Influence of the Hot-Pressing Rate on the Interface Feature and Mechanical Properties of Mg/Al Composite Plates
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
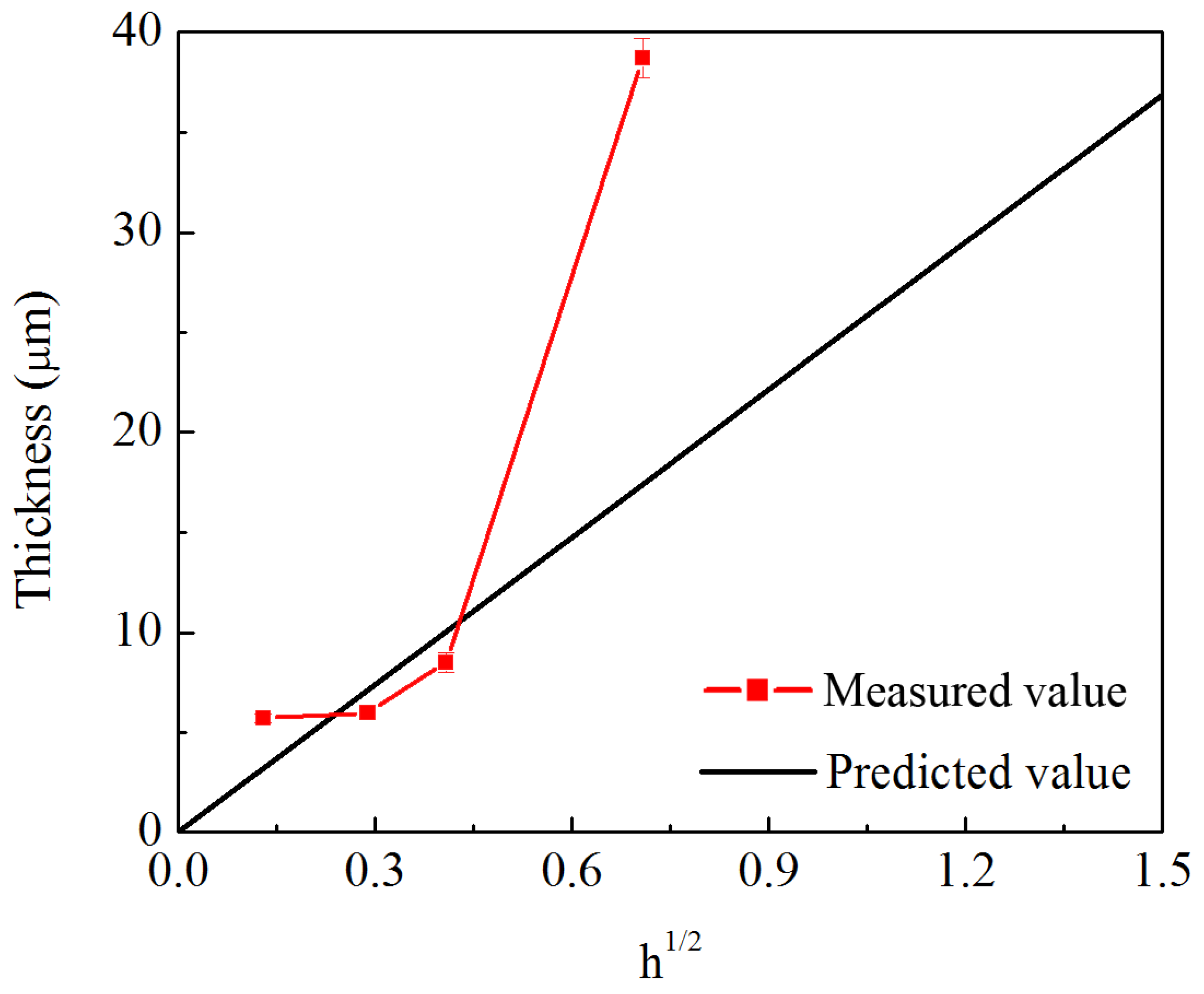
3.1. The Impact of the Strain Rate on Diffusion Thickness
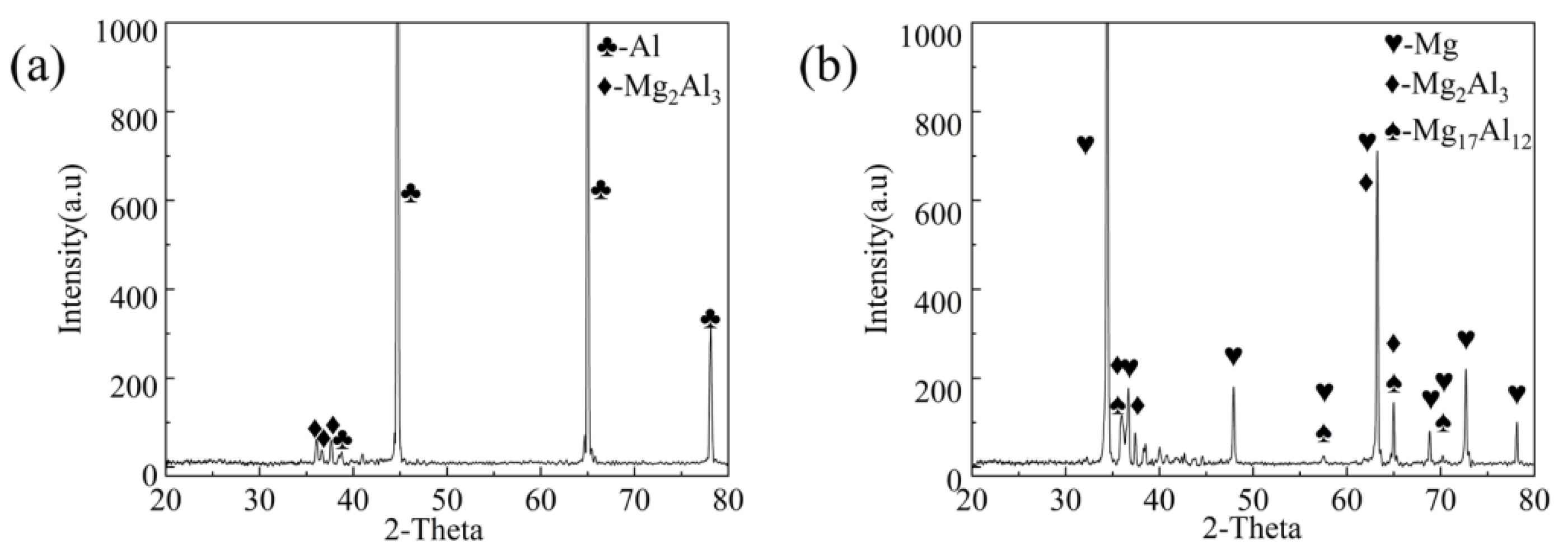
3.2. Phase Compositions at the Interface
3.3. Shear Strength and Fracture Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gurau, G.; Gurau, C.; Fernandes, F.M.B.; Alexandru, P.; Sampath, V.; Marin, M.; Galbinasu, B.M. Structural Characteristics of Multilayered Ni-Ti Nanocomposite Fabricated by High Speed High Pressure Torsion (HSHPT). Metals 2020, 10, 1629. [Google Scholar] [CrossRef]
- Kunčická, L.; Kocich, R. Optimizing electric conductivity of innovative Al-Cu laminated composites via thermomechanical treatment. Mater. Des. 2022, 215, 110441. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, C.; Gao, K.; Liu, B.; Ji, P.; He, J.; Wang, G.; Yin, F. Thickness effect of graphene film on optimizing the interface and mechanical properties of Cu/Ni multilayer composites. Mater. Sci. Eng. A 2020, 798, 140111. [Google Scholar] [CrossRef]
- Peng, H.; Chen, D.; Bai, X.; Wang, P.; Li, D.; Jiang, X. Microstructure and mechanical properties of Mg-to-Al dissimilar welded joints with an Ag interlayer using ultrasonic spot welding. J. Magnes. Alloy. 2020, 8, 552–563. [Google Scholar] [CrossRef]
- Zhu, C.; Xu, S.; Gao, W.; Meng, Y.; Lin, S.; Dai, L. Microstructure characteristics and mechanical properties of Al/Mg joints manufactured by magnetic pulse welding. J. Magnes. Alloy. 2021, 11, 2366–2375. [Google Scholar] [CrossRef]
- Qin, L.; Fan, M.; Guo, X.; Tao, J. Plastic deformation behaviors of Ti-Al laminated composite fabricated by vacuum hot-pressing. Vacuum 2018, 155, 96–107. [Google Scholar] [CrossRef]
- Bai, S.; Liu, L.; Li, K.; Jiang, B.; Huang, G.; Zhang, D.; Pan, F. Investigation into the microstructure, tensile properties and bendability of Mg–Al–Zn/Mg-xGd laminated composite sheets extruded by porthole die. J. Mater. Res. Technol. 2022, 21, 12–29. [Google Scholar] [CrossRef]
- Bai, S.; Wei, L.; He, C.; Liu, L.; Dong, Z.; Liu, W.; Jiang, B.; Huang, G.; Zhang, D.; Xu, J. Effects of layer thickness ratio on the bendability of Mg-Al-Zn/Mg-Gd laminated composite sheet. J. Mater. Res. Technol. 2022, 21, 1013–1028. [Google Scholar] [CrossRef]
- Bi, X.; Hu, Y.; Li, R.; Zhao, H.; Li, T. A novel method for preparing Al/Mg/Al laminated composite material, processing maps and interface diffusion analysis. J. Alloys Compd. 2022, 900, 163417. [Google Scholar] [CrossRef]
- Liu, T.; Song, B.; Huang, G.; Jiang, X.; Guo, S.; Zheng, K.; Pan, F. Preparation, structure and properties of Mg/Al laminated metal composites fabricated by roll-bonding, a review. J. Magnes. Alloy. 2022, 10, 2062–2093. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, T.; Liu, C.; Ma, Y.; Liu, W. Investigation on microstructure, mechanical properties and fracture mechanism of Mg/Al laminated composites. Mater. Sci. Eng. A 2022, 848, 143410. [Google Scholar] [CrossRef]
- Prasad, S.V.S.; Prasad, S.B.; Verma, K.; Mishra, R.K.; Kumar, V.; Singh, S. The role and significance of Magnesium in modern day research-A review. J. Magnes. Alloy. 2022, 10, 1–61. [Google Scholar] [CrossRef]
- Xu, W.; Birbilis, N.; Sha, G.; Wang, Y.; Daniels, J.E.; Xiao, Y.; Ferry, M. A high-specific-strength and corrosion-resistant magnesium alloy. Nat. Mater. 2015, 14, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Rooy, E.L.; Committee, A.H. Introduction to Aluminum and Aluminum Alloys. In Properties and Selection: Nonferrous Alloys and Special-Purpose Materials; ASM International: Almere, The Netherlands, 1990. [Google Scholar]
- Zeng, Y.; Gong, J. The trajectory optimization of spray painting robot for conical surface. Adv. Mater. Res. 2010, 139, 2189–2194. [Google Scholar] [CrossRef]
- Guan, F.; Jiang, W.; Li, G.; Zhu, J.; Wang, J.; Jie, G.; Fan, Z. Effect of vibration on interfacial microstructure and mechanical properties of Mg/Al bimetal prepared by a novel compound casting. J. Magnes. Alloy. 2022, 10, 2296–2309. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, W.; Cao, X.; Wu, J. The effect of annealing on the interface microstructure and mechanical characteristics of AZ31B/AA6061 composite plates fabricated by explosive welding. Mater. Des. (1980–2015) 2015, 65, 1100–1109. [Google Scholar] [CrossRef]
- Zhang, X.; Castagne, S.; Yang, T.; Gu, C.; Wang, J. Entrance analysis of 7075 Al/Mg–Gd–Y–Zr/7075 Al laminated composite prepared by hot rolling and its mechanical properties. Mater. Des. 2011, 32, 1152–1158. [Google Scholar] [CrossRef]
- Kumar, P.; Ghosh, S.K.; Saravanan, S.; Barma, J.D. Significance of the Interlayer in Explosive Welding of Similar and Dissimilar Materials: Review. Combust. Explos. Shock. Waves 2023, 59, 253–278. [Google Scholar] [CrossRef]
- Song, J.; She, J.; Chen, D.; Pan, F. Latest research advances on magnesium and magnesium alloys worldwide. J. Magnes. Alloy. 2020, 8, 1–41. [Google Scholar] [CrossRef]
- Zhu, B.; Liang, W.; Li, X. Interfacial microstructure, bonding strength and fracture of magnesium–aluminum laminated composite plates fabricated by direct hot-pressing. Mater. Sci. Eng. A 2011, 528, 6584–6588. [Google Scholar] [CrossRef]
- Loh, N.; Sia, K. An overview of hot isostatic pressing. J. Mater. Process. Technol. 1992, 30, 45–65. [Google Scholar] [CrossRef]
- Liu, T.; Guo, C.; Tan, S.; Song, B.; Wang, M.; Huang, G.; Zheng, K.; Pan, F. Effect of Hot-Pressing Rate on Interface and Bonding Strength of Mg/Al Composite Sheets with Zn Interlayer. J. Mater. Eng. Perform. 2023, 1–12. [Google Scholar] [CrossRef]
- Cao, M.; Deng, K.-K.; Nie, K.-B.; Wang, C.-J.; Wang, L.; Liang, W. Microstructure, mechanical properties and formability of Ti/Al/Ti laminated composites fabricated by hot-pressing. J. Manuf. Process. 2020, 58, 322–334. [Google Scholar] [CrossRef]
- Mirzadeh, H. High strain rate superplasticity via friction stir processing (FSP): A review. Mater. Sci. Eng. A 2021, 819, 141499. [Google Scholar] [CrossRef]
- Li, X.; Xia, W.; Yan, H.; Chen, J.; Su, B.; Song, M.; Li, Z.; Li, Y. Dynamic recrystallization behaviors of high Mg alloyed Al-Mg alloy during high strain rate rolling deformation. Mater. Sci. Eng. A 2019, 753, 59–69. [Google Scholar] [CrossRef]
- Wang, Y.; Rao, M.; Li, L.; Luo, G.; Shen, Q.; Zhang, L. Accelerated bonding of magnesium and aluminum with a CuNi/Ag/CuNi sandwich interlayer by plasma-activated sintering. Metall. Mater. Trans. A 2016, 47, 631–636. [Google Scholar] [CrossRef]
- Budhe, S.; Banea, M.; De Barros, S.; Da Silva, L. An updated review of adhesively bonded joints in composite materials. Int. J. Adhes. Adhes. 2017, 72, 30–42. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, T.; Castagne, S.; Gu, C.; Wang, J. Proposal of bond criterion for hot roll bonding and its application. Mater. Des. 2011, 32, 2239–2245. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kajihara, M.; Watanabe, Y. Growth behavior of compound layers during reactive diffusion between solid Cu and liquid Al. Mater. Sci. Eng. A 2007, 445, 355–363. [Google Scholar] [CrossRef]
- Wang, P.; Chen, Z.; Hu, C.; Li, B.; Mo, T.; Liu, Q. Effects of annealing on the interfacial structures and mechanical properties of hot roll bonded Al/Mg clad sheets. Mater. Sci. Eng. A 2020, 792, 139673. [Google Scholar] [CrossRef]
- Mahendran, G.; Babu, S.; Balasubramanian, V. Analyzing the effect of diffusion bonding process parameters on bond characteristics of Mg-Al dissimilar joints. J. Mater. Eng. Perform. 2010, 19, 657–665. [Google Scholar] [CrossRef]
- Elsa, M.; Khorram, A.; Ojo, O.O.; Paidar, M. Effect of bonding pressure on microstructure and mechanicalproperties of aluminium/copper diffusion-bonded joint. Sādhanā 2019, 44, 126. [Google Scholar] [CrossRef]
- Oh-Ishi, K.; Edalati, K.; Kim, H.S.; Hono, K.; Horita, Z. High-pressure torsion for enhanced atomic diffusion and promoting solid-state reactions in the aluminum–copper system. Acta Mater. 2013, 61, 3482–3489. [Google Scholar] [CrossRef]
- Varmazyar, J.; Khodaei, M. Diffusion bonding of aluminum-magnesium using cold rolled copper interlayer. J. Alloys Compd. 2019, 773, 838–843. [Google Scholar] [CrossRef]
- Kulkarni, K.N.; Luo, A.A. Interdiffusion and phase growth kinetics in magnesium-aluminum binary system. J. Phase Equilibria Diffus. 2013, 34, 104–115. [Google Scholar] [CrossRef]
- Jafarian, M.; Khodabandeh, A.; Manafi, S. Evaluation of diffusion welding of 6061 aluminum and AZ31 magnesium alloys without using an interlayer. Mater. Des. (1980–2015) 2015, 65, 160–164. [Google Scholar] [CrossRef]
- Vrtnik, S.; Jazbec, S.; Jagodič, M.; Korelec, A.; Hosnar, L.; Jagličić, Z.; Jeglič, P.; Feuerbacher, M.; Mizutani, U.; Dolinšek, J. Stabilization mechanism of γ-Mg17Al12 and β-Mg2Al3 complex metallic alloys. J. Phys. Condens. Matter 2013, 25, 425703. [Google Scholar] [CrossRef]
- Zheng, B.; Zhao, L.; Hu, X.; Dong, S.; Li, H. First-principles studies of Mg17Al12, Mg2Al3, Mg2Sn, MgZn2, Mg2Ni and Al3Ni phases. Phys. B Condens. Matter 2019, 560, 255–260. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, Y.; Zhu, Y.; Li, L. Phase transformation, kinetics and thermodynamics during the combustion synthesis of Mg2Al3 alloy. J. Alloys Compd. 2015, 628, 257–262. [Google Scholar] [CrossRef]
- Li, G.; Jiang, W.; Guan, F.; Zhu, J.; Zhang, Z.; Fan, Z. Microstructure, mechanical properties and corrosion resistance of A356 aluminum/AZ91D magnesium bimetal prepared by a compound casting combined with a novel Ni-Cu composite interlayer. J. Mater. Process. Technol. 2021, 288, 116874. [Google Scholar] [CrossRef]
- Spencer, K.; Zhang, M.X. Heat treatment of cold spray coatings to form protective intermetallic layers. Scr. Mater. 2009, 61, 44–47. [Google Scholar] [CrossRef]
- Yang, H.; Guo, X.; Wu, G.; Wang, S.; Ding, W. Continuous intermetallic compounds coatings on AZ91D Mg alloy fabricated by diffusion reaction of Mg–Al couples. Surf. Coat. Technol. 2011, 205, 2907–2913. [Google Scholar] [CrossRef]
- Lee, K.S.; Yoon, D.H.; Kim, H.K.; Kwon, Y.-N.; Lee, Y.-S. Effect of annealing on the interface microstructure and mechanical properties of a STS–Al–Mg 3-ply clad sheet. Mater. Sci. Eng. A 2012, 556, 319–330. [Google Scholar] [CrossRef]
- Harrigan, W.C. Commercial processing of metal matrix composites. Mater. Sci. Eng. A 1998, 244, 75–79. [Google Scholar] [CrossRef]
- Zhao, K.; Xu, D.; Li, H.; Zhang, J.; Chen, D. Microstructure and mechanical properties of Mg/Mg bimetal composites fabricated by hot-pressing diffusion and co-extrusion. Mater. Sci. Eng. A 2019, 764, 138194. [Google Scholar] [CrossRef]
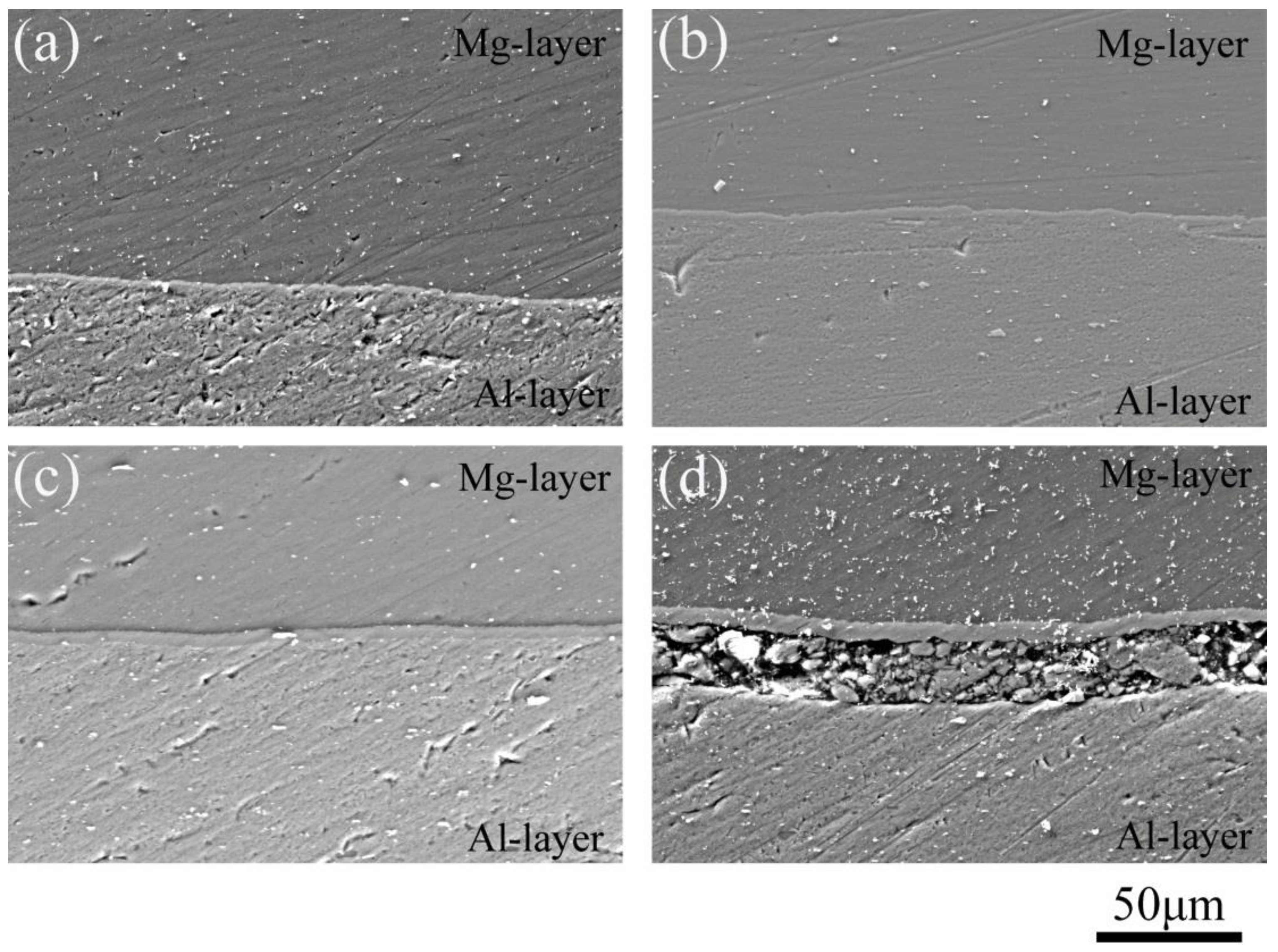
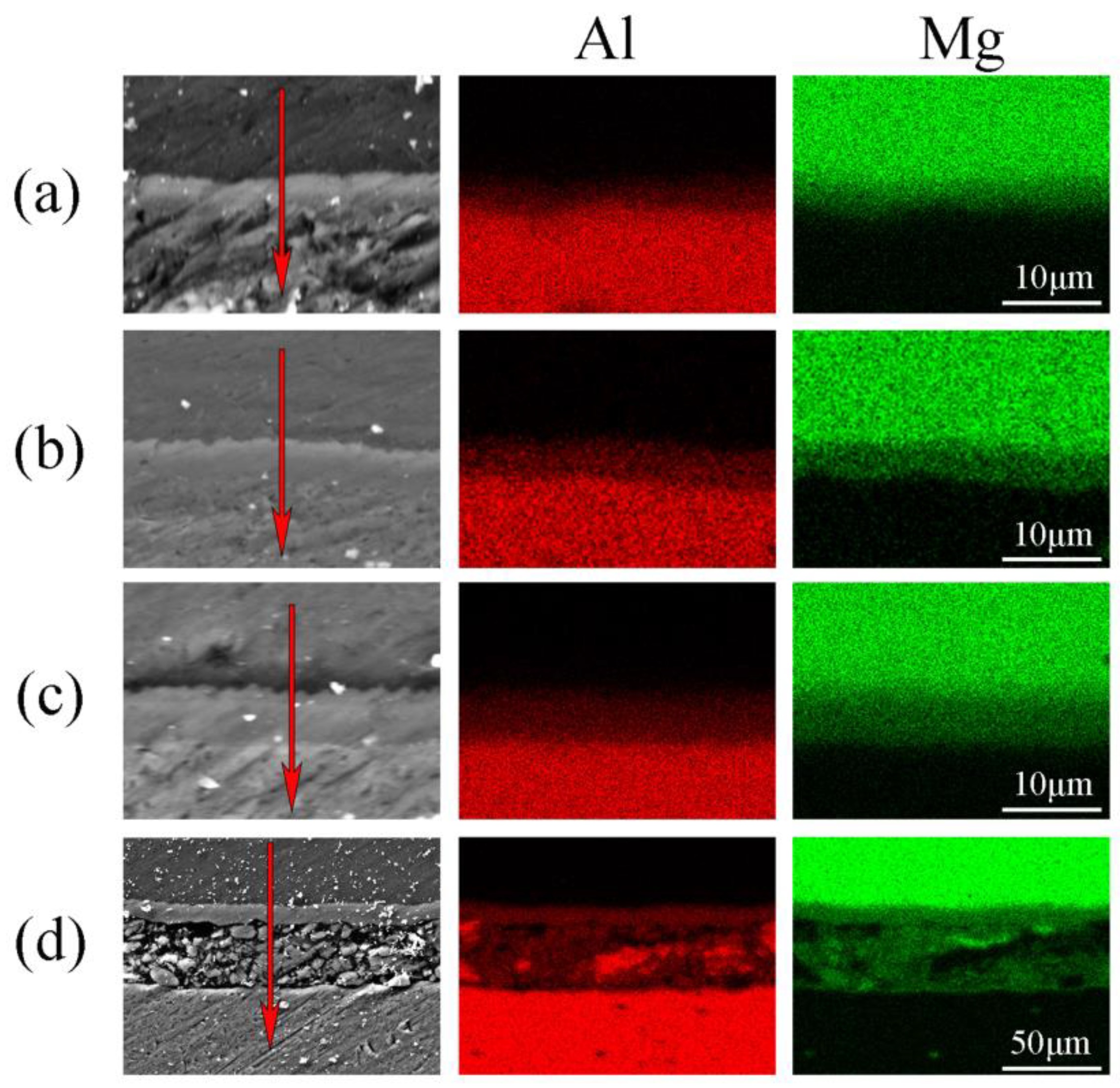
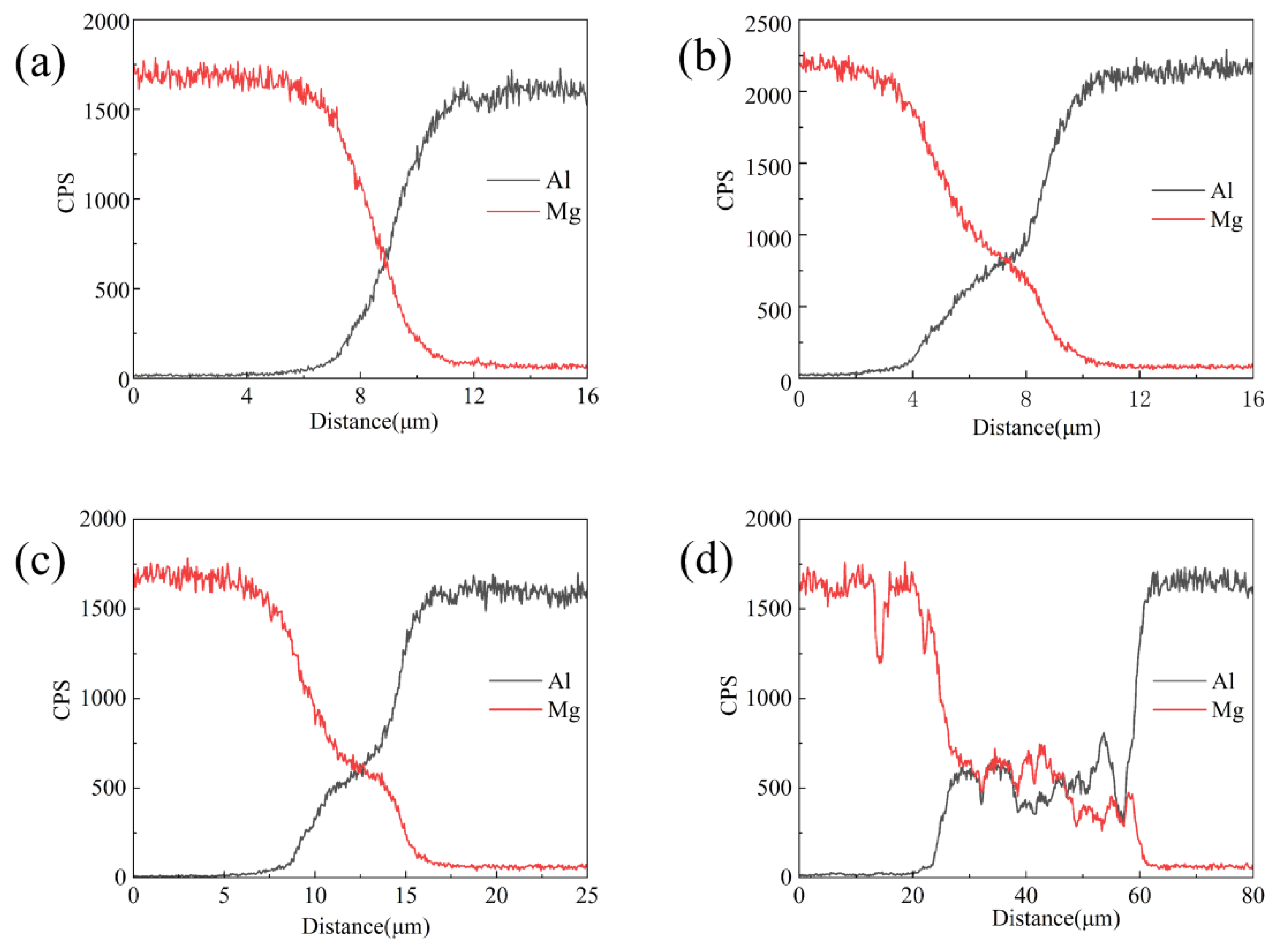

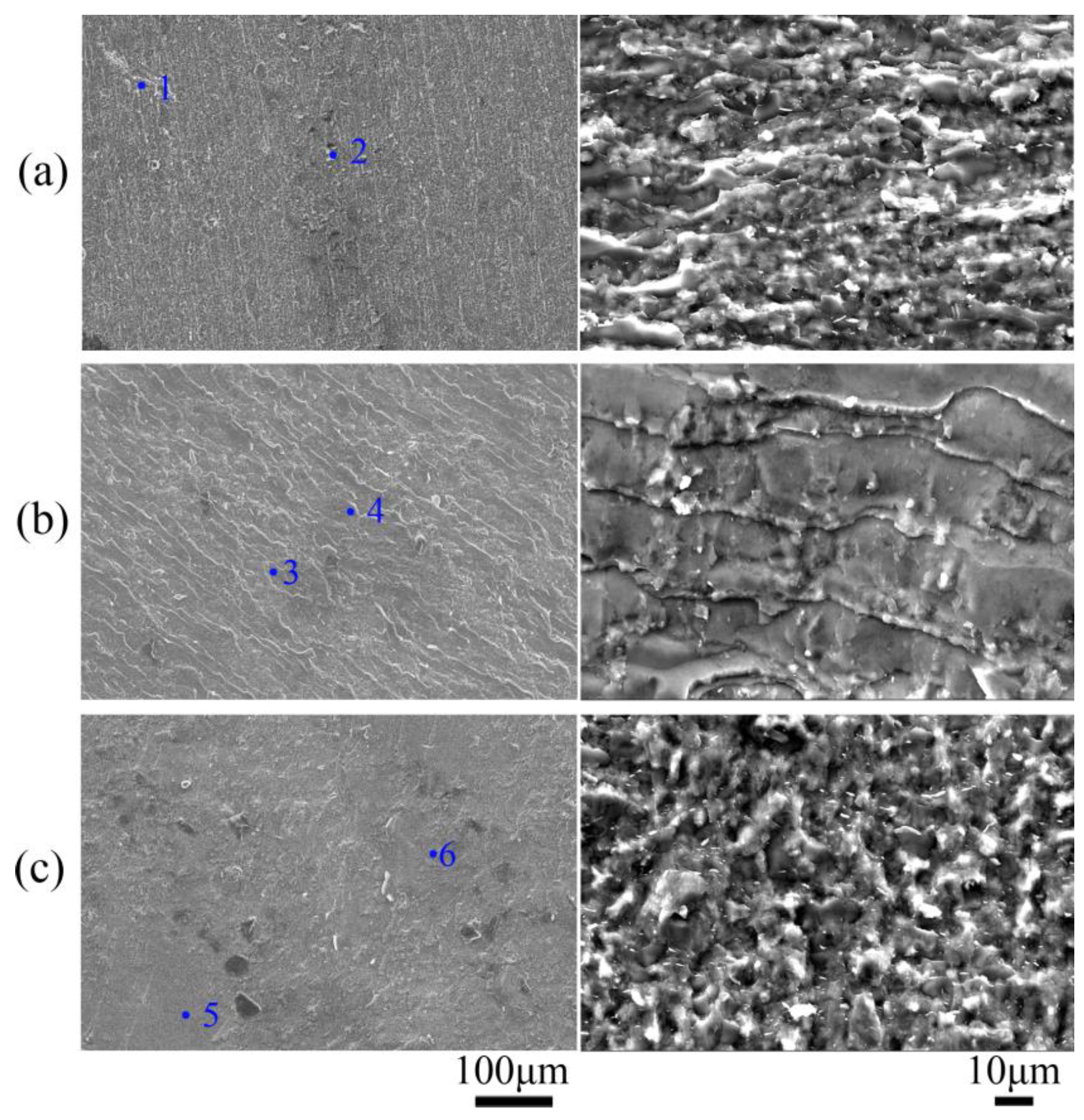
| Sample | Thickness Reduction | Strain Rate | Hot-Pressing Time |
|---|---|---|---|
| P1 | 40% | 1.0 × 10−2 s−1 | 1 min |
| P2 | 40% | 2.0 × 10−3 s−1 | 5 min |
| P3 | 40% | 1.0 × 10−3 s−1 | 10 min |
| P4 | 40% | 3.3 × 10−4 s−1 | 30 min |
| Position | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| Mg | 97.1 | 95.3 | 91.8 | 86 | 69.2 | 51 | 44.4 | 21.8 | 5.7 |
| Al | 2.9 | 4.7 | 8.2 | 14 | 30.8 | 49 | 55.6 | 78.2 | 94.3 |
| Phase | α-Mg | α-Mg + γ | γ + β | β | β + α-Al | α-Al | |||
| Position | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mg | 98.0 | 93.1 | 84.2 | 60.3 | 45.8 | 42 | 38.6 | 31.9 | 13.1 | 5.6 | 2.9 |
| Al | 2.0 | 6.9 | 15.8 | 39.7 | 54.2 | 58 | 61.4 | 68.1 | 86.9 | 94.4 | 97.1 |
| Phase | α-Mg | α-Mg + γ | γ | γ + β | β | β + α-Al | α-Al | ||||
| Position | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Mg | 97 | 84.1 | 40.6 | 38.6 | 32.1 | 3.7 | 2.7 |
| Al | 3 | 15.9 | 59.4 | 61.4 | 67.9 | 96.3 | 97.3 |
| Phase | α-Mg | α-Mg + γ | β | α-Al | |||
| Sample | Shear Strength (MPa) |
|---|---|
| P1 | 2.62 ± 0.53 |
| P2 | 2.47 ± 0.65 |
| P3 | 4.39 ± 1.22 |
| P4 | 2.87 ± 1.19 |
| Position | Mg | Al | Possible Phases |
|---|---|---|---|
| 1 | 8.2 | 91.8 | α-Al |
| 2 | 25.8 | 74.2 | α-Al + β |
| 3 | 36.1 | 63.9 | β |
| 4 | 31.2 | 68.8 | α-Al + β |
| 5 | 40.9 | 59.1 | β |
| 6 | 26.8 | 73.2 | α-Al + β |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, C.; Song, B.; Tan, S.; Xu, H.; Wang, M.; Liu, T.; Guo, N.; Guo, S. Influence of the Hot-Pressing Rate on the Interface Feature and Mechanical Properties of Mg/Al Composite Plates. Metals 2024, 14, 23. https://doi.org/10.3390/met14010023
Guo C, Song B, Tan S, Xu H, Wang M, Liu T, Guo N, Guo S. Influence of the Hot-Pressing Rate on the Interface Feature and Mechanical Properties of Mg/Al Composite Plates. Metals. 2024; 14(1):23. https://doi.org/10.3390/met14010023
Chicago/Turabian StyleGuo, Chuande, Bo Song, Shijun Tan, Haohua Xu, Meng Wang, Tingting Liu, Ning Guo, and Shengfeng Guo. 2024. "Influence of the Hot-Pressing Rate on the Interface Feature and Mechanical Properties of Mg/Al Composite Plates" Metals 14, no. 1: 23. https://doi.org/10.3390/met14010023
APA StyleGuo, C., Song, B., Tan, S., Xu, H., Wang, M., Liu, T., Guo, N., & Guo, S. (2024). Influence of the Hot-Pressing Rate on the Interface Feature and Mechanical Properties of Mg/Al Composite Plates. Metals, 14(1), 23. https://doi.org/10.3390/met14010023







