Abstract
To accurately and conveniently obtain the thermodynamic data of binary liquid alloys, a new method is proposed in this study. It combines ab initio molecular dynamics (AIMD) simulation with a thermodynamic model to estimate the activity and molar excess Gibbs energy of binary liquid alloys. Additionally, two methods of grouping the partial radial distribution function (PRDF) of 5000 steps obtained by simulation are proposed for the first time. The PRDF of Al50Cu50, Al50Ni50, and Al50Fe50 is obtained by AIMD simulation. These PRDF are combined with four thermodynamic models to estimate the activity and molar excess Gibbs energy. Furthermore, the estimation results of the four models are compared with those of the Miedema model. The results show that when the first peak of the PRDF is obtained by the symmetric method, the average relative deviation (ARD) of the activity and molar excess Gibbs energy of the four models are, respectively: 28% and 32% for Molecular Interaction Volume Model (MIVM); 162% and 38% for Regular Solution Model (RSM); 508% and 65% for Wilson model; 562% and 67% for Non-Random Two-Liquid (NRTL). When the first peak of PRDF is obtained by non-symmetric method, the average ARD of the activity and molar excess Gibbs energy of the four models are, respectively: 64% and 20% for MIVM; 115% and 26% for RSM; 661% and 70% for Wilson; 727% and 72% for NRTL. In addition, the average ARD of the activity and molar excess Gibbs energy of the Miedema model are 113% and 33%. These data indicate that the estimation performance of the MIVM model is superior to the other four models, and the symmetric method performs better than the non-symmetric method. The grouping treatment of PRDF data effectively improves estimation performance.
1. Introduction
Thermodynamic data are important parameters for studying structural changes, morphological transformations, and the feasibility of reactions in substances. However, in practice, especially in the field of metallurgy, the determination of thermodynamic data is often very time-consuming, expensive, and difficult to operate. Therefore, accurately and conveniently obtaining thermodynamic data has always been a critical research direction in basic thermodynamics [1]. Owing to the ability of thermodynamic models to derive preliminary approximations and reference values of thermodynamic data in complex liquid mixtures, thermodynamic models have become one of the most cost-effective prediction methods for obtaining thermodynamic data. Meanwhile, it has attracted the attention and favor of researchers by virtue of its obvious convenience, economic feasibility, and satisfactory accuracy. Not only does it provide valuable insights for industrial production, but it also saves a lot of manpower and material resources [2].
Binary aluminum-based alloys are widely used in life production; this paper mainly studies three binary liquid alloys, Al-Cu, Al-Ni, and Al-Fe. Al-Cu alloys can be prepared by Al-Cu alloy flexible nuclear shells and are used in new energy storage materials, which have the advantages of a small footprint, large capacity, and good stability, and are more suitable for high-temperature heat storage [3]. Al-Ni alloy is a typical energy-containing structural material, which has very broad application prospects in both military and civil fields, such as making energy-containing breakers and energy-containing drug masks, etc. [4]. Al-Fe alloy is mainly used for metal welding, which has the advantages of lightweight structure and good overall performance and has a wide range of prospects for application in the aerospace, nuclear energy, automotive, and electrical and electronic industries [5].
In practical experiments, metal smelting needs to meet certain conditions such as high temperature and high pressure, which can easily bring some difficulties and potential safety hazards, while computer simulation can avoid the above problems [6]. In 1985, the AIMD simulation method was proposed by Car R and Parrinello M [7], who used AIMD simulation to compute particle force conditions and trajectory motion. Since then, the successful application of AIMD simulations based on first principles has increased the acceptance of computer simulations as a research method, and AIMD simulations have been widely used to study the structure of melts [8,9]. In 2020, the structural and electronic properties of AlnVn (n = 2–12) clusters were investigated by Han et al. [10] using AIMD, and the poor intermiscibility between the components of Al-V alloys was experimentally explained. In 2023, the molecular dynamics trajectories of PbS and Sb2S3 at various temperatures were obtained by Xie et al. [11] using AIMD and the experimental calculations of PbS and Sb2S3 diffusion coefficients of gas-phase clusters at each temperature. Therefore, it is reasonable to use AIMD to obtain the PRDF of binary liquid alloys in this study.
In order to accurately and conveniently obtain thermodynamic data, such as activity and molar excess Gibbs energy of binary liquid alloys, this study proposes a new method that combines AIMD simulation with thermodynamic models to estimate the activity and molar excess Gibbs energy. Additionally, a method for processing the obtained PRDF data in two different groups, with a total of 5000 steps, is introduced for the first time in this paper. The activity and molar excess Gibbs energy of the alloy are also estimated using the Miedema model. Furthermore, a comparison between the results obtained from five different models and experimental values is conducted, calculating the standard deviation (SD) and average relative deviation (ARD), and comparing the results with those reported in similar literature. Through analysis, the feasibility of this approach is verified, and reference recommendations for selecting models and methods for calculating thermodynamic data of binary liquid alloys are provided.
2. Methods and Models
2.1. Obtaining the PRDF through AIMD Simulations
In this work, the initial configurations of the alloy cells were first established in the Materials Studio simulation software (https://software.umich.edu/titles/materials-studio) using the Packing method, which is mainly used to generate initial models of crystalline or amorphous structures, so that the atoms are randomly arranged in simple cubic cells. Three liquid alloys, Al50Cu50-1400 K, Al50Ni50-1923 K, and Al50Fe50-1823 K with densities of 4.69 g·cm−3 [12], 4.63 g·cm−3 [13], and 4.61 g·cm−3 [13] were set up to form the cell with 122, 128, and 116 atoms, respectively. Next, ab initio molecular dynamics (AIMD) simulation based on density functional theory (DFT) were conducted using the Vienna ab initio simulation package (VASP) software [14], specifically version VASP-5.4.1. VASP simulation encompassed two stages, namely structure optimization and dynamics. The exchange-correlation functional employed the Perdew–Burke–Ernzerhof (PBE) functional [15,16], which is based on the generalized gradient approximation (GGA). The GGA is a widely used approximation in DFT that incorporates electron density and electron density gradients to evaluate the exchange-correlation energy. The PBE functional, as a GGA functional, accurately describes the electronic structure of molecules in terms of both exchange and correlation energies. In this investigation, the ultrasoft pseudopotential (USPP) [17] was employed to characterize the electron–ion interaction within the pseudopotential file. The K-point is set to the Gamma point, and the cutoff energy is chosen to be 1.3 times higher than the maximum cutoff energy provided in the pseudopotential files [14], following the computational guidelines [18]. During the structure optimization stage, the convergence criterion for the electronic step is an energy change smaller than 10−4 eV/Å, while for the ionic step, it is an energy change smaller than 10−3 eV/Å. In the dynamics stage, the simulation temperature is set above the melting point of the alloys, namely 1400 K, 1923 K, and 1823 K, respectively. The simulation uses a canonical ensemble (NVT) that ensures that the number, volume, and temperature of atoms remain constant [19]. To maintain a constant system temperature, the Nose–Hoover thermostat method is employed [20,21]. For the dynamics simulation, a time step of 3 fs is utilized, and the simulation is performed for a total of 5000 steps (15 ps).
In order to comprehensively consider the variations of the partial radial distribution function (g(r)) throughout the entire simulation process and evaluate the differences in the estimation of activity and molar excess Gibbs energy at different step stages, as well as to improve the accuracy of the estimation, two data processing methods were performed on the obtained g(r) data from a total of 5000 steps (taking i-i as an example). The first method involved accumulating the g(r) data every 1000 steps, resulting in a total of five sets of gii(r) data: 0–1000 steps, 0–2000 steps, 0–3000 steps, 0–4000 steps, and 0–5000 steps. The second method involved segmenting the data every 1000 steps, also resulting in five sets of gii(r) data: 0–1000 steps, 1000–2000 steps, 2000–3000 steps, 3000–4000 steps, and 4000–5000 steps. Therefore, each alloy system obtained 5 × 2 × 3 sets of g(r) data.
2.2. Symmetric and Non-Symmetric Methods
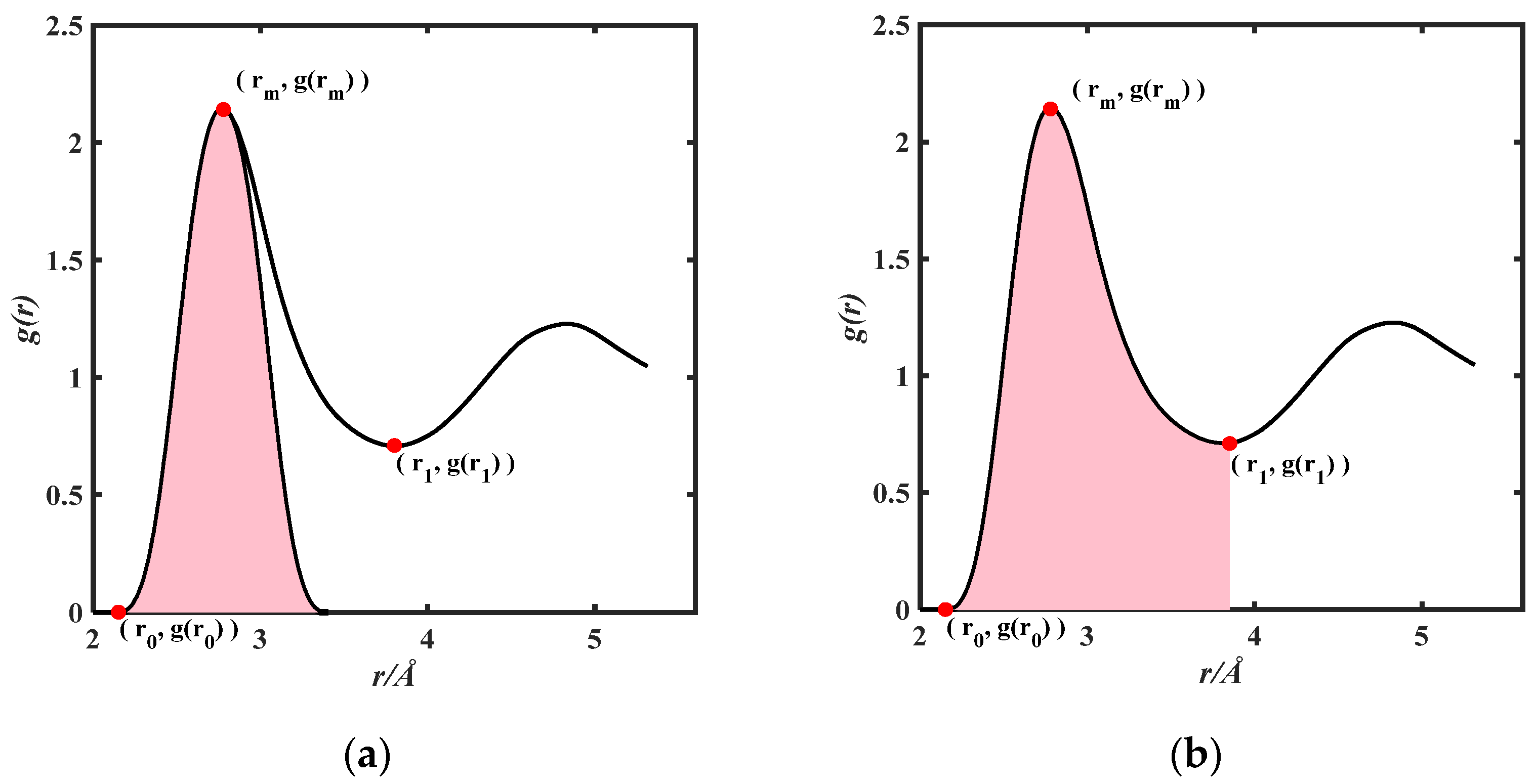
In the subsequent work, the relevant parameters of the thermodynamic model are expressed by equations containing g(r). It is widely acknowledged that the first peak contributes the most to g(r), so various methods have been commonly employed in academia to extract the first peak of g(r). In this study, symmetric and non-symmetric methods were mainly adopted by us. There are three key points for determining the first peak of g(r): r0 represents the abscissa of the starting point of non-zero values, rm represents the abscissa of the first peak’s maximum, and r1 represents the abscissa of the first peak’s minimum.
Regarding the symmetric method [22], the curve on the left side of g(r) is symmetric with the line r = rm as the symmetry axis to get the curve on the right side, and the entire image is the first peak of the PRDF obtained by using the symmetric method. As shown in Figure 1a, the curve in the r0~rm region of g(r) image is symmetric with the line r = rm as the symmetry axis to obtain the curve on the right side, and the pink region represents the first peak of the PRDF obtained by using the symmetric method. As for the non-symmetric method [23], the g(r) curve within the interval from r0~rm represents the first peak obtained using the non-symmetric method. As shown in Figure 1b, the pink region represents the first peak of the PRDF obtained by the non-symmetric method.

Figure 1.
(a) The first peak of partial radial distribution function is obtained by symmetric method; (b) the first peak of partial radial distribution function is obtained by non-symmetric method.
2.3. Thermodynamic Models
2.3.1. Molecular Interaction Volume Model (MIVM)
The Molecular Interaction Volume Model (MIVM) [24] was proposed by Tao in 2000. The MIVM possesses characteristics such as inclusivity, diffusion stability, and thermodynamic consistency. Tao used statistical thermodynamics in the derivation process to obtain configurational partition functions that include both volume and energy terms, which gives the model a clearer physical meaning and better overall prediction results.
For the binary system i-j, the molar excess Gibbs energy of the MIVM model can be expressed as:
The expression for the activity coefficient of component i is:
R is the gas constant (8.314 J/(K.mol)), T is the temperature, xi and xj represent the mole fractions of components i and j in the mixture, Vmi and Vmj represent the molar volumes of pure substances i and j, and Zi and Zj represent the first coordination numbers of pure substances i and j. Bij and Bji are the pair-potential energy interaction parameters, which are defined as:
Here, k is the Boltzmann constant (1.38 × 10−23 J/K). εii, εjj, and εij are the molecular mean pair potentials of the molecule for i-i, j-j, and i-j, respectively.
Wang et al. [25] hypothesized that the equation [26], which relates the molecular pair potential of very dilute gases to the radial distribution function, can be approximately applied to binary liquid alloys. Thus, according to the probability density distribution function and the expectation principle, the expressions for the molecular mean pair potentials, εii, εjj, and εij of the binary liquid alloy can be obtained as:
2.3.2. Regular Solution Model (RSM)
The Regular Solution Model (RSM) [27,28] was proposed by Hildebrand in 1929. This model assumes that the mixture enthalpy of the solution is non-zero, while the mixture entropy is equal to that of an ideal solution. In other words, this model considers the interactions between solvent molecules, but neglects the influence of volume.
For the binary system i-j, the molar excess Gibbs energy of the RSM model can be expressed as:
The expression for the activity coefficient of component i is:
where w is the interaction parameter, and the expression is:
where Z represents the average coordination number, assuming that it can be expressed using local coordination numbers Zii, Zij, Zji, and Zjj.
These local coordination numbers can be expressed using the expressions provided by Hill [26]:
where ρ0 represents the average density of the alloy.
2.3.3. Wilson Model
The Wilson model [29] was proposed by Wilson in 1964. Wilson used the ratio of the Boltzmann distribution to define the “local concentration”, which the local volume fraction was defined and directly substituted into the Flory–Huggins equation [30].
For the binary system i-j, the molar excess Gibbs energy of the Wilson model can be expressed as:
The expression for the activity coefficient of component i is:
where the parameters Aij and Aji are defined as:
2.3.4. Non-Random Two-Liquid Model (NRTL)
The Non-Random Two-Liquid (NRTL) model [31] was proposed by Renon and Prausnitz in 1968. This model was derived by combining a local composition equation, based on the non-random assumption, with a potential energy expression for liquid mixtures from the two-liquid theory. The NRTL model is highly regarded in the field of organic chemistry for its balanced combination of simplicity, accuracy, and rationality.
For the binary system i-j, the molar excess Gibbs energy of the NRTL model can be expressed as:
The expression for the activity coefficient of component i is:
where the parameters τij and τji are defined as:
where α is a non-random factor, its introduction aims to take into account the volume size of the molecule through correction, with a value range of 0.2~0.47, where αij = αji = 0.3.
2.3.5. Miedema Model
The Miedema model [32,33] is a semi-empirical thermodynamic model that was proposed by Miedema et al. in 1973. Considering that the concept of cell is still applicable in alloys, the Wigner–Seitz cell model, which is often used to describe pure metals, is extended to binary alloys.
For the binary system i-j, the mixing enthalpy expression of Miedema model is:
Additionally, considering the relationship proposed by Tanaka [34]:
The molar excess Gibbs energy of the Miedema model can be expressed as:
The expression for the activity coefficient of component i is:
where Vi and Vj are the molar volumes of elements i and j, respectively, Φi and Φj are the electronegativity of elements i and j, respectively, nwsi and nwsj are the electron densities of elements i and j, respectively, and Tmi and Tmj are the melting points of elements i and j, respectively. u, q/p, a, p, r/p are empirical constants of the model, and specific values can be obtained from the literature [32].
2.4. Calculation Process
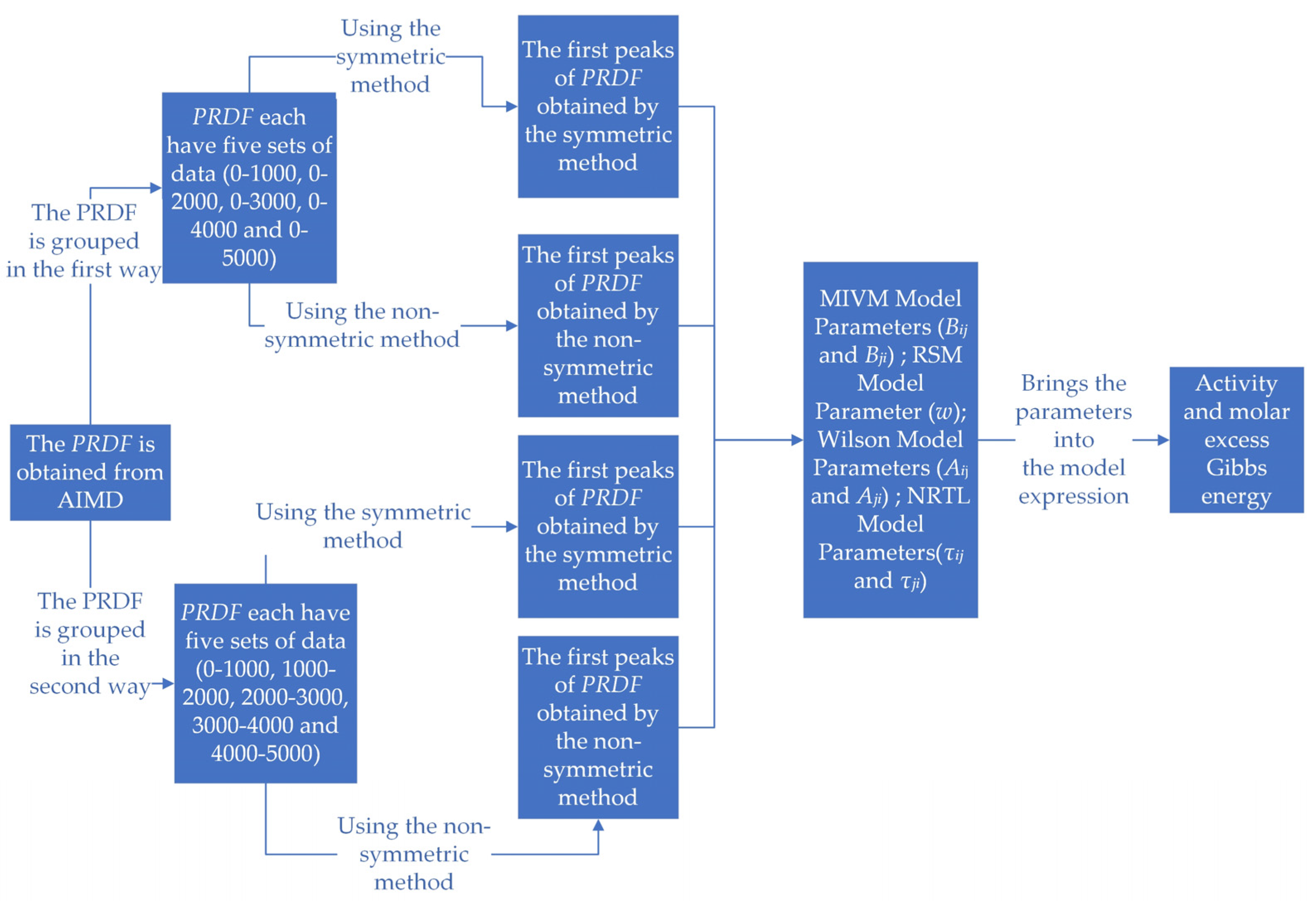
As shown in Figure 2, there are five main steps in the whole calculation process. The PRDF was obtained by AIMD. A total of 5000 steps of PRDF were processed in two groups, and a total of 5 × 2 × 3 groups of PRDF data were obtained for each alloy system. The first peak of PRDF was extracted by symmetric method and non-symmetric method, respectively. The parameters of the four models were calculated with expressions containing PRDF. The activity and molar excess Gibbs energy of the binary liquid alloy system are estimated by taking the obtained parameter values into the expressions of the model activity and molar excess Gibbs energy.
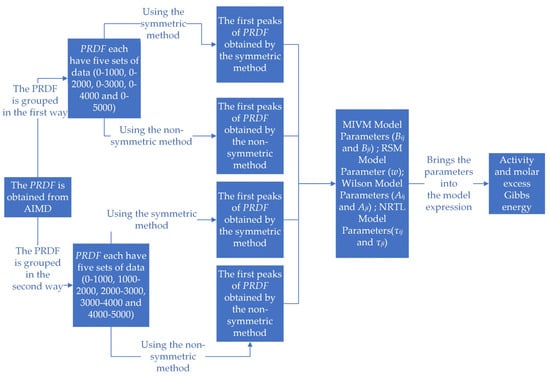
Figure 2.
Calculation process chart.
Meanwhile, the parameter values of the Miedema model can be obtained from the literature [32], which can be used to estimate the activity and molar excess Gibbs energy of binary liquid alloy systems by substituting these values into the formula of the Miedema model. By comparing the results of the Miedema model with those of the other four models, the reliability of the conclusion can be comprehensively evaluated and the accuracy of the conclusion can be verified.
3. Results and Discussion
3.1. The g(r) Simulated by AIMD
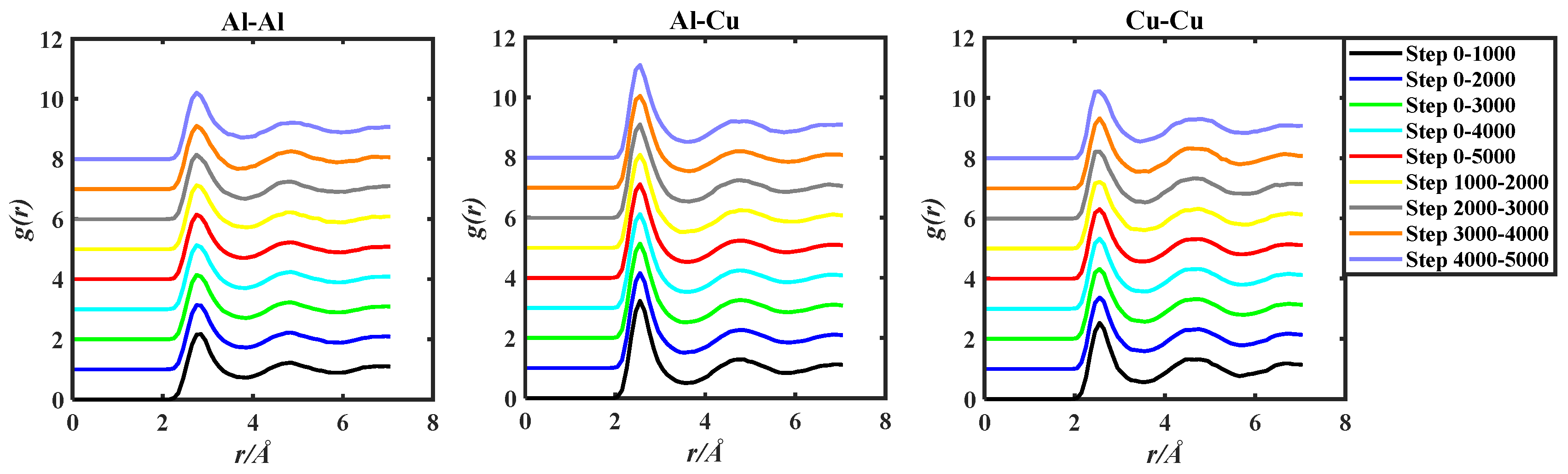
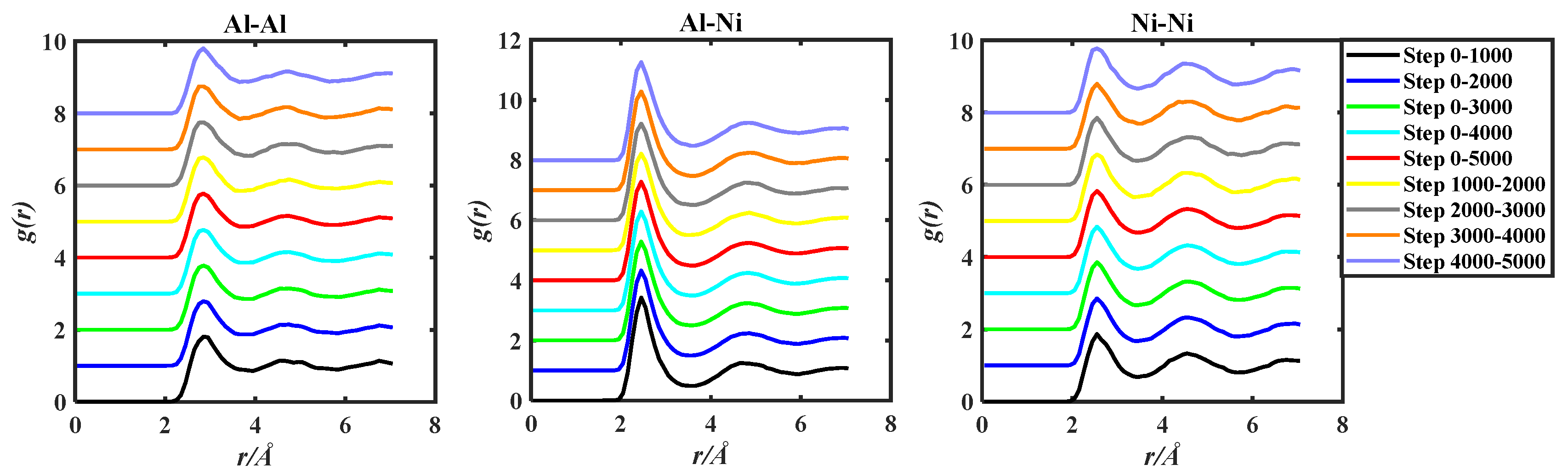
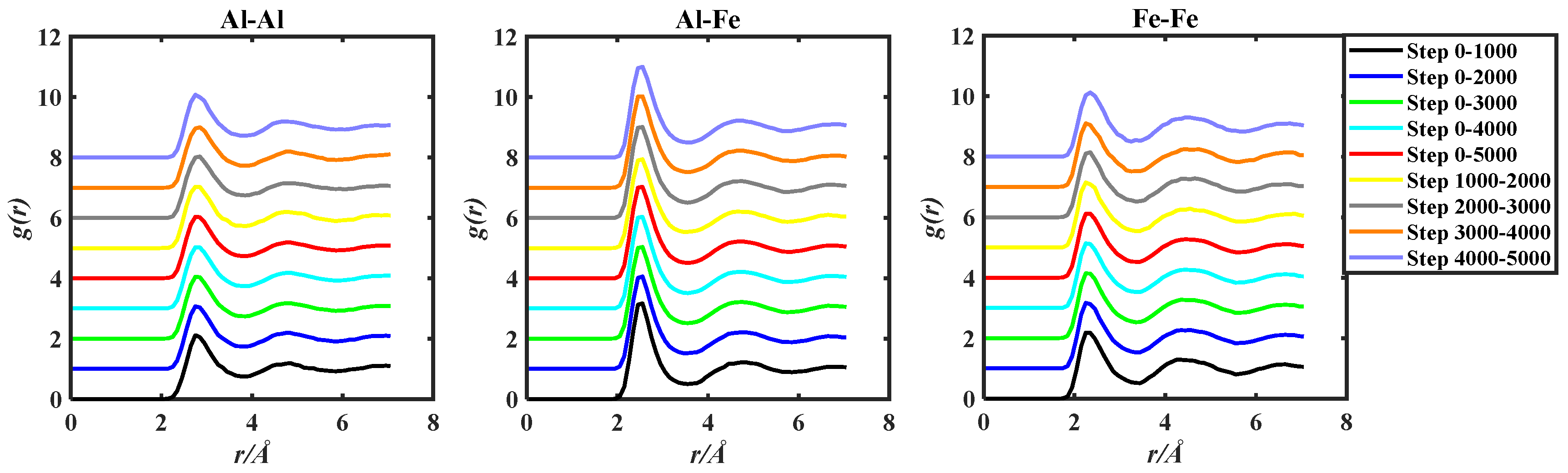
The g(r) of the three alloy systems were obtained by ab initio molecular dynamics simulation. The obtained g(r) data were processed by the two methods in the above Section 2.1, and the obtained images were shown in Figure 3, Figure 4 and Figure 5.

Figure 3.
gAl-Al(r), gAl-Cu(r), and gCu-Cu(r) of the Al50Cu50-1400 K system, grouped by two methods based on 5000 steps PRDF data.
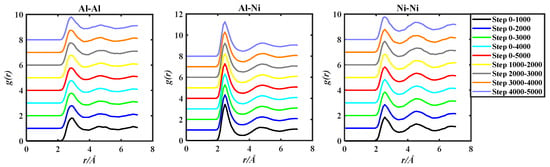
Figure 4.
gAl-Al(r), gAl-Ni(r), and gNi-Ni(r) of the Al50Ni50-1923 K system, grouped by two methods based on 5000 steps PRDF data.

Figure 5.
gAl-Al(r), gAl-Fe(r), and gFe-Fe(r) of the Al50Fe50-1823 K system, grouped by two methods based on 5000 steps PRDF data.
Observing Figure 3, Figure 4 and Figure 5, in order to facilitate the observation of each gii(r), gij(r), and gjj(r) graph, the ordinate of gii(r), gij(r), and gjj(r) is incremented by 1, so that all the graphs do not overlap. By observation, it can be found that the PRDF graphs obtained by AIMD are continuous, smooth, and without sudden large inflection points. This indicates that using the aforementioned parameters for AIMD simulation can obtain relatively accurate PRDF graphs. For the PRDF graphs at different steps, it can be observed that the changes are not very significant, with the main variations occurring at the peak and first peak-valley positions.
There were three key points about the first peak of g(r) presented in Section 2.2 above. The coordinates of the key points for the three systems Al-Cu, Al-Ni, and Al-Fe were presented in Table 1, Table 2 and Table 3, respectively.

Table 1.
The three key coordinate points of the first peak of gAl-Al(r), gAl-Cu(r), and gCu-Cu(r) in the Al50Cu50-1400 K system.

Table 2.
The three key coordinate points of the first peak of gAl-Al(r), gAl-Ni(r), and gNi-Ni(r) in the Al50Ni50-1923 K system.

Table 3.
The three key coordinate points of the first peak of gAl-Al(r), gAl-Fe(r), and gFe-Fe(r) in the Al50Fe50-1823 K system.
Analysis of Table 1, Table 2 and Table 3 reveals that with the increase in simulation steps, the coordinates of the three key points of the first peak of gii(r), gij(r), and gjj(r) for the Al50Cu50-1400 K, Al50Ni50-1923 K, and Al50Fe50-1823 K systems exhibit minimal change. The fluctuation of peak–trough values does not consistently increase or decrease with the number of steps. However, careful analysis indicates that the value of the g(rm) gradually decreases with the number of steps, implying that as the number of steps increases, the peak position gradually shifts downwards.
3.2. Calculating Key Parameters of the Five Models
Expressions (4)–(6) containing g(r) were substituted into model parameter expressions (3) for MIVM, (9) for RSM, (14) for Wilson, and (17) for NRTL. Then, the values of the model parameters were calculated by using the graphical integration method. Obviously, this method is different from the L-PPDF mathematical of Gaussian function fitting adopted by Wang et al. [25], which depends on the fitting parameters u and v. Therefore, no fitting parameter was introduced in the calculation process of model parameters in this study.
The specific calculation of graphical integration utilizes the trapezoidal integration method [35]. This method divides the integration region into multiple small trapezoids and sums their areas to obtain the integral value. The parameter values of each model are calculated by using the symmetric method and the non-symmetric method, as shown in Table 4. Table 5 lists the parameters of Miedema model for the three alloy systems.

Table 4.
Parameters of four models calculated for Al-Cu, Al-Ni, and Al-Fe systems by symmetric and non-symmetric methods.

Table 5.
Parameters for Miedema model calculation of Al-Cu, Al-Ni, and Al-Fe systems.
Through analyzing the parameters of the four thermodynamic models calculated using both symmetric and non-symmetric methods for the Al-Cu, Al-Ni, and Al-Fe systems in Table 4, can conclude that: as the number of simulation steps increases, the changes in parameter values for the four thermodynamic models obtained from both symmetric and non-symmetric calculations are extremely small. Furthermore, there is no discernible pattern or trend in these changes, which contradicts the expected continuous increase or decrease. It is important to note that although the changes in parameter values are very minute, they still hold significance as these subtle variations can potentially impact the accuracy of the results.
3.3. Estimating the Activity and Molar Excess Gibbs Energy
For the three systems of Al-Cu, Al-Ni, and Al-Fe, the SD and ARD estimates for the activity and molar excess Gibbs energy of the five models calculated by using the symmetric method are listed in Table 6 and Table 7, respectively.

Table 6.
The SD and ARD of activity estimated by symmetric method.

Table 7.
The SD and ARD of molar excess Gibbs energy estimated by symmetric method.
Analysis of the results in Table 6 and Table 7 shows that: for the Al-Cu system, the average SD and average ARD of the activity estimates for the five models using the symmetric method are, respectively: 0.055 and 20% for the MIVM model; 0.095 and 205% for the RSM model; 0.159 and 498% for the Wilson model; 0.167 and 550% for the NRTL model; 0.077 and 143% for the Miedema model. The average SD and average ARD of the molar excess Gibbs energy estimates for the five models are, respectively: 1298 J and 13% for the MIVM model; 5775 J and 46% for the RSM model; 8247 J and 69% for the Wilson model; 8453 J and 70% for the NRTL model; 4933 J and 39% for the Miedema model. For the Al-Ni system, the average SD and average ARD of the activity estimates for the five models using the symmetric method are, respectively: 0.072 and 28% for the MIVM model; 0.071 and 252% for the RSM model; 0.159 and 903% for the Wilson model; 0.171 and 1002% for the NRTL model; 0.053 and 167% for the Miedema model. The average SD and average ARD of the molar excess Gibbs energy estimates for the five models are, respectively: 5992 J and 26% for the MIVM model; 11,197 J and 42% for the RSM model; 17,246 J and 66% for the Wilson model; 17,832 J and 68% for the NRTL model; 9361 J and 35% for the Miedema model.
Comparative analysis of the above data shows that when the symmetric method is used, for Al-Cu and Al-Ni systems, the MIVM model has the smallest values of average SD and average ARD for activity estimates and molar excess Gibbs energy estimates, which indicates that the MIVM model has a better estimation effect than the other four models. The average SD and average ARD values for the RSM and Miedema models are in the middle of the range for activity estimates and molar excess Gibbs energy estimates, which indicates that the RSM and Miedema models have ordinary estimation effects.
For the Al-Fe system, the average SD and average ARD of the activity estimates for the five models when using the symmetric method are, respectively: 0.068 and 36% for the MIVM model; 0.034 and 29% for the RSM model; 0.108 and 122% for the Wilson model; 0.116 and 135% for the NRTL model; 0.033 and 29% for the Miedema model. The average SD and average ARD of the molar excess Gibbs energy estimates for the five models are, respectively: 6018 J and 57% for the MIVM model; 2693 J and 25% for the RSM model; 6358 J and 60% for the Wilson model; 6702 J and 63% for the NRTL model; 2647 J and 25% for the Miedema model. Comparative analysis of the above data shows that when the symmetric method is used: for the Al-Fe system, the average ARD of the estimated activity values of the MIVM model, RSM model, and Miedema model were all less than 40%, indicating that the MIVM model, RSM model, and Miedema model all have good estimation effect, of which Miedema model had the better estimation effect.
aest is the estimated value of activity and aexp [36] is the experimental value of activity.
is the estimated value of the molar excess Gibbs energy and
[36] is the experimental value of the molar excess Gibbs energy.
For the three systems of Al-Cu, Al-Ni, and Al-Fe, the SD and ARD estimates for the activity and molar excess Gibbs energy of the five models calculated by using non-symmetric methods are listed in Table 8 and Table 9, respectively.

Table 8.
The SD and ARD of activity estimated by non-symmetric method.

Table 9.
The SD and ARD of molar excess Gibbs energy estimated by non-symmetric method.
Analysis of the results in Table 8 and Table 9 shows that: it can be observed that when the non-symmetric method is employed, for the Al-Cu and Al-Ni systems, the MIVM model exhibits the smallest values for average SD and average ARD in terms of activity estimation and molar excess Gibbs energy estimation. This suggests that the MIVM model provides better estimation performance compared to the other four models. The RSM model and Miedema model show moderate values for average SD and average ARD in terms of activity estimation and molar excess Gibbs energy estimation, indicating ordinary estimation performance for the RSM and Miedema models. The specific data information is as follows. For the Al-Cu system, the average SD and average ARD of the activity estimates for the five models when using the non-symmetric method are, respectively: 0.064 and 68% for the MIVM model; 0.076 and 123% for the RSM model; 0.173 and 580% for the Wilson model; 0.181 and 636% for the NRTL model; 0.077 and 143% for the Miedema model. The average SD and average ARD of the molar excess Gibbs energy estimates for the five models are, respectively: 3041 J and 22% for the MIVM model; 4403 J and 34% for the RSM model; 8652 J and 72% for the Wilson model; 8857 J and 74% for the NRTL model; 4933 J and 39% for the Miedema model. For the Al-Ni system, the average SD and average ARD of the activity estimates for the five models when using the non-symmetric method are, respectively: 0.036 and 111% for the MIVM model; 0.064 and 218% for the RSM model; 0.194 and 1259% for the Wilson model; 0.205 and 1384% for the NRTL model; 0.053 and 167% for the Miedema model. The average SD and average ARD of the molar excess Gibbs energy estimates for the five models are, respectively: 7411 J and 27% for the MIVM model; 10,518 J and 40% for the RSM model; 18,910 J and 73% for the Wilson model; 19,399 J and 75% for the NRTL model; 9361 J and 35% for the Miedema model.
For the Al-Fe system, the average SD and average ARD of the activity estimates for the five models when using the non-symmetric method are, respectively: 0.021 and 13% for the MIVM model; 0.011 and 4% for the RSM model; 0.123 and 146% for the Wilson model; 0.133 and 163% for the NRTL model; 0.033 and 29% for the Miedema model. The average SD and average ARD of the molar excess Gibbs energy estimates for the five models are, respectively: 1268 J and 12% for the MIVM model; 490 J and 4% for the RSM model; 6958 J and 65% for the Wilson model; 7320 J and 69% for the NRTL model; 2647 J and 25% for the Miedema model. Compared with the above data analysis, it can be seen that the average ARD of the estimated activity values of the MIVM model, RSM model, and Miedema model for Al-Fe system is less than 30% when the non-symmetric method is used, indicating that the MIVM model, RSM model, and Miedema model all have good estimation effect, among which the RSM model has better estimation effect.
In conclusion, the average ARD of activity estimation values of the MIVM model, RSM model, Wilson model, NRTL model, and Miedema model are 28%, 162%, 508%, 562%, and 113%, respectively, when the symmetric method was adopted. The average ARD of the molar excess Gibbs energy estimates for the five models are 32%, 38%, 65%, 67%, and 33%, respectively. When the non-symmetric method was used, the average ARD of activity estimation values of the MIVM model, RSM model, Wilson model, NRTL model, and Miedema model are 64%, 115%, 661%, 727%, and 113%, respectively. The average ARD of the molar excess Gibbs energy estimates for the five models are 20%, 26%, 70%, 72%, and 33%, respectively. The results show that the MIVM model performs better than the other four models, the RSM model and Miedema model are second, and the Wilson model and NRTL model are not ideal. At the same time, the first peak of the partial radial distribution function obtained by the symmetric method is better than that by the non-symmetric method.
Comparing with similar studies on estimating the activity and molar excess Gibbs energy of binary liquid alloys, Wang et al. [25] utilized PRDF obtained from the literature and combined it with thermodynamic models to estimate the activity and molar excess Gibbs energy of 19 binary liquid alloys. The MIVM model achieved an ARD of less than 20% for the activity estimation of 15 alloys, while the RSM model achieved an ARD of less than 20% for 10 alloys. Therefore, Wang et al. concluded that the MIVM model performs better than other models, which is consistent with the findings of this study, thus validating the feasibility of our approach and enhancing the credibility of the conclusions.
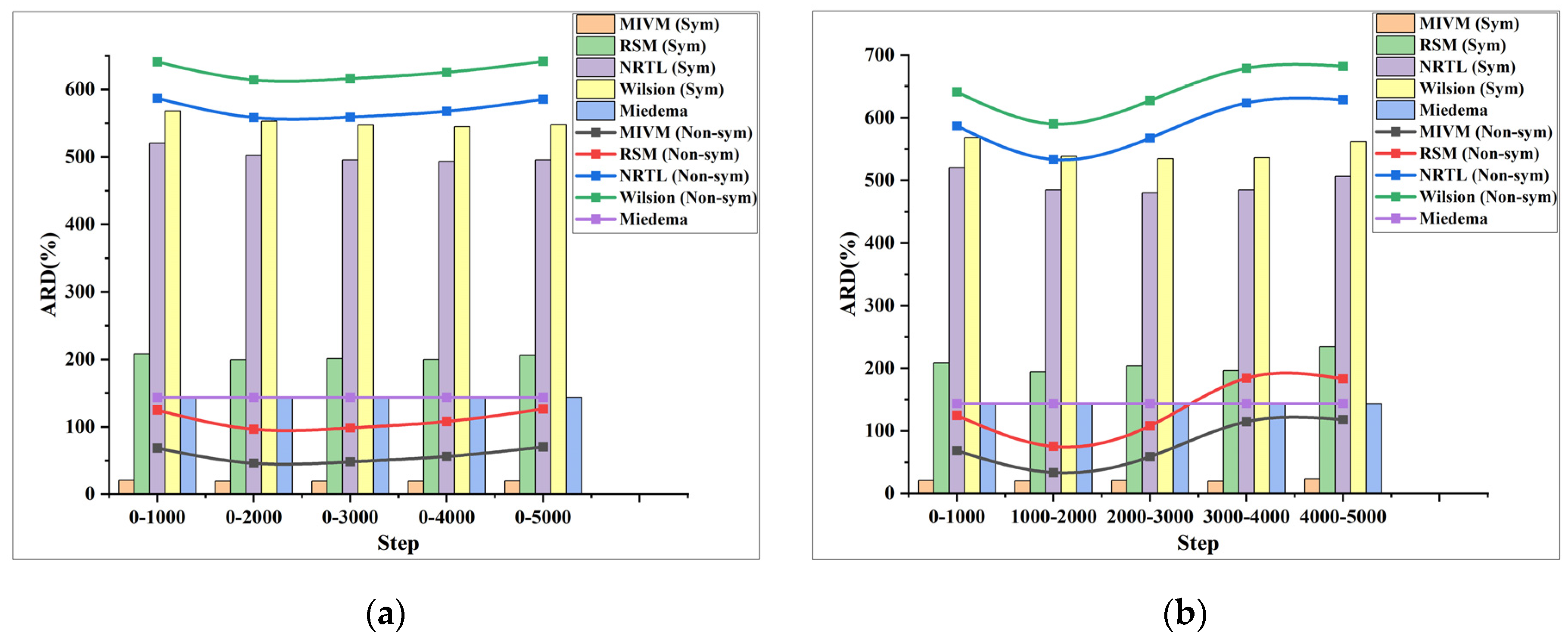
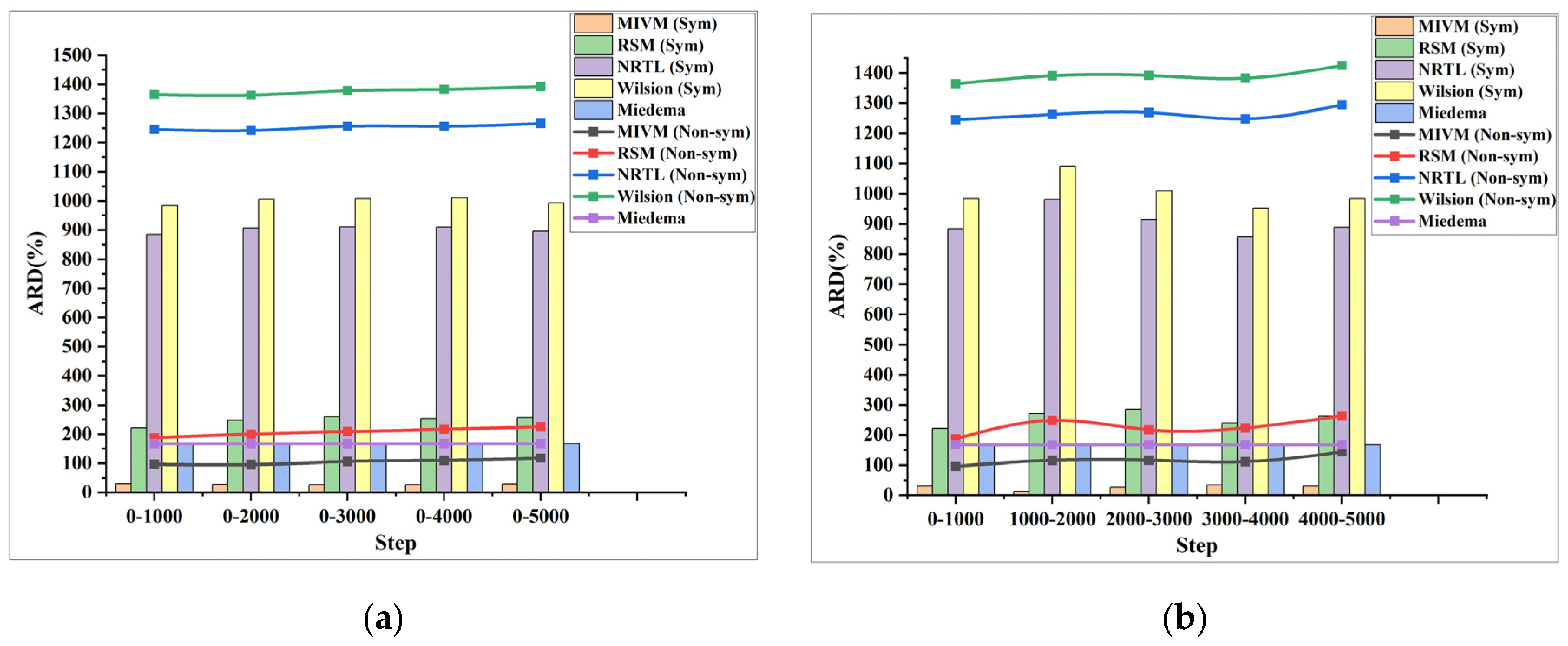
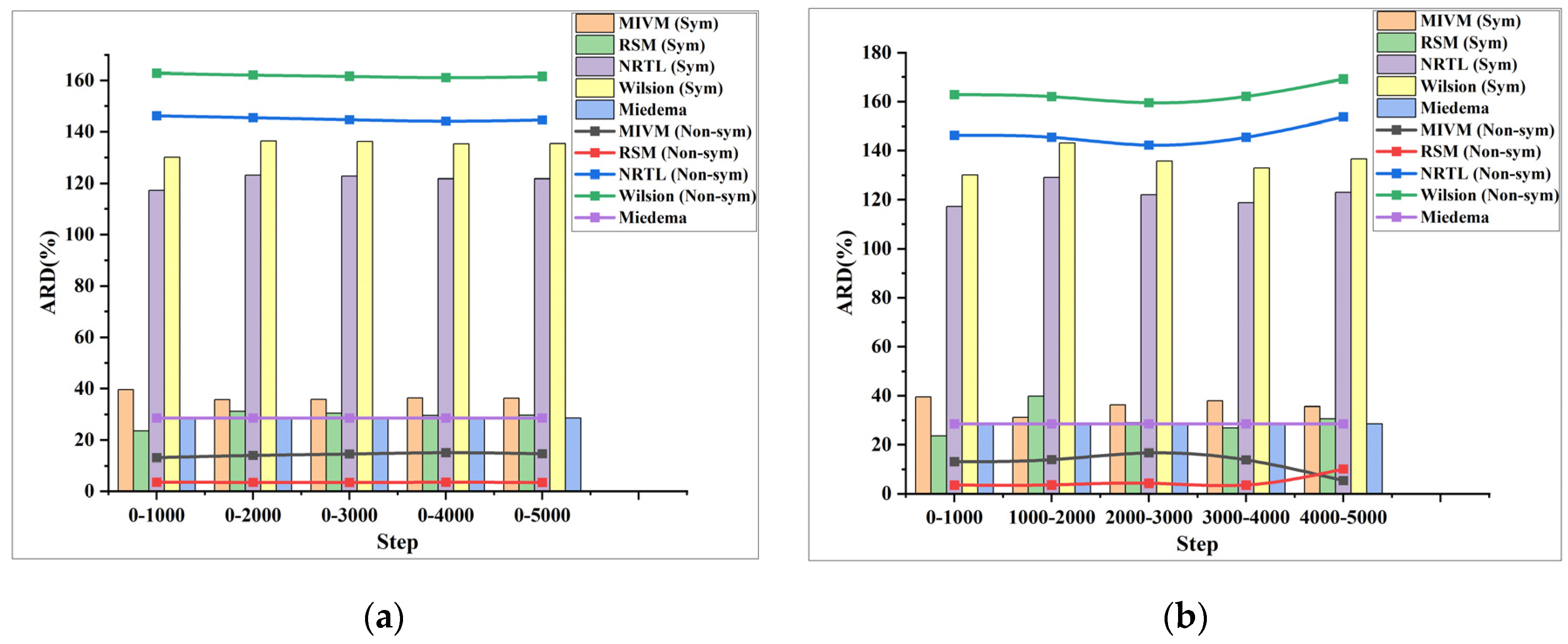
Figure 6, Figure 7 and Figure 8 depict the ARD plots for estimating the activity in the Al-Cu, Al-Ni, and Al-Fe systems. The bar charts display the ARD values obtained using the symmetric method, while the line charts illustrate the ARD values obtained using the non-symmetric method. Specifically, all of the Figures (a) and (b) in Figure 6, Figure 7 and Figure 8 show the ARD of the estimated activity of the three systems using two methods of grouping PRDF data, respectively.

Figure 6.
(a) ARD for the activity of the Al-Cu system estimated by the first PRDF grouping method; (b) ARD for the activity of the Al-Cu system estimated by the second PRDF grouping method.

Figure 7.
(a) ARD for the activity of the Al-Ni system estimated by the first PRDF grouping method; (b) ARD for the activity of the Al-Ni system estimated by the second PRDF grouping method.

Figure 8.
(a) ARD for the activity of the Al-Fe system estimated by the first PRDF grouping method, (b) ARD for the activity of the Al-Fe system estimated by the second PRDF grouping method.
Observing Figure 5, Figure 6 and Figure 7, it can be noted that for the three systems, the ARD and SD of activity estimated using the two proposed methods of stepwise processing of PRDF fluctuate, rather than consistently increasing or decreasing. Specifically, the average ARD of activity and molar excess Gibbs energy estimated by symmetric method are 315% and 47%, respectively. The average ARD of activity and molar excess Gibbs energy estimated by non-symmetric method are 392% and 51%, respectively. Based on the combined analysis of the ARD value and Figure 6, Figure 7 and Figure 8, it is evident that the symmetric method provides a better estimation effect.
4. Conclusions
In this study, PRDF of binary liquid alloys Al-Cu, Al-Ni, and Al-Fe were obtained by AIMD simulation, and five thermodynamic models (MIVM model, RSM model, Wilson model, NRTL model, and Miedema model) were used to estimate the activity and molar excess Gibbs energy of the alloys. The average ARD of the estimated activity of MIVM model is less than 45%, and the average ARD of the estimated activity of the other four models is greater than 110%. In general, the estimated effect of MIVM model is better than the other four models. In addition, the symmetric method is better than the non-symmetric method. The two methods proposed in this paper for PRDF grouping are feasible, and the average ARD and average SD of the activity and molar excess Gibbs energy estimated by using the grouped PRDF data are lower, indicating that grouping the PRDF data before estimation can effectively improve the estimation effect. The experimental results validate the feasibility and applicability of the proposed scheme. It is hoped that it can provide reference for selecting suitable models and methods to estimate thermodynamic data such as activity and molar excess Gibbs energy of binary liquid alloys.
Author Contributions
Conceptualization, Methodology, Review, Theoretical Guidance: D.T.; Writing—Original Draft, Writing—Review and Editing, Conceptualization, Formal analysis, Software: Y.L.; Resources, Conceptualization, Review: X.C.; Validation: T.Z. and J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China under Grant (Grant No. 51464022).
Data Availability Statement
Data are available on request from the authors. The data are not publicly available due to privacy.
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ajayi, A.A.; Oyeniyi, E.; Oshakuade, O.M. Bulk and surface properties of liquid Ag-Cu, Ag-Sb and Cu-Sb alloys. Pramana 2023, 97, 72. [Google Scholar] [CrossRef]
- Agbedor, S.O.; Yang, D.H.; Chen, J.Q.; Wang, L.; Wu, H. Low-Temperature Reactive Sintered Porous Mg-Al-Zn Alloy Foams. Metals 2022, 12, 692. [Google Scholar] [CrossRef]
- Zhou, C.; Jiang, L.; Gu, Z.; Wang, C.; He, L.; Huang, L.; Li, Z.; Li, K. Flexible core–shell structured Al-Cu alloy phase change materials for heat management. Chem. Eng. J. 2023, 471, 144610. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, X.; Chen, H.; Du, N.; Bao, K.; Tan, M. Mesoscale modeling on dynamic behavior of Al-Ni energetic structural materials under shock compression. Chin. J. Energ. Mater. 2020, 28, 984–994. [Google Scholar]
- Yao, Y.; Jing, L.; Wang, S.; Li, G.; Cui, J.; Tang, X.; Jiang, H. Mechanical properties and joining mechanisms of Al-Fe magnetic pulse welding by spot form for automotive application. J. Manuf. Process. 2022, 76, 504–517. [Google Scholar] [CrossRef]
- Shin, S.H.; Kim, S.J. Influence of slag composition on the distribution behavior of Cu between liquid sulfide and Cu-containing multicomponent slag via thermodynamic and kinetic assessment. Metals 2021, 11, 150. [Google Scholar] [CrossRef]
- Car, R.; Parrinello, M. Unified approach for molecular dynamics and density-functional theory. Phys. Rev. Lett. 1985, 55, 2471. [Google Scholar] [CrossRef]
- Wei, H.Q.; Zhang, P.; Tang, Y.Y. Ab Initio Molecular Dynamics Study of the Structure and Properties of Nb-Doped Zr-Cu-Al Amorphous Alloys. Metals 2021, 11, 1821. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Bazlov, A.I. Crystallization of FCC and BCC liquid metals studied by molecular dynamics simulation. Metals 2020, 10, 1532. [Google Scholar] [CrossRef]
- Han, C.; Zhou, Z.; Chen, X.; Xu, Y.; Jiang, W.; Yang, B.; Xu, B.; Liu, D. Structures and electronic properties of AlnVn (n = 2–12) clusters from ab initio calculations. Comput. Theor. Chem. 2020, 1176, 112746. [Google Scholar] [CrossRef]
- Xie, H.; Zhou, Z.; Xiong, H.; Chen, X.; Deng, P.; Wang, Y.; Yang, H.; Meng, C. Theoretical calculation and experimental study on the separation mechanism of PbS-Sb2S3. J. Mol. Struct. 2023, 1273, 134327. [Google Scholar] [CrossRef]
- Kurochkin, A.R.; Popee, P.S.; Yagodin, D.; Borisenko, A.V.; Okhapkin, A.V. Density of copper-aluminum alloys at temperatures up to 1400 °C determined by the gamma-ray technique. High Temp. 2013, 51, 197–205. [Google Scholar] [CrossRef]
- Plevachuk, Y.; Egry, I.; Brillo, J.; Holland-Moritz, D.; Kaban, I. Density and atomic volume in liquid Al-Fe and Al-Ni binary alloys. Int. J. Mater. Res. 2007, 98, 107–111. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1995, 47, 558–561. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244–13249. [Google Scholar] [CrossRef]
- Togo, A.; Tanaka, I. First principles phonon calculations in materials science. Scr. Mater. 2015, 108, 1–5. [Google Scholar] [CrossRef]
- Francis, G.P.; Payne, M.C. Finite basis set corrections to total energy pseudopotential calculations. J. Phys. Condens. Matter 1990, 2, 4395–4404. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef]
- Nosé, S. A molecular dynamics method for simulations in the canonical ensemble. Mol. Phys. 1984, 100, 191–198. [Google Scholar] [CrossRef]
- Guthrie, R.I.; Iida, T. The Physical Properties of Liquid Metals; Clarendon Press: Oxford, UK, 1988; pp. 71–72. [Google Scholar]
- Furukawa, K. The radial distribution curves of liquids by diffraction methods. Rep. Prog. Phys. 1962, 25, 395–440. [Google Scholar] [CrossRef]
- Tao, D. A new model of thermodynamics of liquid mixtures and its application to liquid alloys. Thermochim. Acta 2000, 363, 105–113. [Google Scholar] [CrossRef]
- Wang, C.; Chen, X.; Tao, D. Estimation of Component Activities and Molar Excess Gibbs Energy of 19 Binary Liquid Alloys from Partial Pair Distribution Functions in Literature. Metals 2023, 13, 996. [Google Scholar] [CrossRef]
- Hill, T.L. Statistical Mechanics: Principles and Selected Applications; Courier Corporation: Chelmsford, MA, USA, 1957; p. 185. [Google Scholar]
- Hildebrande, J.H. The Regular Solution Model for Binary Alloy. Proc. Natl. Acad. Sci. USA 1927, 13, 267–272. [Google Scholar]
- Hildebrande, J.H. Solubility VIII Regular Solutions. Am. Chem. Soc. 1929, 51, 66–80. [Google Scholar] [CrossRef]
- Wilson, G.M. Vapor-Liquid Equilibrium. XI. A New Expression for the Excess Free Energy of Mixing. J. Am. Chem. Soc. 1964, 86, 127–130. [Google Scholar] [CrossRef]
- Flory, P.J. Thermodynamics of high polymer solutions. J. Chem. Phys. 1941, 9, 660. [Google Scholar] [CrossRef]
- Renon, H.; Prausnitz, J.M. Local compositions in thermodynamic excess functions for liquid mixtures. AIChE J. 1968, 14, 135–144. [Google Scholar] [CrossRef]
- Miedema, A.R.; Châtel, P.F.; Boer, F.R. Cohesion in alloys-fundamentals of a semi-empirical model. Phys. B 1980, 100, 1–28. [Google Scholar] [CrossRef]
- Miedema, A.R. The electronegativity parameter for transition metals: Heat of formation and charge transfer in alloys. J. Less Common Met. 1973, 32, 117–136. [Google Scholar] [CrossRef]
- Tanaka, T.; Gokcen, N.A.; Spencer, P.J.; Morita, Z.; Tida, T. Evaluation of Interaction Parameters in Dilute Liquid Ternary Alloys by a Solution Model Based on the Free Volume Theory. Int. J. Mater. Res. 1993, 84, 100–105. [Google Scholar] [CrossRef]
- Ciobanu, B.C.; Pop, F.C.; Popescu, P.G. A fast way to compute definite integrals. Soft Comput. 2022, 26, 13485–13488. [Google Scholar] [CrossRef]
- Franke, P.; Neuschütz, D. Binary Systems. Part 1. Elements and Binary Systems from Ag-Al to Au-Tl. In Thermodynamic Properties of Inorganic Materials of Landolt-Börnstein-Group IV Physical Chemistry; Springer: Berlin/Heidelberg, Germany, 2002; Volume 19, p. 151. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).