Abstract
Metallic glass, as an emerging catalytic material, possesses an atomic structure characterized by long-range disorder and short-range order, which creates abundant and accessible active sites that enhance the adsorption and reactivity toward pollutant molecules, particularly dye compounds. In treating highly colored and recalcitrant Reactive Black 5 (RB5) dye wastewater, Mo51Fe34B15 metallic glass wire demonstrate outstanding catalytic degradation performance within a conventional Fenton-like system. Under acidic conditions (pH = 2), the material exhibits a degradation rate constant of 0.698 min−1 for a 20 ppm RB5 dye solution, achieving a degradation efficiency of 98.8% within 10 min. After 10 consecutive cycles, the efficiency remains at 95%, and throughout 15 cycles, it consistently maintains a performance level above 90%. As the reaction proceeds, the degradation rate gradually decreases, primarily due to the accumulation of corrosion products on the catalyst surface, which are predominantly composed of MoO3 and Fe2O3. During the degradation process, metallic Mo0 and Fe0 serve as electron donors that facilitate the decomposition of H2O2, generating highly reactive hydroxyl radicals (•OH). These radicals attack the chromophoric structure of the dye, leading to its structural disruption and enabling rapid decolorization.
1. Introduction
Reactive dyes are widely employed in various industrial sectors, such as textiles, papermaking, plastics, and cosmetics. However, their production and application often lead to substantial pollution of aquatic environments [1,2,3,4]. Among various reactive dyes, Reactive Black 5 (RB5, C26H21N5Na4O19S6), characterized by its typical diazo structure, possesses a complex molecular architecture and high chemical stability, rendering it resistant to degradation by conventional biochemical treatment methods [5,6,7,8,9]. The direct discharge of RB5 wastewater poses significant threats to aquatic ecosystems and can lead to bioaccumulation through the food chain, thereby posing potential risks to human health [10,11,12].
In recent years, Fenton-like technology within advanced oxidation processes (AOPs) has attracted considerable attention due to its high efficiency in degrading refractory organic pollutants and has progressively advanced toward practical engineering applications [13,14,15]. The fundamental mechanism of this technology involves the decomposition of hydrogen peroxide (H2O2) into highly reactive hydroxyl radicals (•OH) under external stimuli, such as catalytic agents, light irradiation, or electrochemical activation [16,17]. These radicals exhibit non-selective reactivity, enabling them to efficiently degrade chemical bonds within organic pollutant molecules [18]. This leads to the cleavage of complex macromolecular structures, followed by their mineralization into simple inorganic compounds such as carbon dioxide and water. Compared to plasma technologies that require continuous high energy input or electrochemical methods dependent on electrical consumption, the Fenton-like system utilizes light and chemical energy under mild conditions, offering the potential for reduced operating costs.
Metallic glasses, also known as amorphous alloys, exhibit distinctive atomic structures characterized by short-range order and long-range disorder, as well as thermodynamic metastability [19,20,21]. These structural and physical features confer unique performance advantages that are difficult to achieve with crystalline materials, including high surface reactivity, abundant active sites, tunable electronic properties, and superior corrosion resistance. As a result, metallic glasses exhibit excellent catalytic activity, selectivity, and stability, making them promising candidates for applications in environmental catalysis and energy conversion [22,23,24]. Currently, their application in wastewater treatment primarily relies on Fenton or Fenton-like reaction mechanisms, in which H2O2 is catalytically decomposed to generate •OH for pollutant degradation [25,26,27]. Recent studies have demonstrated that molybdenum-based metallic glasses exhibit outstanding performance in catalytic degradation, owing to their excellent thermal stability, broad pH tolerance, and high recyclabilit [28,29]. Compared to traditional ribbon or powder forms, wire-shaped catalysts feature a larger specific surface area, which enhances the efficiency of recovery processes [30].
This study successfully developed a novel filamentous Mo51Fe34B15 metallic glass catalyst and established an efficient Fenton-like catalytic system. By elucidating the unique Mo-Fe synergistic mechanism, this work provides a viable strategy to overcome the critical challenges of catalyst stability and pH adaptability, achieving significant advancements in material architecture design and fabrication process optimization with both scientific significance and practical application potential.
2. Materials and Methods
2.1. Preparation and Structural Characterization
High-purity molybdenum (99.9%), iron (99.99%), and boron (99.5%) were employed to fabricate the master alloy ingot using a WK-II type non-consumable vacuum arc melting furnace (voltage: 380 V, power: 30 kW, KYKY, Beijing, China). After complete homogenization of the molten alloy, Mo51Fe34B15 (at.%) metallic glass wire with an average diameter of approximately 60 μm was produced via the melt-spinning technique using the same WK-II apparatus. X-ray diffraction analysis (XRD, SHIMADZU XRD-6100, Cu Kα, Shimadzu, Kyoto, Japan) revealed a broad amorphous scattering peak in the 2θ range of 40° to 48°, with no distinct crystalline diffraction peaks, thereby confirming the fully amorphous microstructure. The thermal behavior was investigated by differential scanning calorimetry (DSC, PE STA 8000) at a heating rate of 20 K/min, which yielded the glass transition temperature (Tg) and onset crystallization temperature (Tx) of 928 K and 1017 K [29], respectively. The surface morphology of the metallic glass wire was analyzed using a field emission scanning electron microscope (FE-SEM, Sigma 500, Carl Zeiss, Oberkochen, Germany).
2.2. Degradation Experiment
This study evaluated the catalytic performance of Mo51Fe34B15 metallic glass wire in degrading RB5 dye wastewater. A stock solution with a concentration of 100 ppm was prepared by dissolving 100 mg of the dye in 1 L of deionized water. Subsequently, working solutions at concentrations of 20, 30, 40, and 50 ppm were obtained through appropriate dilution procedures. The solution temperature was controlled using a water bath, and the pH was adjusted to the target value with 1 M HCl or NaOH after stabilization. Then, 2 g/L of metallic glass wire was added under magnetic stirring, and the reaction was initiated by the addition of H2O2. At selected time intervals (e.g., 2, 5, 8, and 10 min), 3 mL samples were withdrawn and analyzed using a UV-Vis spectrophotometer (Cary 5000, 10401, Agilent Technologies, Shanghai, China) at a scanning speed of 300 nm/min over a wavelength range of 450–700 nm. The validity of pseudo-first-order kinetics for RB5 degradation is demonstrated in Figure S1. Degradation curves were constructed, and the observed rate constant (kobs) was determined using Equation (1) to quantify degradation efficiency [31].
In the equation, C0 represents the initial concentration of the dye solution, Ct denotes the concentration at time t, kobs signifies the observed reaction rate constant, and t stands for the reaction time. The activation energy (Ea) associated with the dye degradation process using metallic glass can be described by the Arrhenius equation [32].
Among the parameters involved, kobs represents the observed reaction rate constant, R denotes the gas constant, T stands for temperature, and lnA refers to the pre-exponential factor.
2.3. Characterization of the Catalytic Mechanism
To determine the surface elemental oxidation states, X-ray photoelectron spectroscopy (XPS, AXIS SUPRA+, Shimadzu, Japan) was performed, with all binding energy values referenced to the standard C 1s peak at 284.8 eV. The presence of free radicals generated during the reaction was detected by electron paramagnetic resonance (EPR, EMXplus, Bruker BioSpin, Rheinstetten, Germany) using 10 mM DMPO as the spin-trapping agent for •OH radicals. Additionally, tert-butyl alcohol (TBA) was used in subsequent radical quenching experiments to confirm the involvement of •OH radicals.
3. Results and Discussion
3.1. Degradation Performance
Figure 1 presents the UV-Vis absorption spectra obtained during the degradation of RB5 dye solution by the Mo51Fe34B15 metallic glass wire. The results show that the maximum absorption peak of the RB5 dye solution is located at 598 nm. With increasing reaction time, the absorption intensity gradually decreases, indicating progressive cleavage of the azo bond (-N=N-) in the dye molecules into amino groups (-NH2) [13]. The decolorization performance is illustrated in the inset of Figure 1a, which demonstrates that the Mo51Fe34B15 metallic glass wire achieves nearly complete decolorization of the dye solution within 10 min, with an observed reaction rate constant of 0.698 min−1 (R2 = 0.99). Figure 1b provides a comparative summary of the observed kobs reported for metallic glass catalysts in wastewater degradation applications [27,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47]. The Mo51Fe34B15 metallic glass wire exhibits significantly enhanced degradation performance compared to conventional Fe-based, Mg-based, and other metallic glass systems (Table S1), highlighting its potential as a highly promising catalyst for treating dye-containing wastewater.
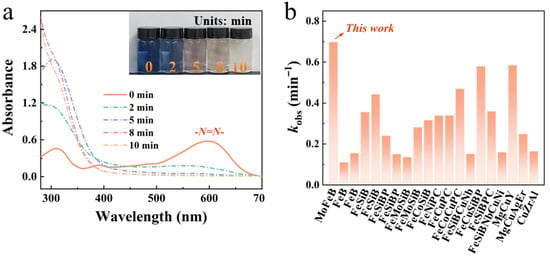
Figure 1.
(a) UV-Vis absorption spectra illustrating the degradation process of RB5 dye solution using Mo51Fe34B15 metallic glass wire. Inset: Schematic diagram of the dye decolorization effect (323 K, pH = 2, 20 ppm RB5, 0.1 M H2O2), and (b) comparative analysis of degradation performance [33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48].
3.2. The Impact of Environmental Factors
3.2.1. Effect of H2O2 Addition
Compared to the use of H2O2 (0.1 M) alone for the oxidative degradation of dye solutions, the incorporation of the Mo51Fe34B15 metallic glass catalyst significantly enhances degradation efficiency. Figure 2 illustrates the effect of varying H2O2 dosages on the degradation performance of the Mo51Fe34B15 metallic glass wire. As the H2O2 concentration increases, the degradation rate also increases. This behavior contrasts with that of conventional Fenton or Fenton-like systems, in which excessive H2O2 may scavenge •OH radicals beyond a certain concentration threshold, leading to reduced or even inhibited treatment efficiency. For example, Fe78Si9B13 and Fe73.5Si13.5B9Cu1Nb3 catalysts [36] exhibit optimal H2O2 concentrations of 1 mM for methylene blue degradation; however, increasing the H2O2 concentration from 1.0 mM to 2.0 mM results in only a marginal improvement in dye removal. Excess H2O2 acts as a scavenger of •OH radicals by converting them into HO2• radicals, which have lower oxidative potential. In contrast, the Mo51Fe34B15-H2O2 system overcomes this limitation. Even when the H2O2 dosage is increased from 0.01 M to 0.1 M, the degradation rate of the Mo51Fe34B15 metallic glass wire for RB5 dye solution continues to increase significantly.
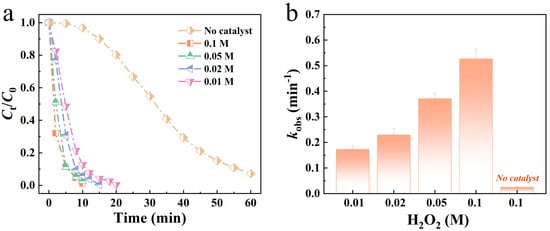
Figure 2.
(a) Mo51Fe34B15 metallic glass wire of different H2O2 addition to degrade the normalized concentration of RB5 solution and (b) reaction rate constant kobs (323 K, pH = 2, 20 ppm RB5).
3.2.2. Effect of pH
During the dye degradation process, the pH value of the solution serves as a critical parameter that significantly influences catalytic degradation efficiency. To systematically investigate the performance of Mo51Fe34B15 metallic glass wire in degrading dye wastewater under varying pH conditions, a series of experiments were conducted using RB5 dye solutions with pH values ranging from 2 to 9. The experimental results are presented in Figure 3. At pH 2, representing a highly acidic environment, the Mo51Fe34B15 metallic glass wire exhibited exceptional degradation efficiency, achieving a dye removal rate exceeding 99%. This indicates that the material possesses high catalytic activity under acidic conditions, enabling efficient dye wastewater treatment. When the pH was increased from 3.5 to 9, the degradation efficiency slightly decreased but remained consistently above 90%. These findings demonstrate that the Mo51Fe34B15 metallic glass wire maintains robust catalytic performance across a broad pH range, highlighting its strong pH adaptability.
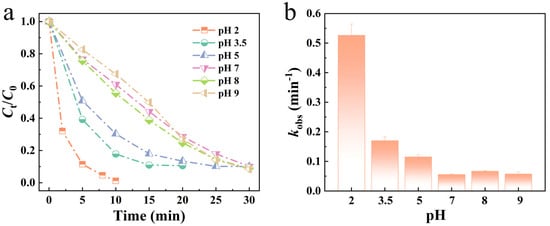
Figure 3.
(a) Mo51Fe34B15 metallic glass wire of different pH values to degrade the normalized concentration of RB5 solution and (b) reaction rate constant kobs (323 K, 20 ppm RB5, 0.1 M H2O2).
3.2.3. Effect of Dye Concentration
The effect of varying concentrations of RB5 dye solution on the degradation performance of Mo51Fe34B15 metallic glass wire is illustrated in Figure 4. Experimental results demonstrate that as the dye concentration increases gradually from 20 ppm to 30 ppm, the degradation efficiency of the Mo51Fe34B15 metallic glass wire significantly improves, with the degradation rate rising from 0.526 min−1 to 0.698 min−1. This enhancement can be attributed to the increased effective collision frequency between dye molecules and active sites on the alloy surface within a certain concentration range, which promotes a higher reaction rate. However, when the dye concentration further increases to 50 ppm, a decline in degradation rate is observed, decreasing from 0.698 min−1 to 0.465 min−1. This decrease may result from the limited availability of active sites, which prevents a large number of dye molecules from interacting effectively with the catalyst surface under high-concentration conditions. Although the reaction rate is reduced, the Mo51Fe34B15 metallic glass wire is still capable of achieving full cleavage of azo bonds within 15 min in a 50 ppm dye solution, demonstrating a decolorization efficiency of over 99%. This indicates that the material maintains excellent degradation capability even at elevated dye concentrations.
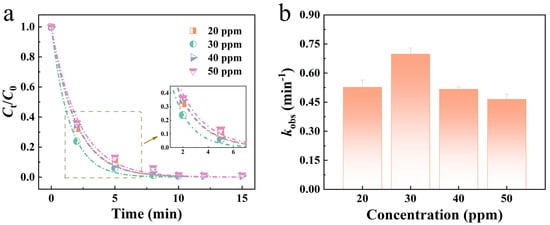
Figure 4.
(a) Mo51Fe34B15 metallic glass wire to degrade the normalized concentration of RB5 solution with different initial concentrations and (b) reaction rate constant kobs (323 K, pH = 2, 0.1 M H2O2).
3.2.4. Effect of Temperature
The effect of varying temperatures on the degradation performance of Mo51Fe34B15 metallic glass wire is illustrated in Figure 5. As the temperature increases from 303 K to 323 K, the degradation rate rises steadily from 0.163 min−1 to 0.526 min−1. This enhancement can be attributed to the increased thermal energy supplied to the reaction system, which elevates the kinetic energy of reactant molecules and thereby enhances both the frequency and effectiveness of molecular collisions. Collectively, these factors contribute to an accelerated catalytic reaction rate. As shown in the inset of Figure 5b, the activation energy of the Mo51Fe34B15 metallic glass wire in the RB5 dye solution is 46.6 kJ/mol, significantly lower than that of crystalline catalysts (60–250 kJ/mol). The reduced activation energy is primarily due to the unique amorphous structure of metallic glass, which lacks long-range ordered lattice arrangements [13]. This structural feature results in higher atomic disorder and a greater number of defect sites, which act as active centers for catalytic reactions. These structural advantages facilitate stronger interactions between reactant molecules and the catalyst surface, thereby promoting the overall catalytic process. The low activation energy enables metallic glass catalysts to exhibit superior catalytic efficiency. Consequently, under identical reaction conditions, metallic glass catalysts can degrade dye molecules more rapidly, leading to shorter wastewater treatment times and enhanced processing capacity.
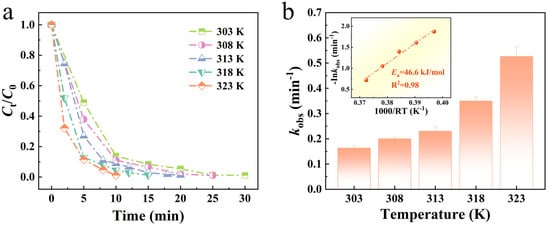
Figure 5.
(a) Mo51Fe34B15 metallic glass wire of different temperatures to degrade the normalized concentration of RB5 solution and (b) reaction rate constant kobs (pH = 2, 20 ppm RB5, 0.1 M H2O2).
3.3. Cycling Stability
Metallic glasses find application in wastewater treatment primarily through chemical reactions that occur between pollutants and the active sites present on their surfaces. However, during prolonged operation, metallic glasses may undergo corrosion or passivation, wherein the surface becomes coated with corrosion products. This phenomenon not only diminishes the number of available active sites but also significantly reduces the overall treatment efficiency. Surface passivation is widely regarded as a critical factor limiting the long-term stability of metallic glasses. For example, Fe-based metallic glasses [48] tend to accumulate substantial reaction products on their surfaces after degrading dye-containing wastewater. These residues are typically removed through acid washing combined with extended ultrasonic treatment to restore catalytic activity and enable reuse in subsequent degradation processes. Nevertheless, as the number of reuse cycles increases, the degradation efficiency tends to decline gradually. Current research focuses on evaluating the cyclic performance and long-term reactivity of metallic glass wires. The degradation efficiency of Mo51Fe34B15 metallic glass wire across varying cycle numbers is illustrated in Figure 6a. It is observed that the degradation efficiency begins to decrease from the 11th cycle, yet remains above 90% even after the 15th cycle. In comparison with the previously reported (Fe0.99Mo0.01)78Si9B13 metallic glass [40], which maintains effectiveness for only up to four reuse cycles, the Mo51Fe34B15 metallic glass wire demonstrates superior long-term reactivity and reusability in the degradation of dye wastewater. The outstanding performance of Mo51Fe34B15 metallic glass wire results from the synergy of composition, structure, and morphology. The Mo-Fe-B system provides high catalytic activity and strong elemental synergy. The amorphous structure enables a high density of active sites and excellent chemical stability. The filamentous shape facilitates efficient electron and mass transfer, reduces active component loss due to friction and wear, and when specifically arranged in the reactor, avoids clogging, agglomeration, and recovery issues commonly associated with powder catalysts.
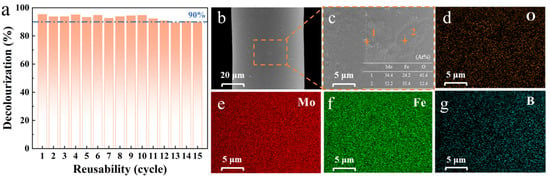
Figure 6.
(a) Cycle stability of Mo51Fe34B15 metallic glass wire in the degradation of RB5 dye solution. SEM images of the post-degradation surface morphology at different magnifications: (b,c) EDS point scanning results are included, as well as (c) the SEM image of the surface morphology along with the EDS elemental distribution map. (d–g) EDS area scanning results pertaining to (c) (323 K, pH = 2, 20 ppm RB5, 0.1 M H2O2).
Considering that the degradation reaction of organic wastewater predominantly occurs at the solid–liquid interface, a comprehensive surface analysis was carried out on the Mo51Fe34B15 metallic glass wire after undergoing 15 degradation cycles, as illustrated in Figure 6b–g, with the aim of elucidating the underlying reaction mechanism. In contrast to the smooth surface of the pristine wire [29], distinct selective corrosion was observed on the surface of the Mo51Fe34B15 metallic glass wire after 15 degradation cycles (Figure 6b,c). The table in Figure 6c presents the results of point-specific EDS analysis for regions 1 and 2, which further confirm that the primary constituents of the corrosion products are oxides of Mo and Fe. The formation of these oxides is likely attributed to chemical interactions between the organic constituents in the wastewater and the alloy surface during the catalytic degradation process, resulting in surface structural modifications. The results of the EDS area scanning are presented in the accompanying Figure 6d–g. The enrichment of oxygen suggests that oxidation plays a crucial role in the corrosion process, while the partial depletion of Mo, Fe, and B elements indicates the potential occurrence of selective elemental dissolution or migration during the reaction. These surface alterations not only influence the morphological characteristics of the catalyst but may also directly affect its catalytic performance. This result further confirms that the catalytic performance of the Mo51Fe34B15 metallic glass wire declines following repeated use.
3.4. XPS Analysis
To further elucidate the reaction mechanism of the Mo51Fe34B15 metallic glass wire, XPS analysis was performed on the degraded samples, with results presented in Figure 7. A considerable quantity of zero-valent metals was found to remain on the surface of the Mo51Fe34B15 metallic glass wire after the reaction. This suggests that not all metallic components were involved in the reaction, with a portion persisting on or near the material surface. In the Mo 3d spectrum of the Mo51Fe34B15 metallic glass (Figure 7a), the peaks at 227.2 eV and 231.1 eV are attributed to Mo0, those at 230.2 eV and 233.4 eV correspond to Mo4+, and the peaks at 231.7 eV and 234.7 eV are assigned to Mo6+. These findings indicate that Mo0 remained the primary electron donor during the degradation process, undergoing oxidation to form MoO2 and MoO3, with MoO3 being the dominant product as inferred from the relative peak areas. In the Fe 2p spectrum (Figure 7b), the peaks at 706.6 eV and 720.3 eV are associated with Fe0, those at 708.7 eV and 722.3 eV correspond to Fe2+, and the peaks at 710.3 eV and 724.4 eV are attributed to Fe3+. The peaks at 712.3 eV and 727.1 eV are identified as satellite peaks, and the primary corrosion product was determined to be Fe2O3. Furthermore, post-degradation analysis revealed that boron in the Mo51Fe34B15 metallic glass predominantly existed in the form of B-O bonds (Figure 7c), while oxygen was mainly detected in metal oxides and organic carbon compounds (Figure 7d).
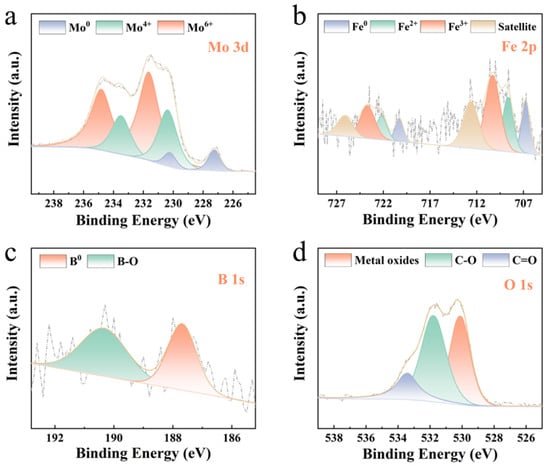
Figure 7.
XPS spectra of the Mo51Fe34B15 metallic glass wire used for the degradation of RB5 solution: (a) Mo 3d, (b) Fe 2p, (c) B 1s, and (d) O 1s spectra (323 K, pH = 2, 20 ppm RB5, 0.1 M H2O2).
3.5. Degradation Mechanism
To gain deeper insights into the catalytic mechanism of the Mo51Fe34B15 metallic glass wire, this study investigated its internal active components through EPR analysis. Under acidic conditions (pH = 2), characteristic •OH signals (1:2:2:1) indicative of a Fenton-like reaction were detected, as illustrated in Figure 8a. The results demonstrated that even a small quantity of TBA could significantly reduce •OH concentration, with a TBA concentration of 100 mM leading to the inhibition of over 70% of the reaction, as shown in Figure 8b,c. Based on these findings, the following reaction pathway is proposed to occur during the degradation process:
Mo0 → Mo4+ + 4e−
Fe0 → Fe2+ + 2e−
Mo4+ + 2H2O2 → Mo6+ + 2OH− + 2•OH
Fe2+ + H2O2 → Fe3+ + OH− + •OH
R-N=N-R’ + •OH → CO2 + H2O
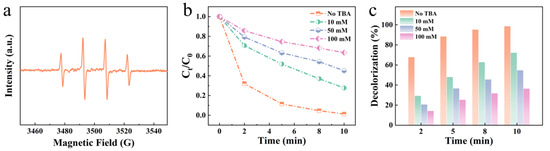
Figure 8.
Mo51Fe34B15 metallic glass wire for degradation of RB5 solution under acidic conditions (pH = 2): (a) Detection of active substances, quenching experiments, (b) normalized concentration and (c) decolorization rate.
The degradation mechanism of the Mo51Fe34B15 metallic glass wire for the treatment of RB5 dye wastewater is illustrated in Figure 9. Under highly acidic conditions, Mo0 and Fe0 serve as electron donors, synergistically promoting the decomposition of H2O2 to produce highly oxidative •OH radicals. These radicals attack the chromophoric groups of RB5 dye molecules, inducing structural disruption and enabling rapid decolorization within 10 min. The simultaneous incorporation of Mo and Fe into metallic glasses is designed to establish a dynamic and efficient bimetallic redox cycle. While Mo acts initially as an electron donor, its more critical function lies in serving as an “accelerator” for the Fe2+/Fe3+ redox cycle, thereby synergistically conferring high catalytic activity, enhanced stability, and superior pH adaptability to the amorphous alloy catalyst. This demonstrates the high degradation efficiency of the Mo51Fe34B15 metallic glass wire under acidic conditions. As the degradation process proceeds, surface corrosion occurs on the material, resulting in the formation of oxidation products such as MoO3 and Fe2O3, which gradually reduce the reaction efficiency. This study elucidates the catalytic mechanism of the Mo51Fe34B15 metallic glass wire, providing a scientific foundation for the development of more efficient and stable catalytic materials.
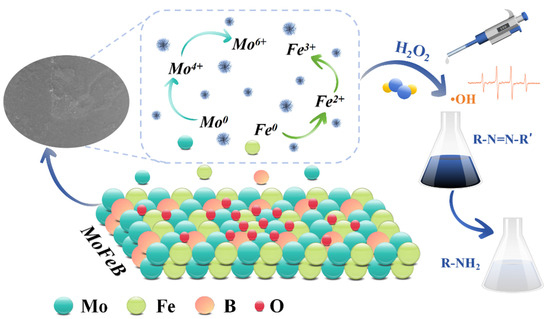
Figure 9.
Schematic diagram of the degradation mechanism by Mo51Fe34B15 metallic glass wire.
4. Conclusions
The Mo51Fe34B15 metallic glass wire was synthesized through compositional design. Its catalytic degradation performance in RB5 dye wastewater was investigated within a conventional Fenton-like oxidation system. The following conclusions were drawn:
- (1)
- The Mo51Fe34B15 metallic glass wires demonstrated a reaction rate constant of 0.698 min−1 in degrading a 20 ppm RB5 dye solution within 10 min, achieving a degradation efficiency of 98.8%.
- (2)
- During the reaction process, corrosion develops on the surface of the Mo51Fe34B15 metallic glass wire, leading to the formation of oxidative products such as MoO3 and Fe2O3, which gradually reduce catalytic efficiency.
- (3)
- The Mo51Fe34B15 metallic glass wire exhibits effective degradation capability toward RB5 dye solutions over a wide pH range from 2 to 9, demonstrating excellent pH adaptability.
- (4)
- The Mo51Fe34B15-H2O2 system overcomes the conventional limitation in which excessive H2O2 acts as a scavenger of •OH radicals. Even when the H2O2 concentration increases from 0.01 M to 0.1 M, the degradation rate of RB5 dye by the Mo51Fe34B15 metallic glass wire is significantly enhanced.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/met15101160/s1, Table S1: Research progress of metallic glass for azo dye wastewater treatment; Figure S1: The pseudo-first order kinetics of RB 5 degradation using Mo51Fe34B15 metallic glass wire.
Author Contributions
Y.-N.C.: Writing—original draft, Validation, Methodology, Investigation, Formal analysis, Data curation. B.S.: Writing—review and editing, Supervision, Methodology, Validation, Forma analysis. C.Z.: Methodology, Validation, Investigation. T.L.: Investigation, Validation, Formal analysis. C.S.: Validation, Investigation, Funding acquisition. S.G.: Writing—review and editing, Supervision, Methodology, Funding acquisition, Conceptualization. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 52471183) and the Fundamental Research Funds for the Central Universities (Nos. SWU-XDJH202313 and SWU-KQ22083).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Acknowledgments
We special acknowledge Hongju Zhang at the Analytical and Testing Center of Southwest University for assistance of XPS and SEM in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- De Benedetto, C.; Macario, A.; Siciliano, C.; Nagy, J.; De Luca, P. Adsorption of Reactive Blue 116 Dye and Reactive Yellow 81 Dye from Aqueous Solutions by Multi-Walled Carbon Nanotubes. Materials 2020, 13, 2757. [Google Scholar] [CrossRef]
- Duman, O.; Tunç, S.; Bozoglan, B.K.; Polat, T.G. Removal of triphenylmethane and reactive azo dyes from aqueous solution by magnetic carbon nanotube-κ-carrageenan-Fe3O4 nanocomposite. J. Alloys Compd. 2016, 687, 370–383. [Google Scholar] [CrossRef]
- Khouni, I.; Marrot, B.; Ben Amar, R. Treatment of reconstituted textile wastewater containing a reactive dye in an aerobic sequencing batch reactor using a novel bacterial consortium. Sep. Purif. Technol. 2012, 87, 110–119. [Google Scholar] [CrossRef]
- M-Ridha, M.J.; Hussein, S.I.; Alismaeel, Z.T.; Atiya, M.A.; Aziz, G.M. Biodegradation of reactive dyes by some bacteria using response surface methodology as an optimization technique. Alex. Eng. J. 2020, 59, 3551–3563. [Google Scholar] [CrossRef]
- De Luca, P.; Nagy, J. Treatment of Water Contaminated with Reactive Black-5 Dye by Carbon Nanotubes. Materials 2020, 13, 5508. [Google Scholar] [CrossRef] [PubMed]
- Satapanajaru, T.; Chokejaroenrat, C.; Pengthamkeerati, P. Removal of Reactive Black 5 and its degradation using combined treatment of nano-zerovalent iron activated persulfate and adsorption processes. Desalin. Water Treat. 2018, 102, 300–311. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Zhao, X.; Dong, Y.; Wang, W.; Lv, Y.; Cao, S.; Wang, L. Enhanced degradation of reactive black 5 via persulfate activation by natural bornite: Influencing parameters, mechanism and degradation pathway. Environ. Technol. 2023, 45, 3961–3973. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.D. Degradation of the azo dye Reactive Black 5 through peroxymonosulfate activation with functional S-doped graphene. Desalin. Water Treat. 2022, 252, 361–370. [Google Scholar] [CrossRef]
- Viana, D.F.; Salazar-Banda, G.R.; Leite, M.S. Electrochemical degradation of Reactive Black 5 with surface response and artificial neural networks optimization models. Sep. Sci. Technol. 2018, 53, 2647–2661. [Google Scholar] [CrossRef]
- Cuervo Lumbaque, E.; Gomes, M.F.; Da Silva Carvalho, V.; de Freitas, A.M.; Tiburtius, E.R.L. Degradation and ecotoxicity of dye Reactive Black 5 after reductive-oxidative process. Environ. Sci. Pollut. Res. 2016, 24, 6126–6134. [Google Scholar] [CrossRef]
- Gupta, S.; Zasońska, B.A.; Acharya, U.; Konefał, M.; Pokorný, V.; Petrovsky, E.; Breitenbach, S.; Unterweger, C.; Bober, P. Magnetoconductive poly(3,4-ethylenedioxythiophene)/maghemite adsorbent for the removal of Reactive Black 5 from aqueous media. Mater. Chem. Phys. 2022, 292, 126753. [Google Scholar] [CrossRef]
- Ferreira, L.C.; Lucas, M.S.; Fernandes, J.R.; Tavares, P.B. Photocatalytic oxidation of Reactive Black 5 with UV-A LEDs. J. Environ. Chem. Eng. 2016, 4, 109–114. [Google Scholar] [CrossRef]
- Zhang, L.C.; Jia, Z.; Lyu, F.; Liang, S.X.; Lu, J. A review of catalytic performance of metallic glasses in wastewater treatment: Recent progress and prospects. Prog. Mater. Sci. 2019, 105, 100576. [Google Scholar] [CrossRef]
- Priyadarshini, M.; Das, I.; Ghangrekar, M.M.; Blaney, L. Advanced oxidation processes: Performance, advantages, and scale-up of emerging technologies. J. Environ. Manag. 2022, 316, 1152925. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.M.; Chen, Z.; Zhang, J.W.; Shan, D.; Wu, Y.; Bai, L.M.; Wang, B.Q. Treatment of industrial dye wastewater and pharmaceutical residue wastewater by advanced oxidation processes and its combination with nanocatalysts: A review. J. Water Process Eng. 2021, 42, 102122. [Google Scholar] [CrossRef]
- Asghar, A.; Raman, A.A.A.; Daud, W. Advanced oxidation processes for in-situ production of hydrogen peroxide/hydroxyl radical for textile wastewater treatment: A review. J. Clean. Prod. 2015, 87, 826–838. [Google Scholar] [CrossRef]
- Goyal, R.; Singh, O.; Agrawal, A.; Samanta, C.; Sarkar, B. Advantages and limitations of catalytic oxidation with hydrogen peroxide: From bulk chemicals to lab scale process. Catal. Rev. 2020, 64, 229–285. [Google Scholar] [CrossRef]
- Yang, W.L.; Deng, Z.J.; Liu, L.B.; Zhou, K.C.; Sharel, P.E.; Meng, L.C.; Ma, L.; Wei, Q.P. Co-generation of hydroxyl and sulfate radicals via homogeneous and heterogeneous bi-catalysis with the EO-PS-EF tri-coupling system for efficient removal of refractory organic pollutants. Water Res. 2023, 243, 120312. [Google Scholar] [CrossRef]
- Bin, S.J.B.; Fong, K.S.; Chua, B.W.; Gupta, M. Mg-based bulk metallic glasses: A review of recent developments. J. Magnes. Alloys 2022, 10, 899–914. [Google Scholar] [CrossRef]
- Pei, L.; Zhang, X.; Yuan, Z. Application of Fe-Based Amorphous Alloy in Industrial Wastewater Treatment: A Review. J. Renew. Mater. 2022, 10, 969–991. [Google Scholar] [CrossRef]
- Jiang, J.L.; Jia, Z.; He, Q.; Wang, Q.; Lyu, F.; Zhang, L.C.; Liang, S.X.; Kruzic, J.J.; Lu, J. Synergistic function of iron and cobalt in metallic glasses for highly improving persulfate activation in water treatment. J. Alloys Compd. 2020, 822, 153574. [Google Scholar] [CrossRef]
- Chen, P.; Hu, X.; Qi, Y.; Wang, X.; Li, Z.; Zhao, L.; Liu, S.; Cui, C. Rapid Degradation of Azo Dyes by Melt-Spun Mg-Zn-Ca Metallic Glass in Artificial Seawater. Metals 2017, 7, 485. [Google Scholar] [CrossRef]
- Chen, Q.; Pang, J.; Yan, Z.C.; Hu, Y.H.; Guo, L.Y.; Zhang, H.; Zhang, L.C.; Wang, W.M. MgZn-based amorphous ribbon as a benign decolorizer in methyl blue solution. J. Non-Cryst. Solids 2020, 529, 119802. [Google Scholar] [CrossRef]
- Lu, S.H.; Wang, M.G.; Zhao, Z.K. Recent advances and future developments in Fe-based amorphous soft magnetic composites. J. Non-Cryst. Solids 2023, 616, 122440. [Google Scholar] [CrossRef]
- Pang, J.; Fu, F.; Li, W.; Zhu, L.; Tang, B. Fe-Mn binary oxide decorated diatomite for rapid decolorization of methylene blue with H2O2. Appl. Surf. Sci. 2019, 478, 54–61. [Google Scholar] [CrossRef]
- Chen, Q.; Yan, Z.; Zhang, H.; Kim, K.; Wang, W. Role of Nanocrystallites of Al-Based Glasses and H2O2 in Degradation Azo Dyes. Materials 2020, 14, 39. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Duan, X.G.; Qin, P.; Zhang, W.C.; Wang, W.M.; Yang, C.; Sun, H.Q.; Wang, S.B.; Zhang, L.C. Disordered Atomic Packing Structure of Metallic Glass: Toward Ultrafast Hydroxyl Radicals Production Rate and Strong Electron Transfer Ability in Catalytic Performance. Adv. Funct. Mater. 2017, 27, 1702258. [Google Scholar] [CrossRef]
- Tang, M.; Lai, L.; Su, C.; Li, C.; Zhang, C.; Guo, S. MoCoB metallic glass microwire catalysts for highly efficient and pH-universal degradation of wastewater. NPJ Mater. Degrad. 2023, 7, 73. [Google Scholar] [CrossRef]
- Chen, Y.N.; Xiao, S.; Yang, Y.; Su, C.; Dai, C.; Tang, J.; Ruan, Y.; Guo, S. The rapid degradation of dye wastewater utilizing MoFeB amorphous alloy wires. Colloids Surf. A Physicochem. Eng. Asp. 2025, 726, 137831. [Google Scholar] [CrossRef]
- Li, R.; Liu, X.J.; Wang, H.; Wu, Y.; Chan, K.C.; Lu, Z.P. Flexible glassy grid structure for rapid degradation of azo dye. Mater. Des. 2018, 155, 346–351. [Google Scholar] [CrossRef]
- Zhou, T.; Wu, X.H.; Zhang, Y.R.; Li, J.F.; Lim, T.T. Synergistic catalytic degradation of antibiotic sulfamethazine in a heterogeneous sonophotolytic goethite/oxalate Fenton-like system. Appl. Catal. B Environ. 2013, 136, 294–301. [Google Scholar] [CrossRef]
- Lv, Z.Y.; Liu, X.J.; Jia, B.; Wang, H.; Wu, Y.; Lu, Z.P. Development of a novel high-entropy alloy with eminent efficiency of degrading azo dye solutions. Sci. Rep. 2016, 6, 34213. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, Q.; Sun, L.G.; Wang, Q.; Zhang, L.C.; Wu, G.; Luan, J.H.; Jiao, Z.B.; Wang, A.D.; Liang, S.X.; et al. Attractive In Situ Self-Reconstructed Hierarchical Gradient Structure of Metallic Glass for High Efficiency and Remarkable Stability in Catalytic Performance. Adv. Funct. Mater. 2019, 29, 1807857. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Yun, L.; Chen, M.X.; Xu, D.D.; Cui, Z.Q.; Zeng, Q.S.; Lin, P.H.; Chu, C.L.; Shen, B.L. Competitive Effects of Structural Heterogeneity and Surface Chemical States on Catalytic Efficiency of FeSiBPCu Amorphous and Nanocrystalline Alloys. ACS Appl. Nano Mater. 2019, 2, 214–227. [Google Scholar] [CrossRef]
- Hou, L.; Wang, Q.; Fan, X.; Miao, F.; Yang, W.; Shen, B. Effect of Co addition on catalytic activity of FePCCu amorphous alloy for methylene blue degradation. New J. Chem. 2019, 43, 6126–6135. [Google Scholar] [CrossRef]
- Jia, Z.; Kang, J.; Zhang, W.C.; Wang, W.M.; Yang, C.; Sun, H.; Habibi, D.; Zhang, L.C. Surface aging behaviour of Fe-based amorphous alloys as catalysts during heterogeneous photo Fenton-like process for water treatment. Appl. Catal. B Environ. 2017, 204, 537–547. [Google Scholar] [CrossRef]
- Tang, Y.; Shao, Y.; Chen, N.; Yao, K.F. Rapid decomposition of Direct Blue 6 in neutral solution by Fe-B amorphous alloys. RSC Adv. 2015, 5, 6215–6221. [Google Scholar] [CrossRef]
- Zhang, C.Q.; Zhu, Z.W.; Zhang, H.F.; Hu, Z.Q. Rapid decolorization of Acid Orange II aqueous solution by amorphous zero-valent iron. J. Environ. Sci. 2012, 24, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Weng, N.; Wang, F.; Qin, F.X.; Tang, W.Y.; Dan, Z.H. Enhanced Azo-Dyes Degradation Performance of Fe-Si-B-P Nanoporous Architecture. Materials 2017, 10, 1001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Q.; Zhang, H.F.; Lv, M.Q.; Hu, Z.Q. Decolorization of azo dye solution by Fe-Mo-Si-B amorphous alloy. J. Non-Cryst. Solids 2010, 356, 1703–1706. [Google Scholar] [CrossRef]
- Zhang, C.Q.; Zhu, Z.W.; Zhang, H.F.; Hu, Z.Q. On the decolorization property of Fe-Mo-Si-B alloys with different structures. J. Non-Cryst. Solids 2012, 358, 61–64. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, Z.; Zhang, H. Effects of the addition of Co, Ni or Cr on the decolorization properties of Fe-Si-B amorphous alloys. J. Phys. Chem. Solids 2017, 110, 152–160. [Google Scholar] [CrossRef]
- Liang, S.X.; Zhang, W.C.; Wang, W.M.; Jia, G.H.; Yang, W.M.; Zhang, L.C. Surface reactivation of FeNiPC metallic glass: A strategy for highly enhanced catalytic behavior. J. Phys. Chem. Solids 2019, 132, 89–98. [Google Scholar] [CrossRef]
- Chen, S.; Yang, G.; Luo, S.; Yin, S.; Jia, J.; Li, Z.; Gao, S.; Shao, Y.; Yao, K. Unexpected high performance of Fe-based nanocrystallized ribbons for azo dye decomposition. J. Mater. Chem. A 2017, 5, 14230–14240. [Google Scholar] [CrossRef]
- Luo, X.K.; Li, R.; Zong, J.Z.; Zhang, Y.; Li, H.F.; Zhang, T. Enhanced degradation of azo dye by nanoporous-copper-decorated Mg-Cu-Y metallic glass powder through dealloying pretreatment. Appl. Surf. Sci. 2014, 305, 314–320. [Google Scholar] [CrossRef]
- Zhang, C.Q.; Zhu, Z.W.; Zhang, H.F. Mg-based amorphous alloys for decolorization of azo dyes. Results Phys. 2017, 7, 2054–2056. [Google Scholar] [CrossRef]
- Zhao, B.; Zhu, Z.; Qin, X.D.; Li, Z.; Zhang, H. Highly efficient and stable CuZr-based metallic glassy catalysts for azo dye degradation. J. Mater. Sci. Technol. 2020, 46, 88–97. [Google Scholar] [CrossRef]
- Zhang, S.D.; Wu, J.; Qi, W.B.; Wang, J.Q. Effect of porosity defects on the long-term corrosion behaviour of Fe-based amorphous alloy coated mild steel. Corros. Sci. 2016, 110, 57–70. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).