Abstract
The use of a Zn/Ag–Cu–Zn/Zn multi-interlayer was observed to avoid the formation of Mg–Al binary intermetallic compounds (IMCs), which cause embrittlement and low strength of bonding when dissimilar metals such as Mg/Al are joined using ultrasound-assisted transient liquid phase bonding (U-TLP). The change in the microstructure and mechanical properties of the AZ31B/LY12 joints at 410, 440, and 460 °C with prolonging ultrasonic treatment (UST) time was investigated. The results showed that the diffusion of Ag and Cu was faster into the brazing seam on the LY12 side than that on the AZ31B side with increasing UST and temperature. The IMCs on both sides of the joints were transformed with the diffusion of Ag and Cu. The transformation made the fracture path shift from the AZ31B side (410, 440 °C) to the LY12 side (460 °C), and the maximum shear strength of the joints from 43.3 (410 °C) to 65.7 (440 °C) to 84.7 MPa (460 °C). The IMCs on the surface of the fracture path corresponding to the joints with optimal mechanical properties changed from Mg7Zn3+MgZn2+α-Mg (410 °C) to MgZnCu+Mg7Zn3 (440 °C) to Al2Cu (460 °C).
1. Introduction
As lightweight, advanced structural materials, magnesium alloys and aluminum alloys are widely used in aviation, aerospace, and other industrial manufacturing fields. Mg/Al welding and joining has inevitably attracted much attention [1,2]. In the last decade, researchers started employing a wide range of welding techniques, including tungsten gas shielded welding [3,4], friction stir welding [5,6,7], laser welding [8,9,10], and ultrasonic spot welding [11,12,13,14]. However, Mg–Al intermetallic compounds (IMCs), such as Al3Mg2 and Al12Mg17, were observed to be produced when these techniques were used to manufacture joints [15,16,17]; this technological limitation of these welding methods was found to seriously affect the mechanical properties of the joints [18,19,20]. Although contact reaction brazing–which uses various filler to join Mg/Al dissimilar alloys–is a very feasible method, its wide application is limited by the long hold time, high welding pressure, and vacuum conditions [21,22,23,24]. Ultrasound-assisted transient liquid phase bonding (U-TLP) is a newly developed joining method that is widely used in dissimilar alloy bonding [25,26,27]. When U-TLP is used, it is easy to break the oxide film layer of the alloys due to the acoustic plastic in solid and cavitation effects in liquid [28,29,30].
Adjusting the formation of IMCs by adding interlayers is currently a topic of discussion in Mg/Al dissimilar metal bonding. Several researchers have conducted studies to understand the regulation of the bonding strength of joints by controlling the production of Al3Mg2 and Al12Mg17 by adding interlayers. Xu et al. successfully joined dissimilar metals Mg/Al by U-TLP using Zn-based brazing material at 220 °C; the results showed that the dissolved Mg and Al easily formed a thicker IMC layer on the surface, resulting in a bonding strength of 23.4 MPa, which was the highest [31]. Hatef Shakeri et al. used physical vapor deposition of the silver layer; the shear strength of the joints reached 31.6 MPa when vapor deposition was used [32]. Peng et al. used ultrasonic spot welding to bond Mg/Al metals using silver foil as the interlayer; when this method was used, the thin diffusion layer (Mg3Ag) consisting of Ag acted as a good barrier to avoid the contact of Mg/Al, and the shear strength of the joints reached the highest value of 62 MPa [33]. Javad et al. used cold-rolled copper sheet as an interlayer in the dissimilar metal bonding of Al/Mg in a vacuum environment and achieved the highest width of the copper diffusion layer and the maximum shear strength of 31.2 MPa of the joints at 90 min [34]. Yang used Ag–Cu–Zn alloy as an interlayer to bond Mg/Al metals by contact reactive brazing in vacuum at a bonding temperature of 500 °C [35]. When this method was used, the Ag–Cu–Zn interlayer acted as a barrier and the shear strength of the joints was found to be 90 MPa. However, employing the contact reactive brazing method not only increased the possibility of softening of the base metal (BM), but also led to the pollution of the vacuum furnace due to the volatilization of zinc at high bonding temperatures. In summary, it was observed that the formation of phases could be changed by the addition of interlayers, which affected the mechanical performance of joints. The current research focuses on avoiding the generation of Mg/Al IMCs, reducing the bonding temperature, and improving the mechanical performance of the joints.
In this study, the above-mentioned challenges were addressed using a Zn/Ag–Cu–Zn/Zn multi-interlayer. Ag and Cu from the Ag–Cu–Zn alloy improved the mechanical properties of the joints. The addition of a Zn foil reduced the bonding temperature, and the molten Zn liquid played a role in breaking the oxide film on the surface of BMs. The evolution of the microstructure of the joints at different bonding temperatures and UST were investigated, and the effect of the formation of IMCs on the mechanical properties of the joints at different temperatures and UST was analyzed.
2. Experimental
For this study, the 20 mm × 20 mm × 3 mm AZ31B magnesium alloy (Mn: 0.2–1.0%, Al: 2.5–3.5%, Zn: 0.6–1.4%, Ca: 0.04%, Si: 0.08%, Cu: 0.01%, Fe: 0.003, Ni: 0.001% mass fraction) and the 20 mm × 20 mm × 3 mm LY12 aluminum alloy (Cu: 3.8–4.9%, Mg: 1.2–1.8%, Fe: 0.5%, Si: 0.5%, Zn: 0.25%, Ti: 0.15%, Ni: 0.5%, Mn: 0–0.9%, Cr: 0.1%, other: 0.15% mass fraction) were taken. Zinc foil (99.99% Zn) and Ag–Cu–Zn alloy foil (Cu: 50%, Zn: 30%, Ag: 20%), with thicknesses of 50 and 100 μm, respectively, were used.
Before the experiment, the surfaces of AZ31B and LY12 were polished using 180#, 320#, 500#, and 800# sandpaper. After polishing of their surfaces, AZ31B and LY12 were placed in 20% NaOH at 75 °C for 20 s for alkaline washing, rinsed with clean water, and finally placed for acid washing in 40% HNO3 for 3 min. The surfaces of AZ31B and LY12 were rinsed with hot water and dried. The Ag–Cu–Zn alloy foil was sized to 20 mm × 20 mm × 0.1 mm and placed for acid washing in 40% HNO3 for 1 min, rinsed in hot water to clean the surface, and dried. The Zn foil was cut into sheets of 20 mm × 20 mm × 0.05 mm and cleaned in petroleum ether and alcohol for 15 min using ultrasound.
The test equipment consists of three parts: ultrasonic generator, induction heating coil, and fixture. The schematic of the equipment is shown in Figure 1a,b and shows the sampling locations of metallographic and shear samples. The AZ31B/Zn/Ag–Cu–Zn/Zn/LY12 structure was placed on the fixture. The top of the fixture was pressed by the ultrasonic generator at a pressure of 0.15 MPa. The specimens were heated for a period of time using the high-frequency induction heating equipment until they reached the bonding temperature. The heating system was switched off and the specimens were left to cool in the atmosphere to complete the bonding. The specimens were then cut at the center using a wire cutter, sandpapered to a mirror finish, and etched with 2% nital for 2–5 s. The microstructure and fracture surface of the joints were analyzed in detail using scanning electron microscopy (SEM; model: JSM-7800F). The phase composition was analyzed using the SEM’s X-MaxN energy spectrometer (EDS). The composition of specific phases and crystallographic data were determined using FIB-SEM (model: FEI Strata 400S). Figure 1a shows the mechanical performance of the joints tested on the WDW-50KN tensile machine by the device. There were three shear samples in each test. The fracture path was analyzed using a Maxima XRD-7000 X-ray diffractometer. The Hysitron TI-PREMIER nana-indentation device was used to measure the hardness of the reactants in the joints.
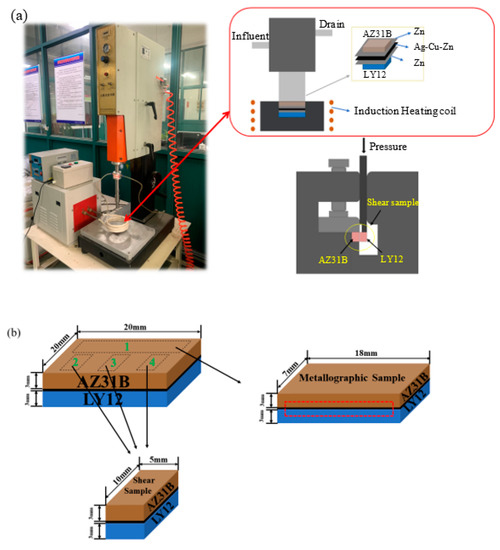
Figure 1.
Schematic of U-TLP and the shear strength test device (a); Sampling schematic diagram of shear sample and metallographic sample (b).
3. Results and Discussion
3.1. Microstructure Evolution of the Joints at 410 °C
Figure 2 shows the microstructure evolution of the joints under different UST at 410 °C. Table 1 shows the EDS results for each point in the joint. At this temperature, the following reactions occurred on the AZ31B and LY12 sides of the joints:
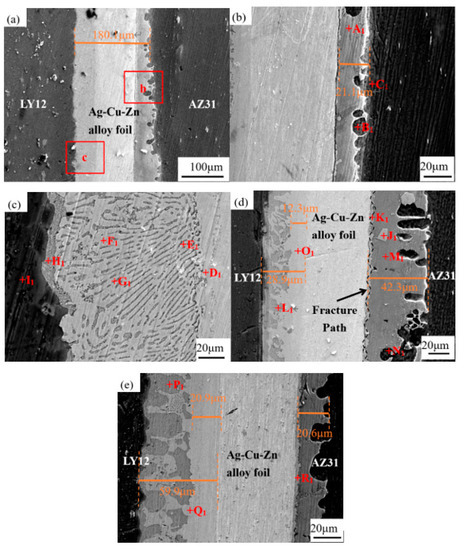
Figure 2.
Microstructure evolution of the joints with increasing UST at 410 °C: (a–c) 5 s; (d) 10 s; and (e) 15 s. Panel (b) refers to area b in (a) and panel (c) refers to area c in (a).

Table 1.
Chemical composition in different regions shown in Figure 2 (at.%).
AZ31B side:
LY12 side:
The reaction on both sides of the joints was even when the UST was 5 s, as shown in Figure 2a. The Ag–Cu–Zn alloy foil acts as a “physical barrier” to block the contact between AZ31B and LY12 BMs, and obvious defects can be observed on the AZ31B side of the joint. Panel (b) is area b in (a). As observed in panel (b), the Zn foil had been consumed completely after 5 s UST. The gray layer of Mg7Zn3 was generated because of the Zn–Mg eutectic reaction (region A1), and a small amount of α-Mg (region B1) grew into the AZ31B side of the joint. Cracks were obvious and could be seen between the Mg7Zn3 IMC and the Ag–Cu–Zn interlayer. The observation shows that it is the Zn foil and not the Ag–Cu–Zn alloy foil that first reacts with the AZ31B BM at 410 °C. Panel (c) is area b in (a). As observed in panel (c), the Zn foil was dissolved completely and the surface of LY12 BM was forced to be wetted by ultrasound [36,37,38], which promoted the bonding between the LY12 BM and the interlayer. A thin, lamellar Zn–Al eutectic (region H1) was formed on the LY12 side of the joint. The joint far from the interface of LY12 mainly comprised large dendritic η-Zn (region F1) and Zn–Al eutectic. In addition, the diffusion of Cu and Ag at the interface of the Ag–Cu–Zn foil (region E1) of the joint was 12.32% and 7.15%, respectively, and the diffusion of Cu and Ag on the LY12 side (region F1) of the joint was 1.64% and 1.23%, respectively. It may thus be concluded that the diffusion of Cu and Ag was faster on the LY12 side of the joint.
The width of the joint on the AZ31B side increased from 21.1 to 42.3 μm when the UST was 10 s, as shown in Figure 2d. This indicates that a large amount of Mg diffused out of AZ31B and reacted with Zn, generating more Mg7Zn3 (region M1) on the AZ31B side of the joint when the UST was 10 s. A bright white material grew between the Ag–Cu–Zn alloy foil and the gray layer of Mg7Zn3. The phase not only contained a large amount of Mg (35.45 at.%) and Zn (41.18 at.%), but also contained 16.41% and 0.21% of Cu and Ag, respectively. Cu first diffused into the brazing seam on the AZ31B side. It is worth noting that the Mg–Zn–Cu mixture (region K1) was exfoliated from region J1 due to the acoustic flow of the ultrasound. On the LY12 side of the joint, a large area of η-Zn disappeared and was replaced by the Zn–Al eutectic (region L1), which increased significantly and changed from strip to block distribution. Meanwhile, a light gray diffusion layer of the Zn–Ag–Cu–Al mixture (region O1) was generated at the interface of the Ag–Cu–Zn alloy foil in the brazing seam on the LY12 side. The diffusion of Cu and Ag into the diffusion layer was 14.07% and 7.85%, respectively, as shown in the EDS data in Table 1. At this time, Al (4.53 at.%) diffused from the LY12 side and solidly dissolved into the diffusion layer.
The width of the joint on the AZ31B side decreased from 42.3 to 20.6 μm when the UST was 15 s, as shown in Figure 2e. This was because a higher UST resulted in the generation of a larger amount of eutectic liquid phase. Since the eutectic liquid phase does not have the ability to withstand longer UST, it was extruded from the brazing seam, which resulted in the Mg7Zn3 layer with a lower width. There was still a uniform layer of Mg–Zn–Cu (region R1) at the interface of the Ag–Cu–Zn interlayer in the brazing seam on the AZ31B side. The thickness of the Mg–Zn–Cu mixture (region K1) at 15 s UST remained the same as that at 10 s UST, which indicated that the Mg–Zn–Cu mixture grew slowly. On the LY12 side of the joint, more Zn–Al eutectic (region P1) was generated. The light gray Zn–Ag–Cu–Al diffusion layer (region Q1) had Cu and Ag diffused to 25.60% and 12.10%, respectively, and its thickness almost doubled from 28.9 to 59.9 μm.
The diffusion of Ag and Cu into the brazing seam on the LY12 side was faster at 410 °C. With longer UST, Mg7Zn3 formed because of the Zn–Mg eutectic reaction on the AZ31B side of the joint, and Mg–Zn–Cu mixture formed at the interface of the Ag–Cu–Zn interlayer on the AZ31B side of the joint. The Zn–Al eutectic on the LY12 side of the joint changed from strip to block with longer UST, and the light gray diffusion layer of Zn–Ag–Cu–Al also widened continuously.
3.2. Microstructure Evolution of the Joints at 440 °C
The microstructure evolution of the joints under different UST time is shown in Figure 2. From the figure, it may be observed that since the diffusion of Cu and Ag was insufficient, their concentrations detected in the AZ31B side of the joint were low. Therefore, the bonding temperature was raised to 440 °C. Due to Cu diffusion, Al2Cu IMC began to form on the LY12 side of the joint.
Figure 3 shows the microstructure evolution of the joints at different UST at 440 °C. Table 2 shows the EDS results for each point in the joint. Compared with 5 s UST at 410 °C, Zn foil reached the melting point at 440 °C. The molten Zn reacted more actively with the Ag–Cu–Zn alloy in the brazing seam on the AZ31B side, and the width of the brazing seam increased from 180.1 to 313.3 μm. The Mg–Zn–Cu mixture (region D2) was generated at the interface of the Ag–Cu–Zn alloy foil in the brazing seam on the AZ31B side. The Mg–Zn–Cu mixture formed in the brazing seam on the AZ31B side was considered to be MgZnCu [39,40,41,42]. At 440 °C, a large number of α-Mg (region A2) attached to the MgZnCu layer. This was because, with the increase of bonding temperature, the Mg–Zn eutectic reaction generated more α-Mg in the brazing seam on the AZ31B side. The Zn–Al eutectic reaction occurred abundantly on the LY12 side of the joint. The concentration of Cu in the Al–Zn–Cu mixture (region E2) increased significantly, with 53.23% on the LY12 side of the joint. It is worth noting that the Al that diffused from the LY12 side at 410 °C was consumed to form a gray/black Al2Cu (region G2) layer on the LY12 side of the joint.
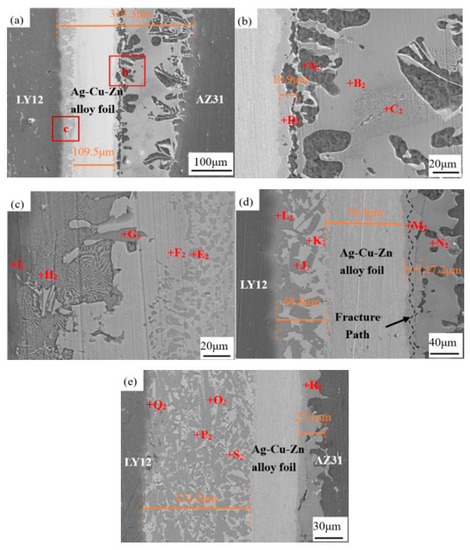
Figure 3.
Microstructure evolution of the joints with increasing UST at 440 °C: (a–c) 5 s; (d) 10 s; and (e) 15 s. Panel (b) refers to area b in (a) and panel (c) refers to area c in (a).

Table 2.
Chemical composition in different regions shown in Figure 3 (at.%).
The thickness of the Ag–Cu–Zn interlayer decreased from 109.5 to 76.3 μm, and that of the MgZnCu layer generated at the interface of the Ag–Cu–Zn interlayer in the brazing seam on the AZ31B side increased from 10.9 to 17.2 μm at 10 s UST. However, tiny cracks were seen to appear in the joints between the MgZnCu and the Mg–Zn layers. Obviously, Mg–Zn IMC was a hard brittle phase. Under external pressure, cracks appeared around Mg–Zn IMC [43,44]. Only a small amount of Cu and Ag were detected in region N2, indicating that the diffusion of Cu and Ag into the brazing seam on the AZ31B side was not sufficient at 10 s UST. The formation of AgZn3 (region K2) indicated that Ag had diffused into the brazing seam on the LY12 side (Ag: 22.96%). The diffusion of Cu also increased significantly, and more massive Al2Cu was formed (region J2).
More liquid phase was extruded from the brazing seam on the AZ31B side, which narrowed the brazing seam on the AZ31B side (25.1 μm) after 15 s UST. In addition, the Ag concentration was very low (only 0.35%), which indicated that Ag had not diffused, or that the diffusion of Ag was extruded out of the brazing seam with the eutectic liquid phase. The Zn–Al eutectic concentration in the brazing seam on the LY12 side decreased (region Q2). More Al combined with Cu to form Al2Cu (region O2), resulting in a sharp increase in the concentration and density of Al2Cu, and a rapid decrease in the concentration of Zn–Al eutectic.
At 440 °C, with increasing UST, the thickness of the MgZnCu layer increased and that of the Mg7Zn3 layer decreased in the brazing seam on the AZ31B side. AgZn3 was also detected in the brazing seam of the LY12 side. Meanwhile, the Zn–Al eutectic phase in the brazing seam on the LY12 side began to decrease and the formation of Al2Cu was initiated.
3.3. Microstructure Evolution of the Joints at 460 °C
At 440 °C, the diffusion of Ag was very low in the brazing seam on the AZ31B side, with the concentration of Ag being only 0.35% at 15 s UST. The diffusion of Ag reached 27.41% on the LY12 side of the joint at 15 s UST. Therefore, the bonding temperature was raised to 460 °C to improve the diffusion of Ag and the mechanical properties of joints.
Figure 4 shows the microstructure evolution of the joints under different UST at 460 °C. Table 3 shows the EDS results for each point in the joint. Panel (b) refers to area b in (a). Obviously, with the increase in temperature, the MgZnCu layer (region A3) at the interface of the Ag–Cu–Zn alloy foil extended into the AZ31B side of the joint. The MgZnCu layer reached a maximum width of 122.6 μm and replaced the Mg7Zn3 layer. Based on the EDS data shown in Table 3, it was found that a short UST resulted in insufficient diffusion of Ag (region A3), that is, the diffusion was only 2.76%. Panel (c) refers to area b in (a). A large amount of Al2Cu (region D3) was generated at 5 s UST and 460 °C, indicating that the temperature rise promoted the reaction of Al2Cu.
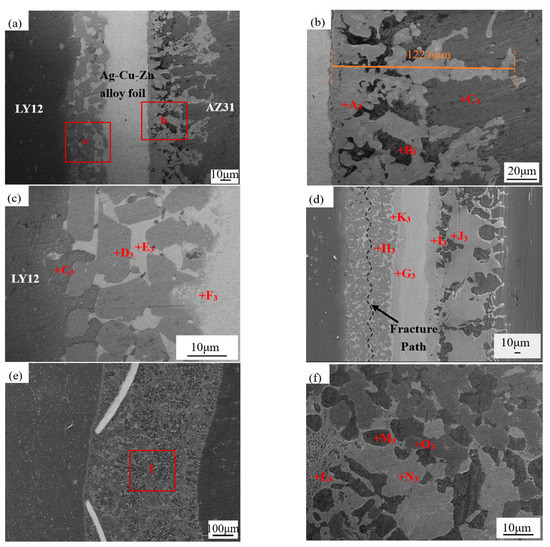
Figure 4.
Microstructure evolution of the joints with increasing UST at 460 °C: (a–c) 5 s; (d) 10 s; and (e,f) 15 s. Panel (b) refers to area b in (a), panel (c) refers to area c in (a), and panel (f) refers to area f in (e).

Table 3.
Chemical composition of different regions shown in Figure 4 (at.%).
The Mg–Zn–Cu–Ag mixture (region I3) containing 12.82% Ag was formed at the interface of the Ag–Cu–Zn foil in the brazing seam of the AZ31B side at 10 s UST. While the concentration of Ag was only 0.46% at 440 °C at 10 s UST, it reached 12.82% at 15 s UST. MgZnCu (region J3) in the brazing seam on the AZ31B side had generated at the interface of Ag–Cu–Zn on the AZ31B side, which changed from the dendritic growth to flake distribution, as shown in Figure 4d. The small amount of Al in region J3 was due to its precipitation from AZ31B BM at a higher temperature and a long UST. The Zn–Cu–Ag–Al mixture layer on the LY12 side (region G3) was also generated at the interface of the Ag–Cu–Zn on the LY12 side of the joint and was found to contain 22.14% of Ag by EDS analysis. Al2Cu+η-Zn+AgZn was detected on the LY12 side of the joint using EDS and XRD data. AgZn is formed at the crystallization temperature below 710 °C:
This was because acoustic streaming by ultrasound accelerated the diffusion of Ag and the damage of cavitation bubbles, causing the local temperature to rise.
The Ag–Cu–Zn interlayer was partially fractured, and, therefore, lost its role as a “physical barrier” to prevent AZ31B/LY12 at 15 s UST (Figure 4e). Combining the EDS results, it was found that Mg–Al IMC (region L3) was formed in the brazing seam and that it would seriously harm the mechanical properties of the joints.
At 460 °C, Ag had diffused into the MgZnCu IMC layer with the formation of Mg–Zn–Cu–Ag mixture in the brazing seam on the AZ31B side at longer UST. The rise in temperature resulted in the precipitation of Al from AZ31B BM with the formation of part of the Mg–Zn–Cu–Al mixture. The formation of AgZn was promoted by the aggregation of Ag by acoustic streaming, and Al2Cu also increased in the brazing seam on the LY12 side.
3.4. Mechanical Performance of the Joints at 410 °C, 440 °C, and 460 °C
The shear strength of the joints and the force–displacement curves are shown in Figure 5. The shear strength increased first and then decreased with increasing UST, at all three temperatures. When the bonding temperature was 410 °C and UST was 10 s, the shear strength of the joints reached a maximum value of 43.3 MPa, and fracture occurred at the brazing seam on the AZ31B side. Figure 6a shows the phase in the fracture path based on XRD analysis; the main components of the fracture are α-Mg, Mg7Zn3, and MgZn2.
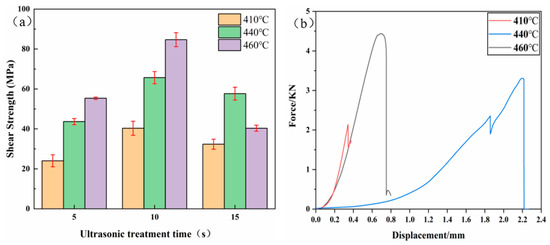
Figure 5.
Shear strength of the joints (a) and force–displacement curves (b) at 410 °C, 440 °C, and 460 °C with increasing UST.
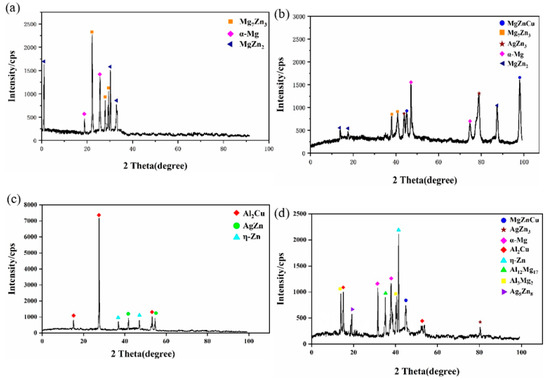
Figure 6.
XRD analysis of the fracture path at 410, 440, and 460 °C. (a) 410 °C, 10 s; (b) 440 °C, 10 s; (c) 460 °C, 10 s; and (d) 460 °C, 15 s.
At bonding temperature 440 °C and UST 10 s, the bonding strength of the joints reached the highest value of 65.7 MPa, and the fracture occurred at the brazing seam on the AZ31B side. Combined with the XRD results shown in Figure 6b, AgZn3 and MgZnCu were first detected at the fracture surface at 10 s UST and 440 °C. The results showed that with an increase in the bonding temperature, the Ag and Cu from the Ag–Cu–Zn alloy foil began to diffuse to both sides of the joint. The formation of Cu and Ag IMCs detected and the bonding strength of the joints from 43.3 MPa (410 °C) to 65.7 MPa (440 °C). Additionally, based on the EDS data in Table 2, the content ratio of Ag and Zn on the LY12 side of the joints was nearly 3:1, which may be inferred as AgZn3.
When the bonding temperature was 460 °C, the fracture path shifted from the interface of Ag–Cu–Zn alloy foil on the AZ31B side to the brazing seam on the LY12 side. The maximum shear strength of the joints reached 84.7 MPa. Based on the XRD results of fracture shown in Figure 6b, Al2Cu, η-Zn, and a small amount of AgZn were detected in the fracture. When the temperature of the brazing seam on the LY12 side was increased, a large amount of Al2Cu was generated due to the diffusion of many Cu atoms into the brazing seam. The diffusion of Ag from the Ag–Cu–Zn alloy foil was faster than that at the other two temperatures, leading to an increase in the concentration of Ag in the brazing seam with the generation of Mg–Zn–Cu–Ag (Ag: 12.82%) mixture at the interface of the Ag–Cu–Zn alloy foil in the brazing seam on the AZ31B side. Figure 7 shows the hardness in different areas of the brazing seam at 410 °C, 440 °C, and 460 °C with 10 s UST. As the temperature goes up, the hardness of the brazing seam on the AZ31B side decreased significantly due to the joints on the AZ31B side at each temperature changed from Mg7Zn3 (410 °C) to MgZnCu (440 °C) to Mg–Zn–Cu–Ag (Ag: 12.82%) mixture (460 °C). Therefore, the fracture path shifted from the interface of Ag–Cu–Zn alloy foil on the AZ31B side to the brazing seam on the LY12 side, which indicated that the IMCs of Ag and Cu can strengthen the mechanical properties of the joints.
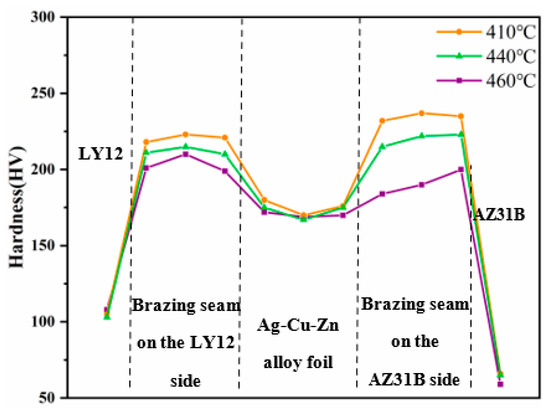
Figure 7.
Hardness in different areas of the joint at 410 °C, 10 s; 440 °C, 10 s; and 460 °C, 10 s.
Al3Mg2 and Ag5Zn8 were detected at the fracture at 15 s UST. The presence of Al3Mg2 indicates that the Ag–Cu–Zn interlayer was broken, resulting in the loss of the physical barrier between Mg/Al BM and creating contact between Mg/Al BM so that the mechanical properties of the joints were reduced to about 40.2 MPa.
4. Conclusions
1. Dissimilar alloys AZ31B/LY12 were successfully joined by Zn/Ag–Cu–Zn/Zn multi-interlayers under three temperature gradients (410 °C, 440 °C, 460 °C) and UST in the atmospheric environment.
2. The diffusion of Ag and Cu into the brazing seam on the LY12 side was faster. The joints on the AZ31B side at each temperature changed from Mg7Zn3 (410 °C) to MgZnCu (440 °C) to Mg–Zn–Cu–Ag (Ag: 12.82%) mixture (460 °C). The joints on the LY12 side at each temperature changed from Zn–Al eutectic (410 °C) to Al2Cu+AgZn3 (440 °C) to Al2Cu+AgZn (460 °C).
3. The IMCs on both sides of the joints were transformed with the diffusion of Ag and Cu. The fracture path of the joints shifted from the interface of the Ag–Cu–Zn alloy foil on the AZ31B side to the brazing seam on the LY12 side at 460 °C, which indicated that the IMCs of Ag and Cu can strengthen the mechanical properties of the joints. The mechanical properties of the joints reached the peak value of 84.7 MPa at 460 °C and 10 s UST.
Author Contributions
Conceptualization, J.G. and Z.S.; methodology, J.G.; software, J.G.; validation, Z.S., J.G. and Y.L.; formal analysis, J.G.; investigation, Y.L. and H.Z.; resources, Y.L.; data curation, Z.Y.; writing—original draft preparation, Z.Y.; writing—review and editing, Y.L.; visualization, Z.Y.; supervision, Z.P.; project administration, Z.P.; funding acquisition, Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by the National Natural Science Foundation of China (51871128, 51875300), Natural Science Foundation of Shandong Province (ZR2018MEE017), local science and technology development projects guided by the central government (YDZX20203700003578), and 2021 major industrial key project of transformation of old and new driving forces in Shandong Province.
Acknowledgments
We thank LetPub (www.letpub.com (accessed on 8 March 2022)) for its linguistic assistance during the preparation of this manuscript.
Conflicts of Interest
The authors declared that they have no conflicts of interest to this work. We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
References
- Mouritz, A.P. Magnesium alloys for aerospace structures. In Introduction to Aerospace Materials; Woodhead Publishing Limited: Cambridge, UK, 2012; pp. 224–231. [Google Scholar]
- Kubásek, J.; Minárik, P.; Hosová, K.; Šašek, S.; Knapek, M.; Veselý, J.; Stráská, J.; Dvorský, D.; Čavojský, M.; Vojtěch, D. Novel magnesium alloy containing Y, Gd and Ca with enhanced ignition temperature and mechanical properties for aviation applications. J. Alloys Compd. 2021, 877, 160089. [Google Scholar] [CrossRef]
- Zeng, X.-Y.; Wang, Y.-X.; Li, X.-Q.; Zhao, T.-J. Effect of inert gas-shielding on the interface and mechanical properties of Mg/Al explosive welding composite plate. J. Manuf. Processes 2019, 45, 166–175. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Z.; Liu, L. Microstructure evolution of Al/Mg butt joints welded by gas tungsten arc with Zn filler metal. Mater. Charact. 2012, 69, 84–89. [Google Scholar] [CrossRef]
- Xu, Y.; Ke, L.; Ouyang, S.; Mao, Y.; Niu, P. Precipitation behavior of intermetallic compounds and their effect on mechanical properties of thick plate friction stir welded Al/Mg joint. J. Manuf. Processes 2021, 64, 1059–1069. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Shigematsu, I.; Saito, N. Dissimilar friction stir welding between magnesium and aluminum alloys. Mater. Lett. 2008, 62, 3827–3829. [Google Scholar] [CrossRef]
- McLean, A.A.; Powell, G.L.F.; Brown, I.H.; Linton, V.M. Friction stir welding of magnesium alloy AZ31B to aluminium alloy 5083. Sci. Technol. Weld. Join. 2013, 8, 462–464. [Google Scholar] [CrossRef]
- Leo, P.; Renna, G.; Casalino, G.; Olabi, A.G. Effect of power distribution on the weld quality during hybrid laser welding of an Al–Mg alloy. Opt. Laser Technol. 2015, 73, 118–126. [Google Scholar] [CrossRef]
- Huang, Y.; Shen, C.; Ji, X.; Li, F.; Zhang, Y.; Hua, X. Effects of Mg content on keyhole behaviour during deep penetration laser welding of Al-Mg alloys. Opt. Laser Technol. 2020, 125, 106056. [Google Scholar] [CrossRef]
- Beiranvand, Z.M.; Ghaini, F.M.; Moosavy, H.N.; Sheikhi, M.; Torkamany, M.J. Solidification cracking susceptibility in pulsed laser welding of Al–Mg alloys. Materialia 2019, 7, 100417. [Google Scholar] [CrossRef]
- Gu, X.; Sui, C.; Liu, J.; Li, D.; Meng, Z.; Zhu, K. Microstructure and mechanical properties of Mg/Al joints welded by ultrasonic spot welding with Zn interlayer. Mater. Des. 2019, 181, 108103. [Google Scholar] [CrossRef]
- Patel, V.K.; Chen, D.L.; Bhole, S.D. Dissimilar ultrasonic spot welding of Mg-Al and Mg-high strength low alloy steel. Theor. Appl. Mech. Lett. 2014, 4, 041005. [Google Scholar] [CrossRef] [Green Version]
- Patel, V.K.; Bhole, S.D.; Chen, D.L. Microstructure and mechanical properties of dissimilar welded Mg–Al joints by ultrasonic spot welding technique. Sci. Technol. Weld. Join. 2013, 17, 202–206. [Google Scholar] [CrossRef]
- Macwan, A.; Chen, D.L. Ultrasonic spot welding of rare-earth containing ZEK100 magnesium alloy to 5754 aluminum alloy. Mater. Sci. Eng. A 2016, 666, 139–148. [Google Scholar] [CrossRef]
- Cheng, K.; Xu, H.; Ma, B.; Zhou, J.; Tang, S.; Liu, Y.; Sun, C.; Wang, N.; Wang, M.; Zhang, L.; et al. An in-situ study on the diffusion growth of intermetallic compounds in the Al–Mg diffusion couple. J. Alloys Compd. 2019, 810, 151878. [Google Scholar] [CrossRef]
- Chen, W.; Wang, W.; Liu, Z.; Zhai, X.; Bian, G.; Zhang, T.; Dong, P. Improvement in tensile strength of Mg/Al alloy dissimilar friction stir welding joints by reducing intermetallic compounds. J. Alloys Compd. 2021, 861, 157942. [Google Scholar] [CrossRef]
- Yamamoto, N.; Liao, J.; Watanabe, S.; Nakata, K. Effect of Intermetallic Compound Layer on Tensile Strength of Dissimilar Friction-Stir Weld of a High Strength Mg Alloy and Al Alloy. Mater. Trans. 2009, 50, 2833–2838. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Ding, Z.; Shen, C.; Chen, Y. Interfacial microstructure and properties of aluminum–magnesium AZ31B multi-pass friction stir processed composite plate. Mater. Des. 2016, 94, 240–252. [Google Scholar] [CrossRef]
- Bae, J.H.; Rao, A.P.; Kim, K.H.; Kim, N.J. Cladding of Mg alloy with Al by twin-roll casting. Scr. Mater. 2011, 64, 836–839. [Google Scholar] [CrossRef]
- Qiao, X.; Li, X.; Zhang, X.; Chen, Y.; Zheng, M.; Golovin, I.S.; Gao, N.; Starink, M.J. Intermetallics formed at interface of ultrafine grained Al/Mg bi-layered disks processed by high pressure torsion at room temperature. Mater. Lett. 2016, 181, 187–190. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Zhang, Z.; Liu, F.; Wang, Z.; Wang, H. Effect of addition of Ce in Sn–30Zn solder on the structure and properties of the Mg/Al-brazed joint. J. Mater. Sci. 2012, 48, 2030–2037. [Google Scholar] [CrossRef]
- Liu, L.M.; Zhao, L.M.; Wu, Z.H. Influence of holding time on microstructure and shear strength of Mg–Al alloys joint diffusion bonded with Zn–5Al interlayer. Mater. Sci. Technol. 2013, 27, 1372–1376. [Google Scholar] [CrossRef]
- Zhao, L.M.; Zhang, Z.D. Effect of Zn alloy interlayer on interface microstructure and strength of diffusion-bonded Mg–Al joints. Scr. Mater. 2008, 58, 283–286. [Google Scholar] [CrossRef]
- Liu, L.; Tan, J.; Liu, X. Reactive brazing of Al alloy to Mg alloy using zinc-based brazing alloy. Mater. Lett. 2007, 61, 2373–2377. [Google Scholar] [CrossRef]
- Lai, Z.; Xie, R.; Pan, C.; Chen, X.; Liu, L.; Wang, W.; Zou, G. Ultrasound-Assisted Transient Liquid Phase Bonding of Magnesium Alloy Using Brass Interlayer in Air. J. Mater. Sci. Technol. 2017, 33, 567–572. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Peng, Z.; Lv, B.; Cui, N.; Yan, J.; Zhang, H.; Zhou, T.; Yu, Z. Evolution of IMC layer and its reinforcing effect in 2024/MB8 dissimilar joints using a multi-interlayer of Cu/Zn via U-TLP bonding. Mater. Sci. Eng. A 2022, 835, 142627. [Google Scholar] [CrossRef]
- Peng, Z.; Zhou, T.; Li, Y.; Cui, Z.; Wu, Z.; Yan, J. Microstructure and mechanical performance of AZ31/2024 dissimilar alloy joints using a multi-interlayer of Ni/Al/Zn via ultrasonic-assisted transient liquid phase bonding. Mater. Des. 2021, 197, 109218. [Google Scholar] [CrossRef]
- Xu, G.; Leng, X.; Jiang, H.; Xiu, Z.; Yan, J. Microstructure and strength of ultrasonic-assisted brazed joints of Si3N4/6061Al composites. J. Manuf. Processes 2020, 54, 89–98. [Google Scholar] [CrossRef]
- Xu, Z.; Li, Z.; Chai, B.; Yan, J. Formation of TiAl3 and its reinforcing effect in TA15 alloy joint ultrasonically brazed with pure Al. J. Alloy. Compd. 2020, 815, 152493. [Google Scholar] [CrossRef]
- Wu, K.; Yuan, X.; Li, T.; Wang, H.; Xu, C.; Luo, J. Effect of ultrasonic vibration on TIG welding–brazing joining of aluminum alloy to steel. J. Mater. Process. Technol. 2019, 266, 230–238. [Google Scholar] [CrossRef]
- Xu, Z.; Li, Z.; Li, J.; Ma, J.; Yan, J. Control Al/Mg intermetallic compound formation during ultrasonic-assisted soldering Mg to Al. Ultrason. Sonochem. 2018, 46, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Shakeri, H.; Mofid, M.A. Physical Vapor Deposition Assisted Diffusion Bonding of Al Alloy to Mg Alloy Using Silver Interlayer. Met. Mater. Int. 2020, 27, 4132–4141. [Google Scholar] [CrossRef]
- Peng, H.; Chen, D.L.; Bai, X.F.; Wang, P.Q.; Li, D.Y.; Jiang, X.Q. Microstructure and mechanical properties of Mg-to-Al dissimilar welded joints with an Ag interlayer using ultrasonic spot welding. J. Magnes. Alloys 2020, 8, 552–563. [Google Scholar] [CrossRef]
- Varmazyar, J.; Khodaei, M. Diffusion bonding of aluminum-magnesium using cold rolled copper interlayer. J. Alloys Compd. 2019, 773, 838–843. [Google Scholar] [CrossRef]
- Yang, T.; Wang, K.; Zhang, D.; Huang, J. Contact-reaction brazing of an AZ31 magnesium/3003 aluminum alloy using a silver-copper-zinc interlayer. J. Mater. Process. Technol. 2017, 249, 531–537. [Google Scholar] [CrossRef]
- Yao, Z.; Kim, G.-Y.; Wang, Z.; Faidley, L.; Zou, Q.; Mei, D.; Chen, Z. Acoustic softening and residual hardening in aluminum: Modeling and experiments. Int. J. Plast. 2012, 39, 75–87. [Google Scholar] [CrossRef]
- Lai, Z.; Chen, X.; Pan, C.; Xie, R.; Liu, L.; Zou, G. Joining Mg alloys with Zn interlayer by novel ultrasonic-assisted transient liquid phase bonding method in air. Mater. Lett. 2016, 166, 219–222. [Google Scholar] [CrossRef]
- Siddiq, A.; Ghassemieh, E. Thermomechanical analyses of ultrasonic welding process using thermal and acoustic softening effects. Mech. Mater. 2008, 40, 982–1000. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Kuang, Y.; Liu, B.; Zhang, K.; Fang, D. Enhanced mechanical properties and degradation rate of Mg-3Zn-1Y based alloy by Cu addition for degradable fracturing ball applications. Mater. Lett. 2017, 195, 194–197. [Google Scholar] [CrossRef]
- He, M.L.; Luo, T.J.; Liu, Y.T.; Lin, T.; Zhou, J.X.; Yang, Y.S. Effects of Cu and Ce co-addition on the microstructure and mechanical properties of Mg-6Zn-0.5Zr alloy. J. Alloys Compd. 2018, 767, 1216–1224. [Google Scholar] [CrossRef]
- Lang, Q.; Wang, Q.; Han, J.; Zhang, W.; Zhang, Y.; Yan, J. Microstructure evolution of Mg/Cu dissimilar metal jointed by ultrasonicinduced transient liquid phase bonding with Zn interlayer. Mater. Charact. 2019, 157, 109897. [Google Scholar] [CrossRef]
- Mao, Z.; Niu, T.; Wang, Q.; Nie, Y.; Yan, J.; Deng, J.; Li, Y. Ultrasound-induced transient liquid-phase bonding of Mg/Cu dissimilar metals with Zn interlayer in air. Mater. Lett. 2020, 268, 127483. [Google Scholar] [CrossRef]
- Xu, Z.; Li, Z.; Peng, L.; Yan, J. Ultrarapid transient liquid phase bonding of Mg alloys within 1 s in air by ultrasonic assistance. Mater. Des. 2019, 161, 72–79. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Z.; Zhu, D.; Ma, Z.; Yan, J. Control of Mg2Sn formation through ultrasonic-assisted transient liquid phase bonding of Mg to Al. J. Mater. Process. Technol. 2018, 255, 524–529. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).