Abstract
316L steel is widely used in various industries and is also one of the metallic materials used for biomedical applications because of its excellent mechanical properties, corrosion resistance, and biocompatibility. This article reports a comprehensive study on the effects of equal channel angular pressing (ECAP) and subsequent recovery treatment on the microstructure and the mechanical, tribological, and corrosion properties of 316L. The process includes an initial annealing at 1050 °C for 2 h to obtain a homogenous microstructure, ECAP at room temperature with a 120° inner angle, and subsequent recovery treatment at 340 °C for 1 h. The microstructure was investigated with an optical microscope and a transmission electron microscope. The mechanical properties were evaluated with hardness and compression tests. The corrosion behavior was analyzed with dynamic polarization tests. The wear test was performed using a scratching tester, and the volume loss was measured with a profilometer. The results of the study demonstrate that the ECAP–recovery sample exhibits improved properties compared to both the annealed sample and the ECAP sample. The corrosion tests show that the ECAP sample has a corrosion resistance higher than that of the annealed sample but lower than that of the ECAP–recovery sample. The ECAP–recovery sample shows the highest wear resistance and corrosive wear resistance among the three samples.
1. Introduction
316L austenitic stainless steel (ASS) has excellent mechanical properties and corrosion resistance [1,2,3], and has found many applications including those in the biomedical field, e.g., artificial joints, spinal fixation fixtures, orthopedic screws and wires, and dental implants, etc. [4,5]. The industrial applications of stainless steel are numerous, such as tubes used for oil and gas transport, petrochemicals, refineries, and conveying tubes, because of its excellent resistance to corrosion and oxidation [6,7,8].
There are several groups of materials used to make orthodontic and orthopedic implants, including chromium and cobalt alloys, austenitic stainless steels, ceramic materials, polymer materials, and popular titanium and its alloys [9,10]. Metallic materials are generally stronger than hard tissues, so their bio-corrosion and biocompatibility are more pressing concerns when they are used for biomedical applications. Williams [10] suggested that three types of corrosion may exist for dental implants:
- Stress cracking (SCC);
- Galvanic corrosion (GC);
- Erosion corrosion (FC).
Several methods are used to produce ultrafine-grained (UFG) alloys, achieved by severe plastic deformation (SPD), such as high-pressure torsion, accumulative roll bonding, and equal channel angular pressing (ECAP) [11,12]. ECAP is widely used for producing ultrafine grains with sizes in the range of several tens of nanometers to 500 nanometers [13,14,15,16,17], which helps improve their mechanical properties [18,19].
D.M. Xu et al. [20] reported that a significant fraction of austenite transformed into martensite after 90% cold reduction. The martensite laths were oriented along the rolling direction. The UFG 316LN austenitic stainless steel exhibited excellent mechanical properties of high yield strength and high plasticity.
Hajizadeh et al. [21] studied the effect of ECAP on the microstructure and mechanical properties of 316L. They found that due to the high stability of the austenite phase in the studied steel, deformation-induced martensite (DIM) was not observed in samples that experienced ECAP using any of the above routes. Based on the X-ray diffraction analysis, the structure after ECAP was completely austenitic. They reported that as-received material showed a very wide range of work hardening, while such a range for the ECAP samples was narrowed.
Guilherme et al. [22] investigated the corrosion behavior of UFG aluminum alloy. They reported that ECAP processing enhances passive corrosion resistance compared to the undeformed sample. However, the improvement in corrosion resistance did not consistently increase with the number of ECAP passes. Factors such as the distribution of high- and low-angle grain boundaries, dislocation density, and the fragmentation and redistribution of coarse dispersoid particles play a significant role in the corrosion behavior post-ECAP.
Awang et al. [23] reported that finer and elongated grains were achieved in 316L after the ECAP process with correspondingly increased hardness. Their findings showed an improvement in the corrosion behavior of ECAP samples in both 0.9% NaCl and E-MEM + 10% NCS.
Xiaoqian Fu et al. [24] investigated the effect of grain size on the corrosion resistance of rolled 316L. They reported that grain uniformity and intermetallic grain boundaries benefited the stability of passive films. There are considerable stress concentrations at grain boundaries after rolling, which can be reduced by annealing. The more grain boundaries, the higher the pitting potential. SenSen Xin et al. [25] studied the temperature and grain size effect on the corrosion behavior of 316L stainless steel in seawater. They showed that fine and coarse grains of 316L stainless steel showed nearly the same corrosion resistance at 25 °C. However, the pitting resistance of the fine-grain steel was reduced greatly and showed lower pitting potential values in comparison with the coarse-grain one. Roston et al. [26] reported that grain refinement improved the corrosion resistance of some alloys such as Mg and Ti. For the other materials, corrosion resistance increased and decreased depending on processing and environmental factors. The reason that grain refinement improved the corrosion resistance is attributed to an improvement in passive film formation and adhesion because of increasing grain boundary density, but a discrepancy exists.
X.Y. Wang et al. [27] investigated a nano-crystalline surface of 304 stainless steel produced by sandblasting and recovery heat treatment. They reported that the nano-crystalline surface showed better corrosion resistance, wear, and corrosive wear properties than the as-received one. Research on metals has reported that ECAP improves wear resistance, but some research has shown opposite results after the ECAP process [28]. For example, an improvement in the wear resistance of pure Ti and TiNi alloy was shown after the ECAP process [29,30]. However, it was also reported that dual-phase steel with an ultra-fine grain showed a higher wear rate than a coarse-grained one [31]. Nagaraj et al. [32] investigated the effect of ECAP on the microstructure and tribo-corrosion characteristics of 316L stainless steel. They found that the 4th pass sample showed minimum wear volume loss and a low coefficient of friction during the tribo-corrosion test due to refined grains that show high resistance to plastic deformation. The open circuit potential (OCP) curve of ECAP-processed samples shifts to a more negative potential.
As shown above, the reported studies are not always consistent, implying that the effect of ECAP on wear, corrosion, and corrosive wear has not been fully understood. Severe plastic deformation (SPD) introduces high-density dislocation cells, which harden the materials but make them more susceptible to corrosion or electrochemical attacks. The recovery treatment can turn the nano-scaled dislocation cells into nano-grains, thus increasing not only the hardness of the material but also its resistance to corrosion [27]. Much research has already been conducted to investigate the effect of the ECAP process on 316L properties. This study was conducted to investigate how the ECAP process affects this material’s properties, including its mechanical, tribological, and electrochemical properties, to further our understanding of the underlying mechanisms. In particular, efforts were made to understand the effects of subsequent recovery treatment (at low temperatures) on the microstructure and corresponding properties of 316L in order to fabricate optimized 316L steel for biomedical and industrial applications.
2. Materials and Methods
For this study, 316L steel is used in rod form with a diameter of 4 mm. The chemical composition of the material is shown in Table 1.

Table 1.
Chemical composition of 316L.
The as-received steel was in an austenite state and annealed at 1050 °C for 2 h in an argon atmosphere (to prevent oxidation) to achieve a homogenous microstructure, and it was cooled in the furnace [33]. The equal channel angular pressing (ECAP) was performed at room temperature by a 50-ton hydraulic press. Graphite powder was used as the lubricant to decrease the friction coefficient between the samples and the die. Samples after two ECAP passes are shown in Figure 1. To gain optimal grain refinement, the Bc route was used based on previous research [11]. The inner contact angle was 120 degrees, while the outer arc of curvature was 60 degrees. The ECAP die was manufactured from two sides, and they were joined together with 10 bolts. The ECAP die was made from H13 steel. The CNC machining process was used to make the die. The channel was completely polished to decrease the friction between the samples and the die. Some of the ECAP samples were then heat treated, i.e., recovery treatment at 340 °C for 1 h in the argon atmosphere with Type F79300 tube furnaces (Barnstead Thermolyne Company, Dubuque, IA, USA), followed by cooling in the furnace. For microstructure observation, samples were polished with sandpaper and then etched with aqua regia solution (nitric acid and hydrochloric acid in a molar ratio of 1:3). Microstructure characterization and grain size measurement were carried out by optical microscopy (OM), and then by transmission electron microscopy (TEM) for more details including microstructure and dislocation density analysis. A JEOL-JEM 2100 (JEOL Company, Tokyo, Japan) equipped with a Gatan imaging filter operated at 200 KV was used for the TEM analysis. TEM foils were prepared by mechanical grinding followed by electrochemical polishing. The grain size was measured based on ASTM E-112 [34]. The Hyen method (line method) was used to measure the grain size.
Figure 1.
The ECAP die (left image) and samples after two ECAP processes (right image).
The X-ray diffraction (XRD) analysis was conducted using a D8-Bruker machine (Bruker Ltd., Coventry, UK) for phase detection. The peak profiles were analyzed using Xpert Highscore software version 5.2.
For the evaluation of the mechanical properties, the compressive and hardness tests were performed. The hardness test was conducted under a 100 kg load. The sample diameter and length for the compression tests were 4 mm and 7 mm, respectively. The compression test was carried out with 0.05 mm/min, and each alloy was tested three times to ensure reproducibility. It may need to be mentioned that in this study, compressive testing rather than tensile testing, which is usually used to evaluate the mechanical properties of materials, was performed due to the following reasons: (1) our samples were not sufficiently large for tensile tests, but were large enough for compressive tests; (2) for dental implants, they may bear more compressive force, which could be predominant.
Yield stress, max stress (i.e., the stress peak before failure), and energy absorption up to the strain of 70% (samples did not fail till the maximum load of the machine) were determined for the three samples.
For the corrosion behavior analysis, electrochemical tests were performed by Gamry PC4/759 (Gamry, Warminster, PA, USA), including measurements of open circuit potential (OCP) and potentiodynamic polarization, using GAMS within a standard three-electrode setup. The setup consists of a platinum sheet as the counter electrode, a calomel electrode as the reference, and a working electrode with a 0.4 cm2 surface area. Potentiodynamic polarization curves were obtained over a potential range of −0.5 to +1.5 V relative to OCP, employing a rate of 0.33 mV/s. The corrosion experiments were performed in both 3.5% NaCl solution and 5% HCl solution at room temperature. The NaCl solution simulates a chloride-containing environment around pH 7.1, while the HCl solution simulates a low-pH environment of pH 0.4. The electrochemical tests were carried out at least three times for data reproducibility.
The wear resistance of a sample was investigated using a scratch test machine (CSEM Tribometer, Peseux, Switzerland). During the test, a four-sided pyramidal Vickers diamond tip reciprocally scratched the surface at a velocity of 0.5 mm/sec for 20 min under different applied loads, 10 and 20 N. The material loss was determined by measuring the volume loss in the worn region using a profilometer (ZE METRICS, A Zygo Company, Middlefield, CT, USA). Corrosive wear tests were performed with the same tribometer using a ceramic ball tip of 6 mm in diameter under a force of 5 N. The corrosive wear tests were performed in 5% HCl and 3.5% NaCl. In addition, dry wear tests were also performed with the ceramic ball tip to compare the results of the corrosive wear and dry wear tests.
3. Results
Figure 2 shows the OM images of steel samples in three states after etching, which are mainly in the austenite phase. The images show that twinning was generated by the ECAP process. Corresponding changes in grain size for the three samples are shown in Figure 3. As observed, the grain size is decreased by the ECAP process, which slightly increases due to the subsequent recovery treatment.

Figure 2.
Optical microscope images of samples (a) annealed, (b) after the ECAP, and (c) after the ECAP–recovery treatment.
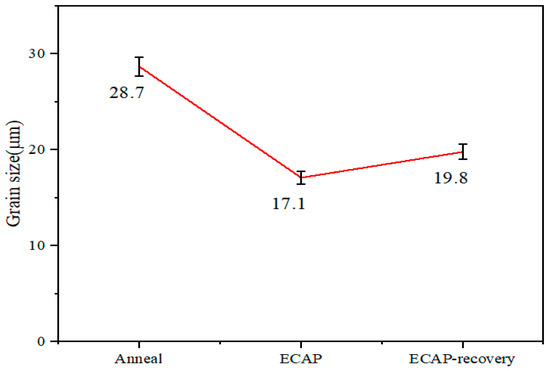
Figure 3.
Grain sizes of the three samples.
For more detailed information on structure and microstructure, such as dislocation density, twins, and possibly formed new phases, a TEM analysis was conducted. Figure 4 displays TEM images of the three samples, showing that the ECAP introduced deformation twins in the austenite structure of the steel (see Figure 5), which remain in the steel after the recovery treatment. No new phase deformation was observed after the ECAP.
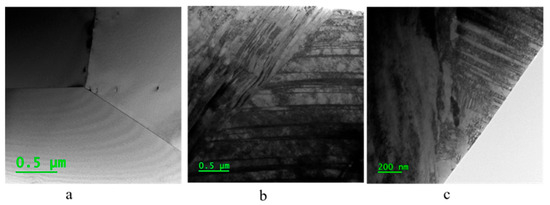
Figure 4.
TEM image results for three samples: (a) Annealed, (b) ECAP, (c) ECAP–recovery.
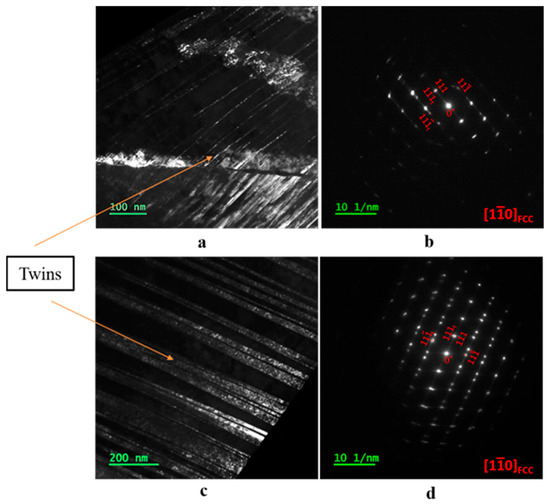
Figure 5.
TEM images of ECAP and ECAP–recovery samples. Twins can be seen clearly. (a,c) ECAP and ECAP–recovery samples, respectively; (b,d) selected area electron diffraction patterns for the ECAP and ECAP–recovery samples, respectively.
Figure 6 illustrates dislocations in the three samples. There are some dislocations in the annealed sample, and the dislocation density is considerably increased by the ECAP, and decreased by the subsequent recovery treatment. Table 2 provides the dislocation density for the three samples. As shown, the dislocation density increases by two orders of magnitude after the ECAP process, and it is decreased to half by the subsequent recovery treatment.
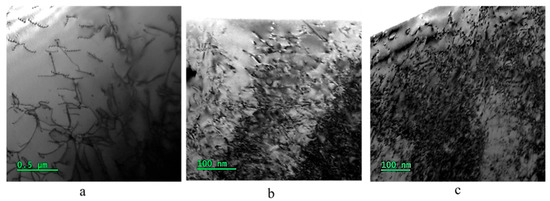
Figure 6.
Dislocations in the three samples: (a) annealed, (b) ECAP, and (c) ECAP–recovery.

Table 2.
Dislocation densities in three samples.
The increase in dislocation density results in an enhanced strain-hardening effect. The hardness of the samples was measured, shown in Figure 7. As demonstrated, the hardness increases from 27.5 to 64.5 (2.34 times) after the ECAP process, and the subsequent recovery treatment further increases it slightly from 64.5 to 65.5. Such a slight increase in hardness, even if the dislocation density decreases as shown in Table 2, implies that the recovery treatment renders the defect configurations more stable or raises barriers to the defects’ generation and movement.
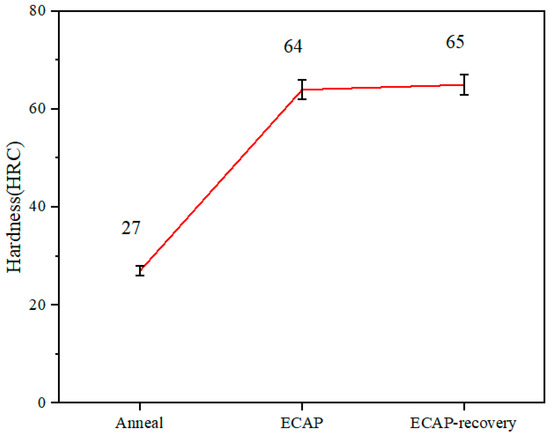
Figure 7.
Hardness measured under a load of 100 g.
For more information on the influences of ECAP and the recovery treatment on the mechanical behavior of the steel, compressive tests were performed. Figure 8 displays stress–strain curves obtained from the compressive tests. None of the samples failed under a compressive force up to 40 KN due to the high formability of 316L steel. The yield stress (YS) values of the three samples are shown in Figure 9.
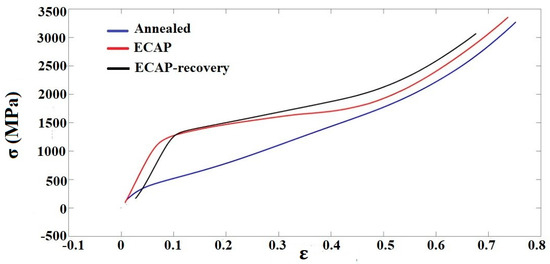
Figure 8.
Compressive stress–strain curves of the three samples.
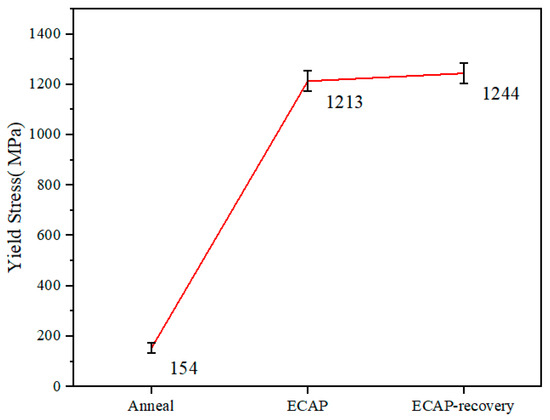
Figure 9.
Compressive yield strengths of the three samples.
According to the compressive tests, the ECAP increases the yield stress from 154 MPa to 1213 MPa (nearly eight times), which further increases to 1244 MPa with the recovery process. The variations in the yield strength are consistent with the hardness values of the three samples, as shown in Figure 8; under the maximum load there was no failure for the samples with three different treatments. The results suggest that the samples possess sufficient ductility, though we could not directly determine the value. The authors would also like to mention that this study is more focused on the material’s resistance to wear and corrosion, so its hardness and electrochemical properties are more of a concern, while less attention was given to the ductility, since the compressive test had already shown that the samples had sufficient ductility (no failure up to compressive strain ~70% under the maximum load).
For wear resistance, the energy absorption ability is beneficial, since it helps dissipate deformation energy and reduce damage to the crystal lattice. In this study, the energy absorption up to the strain of 70% was determined from the compressive stress–strain curves for 316L in different states. As shown in Figure 10, the energy absorption increases from 93.4 MPa to 113.2 MPa after the ECAP process, and it increases a little more after the subsequent recovery treatment.
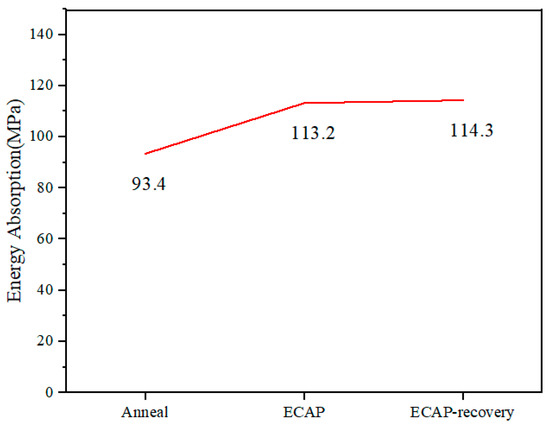
Figure 10.
Energy absorption values of the three samples, determined from the compressive stress–strain curves up to the strain of 70% (Figure 7).
The XRD patterns of the samples in the three states are displayed in Figure 11, with a focus on the 2θ range of 36–56°. As shown, the three samples have similar XRD patterns, characterized by two main peaks of γ phase, meaning that the ECAP and ECAP–recovery processes did not change the structure of the steel, except by introducing dislocations and deformation twinning.
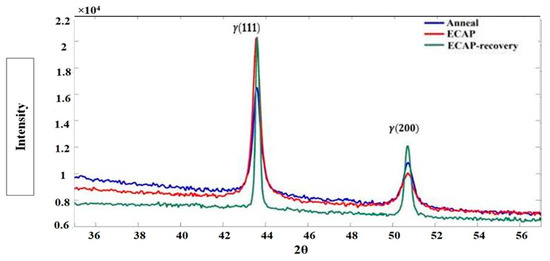
Figure 11.
XRD patterns of 316L steel samples in three states.
Effects of ECAP and ECAP–recovery treatments on the corrosion behavior of the steel were investigated. Potentiodynamic polarization curves of the three samples in 3.5%NaCl and 5%HCl solutions, respectively, were obtained. Figure 12 illustrates the polarization curves of the 316L alloy in three states: annealed, ECAP-treated, and ECAP–recovery-treated. In the 3.5%NaCl solution, the annealed and ECAP-treated samples show similar corrosion potentials while the subsequent recovery treatment markedly increases the corrosion potential or the susceptibility to corrosion. In the acidic solution, the corrosion potentials of the three samples are similar. However, the ECAP and ECAP–recovery treatments decrease the corrosion rates in both solutions (see values given in the table of Figure 12). It is clear that the recovery treatment helps improve the corrosion resistance, since it reduces the strain energy, rendering the material closer to a more stable state, and improves the adherence of surface film to the substrate through reducing the interfacial defects.
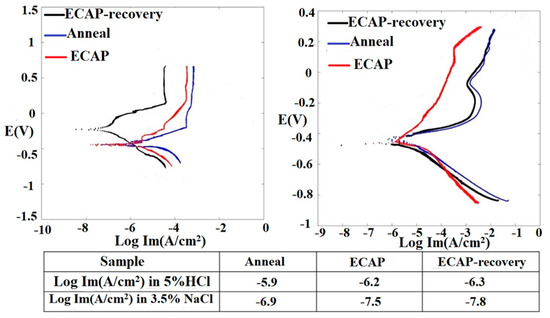
Figure 12.
Dynamic polarization curves of samples in the three states and corresponding Im values, determined in 3.5% NaCl (left) and 5% HCl (right) solutions, respectively. The ECAP–recovery sample performed the best in both solutions.
In this study, scratching wear tests were performed for the three samples. The wear resistance of the samples was evaluated by measuring the volume loss caused by reciprocal scratching with a diamond tip over a certain period. Figure 13 illustrates a sample scratching track, whose dimensions were measured using a profilometer. As shown in Table 3, the measured volume losses caused by reciprocal scratching under a relatively small load of 10 N indicate that the wear of the annealed sample is similar to that of the sample that experienced the ECAP treatment, but the subsequent recovery treatment noticeably enhances the wear resistance. However, under a larger load of 20 N (see Table 4) the ECAP sample performed better than the annealed one. Again, the recovery treatment considerably improves the wear behavior under the larger load. The ECAP–recovery sample shows the highest wear resistance under different loads.
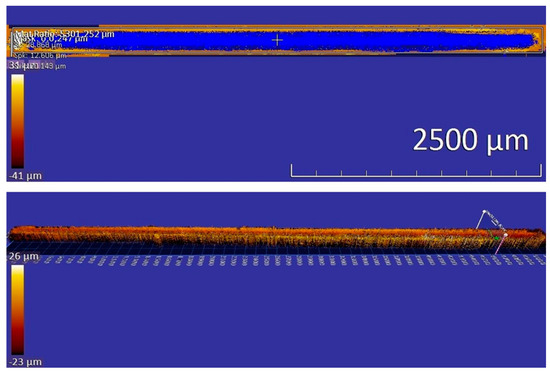
Figure 13.
Volume loss (up image) and max depth (down image) of a sample, determined with a profilometer.

Table 3.
Results of scratch tests under 10 N.

Table 4.
Result of scratch tests under 20 N.
The resistances of the steel in the three states to corrosive wear were also evaluated, respectively, in 3.5% NaCl and 5% HCl solutions under a load of 5N, the results of which are shown in Table 5. As shown, the volume loss caused by corrosive wear decreases with the ECAP, and the ECAP–recovery results in the lowest volume loss.

Table 5.
Volume loss (μm3) × 106 for corrosive wear test under 5 N load in different corrosion environments.
4. Discussion
As shown earlier, the annealed sample has an average grain diameter equal to 28.7 μm, which is decreased to 17.7 μm by the ECAP, and then is slightly increased to 19.87 μm by subsequent recovery treatment. This is understandable since severe plastic deformation can squeeze grains and generate dislocation cells, leading to a reduced average grain size. When the recovery treatment is applied, dislocations may be locally rearranged, slightly increasing the grain size and turning the dislocation cells into nano-grains. According to the Hall–Petch relationship, a smaller grain size increases the hardness of the material. Additionally, the increased dislocation density promotes strain hardening, making important contributions to the hardness of the material. Furthermore, the ECAP-induced deformation twinning may interact with dislocations, thus further enhancing the strain-hardening effect. The high ductility of UFG 316LN austenitic stainless steel was due to twinning, while in commercial steel, strain-induced ά-martensite contributes to high ductility [20]. The above-mentioned factors are responsible for the increases in mechanical strength. As shown, the hardness of the steel increases by 2.34 times after the ECAP process, and it does not change much after the recovery process. During the compressive tests, none of the samples failed under the load up to 40 N. The compressive yield strength increases by 7.8 times after the ECAP process, and it does not change noticeably after the recovery treatment. Energy absorption increases after the ECAP process and it increases slightly after the recovery treatment. For UFG metallic materials processed by severe plastic deformation techniques, the annihilation of mobile dislocations and the clustering of the remaining dislocations into low-angle grain boundaries during annealing can yield hardening. It is also shown that plastic deformation after annealing can cause a restoration of the yield strength and hardness to the same level as observed before annealing. The possible reasons for this deformation-induced softening effect are discussed in detail [35].
ECAP improves the corrosion resistance of the steel in both solutions (see Figure 12), likely due to grain refinement enhancing Cr diffusion along grain boundaries, which accelerates passivation and passive film formation. There are several studies on the effects of ECAP on the corrosion behavior of stainless steel [36,37,38]. Maleki-Ghaleh et al. [35] observed using Ringer’s Solution that the corrosion resistance of Type 316L stainless steel was improved considerably by increasing the number of ECAP passes. However, the situation can be complicated. The increased dislocation density caused by ECAP or severe plastic deformation (SPD) may have a negative influence on corrosion resistance, influenced by ECAP or SPD process conditions and the corrosive environment. The reduced grain size or the increased grain boundary density and increased dislocation density promote Cr diffusion, which benefits the formation of a passive film. However, the high-density dislocations may deteriorate the resistance to corrosion [37,38].
The negative influence of high-density dislocations can be minimized by the subsequent recovery treatment, since it reduces the SPD-induced disordering and the turning of dislocation cells into nano-grains, thus retaining the faster diffusion of Cr along the high-density grain boundaries and enhancing the adherence of passive film to the substrate [26,27].
The wear test results show that the wear resistance of the steel is not affected much by ECAP under 10 N, but the benefit of ECAP on the wear resistance shows up under a larger contact load of 20 N. The beneficial effect of ECAP on wear resistance becoming pronounced under larger loads is understandable, since as the wear attack becomes more severe, the mechanical strength raised by ECAP may render its benefits to wear resistance more pronounced. The higher mechanical strength reduces the contact area and the depth of asperity penetration and, thus, the plowing and delamination processes. As for the subsequent recovery treatment, its benefits to wear resistance are constantly obvious since it decreases stress concentrations and increases toughness. As a result, a combination of hardness and toughness always helps reduce wear, no matter whether the wearing force is large or small.
It is noticed that under a further smaller load of 5 N, the ECAP sample shows a larger volume loss, compared to the annealed one (see Table 5). This could be related to a kind of surface singularity. In the bulk material, dislocations can mutually interact, e.g., pinning and tangling each other, leading to strain hardening and enhanced wear resistance. However, if the wearing force is small, the deformation occurs only in a shallow surface layer. Such mutual interaction of dislocations and other lattice defects could be largely weakened since dislocations and defects are relatively free to move to the surface, bearing in mind that the surface loses the constraint from the other half the lattice. As a result, atoms on the surface could be readily removed layer by layer, especially when the surface has a higher degree of disordering or more accumulated dislocations or defects. In corrosive environments, such damage could be enlarged, as shown in Table 5. On the surface, levels of electron localization and stability are different from those inside the bulk, thus influencing the surface’s mechanical and electrochemical behaviors. Such a singularity of physical surfaces is certainly worth further investigation. The subsequent recovery treatment can reduce disordering and enhance the adherence of the passive film to the substrate as well as the surface singularity, leading to a higher resistance to both dry wear and wear in corrosive solutions regardless of the contact force, as observed in the present study (Table 5). This study demonstrates the necessity of recovery treatment after ECAP for improving the mechanical, tribological, and electrochemical properties of 316L steel, which is also applicable to other passive alloys.
Wear resistance is influenced by multiple factors, e.g., the microstructure and properties of materials, the type and magnitude of contact force, mechanical action, environmental corrosivity, temperature, and their synergy [36,37,38,39,40,41,42,43]. Thus, tailoring microstructures using various approaches, e.g., ECAP, heat treatments, texture control, phase transformations, surface microstructure control, loading conditions, etc. [38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54], is the primary task of materials engineering.
5. Conclusions
In this study, the effects of equal channel angular pressing (ECAP) and subsequent recovery treatment on the microstructure and the mechanical, tribological, and corrosion properties of 316L steel were investigated. The following conclusions are drawn:
- The ECAP increased the dislocation density by two orders of magnitude and introduced considerable deformation twinning, leading to an increase in the compressive yield strength of the steel by about eight times. The subsequent recovery treatment slightly increased the yield strength. The corresponding hardness was increased by more than two-fold.
- The ECAP and ECAP–recovery improved the ability of energy absorption by about 23% at a compressive strain of 70%, which helped enhance the steel’s resistance to wear attacks.
- The ECAP process improved the corrosion behavior. The subsequent recovery treatment markedly increased the corrosion resistance due to lowered dislocation density or strain energy and improved the adherence of surface film to the substrate by reducing the interfacial defects.
- The ECAP was beneficial to the wear resistance of the steel but not under low loads. The benefit became pronounced as the contact load increased. However, ECAP–recovery always markedly enhanced the wear resistance regardless of the contact load. ECAP–recovery also markedly enhanced the corrosive wear resistance of the steel.
- The recovery treatment is essential to reduce the degree of disordering and stress concentrations caused by ECAP, which is effective in improving the steel’s mechanical properties and elevating the resistance of 316L steel to wear, corrosion, and corrosive wear.
Author Contributions
Methodology, A.R.; investigation, A.R., A.H. and G.D.; data curation, A.R.; writing—original draft preparation, A.R.; writing—review and editing, A.R., M.K. and D.L.; supervision, M.K. and D.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rostami, M.; Miresmaeili, R.; Heydari Astaraee, A. Investigation of surface nanostructuring, mechanical performance and deformation mechanisms of AISI 316L stainless steel treated by surface mechanical impact treatment. Met. Mater. Int. 2023, 29, 948–967. [Google Scholar] [CrossRef]
- Kunčická, L.; Kocich, R.; Pagáč, M. Experimental and Numerical Study of Behavior of Additively Manufactured 316L Steel Under Challenging Conditions. Metals 2025, 15, 169. [Google Scholar] [CrossRef]
- Hanawa, T.; Hiromoto, S.; Yamamoto, A.; Kuroda, D.; Asami, K. XPS characterization of the surface oxide film of 316L stainless steel samples that were located in quasi-biological environments. Mater. Trans. 2002, 43, 3088–3092. [Google Scholar] [CrossRef]
- Hermawan, H.; Ramdan, D.; Djuansjah, J.R. Metals for biomedical applications. In Biomedical Engineering—From Theory to Applications; IntechOpen: London, UK, 2011; Volume 1, pp. 411–430. [Google Scholar]
- Fathi, M.H.; Salehi, M.A.; Saatchi, A.; Mortazavi, V.; Moosavi, S.B. In vitro corrosion behavior of bioceramic, metallic, and bioceramic–metallic coated stainless steel dental implants. Dent. Mater. 2003, 19, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Li, J.S.; Gao, W.D.; Cao, Y.; Huang, Z.W.; Gao, B.; Mao, Q.Z.; Li, Y.S. Microstructures and mechanical properties of a gradient nanostructured 316L stainless steel processed by rotationally accelerated shot peening. Adv. Eng. Mater. 2018, 20, 1800402. [Google Scholar] [CrossRef]
- Li, Z.N.; Wei, F.A.; La, P.Q.; Ma, F.L. Enhanced mechanical properties of 316L stainless steel prepared by aluminothermic reaction subjected to multiple warm rolling. Met. Mater. Int. 2018, 24, 633–643. [Google Scholar] [CrossRef]
- Naghizadeh, M.; Mirzadeh, H. Microstructural evolutions during reversion annealing of cold-rolled AISI 316 austenitic stainless steel. Metall. Mater. Trans. A 2018, 49, 2248–2256. [Google Scholar] [CrossRef]
- Biesiekierski, A.; Wang, J.; Gepreel, M.A.; Wen, C. A new look at biomedical Ti-based shape memory alloys. Acta Biomater. 2012, 8, 1661–1669. [Google Scholar] [CrossRef]
- Williams, D.F. Titanium as a metal for implantation. J. Med. Eng. Technol. 1977, 1, 202. [Google Scholar] [CrossRef]
- Langdon, T.G. Twenty-five years of ultrafine-grained materials: Achieving exceptional properties through grain refinement. Acta Mater. 2013, 61, 7035–7059. [Google Scholar] [CrossRef]
- Gebril, M.A.; Omar, M.Z.; Mohamed, I.F.; Othman, N.K.; Aziz, A.M.; Irfan, O.M. The Microstructural Refinement of the A356 Alloy Using Semi-Solid and Severe Plastic-Deformation Processing. Metals 2023, 13, 1843. [Google Scholar] [CrossRef]
- Shin, D.H.; Kim, W.J.; Choo, W.Y. Grain refinement of a commercial 0.15% C steel by equal-channel angular pressing. Scr. Mater. 1999, 41, 259–262. [Google Scholar] [CrossRef]
- Sheremetyev, V.; Derkach, M.; Churakova, A.; Komissarov, A.; Gunderov, D.; Raab, G.; Cheverikin, V.; Prokoshkin, S.; Brailovski, V. Microstructure, mechanical and superelastic properties of Ti-Zr-Nb alloy for biomedical application subjected to equal channel angular pressing and annealing. Metals 2022, 12, 1672. [Google Scholar] [CrossRef]
- Baysal, E.; Koçar, O.; Kocaman, E.; Köklü, U. An overview of deformation path shapes on equal channel angular pressing. Metals 2022, 12, 1800. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Estrin, Y.; Horita, Z.; Langdon, T.G.; Zehetbauer, M.J.; Zhu, Y. Producing bulk ultrafine-grained materials by severe plastic deformation: Ten years later. JOM 2016, 68, 1216–1226. [Google Scholar] [CrossRef]
- Roodposhti, P.S.; Farahbakhsh, N.; Sarkar, A.; Murty, K.L. Microstructural approach to equal channel angular processing of commercially pure titanium—A review. Trans. Nonferr. Met. Soc. China 2015, 25, 1353–1366. [Google Scholar] [CrossRef]
- Radnia, A.; Ketabchi, M.; He, A.; Li, D. Effects of ECAP and subsequent recovery on microstructure, mechanical, tribological and corrosion properties of Ti-6Al-4V alloy. J. Mater. Res. Technol. 2025, 35, 4534–4542. [Google Scholar] [CrossRef]
- Wu, Y.; Dong, H.; Huang, H.; Yuan, T.; Bai, J.; Jiang, J.; Fang, F.; Ma, A. Enhanced Strengthening and Toughening of T6-Treated 7046 Aluminum Alloy through Severe Plastic Deformation. Metals 2024, 14, 10939. [Google Scholar] [CrossRef]
- Xu, D.M.; Li, G.Q.; Wan, X.L.; Xiong, R.L.; Xu, G.; Wu, K.M.; Somani, M.C.; Misra, R.D. Deformation behavior of high yield strength–high ductility ultrafine-grained 316LN austenitic stainless steel. Mater. Sci. Eng. A 2017, 688, 407–415. [Google Scholar] [CrossRef]
- Hajizadeh, K.; Kurzydlowski, K.J. The Effect ECAP Processing Routes on the Microstructure and Mechanical Properties of AISI 316 Austenitic Stainless Steel. Phys. Met. Metallogr. 2024, 125, 1601–1608. [Google Scholar] [CrossRef]
- Vacchi, G.D.; Magalhães, D.C.; Kugelmeier, C.L.; Silva, R.D.; Mendes Filho, A.D.; Kliauga, A.M.; Rovere, C.A. Influence of Long-Term Immersion Tests on the Electrochemical Corrosion Behavior of an Ultrafine-Grained Aluminum Alloy. Metals 2024, 14, 1417. [Google Scholar] [CrossRef]
- Awang Sh’ri, D.N.; Zahari, Z.S.; Yamamoto, A. Effect of ECAP Die Angle on Mechanical Properties and Biocompatibility of SS316L. Metals 2021, 11, 1513. [Google Scholar] [CrossRef]
- Fu, X.; Ji, Y.; Cheng, X.; Dong, C.; Fan, Y.; Li, X. Effect of grain size and its uniformity on corrosion resistance of rolled 316L stainless steel by EBSD and TEM. Mater. Today Commun. 2020, 25, 101429. [Google Scholar] [CrossRef]
- Xin, S.S.; Xu, J.; Lang, F.J.; Li, M.C. Effect of temperature and grain size on the corrosion behavior of 316L stainless steel in seawater. Adv. Mater. Res. 2011, 299, 175–178. [Google Scholar] [CrossRef]
- Mao, X.Y.; Li, D.Y.; Fang, F.; Tan, R.S.; Jiang, J.Q. Does severe plastic deformation (SPD) alone generate a nanocrystalline structure? Phil. Mag. Lett. 2010, 90, 349–360. [Google Scholar] [CrossRef]
- Wang, X.Y.; Li, D.Y. Mechanical, electrochemical and tribological properties of nano-crystalline surface of 304 stainless steel. Wear 2003, 255, 836–845. [Google Scholar] [CrossRef]
- Gao, N.; Wang, C.T.; Wood, R.J.; Langdon, T.G. Tribological properties of ultrafine-grained materials processed by severe plastic deformation. J. Mater. Sci. 2012, 47, 4779–4797. [Google Scholar] [CrossRef]
- Stolyarov, V.V.; Shuster, L.S.; Migranov, M.S.; Valiev, R.Z.; Zhu, Y.T. Reduction of friction coefficient of ultrafine-grained CP titanium. Mater. Sci. Eng. A 2004, 371, 313–317. [Google Scholar] [CrossRef]
- Cheng, X.; Li, Z.; Xiang, G. Dry sliding wear behavior of TiNi alloy processed by equal channel angular extrusion. Mater. Des. 2007, 28, 2218–2223. [Google Scholar] [CrossRef]
- Kim, Y.S.; Seok Yu, H.; Hyuk Shin, D. Low sliding-wear resistance of ultrafine-grained Al alloys and steel having undergone severe plastic deformation. Int. J. Mater. Res. 2009, 100, 871–874. [Google Scholar] [CrossRef]
- Nagaraj, M.; Kumar, D.R.; Suresh, K.S.; Neelakantan, S. Effect of equal channel angular pressing on the microstructure and tribocorrosion characteristics of 316L stainless steel. Vacuum 2023, 210, 111908. [Google Scholar] [CrossRef]
- Herliansyah, M.K.; Dewo, P.; Soesatyo, M.H.; Siswomihardjo, W. The effect of annealing temperature on the physical and mechanical properties of stainless steel 316L for stent application. In Proceedings of the 2015 4th International Conference on Instrumentation, Communications, Information Technology, and Biomedical Engineering (ICICI-BME), Bandung, Indonesia, 2–3 November 2015; pp. 22–26. [Google Scholar]
- ASTM E112-13; Standard Test Methods for Determining Average Grain Size. ASTM: West Conshohocken, PA, USA, 2021.
- Gubicza, J. Annealing-induced hardening in ultrafine-grained and nanocrystalline materials. Adv. Eng. Mater. 2020, 22, 1900507. [Google Scholar] [CrossRef]
- Maleki-Ghaleh, H.; Hajizadeh, K.; Aghaie, E.; Alamdari, S.G.; Hosseini, M.G.; Fathi, M.H.; Ozaltin, K.; Kurzydlowski, K.J. Effect of Equal Channel Angular Pressing Process on the Corrosion Behavior of Type 316L Stainless Steel in Ringer’s Solution. Corrosion 2015, 71, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y. Effects of ECAP and Different Aging Processes on the Corrosion Resistance of Maraging Stainless Steel. Interna-Tional. Core J. Eng. 2025, 11, 187–195. [Google Scholar]
- Marulanda Cardona, D.M.; Castillejo Nieto, F.E. An Overview on the Corrosion Behavior of Steels Processed by Severe Plastic Deformation. Mater. Trans. 2023, 64, 1317–1324. [Google Scholar] [CrossRef]
- Tang, Y.; Li, D.Y. Nano-tribological behavior of high-entropy alloys CrMnFeCoNi and CrFeCoNi under different conditions: A molecular dynamics study. Wear 2021, 476, 203583. [Google Scholar] [CrossRef]
- He, H.-B.; Han, W.-Q.; Li, H.-Y.; Li, D.-Y.; Yang, J.; Gu, T.; Deng, T. Effect of deep cryogenic treatment on machinability and wear mechanism of TiAlN coated tools during dry turning. Int. J. Precis. Eng. Manuf. 2014, 15, 655–660. [Google Scholar] [CrossRef]
- Akonko, S.; Li, D.Y.; Ziomek-Moroz, M. Effects of cathodic protection on corrosive wear of 304 stainless steel. Tribol. Lett. 2005, 18, 405–410. [Google Scholar] [CrossRef]
- Hutchings, I.; Shipway, P. Tribology: Friction and Wear of Engineering Materials; Butterworth-Heinemann: Oxford, UK, 2017. [Google Scholar]
- Budinski, K.G.; Steven, T.; Budinski, T. Properties and Selection for Friction, Wear, and Erosion Applications; ASM International: Materials Park, OH, USA, 2021. [Google Scholar]
- Li, D.Y. Development of novel wear-resistant materials: TiNi-based pseudoelastic tribomaterials. Mater. Des. 2000, 21, 551–555. [Google Scholar] [CrossRef]
- Bunge, H.J. Texture Analysis in Materials Science: Mathematical Methods; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Li, D.Y.; Szpunar, J.A. A possible role for surface packing density in the formation of {111} texture in solidified FCC metals. J. Mater. Sci. Lett. 1994, 13, 1521–1523. [Google Scholar] [CrossRef]
- Ye, H.Z.; Liu, R.; Li, D.Y.; Eadie, R. Development of a new wear-resistant material: TiC/TiNi composite. Scr. Mater. 1999, 41, 1039–1045. [Google Scholar] [CrossRef]
- Ocelík, V.; Janssen, N.; Smith, S.N.; De Hosson, J.T. Additive manufacturing of high-entropy alloys by laser processing. JOM 2016, 68, 1810–1818. [Google Scholar] [CrossRef]
- Ron, T.; Shirizly, A.; Aghion, E. Additive manufacturing technologies of high entropy alloys (HEA): Review and prospects. Materials 2023, 16, 2454. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Y.; Elalem, K.; Anderson, M.J.; Chiovelli, S. A microscale dynamical model for wear simulation. Wear 1999, 225, 380–386. [Google Scholar] [CrossRef]
- Faghihi, S.; Li, D.; Szpunar, J.A. Tribocorrosion behaviour of nanostructured titanium substrates processed by high-pressuretorsion. Nanotechnology 2010, 21, 485703. [Google Scholar] [CrossRef]
- He, Z.F.; Jia, N.; Wang, H.W.; Liu, Y.J.; Li, D.Y.; Shen, Y.F. The effect of strain rate on mechanical properties and microstructure of a metastable FeMnCoCr high entropy alloy. Mater. Sci. Eng. A 2020, 776, 138982. [Google Scholar] [CrossRef]
- Liu, M.; Chen, J.; Lin, Y.; Xue, Z.; Roven, H.J.; Skaret, P.C. Microstructure, mechanical properties and wear resistance of an Al–Mg–Si alloy produced by equal channel angular pressing. Prog. Nat. Sci. Mater. Int. 2020, 30, 485–493. [Google Scholar] [CrossRef]
- Wang, C.T.; Gao, N.; Wood, R.J.; Langdon, T.G. Wear behavior of an aluminum alloy processed by equal-channel angular pressing. J. Mater. Sci. 2011, 46, 123–130. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).