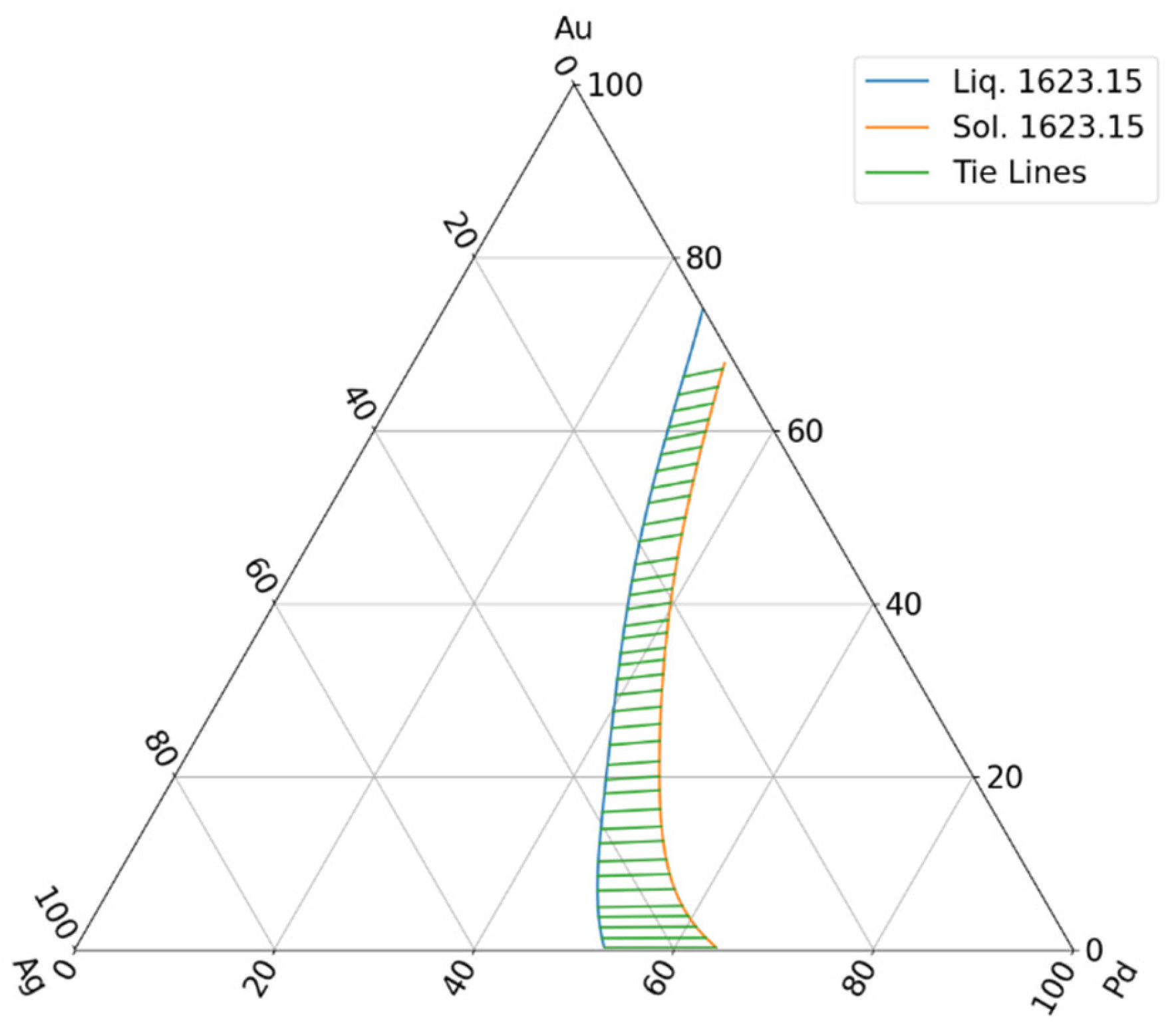
6.2.1. The Tie Lines (The Liquidus and Solidus Concentration Pairs) in TEPD Are Known from the CALPHAD-Type Calculation
6.2.2. If the Tie Lines (Liquidus and Solidus Concentration Pairs) Are Unknown, the TL and Ts Temperatures Are Determined at Many Concentrations Through Digitalisation of the Liquidus and Solidus Isotherms, and Another Method Must Be Followed
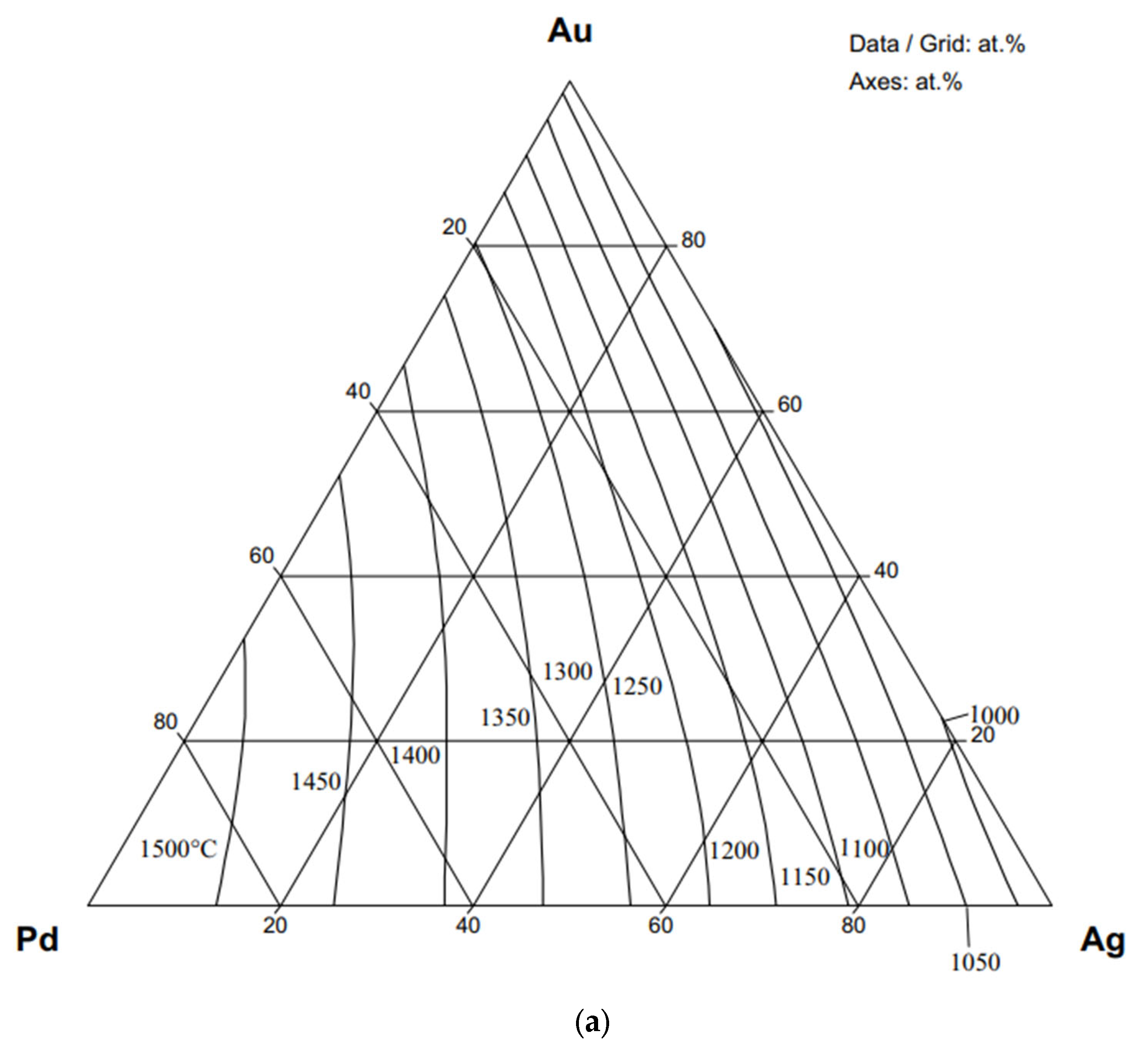
7. An Example for Calculating the Liquidus and Solidus Surfaces, Partition Coefficients, and Slope of the Liquidus Surface of an Isomorphous Ternary Equilibrium Phase Diagram (TEPD)
7.1. Data for Calculation
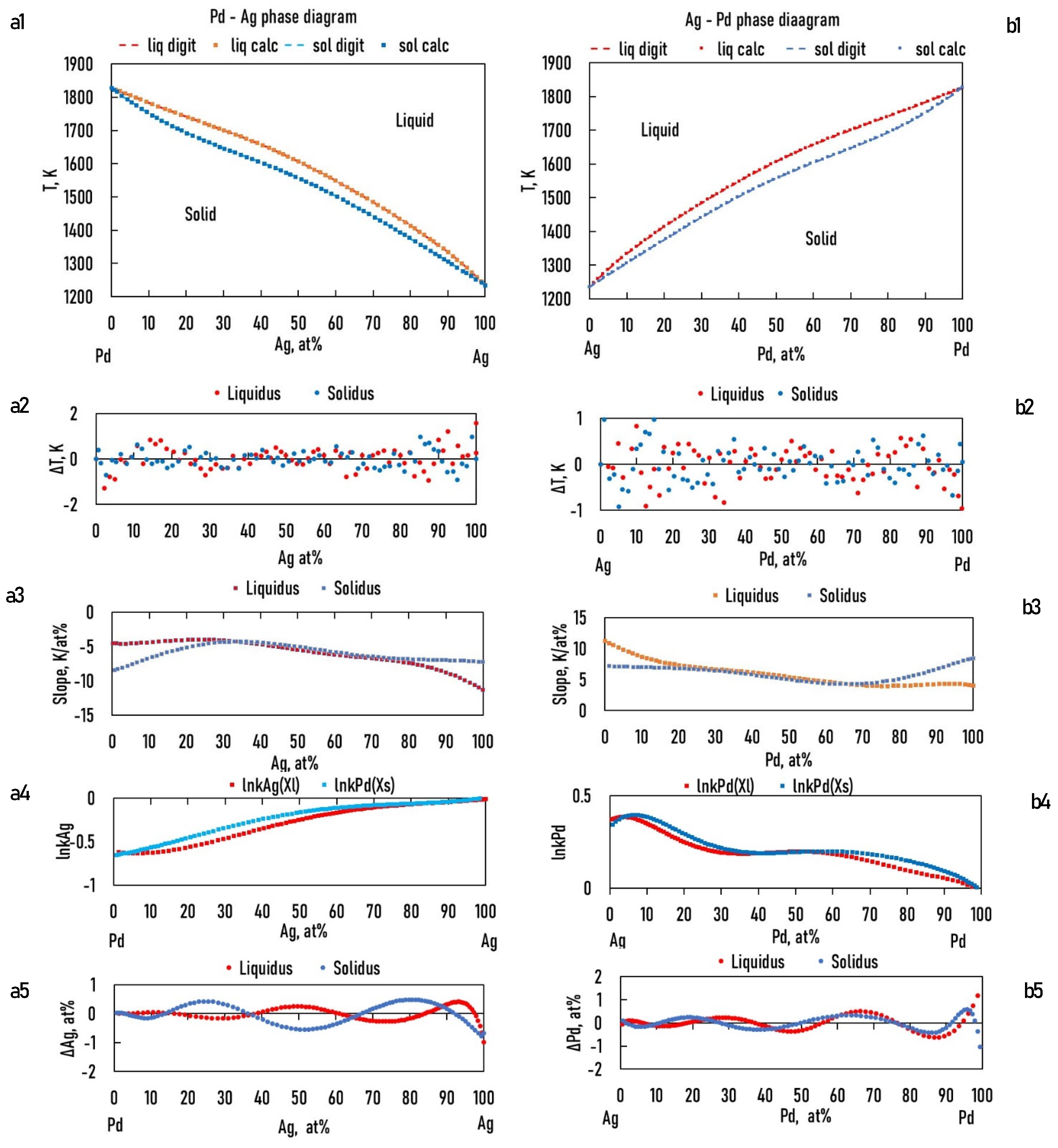
7.2. Calculation of the Liquidus and Solidus Temperatures, Liquidus Slopes, and Partition Ratios of BEPDs
7.2.1. Calculation of the Functions of the Liquidus and Solidus Temperatures of the BEPDs
7.2.2. Calculation of the Functions of the Slope of the Liquidus and Solidus Temperatures of BEPDs
7.2.3. Calculation of the Partition Ratios of the BEPDs
7.3. Calculation of the Liquidus and Solidus Temperatures, Slope of the Liquidus Surface, and Partition Ratios of Au and Pd in AgAuPd TEPDs
7.3.1. Calculation of the Liquidus and Solidus Temperatures
First Estimation
Second Estimation
Third Estimation
Fourth Estimation
Validation by Experiments
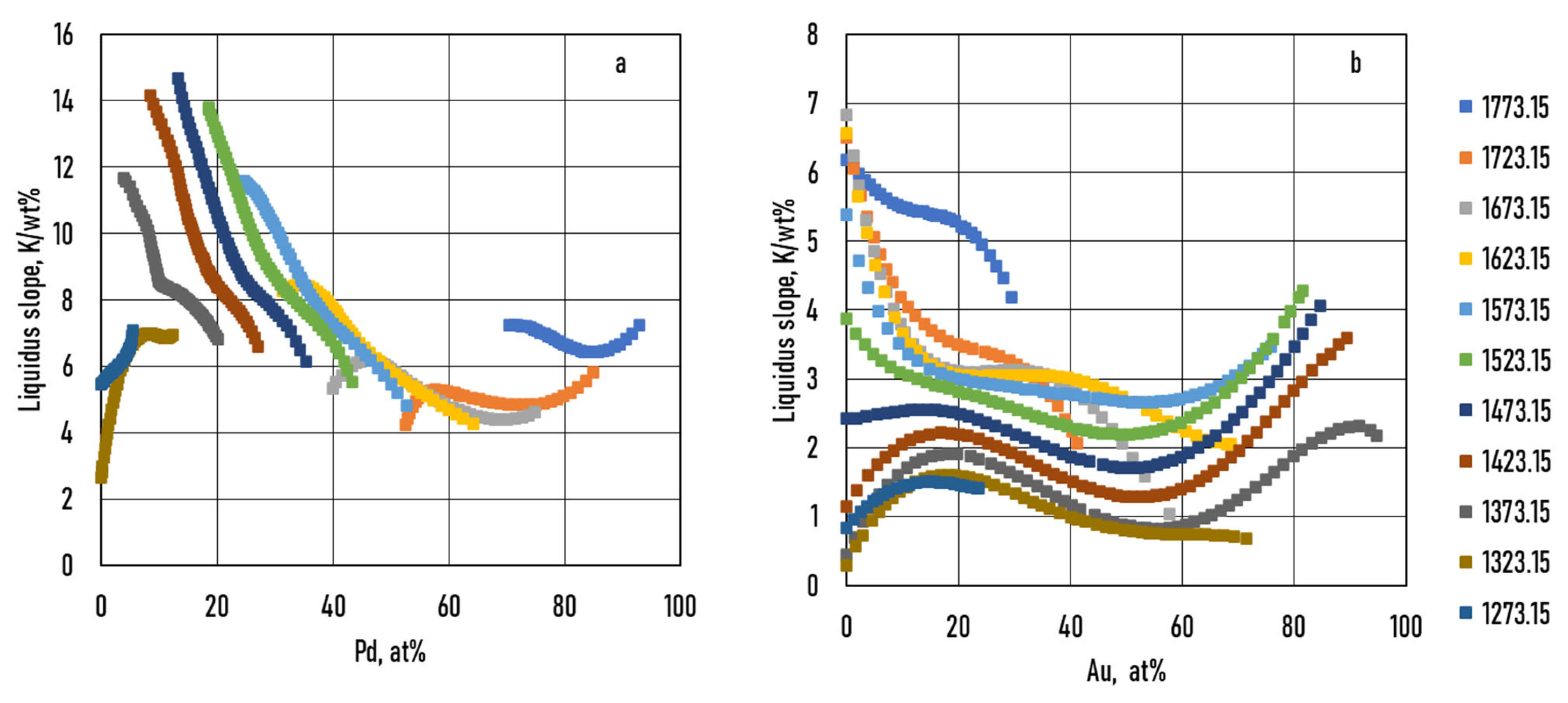
7.3.2. Calculation of the Liquidus and Solidus Slopes
7.3.3. Calculation of the Partition Ratios of the AgAuPd TEPD
First Step
Second Step (First Estimation)
Third Step (Second Estimation)
8. Liquidus and Solidus Temperatures, Liquidus Slopes, and the Partition Ratios
- The absolute maximum and average errors of the calculation of the liquidus and solidus temperatures of the BEPDs are less than 2 K and 0.5 K, respectively (R2 < 0.98). These are less than the error of the temperature measurement by the thermocouple in the temperature range of the investigated alloys. Therefore, the calculation method is suitable for estimating the liquidus and solidus temperatures in the case of binary alloys. Using the derivative of the function, the liquidus slope can be calculated easily. As in this case, the partition ratio can be determined from the phase diagram, and the constants of the function are also calculable.
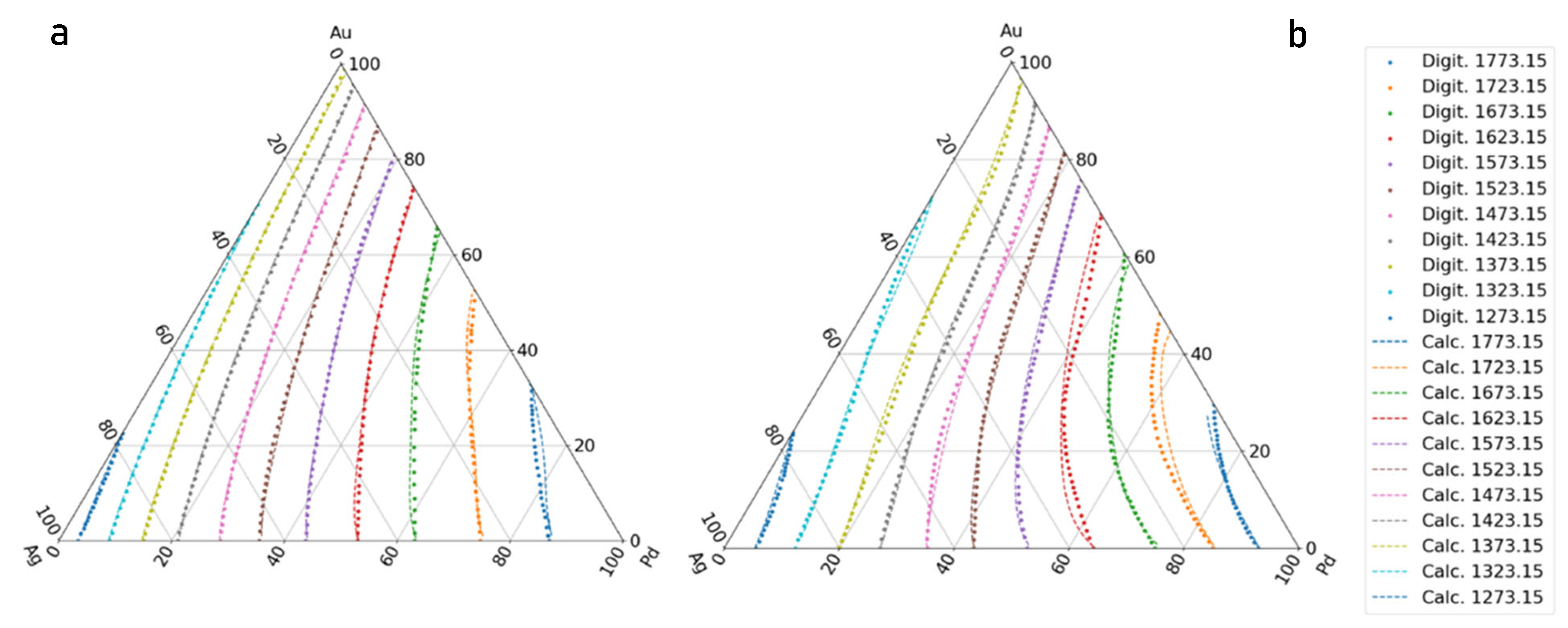
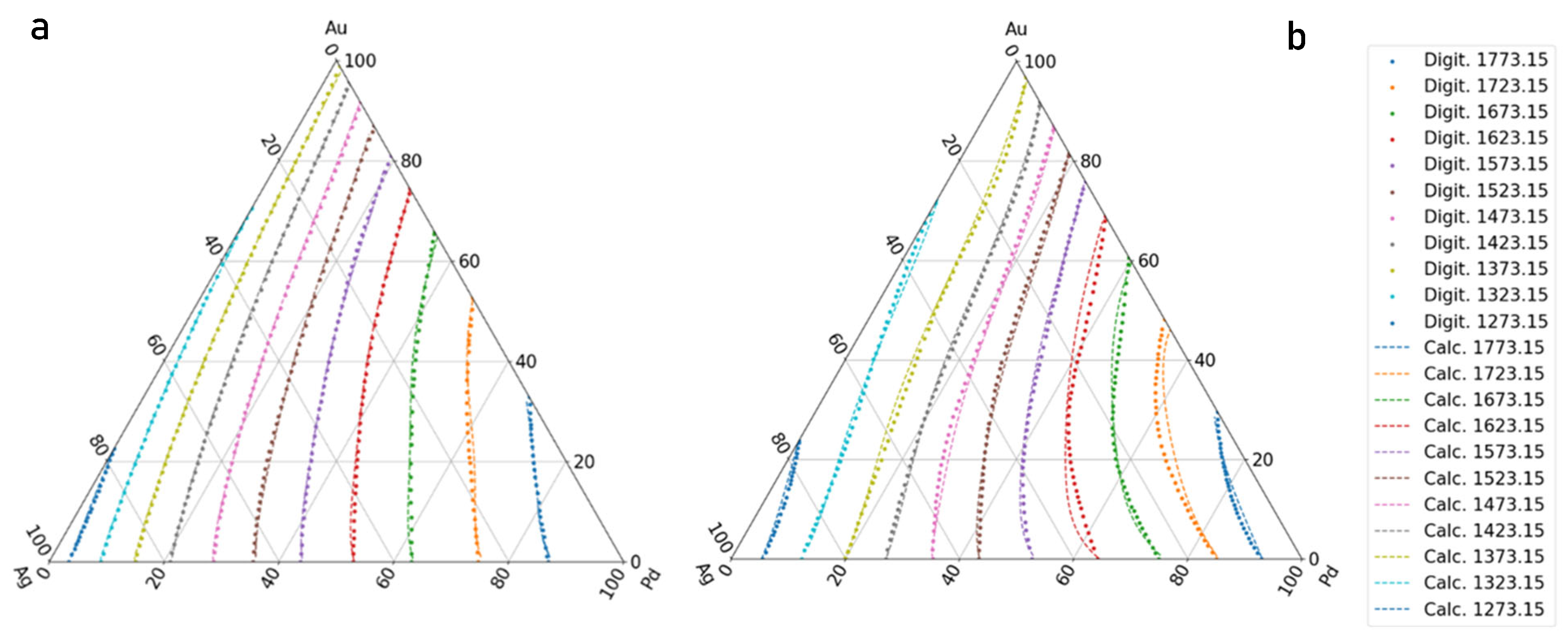
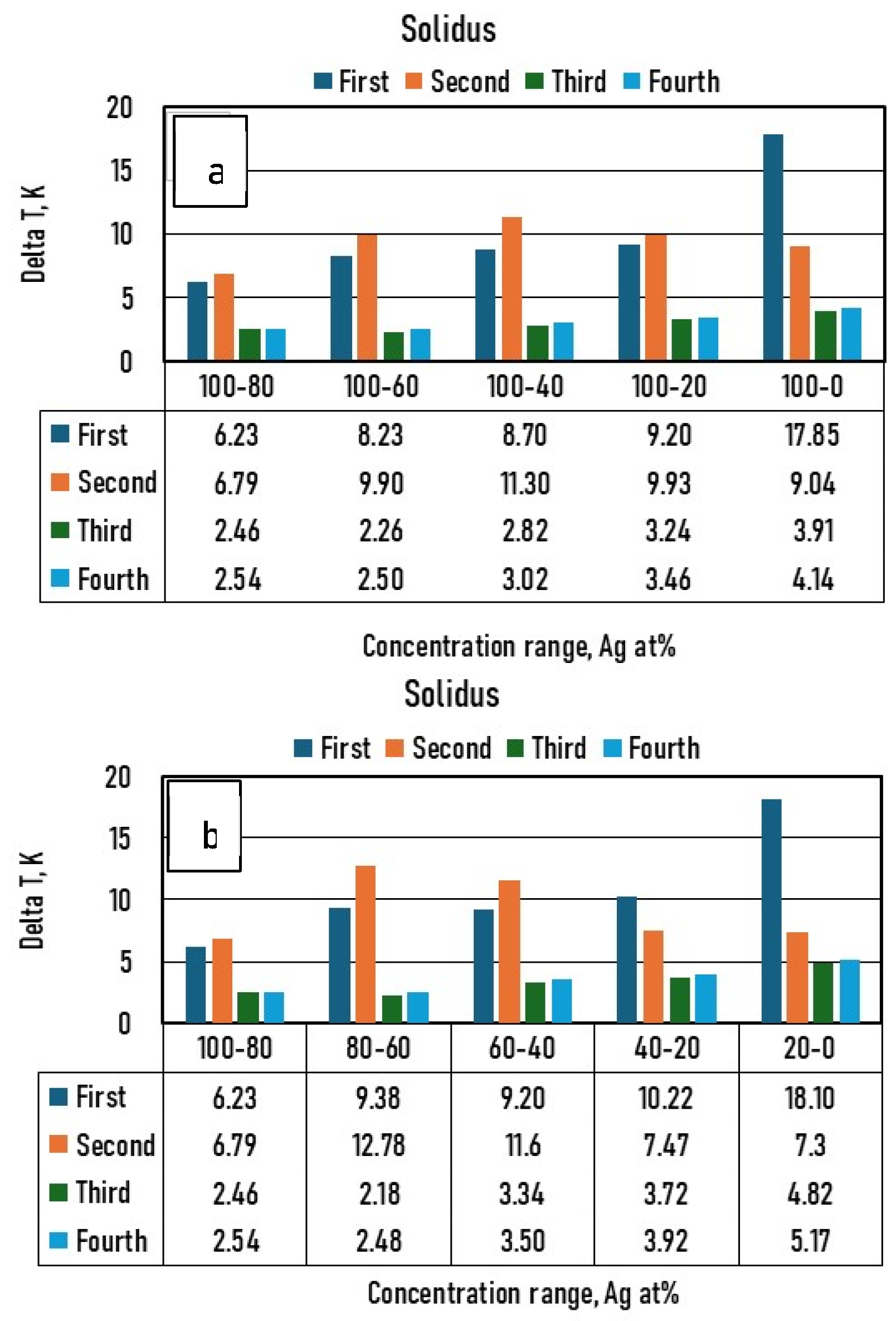
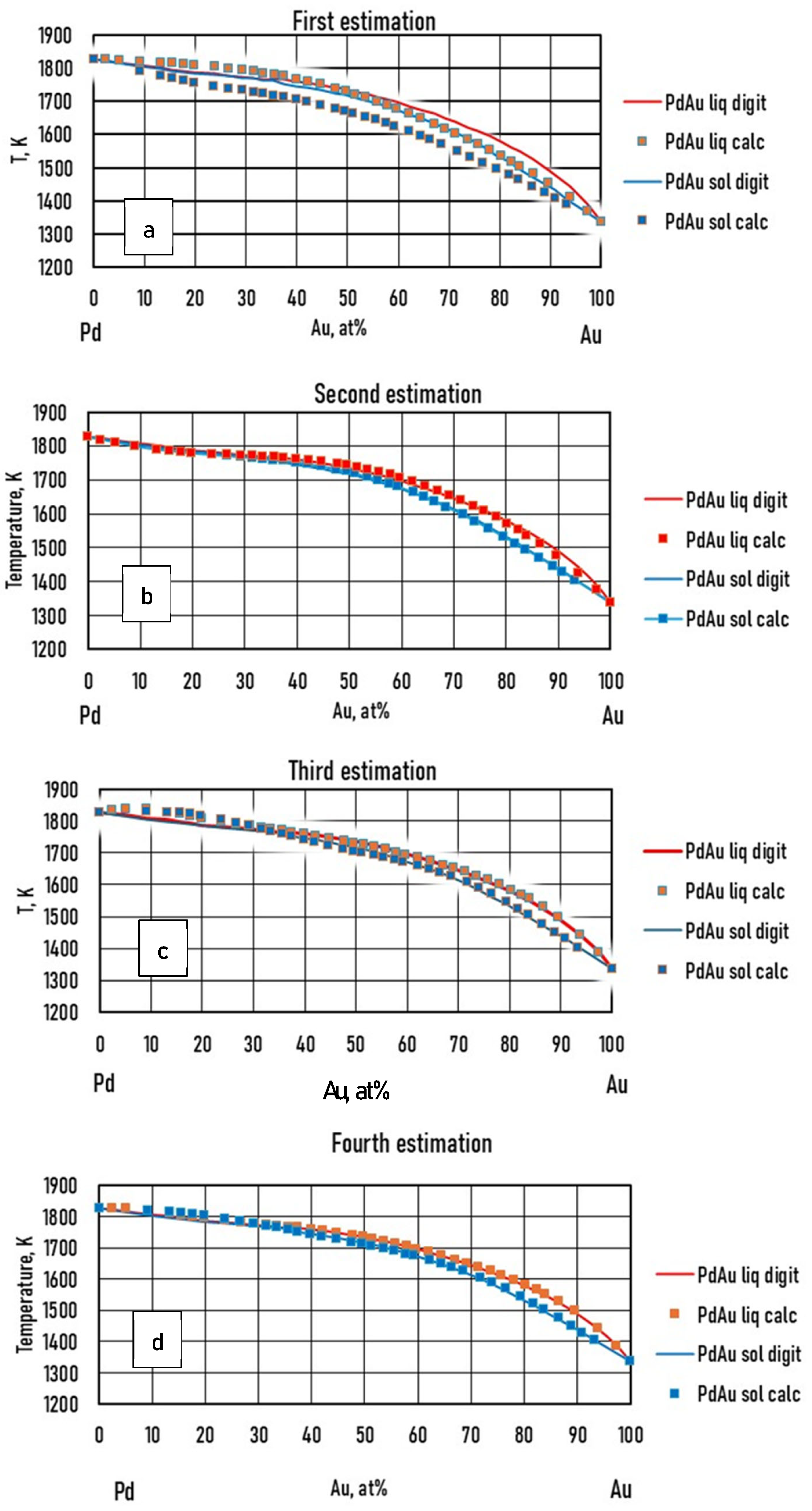
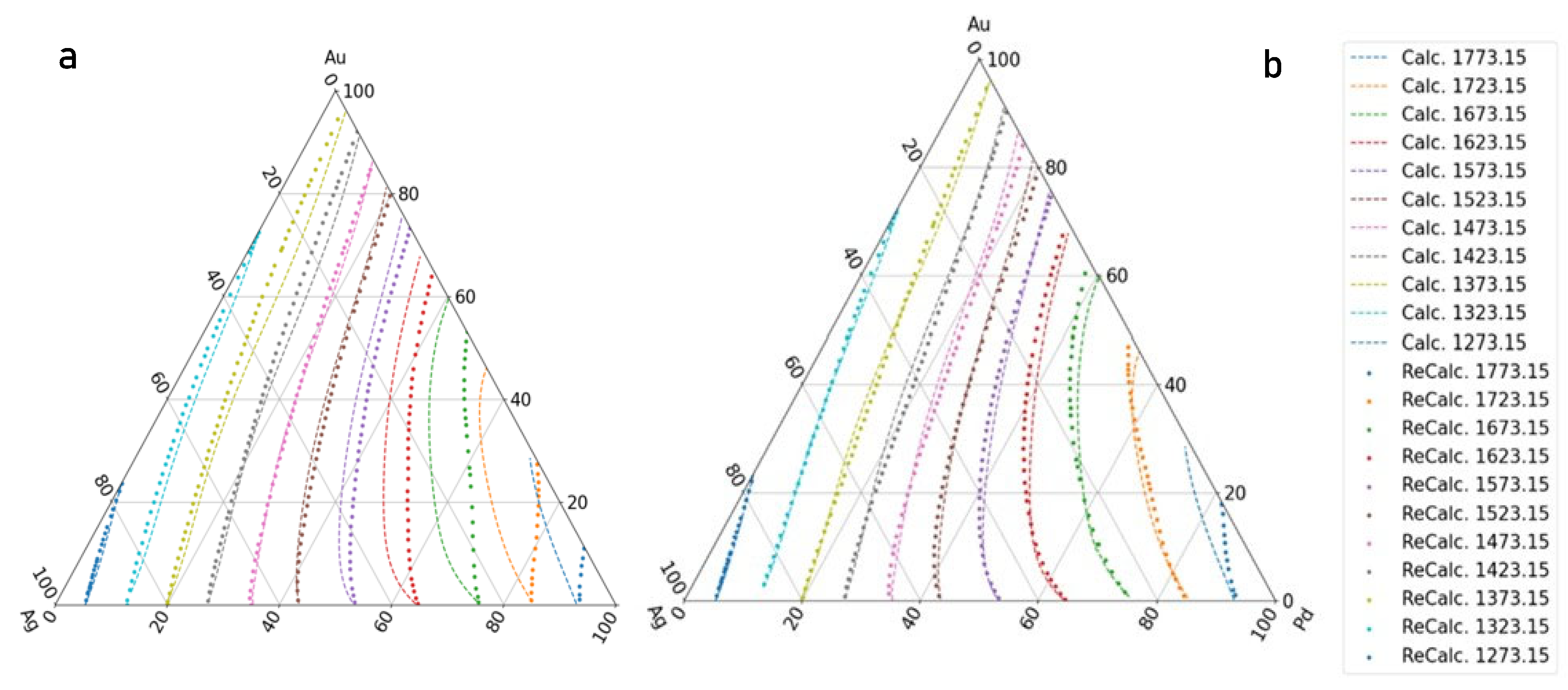
- If the liquidus and solidus isotherms of the TEPD and third BEPD (in this case, the Au-Pd) are unknown, but it is known or presumable that the TEPD is completely isomorphous, using only the functions of the Ag–Au and Ag–Pd BEPDs for the calculation (first estimation), the liquidus (liquidus isotherms) and solidus (solidus isotherms) temperatures can be estimated. In the 100–60 at% Ag range, the average error is less than 2 K for the liquidus (Figure 10a) and 10 K for the solidus (Figure 11a). Far from the Ag corner, the error increased, in the 20–0 at% Ag ranges, 10 K for the liquidus (Figure 10b) and 18,1 K for the solidus (Figure 11b). In the entire range (100–0 at% Ag), it is 9.37 K and 17,85 K in the case of liquidus and solidus, respectively. Consequently, the liquidus temperatures can be calculated with acceptable error near the Ag corner (100–40 at% Ag) because the error of the temperature measurement by the thermocouple is not better than 0.1% (at 1500 K, it is 1.5 K), while in the case of solidus temperatures, the calculation can only give estimated data. With the first estimation, the liquidus and solidus temperatures of the Pd-Au phase diagram can be estimated with relatively high average error, 20.72 K and 37.7 K in the case of liquidus and solidus, respectively (Figure 12a, Table 11). If the third BEPD is unknown, it would be advisable to estimate this BEPD with ~2% relative error. But this is better than nothing.
- If the third BEPD is known (in this case, the Au-Pd) using the functions of the Ag–Au and Ag–Pd BEPDs and the liquidus and solidus temperatures of the third BEPD, the functions are calculable. As a result of this, the error is similar to the error of the first estimation in the 100–80 at% Ag range in both cases. In the entire range (100–0 at% Ag), it decreased in both cases, because in the 20–0 at% range, the error decreased due to the effect of the data of the Au-Pd BEPD. The error of the liquidus and the solidus tempeature at Au-Pd BEPD drastically decreased (4.03 K and 4.56 K (Table 11). With this method, the estimation of the known third BEPD is significantly improved.
- In the third method using the functions of the Ag–Au and Ag–Pd BEPDS and the temperature data given from the liquidus and solidus isotherms of the Ag–Au–Pd TEPD (exept the data of the Au-Pd BEPD) to calculate the functions, the average error of the liquidus temperature is less than 2 K in the entire Ag concentration range. The average error is 1.34 K in the entire range of the liquidus (100–0 at% Ag). The average error of the solidus temperatures is less than 3 K in the 100–40 at% Ag range (Figure 10a), which is less than 0.2% of 1500 K. The average error of the solidus temperature in the entire range of the solidus (100–0 at% Ag, Figure 11a) is 3.91 K, and only near the Au–Pd BEPD (in the 20–0 at% Ag range, Figure 11b) does it increase to 4.82 K. Consequently, the error of the liquidus temperatures is better than the measurable one in the case of the liquidus in the entire concentration range, and then it is usable for the simulation, while the error of the solidus temperatures is slightly inorrect, and only in the 100–40 at% Ag range is precisely suitable.The error of the liquidus and solidus Au-Pd BEPD is greater than the error of the second estimations because the data of this BEPD were not considered (Table 11).
- Using the functions and the temperature data given from the liquidus and solidus isotherms of Ag–Au–Pd TEPD and the data of the Au-Pd BEPD (fourth estimation), the error of the calculated liquidus and solidus temperatures is very similar to the error of the third estimation (Figure 10 and Figure 11). The aim of this version is to improve the calculation of the liquidus and solidus temperature of the Au–Pd BEP, so the error is acceptable when calculating the liquidus and solidus isotherm using the fourth estimation (2.98 K and 5.64 K, Table 11).
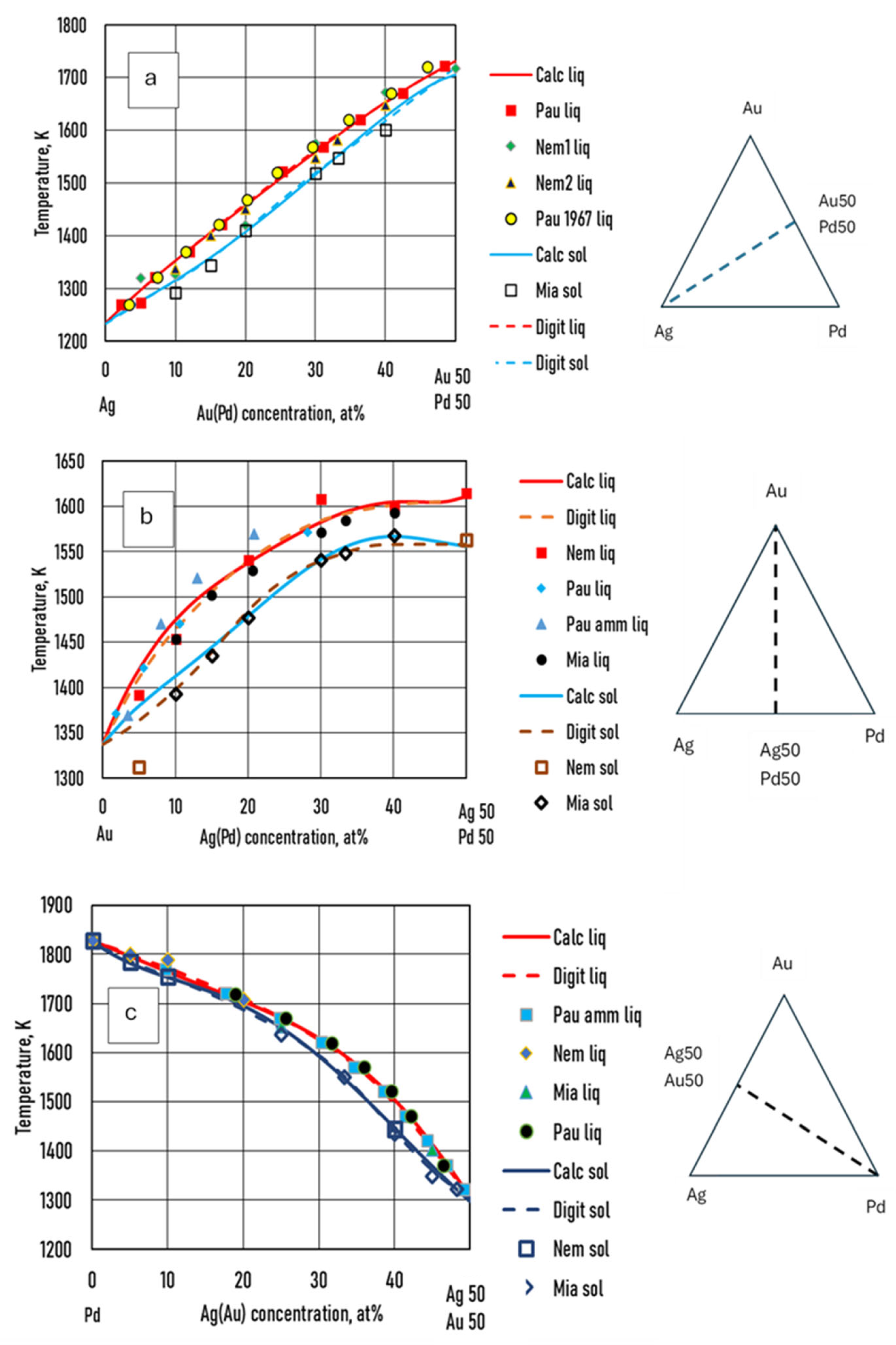
- Some authors [22,23,24] have measured the liquidus and solidus temperature at three sections of the Ag-Au-Pd TEPD: Ag–50at%Au50at%Pd, Au–50at%Ag50at%Pd, and Pd–50at%Ag50at%Au (Figure 12). From these measured data, liquidus and solidus curves were constructed by the authors for these sections. These curves were digitalised (dotted curves) and compared to the ESTPHAD calculations (fourth estimation, continuous curves). The difference between the digitalised and calculated curves is negligible, and the estimation of the measured data using these curves is acceptable, considering that between 1300 and 1800 K, it is difficult to measure the temperature.
- Since the temperature data are not derived from CALPHAD-type calculations, but from the digitalisation of the isotherms of the liquidus and solidus surfaces, there are no congruent liquidus and solidus concentrations, and tie lines are not known. Starting from the partition ratios from the BEPDs, we developed an estimation method in the TEPD to determine the partition ratios. In the first step, we used the BEPD partition ratios to calculate the concentrations of the solid phase (first estimation) in equilibrium with the concentrations of the liquid phase. In the range of 100–40 Ag at%, the error of the calculated temperature is less than 6 K, which, if nothing else, is acceptable as an estimate, but in the range of 40–0 Ag at% the error increases very significantly and cannot be used as an estimate. With the method developed by us (second estimation), the error is around 2 K in the range of 100–60 Ag at%, which causes an error of 0.2 at% at a slope of 10 K/at%, and 0.4 at% at a slope of 5 K/at%, which is acceptable even in simulations. It should be noted, however, that since the procedure contains an approximation, namely that the slope of the tie lines in the TEPD is equal to the slope of the tie lines determined from the BEPD partition ratios. If this approximation is very different from reality (which is not very likely), then the error could have been much more significant.
9. Summary
- (1)
- The liquidus and solidus surfaces of the TEPD can be calculated even in a relatively significant alloying range using the liquidus and solidus functions of the two BEPDs, which contain the base element, when the isotherms of the TEPD are not known (first estimation);
- (2)
- If the third BEPD is known, which does not contain the base element, it can be used to make the calculation more accurate, as in the first estimation, (second estimation);
- (3)
- Knowing the data of the liquid and solidus surfaces (isotherms) of the v, the functions can calculate the liquidus and solidus temperatures in the entire concentration range with the accuracy required for the simulations (third estimation);
- (4)
- Using the third BEPD, if it is known (similarly to the second estimation), the calculation can be further refined (fourth estimation);
- (5)
- The slopes of the liquidus surface can be calculated by deriving the function of the liquid surface;
- (6)
- In the case of graphically known TEPDs, the partition ratios are not known, but by using the partition ratios of the BEPDs and an approximation method developed in this work, a good result can be obtained that can be used in a relatively large concentration range during the solidification simulation;
- (7)
- The functions used for the calculation of liquidus, solidus temperature, liquidus slopes and partition ratios have a hierarchical structure, while in the case of TEPD, the functions used in the calculations contain the functions used in BEPD, completed by delta functions calculated from the TEPD data. As we will show later, this principle can be extended to the calculation of EPDs containing four, five, etc., alloying elements.
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Acronim | |
| CALPHAD | CALculation of PHAse Diagram |
| ESTPHAD | ESTimation of PHAse Diagram |
| EPD | equilibrium phase diagram |
| BEPD | binary equilibrium phase diagram |
| TEPD | ternary equilibrium phase diagram |
| Symbols | |
| free energy of alloy | |
| chemical potential | |
| free energy of A, B, and C elements | |
| free energy of A, B, and C elements in the liquid phase | |
| free energy of A, B, and C elements in the solid phase | |
| concentration of A, B, and C elements of the alloy | |
| concentration of A, B, and C elements in the liquid phase | |
| concentrations of A, B, and C elements in the solid phase | |
| partial molar free energy of A, B, and C elements in the liquid phase | |
| partial molar free energy of A, B, and C elements in solid phase | |
| free enthalpies change of A and B elements at solidification | |
| T | absolute temperature |
| , | enthalpies change of A, B, and C elements at solidification |
| liquidus temperature of pure A, B, and C elements | |
| liquidus and solidus temperature of the A-B-C TEPD | |
| gas constant | |
| partition ratio of B and C elements in the A-B and A-C alloy | |
| , and | partition ratio of B and C elements in the A-B-C alloy*-******* |
| liquidus slope in A-B-C TEPD | |
| numerator in the case of the slope calculation, | |
| and | maps for the calculation of the ), ) and ), ) functions |
| , and | map for the calculation of the functions |
| and | constants of the ) and ) functions |
| and | constants of the ) and ) functions |
| (i) and | constants of the functions |
| , , | maps for the calculation of the , and , functions |
| constants of the and functions | |
| constants of the and functions | |
| , and and | maps for the calculation of the , and and functions |
| (i) and | constants of the and functions |
| (i) and | constants of the and functions |
| Subscripts | |
| m, i | number of constants |
| AB, AC and ABC | A-B, A-C and A-B-C alloys |
| Superscripts | |
| l, s | liquid, solid |
References
- Kaufman, L.; Bernstein, H. Computer Calculation of Phase Diagrams; Academic Press: New York, NY, USA, 1970. [Google Scholar]
- Yan, X.; Chen, S.; Xie, F.; Chang, Y.A. Computational and experimental investigation of microsegregation in an Al-rich Al–Cu–Mg–Si quaternary alloy. Acta. Mater. 2002, 50, 2199–2207. [Google Scholar] [CrossRef]
- Ferreira, I.L.; Garcia, A.; Nestler, B. On macrosegregation in ternary Al-Cu-Si alloys: Numerical and experimental analysis. Scr. Mater. 2004, 50, 407–411. [Google Scholar] [CrossRef]
- Du, Q.; Eskin, D.G.; Katgerman, L. Modeling Macrosegregation during Direct-Chill Casting of Multicomponent Aluminium Alloys. Metall. Mater. Trans. A 2007, 38, 180–189. [Google Scholar] [CrossRef]
- Kattner, U. The Thermodynamic Modelling of Multicomponent Phase Equilibria. JOM 1997, 49, 14–19. [Google Scholar] [CrossRef]
- Greven, K.; Ludwig, A.; Hofmeister, T.; Sahm, R.R. Solidification of Metallic Melts in Research and Technology; Ludwig, A., Ed.; Wiley VCH: Weinheim, Germany, 1999; p. 119. [Google Scholar]
- Grafe, U.; Böttger, B.; Tiaden, J.; Fries, S.G. Coupling of Multicomponent Thermodynamic Databases to a Phase Field Model: Application to Solidification and Solid State Transformations of Superalloys. Scr. Mater. 2000, 42, 1179–1186. [Google Scholar] [CrossRef]
- Boettinger, W.J.; Coriell, S.R.; Greer, A.L.; Karma, A.; Kurz, W.; Rappaz, M.; Trivedi, R. Solidification microstructures: Recent developments, future directions. Acta Mater. 2000, 48, 43–70. [Google Scholar] [CrossRef]
- Zhang, C.; Miao, J.; Chen, S.; Zhang, F.; Luo, A.A. CALPHAD-Based Modelling and Experimental Validation of Microstructural Evaluation and Microsegregation in Magnesium Alloys During Solidification. J. Phase Equilib. Diffus. 2019, 40, 495–507. [Google Scholar] [CrossRef]
- Mikolajczak, P.; Geanau, A.; Ratke, L. Mushy Zone Calculation with Application of CALPHAD Technique. Metals 2017, 7, 363. [Google Scholar] [CrossRef]
- Dore, X.; Combeau, H.; Rappaz, M. Modelling of microsegregation in ternary alloys: Application to the solidification of Al–Mg–Si. Acta. Mater. 2000, 48, 3951–3962. [Google Scholar] [CrossRef]
- Du, Q.; Eskin, D.G.; Katgerman, L. An efficient technique for describing a multi-component open system solidification path. Comput. Coupling Phase Diagr. Thermochem. 2008, 32, 478–484. [Google Scholar] [CrossRef]
- Zhao, G.; Xu, D.; Fu, H. ThermoCalc-based numerical computations for temperature, fraction of solid phase and composition couplings in alloy solidification. Int. J. Mater. Res. 2008, 99, 680–688. [Google Scholar] [CrossRef]
- Zhao, G.; Li, X.Z.; Xu, D.; Guo, J.; Fu, H.; Du, Y.; He, Y. Numerical Computations for Temperature, fraction of Solid Phase and Composition coupling in Ternary Alloy Solidification with Three Different Thermodynamic Data-acquisition Method. Calphad 2012, 36, 155–162. [Google Scholar] [CrossRef]
- Qiu, K.; Wang, R.C.; Peng, C.Q. Mathematical model of Liquidus Temperature in Quaternary Aluminium Phase Diagram. Adv. Mater. Res. 2015, 1095, 545–548. [Google Scholar] [CrossRef]
- Qiu, K.; Wang, R.; Peng, C.; Lu, X.; Wang, N. Polynomial regression and interpolation of thermodynamic data in Al-Si-Mg-Fe system. Comput. Coupling Phase Diagr. Thermochem. 2015, 48, 175–183. [Google Scholar] [CrossRef]
- Djurdjevic, M.B.; Manasijevic, A.; Odonovic, Z.; Dolic, N. Calculation of Liquidus Temperature for Aluminium and Magnesium alloys Applying Method of Equivalency. Adv. Mater. Sci. Eng. 2013, 2013, 170527. [Google Scholar] [CrossRef]
- Robles Hernandez, F.C.; Djurdjevic, M.B.; Kierkus, W.T.; Sokolowski, J.H. Calculation of the liquidus temperature for hypo and hypereutectic aluminium silicon alloys. Mater. Sci. Eng. A 2005, 396, 271–276. [Google Scholar] [CrossRef]
- Farahany, S.; Erfani, M.; Karamoozia, A.; Ourdjini, A.; Idris, M.H. Artificial Neural Networks to Predict of Liquidus Temperature in Hypoeutectic Al-Si Cast Alloys. J. Appl. Sci. 2010, 10, 3243–3249. [Google Scholar] [CrossRef][Green Version]
- Wang, X.; Wang, X.; Wang, B.; Wang, B.; Liu, Q. Differential Calculation Model for Liquidus Temperature of Steel. Steel Res. Int. 2011, 82, 164–168. [Google Scholar] [CrossRef]
- Min, L.; Hongbo, L.; Rongyuan, X.; Xiaorui, C.; Ying, L.; Hao, X.; Caidong, Z.; Zhiqiang, T. Investigation of the liquidus temperature calculation method for medium manganese steel. High Temp. Mater. Process. 2023, 42, 20220285. [Google Scholar] [CrossRef]
- Sertucha, J.; Lacaze, J.; Regordosa, A.; Suarez, R. Polynomial Description of the Fe–C–Si Stable Phase Diagram for up to 4.5 Wt% Si, Including the Effect of Cr, Cu, Mn and P. Int. J. Met. 2025, 19, 1847–1856. [Google Scholar] [CrossRef]
- Vorozhtcov, V.A.; Pavelina, M.E.; Stolyarova, V.L.; Fedorova, A.V.; Sinelshchikova, O.Y.; Almjashev, V.I. Liquidus temperatures in the La2O3-SrO-TiO2-ZrO2-Fe2O3 system: Calculation and experiment. Ceram. Int. 2025, 51, 320–330. [Google Scholar] [CrossRef]
- Afanasyev, O.S.; Afanasyeva, E.A.; Egorov, G.F. Analytical Approximation and Numerical Simulation of Liquid for Nonequilibrium Multicomponent Eutectic Systems. Vestn. Samara State Tech. University. Tech. Sci. Ser. 2022, 30, 6–19. [Google Scholar] [CrossRef]
- Kőrösy, G.; Roósz, A.; Mende, T. The ESTPHAD Concept: An Optimised Set of Simplified equations to Estimate the Equilibrium Liquidus and Solidus Temperatures, Partition Ratio and Liquidus Slope for Quick Access to Equilibrium Data in Solidification Software, Part I: Binary equilibrium phase diagram. Metals 2024, 14, 1266. [Google Scholar]
- Drost, E.; Hausselt, J. Uses of gold in jewellery. Interdiscip. Sci. Rev. 1992, 17, 271–280. [Google Scholar] [CrossRef]
- Kempf, B.; Hausselt, J. Gold, its alloys and their uses in dentistry. Interdiscip. Sci. Rev. 1992, 17, 251–260. [Google Scholar] [CrossRef]
- Kempf, B.; Schmauder, S. Thermodynamic modelling of precious metals alloys. Gold Bull. 1998, 31, 51–57. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.H.; Kang, Y.B.; Melançon, J.; et al. FactSage thermochemical software and databases, 2010–2016. Calphad 2016, 54, 35. [Google Scholar] [CrossRef]
- Prince, A.; Gröbner, J.; Rao, M.V.; Kuznetsov, V. Landolt-Börnstein Book Series: Numerical Data and Functional Relationships in Science and Technology—New Series IV/11B, Ternary Alloy Systems-Silver–Gold–Palladium Phase Diagrams, Crystallographic and Thermodynamic Data; Effenberg, G., Ilyenko, S., Eds.; Springer Nature BV: Dordrecht, The Netherlands, 2006; Volume IV/11B: Noble Metal Systems, pp. 50–54. [Google Scholar]
- Venudhar, Y.C.; Iyengar, L.; Leela, R.; Krishna, K.V. Isoparametric Curves and Vegard’s Law Plots for the Ternary System Palladium-Silver-Gold. Current Sci. 1978, 47, 717–719. [Google Scholar]
- Nemilov, V.A.; Rudnitsky, A.A.; Vidusova, T.A. Investigation of the Au-Pd-Ag System. Izvest. Sekt. Platiny 1946, 20, 225–239. [Google Scholar]
- Pauley, C.L. X-ray Study of the Stacking Fault Density near the Hardness Maximum of the Au-Ag-Pd System. Master’s Thesis, Virginia Polytechnic Institute, Blacksburg, VA, USA, 1967. [Google Scholar]
- Miane, J.M.; Gaune-Escard, M.; Bros, J.P. Liquidus and Solidus Surface of the Ag-Au-Pd Equilibrium Phase Diagram. High Temp. High Press. 1977, 9, 465–469. [Google Scholar]










| BEPD | |||||||
|---|---|---|---|---|---|---|---|
| −0.001525122 | 9.83954E-06 | −2.21084E-08 | |||||
| 0.000275973 | 2.66952E-06 | 2.89592E-08 | |||||
| −0.009145785 | 0.000216506 | −5.02079E-06 | 7.36011E-08 | −5.53729E-10 | 1.62381E-12 | ||
| 0.002466234 | 1.93529E-05 | −1.71905E-06 | 5.15282E-08 | −5.63012E-10 | 2.23671E-12 | ||
| −0.015142951 | 0.0006576 | −2.04085E-05 | 3.81869E-07 | −4.01939E-09 | 2.1859E-11 | −4.77362E-14 | |
| 0.000887203 | 3.54004E-05 | −1.3818E-06 | −6.83248E-09 | 9.61336E-10 | −1.34325E-11 | 5.80735E-14 |
| BEPD | ||||||||
|---|---|---|---|---|---|---|---|---|
| −0.001470912 | 9.08706E-06 | −2.02095E-08 | ||||||
| 0.000296244 | 3.02225E-06 | 2.30809E-08 | ||||||
| −0.005863031 | 5.10683E-05 | −1.02889E-06 | 2.45832E-08 | −2.57412E-10 | 8.95933E-13 | |||
| 0.00464592 | −1.95305E-05 | −1.43628E-06 | 4.45352E-08 | −4.12665E-10 | 1.32031E-12 | |||
| −0.008092053 | 0.000174313 | −1.0007E-05 | 4.35027E-07 | −9.88E-09 | 1.20349E-10 | −7.52722E-13 | 1.899E-15 | |
| 0.002002473 | −8.36957E-05 | 2.89768E-06 | −5.01666E-08 | 4.88935E-10 | 1.76718E-12 |
| BEPD | ||||||
|---|---|---|---|---|---|---|
| −0.001525122 | 2 × 9.83954E-06 | 3 × −2.21084E-08 | ||||
| 0.000275973 | 2 × 2.66952E-06 | 3 × 2.89592E-08 | ||||
| −0.009145785 | 2 × 0.000216506 | 3 × −5.02079E-06 | 4 × 7.36011E-08 | 5 × −5.53729E-10 | 6 × 1.62381E-12 | |
| 0.002466234 | 2 × 1.93529E-05 | 3 × −1.71905E-06 | 4 × 5.15282E-08 | 5 × −5.63012E-10 | 6 × 2.23671E-12 | |
| −0.015142951 | 2 × 0.0006576 | 3 × −2.04085E-05 | 4 × 3.81869E-07 | 5 × −4.01939E-09 | 6 × 2.1859E-11 | |
| 0.000887203 | 2 × 3.54004E-05 | 3 × −1.3818E-06 | 4 × −6.83248E-09 | 5 × 9.61336E-10 | 6 × −1.34325E-11 |
| BEPD | |||||||
|---|---|---|---|---|---|---|---|
| 0.040036927 | −0.000181716 | 1.72324E-06 | −3.9346E-08 | ||||
| −0.102745508 | 0.001980561 | −1.26162E-05 | 3.06916E-08 | ||||
| 0.366998634 | 0.011788084 | −0.002026059 | 8.1556E-05 | −1.43378E-06 | 1.16237E-08 | −3.57194E-11 | |
| −0.613550521 | −0.006202123 | 0.00058256 | −7.83937E-06 | 3.24067E-08 | |||
| 0.761637693 | −0.04593014 | 0.001357858 | −2.06201E-05 | 1.51338E-07 | −4.25186E-10 | ||
| −0.469194667 | 0.051179503 | −0.002457681 | 5.18983E-05 | −4.95942E-07 | 1.76313E-09 |
| Pd-Ag | Pd-Au | Ag-Au | Au-Ag | Ag-Pd | Au-Pd | |
|---|---|---|---|---|---|---|
| Abs. aver. ∆T, liq., K | 0.467 | 0.694 | 0.103 | 0.157 | 0.391 | 0.703 |
| Abs. aver. ∆T, sol., K | 0.283 | 0.905 | 0.094 | 0.12 | 0.283 | 0.792 |
| Pd-Ag | Pd-Au | Ag-Au | Au-Ag | Ag-Pd | Au-Pd | |
|---|---|---|---|---|---|---|
| Abs. max ∆c, liq., at% | 0.98 | 1.914 | 0.012 | 0.004 | 1.173 | 1.148 |
| Abs. aver. ∆c, liq., at% | 0.175 | 0.543 | 0.002 | 0.002 | 0.22 | 0.241 |
| Abs. max ∆c, sol., at% | 0.7873 | 0.9 | 0.012 | 0.013 | 1.029 | 0.762 |
| Abs. aver. ∆c, sol., at% | 0.286 | 0.28 | 0.002 | 0.005 | 0.213 | 0.221 |
| Aver. Delta T Liq. K | Aver. Delta T Sol. K | |
|---|---|---|
| First est. | 20.72 | 37.7 |
| Second est. | 4.03 | 4.56 |
| Third est. | 10.14 | 7.37 |
| Forth est. | 2.98 | 5.64 |
| 0 | −0.009145785 | 0.000216506 | −5.02079E-06 | 7.36011E-08 | −5.53729E-10 | 1.62381E-12 | Biner Ag-Pd | |
| −0.001525122 | 2.73627E-05 | −2.53908E-06 | 5.42026E-08 | −3.34127E-10 | ||||
| 9.83954E-06 | 5.40067E-07 | 2.44466E-08 | −2.97294E-10 | |||||
| −2.21084E-08 | −4.45188E-08 | 4.13816E-11 | ||||||
| 6.57177E-10 | ||||||||
| −3.43842E-12 | ||||||||
| Biner Ag-Au |
| 0 | −0.009145785 | 0.000216506 | −5.02079E-06 | 7.36011E-08 | −5.53729E-10 | 1.62381E-12 | Biner Ag-Pd | |
| −0.001525122 | 2.10522E-05 | −1.98144E-06 | 3.98273E-08 | −2.26295E-10 | ||||
| 9.83954E-06 | 6.31182E-07 | 1.96487E-08 | −2.39973E-10 | |||||
| −2.21084E-08 | −4.41986E-08 | 5.97316E-11 | ||||||
| 6.24807E-10 | ||||||||
| −3.15779E-12 | ||||||||
| Biner Ag-Au |
| 0 | −0.005863031 | 5.10683E-05 | −1.02889E-06 | 2.45832E-08 | −2.57412E-10 | 8.95933E-13 | Binary Ag-Pd | |
| −0.001470912 | 0.000192355 | −9.44422E-06 | 1.3398E-07 | −6.03495E-10 | ||||
| 9.08706E-06 | −7.95952E-06 | 2.91598E-07 | −2.26559E-09 | |||||
| −2.02095E-08 | 1.5897E-07 | −5.22316E-09 | 3.06509E-11 | |||||
| −1.14374E-09 | 2.61784E-11 | |||||||
| 1.54871E-12 | ||||||||
| Binary Ag-Au |
| 0 | −0.005863031 | 5.10683E-05 | −1.02889E-06 | 2.45832E-08 | −2.57412E-10 | 8.95933E-13 | Binary Ag-Pd | |
| −0.001470912 | 0.000199224 | −9.23283E-06 | 1.21293E-07 | −4.97208E-10 | ||||
| 9.08706E-06 | −9.2756E-06 | 3.21833E-07 | −2.36136E-09 | |||||
| −2.02095E-08 | 2.01945E-07 | −6.03414E-09 | 3.2798E-11 | |||||
| −1.66965E-09 | 3.17608E-11 | |||||||
| 3.73822E-12 | ||||||||
| Binary Ag-Au |
| −0.001525122 | 2 × 9.83954E-06 | 3 × −2.21084E-08 | Binary Ag-Au | |||
| 2.10522E-05 | 2 × 6.31182E-07 | 3 × −4.41986E-08 | 4 × 6.24807E-10 | 5 × −3.15779E-12 | ||
| −1.98144E-06 | 2 × 1.96487E-08 | 3 × 5.97316E-11 | ||||
| 3.98273E-08 | 2 × −2.39973E-10 | |||||
| −2.26295E-10 |
| −0.009145785 | 2 × 0.000216506 | 3 × −5.02079E-06 | 4 × 7.36011E-08 | 5 × −5.53729E-10 | 6 × 1.62381E-12 | Binary Ag-Pd | |
| 2.10522E-05 | 2 × 6.31182E-07 | 3 × −4.41986E-08 | 4 × 6.2480E-10 | 5 × −3.15779E-12 | |||
| −1.98144E-06 | 2 × 1.96487E-08 | 3 × 5.97316E-11 | |||||
| 3.98273E-08 | 2 × −2.3997E-10 | ||||||
| −2.26295E-10 |
| −0.001470912 | 2 × 9.08706E-06 | 3 × −2.02095E-08 | Binary Ag-Au | |||
| 0.000199224 | 2 × −9.2756E-06 | 3 × 2.01945E-07 | 4 × −1.66965E-09 | 5 x 3.73822E-12 | ||
| −9.23283E-06 | 2 × 3.21833E-07 | 3 × −6.03414E-09 | 4 × 3.17608E-11 | |||
| 1.21293E-07 | 2 × −2.36136E-09 | 3 × 3.2798E-11 | ||||
| −4.97208E-10 | ||||||
| −0.005863031 | 2 × 5.10683E-05 | 3 × −1.02889E-06 | 4 × 2.45832E-08 | 5 × −2.57412E-10 | 6 × 8.95933E-13 | Binary Ag-Pd | |
| 0.000199224 | 2 × −9.23283E-06 | 3 × 1.21293E-07 | 4 × −4.97208E-10 | ||||
| −9.2756E-06 | 2 × 3.21833E-07 | 3 × −2.36136E-09 | |||||
| 2.01945E-07 | 2 × −6.03414E-09 | 3 × 3.2798E-11 | |||||
| −1.66965E-09 | 2 × 3.17608E-11 | ||||||
| 3.73822E-12 |
| ) | |||||
|---|---|---|---|---|---|
| 0.040036927 | −0.000181716 | 1.72324E-06 | −3.9346E-08 | Binary Ag-Au | |
| 0.001297726 | −9.97448E-06 | 7.81204E-08 | |||
| −5.05785E-05 | −5.85049E-08 | ||||
| 4.19929E-07 |
| 0.3669986 | 0.0117880 | −0.002026 | 8.1556E-05 | −1.43378E-06 | 1.16237E-08 | −3.57194E-11 | Binary Ag-Pd | |
| 0.021171612 | −0.0008127 | 1.45168E-05 | −1.079E-07 | |||||
| −0.0009139 | 1.17625E-06 | 4.18625E-08 | ||||||
| 2.50716E-05 | ||||||||
| −2.94122E-07 | ||||||||
| 1.17629E-09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kőrösy, G.; Roósz, A.; Mende, T. The ESTPHAD Concept: An Optimised Set of Simplified Equations to Estimate the Equilibrium Liquidus and Solidus Temperatures, Partition Ratios, and Liquidus Slopes for Quick Access to Equilibrium Data in Solidification Software Part II: Ternary Isomorphous Equilibrium Phase Diagram. Metals 2025, 15, 803. https://doi.org/10.3390/met15070803
Kőrösy G, Roósz A, Mende T. The ESTPHAD Concept: An Optimised Set of Simplified Equations to Estimate the Equilibrium Liquidus and Solidus Temperatures, Partition Ratios, and Liquidus Slopes for Quick Access to Equilibrium Data in Solidification Software Part II: Ternary Isomorphous Equilibrium Phase Diagram. Metals. 2025; 15(7):803. https://doi.org/10.3390/met15070803
Chicago/Turabian StyleKőrösy, Gergely, András Roósz, and Tamás Mende. 2025. "The ESTPHAD Concept: An Optimised Set of Simplified Equations to Estimate the Equilibrium Liquidus and Solidus Temperatures, Partition Ratios, and Liquidus Slopes for Quick Access to Equilibrium Data in Solidification Software Part II: Ternary Isomorphous Equilibrium Phase Diagram" Metals 15, no. 7: 803. https://doi.org/10.3390/met15070803
APA StyleKőrösy, G., Roósz, A., & Mende, T. (2025). The ESTPHAD Concept: An Optimised Set of Simplified Equations to Estimate the Equilibrium Liquidus and Solidus Temperatures, Partition Ratios, and Liquidus Slopes for Quick Access to Equilibrium Data in Solidification Software Part II: Ternary Isomorphous Equilibrium Phase Diagram. Metals, 15(7), 803. https://doi.org/10.3390/met15070803






