Abstract
Dilatometry was used to simulate and analyze martensite formation in the grain-coarsened heat-affected zone (GCHAZ) of P91 steel for high inter-pass temperatures during multi-pass welding. The inter-pass temperature of 360 °C was within the dual-phase temperature range (~400 °C to 240 °C), but because of the unexpected formation of isothermal martensite, the microstructure at the inter-pass temperature was substantially martensitic and similar in microstructure and hardness to those obtained using lower, conventional inter-pass temperatures (about 250 °C). The results for martensite formation indicate that kinetic classifications for transformation in carbon and alloyed steels should take into account the overlapping effects of the diffusionless transformation and thermally activated processes associated with dislocation motion and the diffusion of interstitial elements. Furthermore, the MS temperature was found to be highly sensitive to the microstructural state of the austenite and the availability of nucleating sites for martensite formation. The data for the kinetics of martensite formation were inconsistent with the widely used Koistinen and Marburger (KM) equation for predicting the volume fraction of martensite as a function of quench temperature. It is concluded that the KM equation has limited applicability
1. Introduction
Steels classified as P91 has high hardenability and form lath martensite even for cooling rates as low as 0.7 °C/s [1]. This characteristic requires carefully controlled welding procedures to limit the hardness of the martensitic structure produced in the heat-affected zone (HAZ) and to prevent hydrogen-assisted cold cracking (HACC). Welding procedures involve the control of weld heat input, the preheat temperature, the inter-pass temperature for multi-pass welding, and subsequent post-weld heat treatment to ”normalize” the microstructure and mechanical properties of the weldment. These procedures facilitate the effusion of hydrogen from the weldment region and can also reduce the possibility of hot cracking, which can be promoted by the presence of Si and Nb [2]. The standards for the multi-pass welding of P91 steel, used for the construction of pressure vessel piping, recommend a minimum inter-pass hold temperature that depends on steel composition and welding conditions [2,3]. P91 typically exhibits a martensite transformation range of about 400 °C to 240 °C, and, at the commonly recommended inter-pass temperature of 250 °C, the alloy is expected to be close to fully martensitic. The current investigation concentrated on the effect of higher inter-pass temperatures within the dual-phase region on the microstructure and hardness of the grain-coarsened heat-affected zone (GCHAZ) after cooling to room temperature.
The kinetics of martensite formation are normally classified as one of three types: athermal, isothermal, and burst transformation [4], and it is generally assumed that athermal kinetics apply to steel such as P91. For a high inter-pass temperature within the martensitic transformation range, a mixture of austenite and martensite would be present. Isothermal holding would be expected to promote the partitioning of C from martensite to austenite and the precipitation of the carbonitrides in the austenite, thereby reducing the hardness of the martensitic weld zone present after cooling to room temperature.
In the current work, simulations of weld cooling profiles within the critical GCHAZ were conducted via dilatometry. As well as providing information relevant to practical aspects of welding procedures, the experimentation allowed the examination of the kinetics of martensite transformation and the consistency of the data with the widely used predictive equation of Koistinen and Marburger (KM) [5] for the volume fraction of martensite formed athermally as a function of the isothermal arrest temperature.
The present investigation followed previous work [6,7] on the same steel that was designed to determine the creep performance of the various sub-zones of the HAZ. Actual multi-pass girth welds were carried out on 50 mm thick pipe steel with an OD of 358 mm using a heat input of 1.6 kJ/mm with a preheat and inter-pass temperature of 250 °C. The root pass weld was made by gas tungsten arc welding (GTAW), and 1.2 mm flux cored arc welding (FCAW) was used for the 30 passes required to fill the vee-shaped weld preparation. Details of the weld procedure are given in ref. [7]. Embedded thermocouples established a cooling time in the HAZ of 20 s from 800 to 500 °C (t8–5), equivalent to a cooling rate of 15 °Cs−1. Weld thermal cycle simulation was performed using dilatometry by heating samples to 1400 °C and cooling at 15 °Cs−1 to 250 °C [7]. The Gleeble technique was also used for simulations on samples large enough for subsequent creep testing [6,7]. The thermal profiles consisted of cooling to 250 °C from peak temperatures of 1367 °C (representing the grain-coarsened HAZ), 1008 °C (grain-refined HAZ) and 868 °C (inter-critical HAZ). The hardness in the critical GCHAZ was about 430 HV0.5, and, after simulating PWHT at 760 °C for 2.5 h, the hardness decreased to 260 HV0.5, approaching the base-plate hardness of 224 HV10, which resulted from the manufacturing treatment of normalizing at 1050 °C and tempering [6,7].
The creep testing performed by Sulaiman [7] on structures that simulated the various sub-zones of actual P91 welds confirmed that the least creep-resistant microstructure is that of the grain-refined heat-affected zone, which is typically the initiation site of Type 4 cracking in welded pressure vessel components [6,7]. The simulations of sub-zone microstructures by both Gleeble and dilatometric techniques also corresponded in terms of microstructure and hardness to those of the corresponding regions of actual welds. These and other experiments confirm the validity of using weld thermal cycle simulations to study the fine structures of actual welds.
Precipitate formation in simulated GCHAZ was examined by Sulaiman [7] using transmission electron microscopy of thin foils and extraction replicas.
The weld thermal cycle simulation using dilatometry involved heating samples to 1400 °C and cooling at 15 °Cs−1 to 250 °C. The same cooling profile was used in the current dilatometric work, except that the time and temperature of the inter-pass hold were varied to examine the effect on the microstructure and the hardness of the GCHAZ. Higher inter-pass temperatures were used with the aim of exploring the gap in knowledge about the effect of holding in the two-phase (austenite plus martensite) condition and the possibility of favorable changes in the microstructure and hardness of the GCHAZ.
The preheat and inter-pass temperature used in the actual welding process was 250 °C, considerably lower than the range of 320–390 °C examined in the current work. The potential advantage of using a higher inter-pass temperature is minimizing the risk of weld cracking by allowing stress relaxation and the more effective effusion of hydrogen from the weld zone, as well as reducing the hardness of the GCHAZ. Such a “pre-tempering” procedure could also lead to a reduction in the temperature and time required for post-weld heat treatment.
2. Materials and Methods
The composition, in weight percent, of the P91 alloy under investigation was 0.09C, 0.4Mn, 0.28Si, 8.5Cr, 0.15Ni, 0.89Mo, 0.16Cu, 0.052N, 0.01S, 0.02P, 0.003O, <0.065Al, <0.01Ti, and <0.005B (wt.%). The as-received pipe material was in the normalized and tempered condition, with a hardness of about 224 HV, and the microstructure consisted of ferrite grains containing relatively coarse carbonitrides, mainly chromium-rich. Welding produced rapid re-austenization and dissolution of the carbonitrides, and weld thermal cycle simulations produced the same microstructure at high temperatures. The low carbon content and high alloy content of P91 ensure a high hardenability for formation of lath martensite upon cooling under heat treatment conditions relevant to welding.
Dilatometer specimens were machined from as-received P91 steel. A high-quench rate dilatometer was used to monitor martensitic transformation. This instrument operated under vacuum, except that pure helium gas could be introduced to control the cooling rate. The dilatometer specimens were hollow cylinders, 10 mm long, with external and internal diameters of 5 mm and 3.5 mm and were heated by induction. As the heat treatments were designed to mimic the thermal cycle in the GCHAZ produced by welding, the peak temperature was set at 1400 °C for 1 s, and cooling was controlled using helium gas at 15 °Cs−1 (to obtain a Δt8–5 value of 20 s).
Three heat treatment profiles were examined:
- Heating to 1400 °C at a rate of 50 °Cs−1, holding for 1 s, then natural cooling to 1100 °C before controlled cooling at 15 °Cs−1 to ambient temperature.
- The same heating and cooling conditions as in Treatment 1, except for holding at 360 °C for 1, 2, 4, 8, 16 and 32 min, before re-cooling at 1 °Cs−1.
- The same heating and cooling conditions as in Treatment 1, except for holding for 4 min at temperatures of 320, 330, 340, 350, 360, 370, and 380 °C, before re-cooling at 1 °Cs−1.
A reference dilatometric treatment using pure nickel was also carried out, which corresponded to Treatment 3, using a hold of 4 min at 340 °C.
3. Results
3.1. Martensitic Transformation on Continuous Cooling
Treatment 1 involved the complete transformation to martensite through continuous cooling at a rate typical of the thermal cycle experienced by the GCHAZ, without any inter-pass hold. The effect of time on temperature and dilatation is shown in Figure 1a, and the dilatometric curve typical of this thermal cycle is presented in Figure 1b. The average hardness of the simulated GCHAZ after this treatment was 438 HV5.
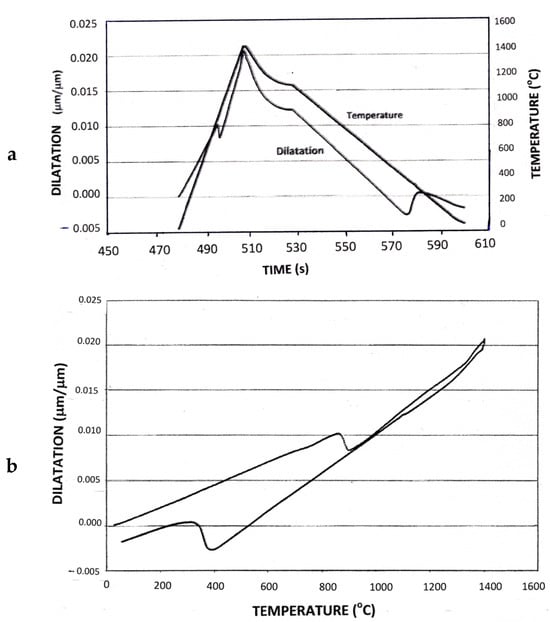
Figure 1.
Dilatometric data for Treatment 1, showing (a) the change in temperature and dilatation with time and (b) change in dilatation as a function of temperature.
Figure 1a records the dilatometric response for Treatment 1, showing the contraction due to austenite formation upon heating at 50 °C s−1 and the expansion upon cooling at 15 °Cs−1 due to martensitic transformation. The total strain produced by the transformation of austenite to martensite in Treatment 1 was estimated using Figure 1b. The ΔL/L value at the estimated MF temperature of 245 °C was compared with the ΔL/L value of a hypothetical structure of 100% austenite at the same temperature by extrapolating the contraction curve for austenite. The estimated volume increase at 245 °C is 1.85%.
The evolution of the volume fraction of martensite (fM) during continuous cooling has been the subject of numerous investigations [5,8,9,10], with many providing support for the KM equation [5]. This equation predicts the volume fraction of martensite (fM) formed at the isothermal arrest temperature, TQ, in iron–carbon alloys that display athermal transformation:
where MS is the transformation start temperature, and α is a constant, with a value of −0.011 for Fe-C alloys with C contents varying in the range of 0.37–1.1% [5]. This equation implies a linear relationship between ln(1 − fM) and (MS − TQ), with a gradient of −0.011. However, variations in α from −0.008 to −0.013 have been reported for other steels [9,10], and the significance of this “constant” remains unclear. More recent analyses of the KM equation [11,12,13] have proposed modifications to better fit the predictions for measured volume fractions of martensite formed during the continuous cooling of a range of plain carbon and alloy steels.
1 − fM = exp[α(MS − TQ)],
In the present dilatometric study of P91 alloy, close-to-linear contraction curves were exhibited before and after transformation (Figure 1b), and, if the volume fraction of martensite present at a given temperature is assumed to be proportional to the sample length change (and hence the volume change), then the volume fraction of martensite is given by x/(x + y), (see Figure 2).
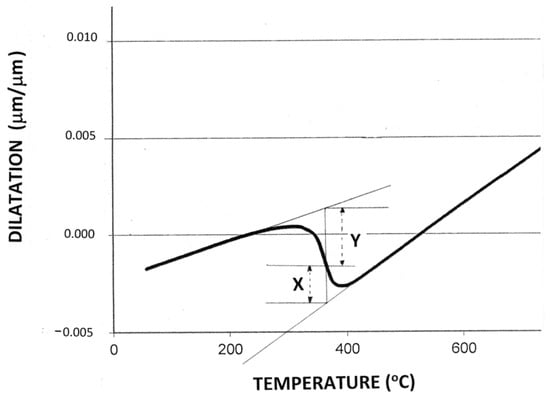
Figure 2.
Dilatation associated with martensitic transformation during continuous cooling of a P91 sample at 15 °C s−1 and the method used to estimate the volume fraction of martensite present at a selected temperature (see text).
Detection of the transformation start temperature can be difficult because the initial increase in length accompanying a continuous decrease in thermal contraction can be sharp or gradual, even for samples of the same alloy. This phenomenon is examined in detail later in this paper. It should be noted that the transformation finish temperature MF can also be difficult to identify precisely as the cooling curve gradually approaches the stage of linear contraction due to the presence of a complete, or an essentially complete, martensitic structure.
The volume fraction of martensite as a function of undercooling below MS can be extracted from the dilatation curve in Figure 1b. The rate of formation of martensite is initially low but rapidly increases to a stage exhibiting transformation that is close to linear with temperature, before trailing off as transformation nears completion. This characteristic sigmoidal form of transformation (see Figure 3a) is not well described by the empirical KM model [5], which, based on Equation (1), implies a transformation curve of the form shown in Figure 3b.
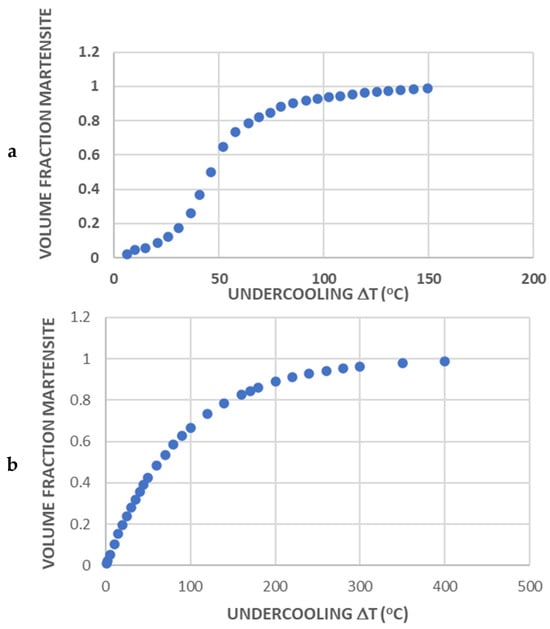
Figure 3.
Evolution of the volume fraction of martensite formed upon continuous cooling of a P91 sample (a) estimated from the dilatation curve shown in Figure 1a and (b) as predicted by the KM equation.
The present data are plotted in Figure 4 using the KM relationship. Parts of the curve in Figure 4 are approximately linear, indicating an exponential relationship; for example, ΔT values between 40 and 200 °C correspond to martensite volume fractions between 0.3 and 1.0. However, the value of the constant α is about −0.033, depending on the choice of data points defining linearity and is significantly different from values quoted for other steels (−0.008 to −0.013 [5,9,10]). These observations are consistent with other reports on the limitations of the KM equation. For example, in a study of low alloy medium carbon steels, Liu et al. [11] reported a “two-stage” transformation process that could not be predicted by the KM analysis. Guimaraes and Rios [13] also reported a two-stage transformation process. These results confirm the conclusion of the present work that the KM equation is not applicable to all steels.
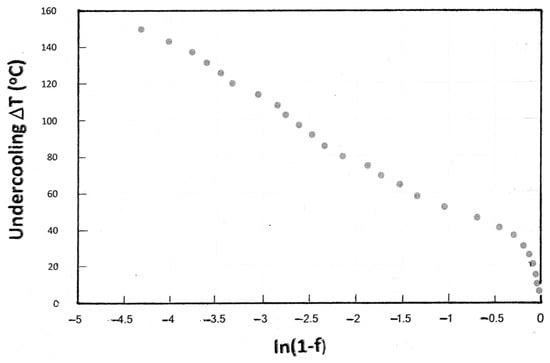
Figure 4.
Plot of ln(1 − f) as a function of undercooling (ΔT or MS − TQ) for the data shown in Figure 3a.
3.2. Variability in MS
The MS temperature recorded for the alloy was variable, despite identical cooling conditions, with an average close to 400 °C. The determination of MS using the data obtained by dilatometry has been discussed in detail by Sourmail and Smanio [14]. The most direct method is identifying the temperature corresponding to the first significant departure from the closely linear thermal contraction curve of austenite during cooling. This is referred to as the “expansion” method because it signals the start of transformation to the martensitic phase (bcc/bct), which has a significantly higher specific volume than the parent austenite (fcc). The identification of MS in this way leads to the problem of significant variability and the frequent observation of a “slow start” phenomenon. Sourmail and Smanio [14] examined the possible origins of this phenomenon and considered the effects of segregation, austenite grain size, and instrumental factors, particularly longitudinal temperature gradients in the specimen. They dismissed the first two factors on the basis that the estimated temperature variations are smaller than those observed. Although they considered the temperature gradient effect as the most likely explanation, two samples of 100CrMnSi6-4 steel heat-treated under identical conditions (their Figure 3 in ref. [14]) showed widely different MS values using the “expansion” method. The expansion method results for MS for 16 samples in the present work showed a variation range of 27 °C and an average value of 404.8 °C with a sample standard deviation of 8.6 °C. The start of transformation was identified as the temperature at which the austenite thermal contraction curve showed a significant departure from the closely linear contraction curve for austenite recorded for temperatures above about 425 °C. The transformation start temperatures higher than the mean were generally consistent with the so-called “slow start” process, whereas MS values lower than the mean were mostly characterized by a sharper increase in dilatation, which indicated a “fast start” of transformation. The recorded values of dilatation as a function of temperature invariably displayed close to a linear relationship for volume fractions of martensite within the range of about 0.2 to 0.6. Extrapolation of this linear segment to the austenite contraction curve was performed to estimate a technical MS that is more likely to reflect the transformation start within the bulk material, which is subject to three-dimensional constraints. The average start temperature was 374.8 °C, with a sample standard deviation of 6.2 °C, about 30 °C lower than the start temperature determined by the expansion method. Yang and Bhadeshia [15] proposed a similar, arbitrary method for defining MS based on the temperature corresponding to the formation of 0.01 volume fraction of martensite.
Since the thermal conditions were identical in each of the 16 tests conducted in the present investigation, the observed variation in the MS could not be attributed to instrumental effects. Although chemical segregation was a possible cause of this variation, the seamless pipe was produced by billet piercing, thus eliminating centerline segregation and extensively hot working the material. Even if interdendritic micro-segregation was present, the scale of the compositional banding identified by Jabar et al. [16] in seamless P91 pipe was unlikely to change the average composition of each of the samples taken for dilatometry. The tubular samples used in the current work were machined from quarter-thickness regions of the pipe parallel to the rolling direction. It is concluded, therefore, that chemical segregation in the extensively heat-treated, hot-formed pipe is unlikely to contribute significantly to the observed variation in MS. The results imply an intrinsic source of variation in the initiation of martensitic transformation, which is widely considered to involve crystalline defect sites in the parent austenite. It is logical that the concentration, distribution, and potency of nucleation sites determine the start temperature. Once plates are initiated, auto-catalysis becomes an important contributor to the rate of transformation. One factor that should be considered is surface nucleation, as the free surface reduces the level of constraint opposing the shear and volume strains accompanying the transformation. This factor could have been especially important for the hollow cylindrical specimens used in the current dilatometer tests as the surface area to volume ratio is particularly high. The surface area to volume ratio (S/V) for the dilatometer samples is 1.77 mm2/mm3. For comparison, a 10 mm long solid cylinder of 5 mm diameter has an S/V value of 1.00 mm2/mm3. Furthermore, the surface of the quenched sample willcool more quickly than the core, establishing a transient temperature gradient.
Suitable nucleating defects in or close to the surface are clearly the preferred sites for the early nucleation of martensite. Annealing twin boundaries in austenite have been observed to be prominent in martensite plate nucleation at the free surface, possibly due to non-coherent steps or ledges in the twin boundary. Mirror-image wedge-shaped clusters of plates are frequently observed across twin boundaries [9,17,18].
In view of the well-documented tendency for martensite plates to form in self-accommodating clusters to compensate for the shear strain accompanying the transformation, the “mirroring” of plate clusters across twin boundaries also likely assists in shear strain minimization. Therefore, twin boundaries intercepting the free surface could provide favorable sites for “early” transformation. Furthermore, the reduced constraint opposing the transformation is accompanied by lower transformation strains in the surrounding austenite, reducing the tendency for auto-catalysis. These two factors are likely to be influential in the slow start phenomenon exhibited by some samples. In other cases, the lack of suitable nucleating sites at the surface would require more significant undercooling, which serves to activate multiple nucleating sites both at the free surface and within the specimen bulk, leading to more pronounced auto-catalysis and a more well-defined start temperature. Since the nucleation of the martensitic phase is a heterogeneous and variable process, it is expected that the expansion method leads to variable results for the determination of the MS. These considerations lead to the conclusion that the empirical equations proposed to predict the MS based solely on alloy composition can only be approximate. Other influential factors include the austenite grain size and the quench rate. Although it is not clear that the cooling rate affects the MS of carbon steels, it has been demonstrated that the cooling rate determines the extent of dynamic stabilization, with faster rates resulting in less retained austenite at room temperature [19,20].
3.3. Hardness as a Function of Inter-Pass Temperature and Time
Figure 5 shows the hardness variation as a function of time at 360 °C. The 95% confidence limits for the hardness values are close to ± 1 point HV5. For continuous cooling (zero hold time at 360 °C), the hardness was 438 HV, with an increase to a peak value of 455 HV5 after holding for 2 min. Under continuous cooling, the extent of auto-tempering would be expected to be relatively low, enhancing the concentration of the interstitial C and N atoms in solution in both austenite and martensite. Sulaiman [7] reported that for continuously cooled P91 samples, fine plate-shaped M2X and M3X carbonitrides were formed, initially rich in Fe and containing minor amounts of Mn, Nb, and V. This type of precipitation was likely during the 1 min hold at 360 °C in the present work, which resulted in a rise in the average hardness to 442 HV5 (Figure 5). Doubling the hold time to 2 min amplified the extent of precipitation hardening, resulting in a peak hardness of 455 HV5. The subsequent decrease in hardness to about 444 HV5 for a hold time of 8 min probably arose from the coarsening and dissolution of the Fe-rich carbonitrides. For longer hold times of 16 and 32 min, the hardness of about 448 HV5 reflected the formation of more stable alloy carbonitrides rich in Cr and Mo, which produced a minor secondary hardening effect [21]. The hardness values shown in Figure 5 are relatively high, contradicting the premise that higher inter-pass hold temperatures reduce the hardness of the GCHAZ.
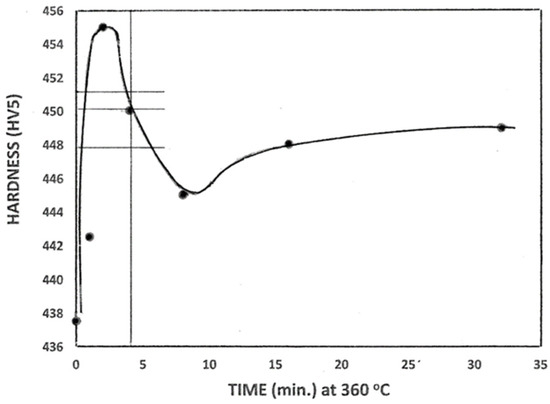
Figure 5.
Hardness of the simulated GCHAZ held at 360 °C for up to 32 min. The intersections of the horizontal lines with the vertical line at 4 min mark the hardness values for hold temperatures of 340, 360, and 380 °C.
Figure 5 also indicates the effect of the variation in the hold temperature from 340 °C to 380 °C for a constant hold time of 4 min on the hardness. At 380 °C, very little martensite formed, and the carbonitride precipitation in the austenite was expected to lower the hardness of the martensite formed on re-cooling after the isothermal arrest (448 HV5). At 340 °C, a substantial amount of martensite was formed before the holding temperature was reached, and the precipitation in the martensite (tempering) was responsible for the higher hardness value of 451 HV5.
3.4. Effect of Isothermal Holding for Various Times at 360 °C
On cooling at 15 °Cs−1, the undershoot of the set control temperature was typically about 0.8 °C, and recovery to the set point occurred within 0.8 s. Nevertheless, a significant increase in sample length followed (Figure 6a,b), and, by inference, there was an increase in the volume fraction of martensite for up to about 10 s of isothermal holding. The validity of this conclusion was confirmed by the dilatometric profile of a pure nickel sample, which showed no increase in sample length after holding at 340 °C for 4 min. Therefore, isothermal transformation of austenite occurred for the P91 steel samples after cooling was interrupted.
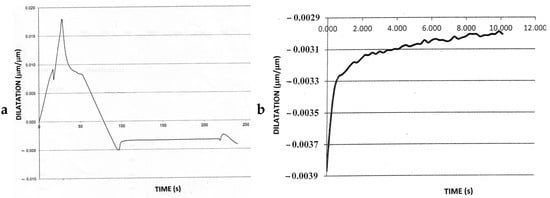
Figure 6.
(a) Dilatometric curve for a sample subjected to Treatment 2 which was held at 360 °C for 2 min before re-cooling. The peak on the right corresponds to transformation of the remnant austenite. (b) Increase in dilatation for the first 10 s at 360 °C for the sample held for 32 min.
Arrest temperatures between 320 °C and 380 °C were within the transformation range, so some martensite already formed before holding (see Figure 1b and Figure 2). The following discussion refers mainly to Treatment 2, which consisted of isothermal holding at 360 °C for durations between 1–32 min. Assuming MS is 400 °C, Figure 3a indicates that for an undercooling of 40 °C (400–360 °C), the approximate volume fraction of martensite present before the isothermal hold is 0.35.
An unexpected dilatation occurred for all hold times (1–32 min) in the form of a significant increase in the sample length within the first 10 s of isothermal holding (Figure 6a). The sample length remained constant thereafter, until re-cooling started. The increase in dilatation on isothermal holding is shown in more detail in Figure 6b, and the length changes due to the formation of both athermal and isothermal martensite are highlighted in Figure 7.
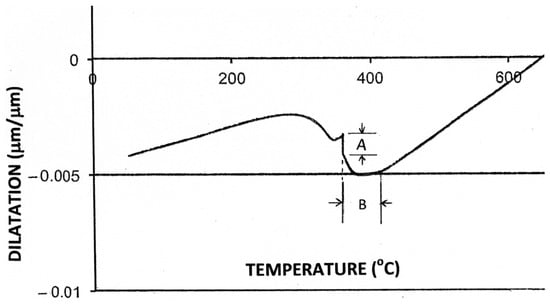
Figure 7.
Dilatation as a function of temperature for a sample held isothermally at 360 °C for a total time of 32 min (Treatment 2). “A” represents the length change due to the formation of isothermal martensite, and “B” marks the temperature range of formation of anisothermal (athermal) martensite prior to holding at 360 °C.
The increase in dilatation can be converted to a volume change and then to a volume fraction of martensite formed by calculating the difference in specific volumes of martensite and austenite at 360 °C using the lattice parameters estimated for austenite and martensite at 360 °C [14]. The difference in specific volume was calculated to be 0.74% and was compared with the volume change implied by the dilatation that occurred upon isothermal holding. The estimated volume fraction of martensite formed for each hold time is recorded in Table 1, together with the corresponding MS temperature.

Table 1.
Percentage isothermal volume change (column 2) and volume fraction of martensite (column 3) after hold times of 1–32 min at 360 °C. Measured MS temperatures are shown (column 4), as well as the estimated total fraction of martensite present at the completion of the isothermal hold (column 5). The static stabilization temperature increase, ΔTss, for restart of transformation on cooling is given in column 6.
The formation of isothermal martensite could be the result of an inertial effect associated with auto-catalysis due to prior martensite formation upon continuous cooling. It is also possible that the rapid interruption of cooling suppresses the process of dynamic stabilization and allows the isothermal formation of martensite until stabilization by interstitial atom locking is re-established. The initial burst of isothermal transformation is however quickly limited by forces opposing transformation, which effectively balance the free energy difference at the arrest temperature. It should be noted that the hold time did not influence the amount of martensite formed isothermally, as the observed increase in volume only occurred within the first 10 s of holding at 360 °C. Furthermore, the volume fraction of isothermal martensite formed during holding at 360 °C (third column of Table 1) did not correlate with the Ms temperature. Therefore, the variations in the volume fraction of martensite formed isothermally must have arisen from other factors, such as variations in the kinetics of the prior athermal transformation before the arrest temperature was reached. The nucleation of martensite is opposed by the intrinsically high shear and volume strains accompanying transformation. Potent nucleation sites need to be activated before transformation can commence and lead to auto-catalysis. In general, the nucleation and growth of martensite plates/laths depend on the structural and compositional characteristics of the parent austenite phase: its grain size, dislocation density, stacking fault energy, annealing twin density and surface area, concentration of vacancies and interstitial defects, concentrations of substitutional and interstitial solute atoms, and amount and size distribution of precipitate species and atomic clusters. Upon continuous cooling, some of these characteristics, including the specific volumes of the austenite and martensite crystal structures, change continuously [22,23]. These factors are also likely more significant in alloyed and microalloyed steels, which contain strong carbide/nitride-forming elements. Therefore, transformation occurs from a dynamically changing parent austenite phase. This situation is likely to apply to P91 alloy steel as this alloy contains C and N atoms and a group of strong carbonitride formers. The solubilities of carbon and nitrogen atoms, atom clustering, and the precipitation of carbonitrides in both austenite and martensite are sensitive to time and temperature during cooling.
At the completion of the hold at 360 °C, the total fraction of martensite present was estimated by adding the amount of martensite formed upon isothermal holding to that formed prior to the hold, resulting from continuous cooling below the MS. The estimated volume fraction of martensite present at the end of the hold at 360 °C is given in column 5 in Table 1 and ranges from 0.74 to 0.90. Therefore, the amount of martensite present after the isothermal hold at 360 °C is significant. The secondary peak in ΔL/L produced following re-cooling from 360 °C accounts for the transformation of at least most of the remnant austenite to martensite. This second peak is only initiated after the cooling of the biphase mixture of austenite and martensite by about 8–20 °C below 360 °C (column 6). It should be noted that Cazziro et al. [24] reported residual austenite in P91 steels at ambient temperature. Therefore, the measured value of MF of about 245 °C in the present work is likely to be approximate. The report of residual austenite was for steel samples cooled at exceptionally low rates (<0.056 °Cs−1), giving rise to the possibility that the rejection of C and N atoms to austenite during martensite formation stabilized the residual austenite and prevented complete transformation. The final stage of transformation in the present investigation occurred for a higher continuous cooling rate of 1 °Cs−1. Even so, the lath shape of martensite crystals is likely to limit space filling, resulting in a small amount of residual austenite.
The unexpected increase in transformation on isothermal holding at 360 °C was also observed for the Treatment 3 samples, which were held for 4 min at temperatures in the range of 380–320 °C. However, the % volume change varied with the hold temperature (Figure 8). At the higher hold temperatures of 370 and 380 °C, a high volume fraction of austenite (0.8–0.9) was present, and the extent of isothermal transformation was low (0.1–0.2). For low temperatures, the fraction of untransformed austenite was low, and again the extent of isothermal transformation was relatively low. It is inferred that for both cases, the forces opposing isothermal transformation are relatively large. For low temperatures in the transformation range, the remnant austenite is likely to be substantially deformed and resistant to isothermal transformation. For higher temperatures, the auto-catalysis of isothermal martensite is likely to be attenuated because of the relatively small volume fraction of athermal martensite, as well as enhanced thermally induced stress relaxation in the surrounding austenite. The intermediate transformation temperature range of 350–360 °C showed the highest % volume changes associated with the formation of isothermal martensite.
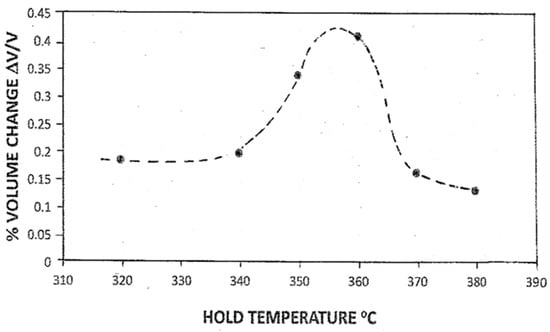
Figure 8.
Percentage volume change associated with formation of isothermal martensite at different hold temperatures (Treatment 3).
The form in Figure 8 is not dissimilar to the data reported by Tsuzuki et al. [25] for isothermal transformation in an Fe-15%Ni alloy, following the prior formation of a 0.05–0.8 volume fraction of martensite upon continuous cooling below the MS temperature of 299 °C. Although the formation of isothermal martensite is typically observed for high-alloy C-free steels with low MS temperatures, it has been reported in C-bearing alloys with elevated MS temperatures, as for the present case for P91 steel (see Table 5.5 in ref. [26]). Furthermore, these results and the current data question the possible undetected presence of isothermal martensite formation in the Fe-C alloys examined by Koistinen and Marburger [5] in their analysis of the volume fraction of athermal martensite formed as a function of undercooling below the MS. Measurements of the volume fraction of martensite or austenite using metallographic, X-ray, and magnetic methods at room temperature after quenching from the hold temperature would not distinguish martensite that formed athermally or isothermally.
3.5. Static Stabilization
Static thermal stabilization was observed during re-cooling after an isothermal hold. A temperature decrease (∆T) was required before martensite formation restarted. Honeycombe and Bhadeshia [21] proposed that stabilization can be induced by any process that temporarily retards the motion of “glissile” interfacial dislocations after re-cooling. Interstitial atoms have been proposed to contribute to stabilization by segregation to austenite/martensite interfaces and diffusion to dislocation substructures in the remnant austenite, reducing their potency as potential nucleation sites [21,27,28].
Figure 9a, based on the data in column 6 in Table 1, indicates that stabilization is more marked for short hold times at a temperature of 360 °C. A possible explanation is that interstitially dissolved carbon and nitrogen atoms are effective in stabilizing the microstructure for short hold times but that subsequent alloy carbonitride formation on isothermal holding ties up carbon and nitrogen, reducing its effectiveness in locking potential nuclei and existing martensite interfaces.
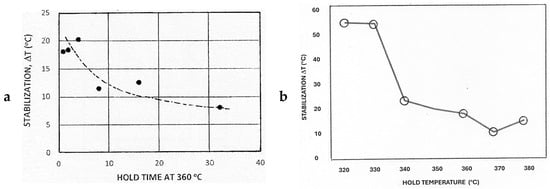
Figure 9.
Effect on static stabilization for (a) various hold times at 360 °C and (b) hold temperature in the range of 320–380 °C for a hold time of 4 min.
The current data are consistent with the trend described by Honeycombe and Bhadeshia [21] of static stabilization increasing as MF is approached (Figure 9b). This observation can be rationalized by an increased resistance to transformation imposed by the enhanced deformation of the remnant austenite when the alloy is substantially transformed to martensite. Overall, the experimental results indicate that static stabilization can result from different forces opposing transformation, depending on the heat treatment time and temperature of an arrest within the MS − MF range.
4. Discussion
The current investigation draws attention to the customary distinctions between the types of kinetics attributed to martensitic transformations: athermal, isothermal, and burst [4,27]. “Athermal” is an adjective describing a process that does not involve heat or a change in temperature. In relation to an athermal phase transformation, the amount of a product phase depends only on temperature and not on the time at that temperature.
Carbon-bearing hardenable steels have long been considered to form via athermal martensitic transformation [5,9]. However, the results of the present investigation indicate that this viewpoint requires some qualification.
Martensitic transformation originates from a free energy difference between the parent austenite phase and the martensitic product phase. However, martensitic transformation causes a major disturbance of the parent austenitic phase, which must accommodate the shear and volume changes imposed by the formation of martensite [29]. The force driving transformation is opposed by forces originating from the surface and volumetric strains produced by martensite formation and possibly from magnetic changes. Although martensitic transformation occurs through the rapid coordinated transfer of atoms from the parent to the product phase, rather than the diffusive transfer of atoms, the presence of mobile interstitial atoms and/or vacancies can influence the kinetics of the overall transformation process via interfacial locking and/or segregation to and locking of potential nuclei. Clearly, the movements of these point defects are thermally activated.
Magee presented an excellent detailed analysis of the nucleation of martensite in 1969 [9], from which he concluded that “martensite formation kinetics in iron alloys are either dynamically stabilized or isothermal”. For C- and N-bearing steels, dynamic stabilization was considered a necessary condition for athermal transformation.
However, the present results show that both dynamically stabilized (athermal) transformation and isothermal transformation can occur in the same alloy. Magee also stressed the importance of auto-catalysis and the prominence of annealing twin boundaries as nucleation sites in many ferrous alloys. These conclusions are relevant to the variation in the MS temperatures observed in the current work.
Apart from the availability of suitable nucleation sites, transformation temperatures can vary depending on the prior austenite grain size and the cooling rate through the transformation range. There is abundant evidence that MS decreases with decreasing austenite grain size. De Souza et al. [30] demonstrated that a decrease in MS occurs with decreasing austenite grain size for a microalloyed steel. Higher alloy steels with sub-zero transformation characteristics also show the same trend [31]. Yang and Bhadeshia [32] reported the effect of austenite grain size on MS for an alloy consisting of 0.125 wt% C, 5.02 wt% Ni, and 2.27 wt% Mn and developed a quantitative model for predicting MS as a function of the prior austenite grain size. Although it is commonly considered that the decrease in MS is related to the austenite strength, which increases through the Hall–Petch effect, Yang and Bhadeshia offered an alternative hypothesis based on the partitioning model of Fisher et al. [33] to predict the MS. It should be recognized, however, that the partitioning model is based on observations of plate martensite in high-alloy steels with low MS temperatures. Even so, Guimaraes and Rios [34] revisited the partitioning model to take auto-catalysis into account and applied their modified equation to the prediction of the volume fraction of martensite formed in a series of high-Ni, low-C alloys and Fe-1.86%C, all of which transformed at low temperatures to plate martensite. The martensite plates formed in these alloys typically have habit planes near {259}F or {3 10 15}F, (where the subscript F refers to fcc austenite). This crystallographic identity does not allow the formation of self-accommodating plate groups. Instead, the morphology is frequently described as consisting of zig-zag groupings of plates [35]. It should be stressed that the measured and predicted habit planes of martensite plates/laths are irrational except for the degenerate case of epsilon martensite in high-alloy austenite with low stacking fault energy, for which the habit plane is exactly {111}F.
The shear strains accompanying martensitic transformation can be mitigated by the co-operative formation of “self-accommodating” plate groups [35], but the dilatational (volumetric) strains cannot be accommodated in this way and require elastic and/or plastic strain in the austenite [17,22,29].
Self-accommodation is frequently observed for many transformations with habit planes of near two-fold {hhk}F symmetry in the austenite, with characteristic four-plate clusters being formed [17,35,36].
The martensite formed in steels with carbon contents in the range of 0.5–1.4% exhibit habit planes close to {225}F. For lower-C (<0.5% C) and some low-carbon, high-alloy steels, the martensite forms in nearly parallel arrays or “lath packets”, with the habit plane being described as {557}F [35], which is close to both two-fold and three-fold symmetry. The observation that habit planes in many iron-based alloys are predominantly planes of nearly two-fold symmetry has important ramifications for the progress of the transformation because it means that plate/lath formation can be coordinated to maximize self-accommodation. As the temperature falls and the driving force for transformation increases, the selection of nuclei is influenced by the strain fields around pre-existing plates, leading to the coordinated development of the macrostructure of the martensitic phase. Just as the detailed atomic movements in the transformation of austenite to martensite are highly coordinated (“military” [27]), coordinated transformation is dominant in alloys with habit planes of the form {hhk}F, as opposed to martensite plates with habit planes of the type {hkl}F, which are characteristic of high-carbon and/or high-alloy steels [35].
As discussed above, Yang and Bhadeshia [32] proposed that the observed decrease in MS with decreasing prior austenite grain size for the C-Ni-Mn alloy that they investigated could be rationalized using Fisher et al.’s [33] partitioning model. However, their alloy was expected to transform to lath martensite—the dominant morphology of most commercial, low- to medium-carbon, hardenable steels. Therefore, the effect of partitioning (or formation of intragrain “pockets” of austenite) on the development of the martensitic structure is likely to be less significant for lath martensites because of their capacity to form self-accommodating groups.
Nevertheless, auto-catalysis is likely to be significant at least to some extent for all martensitic transformations, a generalization that is embedded in Raghavan’s statement [4] that “…. plates do not form randomly in pockets. There is a certain degree of co-operation between plates which tends to increase the efficiency of filling”.
Most of the studies on plate nucleation and growth have been conducted using alloys that undergo isothermal transformation at sub-zero temperatures [4]. Therefore, microstructural evolution has been mapped as a function of time at a constant temperature (equivalently, at a constant free difference between austenite and martensite). However, it is less easy to study transformation under continuous cooling conditions for alloys with elevated transformation temperature ranges, because the chemical driving force increases with time, promoting rapid rates of nucleation and growth of martensite units, and the enhanced possibility of interaction with interstitial atoms, vacancies, and other defects.
Although there is sound evidence that the austenite grain size affects the MS, the effect of the cooling rate on the MS is less well understood. It has been known for many years that the amount of austenite retained in quenched hardenable steels is greater for oil quenching than water quenching, indicating that the cooling rate affects the transformation range [20,37]. In low-alloy hardenable steels, the dynamic stabilization accompanying transformation retards martensite formation, with the effect being more marked at lower cooling rates, leading to a higher volume fraction of retained austenite at room temperature. These trends do not necessarily provide insight into the effect of the cooling rate on the MS. The transformation start temperature is controlled by the nucleation rate, which in turn depends on the condition and characteristics of the austenite, as discussed in Section 3.4.
Recent experimental work on modified P91 steel (9Cr-1.7W-0.4Mo-Co) indicated that MS decreased from 420 °C to 390 °C with increasing cooling rate [1]. In this case, the cooling rates were low (0.167 °C/s to 0.667 °C/s), and changes in the alloy precipitate characteristics in austenite in advance of martensitic transformation are likely to modify the transformation start temperature.
A similar result of the effect of the cooling rate was reported by De Souza et al. [30] for a microalloyed steel (0.1C-0.9Mn-0.7Cr-0.5Mo-0.02Ti-0.04V), using much higher cooling rates (25–100 °C/s). The MS decreased from 520 to 450 °C with increasing cooling rate. They concluded that the cooling rate has an “extrinsic” effect on the MS by affecting the carbide precipitation in austenite prior to and during transformation.
Schastlivtsev et al. [38] reported multi-stage shear-based transformations for a series of Fe-C and Fe-Cr-C alloys that are relevant to the effect of the cooling rate on the MS. Transformation plateaus were recorded for martensite formation, which indicated no change in MS occurred for cooling rates from about 103 K/s up to 105 K/s after austenitizing at 1000 °C in vacuo for 100 s. The data were obtained from the “points of inflection” of the cooling curves produced via the stream quenching of small volumes of 0.15 mm thick samples using argon, alcohol, or distilled water. These results, although frequently quoted in the literature (e.g., [26]), have not been independently confirmed, and it remains uncertain how the cooling curves for such high quench rates can be interpreted to yield definitive MS data, particularly in the likely event of intense recalescence accompanying the transformation that affects the cooling curve profile [39].
Although the austenite grain size and the quench rate were constant in the present case, significant variation occurred in the MS temperature, implying that other factors are influential in initiating martensitic transformation. Clearly, the microstructural state of the austenite and the related availability of viable nucleating sites determine the temperature of the initiation of martensitic transformation. The lath martensite morphology that is characteristic of P91 alloy ensures that auto-catalysis and coordinated transformation play major roles in the rate of transformation to a level that is detectable with dilatometry. There is also likely an effect due to the specimen size and shape, which, in the present case, enhance the surface nucleation of defects such as twin boundaries intercepting the free surface.
P91 alloy steel is designed for high-temperature use, particularly for the fabrication of power generation equipment. The thermal stability and creep resistance are established for this alloy by the presence of 8.5 wt% Cr and 0.89 wt% Mo. The relatively small concentrations of C (0.09 wt%) and N (0.052 wt%) are designed to form creep-resisting alloy carbonitrides, as well as facilitating weld fabrication. The significance of C for welding processes is exemplified by the formulation of carbon-equivalent formulae that are, in effect, estimators of alloy hardenability, which are used to provide weld process guidelines for preheat, inter-pass and post-weld heat treatment temperatures designed to reduce the hardness of the GCHAZ and the possibility of hydrogen-assisted cold cracking [40,41]. These formulae reflect the major influence of carbon on the formation of martensite in rapidly cooled steel [21,26,27]. Carbon strongly depresses the MS temperature and results in a morphological transition from lath martensite (C < 0.5 wt%) to plate martensite (C > 0.8 wt%). For P91, the low C content results in the formation of lath martensite even for relatively slow cooling rates because the hardenability is enhanced by the high alloy content. The C content per se does not influence whether lath martensite forms athermally or isothermally. The different kinetics of transformation are determined by the balance between the forward driving force and the opposing forces, and this balance is temporarily disturbed by an abrupt cessation in cooling.
5. Conclusions
- The current work involved weld thermal cycle simulation of the GCHAZ of P91 steel using relatively high inter-pass temperatures, nominally within the dual-phase temperature range. However, it was found that because of the unexpected formation of a significant volume fraction of isothermal martensite, the microstructure present at the hold temperature was predominantly martensitic.
- Although higher inter-pass temperatures would be expected to minimize weld cracking by allowing stress relaxation and more effective effusion of hydrogen, the hardness of the GCHAZ was not reduced because significant age hardening occurred. Therefore, the cost of maintaining a higher inter-pass temperature than that normally recommended makes this process unattractive for practical multi-pass welding of P91.
- This work confirms that both anisothermal (athermal) and isothermal martensite can occur in P91 steel in the grain-coarsened heat-affected zone under the cooling conditions typical of multi-pass welding.
- The kinetic classifications of martensitic transformation in plain carbon and alloyed steels, particularly those that transform at moderately elevated temperatures, should be interpreted in terms of the overlapping effects of the diffusionless transformation and thermally activated processes primarily associated with the diffusion of interstitial elements.
- The Ms temperature is not a fundamental property of commercial steels since it is sensitive to the condition of the austenite and the availability of appropriate nucleating sites for martensite formation. This caveat applies especially to alloy steels for which the microstructural characteristics of the austenite change continuously during cooling, depending on the cooling rate and the development of precipitates in austenite.
- The characteristics of martensite formation and its stabilization in the alloy studied reflect a delicate balance between the force driving transformation due to the free energy difference and the opposing forces due to the induced strain in austenite, coupled with frictional effects due to the thermally activated motion of interstitial atoms and defects.
- Although the KM equation is a useful empirical model for describing the evolution of the volume fraction of martensite during cooling, it is not generally applicable to alloy steels such as P91.
Author Contributions
Conceptualization, D.D. and E.P.; methodology, D.D.; investigation, H.L.; data analysis, H.L. and D.D.; writing—original draft preparation, D.D.; writing—review and editing, E.P. and H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors thank Morgan Williams for the provision of the experimental data obtained in an undergraduate research project at the University of Wollongong. They also acknowledge the contributions of Liang Chen, University of Wollongong; Samsiah Sulaiman through prior research at the university; and H.K.D.H Bhadeshia, University of Cambridge, for his comments on an early draft of this paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gao, Q.; Yang, Y.; Qu, F. Martensitic Transformation in 9Cr-1.7W-0.4Mo-Co Ferritic Steel. J. Alloys Compd. 2014, 610, 322. [Google Scholar]
- Coleman, K.; Newell, W. P91 and Beyond. Weld. J. 2007, 86, 29–33. [Google Scholar]
- ANSI/AWS D10.11; Recommended Practices for Local Heating of Welds in Piping and Tubing. AWS: Miami, FL, USA.
- Raghavan, V. Kinetics of Martensite Transformations. In Martensite; Olsen, G.B., Owen, W.S., Eds.; ASM Int.: Materials Park, OH, USA, 1992; pp. 197–225. [Google Scholar]
- Koistinen, D.P.; Marburger, R.E. A general equation prescribing the extent of austenite-martensite in iron carbon alloys and plain carbon steels. Acta Metall. 1959, 7, 59–60. [Google Scholar]
- Sulaiman, S.; Li, H.; Dunne, D. Creep testing of simulated HAZ structures in P91 steel. In Proceedings of the IIW Conference, Singapore, 16–17 July 2009; pp. 527–532. [Google Scholar]
- Sulaiman, S. Structure and Properties of the Heat Affected Zone of P91 Creep Resisting Steel. Ph.D. Thesis, University of Wollongong, Wollongong, NSW, Australia, 2008. [Google Scholar]
- Harris, W.J.; Cohen, M. Stabilization of the austenite martensite transformation. Trans. AIME 1949, 180, 447–470. [Google Scholar]
- Magee, C.L. The Nucleation of Martensite. In Phase Transformations; ASM Pub.: Metals Park, OH, USA, 1969; pp. 115–156. [Google Scholar]
- Brook, R.; Entwistle, A.R.; Ibrahim, E.F. The effect of chemical composition on the shape of martensite transformation curves. J. Iron Steel Inst. 1960, 195, 292–298. [Google Scholar]
- Liu, J.H.; Binot, N.; Delagnes, D.; Jahazi, M. Influence of the cooling rate below Ms on the martensitic transformation in a low alloy medium carbon steel. J. Mater. Res. Technol. 2021, 12, 234–242. [Google Scholar]
- Huyan, F.; Hedstrom, P.; Borgenstam, A. Modelling the fraction of martensite in low alloy steels. Mater. Today Proc. 2015, 2, S561–S564. [Google Scholar] [CrossRef]
- Guimaraes, J.R.; Rios, P.R. Microstructural Path Analysis of Martensite Dimensions in FeNiC and FeC Alloys. Mater. Res. 2015, 18, 595–601. [Google Scholar]
- Sourmail, T.; Smanio, V. Determination of Ms temperature: Methods, meaning and influence of ‘slow start’ phenomenon. Mater. Sci. Technol. 2013, 29, 883–888. [Google Scholar]
- Yang, T.; Bhadeshia, H.K.D.H. Designing low carbon, low temperature bainite. Mater. Sci. Technol. 2007, 23, 556–560. [Google Scholar]
- Jabar, S.; Siebert, J.; Strangwood, M.; West, G. The Effect of Micro-Segregation on the Microstructural parameters in Grade 91 Steel. Metall. Mater. Trans. A 2020, 52, 426–437. [Google Scholar] [CrossRef]
- Dunne, D.P.; Wayman, C.M. The Effect of Austenite Ordering on the Martensitic Transformation in Fe-Pt Alloys near the Composition Fe3Pt. Metall. Trans. 1973, 4, 137–145. [Google Scholar] [CrossRef]
- Maki, T. Microstructure and Mechanical Behaviour of Ferrous Martensites. Mater. Sci. Forum 1990, 56, 157–168. [Google Scholar] [CrossRef]
- Zmeskal, O.; Cohen, M. The tempering of two high carbon, high chromium steels. Trans. ASM 1943, 1, 350. [Google Scholar]
- Klier, E.P.; Troiano, A.R. Ar in Chromium Steels. Trans. AIME 1945, 162, 175. [Google Scholar]
- Honeycombe, R.W.K.; Bhadeshia, H.K.D.H. Steels—Microstructure and Properties, 2nd ed.; Edward Arnold: London, UK, 1995; pp. 189–191. [Google Scholar]
- Dunne, D.P. Shape Memory in Ferrous Alloys. In Phase Transformations in Steels; Pereloma, E., Edmonds, D.V., Eds.; Woodhead Publishing: Cambridge, UK, 2012; Volume 2, pp. 83–125. [Google Scholar]
- Dunne, D.P. Martens-ite. Metals 2018, 8, 395. [Google Scholar] [CrossRef]
- Caizzo, D.A.; Besoky, J.I.; Luppo, M.; Danon, C.; Ramos, C.P. Characterization of ASTM A335 P91 ferritic-martensitic steel after continuous cooling at moderate rates. J. Mater. Res. Technol. 2019, 8, 923–934. [Google Scholar]
- Tsuzaki, K.; Fukiage, T.; Maki, T.; Tamura, I. Effect of Ni content on the isothermal character of lath martensite transformation in Fe-Ni alloys. Mater. Sci. Forum 1990, 56, 229–234. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Theory of Transformations in Steels, 1st ed.; CRC Press: Boca Raton, FL, USA, 2021; 574p. [Google Scholar]
- Christian, J.W. Basic crystallography and kinetics. In Martensite Fundamentals and Technology; Petty, E.R., Ed.; Longman Group: London, UK, 1970; pp. 11–42. [Google Scholar]
- Mohany, O.N. On the stabilization of retained austenite: Mechanism and kinetics. Mater. Sci. Eng. 1995, 32, 267–278. [Google Scholar] [CrossRef]
- Dunne, D.P. The Interface Structure of Martensite in Fe3Pt. Scr. Metall. 1978, 12, 143. [Google Scholar] [CrossRef]
- da Silva de Souza, S.; Moreira, P.S.; Faria, G.L. Austenitizing temperature and cooling rate effects on the martensitic transformation in a microalloyed-steel. Mater. Res. 2020, 23, e20190570. [Google Scholar] [CrossRef]
- Abdollah-Zadeh, A. The Investigation of Deformation, Recovery, Recrystallization and Precipitation in Austenitic HSLA Steel Analogue Alloys. Ph.D. Thesis, University of Wollongong, Wollongong, NSW, Australia, 1996. [Google Scholar]
- Yang, T.; Bhadeshia, H.K.D.H. Austenite grain size and the martensite-start temperature. Scr. Metall. 2009, 60, 493–495. [Google Scholar] [CrossRef]
- Fisher, J.C.; Holloman, J.H.; Turnbull, D. Kinetics of the Austenite → Martensite Transformation. JOM 1949, 1, 691–700. [Google Scholar] [CrossRef]
- Guimaraes, J.R.C.; Rios, P.R. Revisiting Fisher’s Analysis of the Martensite Microstructure. Metall. Mater. Trans. A 2011, 42A, 2937–2940. [Google Scholar] [CrossRef]
- McDougall, P.G.; Wayman, C.M. Crystallography and Morphology of Ferrous Martensites. In Martensite; Olsen, G.B., Owen, W.S., Eds.; ASM Int.: Materials Park, OH, USA, 1992; pp. 59–95. [Google Scholar]
- Dunne, D.P. Martensitic Iron-Platinum Alloys. In Proceedings of the International Conference on Displacive Transformationsand Their Applications in Materials Engineering, Urbana, IL, USA, 8–9 May 1996; Inoue, K., Ed.; University of Illinois: Champaign, IL, USA; TMS: Pittsburgh, PA, USA, 1998; pp. 133–140. [Google Scholar]
- Morgan, E.R.; Ko, T. Thermal stabilization of austenite in iron-carbon-nickel alloys. Acta Metall. 1953, 1, 36–48. [Google Scholar] [CrossRef]
- Svastlivsev, V.M.; Mirzayev, D.A.; Karzunov, S.E.; Yakovleva, I.L. New Concepts of Bainitic and Austenitic Transformation in Steels Based on Multistep γ−α Transformation. ISIJ Int. 1995, 35, 955–961. [Google Scholar] [CrossRef]
- Guo, L.; Roelofs, H.; Lemke, M.I.; Bhadeshia, H.K.D.H. Modelling of Recalescence Effect on Austenite Decomposition. Mater. Sci. Technol. 2017, 33, 1258–1267. [Google Scholar] [CrossRef]
- Yurioka, N. Weldability of Modern High Strength Steels. In Proceedings of the American Welding Society First US-Japan Symp. on Advances in Welding Metallurgy, San Francisco, CA, USA, 7–8 June 1990; pp. 79–100. [Google Scholar]
- Lancaster, J.F. Metallurgy of Welding, 6th ed.; Abington Publishing, Woodhead Publishing Limited: Cambridge, UK, 1999; p. 464. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).