Study of the Effect of Tin Addition in Aluminum–Copper Alloys Obtained from Elemental Powders
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussions
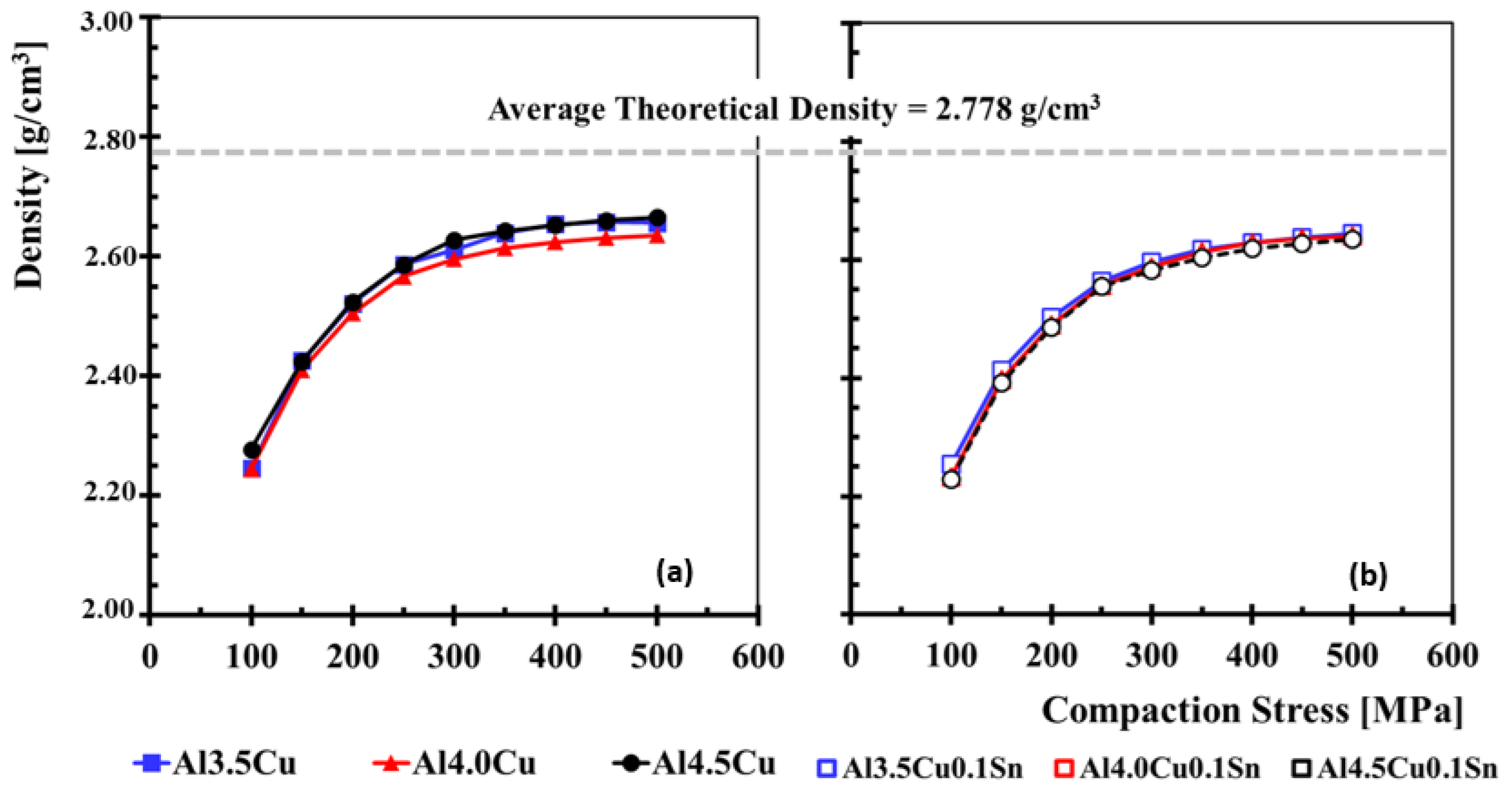
3.1. Sample Compressibility Curves
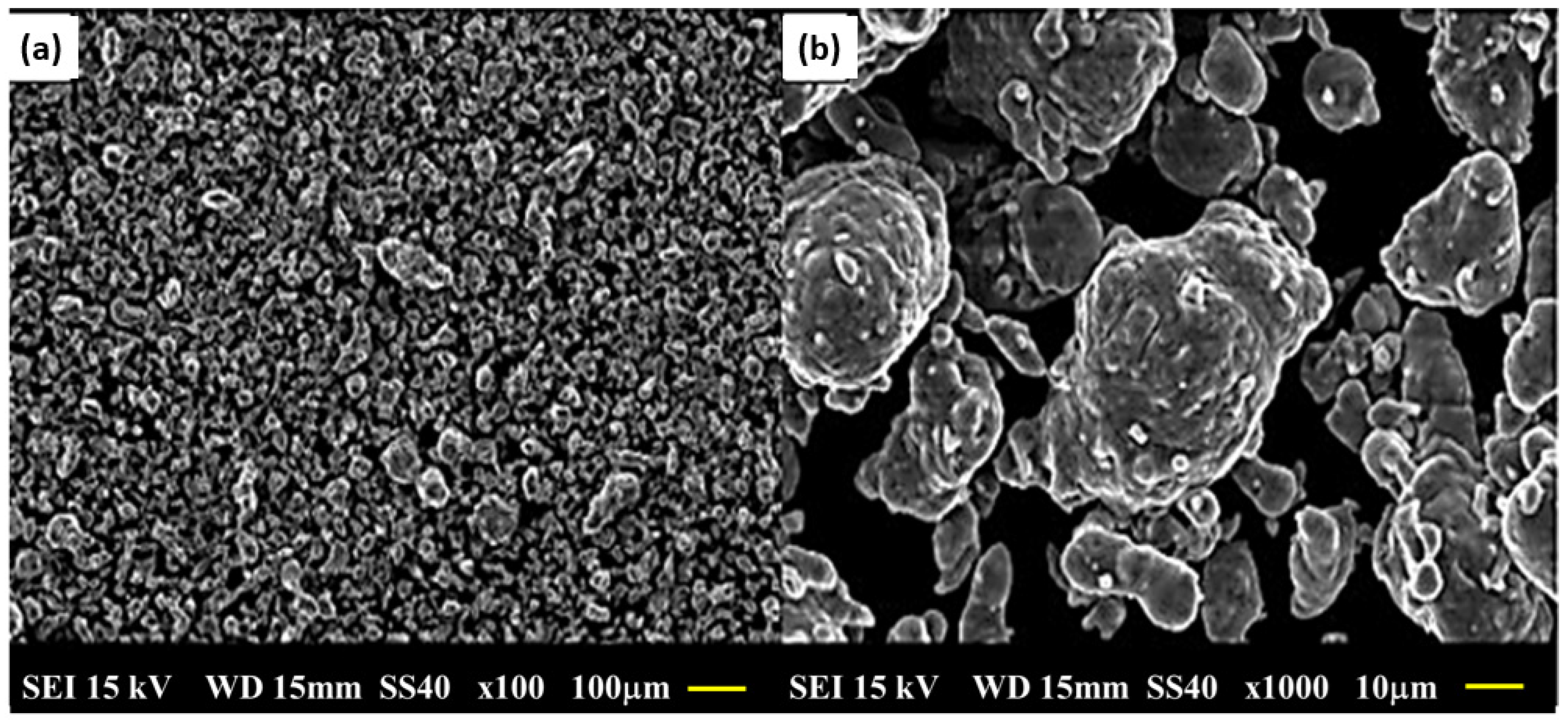
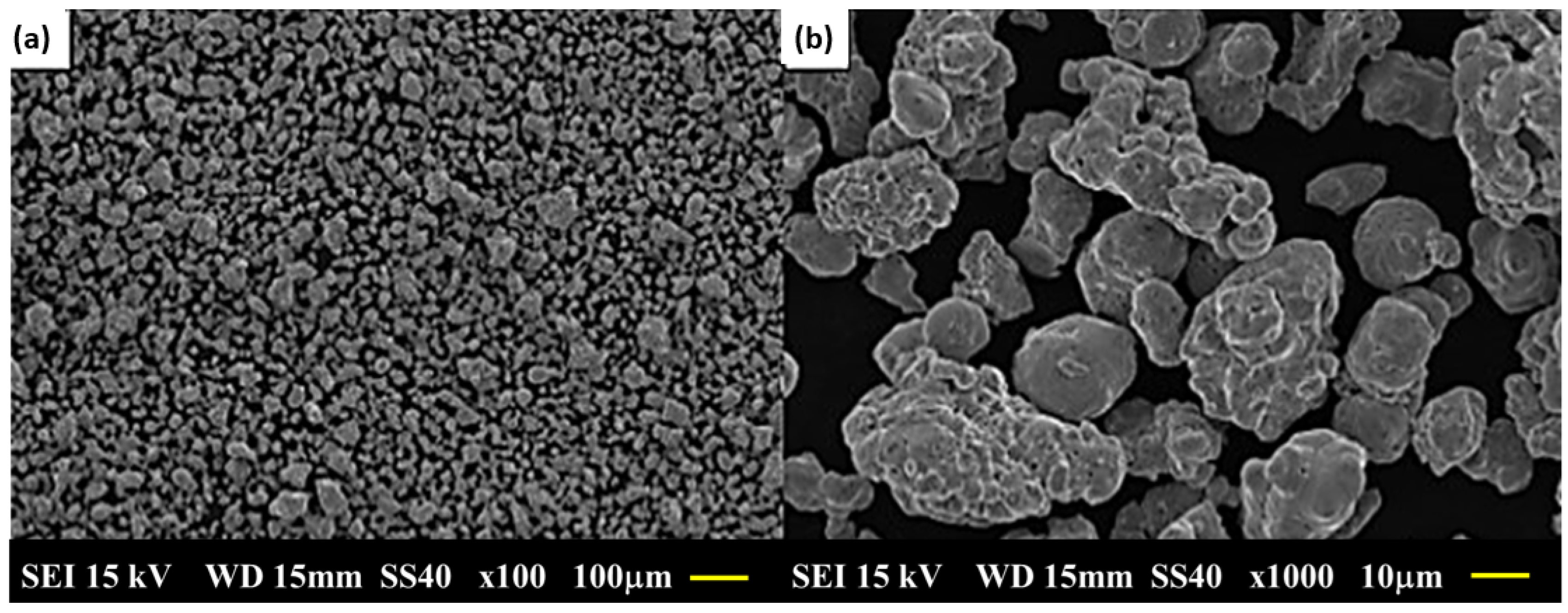
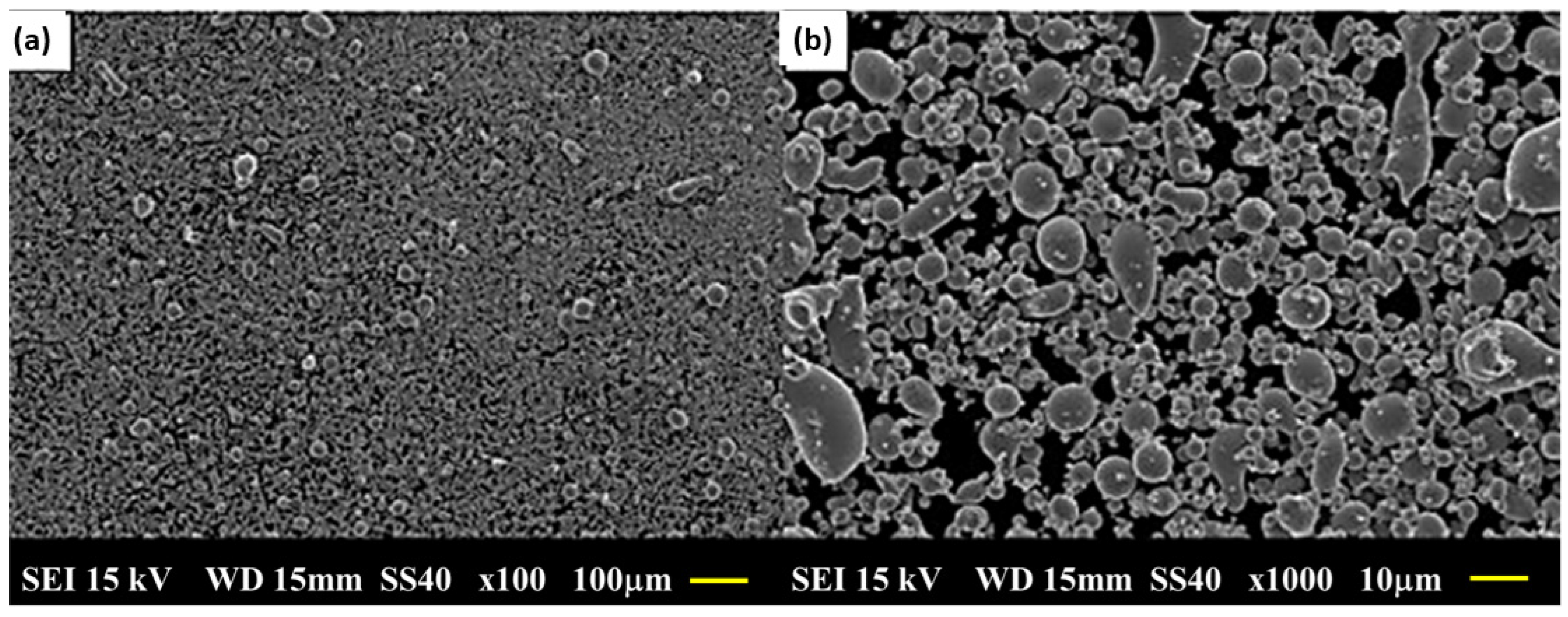
3.2. Particle Size and Morphological Characterization of Elementary Powders
3.3. Density Analysis Before and After Sintering
3.4. Artificial Aging Curves
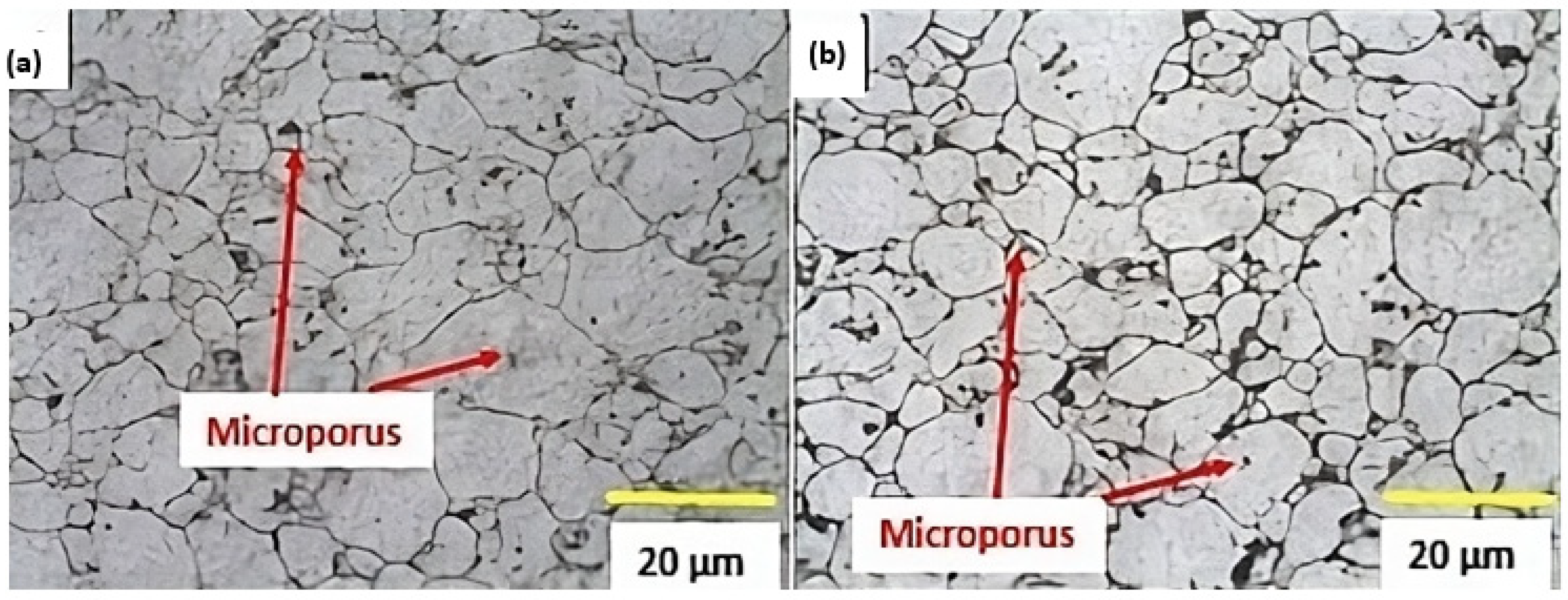
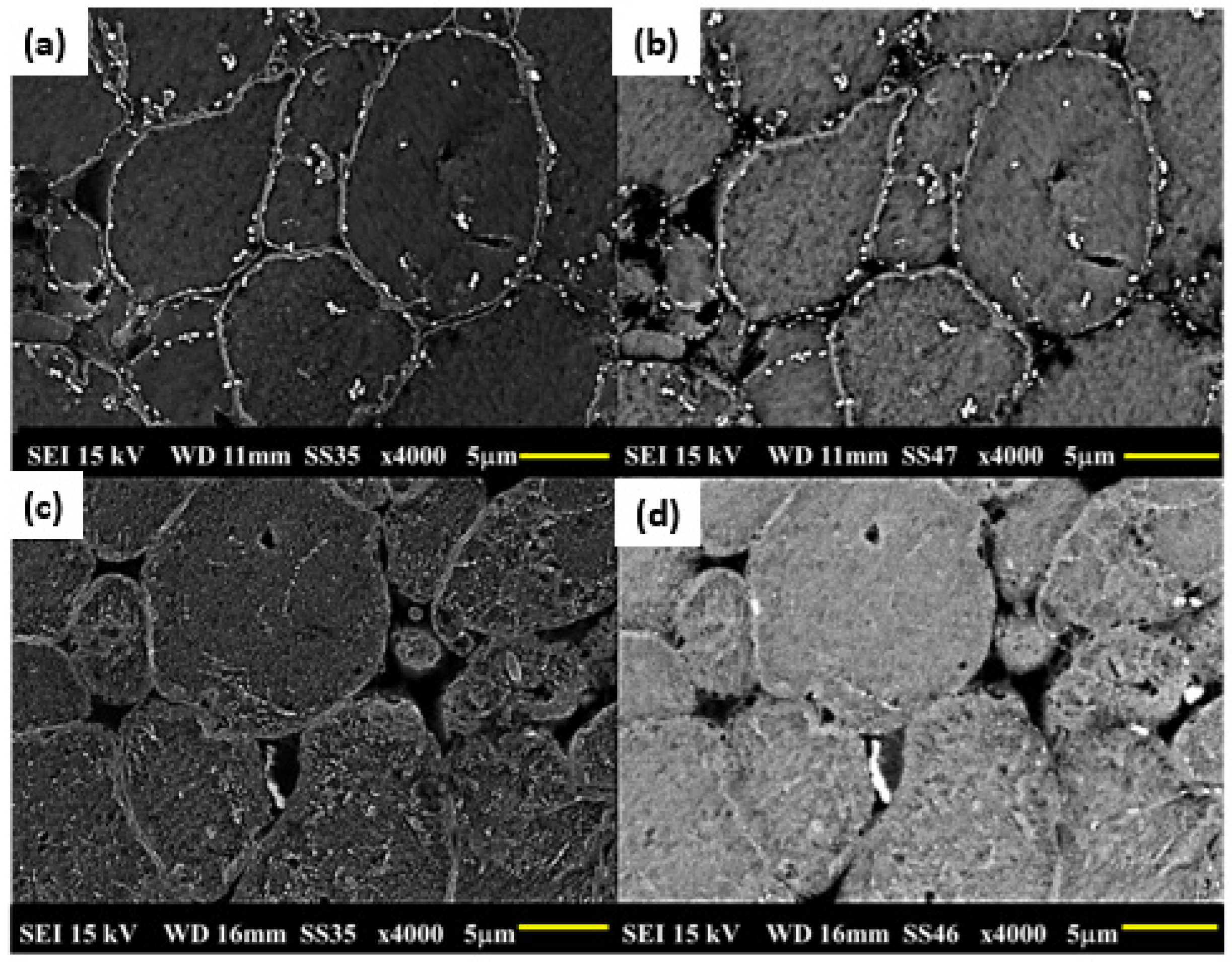
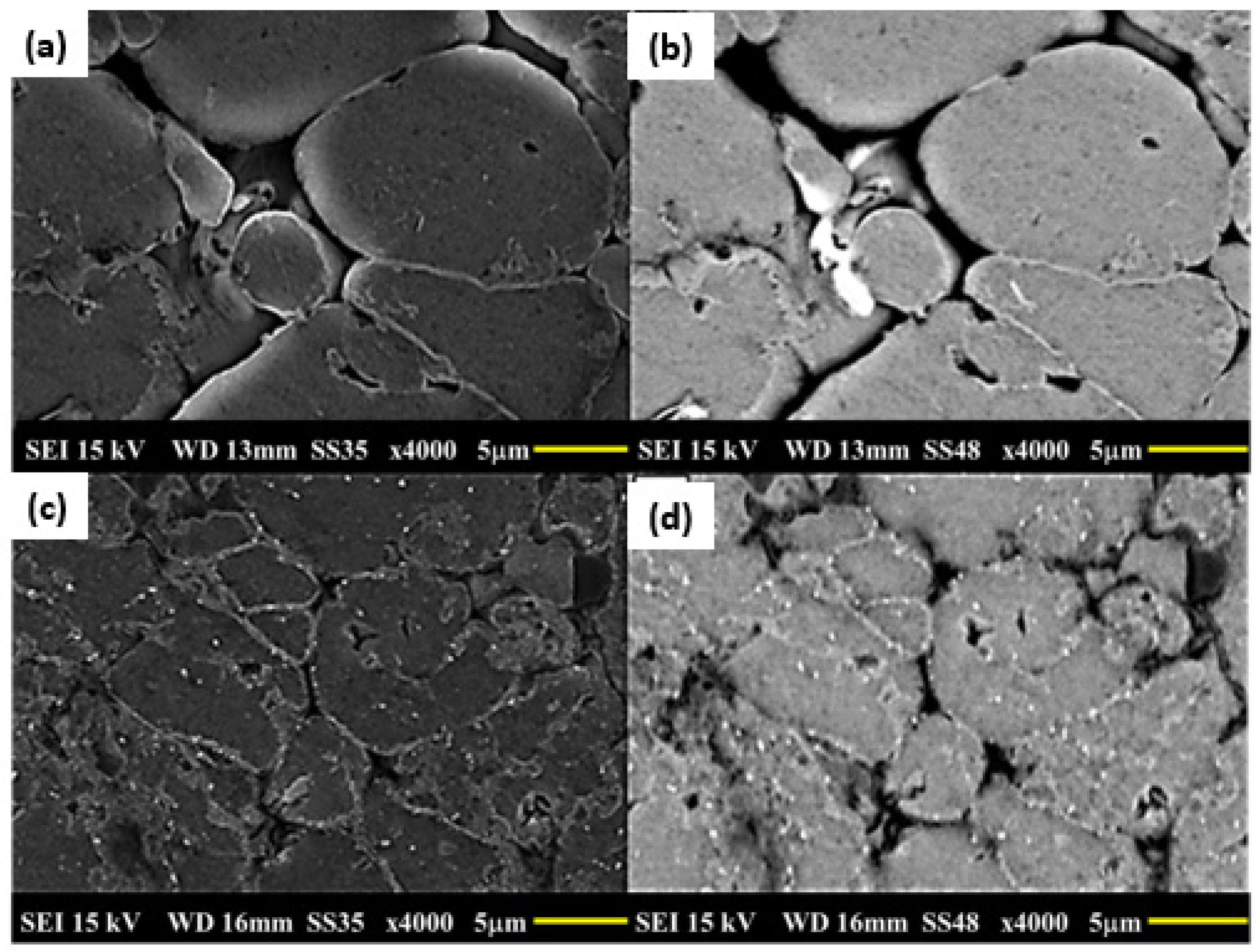
3.5. Microstructures of Sintered Samples and After the T6 Cycle
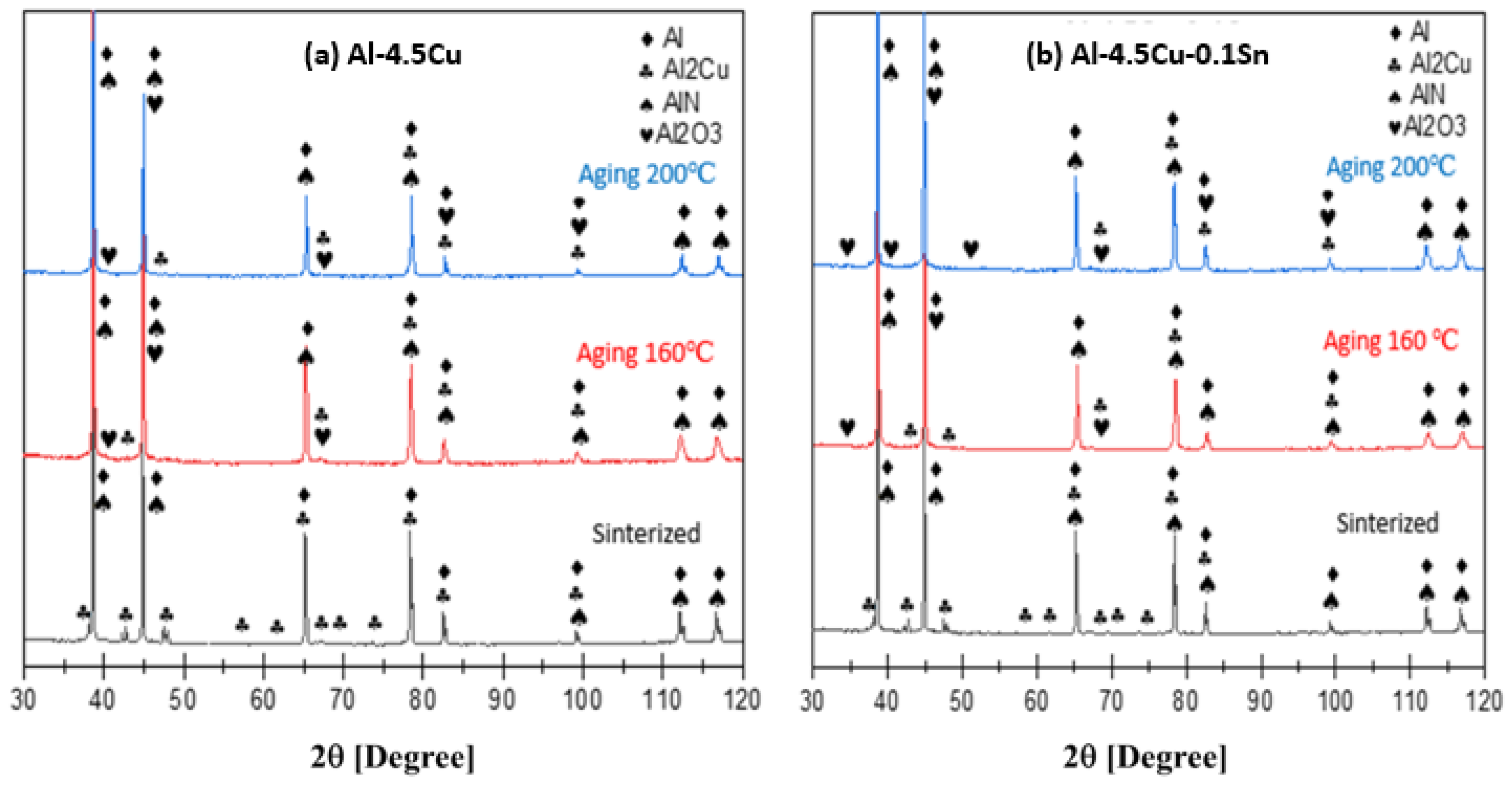
3.6. X-Ray Diffraction of T6 Samples
4. Conclusions
- All the mixtures under study show similar compressibility trends. Specimens compacted at a tension of 200 MPa resulted in average values of 83% of the theoretical density for each alloy.
- For all the mixtures, an increase in density was observed when comparing the specimens compacted in green with those after sintering.
- Intergranular pores smaller than the grains and well distributed in the matrix were observed in the samples without and with added tin.
- The samples with added tin showed a greater response to hardening when subjected to solubilization at 540 °C for 2 h with cooling in a 5% PVP polymer solution and artificial aging at 200 °C for 6 h. It was evident that adding 0.1% tin by mass increased the response to heat treatment. Under this condition, the aging time was shorter for the Al-4.5Cu-0.1Sn samples.
- XRD analysis showed that the Al2Cu phase was formed in all samples with and without the addition of tin.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Emadinia, O.; Vieira, M.T.; Vieira, M.F. Effect of Reinforcement Type and Dispersion on the Hardening of Sintered Pure Aluminium. Metals 2018, 8, 786. [Google Scholar] [CrossRef]
- Ahmad, K.R.; Lee, W.J.; Zaki, R.M.; Noor, M.M.; Wahid, M.F.; Shamsudin, S.R.; Jamaludin, S.B. The microstructure and properties of aluminum composite reinforced with 65 μm alumina particles via powder metallurgy. In Proceedings of the 1st International Conference on Sustainable Materials 2007 (ICoSM2007), Kuala Lumpur, Malaysia, 9–11 June 2007. [Google Scholar]
- Gökçe, A.; Findik, F.; Kurt, A.O. Effects of sintering temperature and time on the properties of Al-Cu PM alloy. Pract. Metallogr. 2017, 54, 533–551. [Google Scholar] [CrossRef]
- Akopyan, T.K.; Shurkin, P.K.; Letyagin, N.V.; Milovich, F.O.; Fortuna, A.S.; Koshmin, A.N. Structure and precipitation hardening response in a cast and wrought Al-Cu-Sn alloy. Mater. Lett. 2021, 300, 130090. [Google Scholar] [CrossRef]
- Upadhyaya, G.S. Powder Metallurgy Technology; Cambridge International Science Publishing: Cambridge, UK, 2014; pp. 1–5. [Google Scholar]
- Neves, E.B. Study of the sintering of an Fe-22, 5Cr-5, 5Ni alloy obtained by mixing elemental powders. Materia 2016, 21, 185–195. [Google Scholar] [CrossRef]
- Motta, C.A.O.; Souza, J.; Martins, V. Enhancing composite materials through fly ash reinforcement in powder metallurgy. Mater. Chem. Phys. 2023, 307, 128124–128132. [Google Scholar] [CrossRef]
- Özay, Ç.; Gencer, E.B.; Gökçe, A. Microstructural properties of sintered Al-Cu-Mg-Sn alloys. J. Therm. Anal. Calorim. 2018, 134, 23–33. [Google Scholar] [CrossRef]
- NEVES, E.C.; do Nascimento, E.G.; Sacilotto, D.G.; Ferreira, J.Z.; Medeiros, J.L.; Biehl, L.V.; Lemos, G.V.; Martins, C.O.; de Jesus Pacheco, D.A. Nondestructive analysis of corrosion in ageing hardened AA6351 aluminum alloys. Mater. Chem. Phys. 2022, 291, 126664. [Google Scholar] [CrossRef]
- Ibrahim, A.; Bishop, D.P.; Kipouros, G.J. Sinterability and characterization of commercial aluminum powder metallurgy alloy Alumix 321. Powder Technol. 2015, 279, 106–112. [Google Scholar] [CrossRef]
- Neves, E.B.; Pollnow, E.N.; Osório, A.G. The effect of microwave energy on sintering of an austenitic stainless steel reinforced with boron carbide. Metall. Mater. Eng. 2022, 28, 515–530. [Google Scholar] [CrossRef]
- Delgado, M.; Ruiz-Navas, E.M.; Gordo, E.; Torralba, J.M. Enhancement of liquid phase sintering through Al-Si additions to Al-Cu systems. J. Mater. Process. Technol. 2005, 162–163, 280–285. [Google Scholar] [CrossRef]
- Schaffer, G.B.; Yao, J.Y.; Bonner, S.J.; Crossin, E.; Pas, S.J.; Hill, A.J. The effect of tin and nitrogen on liquid phase sintering of Al-Cu-Mg-Si alloys. Acta Mater. 2008, 56, 2615–2624. [Google Scholar] [CrossRef]
- Medeiros, J.L.B.; Reguly, A.; Strohaecker, T.R. Applying Oxinitrocarburizing surface strengthening process to corrosion prevention in MIM 17-4 PH stainless steel. Espacios 2015, 36, 21–26. [Google Scholar]
- Schneider, T.H.; Biehl, L.V.; das Neves, E.B.; Medeiros, J.L.B.; de Souza, J.; do Amaral, F.A.D. Method for the determination of parameters in the sintering pross of mixtures of the elemental powders Fe-Cr and Fe-Cr-Ni. MethodsX 2019, 6, 1919–1924. [Google Scholar] [CrossRef] [PubMed]
- Pieczonka, T. Disruption of an alumina layer during sintering of aluminum in nitrogen. Arch. Metall. Mater. 2017, 62, 987–992. [Google Scholar] [CrossRef]
- Dalai, R. Microstructure and property relationship between Al-4.5Cu alloy and Al-4.5Cu-Al2O3 composite developed by mechanical alloying. Mater. Today 2021, 44, 2754–2759. [Google Scholar] [CrossRef]
- Oliveira, M.U.; Biehl, L.V.; Medeiros, J.L.B.; Avellaneda, C.A.O.; Martins, C.O.D.; de Souza, J.; Sporket, F. Manufacturing against corrosion: Increasing materials performance by the combination of cold work and heat treatment for 6063 aluminum alloy. Mater. Sci. 2019, 26, 30–33. [Google Scholar] [CrossRef]
- Olszowka-Myalska, A.; Wrześniowski, P.; Myalska, H.; Godzierz, M.; Kuc, D. Impact of the Morphology of Micro- and Nanosized Powder Mixtures on the Microstructure of Mg-Mg2Si-CNT Composite Sinters. Materials 2019, 12, 3242. [Google Scholar] [CrossRef]
- Cao, H.; Zhan, Z.; Lv, X. Microstructure Evolution and Properties of an In-Situ Nano-Gd2O3/Cu Composite by Powder Metallurgy. Materials 2021, 14, 5021. [Google Scholar] [CrossRef]
- Grützner, S.; Krüger, L.; Radajewski, M.; Schneider, I. Characterization of In-Situ TiB/TiC Particle-Reinforced Ti-5Al-5Mo-5V-3Cr Matrix Composites Synthesized by Solid-State Reaction with B4C and Graphite through SPS. Metals 2018, 8, 377. [Google Scholar] [CrossRef]
- Shahid, R.N.; Scudino, S. Microstructure and Mechanical Behavior of Al-Mg Composites Synthesized by Reactive Sintering. Metals 2018, 8, 762. [Google Scholar] [CrossRef]
- Tochetto, R.; Biehl, L.V.; Medeiros, J.L.B.; Dos Santos, J.C. Evaluation of the space holders technique applied in powder metallurgy process in the use of titanium as biomaterial. Lat. Am. Appl. Res. 2019, 49, 261–268. [Google Scholar] [CrossRef]
- Schaefer, J.L.; Tardio, P.R.; Baierle, I.C.; Nara, E.O.B. GIANN—A Methodology for Optimizing Competitiveness Performance Assessment Models for Small and Medium-Sized Enterprises. Adm. Sci. 2023, 13, 56. [Google Scholar] [CrossRef]
- Vieira, E.D.R.; Biehl, L.V.; Medeiros, J.L.B.; Costa, V.M.; Macedo, R.J. Evaluation of the characteristics of an AISI 1045 steel quenched in different concentration of polymer solutions of polyvinylpyrrolidone. Sci. Rep. 2021, 11, 1313. [Google Scholar] [CrossRef] [PubMed]


| ALLOY | AL % | CU % | SN % |
|---|---|---|---|
| Al-3.5Cu | 96.50 | 3.50 | - |
| Al-4.0Cu | 96.00 | 4.00 | - |
| Al-4.5Cu | 95.50 | 4.50 | - |
| Al-3.5Cu-0.1Sn | 96.40 | 3.50 | 0.10 |
| Al-4.0Cu-0.1Sn | 95.90 | 4.00 | 0.10 |
| Al-4.5Cu-0.1Sn | 95.40 | 4.50 | 0.10 |
| Alloy | Theoretical | Green | True | |||
|---|---|---|---|---|---|---|
| Average | StDev | Average | StDev | Average | StDev | |
| Al-3.5Cu | 2.7676 | 0.0038 | 2.349 | 0.0230 | 2.5095 | 0.0035 |
| Al-4.0Cu | 2.7775 | 0.022 | 2.3468 | 0.0120 | 2.5122 | 0.0027 |
| Al-4.5Cu | 2.7875 | 0.0049 | 2.3374 | 0.0167 | 2.5238 | 0.0049 |
| Al-3.5Cu-0.1Sn | 2.7694 | 0.037 | 2.2879 | 0.0250 | 2.5133 | 0.0026 |
| Al-4.0Cu-0.1Sn | 2.7793 | 0.019 | 2.3149 | 0.0144 | 2.5249 | 0.0052 |
| Al-4.5Cu-0.1Sn | 2.7893 | 0.0012 | 2.3162 | 0.0137 | 2.5256 | 0.0012 |
| Average | 2.7784 | 0.0146 | 2.3254 | 0.0175 | 2.5182 | 0.0033 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elias Junior, P.J.O.; das Neves, E.B.; Biehl, L.V.; Baierle, I.C.; Martins, C.O.D.; Medeiros, J.L.B. Study of the Effect of Tin Addition in Aluminum–Copper Alloys Obtained from Elemental Powders. Metals 2025, 15, 559. https://doi.org/10.3390/met15050559
Elias Junior PJO, das Neves EB, Biehl LV, Baierle IC, Martins COD, Medeiros JLB. Study of the Effect of Tin Addition in Aluminum–Copper Alloys Obtained from Elemental Powders. Metals. 2025; 15(5):559. https://doi.org/10.3390/met15050559
Chicago/Turabian StyleElias Junior, Pedro José Olendski, Ederson Bitencourt das Neves, Luciano Volcanoglo Biehl, Ismael Cristofer Baierle, Carlos Otávio Damas Martins, and Jorge Luis Braz Medeiros. 2025. "Study of the Effect of Tin Addition in Aluminum–Copper Alloys Obtained from Elemental Powders" Metals 15, no. 5: 559. https://doi.org/10.3390/met15050559
APA StyleElias Junior, P. J. O., das Neves, E. B., Biehl, L. V., Baierle, I. C., Martins, C. O. D., & Medeiros, J. L. B. (2025). Study of the Effect of Tin Addition in Aluminum–Copper Alloys Obtained from Elemental Powders. Metals, 15(5), 559. https://doi.org/10.3390/met15050559









