Room-Temperature Superplasticity in a Biodegradable Zn-0.1Mg Alloy
Abstract
1. Introduction
2. Materials and Methods
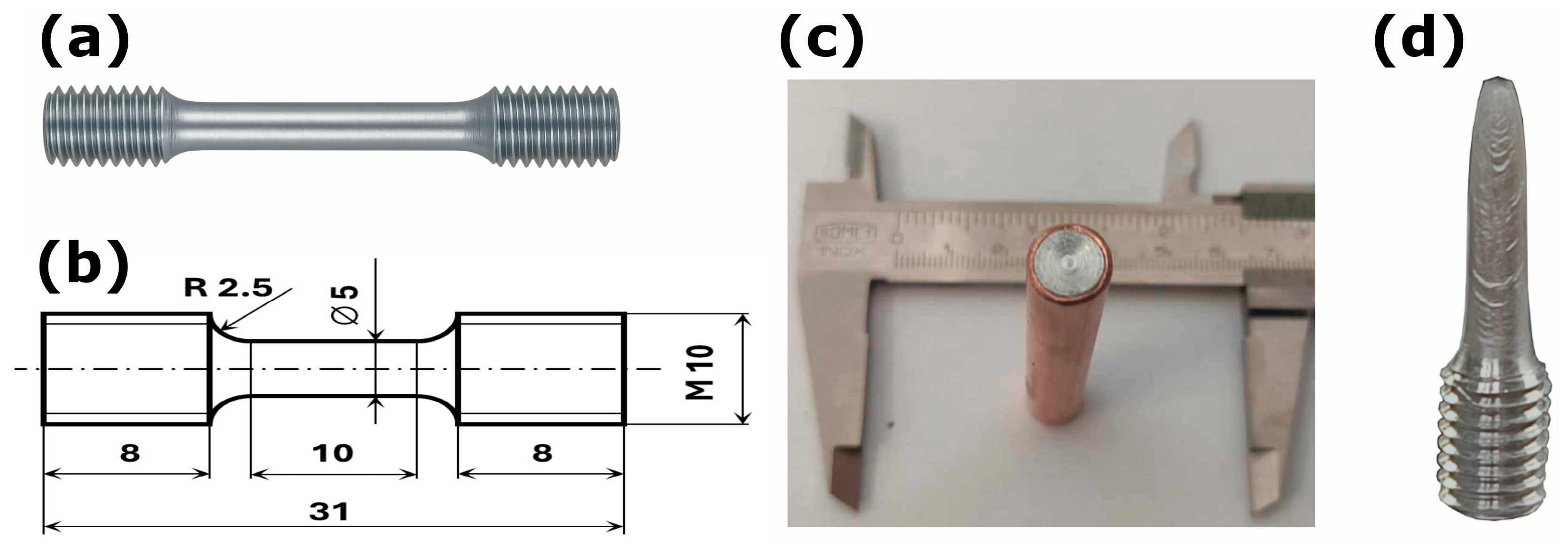
2.1. Material Preparation
2.2. Mechanical Properties Analysis
2.3. X-Ray Diffraction Experiment
2.4. Structure Analysis
- (i)
- Position 0, minimally affected by deformation;
- (ii)
- Position 36, just beneath the fracture surface (see Figure 2).
3. Results
3.1. Mechanical Testing Results
3.2. XRD Structural Analysis
3.3. Analysis of First-Order Elastic Residual Strains and Stresses
3.4. Structure Analysis
4. Discussion
5. Conclusions
Main Findings
- Relative to the annealed baseline (380 °C/2 h + water quench), characterized by a coarse-grained hcp Zn solid solution with grain size > 30 µm, low dislocation density, and minor Mg2Zn11 (see Table 2), the two-step ECAP route (150 °C/RT) produced a concurrent increase in strength and ductility at room temperature.
- At the lowest applied strain rate (0.0001 s−1), an elongation of up to 240% was achieved, representing an exceptional manifestation of superplasticity at room temperature.
- Transmission (Debye-Scherrer) X-ray diffraction using high-energy synchrotron radiation confirmed the presence of the hcp supersaturated Zn solid solution together with minor intermetallic phases Mg2Zn11 and possibly also MgZn2. No transformation of the matrix hcp-Zn phase (space group P63/mmc, No. 194) was detected under severe deformation.
- The ECAP-processed alloy exhibits an ultrafine microstructure with TEM grain size ≈ 0.5–1.0 μm. The XRD line-profile-derived domain (‘crystallite’) size is ≈1 μm and remains unchanged within uncertainty before vs. after tensile testing. Thus, the microstructural refinement is attributed to ECAP, not to the subsequent tensile deformation.
- The activation of non-basal slip systems (prismatic and pyramidal) significantly contributed to the enhanced ductility in the hcp lattice of the supersaturated Zn solid solution.
- The two-step ECAP route is operationally simpler than multi-pass processing and is amenable to larger material volumes. The present mechanical and microstructural results indicate that Zn-Mg alloys are promising candidates for certain bioresorbable applications; however, translational potential remains contingent on targeted degradation and biocompatibility studies and fatigue/fretting-corrosion evaluation.
6. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ECAP | Equal-Channel Angular Pressing |
| XRD | X-ray Diffraction |
| 2D XRD | Two-Dimensional X-ray Diffraction |
| hcp | Hexagonal close-packed |
| ED | Extrusion Direction |
| EBSD | Electron Backscatter Diffraction |
| YS | Yield strength |
| UTS | Ultimate tensile strength |
| FE | Fracture elongation |
| CT | Computed Tomography |
| NMR | Nuclear Magnetic Resonance |
References
- Abd-Elaziem, W.; Darwish, M.A.; Hamada, A.; Daoush, W.M. Titanium-Based Alloys and Composites for Orthopedic Implants Applications: A Comprehensive Review. Mater. Des. 2024, 241, 112850. [Google Scholar] [CrossRef]
- Abd-elrhman, Y.; Gepreel, M.A.H.; Abdel-Moniem, A.; Kobayashi, S. Compatibility Assessment of New V-Free Low-Cost Ti-4.7Mo-4.5Fe Alloy for Some Biomedical Applications. Mater. Des. 2016, 97, 445–453. [Google Scholar] [CrossRef]
- Ziaie, B.; Khalili, S.M.R. Evaluation of Fatigue Life for Dental Implants Using FEM Analysis. Prosthesis 2021, 3, 300–313. [Google Scholar] [CrossRef]
- Hofmann, G.O.; Wagner, F.D. New Implant Designs for Bioresorbable Devices in Orthopaedic Surgery. Clin. Mater. 1993, 14, 207–215. [Google Scholar] [CrossRef]
- Viceconti, M.; Muccini, R.; Bernakiewicz, M.; Baleani, M.; Cristofolini, L. Large-Sliding Contact Elements Accurately Predict Levels of Bone-Implant Micromotion Relevant to Osseointegration. J. Biomech. 2000, 33, 1611–1618. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, W.S.; Sarver, D.; Verstynen, M. Bioresorbable Implants—Practical Considerations. Bone 1996, 19, S109–S119. [Google Scholar] [CrossRef]
- Waksman, R. Update on Bioabsorbable Stents: From Bench to Clinical. J. Interv. Cardiol. 2006, 19, 414–421. [Google Scholar] [CrossRef]
- Bonan, R.; Asgar, A.W. Biodegradable Stents—Where Are We in 2009? US Cardiol. Rev. 2009, 6, 81–84. [Google Scholar] [CrossRef]
- Fischman, D.L.; Leon, M.B.; Baim, D.S.; Schatz, R.A.; Savage, M.P.; Penn, I.; Detre, K.; Veltri, L.; Ricci, D.; Nobuyoshi, M.; et al. A Randomized Comparison of Coronary-Stent Placement and Balloon Angioplasty in the Treatment of Coronary Artery Disease. N. Engl. J. Med. 1994, 331, 496–501. [Google Scholar] [CrossRef]
- Caicedo, M.S.; Pennekamp, P.H.; McAllister, K.; Jacobs, J.J.; Hallab, N.J. Soluble Ions More than Particulate Cobalt-Alloy Implant Debris Induce Monocyte Costimulatory Molecule Expression and Release of Proinflammatory Cytokines Critical to Metal-Induced Lymphocyte Reactivity. J. Biomed. Mater. Res. A 2010, 93, 1312–1321. [Google Scholar] [CrossRef]
- Urban, R.M.; Jacobs, J.J.; Tomlinson, M.J.; Gavrilovic, J.; Black, J.; Peoc’h, M. Dissemination of Wear Particles to the Liver, Spleen, and Abdominal Lymph Nodes of Patients with Hip or Knee Replacement. J. Bone Jt. Surg. 2000, 82, 457–477. [Google Scholar] [CrossRef]
- Kiran, M.; Boscainos, P.J. Adverse Reactions to Metal Debris in Metal-on-Polyethylene Total Hip Arthroplasty Using a Titanium-Molybdenum-Zirconium-Iron Alloy Stem. J. Arthroplast. 2015, 30, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Tande, A.J.; Patel, R. Prosthetic Joint Infection. Clin. Microbiol. Rev. 2014, 27, 302–345. [Google Scholar] [CrossRef]
- Stephen, S. Arthroprosthetic Cobaltism Associated with Metal on Metal Hip Implants. BMJ 2012, 344, 344–430. [Google Scholar] [CrossRef] [PubMed]
- Langkamer, V.G.; Case, C.P.; Heap, P.; Taylor, A.; Collins, C.; Pearse, M.; Solomon, L. Systemic Distribution of Wear Debris after Hip Replacement. A Cause for Concern? J. Bone Jt. Surg.-Ser. B 1992, 74, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, P.F.; Lichstein, P.M.; Shen, C.; Tokarski, A.T.; Parvizi, J. Why Are Total Knee Arthroplasties Failing Today-Has Anything Changed after 10 Years? J. Arthroplast. 2013, 29, 1774–1778. [Google Scholar] [CrossRef]
- Kabir, H.; Munir, K.; Wen, C.; Li, Y. Recent Research and Progress of Biodegradable Zinc Alloys and Composites for Biomedical Applications: Biomechanical and Biocorrosion Perspectives. Bioact. Mater. 2021, 6, 836–879. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable Metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Tahmasebifar, A.; Kayhan, S.M.; Evis, Z.; Tezcaner, A.; Çinici, H.; Koç, M. Mechanical, Electrochemical and Biocompatibility Evaluation of AZ91D Magnesium Alloy as a Biomaterial. J. Alloys Compd. 2016, 687, 906–919. [Google Scholar] [CrossRef]
- Heiden, M.; Walker, E.; Stanciu, L. Magnesium, Iron and Zinc Alloys, the Trifecta of Bioresorbable Orthopaedic and Vascular Implantation—A Review. J. Biotechnol. Biomater. 2015, 5, 1–9. [Google Scholar] [CrossRef]
- Reilly, D.T.; Burstein, A.H. The Elastic and Ultimate Properties of Compact Bone Tissue. J. Biomech. 1975, 8, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.N.; Zheng, Y.F. A Review on Magnesium Alloys as Biodegradable Materials. Front. Mater. Sci. China 2010, 4, 111–115. [Google Scholar] [CrossRef]
- Wang, Z.; Song, J.; Peng, Y. New Insights and Perspectives into Biodegradable Metals in Cardiovascular Stents: A Mini Review. J. Alloys Compd. 2024, 1002, 175313. [Google Scholar] [CrossRef]
- Kraus, T.; Moszner, F.; Fischerauer, S.; Fiedler, M.; Martinelli, E.; Eichler, J.; Witte, F.; Willbold, E.; Schinhammer, M.; Meischel, M.; et al. Biodegradable Fe-Based Alloys for Use in Osteosynthesis: Outcome of an in Vivo Study after 52 Weeks. Acta Biomater. 2014, 10, 3346–3353. [Google Scholar] [CrossRef]
- Yang, H.; Jia, B.; Zhang, Z.; Qu, X.; Li, G.; Lin, W.; Zhu, D.; Dai, K.; Zheng, Y. Alloying Design of Biodegradable Zinc as Promising Bone Implants for Load-Bearing Applications. Nat. Commun. 2020, 11, 401. [Google Scholar] [CrossRef]
- Lu, C.; Song, C.; Yu, Y.; Yang, L.; Zheng, W.; Luo, F.; Xiao, Y.; Luo, J.; Xu, J. Biodegradable Zinc Alloys with High Strength and Suitable Mechanical Integrity as Bone Repair Metals. Sci. Rep. 2024, 14, 30558. [Google Scholar] [CrossRef]
- Li, H.F.; Shi, Z.Z.; Wang, L.N. Opportunities and Challenges of Biodegradable Zn-Based Alloys. J. Mater. Sci. Technol. 2020, 46, 136–138. [Google Scholar] [CrossRef]
- Kubásek, J.; Dvorský, D.; Čapek, J.; Marketa, S.; Klara, H.; Vojtěch, D. Zinc Alloys as Prospective Materials for Biodegradable Medical Devices. Manuf. Technol. 2020, 20, 779–784. [Google Scholar] [CrossRef]
- Young, J.; Reddy, R.G. Synthesis, Mechanical Properties, and in Vitro Corrosion Behavior of Biodegradable Zn-Li-Cu Alloys. J. Alloys Compd. 2020, 844, 156257. [Google Scholar] [CrossRef]
- Duan, J.; Li, L.; Liu, C.; Suo, Y.; Wang, X.; Yang, Y. Novel Zn-2Cu-0.2Mn-XLi (x = 0, 0.1 and 0.38) Alloys Developed for Potential Biodegradable Implant Applications. J. Alloys Compd. 2022, 916, 165478. [Google Scholar] [CrossRef]
- Naik, M.; Narasaiah, N.; Chakravarthy, P.; Kumar, R.A. Hot-Extrusion Behavior of Biodegradable Zn-Mg Alloys. Mater. Today Proc. 2022, 56, 1432–1439. [Google Scholar] [CrossRef]
- Ye, L.; Liu, H.; Sun, C.; Zhuo, X.; Ju, J.; Xue, F.; Bai, J.; Jiang, J.; Xin, Y. Achieving High Strength, Excellent Ductility, and Suitable Biodegradability in a Zn-0.1Mg Alloy Using Room-Temperature ECAP. J. Alloys Compd. 2022, 926, 166906. [Google Scholar] [CrossRef]
- Ji, C.; Ma, A.; Jiang, J.; Song, D.; Liu, H.; Guo, S. Research Status and Future Prospects of Biodegradable Zn-Mg Alloys. J. Alloys Compd. 2024, 993, 174669. [Google Scholar] [CrossRef]
- Pachla, W.; Przybysz, S.; Jarzębska, A.; Bieda, M.; Sztwiertnia, K.; Kulczyk, M.; Skiba, J. Structural and Mechanical Aspects of Hypoeutectic Zn-Mg Binary Alloys for Biodegradable Vascular Stent Applications. Bioact. Mater. 2021, 6, 26–44. [Google Scholar] [CrossRef]
- Xiao, C.; Wang, L.; Ren, Y.; Sun, S.; Zhang, E.; Yan, C.; Liu, Q.; Sun, X.; Shou, F.; Duan, J.; et al. Indirectly Extruded Biodegradable Zn-0.05wt%Mg Alloy with Improved Strength and Ductility: In Vitro and in Vivo Studies. J. Mater. Sci. Technol. 2018, 34, 1618–1627. [Google Scholar] [CrossRef]
- Jarzȩbska, A.; Bieda, M.; Kawałko, J.; Koprowski, P.; Rogal, L.; Chulist, R.; Kania, B.; Sztwiertnia, K.; Pachla, W.; Kulczyk, M. Synergistic Effect of Mg Addition and Hydrostatic Extrusion on Microstructure and Texture of Biodegradable Low-Alloyed Zinc. IOP Conf. Ser. Mater. Sci. Eng. 2018, 375, 012008. [Google Scholar] [CrossRef]
- Kočiško, R.; Kvačkaj, T.; Kováčová, A.; Zemko, M. The Influence of ECAP Geometry on the Effective Strain Distribution. Adv. Mater. Res. 2015, 1127, 135–141. [Google Scholar] [CrossRef]
- Zhang, W.; Ding, H.; Cai, M.; Yang, W.; Li, J. Low-Temperature Superplastic Deformation Mechanism in Ti-6Al-4V Alloy Processed by Friction Stir Processing. Mater. Sci. Eng. A 2019, 764, 138261. [Google Scholar] [CrossRef]
- Nazeer, F.; Long, J.; Yang, Z.; Li, C. Superplastic Deformation Behavior of Mg Alloys: A-Review. J. Magnes. Alloys 2022, 10, 97–109. [Google Scholar] [CrossRef]
- Wang, X.; Meng, B.; Han, J.; Wan, M. Effect of Grain Size on Superplastic Deformation Behavior of Zn-0.033 Mg Alloy. Mater. Sci. Eng. A 2023, 870, 144877. [Google Scholar] [CrossRef]
- Withers, P.J.; Bhadeshia, H.K.D.H. Residual Stress Part 2—Nature and Origins. Mater. Sci. Technol. 2001, 17, 366–375. [Google Scholar] [CrossRef]
- Jarzębska, A.; Bieda, M.; Maj, Ł.; Chulist, R.; Wojtas, D.; Strąg, M.; Sułkowski, B.; Przybysz, S.; Pachla, W.; Sztwiertnia, K. Controlled Grain Refinement of Biodegradable Zn-Mg Alloy: The Effect of Magnesium Alloying and Multi-Pass Hydrostatic Extrusion Preceded by Hot Extrusion. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2020, 51, 6784–6796. [Google Scholar] [CrossRef]
- Nieh, T.G.; Wadsworth, J.; Sherby, O.D. Superplasticity in Metals and Ceramics; Proceedings of the Riso International Symposium on Metallurgy and Materials Science; Cambridge University Press: Cambridge, UK, 1997; pp. 207–231. [Google Scholar] [CrossRef]
- Drakopoulos, M.; Connolley, T.; Reinhard, C.; Atwood, R.; Magdysyuk, O.; Vo, N.; Hart, M.; Connor, L.; Humphreys, B.; Howell, G.; et al. I12: The Joint Engineering, Environment and Processing (JEEP) Beamline at Diamond Light Source. Synchrotron Radiat. 2015, 22, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Toby, B.H.; Von Dreele, R.B. GSAS-II: The Genesis of a Modern Open-Source All Purpose Crystallography Software Package. Appl. Crystallogr. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Rietveld, H.M. A Profile Refinement Method for Nuclear and Magnetic Structures. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Withers, P.J.; Bhadeshia, H.K.D.H. Residual Stress Part 1—Measurement Techniques. Mater. Sci. Technol. 2001, 17, 355–365. [Google Scholar] [CrossRef]
- Fitzpatrick, M.E.; Lodini, A. Analysis of Residual Stress by Diffraction Using Neutron and Synchrotron Radiation; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar] [CrossRef]
- He, B.B. Two-Dimensional X-Ray Diffraction; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 1–426. [Google Scholar] [CrossRef]
- Guo, P.; Li, F.; Yang, L.; Bagheri, R.; Zhang, Q.; Li, B.Q.; Cho, K.; Song, Z.; Sun, W.; Liu, H. Ultra-Fine-Grained Zn-0.5Mn Alloy Processed by Multi-Pass Hot Extrusion: Grain Refinement Mechanism and Room-Temperature Superplasticity. Mater. Sci. Eng. A 2019, 748, 262–266. [Google Scholar] [CrossRef]
- Cao, F.; Li, L.; Suo, Y.; Wang, X.; Yang, Y.; Duan, J. High-Strength and High-Ductility Low-Alloy Biodegradable Zn-Mg-Li Alloy via Hybrid Extrusion-ECAP Processing: In Vitro and in Vivo Study. J. Alloys Compd. 2025, 1038, 182884. [Google Scholar] [CrossRef]
- Nan, X.L.; Wang, H.Y.; Zhang, L.; Li, J.B.; Jiang, Q.C. Calculation of Schmid Factors in Magnesium: Analysis of Deformation Behaviors. Scr. Mater. 2012, 67, 443–446. [Google Scholar] [CrossRef]
- Cepeda-Jiménez, C.M.; Molina-Aldareguia, J.M.; Pérez-Prado, M.T. Origin of the Twinning to Slip Transition with Grain Size Refinement, with Decreasing Strain Rate and with Increasing Temperature in Magnesium. Acta Mater. 2015, 88, 232–244. [Google Scholar] [CrossRef]
- Wątroba, M.; Bednarczyk, W.; Kawałko, J.; Bała, P. Effect of Zirconium Microaddition on the Microstructure and Mechanical Properties of Zn-Zr Alloys. Mater. Charact. 2018, 142, 187–194. [Google Scholar] [CrossRef]








| Property | Vascular Scaffolds | Orthopedic Scaffolds |
|---|---|---|
| Cell response | Support adhesion of vascular endothelial cells | Support bone growth (osteoblasts, osteoclasts) |
| Mechanical integrity | >8 months | >6 months |
| Yield strength | >200 MPa | >230 MPa |
| Tensile strength | >300 MPa | >300 MPa |
| Fracture elongation | >15–18% | >15–18% |
| Elastic modulus | Low, flexible (for vessel bending) | Close to cortical bone (10–20 GPa) |
| Fatigue limit (107 cycles) | >256 MPa | >256 MPa |
| Elastic recoil after expansion | <4% | N/A |
| Hydrogen evolution | <10 μL/cm2/day | <10 μL/cm2/day |
| Sample | YS [MPa] | UTS [MPa] | FE [%] |
|---|---|---|---|
| Annealed, v = 0.00025 s−1 | 70 ± 2.4 | 74 ± 2.9 | 1.8 ± 0.07 |
| 150 °C/RT, v = 0.001 s−1 | 219 ± 8 | 258 ± 9 | 62 ± 2.5 |
| 150 °C/RT, v = 0.00025 s−1 | 165 ± 6 | 212 ± 7 | 150 ± 6 |
| 150 °C/RT, v = 0.0001 s−1 | 147 ± 5 | 173 ± 6 | 240 ± 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saksl, K.; Kočiško, R.; Petroušek, P.; Matvija, M.; Fujda, M.; Csík, D.; Molčanová, Z.; Ballóková, B.; Cuperová, I.; Gáborová, K.; et al. Room-Temperature Superplasticity in a Biodegradable Zn-0.1Mg Alloy. Metals 2025, 15, 1161. https://doi.org/10.3390/met15101161
Saksl K, Kočiško R, Petroušek P, Matvija M, Fujda M, Csík D, Molčanová Z, Ballóková B, Cuperová I, Gáborová K, et al. Room-Temperature Superplasticity in a Biodegradable Zn-0.1Mg Alloy. Metals. 2025; 15(10):1161. https://doi.org/10.3390/met15101161
Chicago/Turabian StyleSaksl, Karel, Róbert Kočiško, Patrik Petroušek, Miloš Matvija, Martin Fujda, Dávid Csík, Zuzana Molčanová, Beáta Ballóková, Iryna Cuperová, Katarína Gáborová, and et al. 2025. "Room-Temperature Superplasticity in a Biodegradable Zn-0.1Mg Alloy" Metals 15, no. 10: 1161. https://doi.org/10.3390/met15101161
APA StyleSaksl, K., Kočiško, R., Petroušek, P., Matvija, M., Fujda, M., Csík, D., Molčanová, Z., Ballóková, B., Cuperová, I., Gáborová, K., Lisnichuk, M., Lupták, M., & Lupták, A. (2025). Room-Temperature Superplasticity in a Biodegradable Zn-0.1Mg Alloy. Metals, 15(10), 1161. https://doi.org/10.3390/met15101161







