Cognitive Fatigue Disrupts Explosive Performance and Vigilance in Trained Individuals
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Design
2.3. Procedures
2.4. Measures
2.5. Statistical Analysis
3. Results
3.1. Performance Measures—Repeated Sprint Ability (RSA)
3.1.1. Best Performance
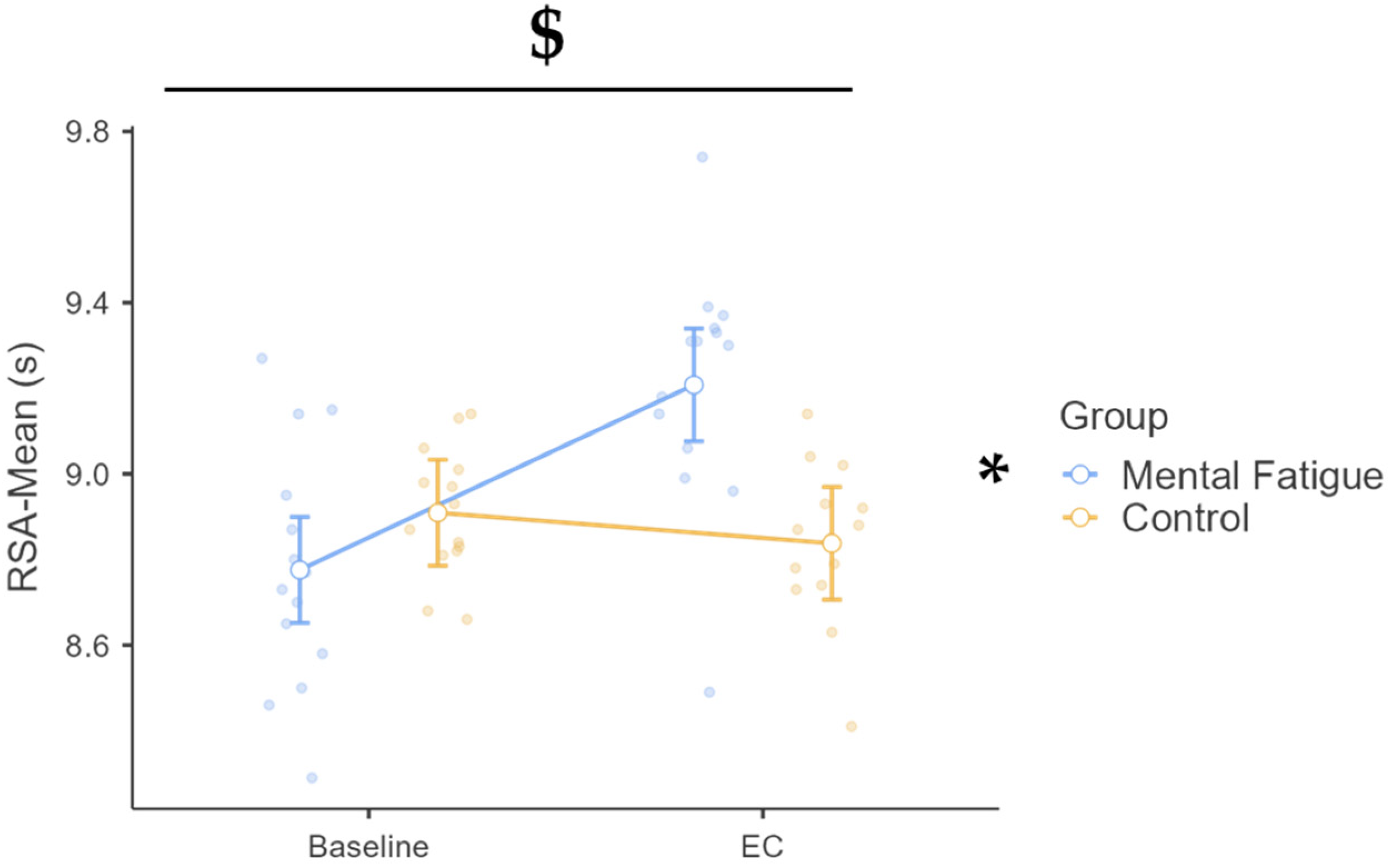
3.1.2. Mean Performance
3.1.3. Total Time
3.1.4. CV and RSA Performance Decrement (Dec%)
3.2. Performance Measures—Countermovement Jump (CMJ)
Height
3.3. Performance Measures—Repeated Jumping Ability (RJA)
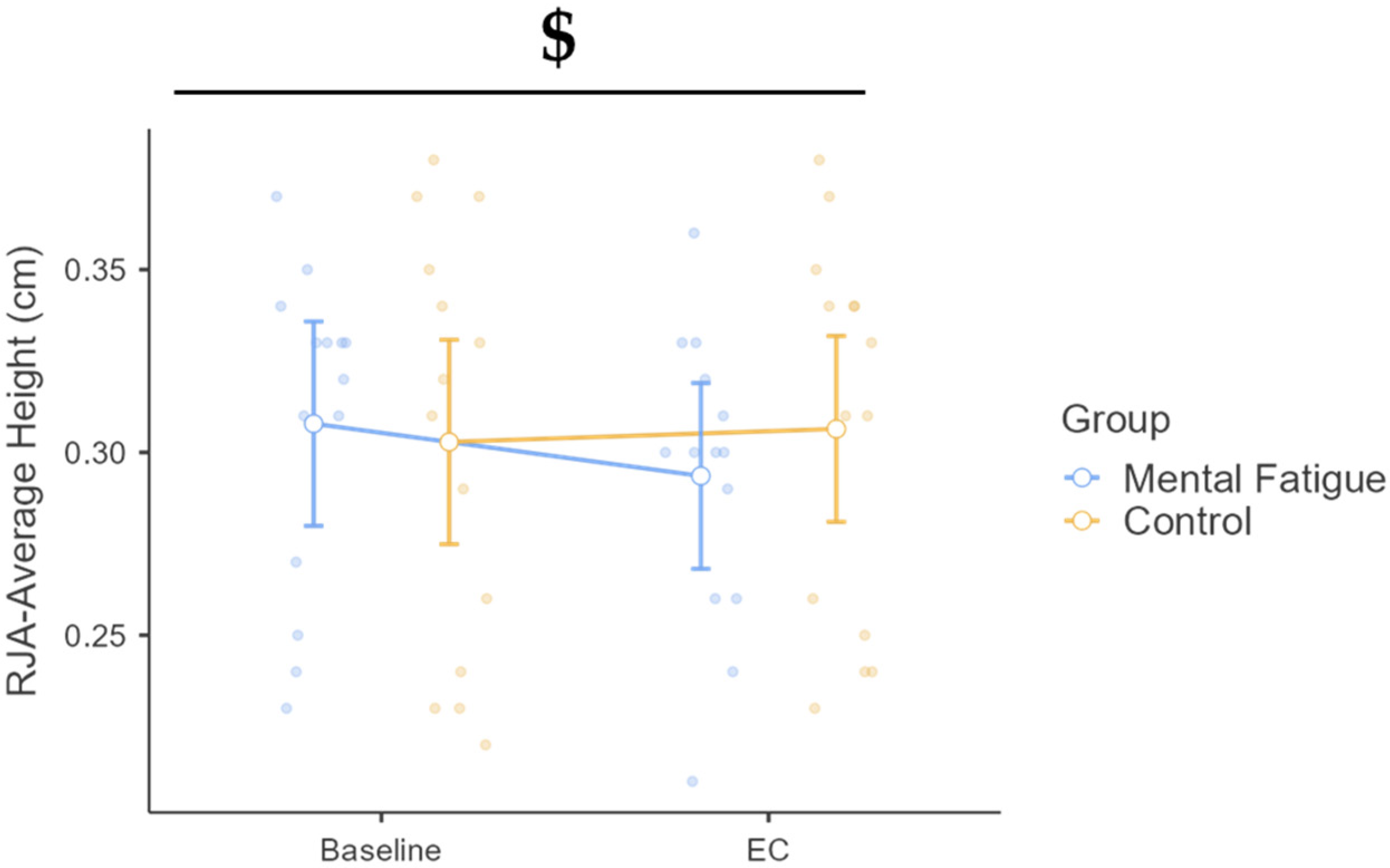
3.3.1. Average Height
3.3.2. Contact Time
3.4. Cognitive and Subjective Measures—PVT
3.4.1. Reaction Time
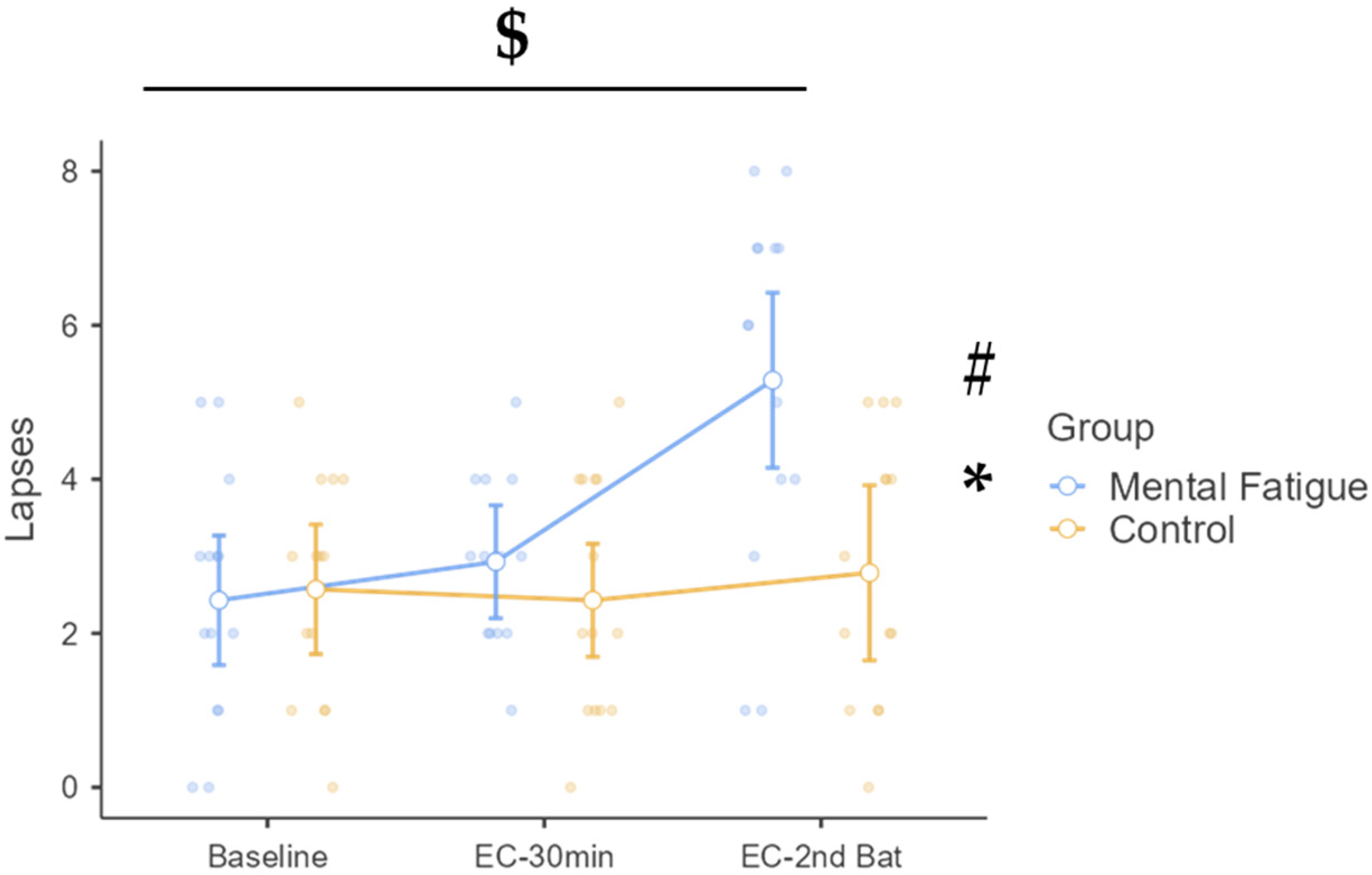
3.4.2. Lapses
3.5. Cognitive and Subjective Measures—Ratings of Perceived Exertion (RPE)
3.6. Cognitive and Subjective Measures—NASA TLX
3.6.1. Mental Demand
3.6.2. Physical Demand
3.6.3. Effort
3.6.4. Frustration
3.7. Cognitive and Subjective Measures—M-Vas
3.8. Cognitive and Subjective Measures—Motivation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Cutsem, J.; Marcora, S.; De Pauw, K.; Bailey, S.; Meeusen, R.; Roelands, B. The Effects of Mental Fatigue on Physical Performance: A Systematic Review. Sports Med. 2017, 47, 1569–1588. [Google Scholar] [CrossRef]
- Boksem, M.A.; Tops, M. Mental Fatigue: Costs and Benefits. Brain Res. Rev. 2008, 59, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Slimani, M.; Znazen, H.; Bragazzi, N.L.; Zguira, M.S.; Tod, D. The Effect of Mental Fatigue on Cognitive and Aerobic Performance in Adolescent Active Endurance Athletes: Insights from a Randomized Counterbalanced, Cross-Over Trial. J. Clin. Med. 2018, 7, 510. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Sun, H.; Soh, K.G.; Mohammadi, A.; Toumi, Z.; Zhang, Z. The Effects of Mental Fatigue on Sport-Specific Motor Performance among Team Sport Athletes: A Systematic Scoping Review. Front. Psychol. 2023, 14, 1143618. [Google Scholar] [CrossRef]
- Pageaux, B.; Lepers, R. The Effects of Mental Fatigue on Sport-Related Performance. Prog. Brain Res. 2018, 240, 291–315. [Google Scholar]
- Stafylidis, A.; Staiano, W.; Mandroukas, A.; Michailidis, Y.; Bonet, L.R.S.; Romagnoli, M.; Metaxas, T.I. Mobile App–Induced Mental Fatigue Affects Strength Asymmetry and Neuromuscular Performance Across Upper and Lower Limbs. Sensors 2025, 25, 4758. [Google Scholar] [CrossRef]
- Queiros, V.S.D.; Dantas, M.; Fortes, L.D.S.; Silva, L.F.D.; Silva, G.M.D.; Dantas, P.M.S.; Cabral, B.G.D.A.T. Mental Fatigue Reduces Training Volume in Resistance Exercise: A Cross-Over and Randomized Study. Percept. Mot. Ski. 2021, 128, 409–423. [Google Scholar] [CrossRef]
- Fortes, L.S.; de Lima-Júnior, D.; Fonseca, F.S.; Albuquerque, M.R.; Ferreira, M.E.C. Effect of Mental Fatigue on Mean Propulsive Velocity, Countermovement Jump, and 100-m and 200-m Dash Performance in Male College Sprinters. Appl. Neuropsychol. Adult 2024, 31, 264–273. [Google Scholar] [CrossRef]
- Staiano, W.; Bonet, L.R.S.; Romagnoli, M.; Ring, C. Mental Fatigue Impairs Repeated Sprint and Jump Performance in Team Sport Athletes. J. Sci. Med. Sport 2024, 27, 105–112. [Google Scholar] [CrossRef]
- Martin, K.; Thompson, K.G.; Keegan, R.; Ball, N.; Rattray, B. Mental Fatigue Does Not Affect Maximal Anaerobic Exercise Performance. Eur. J. Appl. Physiol. 2015, 115, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.P.; Brown, D.M.; Swafford, I.M.; Summerville, B.; Seidi, M.; Hajiaghamemar, M.; Dorgo, S. The Effects of Mental Fatigue on Anaerobic Power and Power Endurance Performance. Sports 2024, 12, 192. [Google Scholar] [CrossRef] [PubMed]
- Pageaux, B.; Lepers, R. Fatigue Induced by Physical and Mental Exertion Increases Perception of Effort and Impairs Subsequent Endurance Performance. Front. Physiol. 2016, 7, 587. [Google Scholar] [CrossRef]
- Gantois, P.; Lima-Júnior, D.D.; Fortes, L.D.S.; Batista, G.R.; Nakamura, F.Y.; Fonseca, F.D.S. Mental Fatigue from Smartphone Use Reduces Volume-Load in Resistance Training: A Randomized, Single-Blinded Cross-Over Study. Percept. Mot. Ski. 2021, 128, 1640–1659. [Google Scholar] [CrossRef]
- Hakim, H.; Khemiri, A.; Chortane, O.G.; Boukari, S.; Chortane, S.G.; Bianco, A.; Marsigliante, S.; Patti, A.; Muscella, A. Mental Fatigue Effects on the Produced Perception of Effort and Its Impact on Subsequent Physical Performances. Int. J. Environ. Res. Public Health 2022, 19, 10973. [Google Scholar] [CrossRef]
- Pitts, J.; Bhatt, T. Effects of Mentally Induced Fatigue on Balance Control: A Systematic Review. Exp. Brain Res. 2023, 241, 13–30. [Google Scholar] [CrossRef]
- Morris, A.J.; Christie, A.D. The Effect of a Mentally Fatiguing Task on Postural Balance Control in Young and Older Women. Exp. Gerontol. 2020, 132, 110840. [Google Scholar] [CrossRef]
- Mortimer, H.; Dallaway, N.; Ring, C. Effects of Isolated and Combined Mental and Physical Fatigue on Motor Skill and Endurance Exercise Performance. Psychol. Sport Exerc. 2024, 75, 102720. [Google Scholar] [CrossRef]
- Fuster, J.; Caparrós, T.; Capdevila, L. Evaluation of Cognitive Load in Team Sports: Literature Review. PeerJ 2021, 9, e12045. [Google Scholar] [CrossRef] [PubMed]
- Bian, C.; Russell, S.; Mali, A.; Lathouwers, E.; De Pauw, K.; Habay, J.; Bogataj, Š.; Roelands, B. Methodological Considerations and Effectiveness for Ecologically Valid Mental Fatigue Inducement in Sports: A Systematic Review. Sports Med. Open 2025, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.J.; Fransen, J.; Skorski, S.; Smith, M.R.; Meyer, T.; Barrett, S.; Coutts, A.J. Mental Fatigue in Football: Is It Time to Shift the Goalposts? An Evaluation of the Current Methodology. Sports Med. 2019, 49, 177–183. [Google Scholar] [CrossRef]
- Musculus, L.; Lautenbach, F.; Knöbel, S.; Reinhard, M.L.; Weigel, P.; Gatzmaga, N.; Pelka, M. An Assist for Cognitive Diagnostics in Soccer: Two Valid Tasks Measuring Inhibition and Cognitive Flexibility in a Soccer-Specific Setting with a Soccer-Specific Motor Response. Front. Psychol. 2022, 13, 867849. [Google Scholar] [CrossRef]
- Mohr, M.; Krustrup, P. Heat Stress Impairs Repeated Jump Ability after Competitive Elite Soccer Games. J. Strength Cond. Res. 2013, 27, 683–689. [Google Scholar] [CrossRef] [PubMed]
- De Pauw, K.; Roelands, B.; Cheung, S.S.; De Geus, B.; Rietjens, G.; Meeusen, R. Guidelines to Classify Subject Groups in Sport-Science Research. Int. J. Sports Physiol. Perform. 2013, 8, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Stafylidis, A.; Michailidis, Y.; Mandroukas, A.; Metaxas, I.; Chatzinikolaou, K.; Stafylidis, C.; Metaxas, T.I. Validity and Reliability of the MyJump 2 Application for Measuring Vertical Jump in Youth Soccer Players Across Age Groups. Appl. Sci. 2025, 15, 6253. [Google Scholar] [CrossRef]
- Puljić, D.; Karavas, C.; Mandroukas, A.; Stafylidis, A. Validity of the Enode Sensor and My Jump 3 App for Assessing Countermovement Jump Performance. Appl. Sci. 2024, 14, 11989. [Google Scholar] [CrossRef]
- Padulo, J.; Bragazzi, N.L.; Nikolaidis, P.T.; Dello Iacono, A.; Attene, G.; Pizzolato, F.; Migliaccio, G.M. Repeated Sprint Ability in Young Basketball Players: Multi-Direction vs. One-Change of Direction (Part 1). Front. Physiol. 2016, 7, 133. [Google Scholar] [CrossRef] [PubMed]
- Daneshfar, A.; Gahreman, D.E.; Koozehchian, M.S.; Amani Shalamzari, S.; Hassanzadeh Sablouei, M.; Rosemann, T.; Nikolaidis, P.T. Multi-Directional Repeated Sprint Is a Valid and Reliable Test for Assessment of Junior Handball Players. Front. Physiol. 2018, 9, 317. [Google Scholar] [CrossRef]
- Ding, L.; Lyu, M.; Chen, Z.; Wu, J.; Wang, Y.; Bishop, C.; Li, Y. Associations between Inter-Limb Asymmetry in Lower Limb Strength and Jump Performance in 14–15-Year-Old Basketball Players. Symmetry 2024, 16, 1421. [Google Scholar] [CrossRef]
- Stroop, J.R. Studies of Interference in Serial Verbal Reactions. J. Exp. Psychol. 1935, 18, 643–662. [Google Scholar] [CrossRef]
- Dinges, D.F.; Powell, J.W. Microcomputer Analyses of Performance on a Portable, Simple Visual RT Task during Sustained Operations. Behav. Res. Methods Instrum. Comput. 1985, 17, 652–655. [Google Scholar] [CrossRef]
- Díaz-García, J.; Clemente-Suárez, V.J.; Fuentes-García, J.P.; Villafaina, S. Combining HIIT Plus Cognitive Task Increased Mental Fatigue but Not Physical Workload in Tennis Players. Appl. Sci. 2023, 13, 7046. [Google Scholar] [CrossRef]
- Dallaway, N.; Mortimer, H.; Gore, A.; Ring, C. Brain Endurance Training Improves Dynamic Calisthenic Exercise and Benefits Novel Exercise and Cognitive Performance: Evidence of Performance Enhancement and Near Transfer of Training. J. Strength Cond. Res. 2024, 38, 1704–1713. [Google Scholar] [CrossRef] [PubMed]
- Staiano, W.; Bonet, L.R.S.; Romagnoli, M.; Ring, C. Mental Fatigue: The Cost of Cognitive Loading on Weight Lifting, Resistance Training, and Cycling Performance. Int. J. Sports Physiol. Perform. 2023, 18, 465–473. [Google Scholar] [CrossRef]
- Hart, S.G.; Staveland, L.E. Development of NASA-TLX (Task Load Index): Results of Empirical and Theoretical Research. In Advances in Psychology; Hancock, P.A., Meshkati, N., Eds.; North-Holland: Amsterdam, The Netherlands, 1988; Volume 52, pp. 139–183. [Google Scholar]
- Borg, G. Ratings of Perceived Exertion and Heart Rates during Short-Term Cycle Exercise and Their Use in a New Cycling Strength Test. Int. J. Sports Med. 1982, 3, 153–158. [Google Scholar] [CrossRef]
- Girard, O.; Mendez-Villanueva, A.; Bishop, D. Repeated-Sprint Ability—Part I: Factors Contributing to Fatigue. Sports Med. 2011, 41, 673–694. [Google Scholar] [CrossRef]
- Glaister, M.; Howatson, G.; Pattison, J.R.; McInnes, G. The Reliability and Validity of Fatigue Measures during Multiple-Sprint Work: An Issue Revisited. J. Strength Cond. Res. 2008, 22, 1597–1601. [Google Scholar] [CrossRef]
- Michailidis, Y.; Motsanos, N.; Metaxas, T. The Effects of a Repeated Sprint Ability Program on Youth Soccer Players’ Physical Performance. Trends Sport Sci. 2022, 29, 85–91. [Google Scholar]
- Del Coso, J.; Muñoz-Fernández, V.E.; Muñoz, G.; Fernández-Elías, V.E.; Ortega, J.F.; Hamouti, N.; Muñoz-Guerra, J. Effects of a Caffeine-Containing Energy Drink on Simulated Soccer Performance. PLoS ONE 2012, 7, e31380. [Google Scholar] [CrossRef]
- Hespanhol, J.E.; Silva Neto, L.G.D.; Arruda, M.D. Reliability of the Four Series 15-Second Vertical Jumping Test. Rev. Bras. Med. Esporte 2006, 12, 95–98. [Google Scholar] [CrossRef]
- Finni, T.; Ikegawa, S.; Kallio, J.; Lepola, V.; Komi, P.V. Vastus Lateral’s Length and Force in Isometric and Stretch-Shortening Cycle Conditions. J. Sports Sci. 2001, 19, 550–551. [Google Scholar]
- Bogataj, Š.; Pajek, M.; Andrašić, S.; Trajković, N. Concurrent Validity and Reliability of My Jump 2 App for Measuring Vertical Jump Height in Recreationally Active Adults. Appl. Sci. 2020, 10, 3805. [Google Scholar] [CrossRef]
- Haynes, T.; Bishop, C.; Antrobus, M.; Brazier, J. The Validity and Reliability of the My Jump 2 App for Measuring the Reactive Strength Index and Drop Jump Performance. J. Sports Med. Phys. Fit. 2019, 59, 253–258. [Google Scholar] [CrossRef]
- Staiano, W.; Romagnoli, M.; Bonet, L.R.S.; Ferri-Caruana, A. Adaptive cognitive tasks for mental fatigue: An innovative paradigm for cognitive loading in human performance. J. Sci. Med. Sport 2024, 27, 883–889. [Google Scholar] [CrossRef]
- Smith, M.R.; Chai, R.; Nguyen, H.T.; Marcora, S.M.; Coutts, A.J. Comparing the Effects of Three Cognitive Tasks on Indicators of Mental Fatigue. J. Psychol. 2019, 153, 759–783. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical Power Analyses Using G*Power 3.1: Tests for Correlation and Regression Analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- IBM Corporation. IBM SPSS Statistics for Windows, Version 29.0.2.0 [Computer Software]; IBM Corporation: Armonk, NY, USA, 2025.
- The Jamovi Project. Jamovi, Version 2.6 [Computer Software]; The Jamovi Project: Sydney, Australia, 2025. Available online: https://www.jamovi.org (accessed on 1 August 2025).
- JASP Team. JASP, Version 0.19.3.0 [Computer Software]; JASP Team: Amsterdam, The Netherlands, 2025. Available online: https://jasp-stats.org (accessed on 1 August 2025).
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Richardson, J.T.E. Eta squared and partial eta squared as measures of effect size in educational research. Educ. Res. Rev. 2011, 6, 135–147. [Google Scholar] [CrossRef]
- Lakens, D. Improving Your Statistical Inferences. 2016. Available online: https://lakens.github.io/statistical_inferences/ (accessed on 1 June 2025).
- Wuensch, K.L. SPSS Programs (Syntax); Department of Psychology, East Carolina University: Greenville, NC, USA, 2017. [Google Scholar]
- Steiger, J.H. Beyond the F Test: Effect Size Confidence Intervals and Tests of Close Fit in the Analysis of Variance and Contrast Analysis. Psychol. Methods 2004, 9, 164–182. [Google Scholar] [CrossRef] [PubMed]
- Smithson, M. Applications in ANOVA and Regression. In Confidence Intervals; SAGE Publications, Inc.: Thousand Oaks, CA, USA, 2003; pp. 42–46. [Google Scholar]
- Smith, M.R.; Zeuwts, L.; Lenoir, M.; Hens, N.; De Jong, L.M.; Coutts, A.J. Mental Fatigue Impairs Soccer-Specific Decision-Making Skill. J. Sports Sci. 2016, 34, 1297–1304. [Google Scholar] [CrossRef]
- Bray, S.R.; Graham, J.D.; Ginis, K.A.M.; Hicks, A.L. Cognitive Task Performance Causes Impaired Maximum Force Production in Human Hand Flexor Muscles. Biol. Psychol. 2012, 89, 195–200. [Google Scholar] [CrossRef]
- Rozand, V.; Pageaux, B.; Marcora, S.M.; Papaxanthis, C.; Lepers, R. Does Mental Exertion Alter Maximal Muscle Activation? Front. Hum. Neurosci. 2014, 8, 755. [Google Scholar] [CrossRef]
- Pageaux, B.; Marcora, S.M.; Rozand, V.; Lepers, R. Mental Fatigue Induced by Prolonged Self-Regulation Does Not Exacerbate Central Fatigue During Subsequent Whole-Body Endurance Exercise. Front. Hum. Neurosci. 2015, 9, 67. [Google Scholar] [CrossRef] [PubMed]
- Marcora, S.M.; Staiano, W.; Manning, V. Mental Fatigue Impairs Physical Performance in Humans. J. Appl. Physiol. 2009, 106, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Habay, J.; Van Cutsem, J.; Verschueren, J.; De Bock, S.; Proost, M.; De Wachter, J.; Tassignon, B.; Meeusen, R.; Roelands, B. Mental fatigue and sport-specific psychomotor performance: A systematic review. Sports Med. 2021, 51, 1527–1548. [Google Scholar] [CrossRef]
- Ding, C.; Soh, K.G.; Sun, H.; Roslan, S.; Cao, S.; Zhao, Y. Does mental fatigue affect performance in racket sports? A systematic review. BMC Sports Sci. Med. Rehabil. 2024, 16, 179. [Google Scholar] [CrossRef]
- Sun, H.; Soh, K.G.; Mohammadi, A.; Toumi, Z. The counteractive effects of interventions addressing mental fatigue on sport-specific performance among athletes: A systematic review with a meta-analysis. J. Sports Sci. 2024, 42, 2279–2291. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Villafaina, S.; García-Calvo, T.; Fuentes-García, J.P. Impact of HIIT sessions with and without cognitive load on cortical arousal, accuracy and perceived exertion in amateur tennis players. Healthcare 2022, 10, 767. [Google Scholar] [CrossRef]
- Migliaccio, G.M.; Di Filippo, G.; Russo, L.; Orgiana, T.; Ardigò, L.P.; Casal, M.Z.; Peyré-Tartaruga, L.A.; Padulo, J. Effects of Mental Fatigue on Reaction Time in Sportsmen. Int. J. Environ. Res. Public Health 2022, 19, 14360. [Google Scholar] [CrossRef]
- Staiano, W.; Díaz-García, J.; García-Calvo, T.; Ring, C. Brain endurance training improves soccer-specific technical skills and cognitive performance in fatigued professional soccer players. J. Sci. Med. Sport 2025, 28, 69–76. [Google Scholar] [CrossRef]
- Invernizzi, P.L.; Signorini, G.; Scurati, R.; Michielon, G.; Benedini, S.; Bosio, A.; Staiano, W. The UP150: A Multifactorial Environmental Intervention to Promote Employee Physical and Mental Well-Being. Int. J. Environ. Res. Public Health 2022, 19, 1175. [Google Scholar] [CrossRef]
- Waldman, H.S.; O’Neal, E.K.; Barker, G.A.; Witt, C.R.; Lara, D.A.; Huber, A.K.; Forsythe, V.N.; Koutnik, A.P.; D’Agostino, D.P.; Staiano, W.; et al. A Ketone Monoester with Carbohydrate Improves Cognitive Measures Postexercise, but Not Performance in Trained Females. Med. Sci. Sports Exerc. 2024, 56, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Kirk, U.; Staiano, W.; Hu, E.; Ngnoumen, C.; Kunkle, S.; Shih, E.; Clausel, A.; Purvis, C.; Lee, L. App-Based Mindfulness for Attenuation of Subjective and Physiological Stress Reactivity in a Population With Elevated Stress: Randomized Controlled Trial. JMIR Mhealth Uhealth 2023, 11, e47371. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Zhao, Y.D.; Yin, F.Q.; Yi, Y.; Geng, L.; Xu, X. Mental fatigue and sports performance of athletes: Theoretical explanation, influencing factors, and intervention methods. Behav. Sci. 2024, 14, 1125. [Google Scholar] [CrossRef] [PubMed]






| Variable | Group | Baseline Mean (SD) | EC-30′ Mean (SD) | EC-2nd Bat. Mean (SD) |
|---|---|---|---|---|
| Mental Demand | MF | 32.9 (14.9) | 72.9 (7.26) | 82.1 (9.75) |
| CON | 34.3 (16.04) | 27.9 (5.79) | 42.1 (9.75) | |
| Physical Demand | MF | 83.6 (11.51) | 10.0 (0.01) | 90.0 (6.79) |
| CON | 85.7 (8.52) | 10.0 (0.01) | 87.1 (7.26) | |
| Effort | MF | 71.4 (11.67) | 40.7 (8.29) | 87.9 (5.79) |
| CON | 72.1 (13.69) | 14.3 (6.46) | 86.4 (4.97) | |
| Frustration | MF | 28.6 (10.27) | 28.6 (9.49) | 40.0 (12.40) |
| CON | 28.6 (11.67) | 24.3 (10.89) | 35.0 (10.92) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stafylidis, A.; Staiano, W.; Mandroukas, A.; Michailidis, Y.; Salazar Bonet, L.R.; Kyranoudis, A.E.; Romagnoli, M.; Ferri-Caruana, A.; Metaxas, T.I. Cognitive Fatigue Disrupts Explosive Performance and Vigilance in Trained Individuals. Sports 2025, 13, 386. https://doi.org/10.3390/sports13110386
Stafylidis A, Staiano W, Mandroukas A, Michailidis Y, Salazar Bonet LR, Kyranoudis AE, Romagnoli M, Ferri-Caruana A, Metaxas TI. Cognitive Fatigue Disrupts Explosive Performance and Vigilance in Trained Individuals. Sports. 2025; 13(11):386. https://doi.org/10.3390/sports13110386
Chicago/Turabian StyleStafylidis, Andreas, Walter Staiano, Athanasios Mandroukas, Yiannis Michailidis, Lluis Raimon Salazar Bonet, Angelos E. Kyranoudis, Marco Romagnoli, Ana Ferri-Caruana, and Thomas I. Metaxas. 2025. "Cognitive Fatigue Disrupts Explosive Performance and Vigilance in Trained Individuals" Sports 13, no. 11: 386. https://doi.org/10.3390/sports13110386
APA StyleStafylidis, A., Staiano, W., Mandroukas, A., Michailidis, Y., Salazar Bonet, L. R., Kyranoudis, A. E., Romagnoli, M., Ferri-Caruana, A., & Metaxas, T. I. (2025). Cognitive Fatigue Disrupts Explosive Performance and Vigilance in Trained Individuals. Sports, 13(11), 386. https://doi.org/10.3390/sports13110386










