Abstract
Arsenopyrite (FeAsS) is often associated with gold, but pre-treatment is necessary prior to gold leaching, mainly due to the gold encapsulation in the matrix of FeAsS. Bio-oxidation is attractive and promising, largely due to its simplicity, low cost and environmental friendliness. A critical problem that still impedes the large-scale applications of this green technology is its slow leaching kinetics. Some metal ions such as Ag+ have previously been found to expedite the bioleaching process. In this paper, the role of Ag+ in the arsenopyrite bioleaching by Acidithiobacillus ferrooxidans was investigated in detail by bioleaching experiments and a series of analyses including thermodynamics, X-ray diffraction (XRD), scanning electron microscopy (SEM) and X-ray photoelectron spectroscopy (XPS). Experimental results suggested that addition of 5 mg/L Ag+ to the leaching system could significantly improve the final As leaching efficiency from 30.4% to 47.8% and shorten the bioleaching period from 19 days to 15 days. Thermodynamic analysis indicates that Ag+ destabilises As2S2, As2S3 and S0 via forming Ag2S, which is confirmed by the XRD analysis on the phase transformation during bioleaching. SEM and XPS analyses further showed that Ag+ removed the passivating film consisting mainly of As2S2, As2S3 and S0 because Ag2S formed on the arsenopyrite surface from the start bioleaching of 36 h. In the presence of Fe3+, Ag2S could easily be dissolved to Ag+ again, likely leading to the establishment of the Ag+/Ag2S cycle. The bacteria utilised the two synergistic cycles of Fe3+/Fe2+ and Ag+/Ag2S to catalyse the bioleaching of arsenopyrite.
1. Introduction
In nature, significant amounts of gold are often found in arsenopyrite (FeAsS) [1,2,3]. However, most gold in the arsenopyrite is difficult to leach, mainly because the gold tends to be encapsulated in the mineral matrix [4,5]. It is, thus, necessary to pre-treat arsenopyrite gold ores before leaching to improve gold extraction. Currently, four pre-treatment methods are commonly used, i.e., oxidative roasting, chemical oxidation, pressure oxidation and biological oxidation [6,7]. Amongst them, bio-oxidation is a simple, low-cost and eco-friendly technology that is appropriate to extract valuable metals from a series of ores/concentrates and waste materials with substantially reduced environmental pollution. In the past decade, this promising green technology has drawn significant attention, and its applications in bio-hydrometallurgy have also been on the increase [8,9,10].
Although, many advantages can be provided from bio-oxidation, the problem of slow leaching kinetics still impedes its large-scale application [8,11]. A range of metal ions, including Cu2+, Bi3+, Co2+, Hg2+ and Ag+ have, therefore, been used as catalysts to enhance the dissolution kinetics and shorten the bioleaching period [12,13]. Much attention has been paid to Ag+ among these metal ions. Significant researches had suggested that Ag+ is an efficient catalyst in improving the leaching yields and kinetics of valuable metals from a wide range of copper sulphide minerals, such as chalcopyrite [14,15,16,17]. Ag+ was also shown to effectively enhance the bioleaching of arsenic sulphide minerals, such as realgar and orpiment [18,19].
However, few research effects have been made to investigate the effects of Ag+ on the bioleaching of arsenopyrite. Research has reported that as 2 mg∙L−1 Ag+ is added to leaching solution, the bioleaching efficiency of As, from arsenopyrite, could be improved by ~23% for the same leaching duration [20]. Maybe the catalytic effect of Ag+ is attributed to an autocatalytic role of Ag+ or formation of an autocatalytic surface. The reason why Ag+ enhanced the bioleaching of arsenopyrite, and how Ag+ played its role in the process, remain unclear.
In our recent research [13,21,22], a compact passivating film was found to form on the surface of arsenopyrite from the beginning stage of bioleaching (<36 h), and this film would severely hinder the subsequent leaching process. The formation of this passivating film that was shown to consist mainly of As2S2, As2S3 and S0 was considered as the main reason for the slow leaching kinetics and long leaching periods. Based on these research findings, this paper reports results from a detailed investigation into the role of Ag+ in the bioleaching of pure arsenopyrite in 9K culture medium containing Acidithiobacillus ferrooxidans (A. ferrooxidans). This investigation starts with a series of bioleaching experiments on natural samples of pure arsenopyrite. The leachate and leached solid samples were respectively analysed with inductively coupled plasma-atomic emission spectrometry (ICP-AES) for the As and Fe concentrations and with X-ray diffraction (XRD), scanning electron microscopy (SEM) and X-ray photoelectron spectroscopy (XPS) for mineralogical phase, morphology and surface composition. In addition, thermodynamic analyses were conducted to elucidate the reasons for the improved bioleaching kinetics by Ag+. The possible mechanisms by which the Ag+ enhanced the bioleaching of arsenopyrite were also proposed in this paper.
2. Thermodynamic Calculations
The software, HSC Chemistry 6.0 [23], was used to construct the Eh−pH diagrams for the FeAsS−H2O and FeAsS−Ag+−H2O systems. The temperature and pressure were set at 25 °C and 1 atm, respectively. Under the leaching conditions of [Fe2+] 0.16 M, [FeAsS] 0.06 M (10 g/L) and [Ag+] 0.046 mM (5 mg/L; when Ag+ was present in the leaching system), the predominance areas for Fe, As and S as well as Ag species on relevant Eh (versus the standard hydrogen electrode (SHE)) and pH scales were presented in Eh−pH diagrams. The available thermodynamic data used in the calculations are listed in Appendix A Table A1.
3. Experimental
3.1. Minerals, Strain and Media
The high-purity arsenopyrite sample investigated had a composition of Fe1.00As0.99S0.97 and only contained impurities of Si 0.132%, Ni 0.062% and Co 0.034% assayed by X-ray fluorescence analysis. Either particles or cuboids of the arsenopyrite samples were used in this study. The arsenopyrite particles (over 90%, −74 μm) were obtained by wet-milling in a ball mill whilst the cuboids (~15 mm × ~15 mm × ~5 mm) were prepared using a cutter. Air-tight plastic bags were used to store the samples to minimise oxidation.
The bacteria of A. ferrooxidans and 9K culture medium containing FeSO4·7H2O 44.8 g/L, (NH4)2SO4 3.0 g/L, K2HPO4·3H2O 0.50 g/L, KNO3 0.14 g/L, Ca(NO3)2·4H2O 0.01 g/L and MgSO4·7H2O 0.5 g/L were utilised for the bioleaching of arsenopyrite. A total of 100 mL 9K culture medium and 15 mL inoculum were first added to 250 mL Erlenmneyer flasks and then shaken in an orbital thermostat shaker under 160 rpm and 30 °C to culture and sub-culture A. ferrooxidans. The medium was adjusted by careful addition of ~3 M H2SO4 to have an initial pH value of 1.8. In the presence of 10 g/L arsenopyrite particles, the bacteria had been sub-cultured for three months before the bioleaching experiments.
Silver nitrate (AgNO3) was utilised as the silver cation (Ag+) source. The reagents used in the bioleaching experiments of arsenopyrite and the cultivation of bacteria were all analytically pure. De-ionised water was utilised throughout all experiments.
3.2. Bioleaching Experiment
Using the same experimental method and conditions for the cultivation of bacteria, the growth of A. ferrooxidans and the bioleaching of arsenopyrite by A. ferrooxidans proceeded simultaneously. The addition of Ag+ to the leaching solution ranged from 1 to 20 mg/L. For the bioleaching of arsenopyrite particles, the pulp density was 10 g/L of FeAsS. Samples from the supernatant were withdrawn at regular intervals during the bioleaching process for chemical analysis. During the bioleaching process, the solution potential and pH values were also measured and recorded. All solution potentials were reported relative to the SHE. The pulp was filtered, washed with copious de-ionised water at least ten times, and dried in a vacuum oven at 35 °C overnight, to attain the residue for the subsequent mineralogical phase study. The morphology and surface composition analyses were conducted on the arsenopyrite cuboids. Prior to each bioleaching experiment, silicon carbide papers of 800, 1500 and 3000 grit were used sequentially to polish the cuboids, and then the contaminants on the surface of cuboids were cleaned ultrasonically in alternate baths of 5 M HCl, methanol and water for 5 min [24,25].
3.3. Analytical Methods
The concentrations of As and Fe in leachate were determined by ICP-AES (PS-6, Baird). Potassium dichromate titration method was used to determine the concentration of Fe2+. The difference between the concentration of the total Fe and Fe2+ obtained the Fe3+ concentration. A pH meter (PHSJ-4A, Shanghai Leici, Shanghai, China) equipped with a Pt electrode and Ag/AgCl (saturated KCl, Orion) reference electrode was used to measure the values of solution potential and pH. An X-ray diffractometer (D/Max 2500, Rigaku, Hiroshima, Tokyo, Japan) was used to determine the mineralogical phases of the bioleached residue. An SEM (Helios NanoLab G3 UC, FEI, Hillsboro, OR, USA) was used to examine the morphology of leached cuboids. The Ag species on the surface of the leached cuboid was analysed with XPS (ESCALAB250Xi, Thermo Fisher, Waltham, MA, USA).
4. Results and Discussion
4.1. Effect of Ag+ Addition on Arsenopyrite Bioleaching
The kinetic results from the arsenopyrite bioleaching are shown in Figure 1. As the leaching behaviour of As from arsenopyrite acts as a critical indicator for the bioleaching of arsenopyrite [13,21,22], the effect of Ag+ on arsenopyrite bioleaching can be evaluated by its influence on the change of As leaching efficiency during bioleaching, as shown in Figure 1a. In contrast with the slow bioleaching kinetics of As without Ag+, the addition of Ag+ to the solution was shown to substantially enhance the bioleaching of As, and thus, arsenopyrite as manifested by the marked rise in the leaching efficiency of As during bioleaching. The optimal concentration of Ag+ was shown to be 5 mg/L. Under this Ag+ level, the final leaching efficiency of As was noticeably increased from 30.4% without Ag+ to 47.8%. The bioleaching period was also shortened from 19 days to 15 days. The presence of Ag+ made the bioleaching of As much faster than the situation without Ag+ from the initial leaching stage. After leaching 1 day, the As leaching efficiency was markedly increased from 2.4% without Ag+ to 12.3% with 5 mg/L Ag+. Obviously, when Ag+ (5 mg/L) was contained in the solution, the passivation of the arsenopyrite surface that took place initially during bioleaching, as reported in our recent researches [13,21], was greatly weakened and even disappeared. When the concentration of Ag+ exceeded 5 mg/L, the bioleaching deteriorated as the As leaching efficiency started to decrease. This phenomenon can be explained by the fact that an excess of Ag+ have detrimentally affected the reproduction and activity of bacteria, and thus, the bioleaching of As.
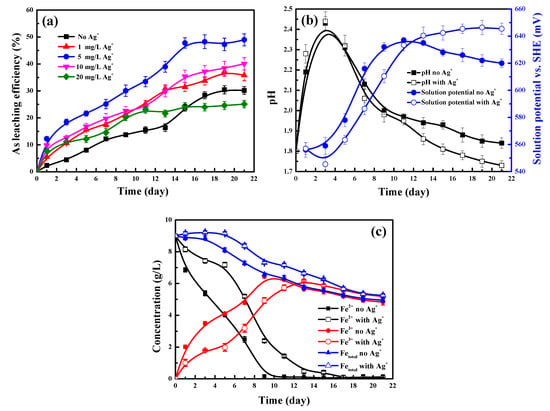
Figure 1.
Effect of Ag+ concentration on the (a) leaching efficiency of As and variation of (b) pH and solution potential and (c) concentrations of Fe3+, Fe2+ and total Fe in the presence and absence of 5 mg/L Ag+ during the bioleaching of arsenopyrite particles.
Under the optimal addition of Ag+ (5 mg/L), Figure 1b,c show the variation of pH and solution potential, as well as concentrations of iron ions including Fe3+, Fe2+ and total Fe during bioleaching. As shown in Figure 1b, a similar variation trend of pH was found regardless if Ag+ was present in the solution. The pH rose noticeably with the growth of bacteria in the initial leaching of 3 days. At the later stage (>3 days), the pH decreased, mainly due to the oxidative dissolution of arsenopyrite [13,21]. The bioleaching process is closely related to the solution potential, which is largely determined by the Fe3+/Fe2+ couple in the leaching system. A similar change trend between the solution potential and the Fe3+ concentration was clearly seen from Figure 1b,c. A greater solution potential can offer a stronger driving force for the bioleaching of arsenopyrite [13,21]. The presence of Ag+ (5 mg/L) was found to cause a much lower Fe3+ concentration and a higher Fe2+ concentration (Figure 1c) and thus a lower level of solution potential (Figure 1b). However, a much higher leaching efficiency of As (Figure 1a) was still achieved, further confirming the catalytic effect of Ag+ (5 mg/L). As will be demonstrated in the following sections, a series of analyses of thermodynamics, mineralogical phase, morphology and surface composition were conducted to elucidate why, and how, Ag+ played this catalytic effect.
4.2. Thermodynamics of Arsenopyrite Dissolution in the Presence of Ag+
The Eh−pH diagrams of FeAsS−H2O and FeAsS−Ag+−H2O systems are presented in Figure 2a–c and 2a'–d', respectively.
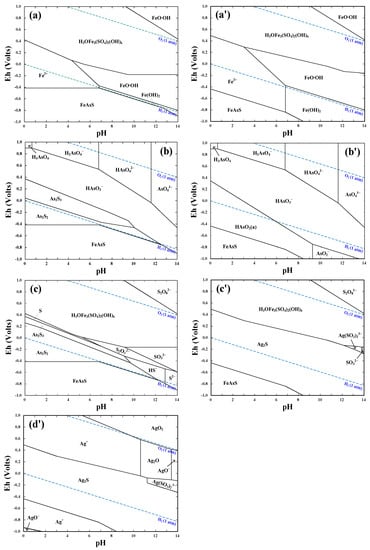
Figure 2.
Eh−pH diagrams of (a)–(c) FeAsS−H2O system and (a')–(d') FeAsS−Ag+−H2O system for (a)/(a') Fe species, (b)/(b') As species, (c)/(c') S species and (d') Ag species. Conditions: [Fe2+] 0.16 M, [FeAsS] 0.06 M, [Ag+] 0.046 mM; 25 °C and 1 atm.
In the pH range of 0–3 that is a typical acidic bioleaching condition, Figure 2a–c show that, in the absence of Ag+, an Eh higher than −0.4 V can drive the oxidative dissolution of arsenopyrite. In the Eh−pH region of −0.4–0.4 V and 0–3, the predominant species of Fe, As and S are Fe2+ (Figure 2a), As2S2/As2S3 (Figure 2b), and As2S2/As2S3/S0 (Figure 2c), respectively. As demonstrated previously [13,21], the solid species of As2S2, As2S3 and S0 are the main intermediate products that would form a passivating film on the arsenopyrite surface, causing a severe hindrance to the bioleaching process. Under an Eh higher than 0.2–0.4 V, H3OFe3(SO4)2(OH)6 in Figure 2a,c is shown to be the main phase for the Fe and S species while HAsO3– and H2AsO4− in Figure 2b are the main phase for the As species. Note that, in the 9K culture medium, H3OFe3(SO4)2(OH)6 and K+ can readily form jarosite [KFe3(SO4)2(OH)6] [26,27].
Comparing Figure 2a–c with 2a'–c', the addition of 0.046 mM (5 mg/L) Ag+ to the FeAsS−H2O system leads to significant changes in the predominance regions for Fe, As and S species. Figure 2a,a' show that, within pH of 0–3, a much lower Eh (> −0.44 – −0.62 V) is required to leach the Fe from FeAsS as Fe2+ to the leaching solution, whilst a much higher Eh (>0.3–0.5 V) is needed for the transformation of Fe2+ to H3OFe3(SO4)2(OH)6. The predominance areas of As and S species, i.e., As2S2, As2S3 and S0 in Figure 2b,c change to those of HAsO2 (a) and Ag2S as shown in Figure 2b',c'. In common with the formation of Fe2+, HAsO2 (a) and Ag2S can also be formed at a lower Eh (> −0.44–−0.62 V). Therefore, thermodynamically, the presence of Ag+ makes As2S2, As2S3 and S0 unstable by forming Ag2S. In addition, Figure 2d' shows that the predominant Ag species is Ag2S, which is readily converted to Ag+ at an Eh higher than 0.3–0.5 V. This means that the oxidative dissolution of Ag2S into the solution can easily take place in the presence of the Fe3+/Fe2+ couple (E° = 0.771 V versus SHE).
4.3. Mineralogical Phase Transformation
To determine the effect of Ag+ (5 mg/L) on the transformation of mineralogical phase, XRD surveys were performed on bioleached arsenopyrite particles for different days. As the passivation took place from the onset of bioleaching [13,21], the phase change was investigated for the initial leaching stage (<3 days). The XRD diffractograms are presented in Figure 3.
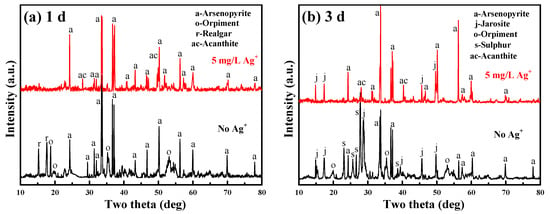
Figure 3.
XRD diffractograms of the bioleached arsenopyrite particles after leaching (a) 1 day and (b) 3 days with and without 5 mg/L Ag+.
Without the addition of Ag+, the phases of realgar (As2S2) and orpiment (As2S3) occurred after bioleaching 1 day (Figure 3a). Elemental sulphur (S0) and jarosite [KFe3(SO4)2(OH)6] were also found after 3 days (Figure 3b). With the addition of Ag+ at 5 mg/L, however, Figure 3a,b shows that the passivating phases of As2S2, As2S3 and S0 disappeared with the formation of acanthite (Ag2S). This agrees well with the thermodynamic predictions in Section 4.2.
4.4. Morphology and Surface Composition Changes
SEM and XPS analyses were further conducted to reveal the effect of Ag+ (5 mg/L) on the morphology and surface composition of the bioleached arsenopyrite cuboids. During the bioleaching process, considerable amounts of jarosite were observed to form after around 5 days whether Ag+ was added to the solution or not. However, our recent research suggested that the precipitates of jarosite only formed a loose and porous overlayer, instead of a passivating film, on the surface of arsenopyrite [13,21]. The surface was investigated just for the initial leaching stage (i.e., 36 h) to avoid the interference from jarosite. The SEM images and XPS spectrum are presented in Figure 4 and Figure 5, respectively.
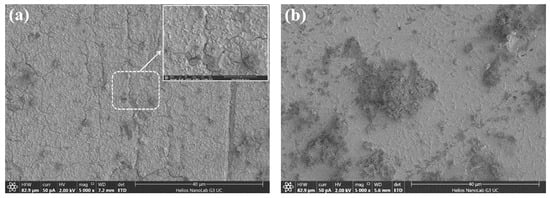
Figure 4.
SEM images for the surfaces of arsenopyrite cuboids after bioleaching 36 h in 9K culture medium (a) without and (b) with 5 mg/L Ag+.
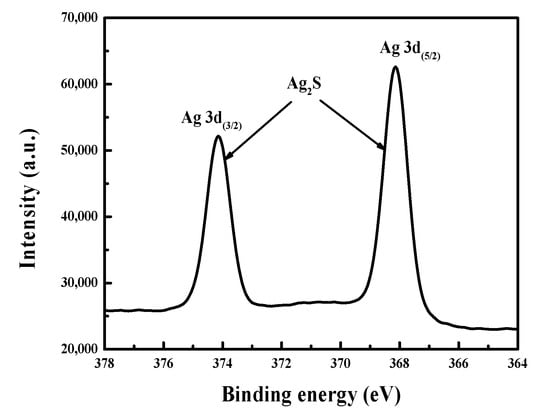
Figure 5.
XPS spectrum of Ag 3d for the surface of arsenopyrite cuboid after bioleaching 36 h in 9K culture medium with 5 mg/L Ag+.
As clearly seen from Figure 4a, a compact passivating film was formed on the arsenopyrite surface. Note that the film was intact in the solution, and the appearance of the cracks occurred only after drying. The film was shown to consist mainly of As2S2, As2S3 and S0 in our recent researches [13,21]. After bioleaching in the presence of Ag+ (5 mg/L), no passivating film was observed on the surface, as presented in Figure 4b. As shown in the above XRD analysis, the disappearance of the phases of As2S2, As2S3 and S0 due to the addition of Ag+ is responsible for the removal of this passivating film.
In addition, Figure 5 shows the XPS spectrum of Ag 3d on the outmost surface of the leached arsenopyrite cuboid at the Ag+ level of 5 mg/L. Two obvious peaks of Ag 3d(3/2) and Ag 3d(5/2) were exhibited. The Ag 3d(5/2) peak centring at the binding energy of 368.1 eV reflects the typical peak of Ag2S [28,29].
In summary, the presence of 5 mg/L Ag+ substantially enhanced the bioleaching efficiency and kinetics (Section 4.1). The XRD, SEM, XPS and thermodynamic results, presented in Section 4.2, Section 4.3 and Section 4.4 demonstrated that the inclusion of 5 mg/L Ag+ in the leaching system could lead to the disappearance of the visible passivating film on the arsenopyrite surface in bioleaching without Ag+. The removal of the passivating film that can form at the initial leaching stage (< 36 h) explains why Ag+ catalyses the whole bioleaching process.
4.5. Possible Mechanisms for the Ag+ Catalysed Bioleaching of Arsenopyrite
Based on the above experimental and analytical results, the possible mechanism for the silver-catalysed bioleaching of arsenopyrite can be proposed as presented in Figure 6. In addition, the possible reactions involved in the bioleaching process are listed in Table 1.
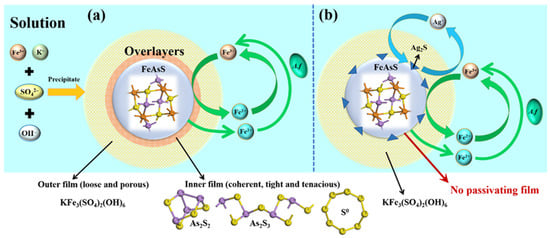
Figure 6.
Schematic diagram of the mechanisms for the bioleaching of arsenopyrite (a) without and (b) with Ag+.

Table 1.
Possible reactions involved in the oxidative leaching of arsenopyrite in the presence of Ag+.
Figure 6a shows that, in the leaching solution without Ag+, the bacteria oxidise the Fe2+ to Fe3+, which will in turn oxidise the arsenopyrite to achieve its leaching, as shown by Equations (1)–(3) in Table 1. With the bioleaching of arsenopyrite, As2S2, As2S3 and S0 are also produced in situ on the surface of arsenopyrite particles. Although these solid products could be further oxidised according to reactions (7)–(9) listed in Table 1, they still formed a passivating film at the initial bioleaching stage, causing a severe impedance to the subsequent leaching process.
With the addition of Ag+ to the solution (Figure 6b), the oxidative dissolution of arsenopyrite would proceed according to Equations (4)–(6) with much lower ∆Gr0 values than those of Equations (1)–(3) (Table 1). In the process, Ag2S was formed on the surface of arsenopyrite, largely preventing the formation of the passivating products (i.e., As2S2, As2S3 and S0) on the basis of Equations (10)–(12), as evidenced by the results from XRD and XPS analyses. In addition, Ag2S itself is a conductor that is beneficial for the transfer of electrons in the leaching process. In the presence of Fe3+, Ag2S can also be oxidised by the Fe3+/Fe2+ couple according to reaction (13), releasing Ag+ back to the solution. Therefore, as presented in Figure 6b, a catalytic cycle of Ag+/Ag2S was also established from the catalytic cycle of Fe3+/Fe2+. The two catalytic cycles led to the elimination of the passivating film, as evidenced by the SEM images, i.e., the much improved bioleaching of arsenopyrite.
5. Conclusions
Experimental results from the arsenopyrite bioleaching by A. ferrooxidans in 9K culture medium suggested that addition of 5 mg/L Ag+ to the solution had no significant influence on the pH, but resulted in a lower level of the solution potential. However, the bioleaching of arsenopyrite was still substantially improved from the onset of leaching. On the one hand, the final leaching efficiency of As was significantly increased from 30.4% to 47.8%; on the other hand, the bioleaching period was noticeably shortened from 19 days to 15 days. This is attributed to the fact that the presence of Ag+ in the solution leads to the removal of the passivating film (being composed mainly of As2S2, As2S3 and S0 [13,21]) that could be formed initially on the surface of arsenopyrite. Thermodynamic and XRD analyses demonstrated that, during the bioleaching of arsenopyrite, Ag+ could destabilise As2S2, As2S3 and S0 through formation of Ag2S, which would easily be dissolved to Ag+ by the Fe3+/Fe2+ couple. SEM and XPS analyses further suggested that the main difference the Ag+ caused was that the formation of the passivating film was prevented by Ag+ via forming Ag2S on the arsenopyrite surface. On the basis of the combined results from bioleaching experiments, thermodynamic and phase analyses, and surface characterisation, a possible mechanism for the silver catalysed bioleaching of arsenopyrite is put forward that the two synergistic cycles of Ag+/Ag2S and Fe3+/Fe2+ were utilised by the bacteria to improve the leaching of arsenopyrite. The results in this paper can also offer useful information for the extraction of valuable metals from other As- and S-bearing materials.
Author Contributions
Y.Z. and Q.L. conceived and designed the study; Q.L. contributed reagents/materials/analysis tools; Y.Z. performed the experiments; X.L. and Y.Z. analysed the data; X.L. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (grant no. 51574284) and the National Key Research and Development Program of China (grant no. 2018YFE0110200).
Acknowledgments
Financial supports from the National Natural Science Foundation of China and the National Key Research and Development Program of China are all gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Free energies of formation (kJ/mol) for relevant species a.
Table A1.
Free energies of formation (kJ/mol) for relevant species a.
| Species | ΔGf°298 | Species | ΔGf°298 | Species | ΔGf°298 | Species | ΔGf°298 |
| /(kJ/mol) | /(kJ/mol) | /(kJ/mol) | /(kJ/mol) | ||||
| FeAsS | −49.7616 | As2S2 | −68.5508 | S | 0 | AgO | 14.50 |
| FeAsO4 | −772.727 | As2S3 | −91.4907 | S2− | 86.00982 | AgO2 | −10.996 |
| Fe3(AsO4)2 | −1766.73 | As2O3 | −576.899 | S22− | 79.76167 | Ag2O | −11.180 |
| Fe(OH)2 | −492.158 | As2O4 | −701.161 | SO32− | −486.755 | Ag2O2 | 27.429 |
| Fe(OH)3 | −705.885 | As2O5 | −782.437 | S2O32− | −518.87 | Ag2O3 | 121.329 |
| FeO·OH | −489.439 | As4O6 | −1152.42 | S2O42− | −600.825 | Ag2S | −40.401 |
| Fe3+ | −17.1907 | AsO2– | −349.991 | S2O52− | −791.217 | Ag2SO3 | −411.615 |
| Fe2+ | −91.5644 | AsO43– | −648.477 | S2O62− | −969.453 | Ag2+ | 268.686 |
| FeOH2+ | −242.064 | As(OH)4– | −824.457 | S2O72− | −795.432 | Ag+ | 77.148 |
| FeOH+ | −275.615 | HAsO3− | −606.638 | S2O82− | −1115.35 | Ag(HS)2− | 0.247 |
| Fe(OH)2+ | −452.391 | HAsO42− | −714.732 | HS2− | 11.51053 | AgO− | −22.762 |
| Fe2(OH)24+ | −467.733 | H2AsO3− | −587.149 | HSO3− | −527.84 | Ag(OH)2− | −260.214 |
| H3OFe3(SO4)2(OH)6 b | −3230.36 | H2AsO4− | −753.399 | HS− | 12.44438 | AgS− | 59.968 |
| H3AsO3 (a) | −638.142 | HS2O3− | −532.363 | Ag(SO3)− | −441.572 | ||
| H2O | −237.177 | H3AsO4 (a) | −764.001 | H2S (a) | −27.656 | Ag(SO3)23− | −946.744 |
| HAsO2 (a) | −402.951 | Ag(SO3)35− | −1434.875 | ||||
| Ag(S2O3)− | −490.262 | ||||||
| Ag(S2O3)23− | −1023.386 | ||||||
| Ag(S2O3)35− | −2241.344 |
a Data from HSC database 6.0 [23] and Thermochemical Data of Pure Substances [30]. b ΔG°298 value was calculated using the equation of ΔGr° = −RT ln K = ∑[νiΔGf°(i)], where ln K was from the Hydrochemical log K Database of HYDRA/MEDUSA software [31] and relevant ΔGf° values are listed in Appendix A.
References
- Corkhill, C.L.; Vaughan, D.J. Arsenopyrite oxidation—A review. Appl. Geochem. 2009, 24, 2342–2361. [Google Scholar] [CrossRef]
- Márquez, M.A.; Ospina, J.D.; Morales, A.L. New insights about the bacterial oxidation of arsenopyrite: A mineralogical scope. Miner. Eng. 2012, 39, 248–254. [Google Scholar] [CrossRef]
- Liu, X.; Xu, B.; Min, X.; Li, Q.; Yang, Y.; Jiang, T.; He, Y.; Zhang, X. Effect of pyrite on thiosulfate leaching of gold and the role of ammonium alcohol polyvinyl phosphate (AAPP). Metals 2017, 7, 278. [Google Scholar] [CrossRef]
- Mikhlin, Y.L.; Romanchenko, A.S.; Asanov, I.P. Oxidation of arsenopyrite and deposition of gold on the oxidized surfaces: A scanning probe microscopy, tunneling spectroscopy and XPS study. Geochim. Cosmochim. Acta 2006, 70, 4874–4888. [Google Scholar] [CrossRef]
- Fomchenko, N.V.; Muravyov, M. Thermodynamic and XRD analysis of arsenopyrite biooxidation and enhancement of oxidation efficiency of gold-bearing concentrates. Int. J. Miner. Process. 2014, 133, 112–118. [Google Scholar] [CrossRef]
- Liu, X.; Li, Q.; Zhang, Y.; Jiang, T.; Yang, Y.; Xu, B.; He, Y. Improving gold recovery from a refractory ore via Na2SO4 assisted roasting and alkaline Na2S leaching. Hydrometallurgy 2019, 185, 133–141. [Google Scholar] [CrossRef]
- Liu, X.; Li, Q.; Zhang, Y.; Jiang, T.; Yang, Y.; Xu, B.; He, Y. Simultaneous removal of S and As from a refractory gold ore in a single stage O2-enriched roasting process. Metall. Mater. Trans. B 2019, 50, 1588–1596. [Google Scholar] [CrossRef]
- Miller, P.; Brown, A.R.G. Bacterial Oxidation of Refractory Gold Concentrates. In Gold Ore Processing: Project Development and Operations; Adams, M.D., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 359–372. [Google Scholar]
- Craw, D.; Falconer, D.; Youngson, J.H. Environmental arsenopyrite stability and dissolution: Theory, experiment, and field observations. Chem. Geol. 2003, 199, 71–82. [Google Scholar] [CrossRef]
- Fantauzzi, M.; Licheri, C.; Atzei, D.; Loi, G.; Elsener, B.; Rossi, G.; Rossi, A. Arsenopyrite and pyrite bioleaching: Evidence from XPS, XRD and ICP techniques. Anal. Bioanal. Chem. 2011, 401, 2237–2248. [Google Scholar] [CrossRef] [PubMed]
- Henao, D.M.O.; Godoy, M.A.M. Jarosite pseudomorph formation from arsenopyrite oxidation using Acidithiobacillus ferrooxidans. Hydrometallurgy 2010, 104, 162–168. [Google Scholar] [CrossRef]
- Pathak, A.; Morrison, L.; Healy, M.G. Catalytic potential of selected metal ions for bioleaching, and potential techno-economic and environmental issues: A critical review. Bioresour. Technol. 2017, 229, 211–221. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Liu, X.; Yin, H.; Yang, Y.; Xu, B.; Jiang, T.; He, Y. The catalytic effect of copper ion in the bioleaching of arsenopyrite by Acidithiobacillus ferrooxidans in 9K culture medium. J. Clean. Prod. 2020, 256, 120391. [Google Scholar] [CrossRef]
- Abdollahi, H.; Shafaei, S.Z.; Noaparast, M.; Manafi, Z.; Niemelä, S.I.; Tuovinen, O.H. Mesophilic and thermophilic bioleaching of copper from a chalcopyrite-containing molybdenite concentrate. Int. J. Miner. Process. 2014, 128, 25–32. [Google Scholar] [CrossRef]
- Feng, S.; Yang, H.; Xin, Y.; Gao, K.; Yang, J.; Liu, T.; Zhang, L.; Wang, W. A novel and highly efficient system for chalcopyrite bioleaching by mixed strains of Acidithiobacillus. Bioresour. Technol. 2013, 129, 456–462. [Google Scholar] [CrossRef]
- Nazari, G.; Dixon, D.G.; Dreisinger, D.B. The mechanism of chalcopyrite leaching in the presence of silver-enhanced pyrite in the Galvanox™ process. Hydrometallurgy 2012, 113–114, 122–130. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, J.; Gan, X.; Hu, M.; Zhang, E.; Qin, W.; Qiu, G. Cooperative bioleaching of chalcopyrite and silver-bearing tailing by mixed moderately thermophilic culture: An emphasis on the chalcopyrite dissolution with XPS and electrochemical analysis. Miner. Eng. 2015, 81, 29–39. [Google Scholar] [CrossRef]
- Guo, P.; Zhang, G.; Cao, J.; Li, Y.; Fang, Z.; Yang, C. Catalytic effect of Ag+ and Cu2+ on leaching realgar (As2S2). Hydrometallurgy 2011, 106, 99–103. [Google Scholar] [CrossRef]
- Zhang, G.; Chao, X.; Guo, P.; Cao, J.; Yang, C. Catalytic effect of Ag+ on arsenic bioleaching from orpiment (As2S3) in batch tests with Acidithiobacillus ferrooxidans and Sulfobacillus sibiricus. J. Hazard. Mater. 2015, 283, 117–122. [Google Scholar] [CrossRef]
- Fang, F.; Zhong, H.; Jiang, F.; Zhan, X. Catalytic effect of silver on bioleaching of arsenopyrite. Int. J. Chem. Eng. Appl. 2014, 5, 474–478. [Google Scholar] [CrossRef][Green Version]
- Liu, X.; Li, Q.; Zhang, Y.; Yang, Y.; Xu, B.; Jiang, T. Formation process of the passivating products from arsenopyrite bioleaching by Acidithiobacillus ferrooxidans in 9K culture medium. Metals 2019, 9, 1320. [Google Scholar] [CrossRef]
- Liu, X.; Li, Q.; Zhang, Y.; Jiang, T.; Yang, Y.; Xu, B.; He, Y. Electrochemical behaviour of the dissolution and passivation of arsenopyrite in 9K culture medium. Appl. Surf. Sci. 2020, 508, 145269. [Google Scholar] [CrossRef]
- Roine, A. Outokumpu HSC Chemistry for Windows: Chemical reaction and equilibrium software with extensive thermochemical database. In User’s Guide, Version 6.0.; Outokumpu Research Oy: Pori, Finland, 2006. [Google Scholar]
- Lázaro, I.; Cruz, R.; González, I.; Monroy, M. Electrochemical oxidation of arsenopyrite in acidic media. Int. J. Miner. Process. 1997, 50, 63–75. [Google Scholar] [CrossRef]
- Cruz, R.; Lázaro, I.; Rodríguez, J.M.; Monroy, M.; González, I. Surface characterization of arsenopyrite in acidic medium by triangular scan voltammetry on carbon paste electrodes. Hydrometallurgy 1997, 46, 303–319. [Google Scholar] [CrossRef]
- Liu, J.Y.; Tao, X.X.; Cai, P. Study of formation of jarosite mediated by thiobacillus ferrooxidans in 9K medium. Procedia Earth Planet. Sci. 2009, 1, 706–712. [Google Scholar] [CrossRef]
- Wang, H.; Bigham, J.M.; Jones, F.S.; Tuovinen, O.H. Synthesis and properties of ammoniojarosites prepared with iron-oxidizing acidophilic microorganisms at 22–65 °C. Geochim. Cosmochim. Acta 2007, 71, 155–164. [Google Scholar] [CrossRef]
- Qorbani, M.; Gholami, M.; Moradlou, O.; Naseri, N.; Moshfegh, A.Z. Optimal Ag2S nanoparticle incorporated TiO2 nanotube array for visible water splitting. RSC Adv. 2014, 4, 7838–7844. [Google Scholar]
- Ristova, M.; Ristov, M. XPS profile analysis on CdS thin film modified with Ag by an ion exchange. Appl. Surf. Sci. 2001, 181, 68–77. [Google Scholar] [CrossRef]
- Barin, I. Thermochemical Data of Pure Substances, 3rd ed.; Wiley-VCH: Weinheim, Germany, 1995. [Google Scholar]
- Puigdomenech, I. Make equilibrium diagrams using sophistacated algorithms (MEDUSA). In Inorganic Chemistry; Royal Institute of Technology: Stockholm, Sweden, 2004. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).