Abstract
Biodegradable metallic implants represent a paradigm shift in implantology, eliminating secondary removal surgeries through predictable controlled degradation. This review systematizes current achievements in selective laser melting (SLM) of biodegradable metals (Mg, Fe, Zn), analyzing how processing parameters influence microstructure, mechanical properties, and degradation kinetics. Key findings demonstrate that SLM-produced Mg alloys achieve bone-matching modulus (40–45 GPa) with moderate degradation (1–3 mm/year); Fe-based systems provide superior strength (400–600 MPa) but slower degradation (0.1–0.5 mm/year); while Zn alloys offer intermediate properties. Design strategies for porous/lattice structures enhancing osseointegration and enabling property gradients are discussed. Major challenges include controlling degradation kinetics, optimizing SLM parameters for reactive metals, standardizing testing methodologies, and regulatory harmonization. This comprehensive analysis provides systematic guidelines for material selection and process optimization, establishing a foundation for developing next-generation personalized biodegradable implants.
1. Introduction
Modern implantology is transitioning to biodegradable implants that are replaced by body tissues. According to a Grand View Research report, the global biodegradable implant market reached USD 5.35 billion in 2022 and is projected to grow to USD 13.1 billion by 2030 with a CAGR of 11.8% [1] This growth is driven by the advantages of biodegradable implants, including elimination of removal surgeries, reduced risk of long-term complications, and more physiological load distribution during healing [2].
Traditional metallic implants (titanium, stainless steel, cobalt-chromium alloys) possess high strength and biocompatibility, but their elastic modulus (100–210 GPa) significantly exceeds that of bone (5–23 GPa) [3,4]. This mismatch causes stress shielding, bone resorption around the implant, loosening, and the need for revisions [5]. Additionally, the permanent presence of the implant may be accompanied by metal ion release, corrosion, inflammatory reactions, and complications [6].
Biodegradable metallic alloys offer an innovative solution by providing temporary mechanical support with precisely controlled degradation kinetics that synchronizes with tissue regeneration, ultimately eliminating the need for implant removal surgeries. Alloys based on magnesium (Mg), iron (Fe), and zinc (Zn) are of particular interest, possessing unique properties and potential for various clinical applications [7].
Magnesium alloys are characterized by low density, elastic modulus close to bone tissue, and relatively rapid degradation capability (3–6 months) [8]. The main corrosion product is magnesium hydroxide, which gradually transforms into water-soluble compounds and is safely excreted from the body. Magnesium is an essential microelement involved in more than 300 biochemical reactions in the body, ensuring a favorable biological response [9].
Iron alloys feature high strength and toughness, making them suitable for implants experiencing significant loads. However, their slow in vivo degradation rate (more than 1 year) limits clinical application, stimulating the development of new compositions with accelerated corrosion [10].
Zinc alloys occupy an intermediate position in terms of mechanical properties and degradation rate between magnesium and iron alloys, demonstrating moderate corrosion rate (3–12 months) and satisfactory strength [11]. Zinc is an important microelement involved in regulating immune function and wound healing, making Zn alloys promising candidates for biodegradable implants [12].
Traditional manufacturing methods (casting, forging, machining) are limited in creating complex geometries and personalized implants. Additive manufacturing (AM), particularly selective laser melting (SLM), opens unique possibilities for overcoming these limitations, allowing the creation of implants with precise geometry, controlled porosity, and individualized properties [13].
The SLM process is based on layer-by-layer deposition of metal powder using a high-energy laser beam that selectively melts and solidifies material according to a digital 3D model [14]. This technology provides unprecedented design freedom, including the creation of complex lattice structures that mimic trabecular bone architecture, promoting osseointegration and controlled implant degradation.
Despite significant progress in developing biodegradable alloys and their additive manufacturing technologies, several unresolved problems remain that require further research. The main challenges include control of degradation rate [15], balance of mechanical properties, technological aspects of working with reactive metals [16], and certification issues for clinical application.
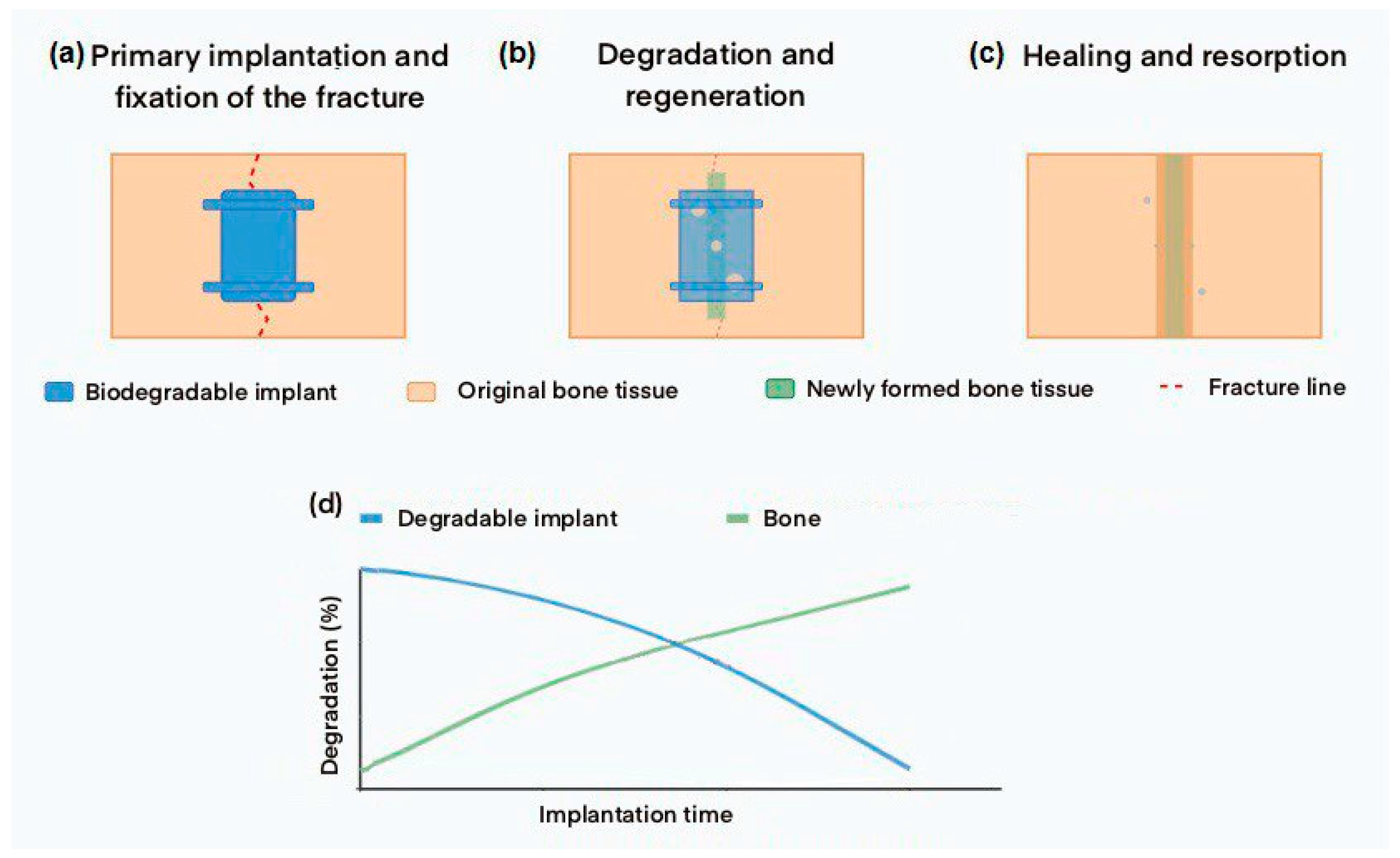
Figure 1 demonstrates the process of temporary functioning of a biodegradable implant and the dynamic relationship between implant degradation and bone tissue regeneration over time.
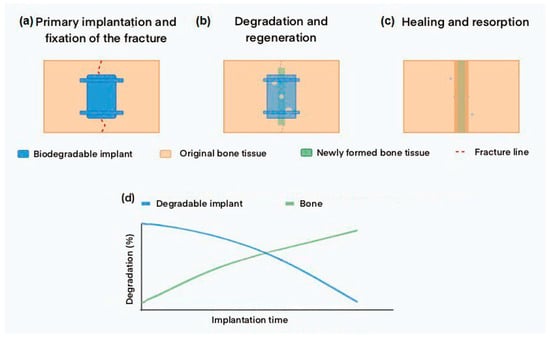
Figure 1.
Schematic of biodegradable implant temporary functioning: (a) Initial implantation and fracture fixation. (b) Gradual implant degradation and new bone tissue formation. (c) Complete fracture healing and implant resorption. (d) Graph showing the relationship between implant degradation and forming bone during rehabilitation.
The purpose of this review is to provide a comprehensive and critical analysis of the current state of research in the field of additive manufacturing of biodegradable metallic implants, with a specific focus on the selective laser melting process. Unlike previous reviews that typically address either biodegradable metals or additive manufacturing separately, this work uniquely integrates both aspects while offering a comparative analysis of Mg, Fe, and Zn-based systems. This review makes several novel contributions to the field by (1) systematizing data on the influence of SLM parameters on microstructure, mechanical properties, and degradation rate of different biodegradable metal systems; (2) critically evaluating porous structure design strategies and their influence on biological response; (3) providing side-by-side comparison of key properties across different biodegradable metal families; and (4) identifying promising research directions to overcome existing limitations for successful clinical translation. The insights presented here will guide researchers, engineers, and clinicians in developing next-generation personalized biodegradable implants with optimized properties and controlled degradation behavior.
2. Additive Manufacturing of Biodegradable Metals: Process Overview
2.1. Technology of the Selective Laser Melting Process
Selective laser melting (SLM) is a promising additive manufacturing (AM) technology for biodegradable metallic implants, based on layer-by-layer melting of metal powder by a high-energy laser. SLM belongs to Powder Bed Fusion (PBF) methods, forming three-dimensional objects through the sequential creation of two-dimensional layers [17].
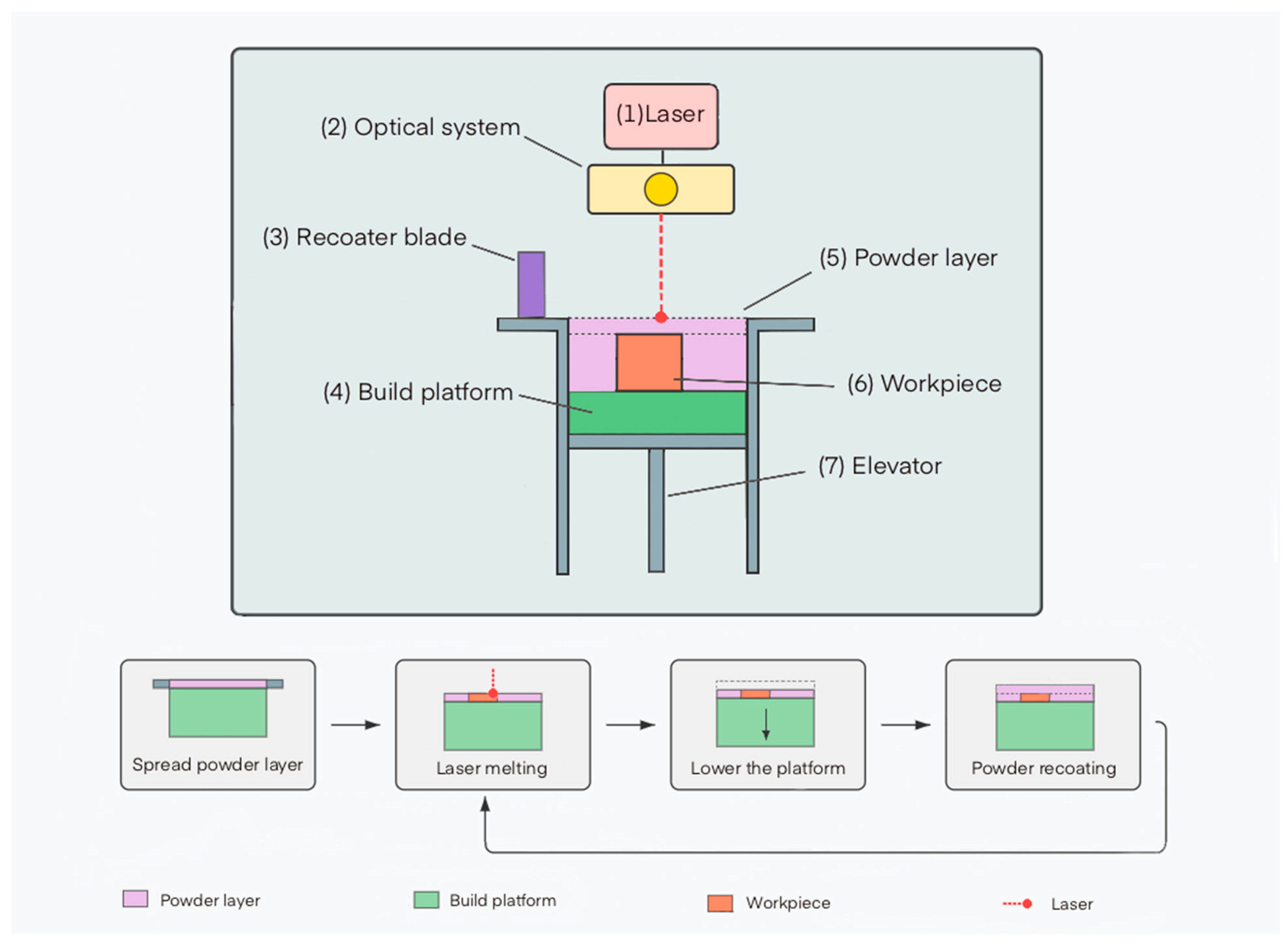
The SLM process includes the following main stages (Figure 2):
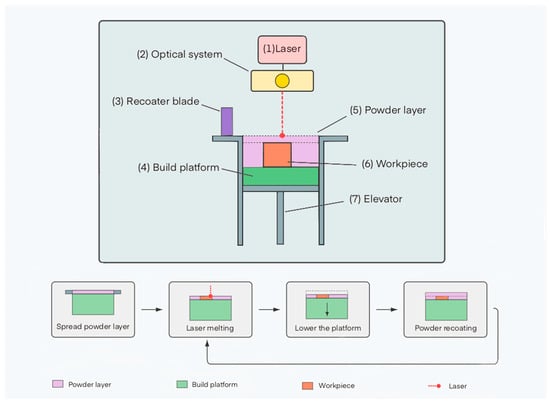
Figure 2.
Schematic representation of the main components of an SLM setup and process stages: (1) laser, (2) focusing and scanning system, (3) powder delivery device, (4) build platform, (5) powder layer, (6) finished part, (7) elevator. The diagram also shows the sequential stages: powder layer deposition, laser beam scanning, platform lowering, and application of a new layer.
- Deposition of a thin layer of metal powder (typically 20–100 μm) on the build platform using a recoater blade or roller mechanism.
- Selective melting of the powder by a laser beam according to the geometry of the current cross-section of the model.
- Lowering the build platform by the height of one layer.
- Applying a new layer of powder and repeating the melting process.
- Sequential repetition of these stages until the complete formation of the product.
2.2. Features of Working with Biodegradable Metals
Working with biodegradable metals in the SLM process has a number of specific features due to their physicochemical properties and requirements for final products. These features must be considered for the successful production of implants with specified characteristics.
2.2.1. Reactivity and Temperature Regime
Biodegradable metals (magnesium, zinc, iron) possess high reactivity and specific thermal properties, complicating their use in SLM processes.
Magnesium and zinc readily oxidize, forming surface oxide films that degrade melting quality and increase product porosity. Studies show that low boiling points of magnesium (1090 °C) and melting of zinc (419.5 °C) create a risk of material evaporation during laser exposure, which can disrupt process stability [18]. In contrast, iron requires significant energy input, but selective evaporation of alloying elements is possible, leading to changes in alloy composition [19].
Thus, processing biodegradable metals by SLM requires strict control of the atmosphere and precise adjustment of laser parameters.
2.2.2. Requirements for Process Parameters and Control of Structural-Phase Composition
Selective laser melting of biodegradable metals requires precise control of parameters that determine optimal characteristics within a narrow “process window.” For magnesium alloys, energy density is critical, ensuring complete melting without evaporation [20] and forming a fine-grained structure that increases strength but potentially accelerates corrosion [21]. For zinc alloys, cooling rate is important, affecting grain size and strength [22]. Iron alloys require high laser power while controlling scanning speed to minimize residual stresses and cracks [23], and SLM parameters allow regulation of phase ratios (γ and ε-martensite) to control biodegradation [24]. Rapid cooling in SLM leads to the formation of non-equilibrium structures with high defect density. SLM of biodegradable metals requires a comprehensive approach that considers the specifics of each alloy and the required properties.
2.2.3. Post-Processing, Sterilization and Degradation Rate Control
SLM implants require specific post-processing to ensure properties and safety. Traditional heat treatment for Mg alloys may be ineffective due to oxidation risk. Micro-arc and plasma electrolytic oxidation, biocompatible coatings (hydroxyapatite, polylactide) are applied to improve corrosion resistance and biocompatibility [25].
Degradation rate management is a key task. For this purpose, alloying (to increase corrosion resistance) [26], surface modification (creation of protective coatings) [27], porosity management (regulation of surface area) [28], and microstructure regulation (phase composition, grain size through SLM parameters) [29] are used. Combining these approaches allows creating implants with predetermined degradation rates.
Critically important is the choice of sterilization method, as biodegradable metals can be sensitive to aggressive environments and high temperatures. Studies show that gamma radiation may be the preferred sterilization method for magnesium implants, as it minimally affects their properties [27].
2.3. Key Process Parameters and Their Influence
The quality and performance characteristics of biodegradable implants manufactured by SLM are largely determined by process parameters. Optimization of these parameters allows achieving the required density, microstructure, mechanical properties, and material degradation rate.
2.3.1. Laser Power
Laser power is one of the key parameters determining the energy input in the SLM process. The optimal power depends on the thermophysical properties of the material and should ensure complete powder melting without excessive overheating and evaporation.
Studies show that for magnesium alloys such as AZ91D, the optimal power range is 100–200 W [30]. Incomplete powder melting and porous structure formation occur when laser power falls below 100 W. Conversely, powers exceeding 200 W cause intensive magnesium evaporation, resulting in chemical composition changes and keyhole defect formation [31].
For iron alloys, which have a higher melting temperature, increased laser power (200–400 W) is required. Ewald et al. (2021) showed that a power of at least 200 W is necessary to achieve optimal density and mechanical properties of the Fe-Mn-Al-Ni alloy (SLM Solutions Group AG, Lübeck, Germany) [32].
Zinc alloys, conversely, require lower power (80–150 W) due to zinc’s low melting temperature. Precise power control is critically important to prevent overheating and excessive evaporation [33].
2.3.2. Scanning Speed
The scanning speed of the laser beam affects the interaction time between the laser and material and, consequently, the penetration depth, melt pool size, and cooling rate.
For magnesium alloys, the optimal scanning speed is in the range of 400–800 mm/s. At lower speeds, excessive material overheating occurs, while at higher speeds, incomplete powder melting occurs [34].
Iron alloys allow higher scanning speeds (600–1200 mm/s) due to their increased heat resistance. Studies by Xie et al. (2024) showed that the optimal scanning speed for the Fe–Mn–Al–C alloy is about 800 mm/s (SLM Solutions, Germany) [35].
Zinc alloys are characterized by lower scanning speeds (300–600 mm/s) due to the need for precise control of thermal exposure [36].
2.3.3. Layer Thickness and Hatch Distance
Layer thickness and distance between scanning tracks determine the spatial distribution of laser energy and the degree of overlap between adjacent melt pools.
The optimal layer thickness for most biodegradable metals is 20–50 μm. Thinner layers provide better accuracy and surface quality but increase build time. Thicker layers increase productivity but can lead to incomplete penetration and reduced mechanical properties [37].
The distance between scanning tracks should ensure sufficient overlap of adjacent melt pools (typically 20–30%) to form a monolithic structure. Typical values are 60–120 μm depending on the material type and laser spot diameter [38].
2.3.4. Scanning Strategy
The scanning strategy (laser beam path scheme) affects heat distribution, residual stresses, and microstructure. Common strategies include raster scanning (in one direction or with rotation between layers), island (chessboard) strategy, and contour scanning. For metals prone to oxidation and evaporation (Mg, Zn), a strategy with rotation of direction between layers is preferable, providing uniform heat distribution and reducing property anisotropy [39].
2.3.5. Energy Density
Energy density is an integral parameter that combines the influence of laser power, scanning speed, hatch distance, and layer thickness. It is calculated using Equation (1),
where E is the volumetric energy density (J/mm3), P is the laser power (W), v is the scanning speed (mm/s), h is the hatch distance (mm), d is the layer thickness (mm).
For each type of biodegradable metal, there is an optimal energy density range that provides a balance between complete powder melting and defect minimization. For magnesium alloys, this range is 120–150 J/mm3, for iron alloys—150–200 J/mm3, for zinc alloys—100–130 J/mm3 [40].
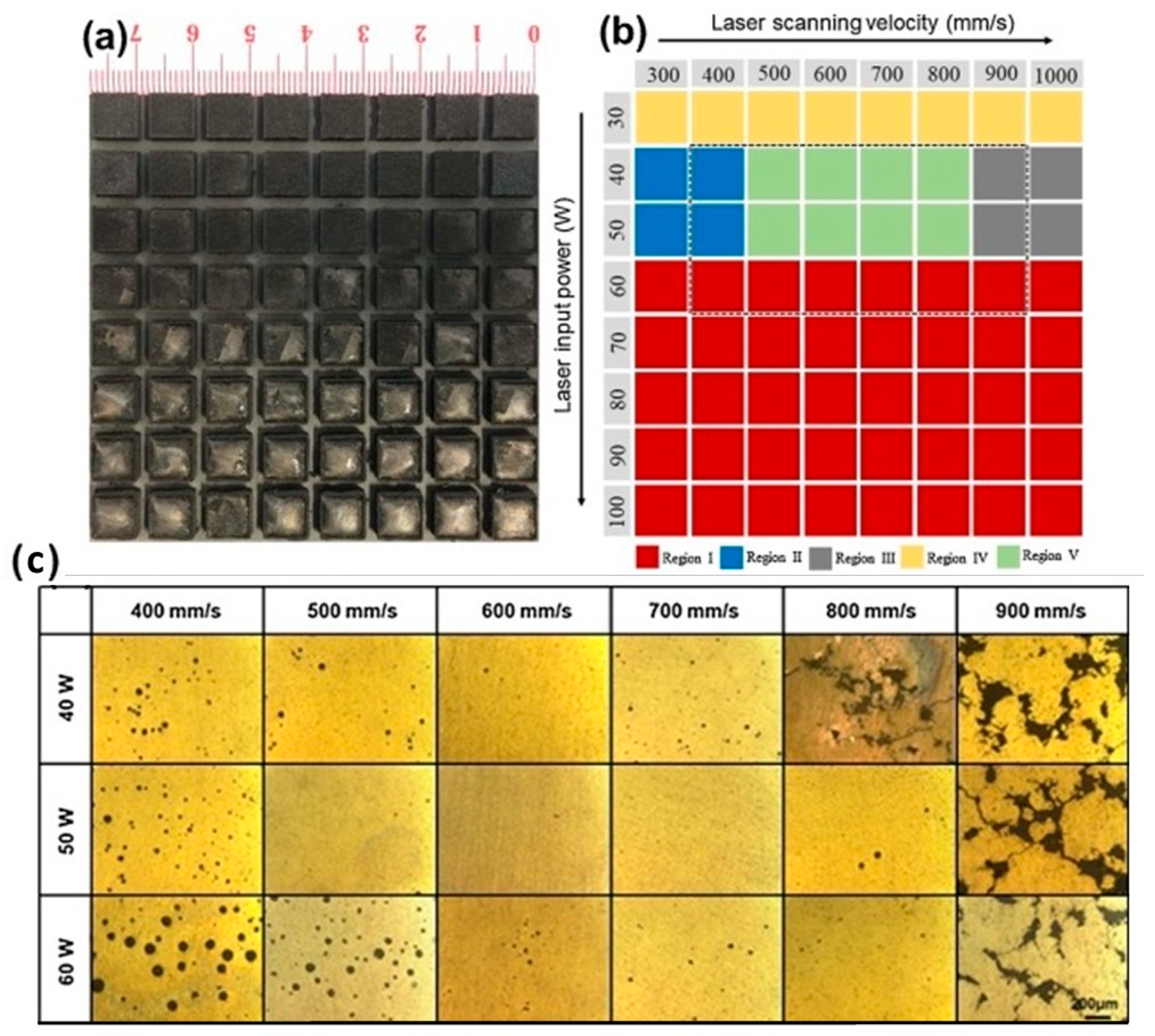
As shown in Figure 3, the quality of SLM processing of ZK60 alloy (SLM 125 H L, SLM Solutions, Lübeck, Germany) significantly depends on laser energy density. With excessive energy (area I), magnesium evaporation and melt splashing occur, leading to an uneven surface. Insufficient energy (areas III–IV) causes incomplete powder melting and defect formation. Optimal formation (area V) is achieved at 40–50 W and 500–800 mm/s, providing homogeneous samples with high dimensional accuracy. These results demonstrate the critical influence of energy density on the morphology and quality of SLM products. As seen from the results, porosity shows a non-monotonic dependence on scanning speed at fixed laser power. The lowest porosity is achieved at a medium power of 50 W, whereas extreme values (40 and 60 W) lead to a significant increase in defects. At low power and high scanning speed, connected pores form due to capillary instability and insufficient track overlap, causing the balling effect. Conversely, the combination of high power and low scanning speed provokes the formation of large spherical pores due to the keyhole effect, associated with vapor trapping in a deep melt pool. Increasing the input energy exacerbates this effect, leading to increased porosity.
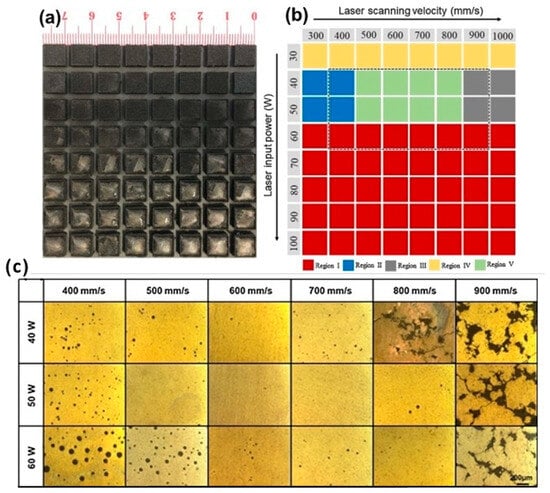
Figure 3.
SLM processing optimization for ZK60 magnesium alloy: (a) macro-morphology in xy plane showing different processing regions, (b) processing parameter map with laser power (40–60 W) and scanning speed (400–900 mm/s), (c) porosity analysis and optical microscopy images corresponding to the marked region in (b), demonstrating the relationship between energy density and part quality. The dotted box in (b) outlines the region corresponding to the samples shown in (c), reprint with permission from Ref. [41], 2021, Elsevier.
2.4. Requirements for Powder Materials for Additive Manufacturing
The quality of metal powders for selective laser melting of biodegradable implants is determined by a set of interrelated characteristics. Optimal parameters include particle size distribution in the range of 15–63 μm (narrow distribution, D10 = 20–25 μm, D50 = 30–40 μm, D90 = 50–60 μm) for a balance between accuracy and flowability. The morphology of particles is critically important, with preference given to spherical particles with a roundness coefficient of at least 0.85 [42]. The chemical purity of powders must meet the strict requirements of international standards ISO 13485 [43] and ASTM F3049 [44].
Various methods are used for powder production, each with its own advantages. Different production methods are used: gas atomization (sphericity and purity), plasma spheroidization (shape correction), mechanical alloying (complex alloys), and hydride–dehydride method (high-purity powders).
For specific metals, specialized technological solutions are recommended. Magnesium powders are optimally obtained by gas atomization in argon followed by spheroidization. Iron powders are preferably produced by vacuum plasma atomization, while for zinc powders, gas atomization with controlled cooling is most suitable. Compliance with these parameters ensures reproducibility of the SLM process and controlled biodegradation characteristics of implants.
2.5. Problems in Additive Manufacturing of Biodegradable Alloys
Despite significant progress in the field of additive manufacturing of biodegradable metallic implants, there are a number of unresolved problems limiting their widespread clinical application.
2.5.1. Degradation Rate Control
One of the key problems is ensuring a predictable and controlled degradation rate of the implant, corresponding to the rate of tissue regeneration. Too rapid degradation leads to loss of mechanical integrity and adverse reactions, while too slow degradation negates the advantages of biodegradation. The complexity of control is exacerbated by differences between in vivo and in vitro, influence of biological factors [45], and patient response [46].
The three main biodegradable metal systems exhibit significantly different degradation kinetics under physiological conditions. Magnesium-based implants typically degrade at rates of 1.0–3.0 mm/year [47], with pure magnesium showing the highest degradation rate (up to 3 mm/year), while alloyed systems demonstrate more moderate rates. Iron-based materials show considerably slower degradation, typically in the range of 0.1–0.5 mm/year [48], with pure iron exhibiting rates as low as 0.008 mm/year in certain environments. Zinc alloys occupy an intermediate position with degradation rates between 0.2–0.5 mm/year [49].
Several key metallurgical and environmental factors influence degradation behavior. The microstructural features of additively manufactured metals—including grain size, phase distribution, and dislocation density—significantly impact corrosion mechanisms. Finer grain structures with increased grain boundary area, typical of SLM-produced materials, generally accelerate degradation compared to coarse-grained counterparts. Phase composition plays an equally critical role; for instance, in Fe-Mn alloys, ε-martensite phases degrade faster than austenitic γ-phases, while in magnesium alloys, the presence of secondary phases like Mg2Ca or Mg17Sr2 can create galvanic couples that accelerate local corrosion.
Environmental factors such as pH, ion concentration, protein adsorption, and fluid flow dynamics profoundly affect degradation kinetics in vivo. The body’s dynamic environment, with variations in mechanical loading, cellular activity, and local chemistry, creates significant complexity in predicting long-term degradation behavior. Static in vitro tests frequently underestimate the degradation rates observed in vivo due to these dynamic physiological factors.
The processing route—particularly for additively manufactured implants—introduces additional variables affecting degradation. SLM processing parameters directly influence microstructural features and residual stresses, which in turn affect corrosion susceptibility. For example, higher cooling rates typically produce finer microstructures with higher dislocation densities that can accelerate corrosion, while processing-induced porosity can lead to localized corrosion phenomena.
2.5.2. Manufacturing Difficulties
Additive manufacturing of biodegradable metals is associated with a number of technological challenges. The high reactivity of magnesium and zinc requires special storage conditions and modifications to SLM systems [50]. Material evaporation leads to smoke and condensate formation, contaminating laser optics and reducing process efficiency [51]. Rapid cooling creates residual stresses, leading to deformation and reduced strength.
2.5.3. Corrosion Fatigue and Stress Corrosion Cracking
The combination of cyclic loads and corrosive environment creates a risk of corrosion fatigue and stress corrosion cracking (SCC) in biodegradable implants [52]. This problem is especially critical for implants working under cyclic loading conditions (e.g., bone plates, screws, cardiovascular stents). Studies have shown that Al-free Mg alloys such as ZX50, developed for biodegradable implants, are susceptible to stress corrosion cracking in m-SBF at a strain rate of 10−7 s−1, resulting in significant loss of plasticity (from 21% in air to 3.8% in m-SBF) [52].
Zinc alloys are characterized by increased sensitivity to SCC, especially in the presence of chlorides, which requires special attention when designing implants for long-term functioning [53].
2.6. Lattice Structures Manufactured by Additive Methods in Tissue Engineering
Additive manufacturing opens unique opportunities for creating complex three-dimensional lattice structures that mimic the architecture of natural tissues and provide optimal biological integration of implants.
2.6.1. Types of Lattice Structures
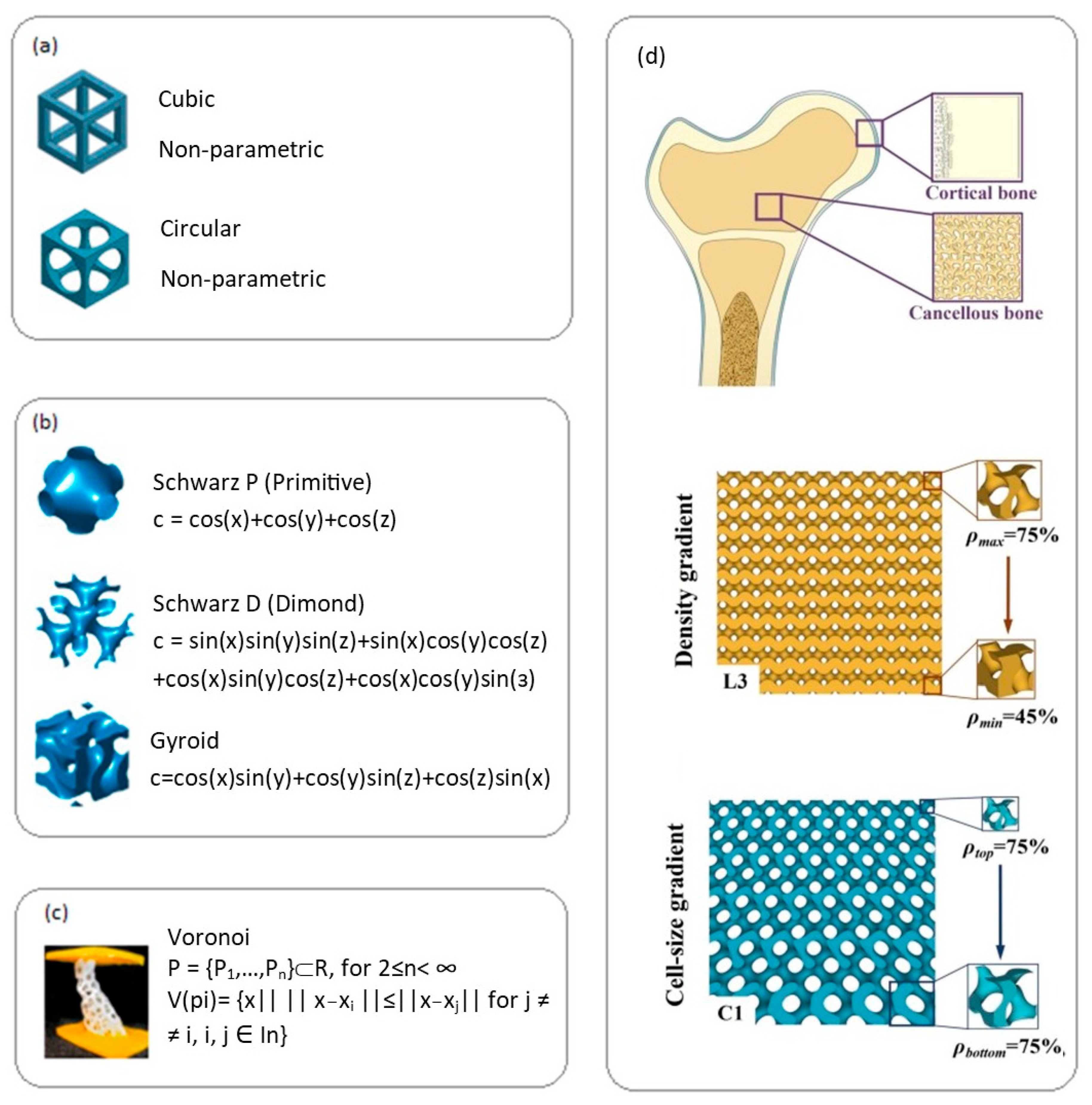
Depending on design and geometry, lattice structures of biodegradable implants are divided into several types. Regular lattice structures (Figure 4a) use repeating cells (cubic, tetrahedral, etc.) for predictable and customizable mechanical properties [54]. Triply periodic minimal surfaces (TPMS) (Figure 4b), such as Schwarz and gyroid structures, provide smooth transitions and good permeability [55]. Voronoi structures (Figure 4c) create stochastic structures and show enhanced cell proliferation [56]. Biomimetic structures recreate the architecture of natural tissues such as bone [57]. Functionally gradient structures (Figure 4d) change parameters (porosity, cell size) to create zones with different properties [57].
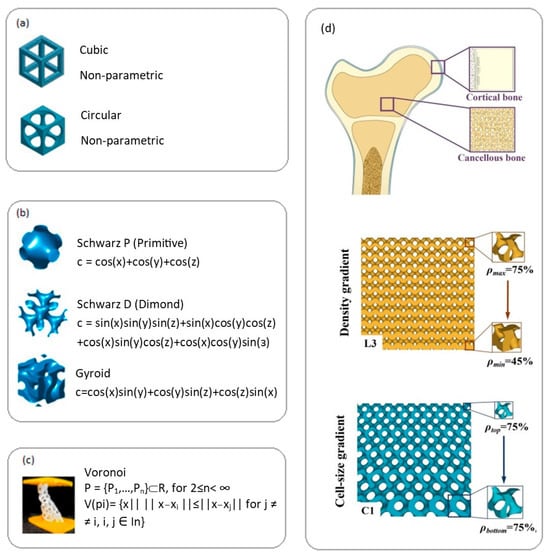
Figure 4.
Main types of lattice structures for biodegradable implant materials: (a) cubic and circular, reprint with permission from Ref. [58], 2024, Elsevier. (b) Triply periodic minimal surfaces—Schwarz P, Schwarz D, gyroid, reprint with permission from Ref. [58], 2024, Elsevier, (c) Voronoi structure reprint with permission from Ref. [58], 2024, Elsevier, (d) schematic diagram depicting natural bone structure with gradient porous structures and gyroid CGPS structures with cell size gradient, reprint with permission from Ref. [57], 2024, Elsevier.
2.6.2. Influence of Lattice Structure Parameters on Mechanical Properties and Biodegradation
The mechanical properties of lattice structures made from biodegradable metals are determined by a combination of material properties and geometric parameters of the lattice. Key factors include porosity, as Schwarz lattices with 42% porosity demonstrated the highest combination of strength and plasticity [59], cell size (affects mechanics/permeability/cell integration; optimally above 300 μm for osseointegration [60]), strut thickness, and topology (determines stiffness/strength [59]). Higher porosity increases surface area, accelerating degradation. For magnesium alloys, interconnected pores promote uniform corrosion, whereas isolated pores lead to localized pitting corrosion [61]. Lattice structures made from biodegradable metals demonstrate improved biological response compared to monolithic implants [62].
Having established the fundamental principles and technical aspects of additive manufacturing for biodegradable metals, we now turn our attention to specific material systems. The following sections examine three primary biodegradable metal families—magnesium, iron, and zinc alloys—in detail. Each section analyzes the unique characteristics, challenges, and opportunities presented by these materials when processed through selective laser melting. We begin with magnesium alloys, which offer the closest mechanical match to bone tissue and have seen the most extensive clinical adoption among biodegradable metals.
3. Magnesium Alloys in Selective Laser Melting
3.1. Historical Overview and Basic Properties
The history of magnesium applications in medicine began in 1878 when Edward C. Huse first used magnesium wire to stop bleeding [63], and in 1900, Payr proposed magnesium plates for joint restoration [64]. Widespread clinical application began later, with CE marking obtained for the DREAMS magnesium stent (Biotronik, Bülach, Switzerland) and MAGNEZIX® screw (Syntellix AG, Hannover, Germany) [65,66]. Modern developments focus on creating new alloys with improved properties and implementing technologies such as selective laser melting, due to the unique characteristics of magnesium that make it promising for biodegradable implants:
- Mechanical properties—magnesium’s elastic modulus (41–45 GPa) is significantly closer to cortical bone (5–23 GPa) than traditional implant materials (titanium alloys—110–120 GPa, stainless steel—200–210 GPa), reducing the risk of stress shielding effects [67].
- Biocompatibility—magnesium is an essential element for the human body, participating in more than 300 biochemical reactions, including protein synthesis and energy metabolism regulation. The daily requirement of an adult for magnesium is 300–400 mg [68].
- Osteogenic properties—magnesium ions released during implant degradation stimulate the proliferation and differentiation of osteoblasts, promoting bone tissue formation [69].
- Degradation in physiological conditions—magnesium undergoes electrochemical corrosion in biological environments with the formation of magnesium hydroxide and hydrogen: Mg + 2H2O → Mg(OH)2 + H2. Magnesium corrosion products are non-toxic and gradually dissolve or are excreted from the body [70].
The use of magnesium alloys as biodegradable implants is associated with several limitations. First, pure magnesium is characterized by a high corrosion rate (up to 3 mm/year), which can lead to premature loss of mechanical integrity of the implant and the formation of gas cavities [71]. Second, magnesium alloys possess relatively low strength, specifically, their yield strength is lower than that of titanium alloys and stainless steel, which limits their application under high load conditions [72]. Third, technological difficulties in processing and additive manufacturing are related to the high reactivity and low boiling point of magnesium [73]. To overcome these limitations, special magnesium alloys with improved properties are being developed, and their additive manufacturing processes are being optimized.
3.2. Influence of Alloying Elements
Magnesium alloying plays a key role in modifying its properties for biomedical applications, allowing control of degradation rate, mechanical characteristics, and biological response. The choice of alloying elements for biodegradable magnesium alloys is determined not only by their influence on material properties but also by biocompatibility, absence of toxicity, and potential therapeutic effect [74]. Table 1 summarizes the influence of alloying elements on the properties of magnesium alloys obtained by SLM.

Table 1.
Influence of alloying elements on the properties of magnesium alloys obtained by SLM.
3.2.1. Calcium (Ca)
Calcium is one of the most important minerals in human bone tissue, playing a key role in its strength and ability to recover after damage. In magnesium alloys, calcium forms the intermetallic compound Mg2Ca, which is distributed predominantly along grain boundaries and affects mechanical properties [87]. The addition of calcium promotes grain refinement, leading to increased strength and plasticity [88,89]. However, calcium content should be controlled, usually less than 1% [80], as exceeding this value can deteriorate the material’s corrosion resistance. Studies show that the Mg-2Zn-0.2Mn alloy with the addition of 0.36 wt.% Ca has optimal corrosion resistance due to the formation of a dense protective film and limited amount of secondary phase. At higher Ca content (0.76% and 1.10%), significant formation of secondary phase is observed, causing intensive galvanic corrosion [90]. In the SLM process, calcium affects the microstructure and properties of magnesium alloys. Modern studies of SLM Mg-Ca alloys demonstrate the possibility of achieving relative density above 99% with optimized process parameters (laser power 150–200 W, scanning speed 400 mm/s) [91].
3.2.2. Zinc (Zn)
Zinc, an important microelement for the human body [92,93,94], participates in solid solution and dispersion strengthening in magnesium alloys, refines grain, and forms the MgZn phase, improving mechanical properties and corrosion resistance at contents up to 5% [95]. However, when exceeding 5–7% Zn, corrosion resistance decreases due to galvanic corrosion, and the microstructure consists of α-Mg and MgZn distributed along grain boundaries. In the SLM process, Mg-Zn alloys demonstrate a fine-grained structure, and the addition of Ca increases strength to 182 MPa and elongation to 9.1% [96].
3.2.3. Strontium (Sr)
Strontium is an important element for bone tissue growth and strengthening. It stimulates osteoblast differentiation while slowing osteoclast activity, also improving calcium absorption by bone tissue [97]. In magnesium alloys, strontium forms secondary phases that improve mechanical properties and corrosion resistance [98]. Binary Mg-Sr alloys predominantly consist of α-Mg and Mg17Sr2 phases [98]. Increasing strontium content (0.5–2.5%) enhances tensile and compressive strength. However, the corrosion resistance of extruded Mg-Sr alloys decreases with increasing strontium content due to galvanic corrosion [3]. In SLM, strontium is of interest for biodegradable implants with osteogenic properties. Adding 0.5–1.0% Sr to magnesium alloys processed by SLM improves the biological response without significantly deteriorating technological properties [99].
3.2.4. Rare Earth Elements (REE)
Rare earth elements (REE) such as gadolinium (Gd), yttrium (Y), neodymium (Nd), and cerium (Ce) improve the mechanical properties and corrosion resistance of magnesium alloys. For biomedical purposes, Gd and Y are of greatest interest [100]. The commercial alloy WE43 (Mg-4Y-3RE-0.5Zr), originally developed for the aerospace industry, has found application in biomedicine due to its optimal properties. This alloy is characterized by enhanced corrosion resistance and mechanical strength, as well as relatively good processability in additive manufacturing [101].
3.2.5. Manganese (Mn) and Zirconium (Zr)
Manganese and zirconium modify the microstructure and neutralize impurities in magnesium alloys. Manganese improves corrosion resistance by binding iron and forming Al-Mn compounds [102]. Zirconium is a powerful grain modifier, promoting the formation of fine-grained structure [102]. In the context of SLM, adding 0.4–0.8% Mn to magnesium alloys helps improve surface quality and reduce porosity of the resulting products [85]. Zirconium, despite its advantages as a structure modifier, can create problems in laser melting due to its high melting temperature (1855 °C) and tendency to oxidize [103]. Recent research shows the promise of the Mg-10Zn-0.8Ca-0.5Zr alloy for the SLM process [104]. The powder of this alloy, obtained by mechanical alloying, is characterized by a homogeneous structure and relatively spherical particle shape. Optimization of SLM parameters allows obtaining samples with high strength, integrity, and minimal porosity (0.64%) at low laser energy density (138 J/mm3) [104].
3.3. Features of the SLM Process for Magnesium Alloys
Selective laser melting of magnesium alloys is associated with a number of specific problems due to the physicochemical properties of magnesium. Understanding these features and developing approaches to overcome them are key to successful additive manufacturing of biodegradable implants from magnesium alloys.
3.3.1. Reactivity and Thermal Properties
Magnesium has high chemical activity, leading to intensive oxidation when heated during the SLM process with the formation of MgO, increasing the risk of ignition [105]. To prevent oxidation, sealed chambers with inert atmosphere (argon/nitrogen, O2 < 10 ppm), evacuation (10−3–10−5 mbar), and oxygen content control (<500 ppm for AZ91D alloy) [106] are used. The low boiling point of magnesium (1090 °C) causes evaporation, pore formation, and changes in alloy composition, while high thermal conductivity (156 W/(m·K)) promotes rapid cooling, leading to fusion defects [107]. These problems are solved by optimizing laser parameters (low power, high scanning speed), powder preheating, and vapor filtration. MgO oxide films (tplan = 2852 °C) on powder particles deteriorate fusion, promoting inclusion formation [108]. To minimize them, powder pre-treatment, increased laser energy, introduction of modifying additives (e.g., Ca), and optimization of scanning modes are applied.
3.3.2. Optimization of SLM Parameters for Magnesium Alloys
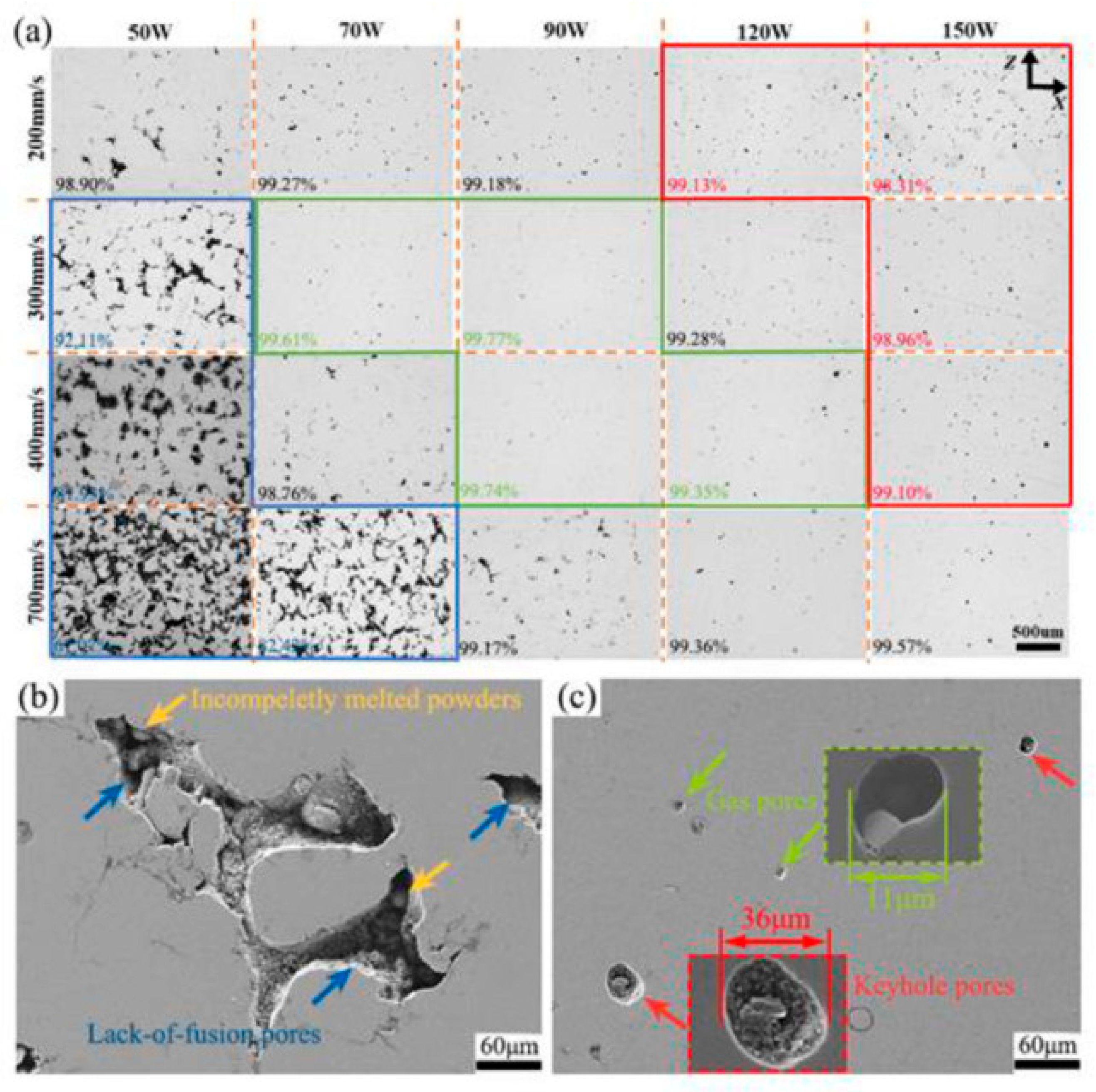
For successful additive manufacturing of magnesium alloys, it is necessary to optimize SLM parameters: laser power (100–200 W, for AZ91D optimally about 150 W) (see Figure 5); scanning speed (high, 400–800 mm/s, for AZ91D optimally about 600–650 mm/s); layer thickness (30 μm); scanning strategy (rotation of direction between layers); energy density (70–120 J/mm3) [106].
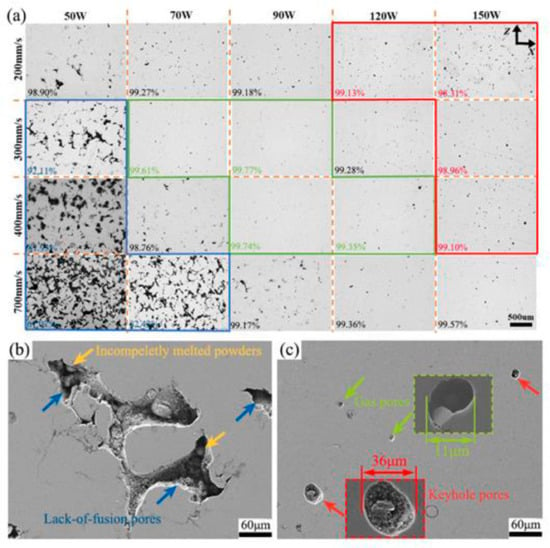
Figure 5.
Typical optical micrographs of vertical planes of AZ91D manufactured at various processing parameters, values marked in the lower left corner represent the corresponding densification (a), SEM micrograph of lack of fusion pores (b), gas pores, keyhole-type pores and corresponding local magnifications (c), Reprint from [106].
Nopová et al. (2023) investigated the influence of SLM parameters on the quality and properties of AZ91D alloy [109]. It was established that optimal process parameters (laser power = 180 W, scanning speed = 612.5 mm/s, hatch distance = 0.133 mm, and layer thickness = 0.05 mm) provide relative density over 99%, low porosity, and homogeneous microstructure. Mechanical tests showed high values of yield strength (181 MPa) and tensile strength (305 MPa), with elongation to failure of 5.2%, which is more than twice the literature data for cast material [109].
Analysis of the microstructure of SLM-manufactured AZ91D showed the dependence of pore morphology on processing parameters (see Figure 3). Lack of fusion areas, keyhole areas, and transition areas were identified, which is consistent with previous studies [18,32,41]. Defects were classified as lack of fusion pores (low energy density), gas pores, and keyhole-type pores (high energy density). Optimal parameters provide densification >99.77%, with predominance of small gas pores associated with Mg and Zn evaporation [110] and argon entrapment.
For SLM WE43 yields two primary defect types: lack-of-fusion (LOF) at low power/high speed, and porosity at high power/low speed. LOF is attributed to insufficient energy density for complete powder melting, while porosity results from metal vapor entrapment at higher energy densities, exacerbated by the high vapor pressure of magnesium. A scan speed of 1000 mm/s and a laser power of 100 W resulted in optimal density (99.88%) [111].
3.4. Microstructure and Mechanical Properties
Additive manufacturing of magnesium alloys ensures the formation of a microstructure significantly different from that obtained by traditional methods (casting, extrusion). These differences are due to the unique crystallization conditions in SLM, characterized by extremely high cooling rates and directional heat removal [93].
3.4.1. Features of SLM-Magnesium Alloys Microstructure
The microstructure of SLM-magnesium alloys is characterized by a fine-grained structure of 5–20 μm, which is significantly smaller than in cast alloys (50–150 μm). The fine-grained structure provides enhanced mechanical properties. It also has a layered morphology (“fish-scale”), growth texture (preferential orientation of crystallographic directions parallel to the build direction), non-equilibrium phase distribution, microsegregation, and high density of crystal lattice defects. Studies have shown that SLM material is characterized by smaller and more uniformly distributed grains, with less anisotropy compared to traditional production methods [51].
3.4.2. Mechanical Properties of SLM-Magnesium Alloys
The mechanical properties of magnesium alloys obtained by SLM usually exceed those of similar materials manufactured by traditional methods, especially in terms of strength. This improvement is due to the fine-grained structure, solid solution strengthening, and high dislocation density.
Table 2 presents the mechanical properties of various magnesium alloys obtained by SLM compared to traditional production methods.

Table 2.
Mechanical properties of magnesium alloys obtained by various methods.
As seen from the table, SLM materials demonstrate increased values of yield strength and tensile strength compared to cast analogues. Particularly impressive improvement is observed for the WE43 alloy, where the yield strength of SLM material (296.3 MPa) is more than twice the value for cast material (145.4 MPa). This is explained by a combination of fine-grained structure, solid solution strengthening, and dispersion strengthening by secondary phases [112].
Of particular interest are the fatigue properties of magnesium alloys obtained by SLM, as they are critically important for implants working under cyclic loading conditions. Studies show that the fatigue limit of SLM materials is 40–45% of the tensile strength, which is comparable to extruded materials and exceeds the values for cast analogues (30–35%) [114].
3.4.3. Effect of Heat Treatment
Heat treatment effectively modifies the microstructure and properties of SLM-magnesium alloys. Applied treatments include: stress relief annealing, homogenization annealing, T4 treatment (solid solution strengthening), and T6 treatment (combination of solid solution strengthening and artificial aging). For example, T4 treatment of WE43 alloy increases plasticity with a moderate decrease in yield strength [115]. Heat treatment of magnesium alloys requires precautions due to their tendency to oxidize, such as protective atmospheres or coatings.
3.5. Biodegradation and Biocompatibility
The rate and mechanism of biodegradation of magnesium alloys obtained by SLM play a key role in their functionality as temporary implants. The unique microstructure and properties of SLM materials significantly affect the corrosion process in the physiological environment.
3.5.1. Mechanism of Biodegradation of Magnesium Alloys
Biodegradation of magnesium alloys in physiological conditions is based on electrochemical corrosion, which can be described by the following reactions:
Anodic reaction: Mg → Mg2+ + 2e−
Cathodic reaction: 2H2O + 2e− → H2 + 2OH−
Hydroxide formation: Mg2+ + 2OH− → Mg(OH)2
Overall reaction: Mg + 2H2O → Mg(OH)2 + H2
In a physiological environment containing chloride ions, the formation of a protective Mg(OH)2 layer can be disrupted due to the formation of soluble MgCl2:
Mg(OH)2 + 2Cl− → MgCl2 + 2OH−
Biodegradation of magnesium alloys in a living organism represents a more complex process, including interaction with proteins, cells, and enzymes. Protein adsorption on the implant surface can either accelerate or slow down corrosion depending on the type of proteins and environmental conditions [116].
3.5.2. Influence of Microstructure on Biodegradation
The microstructure of SLM-magnesium alloys significantly affects the rate and nature of their biodegradation. Fine-grained structure (5–20 μm) increases the area of grain boundaries, which can accelerate corrosion [117]. Non-equilibrium phase distribution and extended solubility limits of alloying elements in SLM materials change their electrochemical behavior—for example, in WE43 alloy, uniform distribution of rare earth elements contributes to the formation of a more stable protective film [118]. High residual stresses characteristic of the SLM process accelerate corrosion due to the mechano-chemical effect, but heat treatment can reduce their negative impact. Present microporosity creates conditions for localized corrosion due to limited mass transfer and local pH changes [119], and the crystallographic texture forming during SLM leads to anisotropy of corrosion behavior, as different crystallographic planes of magnesium have different electrochemical activity [120].
3.5.3. Comparison of Biodegradation Rate of SLM and Traditional Materials
Studies show that the biodegradation rate of SLM-magnesium alloys is higher than that of analogues obtained by traditional methods. For example, for SLM-WE43 alloy, the in vitro corrosion rate is 2.6 ± 1.9 versus 1.0 ± 0.5 mm/year for cast material [121]. This is associated with fine-grained structure, microporosity, and residual stresses, but the corrosion rate can be regulated by optimizing SLM parameters, post-processing, and surface modification.
As evident from the comparative data in Table 3, SLM-produced magnesium alloys consistently demonstrate higher corrosion rates compared to their traditionally manufactured counterparts, with increases ranging from 60% to 160% depending on the specific alloy composition and testing environment. This accelerated degradation can be attributed to several microstructural features unique to the SLM process: (1) the significantly finer grain size (5–20 μm compared to 50–150 μm in cast alloys) increases the total grain boundary area, creating more sites for preferential corrosion; (2) the presence of processing-induced microporosity creates localized areas for corrosion initiation; and (3) high residual stresses from rapid solidification promote stress-assisted corrosion mechanisms.

Table 3.
Comparison of biodegradation rates of magnesium alloys produced by different methods.
The data also reveals that post-processing treatments, particularly heat treatment, can substantially reduce this accelerated degradation. For instance, T4 heat treatment of SLM-WE43 reduced its corrosion rate from 2.6 to 1.8 mm/year by relieving residual stresses and homogenizing the microstructure. This modulation capability highlights the potential to tailor degradation rates to specific clinical requirements through controlled processing and post-processing strategies.
Testing environments significantly impact measured degradation rates, with more complex physiological solutions (containing proteins and various ions) generally resulting in different corrosion behavior compared to simple saline solutions. This underscores the importance of standardized testing protocols when comparing degradation performance across different studies and manufacturing methods.
3.5.4. Methods of Controlling Biodegradation Rate
A complex of methods is applied to regulate the biodegradation rate of SLM-magnesium implants. Alloy composition optimization (addition of RE, Ca, Zn) improves corrosion properties. Heat treatment (e.g., T4 regime for WE43) reduces the corrosion rate by relieving stresses [122]. Surface coatings are effective: MAO (reduces corrosion by 5–10 times) [123], PEO (creates regulated porous layers) [124], and biopolymer coatings. Additionally, porosity is controlled by adjusting SLM parameters, and gradient structures are created for zonal degradation [125]. These approaches allow precise tuning of implant dissolution rate.
3.5.5. Biocompatibility of SLM-Magnesium Implants
The biocompatibility of magnesium alloys obtained by SLM is determined by their chemical composition, microstructure, and surface topography. In vitro studies confirm their good cytocompatibility: they support adhesion, proliferation, and differentiation of osteoblasts, stem cells, and endothelial cells [126].
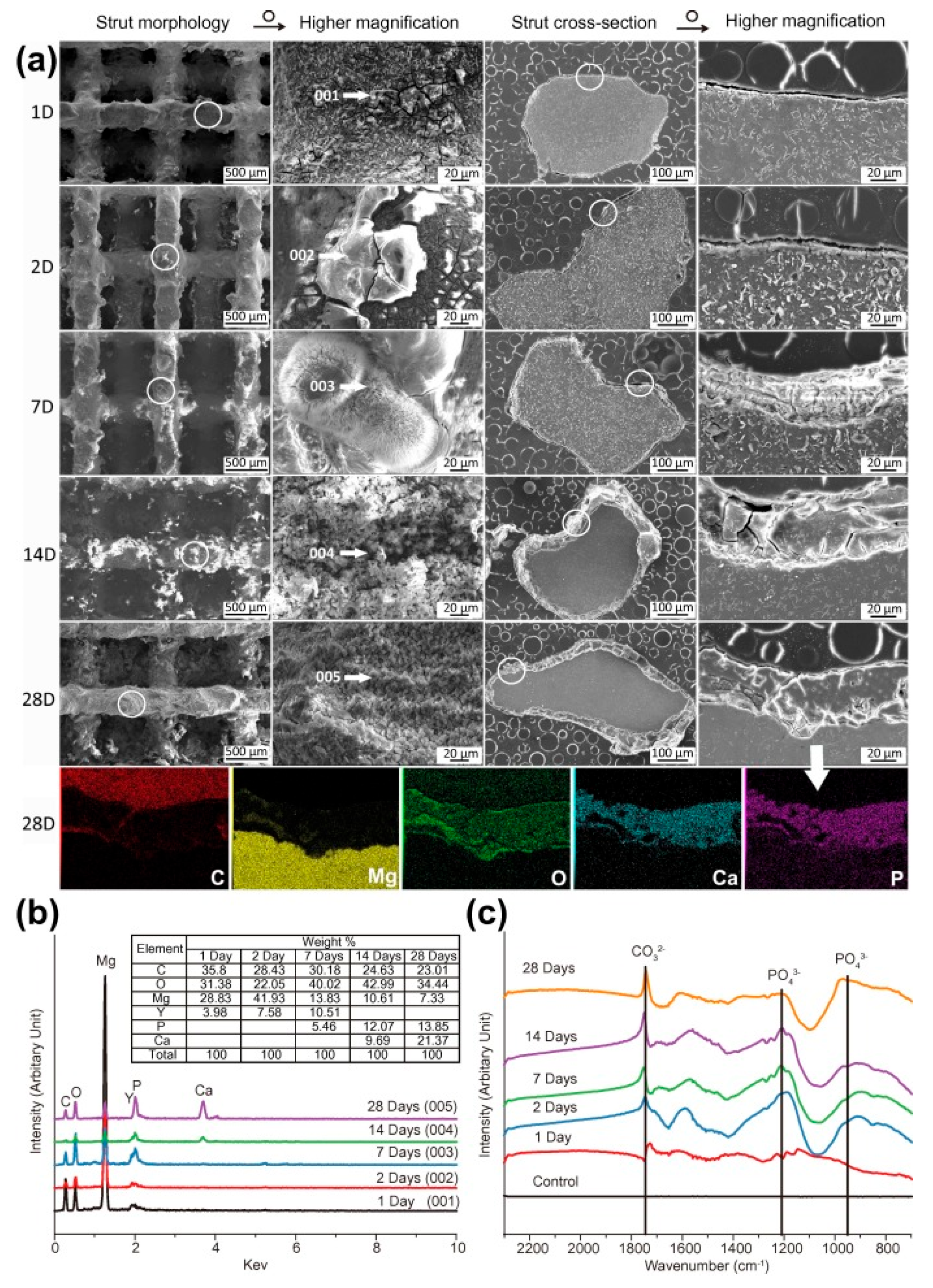
Porous lattice structures (60–70% porosity, 600–800 μm) provide optimal conditions for cell migration and bone tissue formation [57]. Figure 6 shows the Characterization of degradation products on the periphery of scaffolds. In vivo studies on animal models demonstrate active osseointegration and gradual replacement of the implant with bone tissue [127]. First clinical trials of individualized implants made of WE43 show their successful integration and controlled degradation [128].
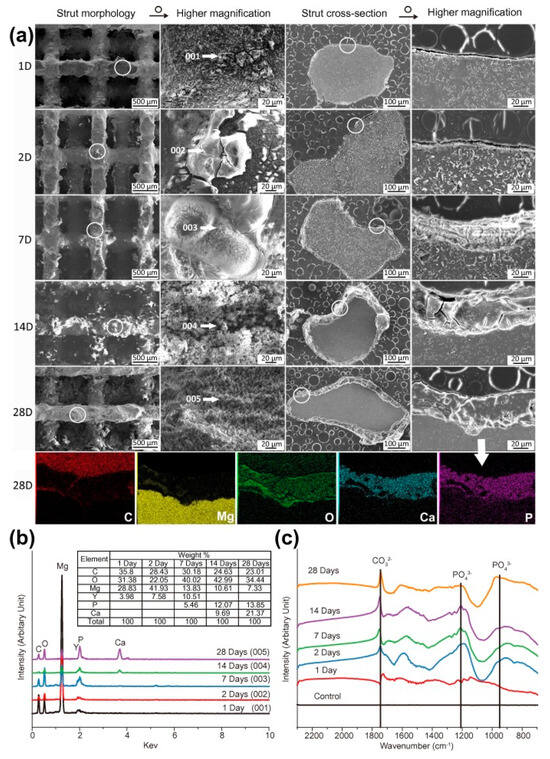
Figure 6.
Characterization of degradation products on the periphery of scaffolds: (a) SEM images and EDS mapping, (b) EDS analysis, and (c) Fourier Transform Infrared Spectroscopy analysis, reproduced from [28], with permission from Elsevier, 2018.
Thus, SLM-magnesium alloys have high potential for clinical application due to the combination of biocompatibility, osteoinductive properties, and regulated degradation rate.
3.6. Clinical Applications and Prospects
SLM–magnesium implants represent a promising direction in orthopedics, craniomaxillofacial and cardiovascular surgery due to the unique possibilities of additive manufacturing and personalization. In orthopedics, they can be used for fracture fixation (screws, plates, rods), providing gradual load transfer and stimulation of osteogenesis [129], as well as bone scaffolds with optimized geometry and osteochondral implants with varying porosity. Clinical studies of MAGNEZIX® screws demonstrate effectiveness comparable to titanium analogues [130]. In craniomaxillofacial surgery, SLM–magnesium finds application in skull reconstruction (personalized implants), maxillofacial reconstruction (implants for defect restoration with improved osseointegration), and as experimental dental implants with controlled degradation rate [131]. In cardiovascular surgery, biodegradable stents (providing temporary support for vessels) and occluders for closing defects are being investigated [131]. Further research is directed towards developing new alloys with optimized properties, surface functionalization [131], creating bioactive implants [28], hybrid structures, improving SLM technology, and integration into personalized medicine platforms. In conclusion, SLM–magnesium alloys present significant potential in the field of biodegradable implants, opening new possibilities through additive manufacturing and magnesium biocompatibility.
While magnesium alloys offer excellent biocompatibility and mechanical similarity to bone, their relatively rapid degradation may be unsuitable for applications requiring prolonged structural support. Iron-based alloys present an alternative with superior strength and slower degradation kinetics, making them potentially valuable for load-bearing applications where longer-term support is necessary. The following section explores how selective laser melting can be applied to iron-based biodegradable systems, addressing both the opportunities and challenges presented by these high-strength alloys.
4. Iron-Based Alloys in Selective Laser Melting
4.1. Historical Overview and Basic Properties
Iron represents a promising material for biodegradable implants due to its combination of high strength, plasticity, excellent biocompatibility, and the possibility of manufacturing complex structures (stents, foils, metal foams) [38]. However, its clinical application is limited by an extremely slow degradation rate: experimental studies in pigs showed that iron stents in the descending aorta maintained structural integrity for 12 months without significant signs of corrosion [10]. Although the natural metabolism of iron in the body ensures good biocompatibility, its excessively low degradation rate makes a pure iron implant unsuitable for temporary clinical application, stimulating the development of methods to accelerate its corrosion.
Iron and its alloys are promising for creating biodegradable implants due to their combination of mechanical and biological properties. Their high strength and stiffness provide the necessary load-bearing capacity in orthopedic applications, while adjustable porosity allows approximating mechanical characteristics to those of natural bone [132,133]. An important advantage is the biocompatibility of iron, which is naturally metabolized in the body, minimizing the risk of undesirable immune reactions [131].
However, the use of iron as a biodegradable material is associated with several problems:
- Low degradation rate—the corrosion rate of pure iron in physiological conditions is 0.1–0.5 mm/year, which is significantly lower than clinically acceptable values for temporary implants (0.5–2.0 mm/year) [134].
- Formation of insoluble corrosion products—iron corrosion products (predominantly oxides and hydroxides) have low solubility and can accumulate around the implant, creating a diffusion barrier that further slows down corrosion [135].
- Potential toxicity—Iron ions, especially when exceeding certain concentrations, can have a cytotoxic effect. Studies have shown that high levels of iron ions can reduce cell proliferation rate and affect metabolic activity [132].
To overcome these limitations, especially the low degradation rate, intensive research is being conducted on the development of new iron-based alloys and optimization of their production technologies, including selective laser melting.
4.2. Influence of Alloying Elements
Alloying of iron plays a key role in modifying its properties for biomedical applications, allowing control of degradation rate, mechanical characteristics, and biological response (Table 4). The choice of alloying elements for biodegradable iron alloys is determined not only by their influence on material properties but also by biocompatibility, absence of toxicity, and potential therapeutic effect.

Table 4.
Influence of alloying elements on the properties of iron alloys obtained by SLM.
4.2.1. Manganese (Mn)
Manganese (Mn) is a promising alloying element for biodegradable iron alloys, affecting phase composition, mechanical properties, and degradation rate. At 20–30% Mn, austenitic (γ, FCC) or austenitic-martensitic (γ + ε, FCC + HCP) structures form, and at >30%—fully austenitic structures [139]. Mn improves strength and enhances plasticity; for example, Fe-30Mn alloys demonstrate yield strength of 200–250 MPa and tensile strength of 430–550 MPa with elongation of 30–40%. Fabricated porous biodegradable SLM Fe-30Mn scaffolds showed mechanical adaptability, biocompatibility, and osseointegration in vivo (48 weeks) [28]. However, in the context of SLM, high reactivity and low evaporation temperature of Mn create technological challenges, such as selective evaporation of Mn and deviation of product composition.
4.2.2. Carbon (C)
Carbon (C) is an important alloying element for iron alloys, affecting mechanical properties and microstructure. It strengthens iron (0.5–1.2% C increases yield strength and strength) [139], stabilizes the austenitic phase, and affects corrosion resistance [141]. Fe-Mn-C possesses high strength and plasticity due to austenitic structure and TRIP effect, as well as accelerated degradation in vitro [140]. In SLM, it is necessary to control the formation of carbides that affect product properties.
4.2.3. Silicon (Si)
Silicon (Si) is an effective alloying element that accelerates the degradation of iron alloys: it increases corrosion rate [141], promotes the formation of duplex ε and γ phases (Fe-28Mn-6Si demonstrates 80% higher degradation rate) [143], improves strength by 70% compared to the base alloy (Fe-30Mn) and improves strain hardening capacity while maintaining good plasticity [144], and at certain compositions induces shape memory effect [142]. Optimal Si content (3–5%) for balance of properties and degradation; Fe-30Mn-5Si showed a degradation rate of 0.80 mm/year [152].
4.2.4. Calcium (Ca) and Magnesium (Mg)
Calcium (Ca) and magnesium (Mg) are biologically active elements that, while accelerating the degradation of iron alloys, can positively affect bone tissue regeneration. They significantly increase corrosion rate [145], and the released Ca2+ and Mg2+ ions can stimulate osteogenesis and angiogenesis [146]. Ca and Mg also affect microstructure and, consequently, alloy properties. Hong et al. (2016) showed that Fe-Mn-Ca/Mg alloys obtained by jet 3D printing accelerate degradation and promote osteoinduction and osteoconduction [153]. In SLM, it is necessary to consider the high reactivity of Ca and Mg and optimize process parameters.
4.2.5. Palladium (Pd) and Copper (Cu)
Palladium (Pd) and copper (Cu) accelerate the degradation of iron alloys and impart additional properties. Minor addition of Pd (0.5–1.0%) to Fe-10Mn increases degradation rate fourfold [149]. Cu-containing alloys possess antimicrobial properties [151], while Pd and Cu can improve mechanical characteristics. Schinhammer et al. (2013) showed that the combination of Mn, C, Pd optimizes degradation rate and mechanical properties [154]. In SLM, differences in melting temperatures and thermophysical properties of Pd and Cu should be considered.
4.3. Features of the SLM Process for Iron Alloys
Selective laser melting of iron alloys for biodegradable implants has a number of features related to both the physicochemical properties of the material and the requirements for final products.
4.3.1. Technological Features of SLM for Iron Alloys
Iron alloys, particularly Fe-Mn systems, possess a number of specific properties affecting the SLM process:
- High melting temperature requires higher laser power and energy density for complete powder melting [155].
- Selective evaporation of alloying elements—In the Fe-Mn SLM process, evaporation of large amounts of Mn will lead to Mn mass loss, defect formation, and chemical composition changes in the final product [156].
- High coefficient of thermal expansion—austenitic Fe-Mn alloys have a relatively high coefficient of thermal expansion [157], which can lead to significant thermal stresses and deformations during SLM.
- Oxidation—although iron alloys are less reactive than magnesium or zinc alloys, they are still subject to oxidation at high temperatures, especially alloys containing Mn, which requires working in a protective atmosphere [158].
4.3.2. Optimization of SLM Parameters for Iron Alloys
Key SLM parameters requiring optimization for iron alloys are:
- Laser power—higher power (200–400 W) is usually required for effective melting of iron alloys compared to magnesium and zinc alloys. Donik et al. (2021) showed that for the Fe-Mn alloy, the optimal power is 250–300 W [159].
- Scanning speed—relatively high scanning speeds (600–1200 mm/s) are applied for iron alloys, allowing reduction in laser interaction time and minimization of selective evaporation of alloying elements. The optimal speed for Fe-Mn alloys is about 800 mm/s [159].
- Hatch distance—for iron alloys, a distance of 70–100 μm is usually used, providing sufficient overlap of tracks for forming a monolithic structure. Donik et al. (2021) used 80 μm [159].
- Layer thickness—typical values for Fe-alloys are 20–40 μm, providing a balance between productivity and quality of the resulting products.
- Scanning strategy—to minimize thermal stresses and property anisotropy, a strategy with rotation of scanning direction between layers (usually by 67° or 90°) is commonly applied [160].
- Energy density—for iron alloys, the optimal energy density is usually 60–150 J/mm3 [161,162].
- Platform preheating temperature—preheating the platform to 500 °C allows reducing thermal gradients and residual stresses, as well as improving product quality [163].
4.3.3. Influence of SLM Parameters on Phase Composition and Microstructure of Fe-Mn Alloys
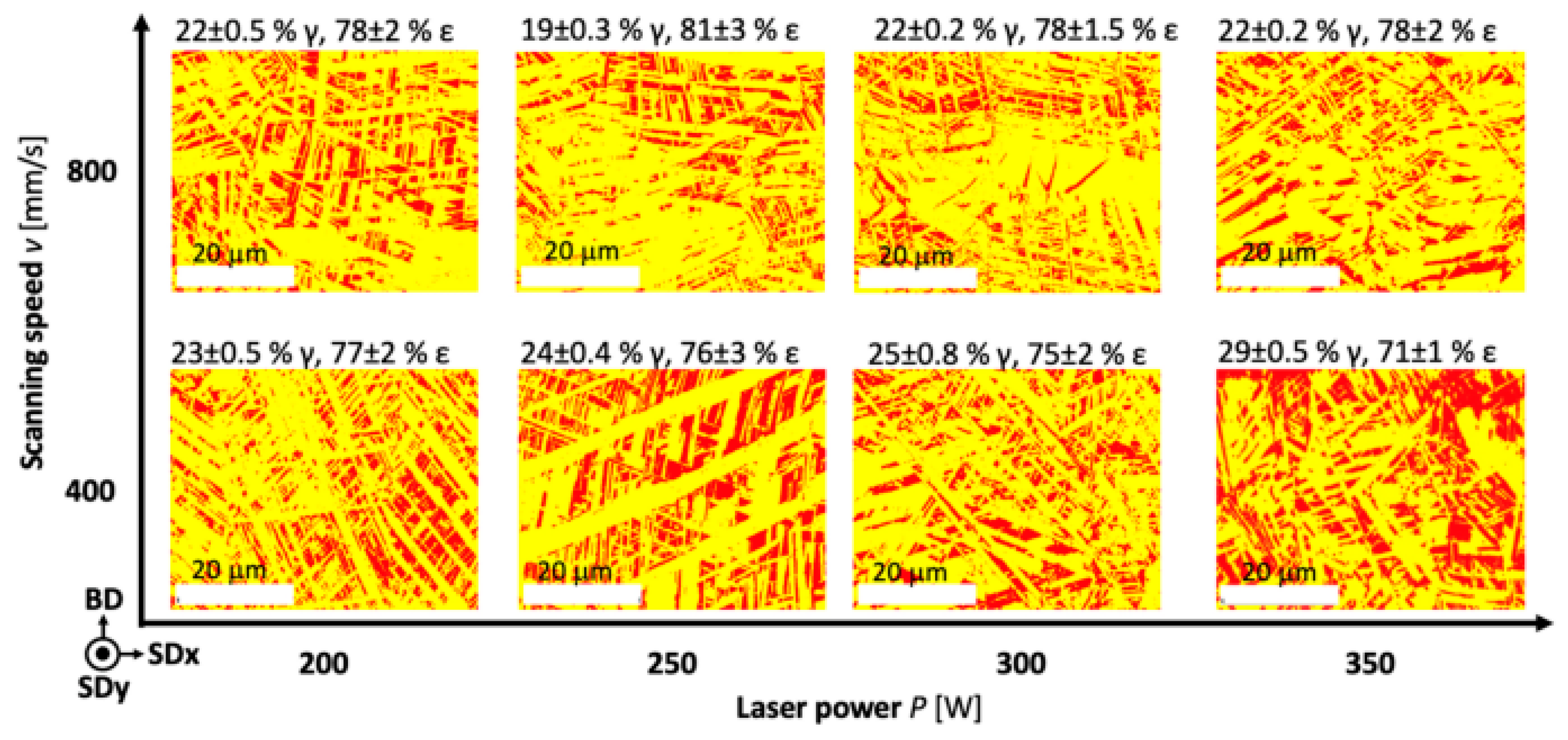
SLM parameters significantly affect phase formation and microstructure of Fe-Mn alloys, determining their mechanical properties and degradation rate. Energy density influences the formation of austenitic (γ) and ε-martensitic phases, with lower densities potentially reducing Mn losses [24]. The formation of γ and ε phases is directly related to Mn content [159]. Thermal effect determines the formation of columnar or equiaxed grains [24]. Rapid cooling leads to non-equilibrium structures with Mn segregation at melt pool boundaries. Donik et al. (2021) established the dependence of ε-phase content on laser energy density, allowing optimization of phase ratio and microstructure for controlled degradation [159].
4.3.4. Post-Processing of SLM Products from Iron Alloys
Post-processing is an important stage in the production of biodegradable implants from iron alloys, allowing modification of microstructure, improvement in mechanical properties, and control of degradation rate:
- Heat treatment—various heat treatment regimes can be applied to reduce residual stresses, homogenize microstructure, and modify phase composition. For Fe-Mn alloys, Mn oxides have several transformations in the temperature range from 700 to 1000 °C, from which heat treatment regimes are often selected [24,164].
- Hot isostatic pressing (HIP)—this method allows eliminating residual porosity and improving mechanical properties of SLM products.
- Surface modification controls degradation and improves biocompatibility: electrochemical polishing, passivation, coating application [165,166]
- Mechanical processing—to achieve the necessary dimensional accuracy and surface quality, finish mechanical processing can be applied. However, for complex porous structures, traditional mechanical processing methods are often inapplicable, requiring the use of specialized approaches such as electric discharge machining or chemical etching.
Figure 7 shows EBSD ε-phase content from 81% (20.5% Mn) to 71% (24.8% Mn). Mn content affects the ε − γ phase transformation, with carbon also playing an important role (Mesquita et al., our previous studies). Despite increasing Mn content in the raw material to 53%, only 32% Mn was achieved in the SLM sample, a dual-phase structure (ε + γ) remained due to manufacturing issues [159].
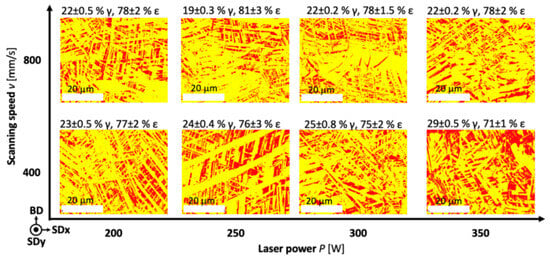
Figure 7.
Phase maps of ε phase—yellow, and γ phase—red for SLM samples produced at different process parameters, reprint from [159].
4.4. Microstructure and Mechanical Properties
The microstructure of iron alloys obtained by SLM differs significantly from that formed by traditional production methods, due to the unique crystallization conditions during laser melting.
4.4.1. Features of SLM-Iron Alloys Microstructure
The microstructure of Fe-Mn alloys obtained by SLM is characterized by a number of features. Phase composition depends on Mn content and SLM parameters: γ-phase (austenite) forms at high Mn content, ε-phase (martensite) at medium content, and α’-phase (martensite) at low Mn content or deformation [159,167]. SLM typically leads to the formation of a fine-grained structure (10–50 μm) with preferential grain orientation, the shape of which depends on process parameters. Microporosity, hot cracks, and residual stresses may be present in SLM materials. The composition of the iron alloy 1.2% C, 32% Mn provides an austenitic structure regardless of method, but SLM parameters affect microstructure, element distribution, grain boundaries, mechanical properties, and corrosion [168].
4.4.2. Mechanical Properties of SLM-Iron Alloys
The mechanical properties of iron alloys obtained by SLM are determined by a combination of chemical composition, phase composition, microstructure, and presence of defects. Table 5 presents the mechanical properties of various Fe alloys obtained by SLM compared to traditional production methods.

Table 5.
Mechanical properties of iron alloys obtained by various methods as well as 316L.
These results highlight the potential of Fe-based biodegradable alloys, particularly those produced via SLM, for applications requiring high strength, though improvements in elongation are needed for broader use in biomedical applications.
4.4.3. Special Mechanical Effects in Fe-Mn Alloys
Fe-Mn alloys, especially with the addition of C and Si, can demonstrate a number of unique mechanical effects that may be useful for biomedical applications:
- TRIP effect (Transformation-Induced Plasticity)—plasticity induced by phase transformation. In Fe-Mn-C alloys with predominantly austenitic structure, mechanical deformation can cause martensite formation, leading to enhanced plasticity and strengthening [174].
- TWIP effect (Twinning-Induced Plasticity)—plasticity induced by twinning. In high-manganese alloys (Fe-Mn with Mn content 25–35%), deformation occurs predominantly through the twinning mechanism, providing high plasticity and strengthening [174].
- Shape memory effect—some Fe-Mn-Si alloys demonstrate shape memory effect associated with reversible martensitic transformation γ ↔ ε. This effect can be used to create implants with functional properties, for example, self-expanding stents [175].
- Superelasticity—Fe-Mn-Si-Al alloys can demonstrate superelastic behavior similar to NiTi alloys, but with better biocompatibility and biodegradability [176].
These effects can be enhanced or modified by optimizing SLM parameters and subsequent heat treatment. For example, by controlling cooling rate and thermal cycles during the SLM process, the ratio of γ and ε phases can be regulated and, consequently, the expression of TRIP/TWIP effects and shape memory effect.
4.4.4. Fatigue Characteristics and Durability
For temporary implants, especially those working under cyclic loading conditions (such as orthopedic fixators or cardiovascular stents), fatigue characteristics and durability are critically important:
- Fatigue limit—for Fe-Mn alloys obtained by SLM, the fatigue limit is usually 40–45% of the tensile strength, which corresponds to 330 MPa for alloys with tensile strength of 839 MPa [177].
- Corrosion fatigue—high fatigue strength (70% of yield strength in air, 65% in r-SBF) due to the plasticity of iron and slow degradation is shown. Cyclic loading accelerated iron degradation, but iron remains a promising bioactive bone implant [178].
- Microstructure influence—fatigue characteristics strongly depend on microstructure and presence of defects. Fine-grained structure with uniform phase distribution usually provides better fatigue strength [179].
- Residual stresses—characteristic for SLM, residual stresses can significantly reduce fatigue strength. Heat treatment for stress relief (heat treatment at 600–700 °C [180]).
4.5. Biodegradation and Biocompatibility
The rate and mechanism of biodegradation of iron alloys obtained by SLM play a key role in their functionality as temporary implants. The unique microstructure and properties of SLM materials significantly affect the corrosion process in the physiological environment.
4.5.1. Mechanism of Biodegradation of Iron Alloys
Biodegradation of iron alloys in physiological conditions is based on electrochemical corrosion, which can be described by the following reactions:
Anodic reaction: Fe → Fe2+ + 2e−
Cathodic reaction: O2 + 2H2O + 4e− → 4OH−
Hydroxide formation: Fe2+ + 2OH− → Fe(OH)2
Further oxidation: 4Fe(OH)2 + O2 + 2H2O → 4Fe(OH)3
Fe(OH)3 can then dehydrate to form various forms of iron oxides (Fe2O3, Fe3O4).
Unlike magnesium alloys, during corrosion of iron alloys, a significant amount of gaseous hydrogen is usually not formed, since the main cathodic reaction is oxygen reduction [181].
An important feature of iron alloy corrosion is the formation of insoluble corrosion products (iron oxides and hydroxides), which form a diffusion barrier slowing down further corrosion. This explains the relatively low degradation rate of pure iron in vivo and the need for alloying to accelerate corrosion [182].
In a physiological environment, the corrosion process is further complicated by interaction with proteins, cells, and enzymes. Protein adsorption on the implant surface can either accelerate or slow down corrosion depending on the type of proteins and environmental conditions [183].
4.5.2. Influence of Microstructure on Biodegradation
The microstructure of SLM-iron alloys affects biodegradation, determined by grain size (fine-grainedness increases the area of electrochemically active boundaries, phase composition (different electrochemical activity of γ, ε, α’ phases, where ε is more active than γ) [184], distribution of alloying elements, residual stresses (accelerate corrosion mechano-chemically). SLM-Fe-Mn alloys corrode faster than traditional ones, which is related to process parameters and microstructure [185].
4.5.3. Comparison of Biodegradation Rate of SLM and Traditional Materials
The biodegradation rate of SLM-iron alloys is usually higher than that of analogues manufactured traditionally. They compared the corrosion rate of pure iron obtained by laser melting, which was 13 times higher than that of cast iron [186]. For example, the corrosion rate of the Fe-Mn alloy (SLM) was 0.22 mm/year versus 0.008 mm/year for pure iron (cast) [187]. This is explained by fine-grained structure, non-uniform distribution of alloying elements, microporosity, and residual stresses.
4.5.4. Methods of Controlling Biodegradation Rate
To optimize the biodegradation rate of SLM-iron implants, the following are used: alloying; phase composition control (regulation of γ and ε phase ratio [159]); creation of galvanic pairs (introduction of Pd, Cu, Ag [188,189]); surface modification [165,166]; porous structure design.
4.5.5. Biocompatibility of SLM-Iron Implants
The biocompatibility of iron alloys obtained by SLM is determined by a combination of factors, including chemical composition, microstructure, surface topography, and degradation products. In vitro studies show that SLM materials based on Fe-Mn usually demonstrate good cytocompatibility, supporting adhesion, proliferation, and differentiation of various cell types, including osteoblasts, mesenchymal stem cells, and endothelial cells [190].
The influence of iron ions released during implant degradation on cell metabolism depends on their concentration. At low and moderate concentrations, Fe2+ ions can stimulate proliferation and differentiation of osteoblasts, whereas high concentrations can cause oxidative stress and cytotoxicity [191].
Of particular interest is the influence of alloying elements on biological response. Manganese at low concentrations is necessary for normal cell functioning, however, its excess can cause neurotoxic effects. Calcium and magnesium, on the contrary, have a positive effect on osteogenesis and angiogenesis, stimulating bone tissue formation [192].
In vivo studies on animal models (rats, rabbits, pigs) confirm the biocompatibility of SLM-iron implants. Good integration with surrounding tissues, minimal inflammatory reaction, and gradual degradation with replacement by new tissue are observed [193].
An important aspect of biocompatibility is control of local concentration of degradation products. The porous structure of SLM implants promotes uniform distribution of ions and prevents their local accumulation in toxic concentrations [125].
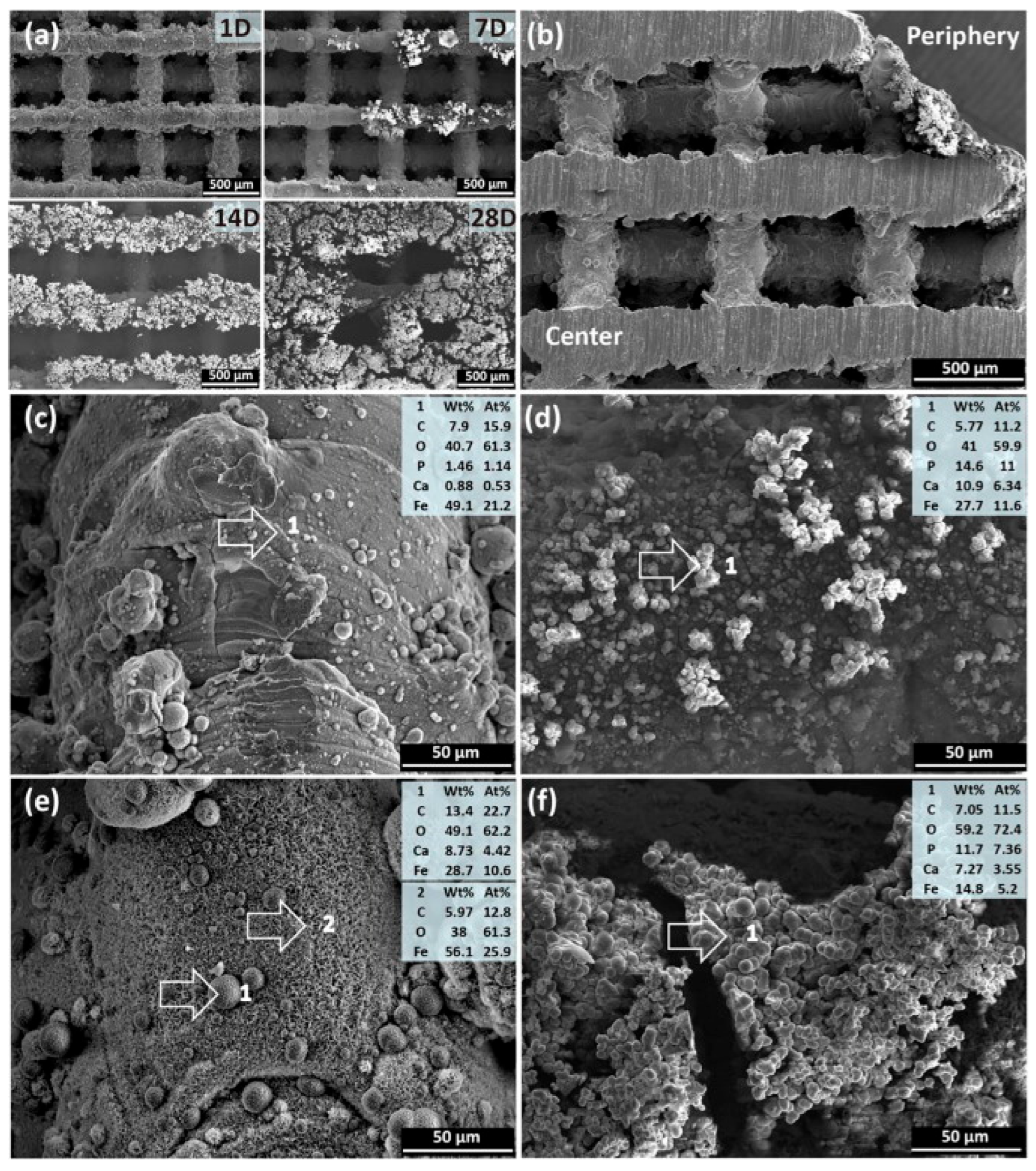
In Figure 8, the SEM showed degradation of struts with the formation of white products. By day 28, the surface is covered with loose products. Degradation is non-uniform: products in the center are thinner and denser than on the periphery. The composition of products (C, O, P, Ca, Fe) differs: more P and Ca on the periphery. By day 28, crystalline structures (spherical and feather-like) form in the center, without P. On the periphery, the composition is similar to day 7, but with increased O and decreased Fe [125].
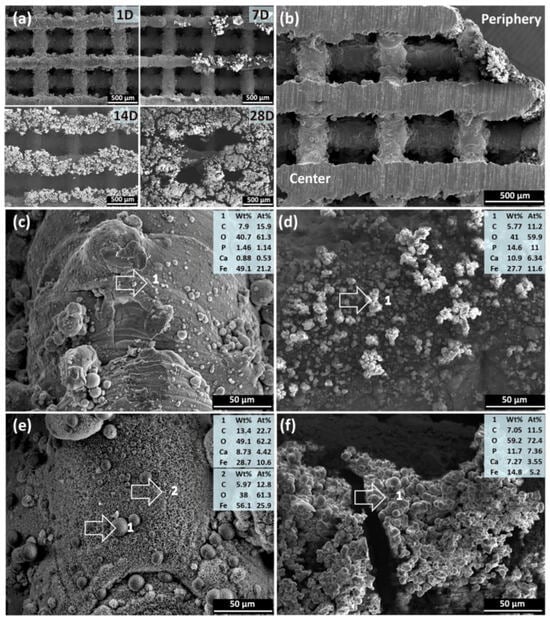
Figure 8.
SEM and EDS analyses of degradation products from the scaffold periphery to the center: (a) degradation products on the periphery at different immersion time points, (b) cross section of the scaffolds after 7-day immersion, (c) degradation products in the center and (d) on the periphery after 7-day immersion, (e) degradation products in the center and (f) on the periphery after 28-day immersion. The numbers 1 and 2 indicates the spot where EDS analysis was performed [125].
4.6. Clinical Applications and Prospects
SLM-iron implants are promising in cardiovascular, orthopedic, and reconstructive surgery due to their mechanical properties and controlled degradation rate. In cardiovascular surgery, they are used for biodegradable stents (coronary, peripheral), the design of which is optimized using SLM [194]. Clinical studies demonstrate good biocompatibility, and the Fe-35Mn stent showed minimal inflammatory reaction and gradual degradation [195]. In orthopedics, SLM-iron is applied for fracture fixation (screws, plates, rods), optimizing design with SLM, for bone scaffolds, providing osseointegration and controlled degradation, and for intervertebral implants. Further research is directed toward developing new alloys, functionally gradient materials, bioactive implants, composite materials, and improving SLM technology. In conclusion, SLM–iron alloys are a promising direction in developing biodegradable implants, combining strength, biocompatibility, and controlled degradation. SLM opens possibilities for creating personalized structures with optimized properties.
Having examined both magnesium alloys with their rapid degradation profiles and iron alloys with their high strength but slow corrosion rates, we now focus on zinc-based systems. Zinc alloys occupy an intermediate position between magnesium and iron in terms of both mechanical properties and degradation behavior, potentially offering a balanced solution for certain biomedical applications. The following section investigates how selective laser melting can be applied to zinc alloys to create biodegradable implants with tailored properties.
5. Zinc Alloys in Selective Laser Melting
5.1. Historical Overview and Basic Properties
Zinc occupies a unique position among biodegradable metals, demonstrating intermediate properties between magnesium and iron, possessing a degradation rate of 0.2 mm/year [196] and good biocompatibility of corrosion products [197]. However, the application of zinc is limited by low mechanical strength [198], brittleness, technological complexities, and creep at body temperature [199], which requires the development of special zinc alloys and optimization of their production technologies, including selective laser melting.
5.2. Influence of Alloying Elements
Alloying of zinc plays a key role in improving its properties for biomedical applications, allowing enhanced mechanical strength, improved plasticity, and controlled degradation rate. The choice of alloying elements for biodegradable zinc alloys is determined not only by their influence on material properties but also by biocompatibility, absence of toxicity, and potential therapeutic effect.
5.2.1. Magnesium (Mg)
Magnesium is a promising alloying element for zinc alloys, affecting: mechanical properties (significantly increases strength) [200], microstructure [201], degradation rate (depends on concentration) [201], and biocompatibility. Studies have shown that the optimal magnesium content is 1–2%, providing a balance between improved mechanical properties, controlled degradation rate, and good processability in SLM [202]. Corrosion rates of Zn alloys are approximately 14–30 μm/year [203], these values are comparable to bone regeneration rate. The results of the study suggest that magnesium addition contributes to enhanced cytocompatibility of the Zn-3Cu alloy [204].
5.2.2. Calcium (Ca)
Calcium, being a biocompatible element, plays an important role in bone tissue formation and regeneration [205]. In zinc alloys, calcium moderately increases strength, contributes to grain refinement, and formation of a more homogeneous microstructure [206], and typically accelerates zinc corrosion. Calcium ions released during alloy degradation stimulate proliferation and differentiation of osteoblasts, promoting bone tissue formation [207]. Due to these properties, Zn-Ca alloys exhibit pronounced osteogenic properties and are considered promising for creating temporary implants used in orthopedics and maxillofacial surgery.
5.2.3. Strontium (Sr)
Strontium, structurally and chemically similar to calcium, has a positive effect on bone tissue formation [208]. Strontium moderately increases the strength of zinc (yield strength 130–230 MPa, tensile strength 220–360 MPa for Zn-(0.5–2%)Sr alloys [209]) through solid solution strengthening, promotes grain refinement and formation of intermetallic phases affecting mechanical properties and corrosion behavior. Strontium typically accelerates zinc corrosion due to the formation of microgalvanic pairs [210]. It stimulates osteoblasts and inhibits osteoclasts, and strontium ranelate is used for osteoporosis treatment [211]. Studies of Zn-Sr alloys for additive manufacturing are promising for orthopedic applications.
5.2.4. Copper (Cu)
Copper, an essential microelement with antibacterial properties, significantly increases zinc’s strength and plasticity (yield strength 225 ± 9 MPa, tensile strength 330 ± 12 MPa, Zn3Cu [212]). Degradation rates of Zn-xCu alloys in c-SBF solution are at a relatively low level, ranging from 22.1 ± 4.7 to 33.0 ± 1.0 μm/year [213], with a trend towards a slight increase in corrosion rate with increasing copper content compared to pure zinc. The study [213] found that the best antibacterial properties are exhibited by alloys with copper content above 2%.
5.2.5. Silver (Ag)
Silver, due to its pronounced antibacterial properties, is of interest as an alloying element for biodegradable implants. Silver moderately increases zinc strength by forming solid solutions and intermetallic compounds [214]. Zn-Ag alloys corrode faster than pure Zn; silver typically accelerates zinc corrosion through the galvanic effect [215]. The bone marrow cavities of rat femur models were implanted and inoculated with S. aureus and E. coli; results showed that the Zn-Ag alloy exhibited optimal antibacterial properties [216].
5.2.6. Multi-Component Alloys
To optimize properties, multi-component zinc alloys are being developed: Zn-Cu-Ag (improved mechanical and antibacterial properties [217]); Zn-Mg-Sr (strengthening and hemocompatibility [218]); Zn-Li-Mg (improved plasticity, strength, and degradation rate [219]). Multi-component alloys expand optimization possibilities but require strict control of composition and microstructure to ensure reproducibility and biocompatibility.
5.3. Features of the SLM Process for Zinc Alloys
Selective laser melting of zinc alloys is associated with a number of specific problems and requires careful optimization of process parameters to ensure high quality of the resulting products.
5.3.1. Technological Features and Optimization of SLM Parameters for Zinc Alloys
Zinc and its alloys possess specific properties affecting SLM: low melting temperature (419.5 °C) requires control of energy parameters; high vapor pressure creates a risk of evaporation and defects [220]; high thermal conductivity (116 W/(m·K)) promotes rapid heat removal; high reflectivity reduces laser energy absorption efficiency; easy oxidation leads to oxide film formation [221].
5.3.2. Optimization of SLM Parameters for Zinc and Zinc Alloys
For SLM of zinc and zinc alloys, the following are optimized: laser power (pure Zn 50 W, for Zn-10Mg optimally 70 W [222]); scanning speed (pure Zn 700 mm/s, for Zn-10Mg optimally 600 mm/s [222]); hatch distance (600–800 μm [222]); layer thickness (30 μm [222]); scanning strategy (with 67° rotation between layers [223]); platform preheating temperature (120 °C [223]). Optimization of these parameters allows obtaining zinc and zinc alloy products with desired mechanical properties, density, and microstructure necessary for specific biomedical applications.
5.3.3. Influence of SLM Parameters on Microstructure of Zinc Alloys
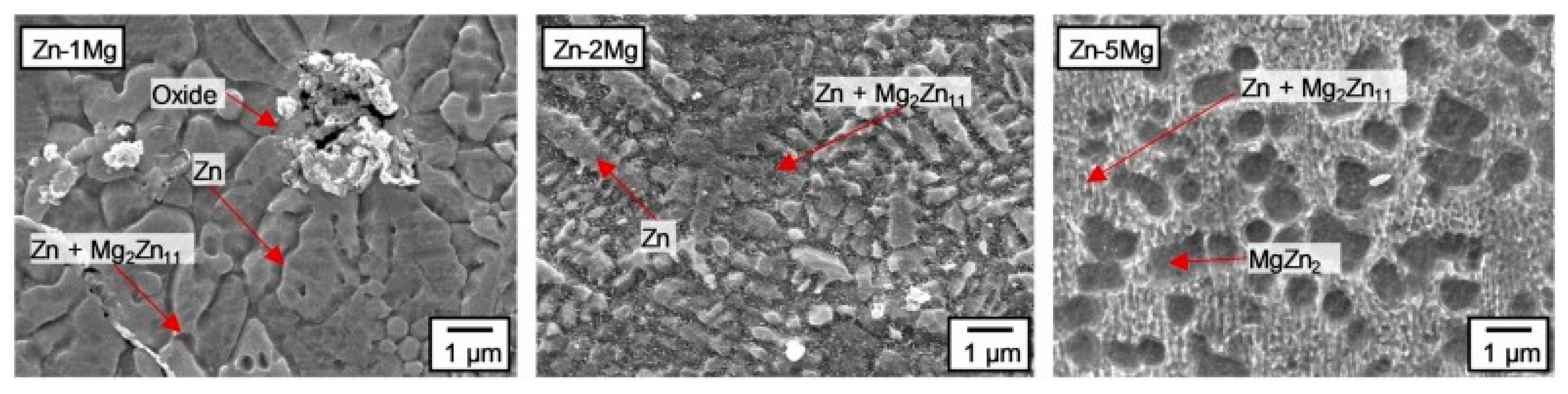
SLM parameters affect the microstructure of zinc alloys and, consequently, their mechanical properties and degradation rate: high cooling rates lead to fine-grained structure (5–30 μm, with average grain size of 27.1 μm) [223]; metastable phases may form (e.g., Mg2Zn11 in Zn-Mg alloys) [223]; porosity control is critical (high density >99% or controlled porosity is achieved) [224].
5.3.4. Post-Processing of SLM Products from Zinc Alloys
Post-processing is important for biodegradable implants from zinc alloys, as it allows modifying microstructure, improving mechanical properties, and controlling degradation rate. Heat treatment is applied to relieve internal stresses, improve microstructure, and mechanical properties. Heat treatment helps stabilize the material and reduce porosity [225]. Despite growing interest in additive manufacturing of biodegradable zinc implants by selective laser melting (SLM), issues related to post-processing of such products to improve their mechanical properties, biocompatibility, and degradation kinetics remain understudied, highlighting the need for further systematic research in this area.
5.4. Microstructure and Mechanical Properties
The microstructure and, consequently, mechanical properties of zinc alloys obtained by SLM significantly differ from those of similar materials manufactured by traditional methods, due to the unique crystallization conditions during laser melting.
5.4.1. Features of SLM Zinc and Zinc Alloys Microstructure
The microstructure of SLM-obtained pure zinc is characterized by equiaxed grains and lamellar structure with grain size of 8–10 μm, as well as the presence of oxides at grain boundaries, which is explained by the low temperature gradient and inhibiting effect of oxides on grain growth [226].
Figure 9 shows the microstructure of SLM Zn-xMg alloy samples, where Zn-xMg consists mainly of HCP phases (fewer slip systems), with Mg2Zn11 precipitates being BCC, which reduces plasticity. Zn-1Mg shows fine α-Zn with Mg precipitates/oxides at boundaries (grain size ~2 μm). With increasing Mg, Mg2Zn11 increases. In pure Zn, only α-Zn is present (size ~6 μm). In Zn-2Mg, even finer α-Zn grains are observed. Zn-5Mg consists of MgZn2(~1 μm) and α-Zn + Mg2Zn11 [227]. Zn-xMg consists mainly of HCP phases (fewer slip systems), with Mg2Zn11 precipitates being BCC, which reduces plasticity [227].

Figure 9.
Microstructure of SLM Zn-xMg alloy samples, reprint from [227].
5.4.2. Mechanical Properties of SLM–Zinc Alloys
The mechanical properties of zinc alloys obtained by SLM usually significantly exceed those of similar materials manufactured by traditional methods, especially in terms of strength indicators. Table 6 presents the mechanical properties of various zinc alloys obtained by SLM compared to traditional production methods.

Table 6.
Mechanical properties of zinc alloys obtained by various methods.
Table 6 reveals that zinc-based biodegradable alloys demonstrate significantly enhanced mechanical properties when processed via SLM compared to traditional casting methods. Pure zinc processed by SLM exhibits remarkable improvements, with yield strength increasing from 17 MPa (casting) to 84 MPa (SLM)—nearly a five-fold enhancement. Similarly, tensile strength improves from 20 MPa to 95 MPa, while elongation increases dramatically from 0.2% to 11.7%, indicating substantially improved ductility.
Alloying zinc with magnesium and copper further enhances mechanical performance. Zn-3Cu (SLM) demonstrates the highest strength among the zinc alloys tested, achieving 152 MPa yield strength and 222 MPa tensile strength—representing 137% and 165% improvements over cast Zn-3Cu, respectively. The Zn-2Mg system shows more moderate but still significant improvements when processed by SLM. These results highlight the effectiveness of SLM processing in refining microstructure and enhancing the mechanical properties of zinc alloys, making them more viable candidates for load-bearing biodegradable implant applications. However, elongation values remain relatively low compared to other biodegradable metal systems, indicating that further alloy development and processing optimization are needed to improve ductility for broader biomedical applications.
5.4.3. Fatigue Characteristics and Creep
For temporary implants, fatigue characteristics and creep resistance are important. The fatigue strength of porous zinc in air is 70% of yield strength, and in simulated physiological environment (r-SBF)—80% [232]. This is associated with the formation of corrosion products that strengthen the connections of the structure struts. To minimize creep, it is recommended to use multi-component alloys with thermally stable phases [219], optimized heat treatment, and to consider the creep factor in design.
5.5. Biodegradation and Biocompatibility
The rate and mechanism of biodegradation of zinc alloys obtained by SLM play a key role in their functionality as temporary implants. The unique microstructure and properties of SLM materials significantly affect the corrosion process in the physiological environment.
5.5.1. Mechanism of Biodegradation of Zinc Alloys
Biodegradation of zinc alloys in physiological conditions is based on electrochemical corrosion, which can be described by the following reactions:
Anodic reaction: Zn → Zn2+ + 2e−
Cathodic reaction: O2 + 2H2O + 4e− → 4OH−
Hydroxide formation: Zn2+ + 2OH− → Zn(OH)2
Further reactions: Zn(OH)2 → ZnO + H2O
An important feature of zinc corrosion is the formation of relatively dense and stable corrosion products (oxides, hydroxides, carbonates, phosphates), which can form a protective layer slowing down further corrosion. However, in a physiological environment, this protective layer is usually less stable than in atmospheric conditions, due to the presence of chloride ions, which can cause pitting corrosion [232].
In a living organism, the corrosion process is further complicated by interaction with proteins, cells, and enzymes. Protein adsorption on the implant surface can either accelerate or slow down corrosion depending on the type of proteins and environmental conditions [233].
5.5.2. Influence of Microstructure on Biodegradation
The microstructure of SLM–zinc alloys significantly affects biodegradation: fine-grainedness increases the area of grain boundaries, accelerating corrosion, but uniform distribution of alloying elements can compensate for this effect [230]; different phases have different electrochemical activity (intermetallics, such as MgZn2, CaZn13, accelerate corrosion [234]); non-uniform distribution of alloying elements creates microgalvanic pairs; porosity promotes localized corrosion; grain orientation affects corrosion anisotropy [235].
5.5.3. Comparison of Biodegradation Rate of SLM and Traditional Materials
SLM technology allows significantly reducing the corrosion rate of zinc when alloyed with magnesium (up to 0.09 mm/year for the Zn-3Mg alloy [236]), demonstrating a clear advantage of additive manufacturing over traditional casting, which is characterized by a higher corrosion rate for the same alloy (0.21 mm/year [237]). This is explained by fine-grained structure, larger area of grain boundaries, microgalvanic pairs, and residual stresses.
5.5.4. Methods of Controlling Biodegradation Rate
To optimize the biodegradation rate of SLM-zinc implants, the following are used: alloy composition optimization (alloying with Mg, Ca, Cu, Ag) [238]; heat treatment (reduces corrosion rate due to homogenization and stress reduction) [239]; surface modification (anodizing, micro-arc oxidation, application of biopolymer coatings) [240]; porous structure design.
5.5.5. Biocompatibility of SLM-Zinc Implants
The biocompatibility of SLM-zinc alloys is determined by chemical composition, microstructure, surface topography, and degradation products. Low and moderate concentrations of Zn2+ ions (10–50 μM) stimulate osteoblast proliferation and mineralization, while high concentrations (>100 μM) can be cytotoxic [241]. Alloying elements also affect biocompatibility: magnesium and calcium stimulate osteogenesis, copper possesses antibacterial and angiogenic properties, and silver enhances antibacterial effects. However, in vivo studies on animals are needed to confirm biocompatibility (good integration, minimal inflammation, controlled degradation with tissue replacement). Zn-based alloys (AMed Zn-Li, Zn-Se) effectively suppress osteosarcoma and promote bone regeneration [238].
5.6. Clinical Applications and Prospects
SLM–zinc implants are promising in cardiovascular surgery; the antibacterial properties of Zn-Ag alloys help prevent infection, a serious problem in implants made from degradable metal, making Zn-Ag alloys promising candidates for vascular stents [215]. Zn-Mg and Zn-Li alloys in vitro and in vivo stimulate bone cell proliferation and new bone formation, making them promising for bone implants, fracture fixation, and dentistry [215]. Future research is directed towards applying new materials (coating application [242], use of nanoparticles [243], etc.), improving SLM process parameters, and studying in vivo degradation. SLM-zinc alloys represent a promising but still at early stages of clinical application direction in creating biodegradable implants.
6. Comparative Analysis of Biodegradable Metallic Systems
The preceding sections have individually examined magnesium, iron, and zinc-based biodegradable metals processed through selective laser melting. Each system presents distinct advantages and limitations regarding mechanical properties, degradation behavior, biocompatibility, and processing challenges. To facilitate material selection for specific biomedical applications, it is essential to directly compare these three metal systems across key parameters. The following comparative analysis synthesizes the findings from previous sections to provide a comprehensive overview of the relative performance of these biodegradable metallic systems, highlighting their respective strengths and weaknesses for various implant applications. Table 7 shows the comparison of key characteristics of biodegradable metallic alloys.

Table 7.
Comparison of key characteristics of biodegradable metallic alloys for SLM.
Magnesium alloys demonstrate the most balanced properties for biomedical implants. Their elastic modulus (40–45 GPa) is close to bone tissue, minimizing the stress shielding effect. Degradation rate (1–3 mm/year) corresponds to healing times, and alloying (Ca, Zn, REE) allows controlling corrosion and mechanical properties. However, the high reactivity of magnesium and risk of excessive gas formation require careful optimization of SLM parameters, including protective atmosphere and platform preheating.
Iron alloys possess outstanding strength (yield strength 400–600 MPa) and plasticity, but their extremely slow degradation (0.1–0.5 mm/year) limits application. Alloying with Mn, C, and Si accelerates corrosion, and SLM provides a fine-grained structure, improving mechanical characteristics. However, the necessity of post-processing (e.g., HIP) and risk of oxide accumulation complicate their use.
Zinc alloys occupy an intermediate position: their degradation rate (0.2–0.5 mm/year) is closer to magnesium, and strength (180–280 MPa)—to iron. Alloying with Mg and Cu improves mechanical properties and antibacterial activity. SLM allows achieving high density (>99%), but the low melting temperature of zinc requires precise control of parameters (laser power 50–70 W, platform preheating to 120 °C) to minimize evaporation and porosity.
Table 8 presents the main SLM process parameters for various biodegradable materials.

Table 8.
Comparison of key SLM process parameters for various biodegradable metals.
The comparative analysis of magnesium, iron, and zinc-based biodegradable systems highlights the significant progress made in additive manufacturing of metallic implants. However, numerous challenges still impede the widespread clinical application of these promising materials. The following section identifies key technological, biological, regulatory, and economic barriers that must be overcome, while also exploring emerging research directions that may address these limitations. Understanding these challenges and opportunities is crucial for advancing the field toward successful clinical translation of additively manufactured biodegradable implants.
7. Current Problems and Prospects
7.1. Current Development Problems
Despite progress, additive manufacturing of biodegradable metallic implants faces technological, biological, regulatory, and economic challenges. Key technological challenges include control of degradation rate (differing in vitro and in vivo) [7,244], achieving balance between mechanical strength and degradation [245,246], protection of powders from oxidation [16,247], as well as optimization of SLM parameters to minimize defects, residual stresses, and selective evaporation of alloying elements [248,249,250,251,252]. An important task remains the development of accurate models for predicting degradation in vivo, considering the interaction of various factors [253,254].
A serious problem is the insufficient understanding of long-term effects of biodegradable metal degradation products, especially for new alloys, exacerbated by the variability of biological response [89,90], potential toxicity of alloying elements [100], and immune response [101,102]. Degradation in vivo includes complex interaction of corrosion, mechanical stresses, and biological processes [7,103], as well as mechano-corrosion effects [104]. The key task is optimizing implant-tissue interaction [105,106,107].
Regulatory challenges include compliance with clinical trial requirements, obtaining manufacturing certification, and standardization, which is particularly problematic for additively manufactured implants due to the lack of specialized standards [7], complicating obtaining approvals [255]. Economic challenges are due to high equipment and material costs, scaling problems, and competition with traditional implants. Proposed solutions include interdisciplinary collaboration and development of new biocompatible materials. Successful transition to clinical application requires a comprehensive solution to these problems. Quality control of small-batch production also presents a challenge [256].
Figure 10 shows the relationship between the different categories of challenges in additive manufacturing of biodegradable implants.
Figure 10.
Comprehensive diagram illustrating the interrelationship between different categories of problems (technological, biological, regulatory, and economic) in the field of additive manufacturing of biodegradable implants. Includes examples of key challenges in each category and their mutual influence, as well as potential solutions.
7.2. Promising Research Directions
Additive manufacturing of biodegradable metallic implants is a rapidly developing field with the potential to overcome limitations and expand clinical applications. Main directions include: development of multi-component, composite, and functionally gradient materials with optimized properties [257,258,259]. An important direction is improvement of technological processes: use of hybrid additive manufacturing [260], improvement of SLM processes, and development of post-processing for controlling degradation and improving biological response [261,262]. Also considered are development of integrated models linking microstructure, properties, and degradation [263], application of AI for optimization and prediction [264], creation of digital twins for modeling implant behavior [265]. Development of methodology for fully personalized implants: development of personalized implants considering individual characteristics [266], minimally invasive solutions [267,268], and development of multifunctional implants combining mechanical support with controlled drug release and integrated sensors for monitoring the healing process [269].
8. Conclusions
Additive manufacturing of biodegradable metallic implants has emerged as a rapidly evolving, interdisciplinary field that leverages selective laser melting (SLM) technology to create personalized biomedical devices from magnesium, iron, and zinc alloys. This systematic review has demonstrated both the substantial progress achieved and the critical challenges that remain for successful clinical implementation.
Material science has advanced biodegradable alloys. Magnesium alloys (WE43, AZ91D, ZK60) are biocompatible, bone-like, but exhibit low degradation/strength balance. SLM boosts magnesium alloy degradation (by 60–160%) and strength (by up to 250%). Iron-based alloys (Fe-Mn, etc.) are strong, ductile, but degrade too slowly, which is why they require alloying. Zinc exhibits ideal degradation kinetics, improving mechanics via alloying.
SLM excels at complex geometries and controlled porosity, surpassing traditional methods. SLM’s fine microstructure enhances mechanics but also speeds degradation. Topology optimization and biomimicry (TPMS) enable personalized implants with tailored property gradients.
However, additive manufacturing faces substantial limitations that impede widespread adoption. High equipment and material costs, strict powder quality requirements, and process complexity requiring extensive parameter optimization create significant barriers. Additionally, mandatory post-processing, batch-to-batch variability in small-scale production, and limited standardized testing protocols complicate quality control and certification for medical applications.
Despite clinical promise (e.g., MAGNEZIX® screws), widespread use remains limited due to some key challenges. Optimizing the materials’ composition/microstructure for mechanics/degradation, as well as managing reactivity and controlling evaporation/stress during SLM is difficult. Additionally, long-term degradation effects and tissue responses are unclear, while regulatory criteria lack standardization. Finally, the high material costs currently lead to limited production.
Future AM biodegradable implants require the following: (1) multi-material and graded designs; (2) AI/ML-driven optimization; (3) interdisciplinary collaboration; (4) international regulatory harmonization; and (5) smart functionalities (drug delivery, sensors).
Prioritizing research and setting timelines is key. Short term (1–3 years): standardize tests, model degradation, optimize SLM process. Medium term (3–5 years): multi-material implants, real-time SLM monitoring, producing new alloys. Long term (5–10 years): automated manufacturing, “smart” implants, regulatory harmonization. This approach streamlines resource allocation.
AM biodegradable implants are transformative for personalized treatment. Understanding composition-structure-property-biology, standardizing evaluation, and interdisciplinary collaboration are key to overcoming limitations and achieving clinical adoption.
Author Contributions
Conceptualization, A.P. and I.P.; methodology, I.P.; formal analysis, A.G. and I.P.; investigation, A.G.; resources, A.P.; data curation, A.G.; writing—original draft preparation, A.G.; writing—review and editing, I.P. and A.P.; visualization, A.G.; supervision, I.P. and A.P.; project administration, A.P.; funding acquisition, A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Ministry of Science and Higher Education of the Russian Federation (agreement No. 075-15-2024-562).
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AI | Artificial Intelligence |
| AM | Additive Manufacturing |
| ASTM | American Society for Testing and Materials |
| BCC | Body-Centered Cubic |
| CAGR | Compound Annual Growth Rate |
| CE | Conformité Européenne |
| EBSD | Electron Backscatter Diffraction |
| FCC | Face-Centered Cubic |
| HBSS | Hank’s Balanced Salt Solution |
| HCP | Hexagonal Close-Packed |
| HIP | Hot Isostatic Pressing |
| ISO | International Organization for Standardization |
| MAO | Micro-Arc Oxidation |
| ML | Machine Learning |
| OM | Optical Microscopy |
| PBF | Powder Bed Fusion |
| PBS | Phosphate Buffered Saline |
| PEO | Plasma Electrolytic Oxidation |
| PM | Powder Metallurgy |
| REE | Rare Earth Elements |
| SBF | Simulated Body Fluid |
| SCC | Stress Corrosion Cracking |
| SEM | Scanning Electron Microscopy |
| SLM | Selective Laser Melting |
| TPMS | Triply Periodic Minimal Surfaces |
| TRIP | Transformation-Induced Plasticity |
| TWIP | Twinning-Induced Plasticity |
References
- Grand View Research. Orthopedic Implants Market Size, Share & Trends Analysis Report By Product (Lower Extremity Implants, Spinal Implants, Dental Implants, Upper Extremity Implants), By End Use (Hospitals, Outpatient Specialties), By Region, And Segment Forecasts, 2025–2030. Available online: https://www.researchandmarkets.com/reports/5899491/orthopedic-implants-market-size-share-and-trends?srsltid=AfmBOoohp1h9BX4o9SoqB78gB-6JwVdlyL84saMdYnVP8u5KmxCDEnF1 (accessed on 22 April 2025).
- Li, J.; Liu, Y.; Hermansson, L.; Soremark, R. Evaluation o biocompatibility of various ceramic powders with human fibroblasts in vitro. Clin. Mater. 1993, 12, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wen, J.; Liu, Y.; He, J.; Shi, H.; Tian, P. Progress in Research on Biodegradable Magnesium Alloys: A Review. Adv. Eng. Mater. 2020, 22, 2000213. [Google Scholar] [CrossRef]
- Li, J.; Qin, L.; Yang, K.; Ma, Z.; Wang, Y.; Cheng, L.; Zhao, D. Materials evolution of bone plates for internal fixation of bone fractures: A review. J. Mater. Sci. Technol. 2020, 36, 190–208. [Google Scholar] [CrossRef]
- Joshi, M.G.; Advani, S.G.; Miller, F.; Santare, M.H. Analysis of a femoral hip prosthesis designed to reduce stress shielding. J. Biomech. 2000, 33, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tan, L.; Yu, X.; Etim, I.P.; Ibrahim, M.; Yang, K. Mechanical properties of magnesium alloys for medical application: A review. J. Mech. Behav. Biomed. Mater. 2018, 87, 68–79. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Witte, F.; Fischer, J.; Nellesen, J.; Crostack, H.-A.; Kaese, V.; Pisch, A.; Beckmann, F.; Windhagen, H. In vitro and in vivo corrosion measurements of magnesium alloys. Biomaterials 2006, 27, 1013–1018. [Google Scholar] [CrossRef]
- de Baaij, J.H.F.; Hoenderop, J.G.J.; Bindels, R.J.M. Magnesium in Man: Implications for Health and Disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef]
- Peuster, M.; Hesse, C.; Schloo, T.; Fink, C.; Beerbaum, P. Long-term biocompatibility of a corrodible peripheral iron stent in the porcine descending aorta. Biomaterials 2006, 27, 4955–4962. [Google Scholar] [CrossRef]
- Kádár, C.; Gorejová, R.; Kubelka, P.; Oriňaková, R.; Orbulov, I.N. Mechanical and Degradation Behavior of Zinc-Based Biodegradable Metal Foams. Adv Eng Mater 2024, 26, 2301496. [Google Scholar] [CrossRef]
- John, E.; Laskow, T.C.; Buchser, W.J.; Pitt, B.R.; Basse, P.H.; Butterfield, L.H.; Kalinski, P.; Lotze, M.T. Zinc in innate and adaptive tumor immunity. J. Transl. Med. 2010, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Hüner, B.; Kıstı, M.; Uysal, S.; Uzgören, İ.N.; Özdoğan, E.; Süzen, Y.O.; Demir, N.; Kaya, M.F. An Overview of Various Additive Manufacturing Technologies and Materials for Electrochemical Energy Conversion Applications. ACS Omega 2022, 7, 40638–40658. [Google Scholar] [CrossRef] [PubMed]
- Sing, S.L.; An, J.; Yeong, W.Y.; Wiria, F.E. Laser and electron-beam powder-bed additive manufacturing of metallic implants: A review on processes, materials and designs. J. Orthop. Res. 2016, 34, 369–385. [Google Scholar] [CrossRef]
- Wadge, M.D.; McGuire, J.; Hanby, B.V.T.; Felfel, R.M.; Ahmed, I.; Grant, D.M. Tailoring the degradation rate of magnesium through biomedical nano-porous titanate coatings. J. Magnes. Alloys 2021, 9, 336–350. [Google Scholar] [CrossRef]
- Qin, Y.; Wen, P.; Guo, H.; Xia, D.; Zheng, Y.; Jauer, L.; Poprawe, R.; Voshage, M.; Schleifenbaum, J.H. Additive manufacturing of biodegradable metals: Current research status and future perspectives. Acta Biomater. 2019, 98, 3–22. [Google Scholar] [CrossRef]
- Soni, N.; Renna, G.; Leo, P. Advancements in Metal Processing Additive Technologies: Selective Laser Melting (SLM). Metals 2024, 14, 1081. [Google Scholar] [CrossRef]
- Liu, S.; Yang, W.; Shi, X.; Li, B.; Duan, S.; Guo, H.; Guo, J. Influence of laser process parameters on the densification, microstructure, and mechanical properties of a selective laser melted AZ61 magnesium alloy. J. Alloys Compd. 2019, 808, 151160. [Google Scholar] [CrossRef]
- Kruth, J.P.; Froyen, L.; Van Vaerenbergh, J.; Mercelis, P.; Rombouts, M.; Lauwers, B. Selective laser melting of iron-based powder. J. Mater. Process Technol. 2004, 149, 616–622. [Google Scholar] [CrossRef]
- Li, K.; Ji, C.; Bai, S.; Jiang, B.; Pan, F. Selective laser melting of magnesium alloys: Necessity, formability, performance, optimization and applications. J. Mater. Sci. Technol. 2023, 154, 65–93. [Google Scholar] [CrossRef]
- Gajanan, M.N.; Narendranath, S.; Satheesh Kumar, S.S. Effect of grain refinement on mechanical and corrosion behavior of AZ91 magnesium alloy processed by ECAE. IOP Conf. Ser. Mater. Sci. Eng. 2019, 591, 012015. [Google Scholar] [CrossRef]
- Qin, Y.; Wen, P.; Xia, D.; Guo, H.; Voshage, M.; Jauer, L.; Zheng, Y.; Schleifenbaum, J.H.; Tian, Y. Effect of grain structure on the mechanical properties and in vitro corrosion behavior of additively manufactured pure Zn. Addit. Manuf. 2020, 33, 101134. [Google Scholar] [CrossRef]
- Mugwagwa, L.; Dimitrov, D.; Matope, S.; Muvunz, R. Residual Stresses and Distortions in Selective Laser Melting—A Review. In Proceedings of the 17th Rapid Product Development Association of South Africa, Vanderbijlpark, South Africa, 2–4 November 2016. [Google Scholar]
- Kraner, J.; Medved, J.; Godec, M. Thermodynamic Behavior of Fe-Mn and Fe-Mn-Ag Powder Mixtures during Selective Laser Melting. Metals 2021, 11, 234. [Google Scholar] [CrossRef]
- Jo, J.-H.; Hong, J.-Y.; Shin, K.-S.; Kim, H.-E.; Koh, Y.-H. Enhancing biocompatibility and corrosion resistance of Mg implants via surface treatments. J. Biomater. Appl. 2012, 27, 469–476. [Google Scholar] [CrossRef]
- Gao, C.; Li, S.; Liu, L.; Bin, S.; Yang, Y.; Peng, S.; Shuai, C. Dual alloying improves the corrosion resistance of biodegradable Mg alloys prepared by selective laser melting. J. Magnes. Alloys 2021, 9, 305–316. [Google Scholar] [CrossRef]
- Seitz, J.; Collier, K.; Wulf, E.; Bormann, D.; Angrisani, N.; Meyer-Lindenberg, A.; Bach, F. The Effect of Different Sterilization Methods on the Mechanical Strength of Magnesium Based Implant Materials. Adv. Eng. Mater. 2011, 13, 1146–1151. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.; Pavanram, P.; Leeflang, M.A.; Fockaert, L.I.; Pouran, B.; Tümer, N.; Schröder, K.U.; Mol, J.M.C.; Weinans, H.; et al. Additively Manufactured Biodegradable Porous Magnesium. Acta Biomater. 2018, 67, 378–392. [Google Scholar] [CrossRef]
- Han, H.-S.; Minghui, Y.; Seok, H.-K.; Byun, J.-Y.; Cha, P.-R.; Yang, S.-J.; Kim, Y.C. The modification of microstructure to improve the biodegradation and mechanical properties of a biodegradable Mg alloy. J. Mech. Behav. Biomed. Mater. 2013, 20, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Guo, H. A Review of SLMed Magnesium Alloys: Processing, Properties, Alloying Elements and Postprocessing. Metals 2020, 10, 1073. [Google Scholar] [CrossRef]
- Palanivel, S.; Nelaturu, P.; Glass, B.; Mishra, R.S. Friction stir additive manufacturing for high structural performance through microstructural control in an Mg based WE43 alloy. Mater. Des. (1980–2015) 2015, 65, 934–952. [Google Scholar] [CrossRef]
- Ewald, F.C.; Brenne, F.; Gustmann, T.; Vollmer, M.; Krooß, P.; Niendorf, T. Laser Powder Bed Fusion Processing of Fe-Mn-Al-Ni Shape Memory Alloy—On the Effect of Elevated Platform Temperatures. Metals 2021, 11, 185. [Google Scholar] [CrossRef]
- Deng, Q.; Wu, Y.; Luo, Y.; Su, N.; Xue, X.; Chang, Z.; Wu, Q.; Xue, Y.; Peng, L. Fabrication of high-strength Mg-Gd-Zn-Zr alloy via selective laser melting. Mater. Charact. 2020, 165, 110377. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, L.; Feng, Z.; Chen, Y. Research progress on selective laser melting (SLM) of magnesium alloys: A review. Optik 2020, 207, 163842. [Google Scholar] [CrossRef]
- Xie, T.; Liu, J.; Xiao, L.; Xie, Y.; Qian, S.; Dai, Y.; Wu, J. Additive Manufacturing of Fe–Mn–Al–C Lightweight Steel by Laser Powder Bed Fusion: The Role of Laser Scanning Speed on Forming Quality, Microstructure and Properties. J. Mater. Res. Technol. 2024, 33, 6610–6621. [Google Scholar] [CrossRef]
- Pola, A.; Tocci, M.; Goodwin, F.E. Review of Microstructures and Properties of Zinc Alloys. Metals 2020, 10, 253. [Google Scholar] [CrossRef]
- Venezuela, J.; Dargusch, M.S. Addressing the slow corrosion rate of biodegradable Fe-Mn: Current approaches and future trends. Curr. Opin. Solid. State Mater. Sci. 2020, 24, 100822. [Google Scholar] [CrossRef]
- Rabeeh, V.P.M.; Hanas, T. Progress in manufacturing and processing of degradable Fe-based implants: A review. Prog. Biomater. 2022, 11, 163–191. [Google Scholar] [CrossRef]
- Bai, J.; Wang, Q.; Men, Z.; Chen, W.; Huang, H.; Ji, C.; Li, Y.; Wang, L.; Zhu, L.; Li, K.; et al. Generation Mechanism of Anisotropy in Mechanical Properties of WE43 Fabricated by Laser Powder Bed Fusion. Micromachines 2024, 15, 976. [Google Scholar] [CrossRef]
- Mouzou, E.; Paternoster, C.; Tolouei, R.; Purnama, A.; Chevallier, P.; Dubé, D.; Prima, F.; Mantovani, D. In vitro degradation behavior of Fe–20Mn–1.2C alloy in three different pseudo-physiological solutions. Mater. Sci. Eng. C 2016, 61, 564–573. [Google Scholar] [CrossRef]
- Wu, C.L.; Zai, W.; Man, H.C. Additive manufacturing of ZK60 magnesium alloy by selective laser melting: Parameter optimization, microstructure and biodegradability. Mater. Today Commun. 2021, 26, 101922. [Google Scholar] [CrossRef]
- Choi, J.-P.; Shin, G.-H.; Lee, H.-S.; Yang, D.-Y.; Yang, S.; Lee, C.-W.; Brochu, M.; Yu, J.-H. Evaluation of Powder Layer Density for the Selective Laser Melting (SLM) Process. Mater. Trans. 2017, 58, 294–297. [Google Scholar] [CrossRef]
- ISO 13485:2016; Medical Devices—Quality Management Systems—Requirements for Regulatory Purposes. International Organization for Standardization (ISO): Geneva, Switzerland, 2016.
- ASTM International. Standard Guide for Characterizing Properties of Metal Powders Used for Additive Manufacturing Processes; ASTM: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Jana, A.; Das, M.; Balla, V.K. In vitro and in vivo degradation assessment and preventive measures of biodegradable Mg alloys for biomedical applications. J. Biomed. Mater. Res. A 2022, 110, 462–487. [Google Scholar] [CrossRef]
- Pogorielov, M.; Husak, E.; Solodivnik, A.; Zhdanov, S. Magnesium-based biodegradable alloys: Degradation, application, and alloying elements. Interv. Med. Appl. Sci. 2017, 9, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, N.; Nakao, Y.; Ishikawa, R.; Tsuchida, D.; Iijima, M. Degradation and Biocompatibility of AZ31 Magnesium Alloy Implants In Vitro and In Vivo: A Micro-Computed Tomography Study in Rats. Materials 2020, 13, 473. [Google Scholar] [CrossRef]
- Li, Y.; Jahr, H.; Lietaert, K.; Pavanram, P.; Yilmaz, A.; Fockaert, L.I.; Leeflang, M.A.; Pouran, B.; Gonzalez-Garcia, Y.; Weinans, H.; et al. Additively manufactured biodegradable porous iron. Acta Biomater. 2018, 77, 380–393. [Google Scholar] [CrossRef]
- Kabir, H.; Munir, K.; Wen, C.; Li, Y. Recent research and progress of biodegradable zinc alloys and composites for biomedical applications: Biomechanical and biocorrosion perspectives. Bioact. Mater. 2021, 6, 836–879. [Google Scholar] [CrossRef] [PubMed]
- Manjhi, S.K.; Sekar, P.; Bontha, S.; Balan, A.S.S. Additive manufacturing of magnesium alloys: Characterization and post-processing. Int. J. Lightweight Mater. Manuf. 2024, 7, 184–213. [Google Scholar] [CrossRef]
- Esmaily, M.; Zeng, Z.; Mortazavi, A.N.; Gullino, A.; Choudhary, S.; Derra, T.; Benn, F.; D’Elia, F.; Müther, M.; Thomas, S.; et al. A detailed microstructural and corrosion analysis of magnesium alloy WE43 manufactured by selective laser melting. Addit. Manuf. 2020, 35, 101321. [Google Scholar] [CrossRef]
- Jafari, S.; Harandi, S.E.; Singh Raman, R.K. A review of stress-corrosion cracking and corrosion fatigue of magnesium alloys for biodegradable implant applications. JOM Miner. Met. Mater. Soc. 2015, 67, 1143–1153. [Google Scholar] [CrossRef]
- Singh Raman, R.K.; Wen, C.; Löffler, J.F. Human Body-Fluid-Assisted Fracture of Zinc Alloys as Biodegradable Temporary Implants: Challenges, Research Needs and Way Forward. Materials 2023, 16, 4984. [Google Scholar] [CrossRef]
- Ali, M.; Sajjad, U.; Hussain, I.; Abbas, N.; Ali, H.M.; Yan, W.M.; Wang, C.C. On the assessment of the mechanical properties of additively manufactured lattice structures. Eng. Anal. Bound. Elem. 2022, 142, 93–116. [Google Scholar] [CrossRef]
- Feng, J.; Fu, J.; Lin, Z.; Shang, C.; Niu, X. Layered infill area generation from triply periodic minimal surfaces for additive manufacturing. Comput.-Aided Des. 2019, 107, 50–63. [Google Scholar] [CrossRef]
- Liang, H.; Yang, Y.; Xie, D.; Li, L.; Mao, N.; Wang, C.; Tian, Z.; Jiang, Q.; Shen, L. Trabecular-like Ti-6Al-4V scaffolds for orthopedic: Fabrication by selective laser melting and in vitro biocompatibility. J. Mater. Sci. Technol. 2019, 35, 1284–1297. [Google Scholar] [CrossRef]
- Chen, J.; Song, C.; Deng, Z.; Huang, J.; Han, C.; Yang, Y.; Wang, J.; Xu, K. Functional gradient design of additive manufactured gyroid tantalum porous structures: Manufacturing, mechanical behaviors and permeability. J. Manuf. Process 2024, 125, 202–216. [Google Scholar] [CrossRef]
- Ataollahi, S. A review on additive manufacturing of lattice structures in tissue engineering. Bioprinting 2023, 35, e00304. [Google Scholar] [CrossRef]
- Dargusch, M.S.; Soro, N.; Demir, A.G.; Venezuela, J.; Sun, Q.; Wang, Y.; Abdal-hay, A.; Alali, A.Q.; Ivanovski, S.; Previtali, B.; et al. Optimising degradation and mechanical performance of additively manufactured biodegradable Fe–Mn scaffolds using design strategies based on triply periodic minimal surfaces. Smart Mater. Med. 2024, 5, 127–139. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Demir, C.D.; Bermingham, A.G.; Dargusch, M.J. Challenges and Opportunities in the Selective Laser Melting of Biodegradable Metals for Load-Bearing Bone Scaffold Applications. Metall. Mater. Trans. A 2020, 51, 3311–3334. [Google Scholar]
- Bandyopadhyay, A.; Mitra, I.; Goodman, S.B.; Kumar, M.; Bose, S. Improving biocompatibility for next generation of metallic implants. Prog. Mater. Sci. Pergamon 2023, 133, 101053. [Google Scholar] [CrossRef]
- Huse, E.C. A New Ligature. Chic. Med. J. Exam. 1878, 37, 171–172. [Google Scholar]
- Rostock, P. Ist das Magnesium als Naht-und Schienungsmaterial für Knochenoperationen geeignet? Arch. Orthop. Unfallchir. 1937, 38, 486–492. [Google Scholar] [CrossRef]
- Plaass, C.; Ettinger, S.; Sonnow, L.; Koenneker, S.; Noll, Y.; Weizbauer, A.; Reifenrath, J.; Claassen, L.; Daniilidis, K.; Stukenborg-Colsman, C.; et al. Early results using a biodegradable magnesium screw for modified chevron osteotomies. J. Orthop. Res. 2016, 34, 2207–2214. [Google Scholar] [CrossRef] [PubMed]
- Windhagen, H.; Radtke, K.; Weizbauer, A.; Diekmann, J.; Noll, Y.; Kreimeyer, U.; Schavan, R.; Stukenborg-Colsman, C.; Waizy, H. Biodegradable magnesium-based screw clinically equivalent to titanium screw in hallux valgus surgery: Short term results of the first prospective, randomized, controlled clinical pilot study. Biomed. Eng. Online 2013, 12, 62. [Google Scholar] [CrossRef] [PubMed]
- Cha, P.-R.; Han, H.-S.; Yang, G.-F.; Kim, Y.-C.; Hong, K.-H.; Lee, S.-C.; Jung, J.-Y.; Ahn, J.-P.; Kim, Y.-Y.; Cho, S.-Y.; et al. Biodegradability engineering of biodegradable Mg alloys: Tailoring the electrochemical properties and microstructure of constituent phases. Sci. Rep. 2013, 3, 2367. [Google Scholar] [CrossRef] [PubMed]
- Strumińska-Parulska, D.; Strumińska-Parulska, D.; Moniakowska, A.; Block, K. Radiation safety of calcium and magnesium diet supplements. J. Elem. 2023, 29, 679–691. [Google Scholar] [CrossRef]
- Wu, J.; Cheng, X.; Wu, J.; Chen, J.; Pei, X. The development of magnesium-based biomaterials in bone tissue engineering: A review. J. Biomed. Mater. Res. B Appl. Biomater. 2024, 112, e35326. [Google Scholar] [CrossRef]
- Wang, C.; Sun, M.; Yang, C.; Wang, H.; Wang, J.; Mao, L.; Yang, Y.; Ying, T.; Chu, P.K.; Zeng, X. Degradation behavior of pure Mg in the physiological medium and growth mechanism of surface corrosion product films. J. Magnes. Alloys 2024, 13, 1523–1535. [Google Scholar] [CrossRef]
- Mei, D.; Li, Y.; Tian, Y.; Zhang, Q.; Liu, M.; Zhu, S.; Wang, L.; Guan, S. The effect of selected corrosion inhibitors on localized corrosion of magnesium alloy: The expanded understanding of “inhibition efficiency”. Corros. Sci. 2024, 226, 111650. [Google Scholar] [CrossRef]
- Pan, H.; Qin, G.; Huang, Y.; Ren, Y.; Sha, X.; Han, X.; Liu, Z.-Q.; Li, C.; Wu, X.; Chen, H.; et al. Development of low-alloyed and rare-earth-free magnesium alloys having ultra-high strength. Acta Mater. 2018, 149, 350–363. [Google Scholar] [CrossRef]
- Çam, G.; Günen, A. Challenges and Opportunities in the Production of Magnesium Parts by Directed Energy Deposition Processes. J. Magnes. Alloys 2024, 12, 1663–1686. [Google Scholar] [CrossRef]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef]
- Jung, Y.-G.; Yang, W.; Kim, Y.J.; Kim, S.K.; Yoon, Y.-O.; Lim, H.; Kim, D.H. Effect of Ca addition on the microstructure and mechanical properties of heat-treated Mg-6.0Zn-1.2Y-0.7Zr alloy. J. Magnes. Alloys 2021, 9, 1619–1631. [Google Scholar] [CrossRef]
- Chaudry, U.M.; Farooq, A.; bin Tayyab, K.; Malik, A.; Kamran, M.; Kim, J.-G.; Li, C.; Hamad, K.; Jun, T.-S. Corrosion behavior of AZ31 magnesium alloy with calcium addition. Corros. Sci. 2022, 199, 110205. [Google Scholar] [CrossRef]
- Lin, X.; Saijilafu; Wu, X.; Wu, K.; Chen, J.; Tan, L.; Witte, F.; Yang, H.; Mantovani, D.; Zhou, H.; et al. Biodegradable Mg-based alloys: Biological implications and restorative opportunities. Int. Mater. Rev. 2023, 68, 365–403. [Google Scholar] [CrossRef]
- Cai, S.; Lei, T.; Li, N.; Feng, F. Effects of Zn on microstructure, mechanical properties and corrosion behavior of Mg–Zn alloys. Mater. Sci. Eng. C 2012, 32, 2570–2577. [Google Scholar] [CrossRef]
- Yu, F.; Guohua, W.; Chunquan, Z. Effect of Strontium on Mechanical Properties and Corrosion Resistance of AZ91D. Materials Science Forum 2007, 546–549, 567–570. [Google Scholar]
- Ding, Y.; Wen, C.; Hodgson, P.; Li, Y. Effects of alloying elements on the corrosion behavior and biocompatibility of biodegradable magnesium alloys: A review. J. Mater. Chem. B 2014, 2, 1912–1933. [Google Scholar] [CrossRef]
- Tang, C.; Wu, K.; Liu, W.; Feng, D.; Wang, X.; Miao, G.; Yang, M.; Liu, X.; Li, Q. Effects of Gd, Y Content on the Microstructure and Mechanical Properties of Mg-Gd-Y-Nd-Zr Alloy. Metals 2018, 8, 790. [Google Scholar] [CrossRef]
- Yang, Y.; Ling, C.; Yang, M.; Yang, L.; Wang, D.; Peng, S.; Shuai, C. Selective Laser Melted Rare Earth Magnesium Alloy with High Corrosion Resistance. Int. J. Bioprint 2022, 8, 574. [Google Scholar] [CrossRef]
- Shi, L.; Yan, Y.; Shao, C.; Yu, K.; Zhang, B.; Chen, L. The influence of yttrium and manganese additions on the degradation and biocompatibility of magnesium-zinc-based alloys: In vitro and in vivo studies. J. Magnes. Alloys 2024, 12, 608–624. [Google Scholar] [CrossRef]
- Yu, Z.; Tang, A.; Li, C.; Liu, J.; Pan, F. Effect of manganese on the microstructure and mechanical properties of magnesium alloys. Int. J. Mater. Res. 2019, 110, 1016–1024. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, K.; Liang, Y.; Cheng, J.; Dai, Y. Selective Laser Melted Magnesium Alloys: Fabrication, Microstructure and Property. Materials 2022, 15, 7049. [Google Scholar] [CrossRef] [PubMed]
- Mousavian, S.M.H.; Tabaian, S.H.; Badihehaghdam, M. Effects of zirconium addition on electrochemical and mechanical properties of Mg-3Zn-1Ca-1RE alloy. Anti-Corros. Methods Mater. 2020, 67, 583–591. [Google Scholar] [CrossRef]
- Seong, J.W.; Kim, W.J. Development of biodegradable Mg–Ca alloy sheets with enhanced strength and corrosion properties through the refinement and uniform dispersion of the Mg2Ca phase by high-ratio differential speed rolling. Acta Biomater. 2015, 11, 531–542. [Google Scholar] [CrossRef]
- Du, Y.Z.; Qiao, X.G.; Zheng, M.Y.; Wang, D.B.; Wu, K.; Golovin, I.S. Effect of microalloying with Ca on the microstructure and mechanical properties of Mg-6 mass%Zn alloys. Mater. Des. 2016, 98, 285–293. [Google Scholar] [CrossRef]
- Erdmann, N.; Angrisani, N.; Reifenrath, J.; Lucas, A.; Thorey, F.; Bormann, D.; Meyer-Lindenberg, A. Biomechanical testing and degradation analysis of MgCa0.8 alloy screws: A comparative in vivo study in rabbits. Acta Biomater. 2011, 7, 1421–1428. [Google Scholar] [CrossRef]
- Zuo, Y.B.; Fu, X.; Mou, D.; Zhu, Q.F.; Li, L.; Cui, J.Z. Study on the role of Ca in the grain refinement of Mg–Ca binary alloys. Mater. Res. Innov. 2015, 19, S1-94–S1-97. [Google Scholar] [CrossRef]
- Ahmadi, M.; Tabary, S.A.A.B.; Rahmatabadi, D.; Ebrahimi, M.S.; Abrinia, K.; Hashemi, R. Review of selective laser melting of magnesium alloys: Advantages, microstructure and mechanical characterizations, defects, challenges, and applications. J. Mater. Res. Technol. 2022, 19, 1537–1562. [Google Scholar] [CrossRef]
- Nagata, M.; Lönnerdal, B. Role of zinc in cellular zinc trafficking and mineralization in a murine osteoblast-like cell line. J. Nutr. Biochem. 2011, 22, 172–178. [Google Scholar] [CrossRef]
- Xi, H.; Jihua, C.; Hongge, Y.; Bin, S.; Guanghao, Z.; Chongming, M. Effects of minor Sr addition on microstructure and mechanical properties of the as-cast Mg–4.5Zn–4.5Sn–2Al-based alloy system. J. Alloys Compd. 2013, 579, 39–44. [Google Scholar] [CrossRef]
- Peng, Q.; Li, X.; Ma, N.; Liu, R.; Zhang, H. Effects of backward extrusion on mechanical and degradation properties of Mg–Zn biomaterial. J. Mech. Behav. Biomed. Mater. 2012, 10, 128–137. [Google Scholar] [CrossRef]
- Shuai, C.; He, C.; Feng, P.; Guo, W.; Gao, C.; Wu, P.; Yang, Y.; Bin, S.; Feng, P. Biodegradation mechanisms of selective laser-melted Mg-xAl-Zn alloy: Grain size and intermetallic phase. Virtual Phys. Prototyp. 2018, 13, 59–69. [Google Scholar] [CrossRef]
- Zhang, B.; Hou, Y.; Wang, X.; Wang, Y.; Geng, L. Mechanical properties, degradation performance and cytotoxicity of Mg–Zn–Ca biomedical alloys with different compositions. Mater. Sci. Eng. C 2011, 31, 1667–1673. [Google Scholar] [CrossRef]
- Atkins, G.J.; Welldon, K.J.; Halbout, P.; Findlay, D.M. Strontium ranelate treatment of human primary osteoblasts promotes an osteocyte-like phenotype while eliciting an osteoprotegerin response. Osteoporos. Int. 2009, 20, 653–664. [Google Scholar] [CrossRef]
- Zhao, C.; Pan, F.; Zhang, L.; Pan, H.; Song, K.; Tang, A. Microstructure, mechanical properties, bio-corrosion properties and cytotoxicity of as-extruded Mg-Sr alloys. Mater. Sci. Eng. C 2017, 70, 1081–1088. [Google Scholar] [CrossRef]
- Bornapour, M.; Celikin, M.; Cerruti, M.; Pekguleryuz, M. Magnesium implant alloy with low levels of strontium and calcium: The third element effect and phase selection improve bio-corrosion resistance and mechanical performance. Mater. Sci. Eng. C 2014, 35, 267–282. [Google Scholar] [CrossRef]
- Li, H.; Wang, P.; Lin, G.; Huang, J. The role of rare earth elements in biodegradable metals: A review. Acta Biomater. 2021, 129, 33–42. [Google Scholar] [CrossRef]
- Wang, L.; Hu, J.; Guo, E.; Li, Y.; Kang, H.; Feng, Y. Microstructure and Mechanical Properties of Thick-Walled WE43A Alloy Fabricated by Wire Arc Additive Manufacturing. J. Mater. Eng. Perform. 2024. [Google Scholar] [CrossRef]
- Davis, J.R. (Ed.) Magnesium and Magnesium Alloys. In Alloying; ASM International: Almere, The Netherlands, 2001; pp. 432–453. [Google Scholar] [CrossRef]
- Cox, B. Oxidation of Zirconium and its Alloys. In Advances in Corrosion Science and Technology; Springer: Boston, MA, USA, 1976; pp. 173–391. [Google Scholar]
- Hendea, R.E.; Raducanu, D.; Claver, A.; García, J.A.; Cojocaru, V.D.; Nocivin, A.; Stanciu, D.; Serban, N.; Ivanescu, S.; Trisca-Rusu, C.; et al. Biodegradable Magnesium Alloys for Personalised Temporary Implants. J. Funct. Biomater. 2023, 14, 400. [Google Scholar] [CrossRef]
- Ng, C.C.; Savalani, M.; Man, H.C. Fabrication of Magnesium Using Selective Laser Melting Technique. Rapid Prototyp. J. 2011, 17, 479–490. [Google Scholar]
- Li, X.; Fang, X.; Wang, S.; Wang, S.; Zha, M.; Huang, K. Selective laser melted AZ91D magnesium alloy with superior balance of strength and ductility. J. Magnes. Alloys 2023, 11, 4644–4658. [Google Scholar] [CrossRef]
- Liu, S.H.; Liu, X.J.; Liu, B.; Liu, L.M.; Jin, W.Z.; Hu, X.J. Effect of some alloying elements on boiling point of magnesium. Mater. Sci. Technol. 2005, 21, 735–738. [Google Scholar] [CrossRef]
- Lun Sin, S.; Elsayed, A.; Ravindran, C. Inclusions in magnesium and its alloys: A review. Int. Mater. Rev. 2013, 58, 419–436. [Google Scholar] [CrossRef]
- Nopová, K.; Jaroš, J.; Červinek, O.; Pantělejev, L.; Gneiger, S.; Senck, S.; Koutný, D. Processing of AZ91D Magnesium Alloy by Laser Powder Bed Fusion. Appl. Sci. 2023, 13, 1377. [Google Scholar] [CrossRef]
- Wei, K.; Wang, Z.; Zeng, X. Influence of element vaporization on formability, composition, microstructure, and mechanical performance of the selective laser melted Mg–Zn–Zr components. Mater. Lett. 2015, 156, 187–190. [Google Scholar] [CrossRef]
- Ji, C.; Li, K.; Liao, R.; Li, Z.; Yin, B.; Wen, P.; Jiang, B.; Murr, L.E.; Pan, F. Tensile creep mechanisms of laser powder bed fused WE43 alloy with heterogeneous microstructure: Evolution in dislocations and precipitates. J. Mater. Sci. Technol. 2025, 238, 209–229. [Google Scholar] [CrossRef]
- Zumdick, N.A.; Jauer, L.; Kersting, L.C.; Kutz, T.N.; Schleifenbaum, J.H.; Zander, D. Additive manufactured WE43 magnesium: A comparative study of the microstructure and mechanical properties with those of powder extruded and as-cast WE43. Mater. Charact. 2019, 147, 384–397. [Google Scholar] [CrossRef]
- Neh, K.; Ullmann, M.; Kawalla, R. Twin-Roll-Casting and Hot Rolling of Magnesium Alloy WE43. Procedia. Eng. 2014, 81, 1553–1558. [Google Scholar] [CrossRef]
- Cosma, C.; Kessler, J.; Gebhardt, A.; Campbell, I.; Balc, N. Improving the Mechanical Strength of Dental Applications and Lattice Structures SLM Processed. Materials 2020, 13, 905. [Google Scholar] [CrossRef]
- Hu, Q.; Chen, C.; Liu, M.; Chang, C.; Yan, X.; Dai, Y. Improved corrosion resistance of magnesium alloy prepared by selective laser melting through T4 heat treatment for biomedical applications. J. Mater. Res. Technol. 2023, 27, 813–825. [Google Scholar] [CrossRef]
- Höhn, S.; Virtanen, S.; Boccaccini, A.R. Protein adsorption on magnesium and its alloys: A review. Appl. Surf. Sci. 2019, 464, 212–219. [Google Scholar] [CrossRef]
- Yao, H.; Wen, J.-B.; Xiong, Y.; Lu, Y.; Huttula, M. Microstructure Evolution in Mg-Zn-Zr-Gd Biodegradable Alloy: The Decisive Bridge Between Extrusion Temperature and Performance. Front. Chem. 2018, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Mraied, H.; Wang, W.; Cai, W. Influence of chemical heterogeneity and microstructure on the corrosion resistance of biodegradable WE43 magnesium alloys. J. Mater. Chem. B 2019, 7, 6399–6411. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhou, X.; Curioni, M.; Pawar, S.; Liu, H.; Fan, Z.; Scamans, G.; Thompson, G. Corrosion Behavior of Pure Magnesium with Low Iron Content in 3.5 wt% NaCl Solution. J. Electrochem. Soc. 2015, 162, 362–368. [Google Scholar] [CrossRef]
- Yuwono, J.A.; Birbilis, N.; Williams, K.S.; Medhekar, N.V. Electrochemical stability of magnesium surfaces in an aqueous environment. J. Phys. Chem. C Am. Chem. Soc. 2016, 120, 26922–26933. [Google Scholar] [CrossRef]
- Lovašiová, P.; Lovaši, T.; Kubásek, J.; Jablonská, E.; Msallamová, Š.; Michalcová, A.; Vojtěch, D.; Suchý, J.; Koutný, D.; Ghassan Hamed Alzubi, E. Biodegradable WE43 Magnesium Alloy Produced by Selective Laser Melting: Mechanical Properties, Corrosion Behavior, and In-Vitro Cytotoxicity. Metals 2022, 12, 469. [Google Scholar] [CrossRef]
- Ling, C.; Li, Q.; Zhang, Z.; Yang, Y.; Zhou, W.; Chen, W.; Dong, Z.; Pan, C.; Shuai, C. Influence of heat treatment on microstructure, mechanical and corrosion behavior of WE43 alloy fabricated by laser-beam powder bed fusion. Int. J. Extrem. Manuf. 2023, 6, 015001. [Google Scholar] [CrossRef]
- Shi, X.; Wang, Y.; Li, H.; Zhang, S.; Zhao, R.; Li, G.; Zhang, R.; Sheng, Y.; Cao, S.; Zhao, Y.; et al. Corrosion resistance and biocompatibility of calcium-containing coatings developed in near-neutral solutions containing phytic acid and phosphoric acid on AZ31B alloy. J. Alloys Compd. 2020, 823, 153721. [Google Scholar] [CrossRef]
- Dou, J.; Wang, J.; Lu, Y.; Chen, C.; Yu, H.; Ma, R.L.-W. Bioactive MAO/CS composite coatings on Mg-Zn-Ca alloy for orthopedic applications. Prog. Org. Coat. 2021, 152, 106112. [Google Scholar] [CrossRef]
- Li, Y.; Jahr, H.; Pavanram, P.; Bobbert, F.S.L.; Puggi, U.; Zhang, X.Y.; Pouran, B.; Leeflang, M.A.; Weinans, H.; Zhou, J.; et al. Additively manufactured functionally graded biodegradable porous iron. Acta Biomater. 2019, 96, 646–661. [Google Scholar] [CrossRef]
- Charyeva, O.; Dakischew, O.; Sommer, U.; Heiss, C.; Schnettler, R.; Lips, K.S. Biocompatibility of magnesium implants in primary human reaming debris-derived cells stem cells in vitro. J. Orthop. Traumatol. 2016, 17, 63–73. [Google Scholar] [CrossRef]
- Nourisa, J.; Zeller-Plumhoff, B.; Helmholz, H.; Luthringer-Feyerabend, B.; Ivannikov, V.; Willumeit-Römer, R. Magnesium ions regulate mesenchymal stem cells population and osteogenic differentiation: A fuzzy agent-based modeling approach. Comput. Struct. Biotechnol. J. 2021, 19, 4110–4122. [Google Scholar] [CrossRef] [PubMed]
- Siring, J.; Cökelek, A.; Mohnfeld, N.; Wester, H.; Behrens, B.A. Evaluating the Degradation of WE43 for Implant Applications: Optical and Mechanical Insights. Appl. Sci. 2025, 15, 3300. [Google Scholar] [CrossRef]
- Jayasathyakawin, S.; Ravichandran, M.; Naveenkumar, R.; Radhika, N.; Ismail, S.O.; Mohanavel, V. Recent advances in magnesium alloys for biomedical applications: A review. Mater. Today Commun. 2025, 42, 111239. [Google Scholar] [CrossRef]
- Coheña-Jiménez, M.; Prieto-Domínguez, R.; Pérez-Belloso, A.J.; Muriel-Sánchez, J.M.; Gómez-Carrión, Á.; Montaño-Jiménez, P. Comparison of Resorbable and Non-Resorbable Osteosynthesis Material in Hallux Surgery: A Systematic Review. Life 2023, 13, 2018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, W.; Liu, J.; Wang, L.; Tang, Y.; Wang, K. A review on magnesium alloys for biomedical applications. Front. Bioeng. Biotechnol. 2022, 10, 953344. [Google Scholar] [CrossRef] [PubMed]
- Wegener, B.; Sichler, A.; Milz, S.; Sprecher, C.; Pieper, K.; Hermanns, W.; Jansson, V.; Nies, B.; Kieback, B.; Müller, P.E.; et al. Development of a novel biodegradable porous iron-based implant for bone replacement. Sci. Rep. 2020, 10, 9141. [Google Scholar] [CrossRef]
- Salama, M.; Vaz, M.F.; Colaço, R.; Santos, C.; Carmezim, M. Biodegradable Iron and Porous Iron: Mechanical Properties, Degradation Behaviour, Manufacturing Routes and Biomedical Applications. J. Funct. Biomater. 2022, 13, 72. [Google Scholar] [CrossRef]
- Shuai, C.; Li, S.; Peng, S.; Feng, P.; Lai, Y.; Gao, C. Biodegradable metallic bone implants. Mater. Chem. Front. 2019, 3, 544–562. [Google Scholar] [CrossRef]
- Md Yusop, A.H.; Al Sakkaf, A.; Nur, H. Modifications on porous absorbable Fe-based scaffolds for bone applications: A review from corrosion and biocompatibility viewpoints. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 18–44. [Google Scholar] [CrossRef]
- Hermawan, H.; Purnama, A.; Dube, D.; Couet, J.; Mantovani, D. Fe–Mn alloys for metallic biodegradable stents: Degradation and cell viability studies. Acta Biomater. 2010, 6, 1852–1860. [Google Scholar] [CrossRef]
- Hermawan, H.; Alamdari, H.; Mantovani, D.; Dubé, D. Iron–manganese: New class of metallic degradable biomaterials prepared by powder metallurgy. Powder Metall. 2008, 51, 38–45. [Google Scholar] [CrossRef]
- Li, L.; Yang, X. The Essential Element Manganese, Oxidative Stress, and Metabolic Diseases: Links and Interactions. Oxid. Med. Cell. Longev. 2018, 2018, 7580707. [Google Scholar] [CrossRef]
- Seol, J.B.; Jung, J.E.; Jang, Y.W.; Park, C.G. Influence of carbon content on the microstructure, martensitic transformation and mechanical properties in austenite/ε-martensite dual-phase Fe–Mn–C steels. Acta Mater. 2013, 61, 558–578. [Google Scholar] [CrossRef]
- Dai, Y.J.; Mi, Z.L. Influence of Carbon on Mechanical Behavior of Fe-Mn-C System Alloys. Adv. Mat. Res. 2014, 941–944, 1469–1472. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, Y.F. Effects of alloying elements (Mn, Co, Al, W, Sn, B, C and S) on biodegradability and in vitro biocompatibility of pure iron. Acta Biomater. 2011, 7, 1407–1420. [Google Scholar] [CrossRef] [PubMed]
- Roman, A.M.; Cimpoeșu, R.; Pricop, B.; Cazacu, M.M.; Zegan, G.; Istrate, B.; Cocean, A.; Chelariu, R.; Moscu, M.; Bădărău, G.; et al. Investigations on the Degradation Behavior of Processed FeMnSi-xCu Shape Memory Alloys. Nanomaterials 2024, 14, 330. [Google Scholar] [CrossRef]
- Xu, Z.; Hodgson, M.A.; Cao, P. Microstructure and degradation behavior of forged Fe-Mn-Si alloys. Int. J. Mod. Phys. B. World Sci. 2015, 29, 10–11. [Google Scholar] [CrossRef]
- Fiocchi, J.; Lemke, J.N.; Zilio, S.; Biffi, C.A.; Coda, A.; Tuissi, A. The effect of Si addition and thermomechanical processing in an Fe-Mn alloy for biodegradable implants: Mechanical performance and degradation behavior. Mater Today Commun. 2021, 27, 102447. [Google Scholar] [CrossRef]
- Hermawan, H.; Dubé, D.; Mantovani, D. Development of Degradable Fe-35Mn Alloy for Biomedical Application. Adv. Mat. Res. 2006, 15–17, 107–112. [Google Scholar]
- Park, J.W.; Hanawa, T.; Chung, J.H. The relative effects of Ca and Mg ions on MSC osteogenesis in the surface modification of microrough Ti implants. Int. J. Nanomed. 2019, 14, 5697–5711. [Google Scholar] [CrossRef]
- Alsakkaf, A.; Md Yusop, A.H.; Idris, H.; Hassan, A.G.; Iqbal, N.; Unal, R.; Sudin, I. Development of Fe-Mg alloys with intermediate degradation kinetics as potential biodegradable bone implants. Mater. Today Commun. 2024, 41, 110609. [Google Scholar] [CrossRef]
- Čapek, J.; Msallamová, Š.; Jablonská, E.; Lipov, J.; Vojtěch, D. A novel high-strength and highly corrosive biodegradable Fe-Pd alloy: Structural, mechanical and in vitro corrosion and cytotoxicity study. Mater. Sci. Eng. C 2017, 79, 550–562. [Google Scholar] [CrossRef]
- Schinhammer, M.; Hänzi, A.C.; Löffler, J.F.; Uggowitzer, P.J. Design strategy for biodegradable Fe-based alloys for medical applications. Acta Biomater. 2010, 6, 1705–1713. [Google Scholar] [CrossRef]
- Wei, S.; Ma, Z.; Tan, L.; Chen, J.; Misra, R.D.K.; Yang, K. Effect of copper content on the biodegradation behavior of Fe-Mn-C alloy system. Mater. Technol. 2022, 37, 1109–1119. [Google Scholar] [CrossRef]
- Paul, B.; Kiel, A.; Otto, M.; Gemming, T.; Hoffmann, V.; Giebeler, L.; Kaltschmidt, B.; Hütten, A.; Gebert, A.; Kaltschmidt, B.; et al. Inherent Antibacterial Properties of Biodegradable FeMnC(Cu) Alloys for Implant Application. ACS Appl Bio Mater. Am. Chem. Soc. 2024, 7, 839–852. [Google Scholar] [CrossRef] [PubMed]
- Prokoshkin, S.; Pustov, Y.; Zhukova, Y.; Kadirov, P.; Karavaeva, M.; Prosviryakov, A.; Dubinskiy, S. Effect of Thermomechanical Treatment on Structure and Functional Fatigue Characteristics of Biodegradable Fe-30Mn-5Si (wt%) Shape Memory Alloy. Materials 2021, 14, 3327. [Google Scholar] [CrossRef]
- Hong, D.; Chou, D.T.; Velikokhatnyi, O.I.; Roy, A.; Lee, B.; Swink, I.; Issaev, I.; Kuhn, H.A.; Kumta, P.N. Binder-jetting 3D printing and alloy development of new biodegradable Fe-Mn-Ca/Mg alloys. Acta Biomater. 2016, 45, 375–386. [Google Scholar] [CrossRef]
- Schinhammer, M.; Steiger, P.; Moszner, F.; Löffler, J.F.; Uggowitzer, P.J. Degradation performance of biodegradable FeMnC(Pd) alloys. Mater. Sci. Eng. C 2013, 33, 1882–1893. [Google Scholar] [CrossRef]
- Chen, D.; Wang, P.; Pan, R.; Zha, C.; Fan, J.; Liang, D.; Zhao, Y. Characteristics of Metal Specimens Formed by Selective Laser Melting: A State-of-the-Art Review. J. Mater. Eng. Perform. 2020, 30, 7073–7100. [Google Scholar] [CrossRef]
- Block-Bolten, A.; Eagar, T.W. Metal vaporization from weld pools. Metall. Trans. B 1984, 15, 461–469. [Google Scholar] [CrossRef]
- Cabañas, N.; Akdut, N.; Penning, J.; De Cooman, B.C. High-temperature deformation properties of austenitic Fe-Mn alloys. Met. Mater. Trans. A Phys. Met. Mater. Sci. 2006, 37, 3305–3315. [Google Scholar] [CrossRef]
- Wang, F.; Tan, Q.; Liu, T.; Venezuela, J.; Shi, Z.; Hurley, S.; Ly, A.; Xu, C.; Erbulut, D.; Yin, J.; et al. Reassessing the Biodegradation Behavior of Additively Manufactured Pure Iron and Iron-Manganese Alloys. 2025. Available online: https://ssrn.com/abstract=5135577 (accessed on 22 April 2025).
- Donik, Č.; Kraner, J.; Kocijan, A.; Paulin, I.; Godec, M. Evolution of the ε and γ phases in biodegradable Fe–Mn alloys produced using laser powder-bed fusion. Sci. Rep. 2021, 11, 19506. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Sun, B.; Mao, L. Effect of Scanning Strategy on the Manufacturing Quality and Performance of Printed 316L Stainless Steel Using SLM Process. Materials 2024, 17, 1189. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.N.; Cherqaoui, A.; Henrique Michelin Beraldo, C.; Paternoster, C.; Gélinas, S.; Blais, C.; Mengucci, P.; Mantovani, D. Effect of Volumetric Energy Density on the Evolution of the Microstructure and the Degradation Behavior of 3D-Printed Fe-Mn-C Alloys from Water-Atomized Powders. Metals 2025, 15, 101. [Google Scholar] [CrossRef]
- Gökhan, A.; Carluccio, D.; Bermingham, M.; Dargusch, M.; Gökhan Demir, A.; Caprio, L.; Previtali, B. Selective laser melting Fe and Fe-35Mn for biodegradable implants. Int. J. Mod. Phys. B 2020, 34, 2040034. [Google Scholar]
- Mugwagwa, L.; Dimitrov, D.; Matope, S.; Yadroitsev, I. Influence of process parameters on residual stress related distortions in selective laser melting. Procedia Manuf. 2018, 21, 92–99. [Google Scholar] [CrossRef]
- Nie, Y.; Yuan, B.; Liang, J.; Deng, T.; Li, X.; Chen, P.; Zhang, K.; Li, X.; Li, K.; Peng, H.; et al. Mechanical and functional properties of Fe–Mn–Si biodegradable alloys fabricated by laser powder bed fusion: Effect of heat treatment. Mater. Sci. Eng. A 2024, 908, 146725. [Google Scholar] [CrossRef]
- de Andrade, L.M.; Paternoster, C.; Chevallier, P.; Gambaro, S.; Copes, F.; de Oliveira Sales, V.F.; Mantovani, D. Electropolishing Fe-based biodegradable metals for vascular applications: Impact on surface properties, corrosion and cell viability. RSC Applied Interfaces. R. Soc. Chem. 2025, 2, 420–438. [Google Scholar] [CrossRef]
- Huang, S.; Ulloa, A.; Nauman, E.; Stanciu, L. Collagen Coating Effects on Fe–Mn Bioresorbable Alloys. J. Orthop. Res. 2020, 38, 523–535. [Google Scholar] [CrossRef]
- Liu, J.; Xie, T.; Xie, Y.; Xiao, L.; Lin, Y.; Dai, Y.; Wu, J. Microstructure and Mechanical Properties of Fe–30Mn–9Al–C–3Ni Low-Density Steel Manufactured by Selective Laser Melting. J. Mater. Res. Technol. 2024, 33, 4280–4289. [Google Scholar] [CrossRef]
- Jafarian, H.R.; Sabzi, M.; Mousavi Anijdan, S.H.; Eivani, A.R.; Park, N. The influence of austenitization temperature on microstructural developments, mechanical properties, fracture mode and wear mechanism of Hadfield high manganese steel. J. Mater. Res. Technol. 2021, 10, 819–831. [Google Scholar] [CrossRef]
- Hufenbach, J.; Sander, J.; Kochta, F.; Pilz, S.; Voss, A.; Kühn, U.; Gebert, A. Effect of Selective Laser Melting on Microstructure, Mechanical, and Corrosion Properties of Biodegradable FeMnCS for Implant Applications. Adv. Eng. Mater. 2020, 22, 2000182. [Google Scholar] [CrossRef]
- Shi, Z.-Z.; Gao, X.-X.; Zhang, H.-J.; Liu, X.-F.; Li, H.-Y.; Zhou, C.; Yin, Y.-X.; Wang, L.-N. Design biodegradable Zn alloys: Second phases and their significant influences on alloy properties. Bioact. Mater. 2020, 5, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Dehestani, M.; Trumble, K.; Wang, H.; Wang, H.; Stanciu, L.A. Effects of microstructure and heat treatment on mechanical properties and corrosion behavior of powder metallurgy derived Fe–30Mn alloy. Mater. Sci. Eng. A 2017, 703, 214–226. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, Y.F.; Ruan, L. In vitro investigation of Fe30Mn6Si shape memory alloy as potential biodegradable metallic material. Mater. Lett. 2011, 65, 540–543. [Google Scholar] [CrossRef]
- Tolosa, I.; Garciandía, F.; Zubiri, F.; Zapirain, F.; Esnaola, A. Study of mechanical properties of AISI 316 stainless steel processed by “selective laser melting”, following different manufacturing strategies. Int. J. Adv. Manuf. Technol. 2010, 51, 639–647. [Google Scholar] [CrossRef]
- Grässel, O.; Krüger, L.; Frommeyer, G.; Meyer, L.W. High strength Fe–Mn–(Al, Si) TRIP/TWIP steels development—Properties—Application. Int. J. Plast. 2000, 16, 1391–1409. [Google Scholar] [CrossRef]
- Sawaguchi, T.; Bujoreanu, L.G.; Kikuchi, T.; Ogawa, K.; Koyama, M.; Murakami, M. Mechanism of reversible transformation-induced plasticity of Fe–Mn–Si shape memory alloys. Scr. Mater. 2008, 59, 826–829. [Google Scholar] [CrossRef]
- Omori, T.; Ando, K.; Okano, M.; Xu, X.; Tanaka, Y.; Ohnuma, I.; Kainuma, R.; Ishida, K. Superelastic Effect in Polycrystalline Ferrous Alloys. Science 2011, 333, 68–71. [Google Scholar] [CrossRef]
- Liu, P.; Wu, H.; Liang, L.; Song, D.; Liu, J.; Ma, X.; Li, K.; Fang, Q.; Tian, Y.; Baker, I. Microstructure, mechanical properties and corrosion behavior of additively-manufactured Fe–Mn alloys. Mater. Sci. Eng. A 2022, 852, 143585. [Google Scholar] [CrossRef]
- Li, Y.; Lietaert, K.; Li, W.; Zhang, X.Y.; Leeflang, M.A.; Zhou, J.; Zadpoor, A.A. Corrosion fatigue behavior of additively manufactured biodegradable porous iron. Corros Sci. 2019, 156, 106–116. [Google Scholar] [CrossRef]
- Han, S.Z.; Choi, E.A.; Lim, S.H.; Kim, S.; Lee, J. Alloy design strategies to increase strength and its trade-offs together. Prog. Mater. Sci. 2021, 117, 100720. [Google Scholar] [CrossRef]
- Shiomi, M.; Osakada, K.; Nakamura, K.; Yamashita, T.; Abe, F. Residual stress within metallic model made by selective laser melting process. CIRP Ann. Manuf. Technol. 2004, 53, 195–198. [Google Scholar] [CrossRef]
- Gorejová, R.; Haverová, L.; Oriňaková, R.; Oriňak, A.; Oriňak, M. Recent advancements in Fe-based biodegradable materials for bone repair. J. Mater. Sci. 2019, 54, 1913–1947. [Google Scholar] [CrossRef]
- Zhang, E.; Chen, H.; Shen, F. Biocorrosion properties and blood and cell compatibility of pure iron as a biodegradable biomaterial. J. Mater. Sci. Mater. Med. 2010, 21, 2151–2163. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. On the Mechanisms of Biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef] [PubMed]
- Kadirov, P.; Zhukova, Y.; Gunderov, D.; Antipina, M.; Teplyakova, T.; Tabachkova, N.; Baranova, A.; Gunderova, S.; Pustov, Y.; Prokoshkin, S. Effect of Accumulative High-Pressure Torsion on Structure and Electrochemical Behavior of Biodegradable Fe-30Mn-5Si (wt.%) Alloy. Crystals 2025, 15, 351. [Google Scholar] [CrossRef]
- Li, Y.; Jahr, H.; Zhou, J.; Zadpoor, A.A. Additively Manufactured Biodegradable Porous Metals. Acta Biomater. 2020, 115, 29–50. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, J.; Yu, H.; Lin, W.; Li, X.; Tian, Y.; Zhao, M. Comparative Study on Biodegradation of Pure Iron Prepared by Microwave Sintering and Laser Melting. Materials 2022, 15, 1604. [Google Scholar] [CrossRef]
- Cheng, J.; Zheng, Y.F. In vitro study on newly designed biodegradable Fe-X composites (X = W, CNT) prepared by spark plasma sintering. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 485–497. [Google Scholar] [CrossRef]
- Rybalchenko, O.; Anisimova, N.; Martynenko, N.; Rybalchenko, G.; Kiselevskiy, M.; Tabachkova, N.; Shchetinin, I.; Raab, A.; Dobatkin, S. Structure Optimization of a Fe–Mn–Pd Alloy by Equal-Channel Angular Pressing for Biomedical Use. Materials 2022, 16, 45. [Google Scholar] [CrossRef] [PubMed]
- Rivkin, B.; Akbar, F.; Otto, M.; Beyer, L.; Paul, B.; Kosiba, K.; Gustmann, T.; Hufenbach, J.; Medina-Sánchez, M. Remotely Controlled Electrochemical Degradation of Metallic Implants. Small 2024, 20, 2307742. [Google Scholar] [CrossRef]
- Aufa, A.N.; Hassan, M.Z.; Ismail, Z.; Ramlie, F.; Jamaludin, K.R.; Md Daud, M.Y.; Ren, J. Current trends in additive manufacturing of selective laser melting for biomedical implant applications. J. Mater. Res. Technol. 2024, 31, 213–243. [Google Scholar] [CrossRef]
- Chen, L.; Shen, Q.; Liu, Y.; Zhang, Y.; Sun, L.; Ma, X.; Song, N.; Xie, J. Homeostasis and metabolism of iron and other metal ions in neurodegenerative diseases. Signal Transduct. Target. Ther. 2025, 10, 31. [Google Scholar]
- Hu, J.; Shao, J.; Huang, G.; Zhang, J.; Pan, S. In Vitro and In Vivo Applications of Magnesium-Enriched Biomaterials for Vascularized Osteogenesis in Bone Tissue Engineering: A Review of Literature. J. Funct. Biomater. 2023, 14, 326. [Google Scholar] [CrossRef] [PubMed]
- Tai, C.-C.; Huang, Y.-M.; Liaw, C.-K.; Yang, K.-Y.; Ma, C.-H.; Huang, S.-I.; Huang, C.-C.; Tsai, P.-I.; Shen, H.-H.; Sun, J.-S.; et al. Biocompatibility and Biological Performance of Additive-Manufactured Bioabsorbable Iron-Based Porous Interference Screws in a Rabbit Model: A 1-Year Observational Study. Int. J. Mol. Sci. 2022, 23, 14626. [Google Scholar] [CrossRef]
- Flege, C.; Vogt, F.; Höges, S.; Jauer, L.; Borinski, M.; Schulte, V.A.; Hoffmann, R.; Poprawe, R.; Meiners, W.; Jobmann, M.; et al. Development and characterization of a coronary polylactic acid stent prototype generated by selective laser melting. J. Mater. Sci. Mater. Med. 2013, 24, 241–255. [Google Scholar] [CrossRef]
- Sing, N.B.; Mostavan, A.; Hamzah, E.; Mantovani, D.; Hermawan, H. Degradation behavior of biodegradable Fe35Mn alloy stents. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Bowen, P.K.; Shearier, E.R.; Zhao, S.; Guillory, R.J.; Zhao, F.; Goldman, J.; Drelich, J.W. Biodegradable Metals for Cardiovascular Stents: From Clinical Concerns to Recent Zn-Alloys. Adv. Health Mater. 2016, 5, 1121–1140. [Google Scholar] [CrossRef]
- Bowen, P.K.; Guillory, R.J.; Shearier, E.R.; Seitz, J.-M.; Drelich, J.; Bocks, M.; Zhao, F.; Goldman, J. Metallic zinc exhibits optimal biocompatibility for bioabsorbable endovascular stents. Mater. Sci. Eng. C 2015, 56, 467–472. [Google Scholar] [CrossRef]
- Kong, L.; Heydari, Z.; Lami, G.H.; Saberi, A.; Baltatu, M.S.; Vizureanu, P. A Comprehensive Review of the Current Research Status of Biodegradable Zinc Alloys and Composites for Biomedical Applications. Materials 2023, 16, 4797. [Google Scholar] [CrossRef]
- Zhu, S.; Wu, C.; Li, G.; Zheng, Y.; Nie, J.-F. Creep properties of biodegradable Zn-0.1Li alloy at human body temperature: Implications for its durability as stents. Mater. Res. Lett. 2019, 7, 347–353. [Google Scholar] [CrossRef]
- Domínguez López, G.; Williams, P.L.; Llorca, J.; Echeverry-Rendón, M.; Echeverry-Rendon, M. Screening of Zn Alloys for Cardiovascular and Bone Applications: Effect of the Alloying Elements. Available online: https://ssrn.com/abstract=5027614 (accessed on 14 March 2025).
- Imtiaz, H.; Riaz, M.; Anees, E.; Bashir, F.; Hussain, T. Biodegradable zinc–magnesium alloys for bone fixation: A study of their structural integrity, corrosion resistance, and mechanical properties. Mater. Chem. Phys. 2025, 334, 130429. [Google Scholar] [CrossRef]
- Cao, X.; Wang, X.; Chen, J.; Geng, X.; Tian, H. 3D Printing of a Porous Zn-1Mg-0.1Sr Alloy Scaffold: A Study on Mechanical Properties, Degradability, and Biosafety. J. Funct. Biomater. 2024, 15, 109. [Google Scholar] [CrossRef]
- Yang, H.; Jia, B.; Zhang, Z.; Qu, X.; Li, G.; Lin, W.; Zhu, D.; Dai, K.; Zheng, Y. Alloying design of biodegradable zinc as promising bone implants for load-bearing applications. Nat. Commun. 2020, 11, 401. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Huang, H.; Niu, J.; Zhang, L.; Zhang, H.; Pei, J.; Tan, J.; Yuan, G. Design and characterizations of novel biodegradable Zn-Cu-Mg alloys for potential biodegradable implants. Mater. Des. 2017, 117, 84–94. [Google Scholar] [CrossRef]
- Zhu, K.; Prince, R.L. Calcium and bone. Clin. Biochem. 2012, 45, 936–942. [Google Scholar] [CrossRef]
- Čapek, J.; Kubásek, J.; Pinc, J.; Drahokoupil, J.; Čavojský, M.; Vojtěch, D. Extrusion of the biodegradable ZnMg0.8Ca0.2 alloy–The influence of extrusion parameters on microstructure and mechanical characteristics. J. Mech. Behav. Biomed. Mater. 2020, 108, 103796. [Google Scholar] [CrossRef]
- Zhang, Z.; Jia, B.; Yang, H.; Han, Y.; Wu, Q.; Dai, K.; Zheng, Y. Biodegradable ZnLiCa ternary alloys for critical-sized bone defect regeneration at load-bearing sites: In vitro and in vivo studies. Bioact. Mater. 2021, 6, 3999–4013. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, W.E.; Schrooten, I.; De Broe, M.E.; D’Haese, P.C. Strontium and Bone. J. Bone Miner. Res. 1999, 14, 661–668. [Google Scholar] [CrossRef]
- Mostaed, E.; Sikora-Jasinska, M.; Drelich, J.W.; Vedani, M. Zinc-based alloys for degradable vascular stent applications. Acta Biomater. 2018, 71, 1–23. [Google Scholar] [CrossRef]
- Song, J.; Gao, Y.; Liu, C.; Chen, Z. The effect of Sr addition on the microstructure and corrosion behaviour of a Mg-Zn-Ca alloy. Surf. Coat. Technol. 2022, 437, 128328. [Google Scholar] [CrossRef]
- Reginster, J. Strontium Ranelate in Osteoporosis. Curr. Pharm. Des. 2005, 8, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Palai, D.; Roy, T.; Prasad, P.S.; Hazra, C.; Dhara, S.; Sen, R.; Das, S.; Das, K. Influence of Copper on the Microstructural, Mechanical, and Biological Properties of Commercially Pure Zn-Based Alloys for a Potential Biodegradable Implant. ACS Biomater. Sci. Eng. Am. Chem. Soc. 2022, 8, 1443–1463. [Google Scholar] [CrossRef]
- Tang, Z.; Niu, J.; Huang, H.; Zhang, H.; Pei, J.; Ou, J.; Yuan, G. Potential biodegradable Zn-Cu binary alloys developed for cardiovascular implant applications. J. Mech. Behav. Biomed. Mater. 2017, 72, 182–191. [Google Scholar] [CrossRef]
- Čákyová, V.; Gorejová, R.; Kupková, M.; Sopčák, T.; Strečková, M.; Fáberová, M.; Özaltin, K.; Džupon, M.; Oriňaková, R. Study of Zn-Ag Alloys Prepared via Powder Metallurgy. 2025. Available online: https://ssrn.com/abstract=5115727 (accessed on 17 March 2025).
- Xiao, X.; Liu, E.; Shao, J.; Ge, S. Advances on biodegradable zinc-silver-based alloys for biomedical applications. J. Appl. Biomater. Funct. Mater. 2021, 19, 22808000211062407. [Google Scholar] [CrossRef]
- Xie, Z.; Yang, M.Z.; Xue, J.B.; Chen, Y.; Peng, L.J.; Cai, B.; Zhang, Y.Y. Antibacterial properties of biodegradable zinc alloys in vivo. Chinese Journal of Tissue Engineering Research. Chin. J. Tissue Eng. Res. 2019, 23, 2196–2201. [Google Scholar]
- Di, T.; Xu, Y.; Liu, D.; Sun, X. Microstructure, Mechanical Performance and Anti-Bacterial Activity of Degradable Zn-Cu-Ag Alloy. Metals 2022, 12, 1444. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J.; Yang, Y.; Zhou, F.; Pu, Z.; Li, L.; Zheng, Y. Microstructure, mechanical properties, in vitro degradation behavior and hemocompatibility of novel Zn–Mg–Sr alloys as biodegradable metals. Mater. Lett. 2016, 162, 242–245. [Google Scholar] [CrossRef]
- Waqas, M.; He, D.; Wu, X.; Tan, Z.; Shao, W.; Guo, X. Investigation on the preparation, microstructure, mechanical and degradation properties of laser additive manufactured Zn–Li–Mg alloy for bioresorbable application. J. Mater. Res. Technol. 2023, 26, 8509–8526. [Google Scholar] [CrossRef]
- Meng, F.; Du, Y. Research Progress on Laser Powder Bed Fusion Additive Manufacturing of Zinc Alloys. Materials 2024, 17, 4309. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Li, Z.W.; Harikisun, R.; Chang, S.S. Zinc oxide films formed by oxidation of zinc under low partial pressure of oxygen. Mater. Lett. 2003, 57, 1435–1440. [Google Scholar] [CrossRef]
- Waqas, M.; He, D.; Tan, Z.; Yang, P.; Gao, M.; Guo, X. A study of selective laser melting process for pure zinc and Zn10mg alloy on process parameters and mechanical properties. Artic. Rapid Prototyp. J. 2023, 29, 1923–1939. [Google Scholar] [CrossRef]
- Cui, Z.; Zhang, Y.; Wang, J.; Zhou, L.; Hao, X.; Wang, W.; Li, W.; Cheng, W.; Chang, C. Comparison on microstructure and properties of Zn-10 Mg alloy prepared by SPS and SLM techniques. Mater Lett. 2025, 389, 138327. [Google Scholar] [CrossRef]
- Cockerill, I.; Su, Y.; Sinha, S.; Qin, Y.X.; Zheng, Y.; Young, M.L.; Zhu, D. Porous zinc scaffolds for bone tissue engineering applications: A novel additive manufacturing and casting approach. Mater. Sci. Eng. C 2020, 110, 110738. [Google Scholar] [CrossRef]
- Mahmood, M.A.; Chioibasu, D.; Rehman, A.U.; Mihai, S.; Popescu, A.C. Post-Processing Techniques to Enhance the Quality of Metallic Parts Produced by Additive Manufacturing. Metals 2022, 12, 77. [Google Scholar] [CrossRef]
- Wang, C.; Hu, Y.; Zhong, C.; Lan, C.; Li, W.; Wang, X. Microstructural evolution and mechanical properties of pure Zn fabricated by selective laser melting. Mater. Sci. Eng. A 2022, 846, 143276. [Google Scholar] [CrossRef]
- Voshage, M.; Megahed, S.; Schückler, P.G.; Wen, P.; Qin, Y.; Jauer, L.; Poprawe, R.; Schleifenbaum, J.H. Additive manufacturing of biodegradable Zn-xMg alloys: Effect of Mg content on manufacturability, microstructure and mechanical properties. Mater. Today Commun. 2022, 32, 103805. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, F.; Gao, C.; Feng, P.; Xue, L.; He, S.; Shuai, C. A combined strategy to enhance the properties of Zn by laser rapid solidification and laser alloying. J. Mech. Behav. Biomed. Mater. 2018, 82, 51–60. [Google Scholar] [CrossRef]
- Galib, R.H.; Sharif, A. Development of Zn-Mg Alloys as a Degradable Biomaterial. Columbia Int. Publ. Adv. Alloys Compd. 2016, 1, 1–7. [Google Scholar]
- Dambatta, M.S.; Izman, S.; Kurniawan, D.; Hermawan, H. Processing of Zn-3Mg alloy by equal channel angular pressing for biodegradable metal implants. J. King Saud. Univ. Sci. 2017, 29, 455–461. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J.; Qiu, K.; Yang, Y.; Pu, Z.; Li, L.; Zheng, Y. Effects of alloying elements (Ca and Sr) on microstructure, mechanical property and in vitro corrosion behavior of biodegradable Zn–1. 5Mg alloy. J. Alloys Compd. 2016, 664, 444–452. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Bobbert, F.S.L.; Lietaert, K.; Dong, J.H.; Leeflang, M.A.; Zhou, J.; Zadpoor, A.A. Corrosion fatigue behavior of additively manufactured biodegradable porous zinc. Acta Biomater. 2020, 106, 439–449. [Google Scholar] [CrossRef]
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Chen, W.; Wu, C.; Liu, Y.; Su, X.; Wang, J. Effect of calcium on microstructure and corrosion resistance of Zn-6Al-3Mg alloy coatings. Mater. Today Commun. 2025, 46, 112495. [Google Scholar] [CrossRef]
- Chao, Z.; Wang, B.; Xu, C.; Li, Y. Study of grain orientation effect on the corrosion behavior of biocompatible magnesium alloy Mg–2Zn-0.5Ca. Mater. Chem. Phys. 2024, 328, 130039. [Google Scholar] [CrossRef]
- Ning, J.; Ma, Z.-X.; Zhang, L.-J.; Wang, D.-P.; Na, S.-J. Effects of magnesium on microstructure, properties and degradation behaviors of zinc-based alloys prepared by selective laser melting. Mater. Res. Express. 2022, 9, 086511. [Google Scholar] [CrossRef]
- Dambatta, M.S.; Izman, S.; Kurniawan, D.; Farahany, S.; Yahaya, B.; Hermawan, H. Influence of thermal treatment on microstructure, mechanical and degradation properties of Zn–3Mg alloy as potential biodegradable implant material. Mater. Des. 2015, 85, 431–437. [Google Scholar] [CrossRef]
- Bandekian, S.; Baghbaderani, M.Z.; Drelich, J.W.; Sharif, S.; Ismail, A.F.; Bakhsheshi-Rad, H.R. Additive manufacturing of zinc-based biomaterials: Fabrication, performance and property evaluation. J. Mater. Res. Technol. 2025, 36, 5484–5508. [Google Scholar] [CrossRef]
- Pham, D.N.; Hiromoto, S.; Kobayashi, E. Influences of Zinc Content and Solution Heat Treatment on Microstructure and Corrosion Behavior of Mg-Zn Binary Alloys. Corrosion 2021, 77, 323–338. [Google Scholar] [CrossRef]
- Yuan, W.; Xia, D.; Wu, S.; Zheng, Y.; Guan, Z.; Rau, J.V. A review on current research status of the surface modification of Zn-based biodegradable metals. Bioact. Mater. 2022, 7, 192–216. [Google Scholar] [CrossRef] [PubMed]
- Shearier, E.R.; Bowen, P.K.; He, W.; Drelich, A.; Drelich, J.; Goldman, J.; Zhao, F. In Vitro Cytotoxicity, Adhesion, and Proliferation of Human Vascular Cells Exposed to Zinc. ACS Biomater. Sci. Eng. Am. Chem. Soc. 2016, 2, 634–642. [Google Scholar] [CrossRef]
- Du, C.; Zuo, K.; Ma, Z.; Zhao, M.; Li, Y.; Tian, S.; Lu, Y.; Xiao, G. Effect of Substrates Performance on the Microstructure and Properties of Phosphate Chemical Conversion Coatings on Metal Surfaces. Molecules 2022, 27, 6434. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Linsley, C.S.; Pan, S.; Yao, G.; Wu, B.M.; Levi, D.S.; Li, X. Zn-Mg-WC Nanocomposites for Bioresorbable Cardiovascular Stents: Microstructure, Mechanical Properties, Fatigue, Shelf Life, and Corrosion. ACS Biomater. Sci. Eng. Am. Chem. Soc. 2022, 8, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Hermawan, H.; Dubé, D.; Mantovani, D. Developments in metallic biodegradable stents. Acta Biomater. 2010, 6, 1693–1697. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Chen, X.; Yang, J.; Pan, H.; Chen, D.; Wang, L.; Zhang, J.; Zhu, D.; Wu, S.; et al. Fundamental Theory of Biodegradable Metals—Definition, Criteria, and Design. Adv. Funct. Mater. 2019, 29, 1805402. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Smith, C.; Sankar, J. Recent advances on the development of magnesium alloys for biodegradable implants. Acta Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef]
- Zhang, E.; Yang, L. Microstructure, mechanical properties and bio-corrosion properties of Mg–Zn–Mn–Ca alloy for biomedical application. Mater. Sci. Eng. A 2008, 497, 111–118. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Y.; Bai, Q. Defect Formation Mechanisms in Selective Laser Melting: A Review. Chin. J. Mech. Eng. 2017, 30, 515–527. [Google Scholar] [CrossRef]
- Gu, D.D.; Meiners, W.; Wissenbach, K.; Poprawe, R. Laser additive manufacturing of metallic components: Materials, processes and mechanisms. Int. Mater. Rev. 2012, 57, 133–164. [Google Scholar] [CrossRef]
- Mercelis, P.; Kruth, J. Residual stresses in selective laser sintering and selective laser melting. Rapid Prototyp. J. 2006, 12, 254–265. [Google Scholar] [CrossRef]
- Wang, D.; Yang, Y.; Liu, R.; Xiao, D.; Sun, J. Study on the designing rules and processability of porous structure based on selective laser melting (SLM). J. Mater. Process Technol. 2013, 213, 1734–1742. [Google Scholar] [CrossRef]
- Yasa, E.; Kruth, J.-P. Microstructural investigation of Selective Laser Melting 316L stainless steel parts exposed to laser re-melting. Procedia Eng. 2011, 19, 389–395. [Google Scholar] [CrossRef]
- Mellal, A.; Wiskott, H.W.A.; Botsis, J.; Scherrer, S.S.; Belser, U.C. Stimulating effect of implant loading on surrounding bone. Clin. Oral. Implant. Res. 2004, 15, 239–248. [Google Scholar] [CrossRef]
- Jahr, H.; Li, Y.; Zhou, J.; Zadpoor, A.A.; Schröder, K.-U. Additively Manufactured Absorbable Porous Metal Implants–Processing, Alloying and Corrosion Behavior. Front. Mater. 2021, 8, 628633. [Google Scholar] [CrossRef]
- Fazel-Rezai, R. Biomedical Engineering–From Theory to Applications Edited by Reza Fazel-Rezai; InTechOpen: London, UK, 2011; Volume 498. [Google Scholar]
- Doll, K.; Doll, P.W.; Doll, C.; Doll, T. Importance of interdisciplinary training for successful development of innovative biomedical implants. Curr. Dir. Biomed. Eng. 2022, 8, 761–764. [Google Scholar] [CrossRef]
- Khan, A.R.; Grewal, N.S.; Zhou, C.; Yuan, K.; Zhang, H.-J.; Jun, Z. Recent advances in biodegradable metals for implant applications: Exploring in vivo and in vitro responses. Results Eng. 2023, 20, 101526. [Google Scholar] [CrossRef]
- Rashidy Ahmady, A.; Ekhlasi, A.; Nouri, A.; Haghbin Nazarpak, M.; Gong, P.; Solouk, A. High entropy alloy coatings for biomedical applications: A review. Smart Mater. Manuf. 2023, 1, 100009. [Google Scholar] [CrossRef]
- Pompe, W.; Worch, H.; Epple, M.; Friess, W.; Gelinsky, M.; Greil, P.; Hempel, U.; Scharnweber, D.; Schulte, K. Functionally graded materials for biomedical applications. Mater. Sci. Eng. A 2003, 362, 40–60. [Google Scholar] [CrossRef]
- Dilberoglu, U.M.; Gharehpapagh, B.; Yaman, U.; Dolen, M. Current trends and research opportunities in hybrid additive manufacturing. Int. J. Adv. Manuf. Technol. 2021, 113, 623–648. [Google Scholar] [CrossRef]
- Gao, B.; Zhao, H.; Peng, L.; Sun, Z. A Review of Research Progress in Selective Laser Melting (SLM). Micromachines 2022, 14, 57. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fan, H.; Li, H.; Hua, L.; Du, J.; He, Y.; Jin, Y. Recent Advancements in the Surface Modification of Additively Manufactured Metallic Bone Implants. Addit. Manuf. Front. 2025, 4, 200195. [Google Scholar] [CrossRef]
- Yi Wang, W.; Li, J.; Liu, W.; Liu, Z.-K. Integrated computational materials engineering for advanced materials: A brief review. Comput. Mater. Sci. 2019, 158, 42–48. [Google Scholar] [CrossRef]
- Ng, W.L.; Goh, G.L.; Goh, G.D.; Ten, J.S.J.; Yeong, W.Y. Progress and Opportunities for Machine Learning in Materials and Processes of Additive Manufacturing. Adv. Mater. 2024, 36, e2310006. [Google Scholar] [CrossRef]
- Diniz, P.; Grimm, B.; Garcia, F.; Fayad, J.; Ley, C.; Mouton, C.; Oeding, J.F.; Hirschmann, M.T.; Samuelsson, K.; Seil, R. Digital twin systems for musculoskeletal applications: A current concepts review. Knee Surgery, Sports Traumatology. Arthroscopy 2025, 33, 1892–1910. [Google Scholar]
- Maintz, M.; Tourbier, C.; de Wild, M.; Cattin, P.C.; Beyer, M.; Seiler, D.; Honigmann, P.; Sharma, N.; Thieringer, F.M. Patient-specific implants made of 3D printed bioresorbable polymers at the point-of-care: Material, technology, and scope of surgical application. 3D Print. Med. 2024, 10, 13. [Google Scholar] [CrossRef]
- Merces, L.; Ferro, L.M.M.; Thomas, A.; Karnaushenko, D.D.; Luo, Y.; Egunov, A.I.; Zhang, W.; Bandari, V.K.; Lee, Y.; McCaskill, J.S.; et al. Bio-Inspired Dynamically Morphing Microelectronics toward High-Density Energy Applications and Intelligent Biomedical Implants. Adv. Mater. 2024, 36, e2313327. [Google Scholar] [CrossRef]
- Xue, L.; Dai, S.; Li, Z. Biodegradable shape-memory block co-polymers for fast self-expandable stents. Biomaterials 2010, 31, 8132–8140. [Google Scholar] [CrossRef]
- Kadhim, A.C.; Azzahrani, A.S.; Alsheikhy, A.A.; Resen, D.A. Smart Bandages with Integrated Sensors for Real-Time Monitoring of Wound Inflammation and Infection. J. Opt. 2023. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).