Abstract
This paper aims to investigate the diffusion bonding of Ti6Al4V to Al2O3. The potential of the use of reactive nanolayered thin films will also be investigated. For this purpose, Ni/Ti multilayer thin films with a 50 nm modulation period were deposited by magnetron sputtering onto the base materials. Diffusion bonding experiments were performed at 800 °C, under 50 MPa and a dwell time of 60 min, with and without interlayers. Microstructural characterization of the interface was conducted through scanning electron microscopy (SEM) with energy-dispersive X-ray spectroscopy (EDS). The joints experiments without interlayer were unsuccessful. The interface is characterized by the presence of a crack close to the Al2O3 base material. The results revealed that the Ni/Ti reactive multilayers improved the diffusion bonding process, allowing for sound joints to be obtained at 800 °C for 60 min. The interface produced is characterized by a thin thickness and is mainly composed of NiTi and NiTi2 reaction layers. Mechanical characterization of the joint was assessed by hardness and reduced Young’s modulus distribution maps that enhance the different phases composing the interface. The hardness maps showed that the interface exhibits a hardness distribution similar to the Al2O3, which can be advantageous to the mechanical behavior of the joints.
Keywords:
multilayers; diffusion bonding; Ti6Al4V alloys; Al2O3; microstructure; interface; sputtering 1. Introduction
In recent years, research into the development of similar and dissimilar joining technologies of titanium alloys has attracted much attention in the scientific community [1,2,3,4,5]. This is due to the attractive properties of these alloys, such as low density, high temperature properties and excellent creep and corrosion resistance [6,7,8]. Ti6Al4V, TiAl and TiNi are the most attractive titanium alloys for the aerospace industry. However, the major challenge for their application in the aerospace industry is related to the service temperature. The joining of these alloys to other materials will enhance the successful implementation of these alloys since a combination of unique properties will occur.
The successful joining of metal to advanced ceramics can contribute to overcome the challenges that titanium alloys present when the application requires operating temperatures above 550 °C [7]. Besides, due to the properties of advanced ceramics, such as high wear resistance and high thermal stability [9,10], this dissimilar joining will promote the formation of components with a combination of desirable properties.
However, joining materials with such different properties is quite demanding, making it a challenging task to obtain sound joints with good mechanical properties. One of the main difficulties is the development of residual stresses at the interface. The coefficients of thermal expansion (CTE) of ceramics are generally considerably lower than the CTE of metals. This CTE mismatch and the different mechanical behavior induce the formation of residual stresses at the interface of the joint during cooling. Hence, in recent years, researchers have been working to understand the mechanisms that affect the metal to ceramic joining process and propose alternatives [11,12,13,14,15,16,17,18,19,20,21].
Brazing [11,12,13,14] and diffusion bonding [15,16,17,18,19,20,21] are the most reported technologies for metal to ceramic joining. Brazing is a very attractive process since it is a cost-effective process, and a correct selection of the filler metal promotes the successful joining of titanium alloys to ceramics such as Al2O3 and ZrO2. The most-reported fillers used are Ti-based and Ag-based alloys. Ag-based alloys promote the formation of a significant extension of (Ag), which degrades the service temperature of the joints, while the Ti-based alloys require high processing temperatures that promote microstructural changes of base materials such as Ti6Al4V alloy.
Diffusion bonding [15,16,17,18,19,20,21] has the potential of obtaining sound joints without requiring the use of Ag-based fillers, and also the possibility of using lower bonding temperatures than those required with Ti-based fillers. However, in the conventional diffusion bonding process, high temperatures are required for joint production success during long durations. Several approaches have been developed in order to reduce the bonding conditions for the diffusion bonding of titanium alloys. The use of varying pressure, interlayers with ceramic nanoparticles or reactive interlayers has been referred to as a potential approach for overcoming the problems in dissimilar diffusion bonding [22,23,24,25,26,27,28]. The use of interlayers in diffusion bonding can reduce the processing conditions and promote the formation of a joint with good mechanical properties. In recent years, the joining of dissimilar metals using interlayers has evolved into reactive multilayer foils/films with nanometric bilayer thickness (modulation period) and chemical elements that react exothermically with each other, favoring diffusivity and reactivity, as well as acting as a localized source of heat/energy, e.g., [5,25,26,27,28]. The use of reactive multilayers for ceramic/metal joining is scarce, and only a few works have been reported so far [20,21].
Yi et al. [20] investigated the diffusion bonding of Al2O3 to copper assisted by Al/Ti reactive nano multilayers with different modulation periods. The diffusion bonding experiments were performed at 900 °C for 10 min. The results demonstrated that a sound joint can be obtained. Several intermetallic compounds were formed at the interface. The joint strength is strongly dependent on the modulation period of the multilayers. The use of reactive multilayers proves to be an effective approach in the joining of Cu to Al2O3.
Cao et al. [21] investigated the diffusion bonding of TiAl to TiC using Ni/Al multilayers with a total thickness of ~30 µm. Microstructural characterization revealed that the interface consists of a layered structure; a Ni3(AlTi) layer, a Ni2AlTi layer, a (Ni, Al, Ti) layer and a Ni diffusion layer were observed from the interlayer towards the TiAl base material. Ni/Al multilayers’ application improved the joints’ quality based on the shear strength values obtained.
The applicability of different reactive multilayer systems produced by magnetron sputtering has been studied for similar and dissimilar joints of NiTi, Ti6Al4V and TiAl alloys, as well as for Ni-based superalloys and steels [5,25,26,27,28]. The similar and dissimilar joints were produced with success for these base materials and under less demanding bonding conditions (time, temperature and/or pressure) than the ones required without multilayers. Nevertheless, the conditions are dependent on the reactive multilayer system used and the microstructure and mechanical properties at the interface are strongly influenced by the chemical composition of the base materials. Reliable joints produced for these base materials led us to consider an excellent approach to use these multilayers to join ceramic and titanium alloys.
In this context, the objective of the present work consists in the study of the feasibility of joining Ti6Al4V to Al2O3 by diffusion bonding using Ni/Ti reactive multilayers prepared by magnetron sputtering. The microstructure analysis of the interface was performed by scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDS)) in order to evaluate the integrity of the joint. Nanoindentation tests across the joint interface were carried out to understand the role of the reaction phases at the interface. Diffusion bonding experiments without multilayers were also conducted in order to evaluate the potential of this interlayer in dissimilar Ti6Al4V/Al2O3 joints.
2. Materials and Methods
2.1. Base Materials
Ti6Al4V alloy and Al2O3, supplied by Goodfellow in rods with diameters of 7 and 4 mm, respectively, were cut 5 mm in length and ground and polished down to 1 µm diamond suspension. The base materials were cleaned with acetone, ethanol and deionized water in an ultrasonic bath and dried with heat blow air before the multilayer’s deposition. The quality of the polished surfaces was assessed by optical microscopy (OM) (DM4000, Leica Microsystems (Leica Microsystems, Wetzlar, Germany)) and by the determination of the average roughness (Ra) of the polished base materials obtained by profilometry (Perthometer SP4, with laser probe (Mahr Perthometer SP4, Göttingen, Germany)). This evaluation was crucial for Al2O3 due to the presence of porosity. The polished process was adjusted in order to obtain different Ra values for Al2O3 (from 0.10 to 1.27 µm).
2.2. Deposition of Ni/Ti Multilayer Thin Films
Nickel and Titanium nanolayers were deposited alternately onto the polished surfaces of the base materials (substrates) by direct current magnetron sputtering using titanium (99.99% pure) and nickel (Ni-7V wt.%) targets (150 mm × 150 mm × 3–7 mm thick). After achieving a base pressure below 5 × 10−4 Pa in the sputtering chamber, Ar was introduced (P ≈ 1.5 × 10−1 Pa), and the substrate materials were cleaned by heating followed by Ar+ (current of 20A) etching using an ion gun. The depositions, carried out at a 4 × 10−1 Pa Ar pressure, started immediately after the substrates’ cleaning. To avoid substrate heating and thus prevent reactions during the deposition process, a thick copper block was used as substrate holder, which acted as a heat sink. The power density applied to Ti and Ni targets was ~7.20 × 10−2 Wmm−2 and ~2.8 × 10−2 Wmm−2, respectively, to obtain a near equiatomic average chemical composition (50 at. % Ni). According to the literature, in B2-NiTi vanadium can be located in Ni-substitutional site, as well as in Ti-substitutional site [29]. Therefore, a Ni:Ti atomic ratio close to 1 should be obtained, in order to guarantee that upon heating B2-NiTi is formed. The substrate holder’s rotation speed defines the time that the rotating substrates are in front of each target, determining the thickness of the individual layers, and, consequently, the period or bilayer thickness. In this work, a substrates’ rotation speed of ~2.0 rpm was used to achieve ≈ 50 nm of modulation period, and a deposition time of ~30 min was selected for a total thickness close to 3.0 µm. The initial layer was Ti to assure a good adhesion between the base materials and the multilayer thin films. This is particularly relevant for the Al2O3 base material because the lack of adhesion can compromise the diffusion bonding process. The top layer was Ni to prevent oxidation.
The adhesive strength between multilayer film and Al2O3 was evaluated by a procedure similar to that used by Lim et al. [30]. The adhesion strength between the film and substrate was measured using a mechanical tester with a load cell of 500 N. The mechanical pull test was carried out with a loading speed of 10.0 μm/min. The bonding strength is calculated as the average tensile stress at failure.
2.3. Diffusion Bonding Process
Ti6Al4V/Al2O3 diffusion bonding was performed in an apparatus consisting of a mechanical testing machine (LLOYD Instruments LR 30K (AMETEK Test & Calibration Instruments Lloyd Materials Testing, West Sussex, UK)), vertical infrared radiation furnace, molybdenum punctures (to apply pressure), quartz tube and vacuum system, as described in previous works [26,27].
Diffusion bonding experiments were performed at a vacuum level better than 10−2 Pa. The dissimilar base materials were joined with and without the reactive multilayers to evaluate the interlayer’s potential in the joining process. The diffusion bonding experiments were carried out at 800 °C, by applying a pressure of 50 MPa during 60 min. Different rates of heating and cooling were investigated. In the first step, the rate of heating and cooling was 10 °C/min and in a second step, the heating rate was 10 °C/min up to 800 °C, and the cooling rate was 5 °C/min up to 500 °C, followed by 3 °C/min down to room temperature.
2.4. Microstructural Characterization
Microstructural and chemical characterization of the multilayer thin films and diffusion bonding joints were carried out by scanning electron microscopy (SEM) (FEI Quanta 400FEG ESEM/EDAX Genesis X4M (FEI Company, Hillsboro, OR, USA)) operating at an accelerating voltage of 15 keV, coupled with energy-dispersive X-ray spectroscopy (EDS) (Oxford Instrument, Oxfordshire, UK). The EDS measurements were made at an accelerating voltage of 15 keV by the standardless quantification method. The results obtained by this method provide a fast quantification with automatic background subtraction, matrix correction, and normalization to 100% for all the elements in the peak identification list. The cross-sections of the films and of the joints’ interfaces were prepared using standard metallographic procedures.
2.5. Mechanical Characterization
Nanoindentation tests were carried out across the joints’ interface by using a Micro Materials-NanoTest equipment with a Berkovich diamond indenter. The depth-sensing indentation tests were performed in load control mode, at a maximum load of 5 mN. Several indentation matrixes formed by 8 rows and 12 columns (96 measurements) were defined along the joint interface in order that each matrix starts on the Ti6Al4V side, cross the interface, and finish on the alumina side. The distance between columns was 3 µm to guarantee that some indentations fall in the region corresponding to the interlayer thin films, and the distance between rows was 5 µm. Fused quartz was used as reference material to determine the Berkovich tip area function. Hardness and reduced Young’s modulus were determined by the Oliver and Pharr method [31]. Before the indentation tests, the joints were polished using standard metallographic procedures.
3. Results and Discussion
3.1. Characterization of Ni/Ti Multilayer Thin Films Deposited onto Al2O3
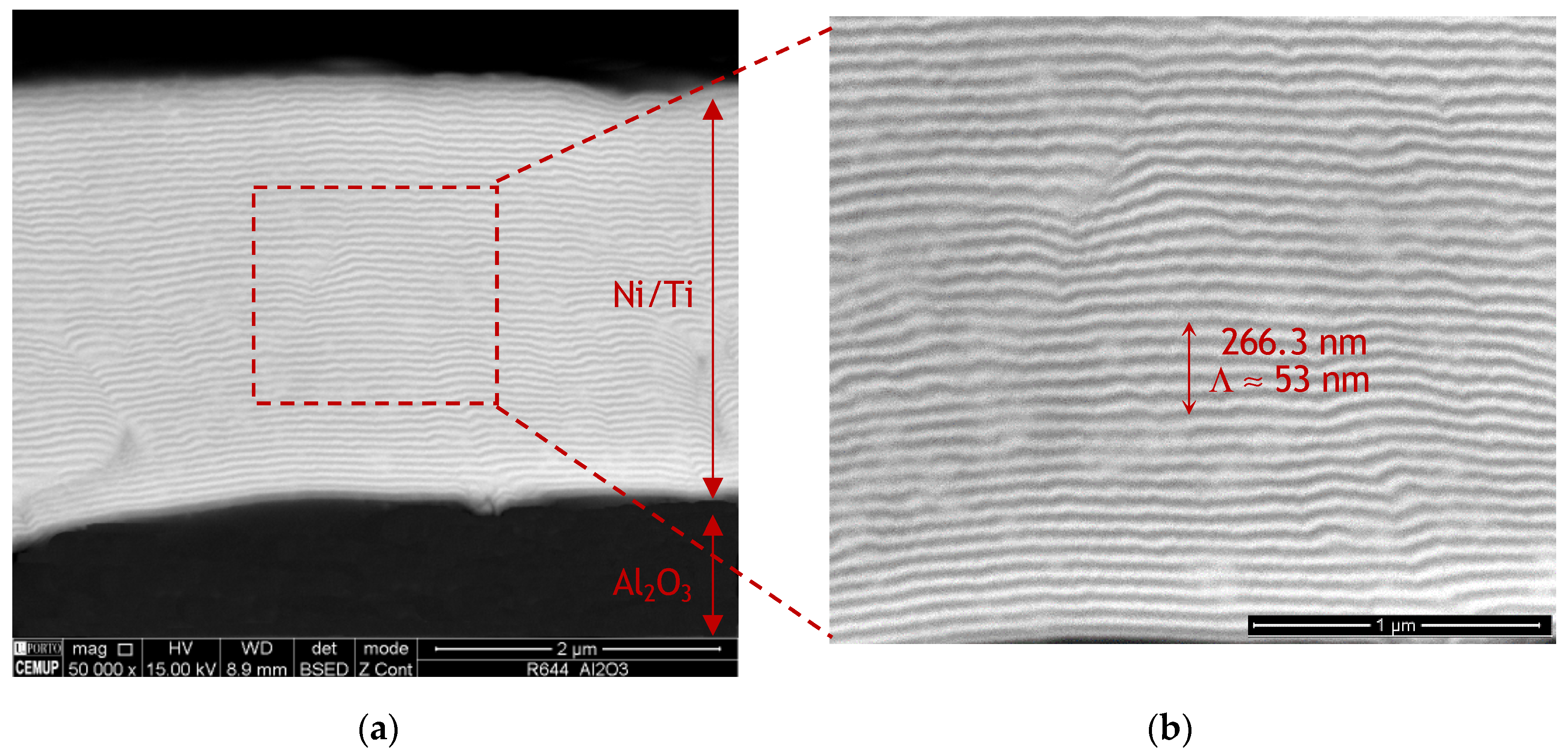
Figure 1 shows SEM images of an as-deposited Ni/Ti multilayer thin film deposited onto the Al2O3 base material. During the sputtering process, multilayer reaction was not detected. SEM images revealed that the multilayer’s structure was preserved, being possible to observe the Ni- (light grey) and Ti-rich (dark grey) alternated nanolayers clearly present in the high magnification SEM image of Figure 1b. The Ni/Ti multilayer film’s total thickness is approximately 3.0 µm and the modulation period (Λ) is close to 50 nm.
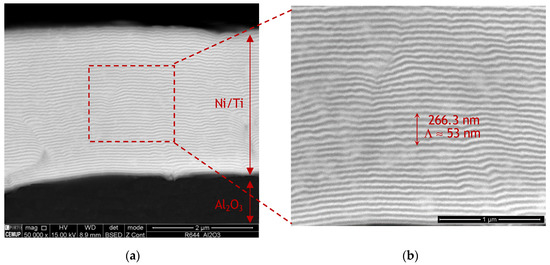
Figure 1.
(a) Scanning electron microscopy (SEM) images of a Ni/Ti multilayer thin film with ~50 nm modulation period (Λ) deposited onto Al2O3 and (b) higher magnification of the region marked in (a).
The success of obtaining sound joints depends strongly on the multilayer thin films’ good adhesion to the base materials. Due to the high challenges for a good preparation of the Al2O3 surface, an evaluation of the adhesion and morphology of the films deposited onto this base material was crucial.
The multilayer thin films’ adhesion to the Al2O3 base material is strongly influenced by the surface topography. In contrast to the observed average roughness for Ti6Al4V (Ra = 0.06 ± 0.01 µm) prepared using the standard metallographic procedure, alumina prepared by the same process exhibits a Ra of 1.27 ± 0.01 µm. Although due to this higher value the Al2O3 surface peaks could act as mechanical anchoring zones to obtain good ceramic/multilayer film adhesion, the value is higher than that considered adequate for the diffusion bonding process [30,32,33]. To obtain an Al2O3 surface with less Ra, an alternative approach to the metallographic procedure was conducted using solutions with high diamond concentration (75, 6 and 1 µm), which resulted in Ra close to 0.10 ± 0.01 µm.
The adhesive strength between multilayer film and ceramic substrate was measured. Pull-off tests were done to evaluate the adhesive strength between Al2O3 and Ni/Ti multilayer thin films with low (0.1 µm) and high (1.27 µm) Ra. As referred in the literature [33], a good value of adhesive strength should be around 10 MPa. As expected, the adhesion is better for the base material with higher Ra (16 MPa). For lower Ra values, an adhesive strength between 0 to 1 MPa was observed. It means that reducing the roughness of the ceramic surface impairs its adhesion to the Ni/Ti reactive multilayers. Therefore, in this work, ceramic base materials with high roughness were used.
3.2. Characterization of Diffusion Bonding Joints
The effectiveness of using Ni/Ti multilayer thin films to reaction-assist the diffusion bonding of Ti6Al4V alloy to Al2O3 was assessed through microstructural and mechanical characterization. Joining of Ti6Al4V to Al2O3 was processed at 800 °C, applying a pressure of 50 MPa for 60 min, without and with Ni/Ti multilayer thin films as interlayer material. Experiments conducted using base materials without interlayers allowed the multilayer thin films’ role in these dissimilar joints to be understood.
3.2.1. Diffusion Bonding without Interlayer
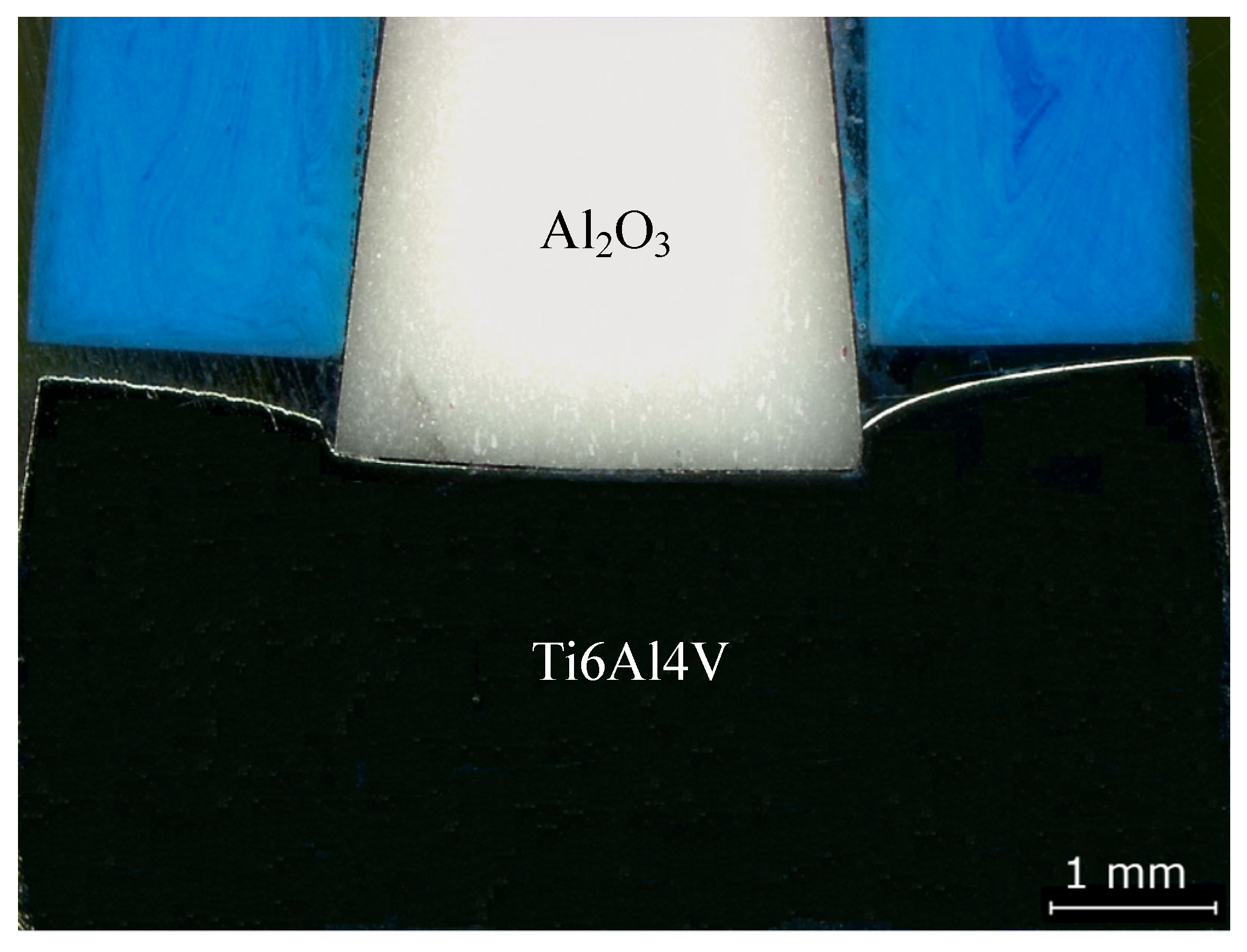
Initially, the experiments were carried out with heating and cooling rates of 10 °C/min. Observations in optical microscopy (OM) revealed a high deformation in the Ti6Al4V alloy after joining without a multilayer. Figure 2 shows OM images where plastic deformation is clearly observed for the joint produced. In order to reduce the residual stresses, a heating rate of 10 °C/min up to 800 °C, and cooling rate of 5 °C/min up to 500 °C, followed by 3 °C/min down to room temperature, were adopted for all the subsequent diffusion bonding experiments.
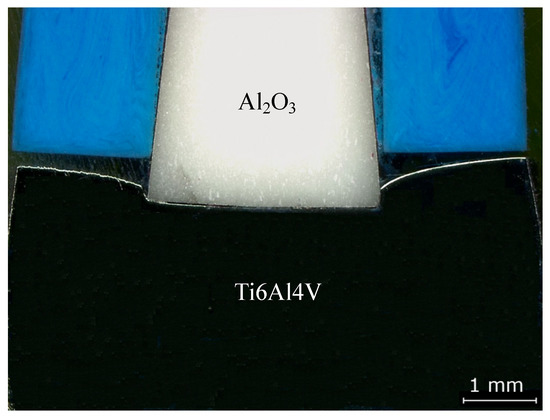
Figure 2.
Optical microscopy (OM) image of the Ti6Al4V/Al2O3 joint processed at 800 °C under 50 MPa for 60 min with heating and cooling rates of 10 °C/min.
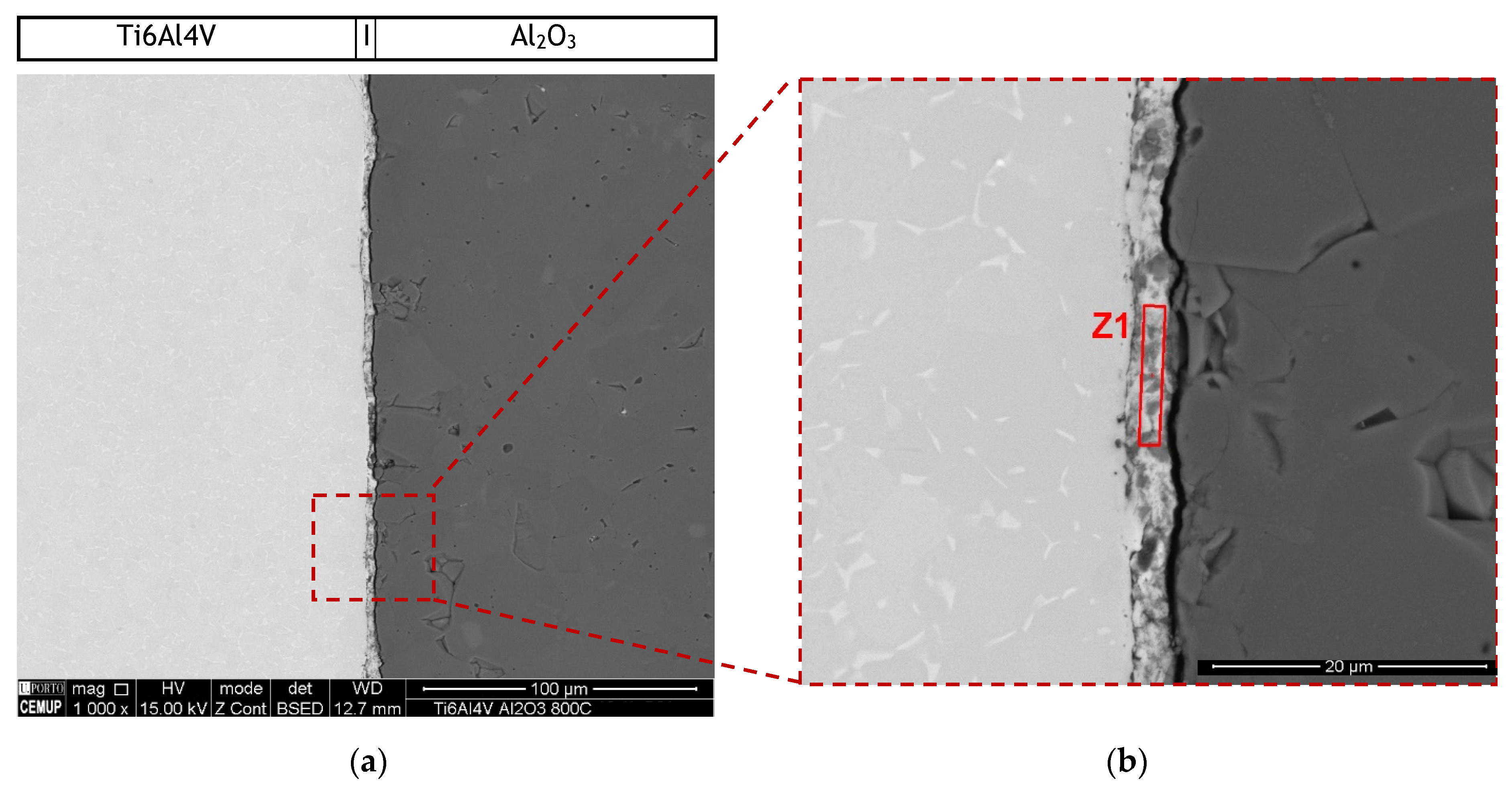
The microstructure of the interface of the joints produced without interlayer was characterized by SEM. Backscattered electron (BSE) SEM images of the joint produced can be observed in Figure 3. Although the sample was apparently bonded at the end of the diffusion bonding process, an unbonded layer can be observed after metallographic preparation. This may have been due to residual stresses that occurred during cooling leading to the detachment of the base materials or even to the formation of a brittle phase that resulted in crack nucleation and propagation during sample preparation. Anyway, a reaction layer is observed close to the Ti6Al4V base material. SEM observation revealed that this reaction zone exhibits a thickness of 3.1 μm.
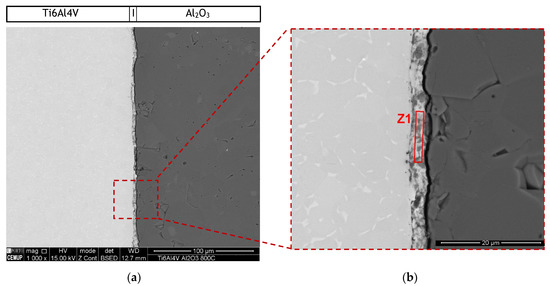
Figure 3.
(a) Backscattered electron (BSE) SEM images of diffusion bonded Ti6Al4V/Al2O3 joint processed at 800 °C and (b) higher magnification of the region marked in (a).
The chemical composition of reaction zone Z1 was obtained by EDS, resulting in 48.5% Ti, 39.3% O, 11.3% Al and 0.9% V (atomic%). This zone is due to the reaction between the elements that diffused from the base materials to the center of the interface. Although the EDS does not allow a correct analysis of the oxygen, based on these results it is clear that in this layer reaction occurred between the elements of the Ti base material with some of the oxygen, and eventually Al, coming from the ceramic base material. According to these results, at 800 °C it is not possible to diffusion bond Ti6Al4V to Al2O3, even by applying pressures up to 50 MPa during 60 min.
3.2.2. Diffusion Bonding Using Ni/Ti Reactive Multilayers
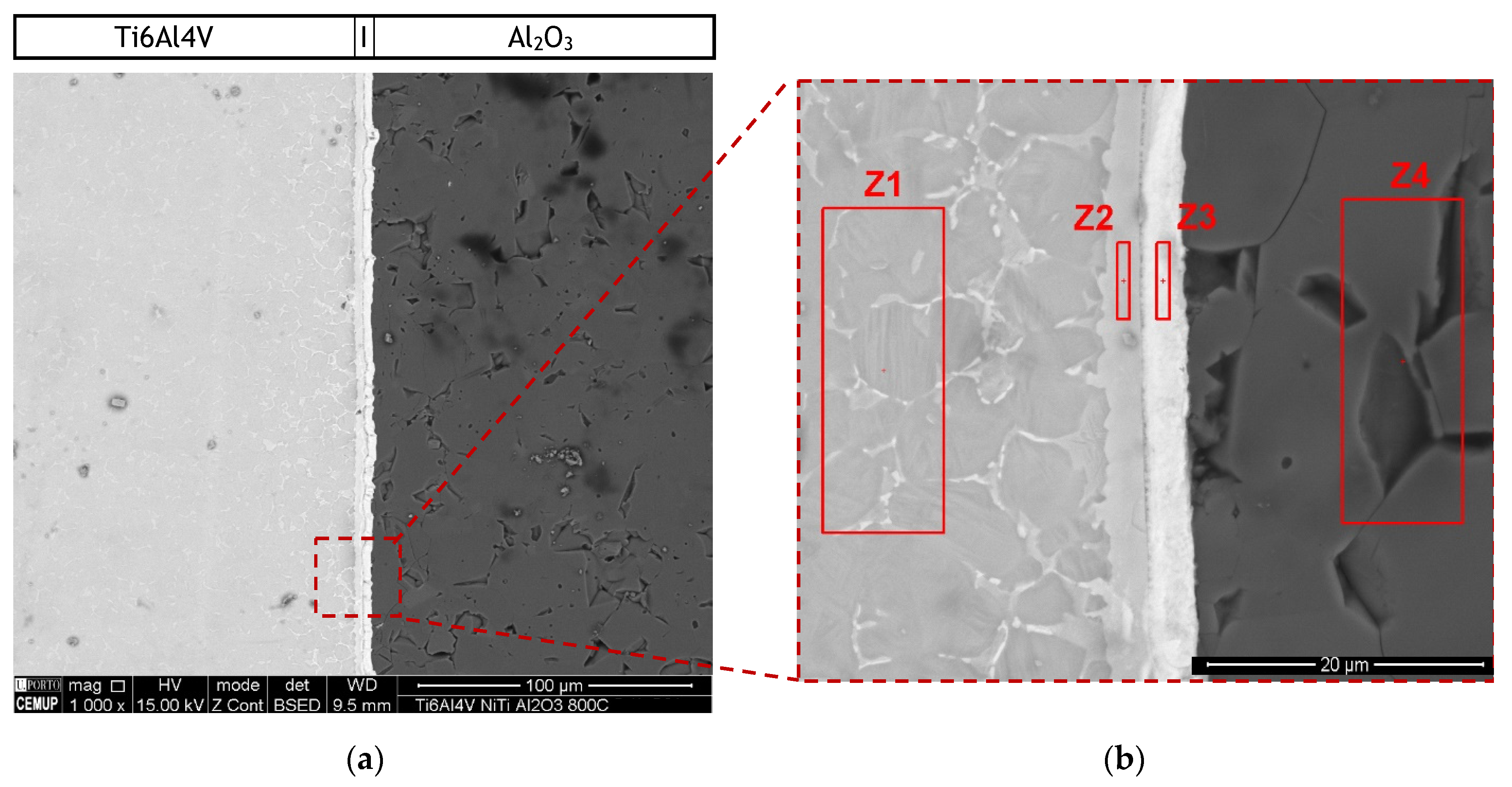
Diffusion bonding of Ti6Al4V to Al2O3 was processed under the same conditions using Ni/Ti reactive multilayers. Nanometric interlayers on the contact surfaces of the base materials aim to improve the diffusivity through the interface e.g., [5,26,27], but can also contribute to reduce or eliminate the formation of residual stresses. Microstructural characterization reveals that interfaces with apparent soundness were produced during solid-state diffusion bonding of Ti6Al4V to Al2O3 using Ni/Ti multilayer thin films. The cross-section of the joint was observed and analysed by SEM. The BSE images in Figure 4 show an interface with a thickness of 6.4 ± 0.2 µm without voids or cracks along the interface. Although pores characterized the Al2O3 base material, they did not affect the effectiveness of the joining process. Two regions can be distinguished at the interface, one darker zone adjacent to the Ti6Al4V alloy and a brighter zone adjacent to the Al2O3. The bondline is perceptible at the center of the interface. Previous work [25] demostrated that the interface of similar TiAl joints using Ni/Ti reactive multilayers exibith a line rich in Ti that divided the interface. The identification of the possible phases at the interface was performed taking into account the chemical composition obtained by EDS in the regions highlighted in red in Figure 4b, combined with the phase diagrams [34,35]. The EDS results are presented in Table 1. During the diffusion bonding process, Ni and Ti from the multilayer thin film reacted to form intermetallic compounds. According to previous works [34,35] and considering the average chemical composition (Ni:Ti atomic ratio close to 1), austenitic NiTi should form. However, due to diffusion, a Ti enrichment is observed at the interface (zone Z2 in Figure 4b). Cavaleiro et al. [36] studied the interaction between Ni/Ti multilayer thin films and Ti6Al4V substrates during heat treatment and confirmed the diffusion of Ni from the thin films towards the β-phase of the substrate. In fact, when compared to the Ti6Al4V in Figure 3, in Figure 4 the Ti6Al4V base material (zone Z1) has more bright phase along the α-Ti grain boundaries, which should correspond to β–Ti with a higher concentration of β-stabilizer elements, such as Ni and V [26,35,36,37].
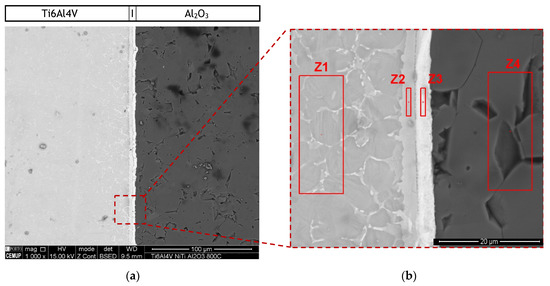
Figure 4.
(a) BSE SEM images of diffusion bonded Ti6Al4V/Al2O3 joint processed at 800 °C using a Ni/Ti multilayer and (b) higher magnification of the region marked in (a).

Table 1.
Chemical composition obtained by EDS of the regions highlighted in Figure 4.
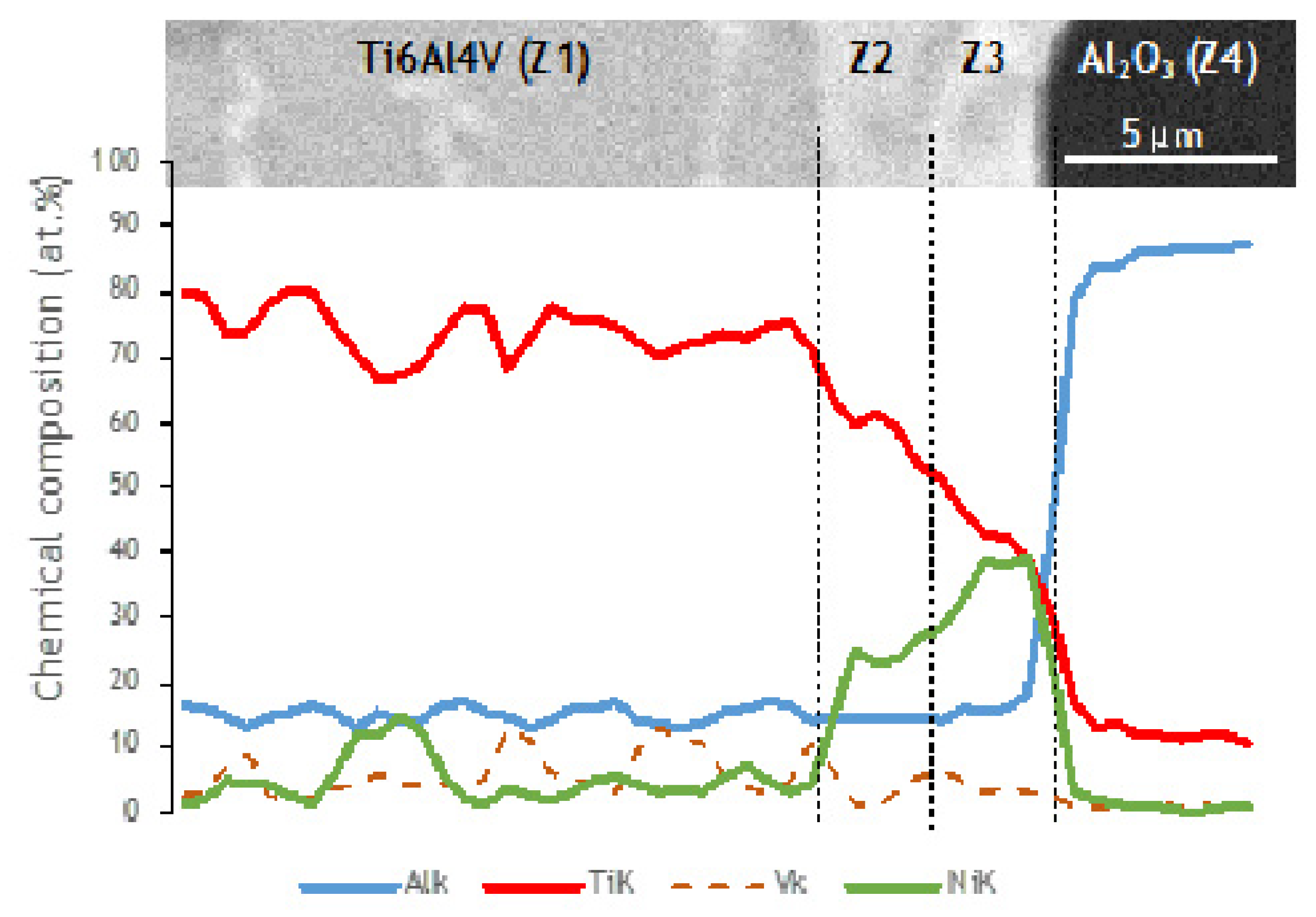
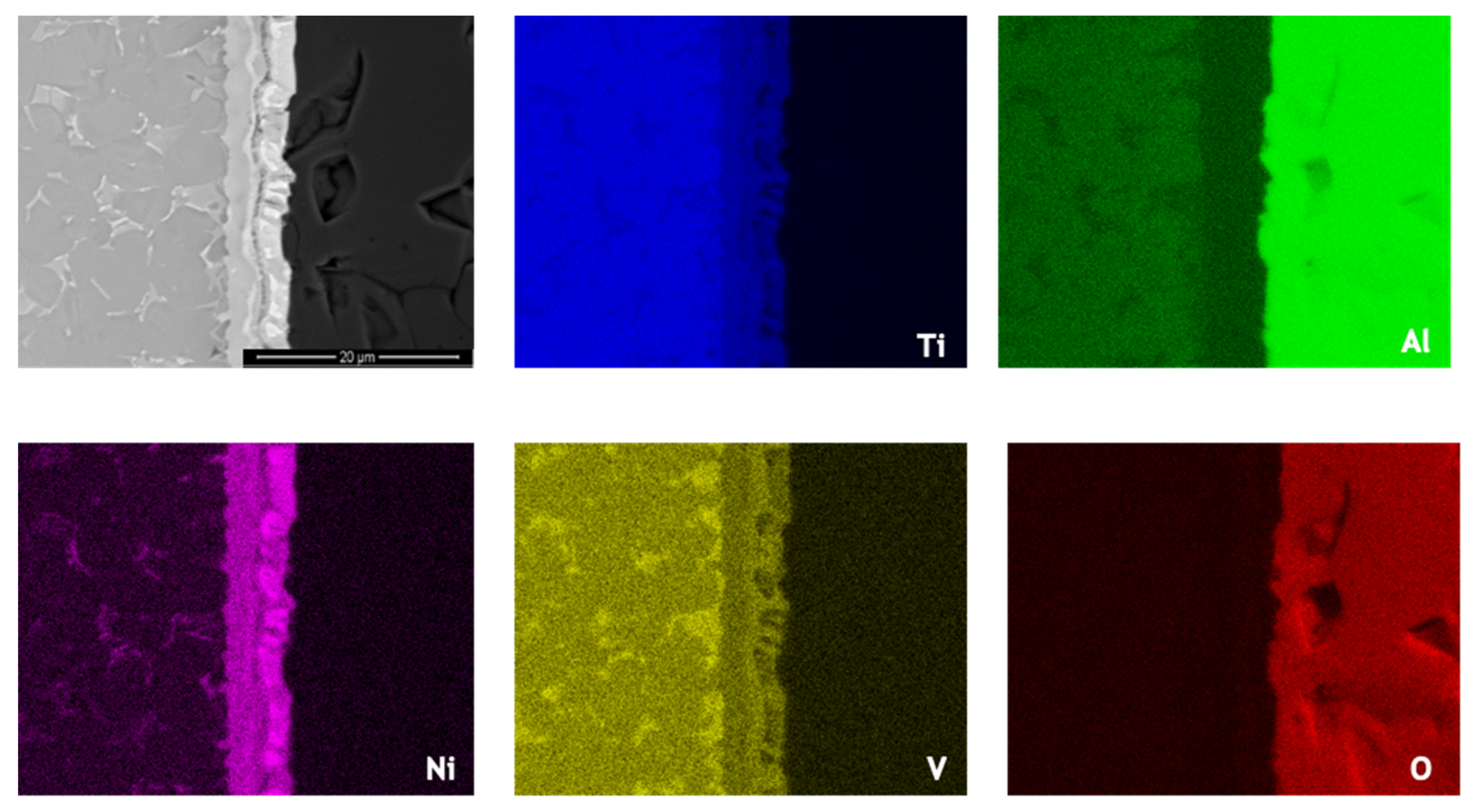
Ni and V diffused from the interlayer towards the Ti6Al4V, as can be observed in the elemental distribution profiles shown in Figure 5, as well as in the elemental distribution maps of Figure 6. The vanadium content in the interlayer is due to its presence in the Ni target used for producing the Ni/Ti multilayer thin films, as referred in Section 2.2. The chemical composition of zone Z1, combined with the Ti-Al-V and Ti-Al-Ni ternary phase diagrams [38,39], indicates as possible phases α-Ti and β-Ti (Table 1). The presence of Ni and V along the α–Ti grain boundaries is confirmed by their distribution on the Ti6Al4V side (Figure 6). The decrease of the Ni content in zone Z2 (compared to zone Z3) is explained due to the high diffusion coefficient of Ni in Ti6Al4V [40], i.e., Ni diffused from the interlayer thin film towards the base metal, as explained above. The content of Ti and Ni available in Z2, combined with the Ni-Ti phase diagram, points to the presence of the NiTi2 phase, and is also reported in the literature [26,36].
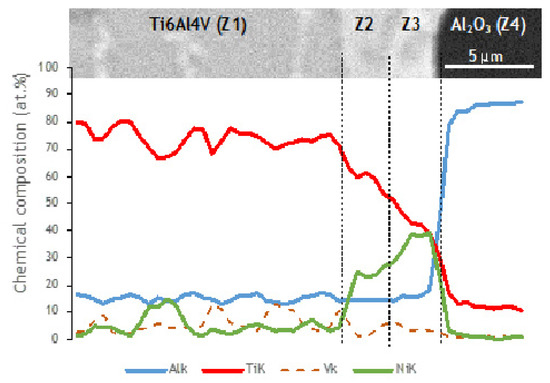
Figure 5.
SEM image and EDS profiles across the joint processed at 800 °C using a Ni/Ti multilayer.
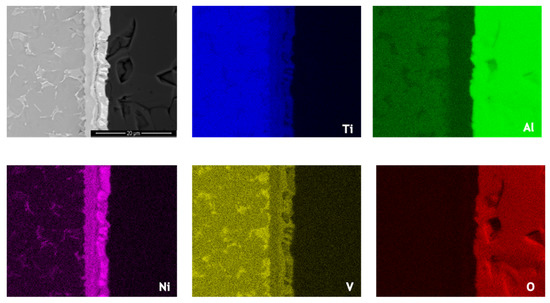
Figure 6.
SEM image and EDS elemental distribution maps across the joint processed at 800 °C using an Ni/Ti multilayer.
The chemical composition of zone Z3 is similar to the as-deposited multilayer thin film (~49 at.% Ni, 47 at.% Ti and 4 at.% V). Apparently no significant interdiffusion occurred between multilayer and Al2O3 base material, which can be explained by the joint temperature (800 °C) being not enough [40,41,42]. At 800 °C, the diffusion coefficient of Ni in NiTi2 (Z2) is lower than in Ti6Al4V; as a result, NiTi2 works like a barrier, retarding the diffusion of Ni [40]. Chemical composition in zone Z3, combined with the Ni-Ti [43] phase diagram, confirms the presence of B2-NiTi phase. Zone Z4 in Figure 4 is located far from the interface, so its chemical composition (Table 1) corresponds to the alumina base material. As expected, on the alumina side the EDS maps confirm the presence of Al and O.
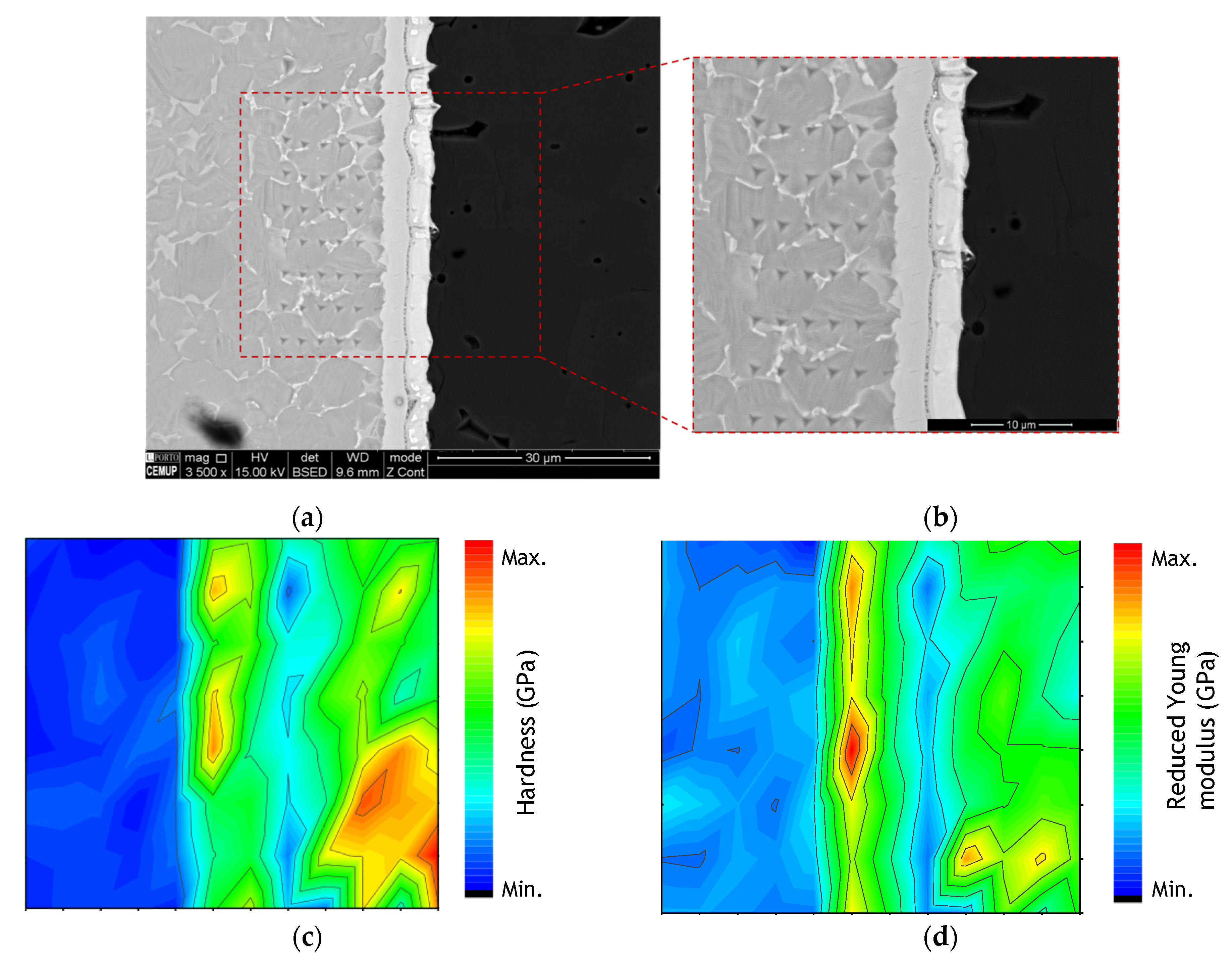
Nanoindentation tests were performed across the interface and adjacent base materials. Figure 7 shows the SEM image of a nanoindentation matrix and the corresponding hardness and reduced Young’s modulus (Er) maps. The residual imprints of the indenter cannot be clearly seen in Al2O3 and zone Z2 (interface adjacent to the Ti6Al4V base material) because the indentations are too small due to the high hardness values. Nevertheless, part of the matrix is perceptible in Figure 7 with the 8 rows distinguishable on the Ti6Al4V side, while several smaller indentations can be observed in zone Z3 (interface adjacent to the Al2O3 base material).
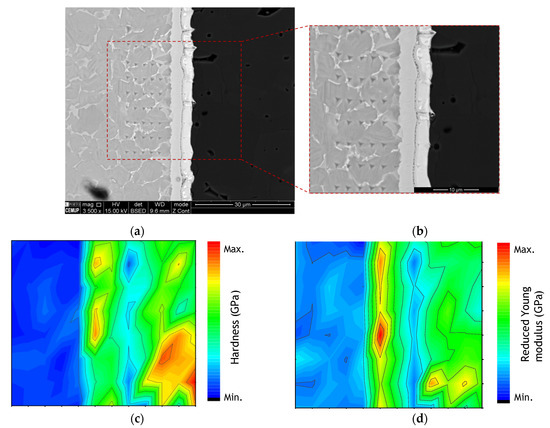
Figure 7.
(a,b) SEM images of the nanoindentation matrix, (c) hardness and (d) reduced Young’s modulus map across the joint processed at 800 °C using a Ni/Ti multilayer thin film.
The hardness and reduced Young’s modulus (Er) maps clearly exhibit the differences between the different zones composing the interface and the base materials. As expected, the metallic base material has significantly lower hardness and Er values than the alumina base material. At the interface, zone Z3, adjacent to the alumina, has rather low hardness and Er values, while zone Z2 is characterized by high hardness and Er values. The mechanical properties at the joint interface corroborate the phase identification based on the EDS results, i.e., NiTi2 and B2-NiTi identified at zones Z2 and Z3, respectively. In a recent work, a higher hardness was also measured by nanoindentation in the region corresponding to the NiTi2 phase at the interface of NiTi to Ti6Al4V diffusion bonds assisted by Ni/Ti multilayer thin films [44]. The high hardness value of the NiTi2 phase confirms its brittle character and can compromise the mechanical strength of the joints. Brittle behavior of the NiTi2 was also confirmed by Hu et al. [45]. It should be noted that the interface is characterized by mechanical properties close to the Al2O3 base material. In the essential, the nanoindentation results obtained for the different matrixes are similar, meaning that the results presented are representative of the mechanical properties of the ceramic/metal joint.
To summarize, the diffusion bonding of Ti6Al4V to Al2O3 can be improved by using Ni/Ti multilayer thin films. At 800 °C, the joining between these dissimilar materials was unsuccessful without multilayers. The interface obtained is characterized by a reaction layer close to Ti6Al4V and a crack along the interface with Al2O3. This may have happened due to crack nucleation and propagation during sample preparation resulting in the formation of a brittle phase or residual stresses. The use of Ni/Ti multilayer thin films shows to be effective, and it was possible to obtain a sound joint between Ti6Al4V and Al2O3, at a rather low temperature. The multilayer reaction and the interdiffusion between the elements of the base and interlayer materials promoted the formation of two reaction layers at the interface. The hardness values at the interface are close to the Al2O3 base material, confirming that the use of these multilayers have potential to be applied in the diffusion bonding between metals and ceramic materials.
4. Conclusions
Diffusion bonding of Ti6Al4V to Al2O3 was processed at 800 °C for 60 min without and with Ni/Ti multilayer thin film as interlayer material. The joining experiments without interlayer were unsuccessful. The interfaces are characterized by the presence of a crack close to the Al2O3 base material. The heating and cooling rates during the diffusion bonding have a significant effect on the plastic deformation of the Ti6Al4V base material. In order to decrease the deformation of the base material, slow cooling rates need to be applied. The use of a Ni/Ti multilayer to assist the diffusion bonding of Ti6Al4V to Al2O3 proves to be a good approach. Sound interfaces were obtained using this multilayer thin film with a modulation period close to 50 nm. The interfaces are mainly composed of two reaction layers. Due to the diffusion of Ni towards Ti6Al4V, the zone adjacent to this base material is enriched in Ti, corresponding to NiTi2. The interdiffusion between alumina and interlayer material is insignificant at 800 °C; thus, the zone adjacent to Al2O3 is constituted by NiTi, due to the reaction of Ni and Ti from the multilayer and according to its average chemical composition. The hardness and reduced Young modulus maps obtained by nanoindentation corroborate the microstructural characterization and clearly distinguished the different reaction layers composing the interface. The hardness maps showed that the interface exhibited hardness values close to the Al2O3 base material, which can be favorable to the mechanical behavior of the joints.
Author Contributions
Conceptualization, M.S.J.; methodology, S.S., A.S.R. and M.T.V.; validation, S.S., A.S.R. and M.T.V.; investigation, M.S.J., S.S., A.S.R. and M.T.V.; writing—original draft preparation M.S.J.; writing—review and editing, S.S. and A.S.R.; funding acquisition, S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Project PTDC/CTM-CTM/31579/2017—POCI-01- 0145-FEDER- 031579- funded by FEDER funds through COMPETE2020—Programa Operacional Competitividade e Internacionalização (POCI) and by national funds (PIDDAC) through FCT/MCTES. This research was also supported by FEDER funds through the program COMPETE —Programa Operacional Factores de Competitividade, and by national funds through FCT—Fundação para a Ciência e a Tecnologia, under the project UIDB/EMS/00285/2020.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to CEMUP-Centre of Materials of University of Porto for expert assistance with SEM.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cao, J.; Qi, J.; Song, X.; Feng, J. Welding and joining of titanium aluminides. Materials 2014, 7, 4930–4962. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Sun, J.H.; Chen, J.H. Mechanisms of joining aluminium A6061- T6 and titanium Ti–6Al–4V alloys by cold metal transfer technology. Sci. Technol. Weld. Join. 2013, 18, 425–433. [Google Scholar] [CrossRef]
- Tomashchuk, I.; Sallamand, P. Metallurgical strategies for the joining of titanium alloys with steels. Adv. Eng. Mater. 2018, 20, 1700764. [Google Scholar] [CrossRef]
- Xue, Z.; Yang, Q.; Gu, L.; Hao, X.; Ren, Y.; Geng, Y. Diffusion bonding of TiAl based alloy to Ti-Al-4V alloy using amorphous interlayer. Mater. Wiss. Werkstofftech 2015, 46, 40–46. [Google Scholar] [CrossRef]
- Simões, S.; Ramos, A.S.; Viana, F.; Vieira, M.T.; Vieira, M.F. Joining of TiAl to steel by diffusion bonding with Ni/Ti reactive multilayers. Metals 2016, 6, 96. [Google Scholar] [CrossRef]
- Leyens, C.; Peters, M. Titanium and Titanium Alloys: Fundamentals and Applications; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003; pp. 89–145. ISBN 9783527305346. [Google Scholar]
- Dai, J.; Zhu, J.; Chem, C.; Weng, F. High temperature oxidation behavior and research status of modifications on improving high temperature oxidation resistance of titanium alloys and titanium aluminides: A review. J. Alloys Compd. 2016, 685, 784–798. [Google Scholar] [CrossRef]
- Dimiduk, D.M. Gamma titanium aluminide alloys—An assessment within the competition of aerospace structural materials. Mater. Sci. Eng. A 1999, 263, 281–288. [Google Scholar] [CrossRef]
- Aldinger, F.; Weberruss, V.A. Advanced Ceramics and Future Materials: An Introduction to Structures, Properties, Technologies, Methods; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010; pp. 170–260. ISBN 978-3-527-32157-5. [Google Scholar]
- Richerson, D.W.; Lee, W.E. Modern Ceramic Engineering: Properties, processing, and Use in Design, 4th ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2018; ISBN 9781498716918. [Google Scholar]
- Yang, Z.; Lin, J.; Wang, Y.; Wang, D. Characterization of microstructure and mechanical properties of Al2O3/TiAl joints vacuum-brazed with Ag-Cu-Ti+W composite filler. Vacuum 2017, 143, 294–302. [Google Scholar] [CrossRef]
- Niu, G.B.; Wang, D.P.; Yang, Z.W.; Wang, Y. Microstructure and mechanical properties of Al2O3 ceramic and TiAl alloy joints brazed with Ag-Cu-Ti filler metal. Ceram. Int. 2016, 42, 6924–6934. [Google Scholar] [CrossRef]
- Dai, X.; Cao, J.; Wang, Z.; Wang, X.; Chen, L.; Huang, Y.; Feng, J. Brazing ZrO2 ceramic and TC4 alloy by novel WB reinforced Ag-Cu composite filler: Microstructure and properties. Ceram. Int. 2017, 43, 15296–15305. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, J.; Shen, P.; Guo, Z.; Liu, H. Effect of fabrication parameters on interface of zirconia and Ti-6Al-4V joints using Zr55Cu30Al10Ni5 amorphous filler. J. Mater. Eng. Perform. 2013, 22, 2602–2609. [Google Scholar] [CrossRef]
- Travessa, D.; Ferrante, M. The Al2O3-titanium adhesion in the view of the diffusion bonding process. J. Mater. Sci. 2002, 37, 4385–4390. [Google Scholar] [CrossRef]
- Correia, R.N.; Emiliano, J.V.; Moretto, P. Microstructure of diffusional zirconia-titanium and zirconia-(Ti-6wt% Al-4 wt% V) alloy joints. J. Mater. Sci. 1998, 33, 215–221. [Google Scholar] [CrossRef]
- Barrena, M.I.; Matesanz, L.; Gómez de Salazar, J.M. Al2O3/Ti6Al4V diffusion bonding joints using Ag-Cu interlayer. Mater. Charact. 2009, 60, 1263–1267. [Google Scholar] [CrossRef]
- Cao, J.; Liu, J.; Song, X.; Lin, X.; Feng, J. Diffusion bonding of TiAl intermetallic and Ti3AlC2 ceramic: Interfacial microstructure and joining properties. Mater. Des. 2014, 56, 115–121. [Google Scholar] [CrossRef]
- Liu, J.; Cao, J.; Song, X.; Wang, Y.; Feng, J. Evaluation on diffusion bonded joints of TiAl alloy to Ti3SiC2 ceramic with and without Ni interlayer: Interfacial microstructure and mechanical properties. Mater. Des. 2014, 57, 592–597. [Google Scholar] [CrossRef]
- Yi, J.L.; Zhang, Y.P.; Wang, X.X.; Dong, C.L.; Hu, H.C. Characterization of Al/Ti nano multilayer as a jointing material at the interface between Cu and Al2O3. Mater. Trans. 2016, 57, 1494–1497. [Google Scholar] [CrossRef]
- Cao, J.; Song, X.G.; Wu, L.Z.; Qi, J.L.; Feng, J.C. Characterization of Al/Ni multilayers and their application in diffusion bonding of TiAl to TiC cermet. Thin Solid Films 2012, 520, 3528–3531. [Google Scholar] [CrossRef]
- AlHazaa, A.; Haneklaus, N.; Almutairi, Z. Impulse pressure-assisted diffusion bonding (IPADB): Review and outlook. Metals 2021, 11, 323. [Google Scholar] [CrossRef]
- Cooke, K.O.; Atieh, A.M. Current trends in dissimilar diffusion bonding of titanium alloys to stainless steels, aluminium and magnesium. J. Manuf. Mater. Process. 2020, 4, 39. [Google Scholar] [CrossRef]
- Hu, A.; Janczak-Rusch, J.; Sano, T. Joining technology innovations at the macro, micro and nano levels. Appl. Sci. 2019, 9, 3568. [Google Scholar] [CrossRef]
- Simões, S.; Viana, F.; Ramos, A.S.; Vieira, M.T.; Vieira, M.F. TiAl diffusion bonding using Ni/Ti multilayers. Weld. World 2017, 61, 1267–1273. [Google Scholar] [CrossRef]
- Simões, S.; Viana, F.; Ramos, A.S.; Vieira, M.T.; Vieira, M.F. Reaction zone formed during diffusion bonding of TiNi to Ti6Al4V using Ni/Ti nanolayers. J. Mater. Sci. 2013, 58, 7718–7727. [Google Scholar] [CrossRef]
- Simões, S.; Viana, F.; Ramos, A.S.; Vieira, M.T.; Vieira, M.F. Diffusion bonding of TiAl using reactive Ni/Al nanolayers and Ti and Ni foils. Mater. Chem. Phys. 2011, 128, 202–207. [Google Scholar] [CrossRef]
- Ramos, A.S.; Vieira, M.T.; Simões, S.; Viana, F.; Vieira, M.F. Reaction-assisted diffusion bonding of advanced materials. Defect Diffus. Forum 2010, 297–301, 972–977. [Google Scholar] [CrossRef]
- Yamamoto, S.; Yokomine, T.; Sato, K.; Terai, T.; Fukuda, T.; Kakeshita, T. Ab initio Prediction of Atomic Location of Third Elements in B2-Type TiNi. Mater. Trans. 2018, 59, 353–358. [Google Scholar] [CrossRef]
- Lim, J.D.; Lee, P.M.; Rhee, D.M.W.; Leong, K.C.; Chen, Z. Effect of surface treatment on adhesion strength between magnetron sputtered copper thin films and alumina substrate. Appl. Surf. Sci. 2015, 355, 509–515. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. Improved technique for determining hardness and elastic modulus using load and displacements sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Uday, M.B.; Ahmad-Fauzi, M.N.; Noor, A.M.; Rajoo, S. Current Issues and Problems in the Joining of Ceramic to Metal. In Joining Technologies; Ishak, M., Ed.; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Lim, J.D.; Lee, P.M.; Chen, Z. Understanding the bonding mechanisms of directly sputtered copper thin film on an alumina substrate. Thin Solid Films 2017, 634, 6–14. [Google Scholar] [CrossRef]
- Cavaleiro, A.J.; Santos, R.J.; Ramos, A.S.; Vieira, M.T. In-situ thermal evolution of Ni/Ti multilayer thin films. Intermetallics 2014, 51, 11–17. [Google Scholar] [CrossRef]
- Cavaleiro, A.J.; Ramos, A.S.; Martins, R.M.S.; Fernandes, F.M.B.; Morgiel, J.; Baehtz, C.; Vieira, M.T. Phase transformations in Ni/Ti multilayers investigated by synchrotron radiation-based X-ray diffraction. J. Alloys Compd. 2015, 646, 1165–1171. [Google Scholar] [CrossRef]
- Cavaleiro, A.; Ramos, A.S.; Fernandes, F.; Baehtz, C.; Vieira, M.T. Interaction between Ni/Ti nanomultilayers and bulk Ti-6Al-4V during heat treatment. Metals 2018, 8, 878. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, M.; Zhang, Q.; Hu, T.; Wang, J.; Zheng, K. Microstructure evolution and bonding strength of the Al2O3/Al2O3 interface brazed via Ni-Ti intermetallic phases. J. Eur. Ceram. Soc. 2020, 40, 1496–1504. [Google Scholar] [CrossRef]
- Hayes, F.H. The Al-Ti-V (aluminum-titanium-vanadium) system. J. Phase Equilibria 1995, 16, 163–176. [Google Scholar] [CrossRef]
- Humeau, B.; Rogl, P.; Zeng, K.; Schmid-Fetzer, R.; Bohn, M.; Bauer, J. The ternary system Al-Ni-Ti Part I: Isothermal section at 900 °C.; Experimental investigation and thermodynamic calculation. Intermetallics 1999, 7, 1337–1345. [Google Scholar] [CrossRef]
- He, P.; Liu, D. Mechanism of forming interfacial intermetallic compounds at interface for solid state diffusion bonding of dissimilar materials. Mater. Sci. Eng. A 2006, 437, 430–435. [Google Scholar] [CrossRef]
- Hatakeyama, F.; Suganuma, K.; Okamoto, T. Solid-state bonding of alumina to austenitic stainless steel. J. Mater. Sci. 1986, 21, 2455–2461. [Google Scholar] [CrossRef]
- Misra, A.K. Reaction of Ti and Ti-Al alloys with alumina. Metall. Trans. A 1991, 22, 715–721. [Google Scholar] [CrossRef]
- Hornbuckle, B.C.; Xiao, X.Y.; Noebe, R.D.; Martens, R.; Weaver, M.L.; Thompson, G.B. Hardening behavior and phase decomposition in very Ni-rich Nitinol alloys. Mater. Sci. Eng. A 2015, 639, 336–344. [Google Scholar] [CrossRef]
- Cavaleiro, A.J.; Ramos, A.S.; Braz Fernandes, F.M.; Schell, N.; Vieira, M.T. Follow-up structural evolution of Ni/Ti reactive nano and microlayers during diffusion bonding of NiTi to Ti6Al4V in a synchrotron beamline. J. Mater. Process. Technol. 2020, 275, 116354. [Google Scholar] [CrossRef]
- Hu, L.; Xue, Y.; Shi, F. Intermetallic formation and mechanical properties of Ni-Ti diffusion couples. Mater. Des. 2017, 130, 175–182. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).