Effects of Vanadium and Niobium on the Mechanical Properties and High-Temperature Oxidation Behavior of Austenitic Stainless Steels
Abstract
1. Introduction
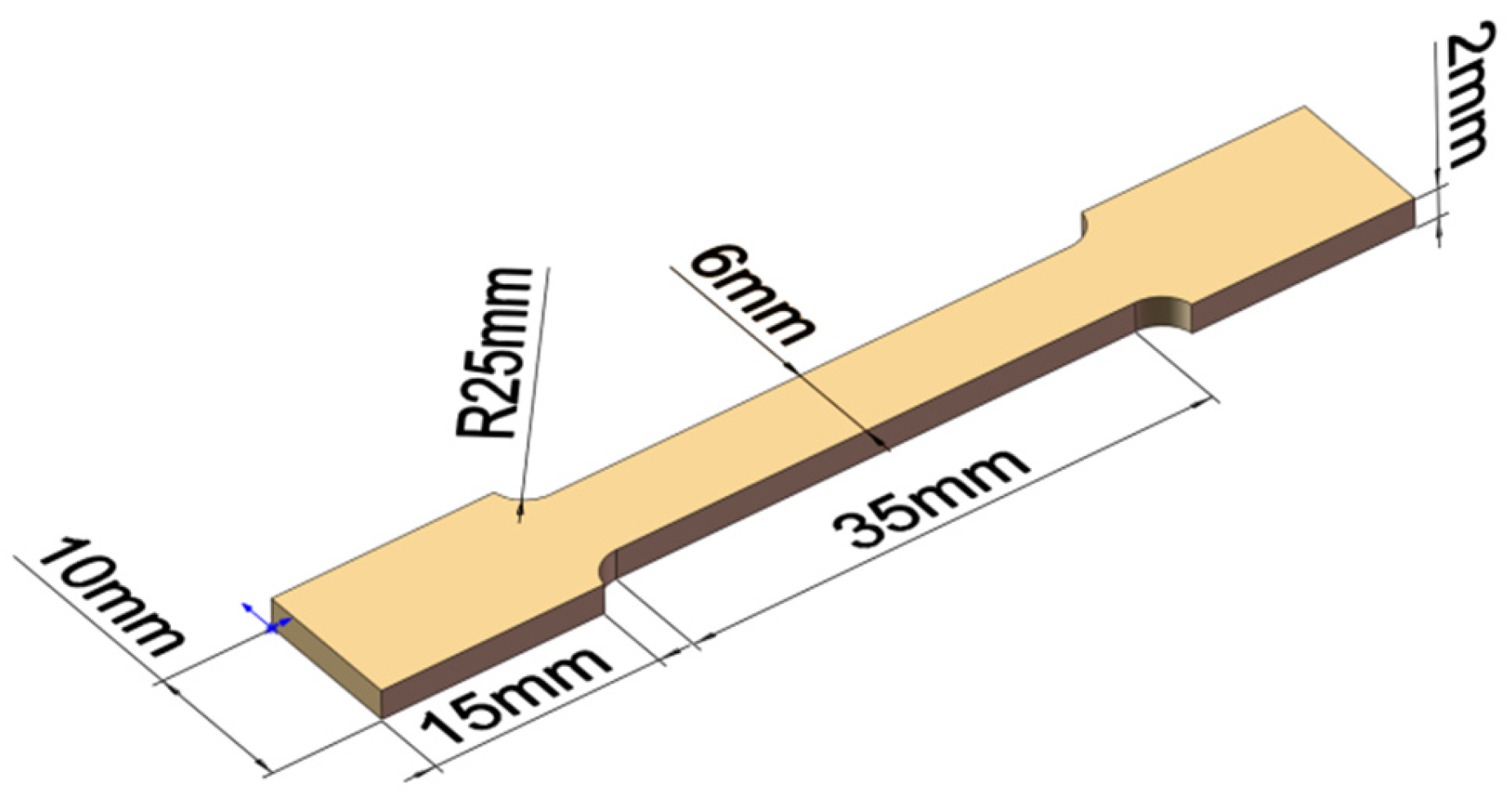
2. Materials and Methods
3. Results and Discussion
3.1. Mechanical Properties
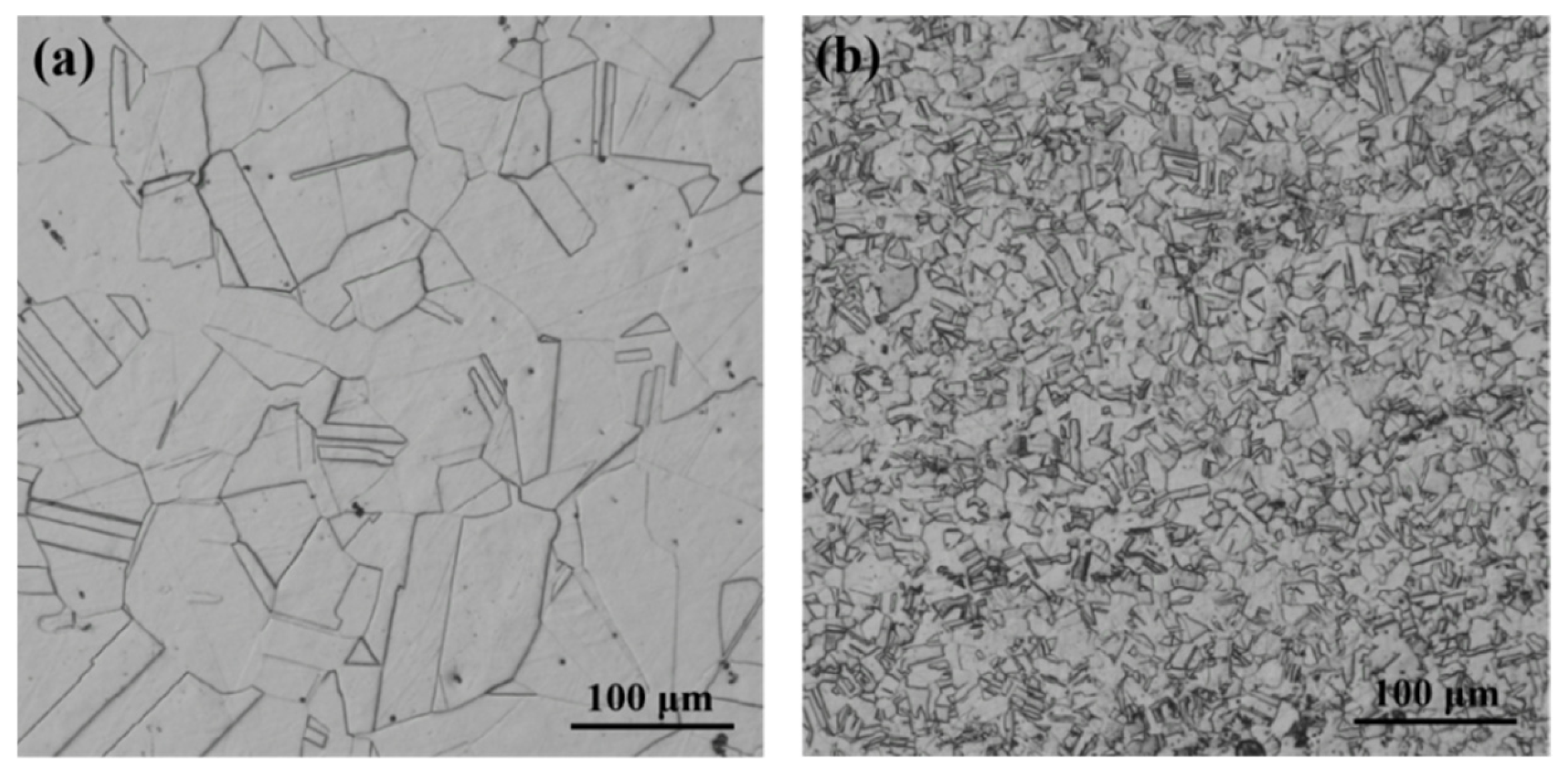
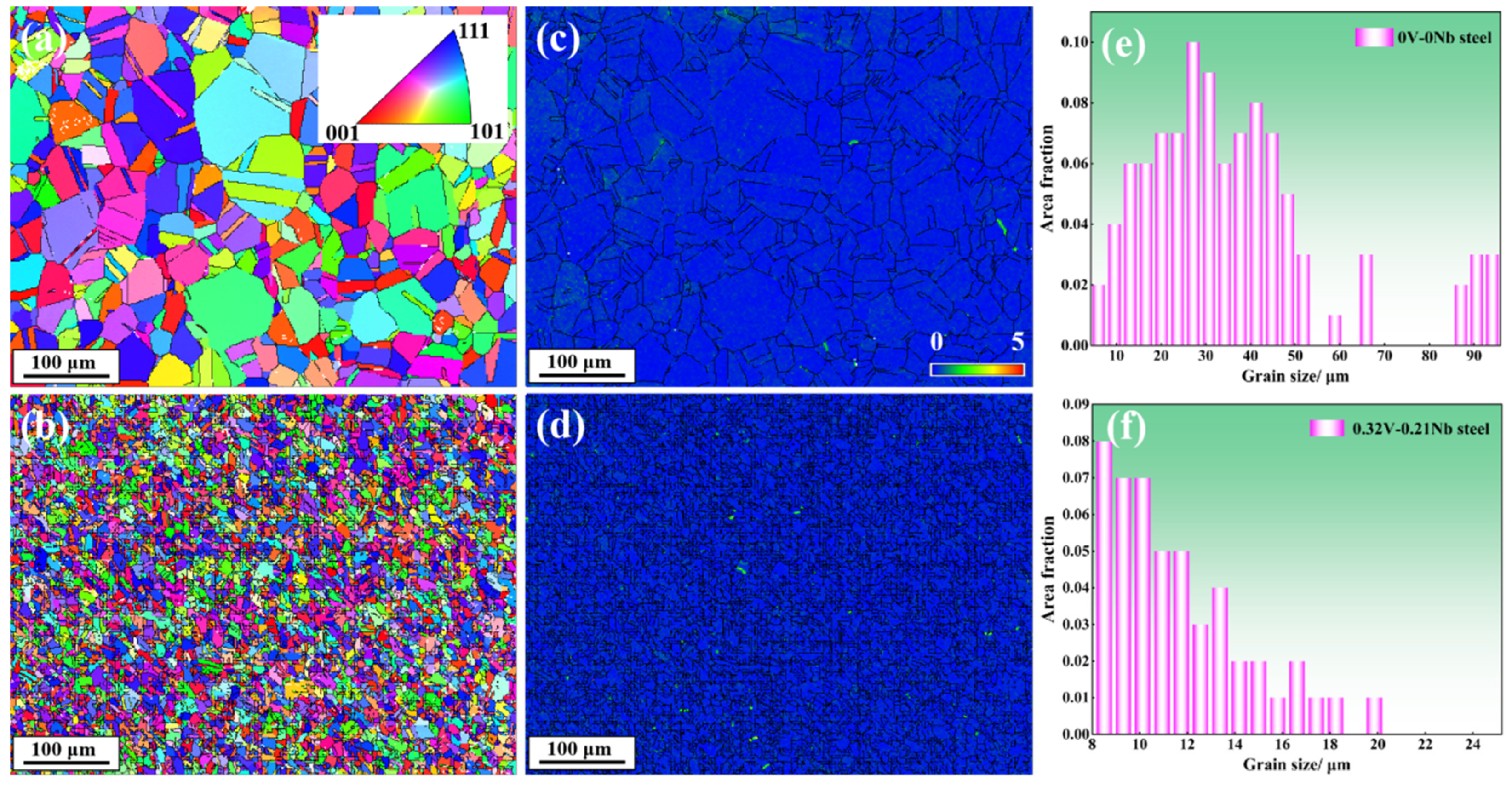
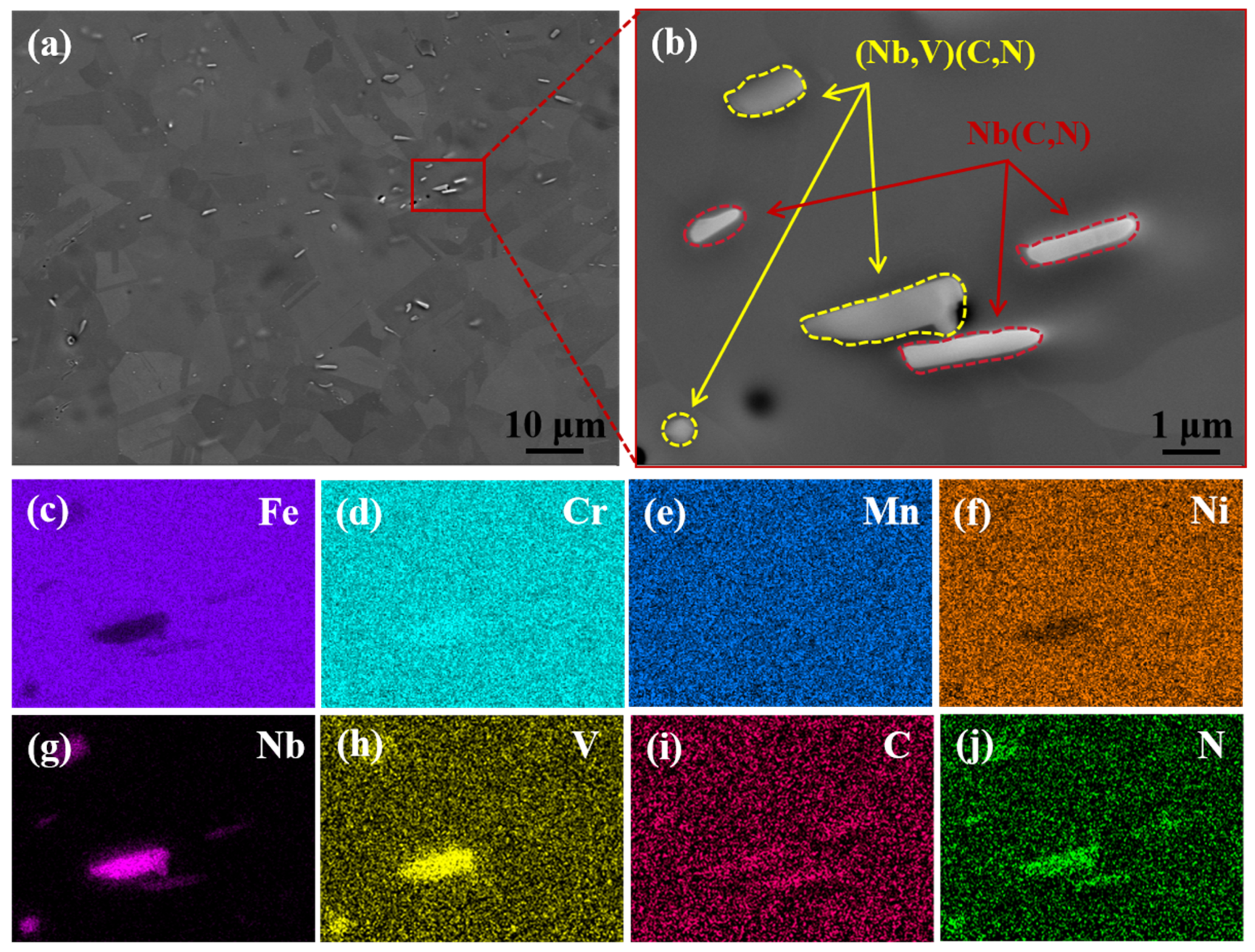
3.1.1. Microstructure
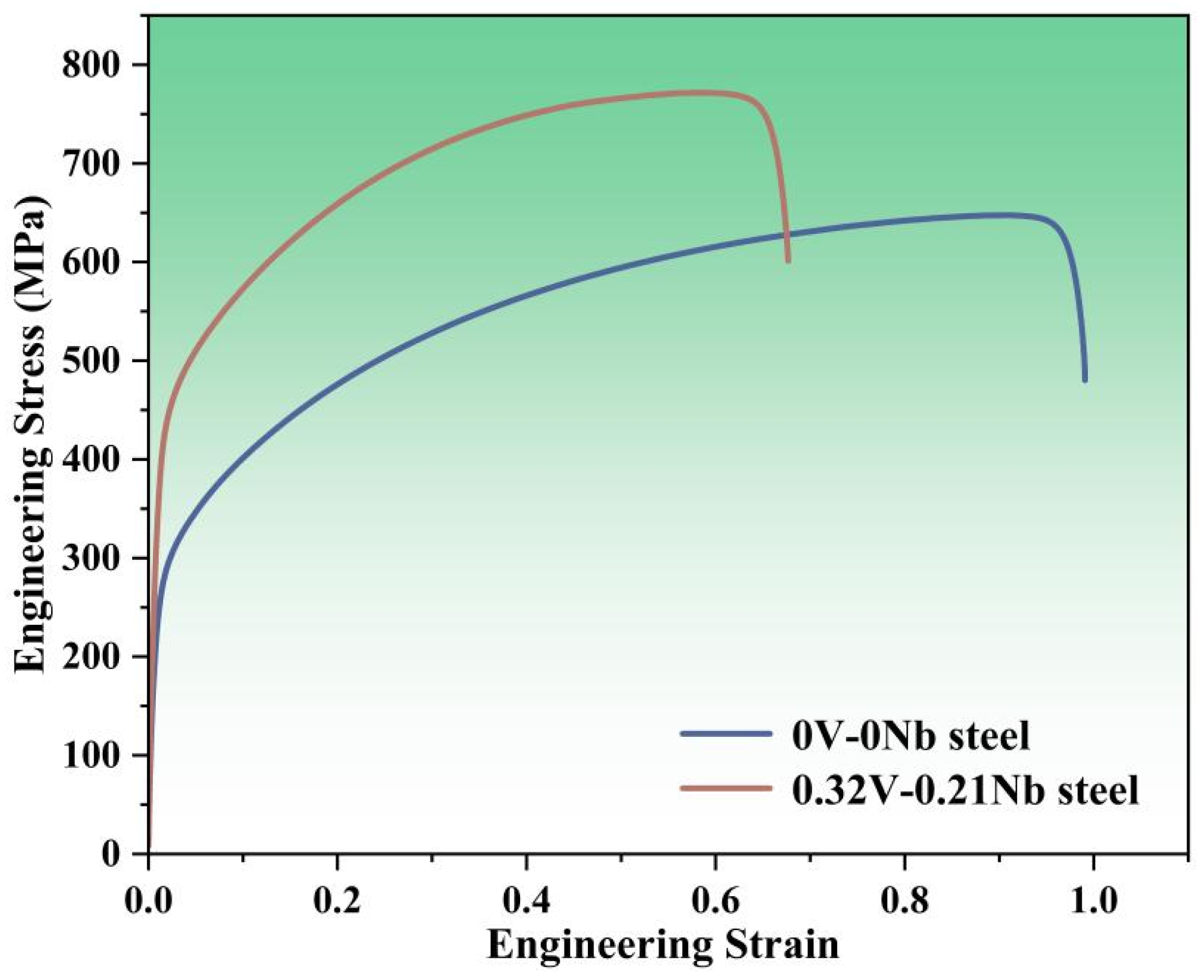
3.1.2. Tensile Properties
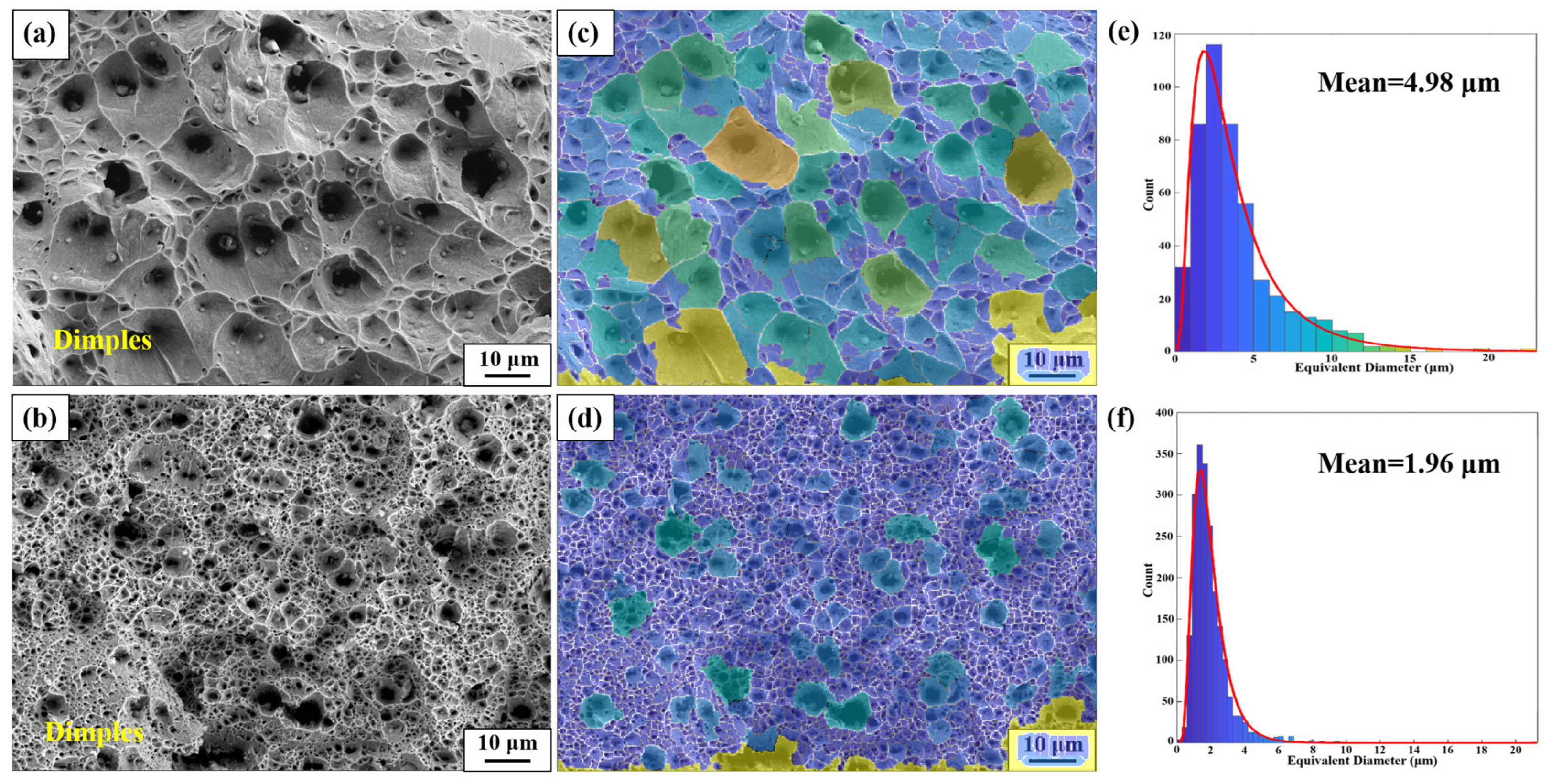
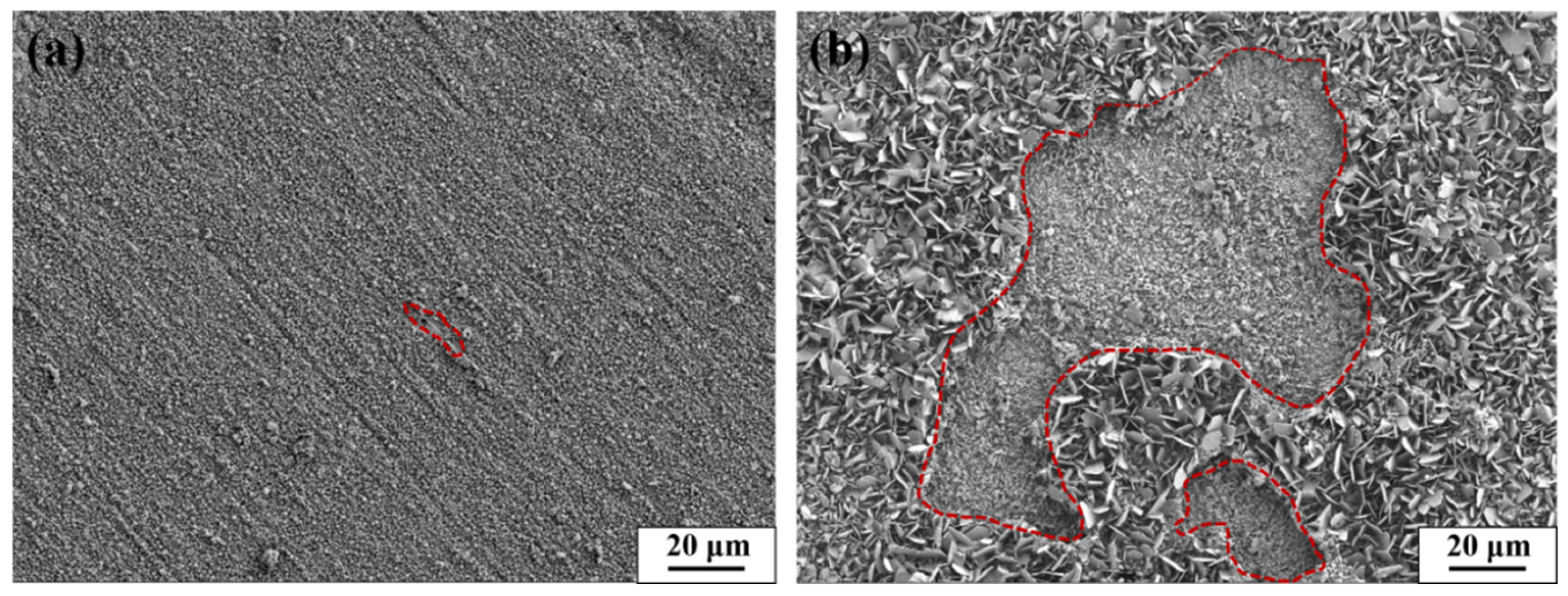
3.1.3. Fracture Analysis
3.2. High-Temperature Oxidation Performance
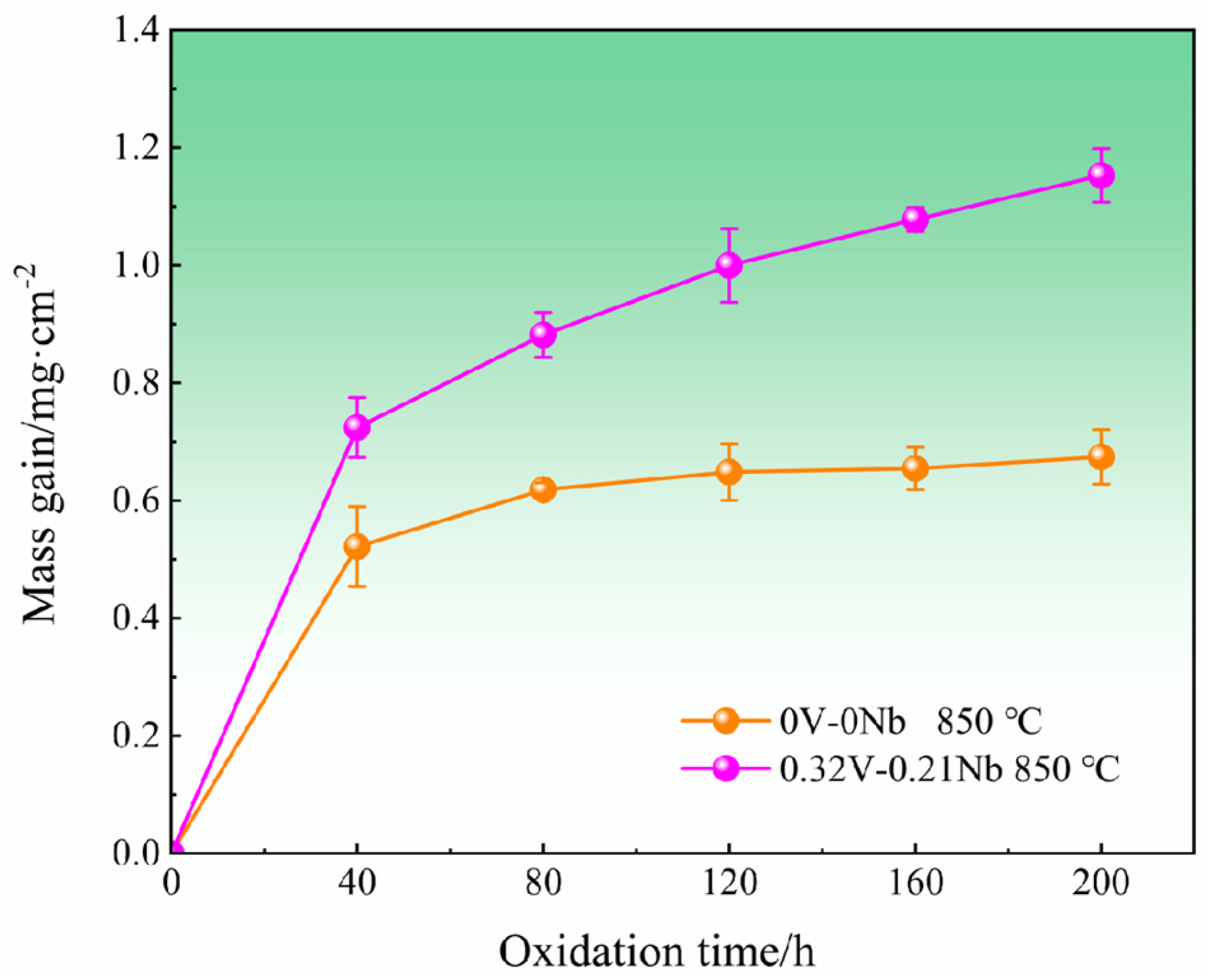
3.2.1. Oxidation Kinetics
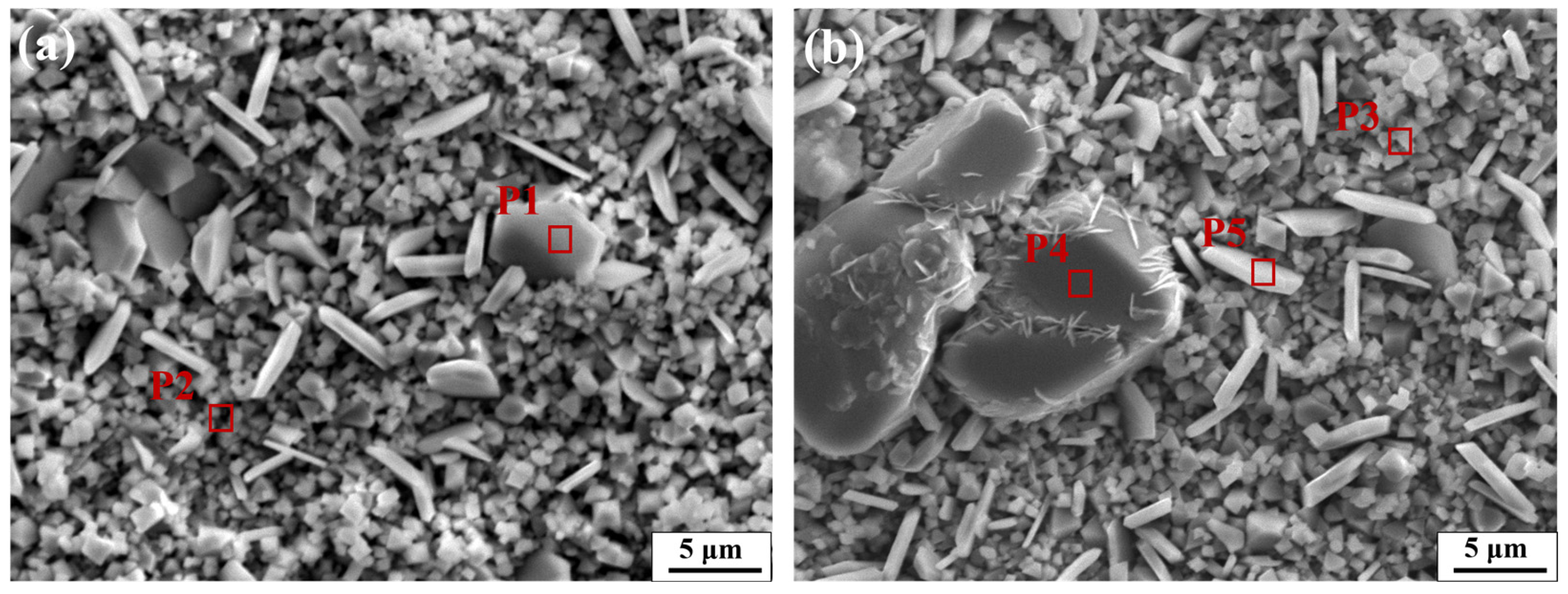
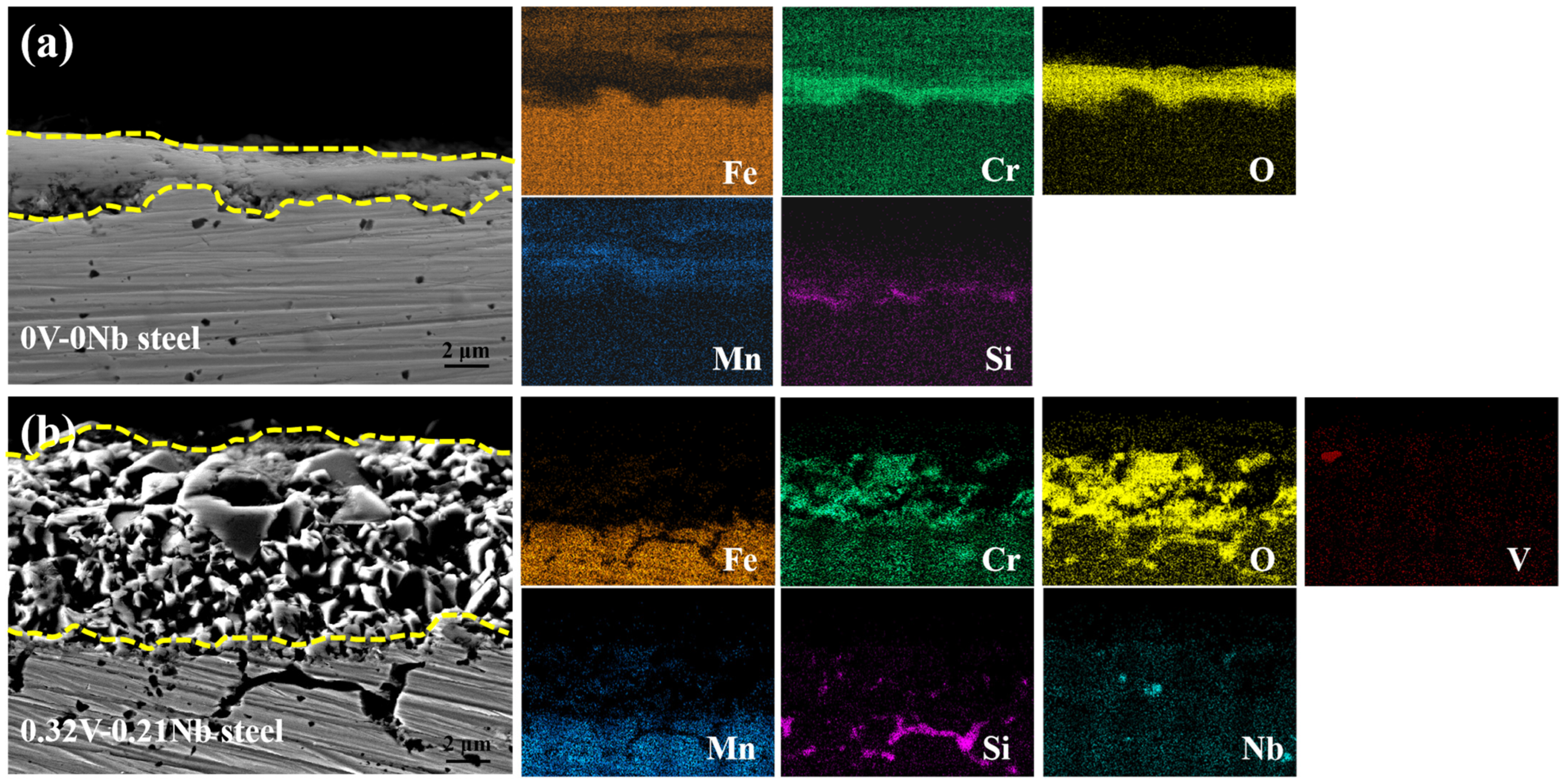
3.2.2. Morphology of Oxide Layer
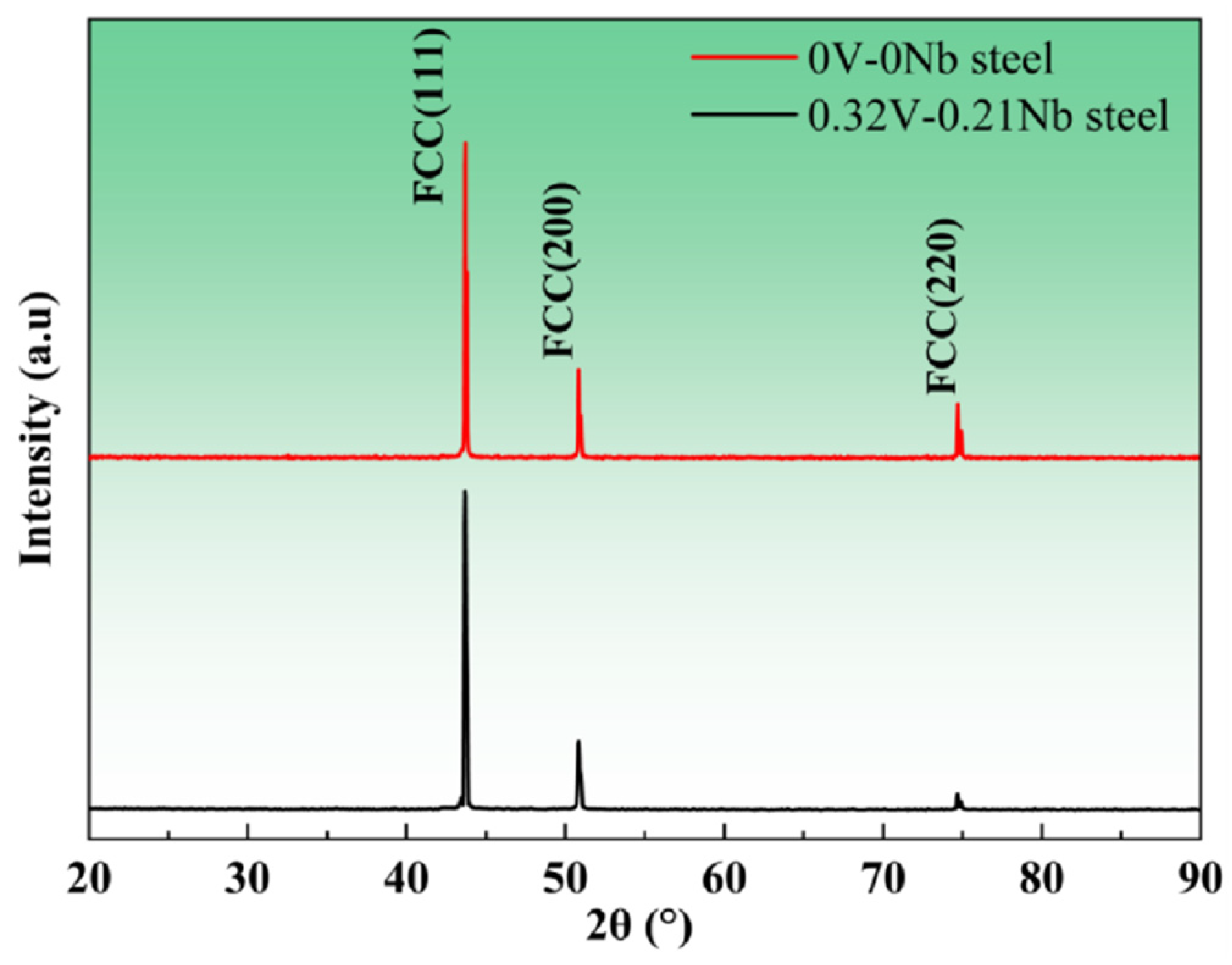
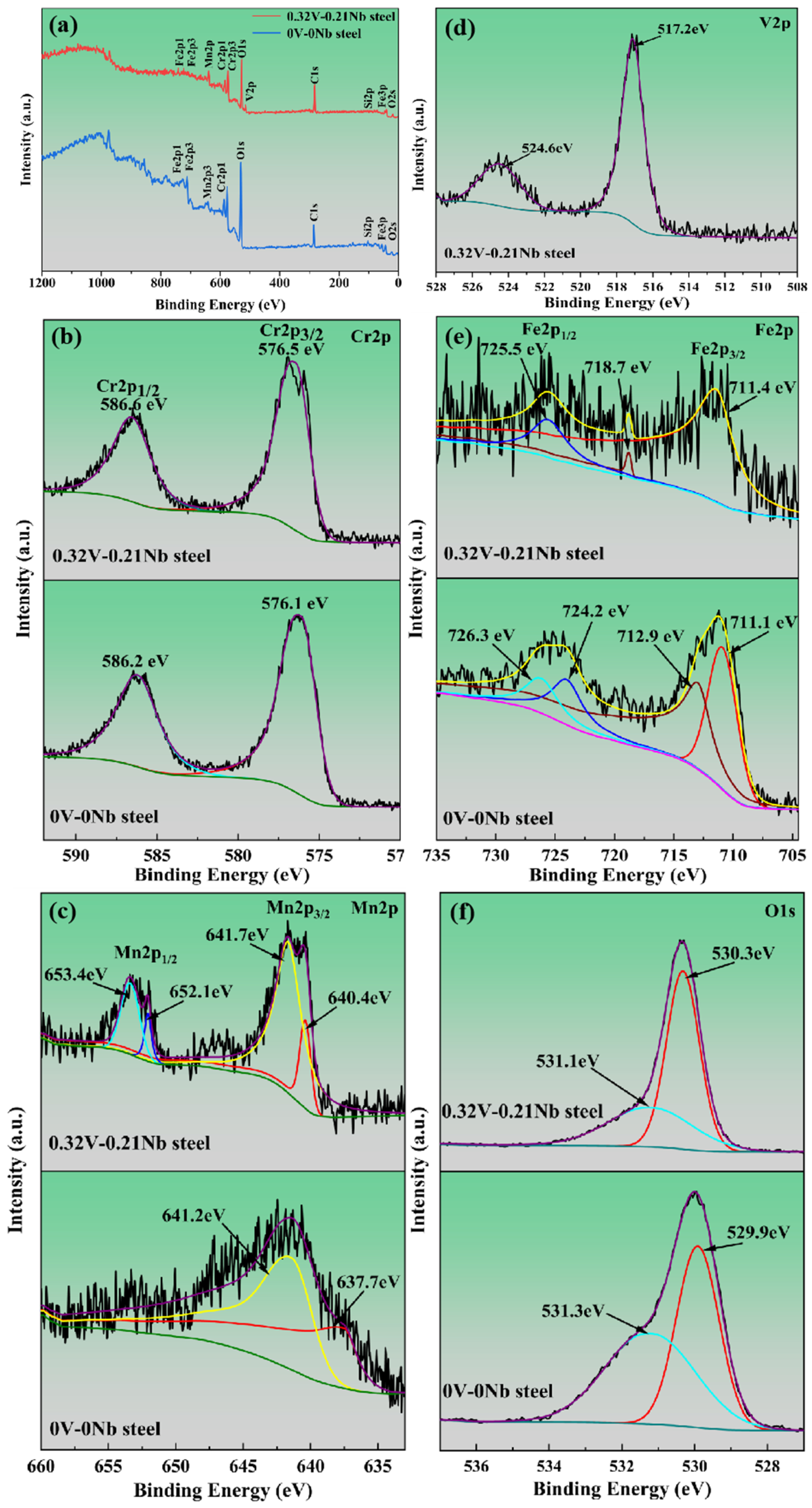
3.2.3. Oxide Phase Analysis
4. Conclusions
- (1)
- The microstructure of the test steel after the addition of V and Nb remained a single-phase austenitic organization. The microalloying elements V and Nb reduced the size of the austenitic grains and refined the grains.
- (2)
- The V and Nb elements play an effective role in fine-grain strengthening. According to theoretical calculations and experimental results, the contribution of fine-grain strengthening to the room-temperature yield strength was approximately 42.9%.
- (3)
- The weight gain and oxide film scattering increased significantly with increasing V and Nb contents after the high-temperature oxidation. During high-temperature oxidation, the dissolution of V oxide (V2O5) further increased the stress in the oxide layer and promoted the cracking and peeling of the oxide film.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.T.; Du, H.Y.; Wei, Y.H.; Hou, L.F.; Liu, X.D.; Wei, H.; Liu, B.S.; Jia, J.W. Precipitation and Properties at Elevated Temperature in Austenitic Heat-Resistant Steels-A Review. Steel Res. Int. 2021, 92, 2000378. [Google Scholar] [CrossRef]
- Nikulin, I.; Kipelova, A.; Kaibyshev, R. Effect of high-temperature exposure on the mechanical properties of 18Cr–8Ni–W–Nb–V–N stainless steel. Mater. Sci. Eng. A 2012, 554, 61–66. [Google Scholar] [CrossRef]
- Wang, F.; Zou, D.N.; Wang, Q.S.; Zhang, X.M.; Tong, L.B.; Liang, X.L.; Li, Y.; Jiang, Y.C. The negative effect of V content on high temperature oxidation resistance of austenitic stainless steels at 850 °C. Mater. Charact. 2024, 212, 113967. [Google Scholar] [CrossRef]
- Huang, J.Z.; Wang, J.Y.; Yang, L.J.; Du, W.W.; Wu, M.H.; Zhang, Q.K.; Song, Z.L. Comparative study on microstructure and mechanical properties of a novel nano-composite strengthening heat-resistant steel and two typical heat-resistant steels. Mater. Today Commun. 2023, 36, 106679. [Google Scholar] [CrossRef]
- Xu, W.J.; Jia, G.D.; Pan, J.; Wang, Z.X.; Li, J.; Xiao, X.S. Effects of Nb on Creep Properties and Hot Corrosion Resistance of New Alumina-Forming Austenitic Steels at 700 °C. Metals 2024, 14, 870. [Google Scholar] [CrossRef]
- Dai, H.B.; Huang, G.Q.; Zeng, H.B. Multi-objective optimal dispatch strategy for power systems with Spatio-temporal distribution of air pollutants. Sustain. Cities Soc. 2023, 98, 104801. [Google Scholar] [CrossRef]
- Bugge, J.; Kjær, S.; Blum, R. High-efficiency coal-fired power plants development and perspectives. Energy 2006, 31, 1437–1445. [Google Scholar] [CrossRef]
- Jia, J.W.; Li, H.; Du, H.Y.; Ren, J.N.; Liang, H.M.; Wei, H.; Hou, H.; Liu, X.D. Microstructure and Oxidation Behavior of C-HRA-5 Austenitic Heat-Resistant Steel in Air at the Temperature Range of 650–750 °C. Adv. Eng. Mater. 2024, 26, 2301622. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, B.K.; Kim, D.I.; Choi, P.P.; Raabe, D.; Yi, K.W. The role of grain boundaries in the initial oxidation behavior of austenitic stainless steel containing alloyed Cu at 700 °C for advanced thermal power plant applications. Corros. Sci. 2015, 96, 52–66. [Google Scholar] [CrossRef]
- Wu, H.B.; Ju, B.; Tang, D.; Hu, R.R.; Guo, A.M.; Kang, Q.; Wang, D. Effect of Nb addition on the microstructure and mechanical properties of an 1800 MPa ultrahigh strength steel. Mater. Sci. Eng. A 2015, 622, 61–66. [Google Scholar] [CrossRef]
- Gong, P.; Palmiere, E.J.; Rainforth, W.M. Dissolution and Precipitation Behaviour in Steels Microalloyed with Niobium During Thermomechanical Processing. Acta Mater. 2015, 97, 392–403. [Google Scholar] [CrossRef]
- Okayasu, M.; Wu, S.; Noda, K.; Lin, D.Y.; Yang, S.M. Mechanical properties of austenitic stainless steel with high niobium contents. Mater. Sci. Technol. 2016, 32, 1382–1394. [Google Scholar] [CrossRef]
- Cai, Z.; Mao, X.; Bao, S.; Zhao, G.; Xu, Y. Influence of Vanadium Microalloying on Deformation-Induced Pearlite Transformation of Eutectoid Steel. Metals 2019, 9, 268. [Google Scholar] [CrossRef]
- Gladman, T. The Physical Metallurgy of Microalloyed Steels; The Institute of Materials: London, UK, 1997. [Google Scholar]
- Zhang, H.W.; Xu, Y.L.; Hu, P.F.; Xu, W.J.; Li, J.; Li, K.F.; Xiao, X.S. Strengthening behaviors of V and W modified Cr19 series duplex stainless steels with transformation induced plasticity. Mater. Sci. Eng. A 2017, 705, 134–141. [Google Scholar] [CrossRef]
- Liu, H.H.; Fu, P.X.; Liu, H.W.; Sun, C.; Du, N.Y.; Li, D.Z. Simultaneously enhancing strength and toughness of medium-carbon martensitic steel via nano precipitates and fine-grained structure. Mater. Sci. Eng. A 2022, 842, 143030. [Google Scholar] [CrossRef]
- Wen, T.; Hu, X.F.; Song, Y.Y.; Yan, D.S.; Rong, L.J. Carbides and mechanical properties in a Fe–Cr–Ni–Mo high-strength steel with different V contents. Mater. Sci. Eng. A 2013, 588, 201–207. [Google Scholar] [CrossRef]
- Park, D.B.; Huh, M.Y.; Jung, W.S.; Suh, J.Y.; Shim, J.H.; Lee, S.C. Effect of vanadium addition on the creep resistance of 18Cr9Ni3CuNbN austenitic stainless heat resistant steel. J. Alloys Compd. 2023, 574, 532–538. [Google Scholar] [CrossRef]
- Chen, W.J.; Gao, P.F.; Wang, S.; Zhao, X.L.; Zhao, Z.Z. Strengthening mechanisms of Nb and V microalloying high strength hot-stamped steel. Mater. Sci. Eng. A 2020, 797, 140115. [Google Scholar] [CrossRef]
- Ye, L.; Wang, J.F.; Zeng, Z.L.; Wang, W.D.; Song, S.X.; Kolan, M.R.; Wang, L.; Wang, X.D. Realization of Selective Strengthening of Ferrite by Nb/V Microalloying in a Medium Carbon Lightweight δ-TRIP Steel. Metall. Mater. Trans. A 2020, 51, 2460–2468. [Google Scholar] [CrossRef]
- Ran, M.; Wang, Q.; He, Y.; Zhou, H.; Huang, Y.; Zheng, W.; Tang, R. Synergistic Roles of Nb and Mo in the Formation of Oxides on Fe-20Cr-25Ni-Nb Stainless Steels in High-Temperature CO2. Metals 2023, 13, 665. [Google Scholar] [CrossRef]
- GB/T 228.1-2010; Metallic materials—Tensile testing—Part 1: Method of Test at Room Temperature. National Standard of the People’s Republic of China: Beijing, China, 2010.
- GB/T 13303-1991; Steels—Determination Method of Oxidation Resistance. National Standard of the People’s Republic of China: Beijing, China, 1991.
- Kim, D.W.; Kwon, D.Y.; Kang, J.H. Strengthening of high nitrogen austenitic stainless steel by Nb addition. Mater. Charact. 2024, 209, 113776. [Google Scholar] [CrossRef]
- Erneman, J.; Schwind, M.; Liu, P.; Nilsson, J.O.; Andrén, H.O.; Ågren, J. Precipitation reactions caused by nitrogen uptake during service at high temperatures of a niobium stabilised austenitic stainless steel. Acta Mater. 2004, 52, 4337–4350. [Google Scholar] [CrossRef]
- Li, Y.M.; Liu, Y.C.; Liu, C.X.; Li, C.; Li, H.J. Mechanism for the formation of Z-phase in 25Cr-20Ni-Nb-N austenitic stainless steel. Mater. Lett. 2018, 233, 16–19. [Google Scholar] [CrossRef]
- Vodárek, V. Stability of Z-phase and M6X in creep-resistant steels. Scr. Mater. 2012, 66, 678–681. [Google Scholar] [CrossRef]
- Zhao, T.; Chen, C.; Wang, Y.F.; Wang, T.S.; Sheng, G.L.; He, Y.M. Effect of Nb–V microalloying on low-cycle fatigue property of Fe–Mn–Al–C austenitic steel. J. Mater. Res. Technol. 2023, 23, 3711–3725. [Google Scholar] [CrossRef]
- Liu, J.; Guo, K.; Ma, H.; He, J.; Wang, J.; Zhang, C.; Wang, T.; Wang, Q. Microstructural Evolution and Tensile Properties of Nb-V-Ti-N Microalloyed Steel with Varying Nitrogen Contents. Metals 2025, 15, 266. [Google Scholar] [CrossRef]
- Wen, H.; Wang, Q.; Dou, Y.; Wang, Q.; Xu, X.; Wang, Q. Comparison of Strengthening Mechanism of the Nb, V, and Nb-V Micro-Alloyed High-Strength Bolt Steels Investigated by Microstructural Evolution and Strength Modeling. Metals 2024, 14, 1309. [Google Scholar] [CrossRef]
- Han, J.; Li, H.J.; Frank, B.; Jiang, L.Z.; Zhu, Z.X.; Xu, H.G.; Ma, L. Precipitation and impact toughness of Nb-V stabilised 18Cr-2Mo ferritic stainless steel during isothermal aging. Mater. Sci. Eng. A 2014, 612, 63–70. [Google Scholar] [CrossRef]
- Yi, H.Y.; Liang, T.; Wang, M.; Zha, X.D.; Ma, Y.C.; Liu, K. Effects of Silicon on the Microstructure and Mechanical Properties of 15-15Ti Stainless Steel. Acta Metall. Sin-Engl. 2020, 33, 583–1590. [Google Scholar] [CrossRef]
- Shakhova, I.; Dudko, V.; Belyakov, A.; Tsuzaki, K.; Kaibyshev, R. Effect of large strain cold rolling and subsequent annealing on microstructure and mechanical properties of an austenitic stainless steel. Mater. Sci. Eng. A 2012, 545, 176–186. [Google Scholar] [CrossRef]
- Odnobokova, M.V.; Belyakov, A.N.; Dolzhenko, P.D.; Kostina, M.V.; Kaibyshev, R.O. On the strengthening mechanisms of high nitrogen austenitic stainless steels. Mater. Lett. 2023, 331, 133502. [Google Scholar] [CrossRef]
- Chen, G.; Wang, H.; Sun, H.Y.; Zhang, Y.Y.; Cao, P.; Wang, J. Effects of Nb-doping on the mechanical properties and high-temperature steam oxidation of annealing FeCrAl fuel cladding alloys. Mater. Sci. Eng. A 2021, 803, 140500. [Google Scholar] [CrossRef]
- Wang, F.; Xiao, G.Z.; Zou, D.N.; Zhang, K.X.; Zhang, X.M.; Li, Y.; Tong, L.B. Effect of Nb on high-temperature oxidation of austenitic stainless steel at 850 °C. J. Iron Steel Res. Int. 2024. [Google Scholar] [CrossRef]
- Takahashi, T.; Minamino, Y.; Hirasawa, H.; Ouchi, T. High-temperature oxidation and its kinetics study of TiAl and TiVAlloys in air. Mater. Trans. 2014, 55, 290–297. [Google Scholar] [CrossRef]
- DiStefano, J.R.; DeVan, J.H. Reactions of oxygen with V-Cr-Ti alloys. J. Nucl. Mater. 1997, 249, 150–158. [Google Scholar] [CrossRef]
- Jain, U.; Sonber, J.; Tewaria, R. High temperature oxidation behaviour of V-Ti-Ta alloys. Fusion Eng. Des. 2019, 144, 125–132. [Google Scholar] [CrossRef]
- Wilson, J.R.; Lewis, M.E. Oxidation of vanadium in dry and moist oxygen–argon mixtures. Nature 1965, 206, 1350. [Google Scholar] [CrossRef]
- Shen, Q.; Sun, X.J.; Chen, S. Electrode Doping and Dielectric Effect in Hole Injection into Organic Semiconductors through High Work-Function Oxides. J. Phys. Chem. Lett. 2023, 14, 4830–4836. [Google Scholar] [CrossRef]

| Test Steels | C | Si | Mn | Cr | Ni | N | V | Nb | Fe |
|---|---|---|---|---|---|---|---|---|---|
| 0 V–0 Nb | 0.034 | 0.45 | 1.18 | 19.08 | 8.50 | 0.14 | — | — | Bal |
| 0.32 V–0.21 Nb | 0.035 | 0.47 | 1.13 | 18.85 | 8.30 | 0.14 | 0.32 | 0.21 | Bal |
| Test Steels | Yield Strength (MPa) | Tensile Strength (MPa) | Elongation (%) |
|---|---|---|---|
| 0 V–0 Nb | 329.5 ± 9.6 | 654.6 ± 7.1 | 96.2 ± 5.8 |
| 0.32 V–0.21 Nb | 464.6 ± 17.1 | 768.8 ± 14.0 | 55.8 ± 2.9 |
| Test Steels | Position | Elements (wt%) | ||||||
|---|---|---|---|---|---|---|---|---|
| O | Mn | Cr | Fe | Si | V | Nb | ||
| 0 V–0 Nb | P1 | 35.78 | 2.49 | 54.61 | 6.97 | 0.15 | — | — |
| P2 | 19.54 | 12.41 | 46.56 | 5.49 | 0.21 | — | — | |
| 0.32 V–0.21 Nb | P3 | 35.48 | 4.11 | 56.54 | 2.94 | 0.34 | 0.34 | 0.25 |
| P4 | 28.06 | 33.62 | 2.78 | 3.87 | 0.39 | 31.14 | 0.14 | |
| P5 | 34.92 | 3.02 | 46.78 | 14.46 | 0.13 | 0.59 | 0.10 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Zhang, Z.; Xiao, G.; Zou, D. Effects of Vanadium and Niobium on the Mechanical Properties and High-Temperature Oxidation Behavior of Austenitic Stainless Steels. Metals 2025, 15, 347. https://doi.org/10.3390/met15040347
Wang F, Zhang Z, Xiao G, Zou D. Effects of Vanadium and Niobium on the Mechanical Properties and High-Temperature Oxidation Behavior of Austenitic Stainless Steels. Metals. 2025; 15(4):347. https://doi.org/10.3390/met15040347
Chicago/Turabian StyleWang, Fan, Zheng Zhang, Guizhi Xiao, and Dening Zou. 2025. "Effects of Vanadium and Niobium on the Mechanical Properties and High-Temperature Oxidation Behavior of Austenitic Stainless Steels" Metals 15, no. 4: 347. https://doi.org/10.3390/met15040347
APA StyleWang, F., Zhang, Z., Xiao, G., & Zou, D. (2025). Effects of Vanadium and Niobium on the Mechanical Properties and High-Temperature Oxidation Behavior of Austenitic Stainless Steels. Metals, 15(4), 347. https://doi.org/10.3390/met15040347





