The Effect of Oral Adenosine Triphosphate (ATP) Supplementation on Anaerobic Exercise in Healthy Resistance-Trained Individuals: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection of Studies
2.3. Data Extraction
2.4. Risk of Bias Assessment
2.5. Study Quality Assessment
2.6. Statistical Analysis
3. Results
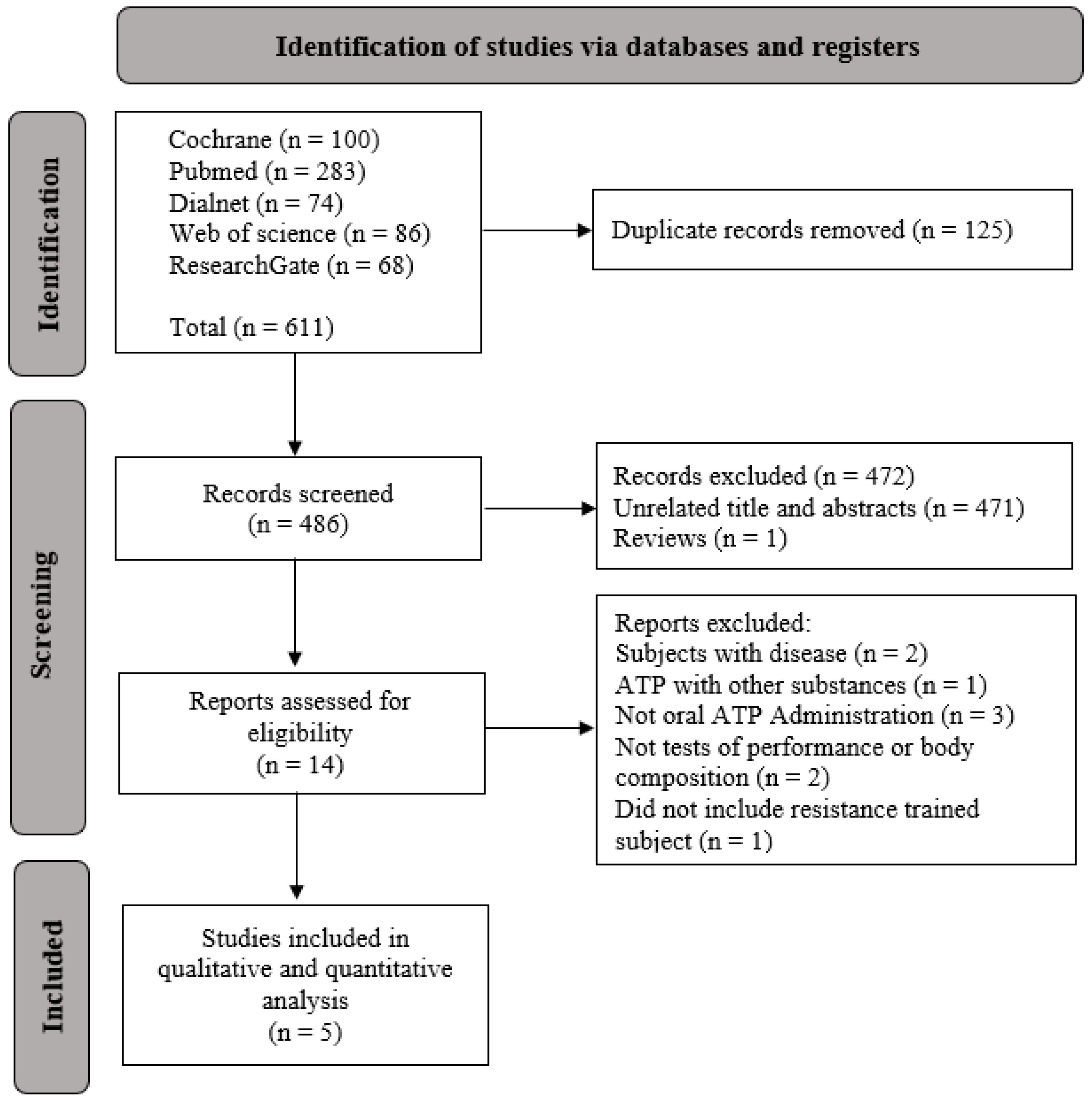
3.1. Study Selection
3.2. Study Characteristics
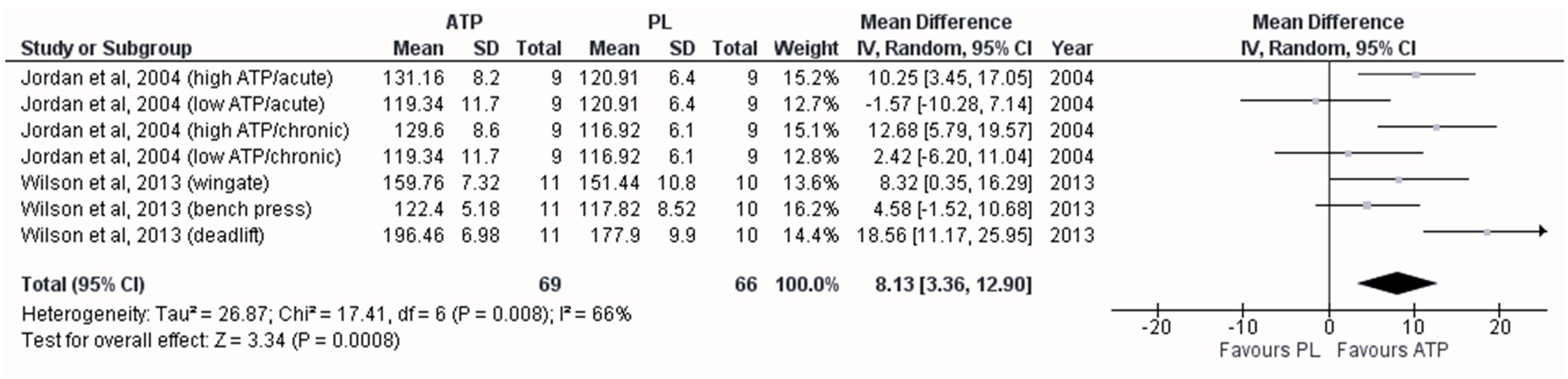
3.3. Effect on Maximal Strength
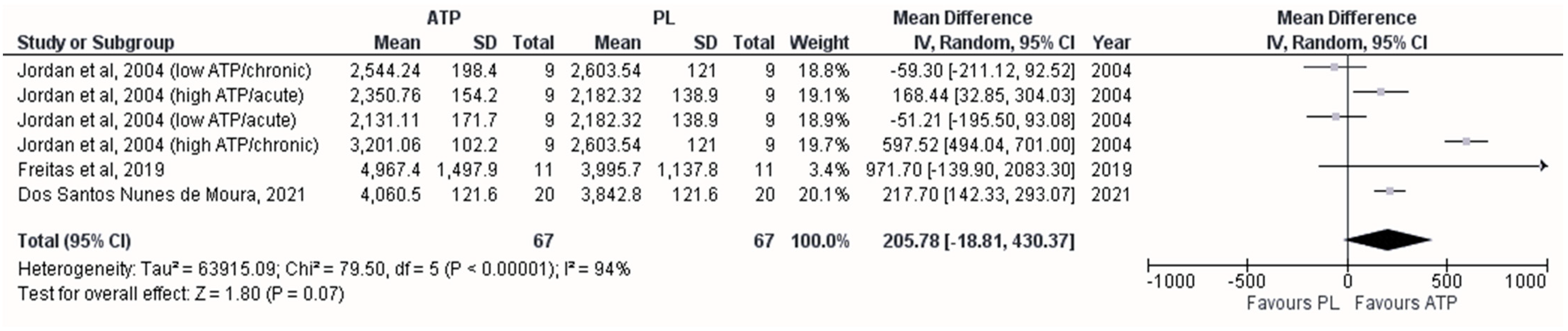
3.4. Effect on the Maximum Number of Repetitions
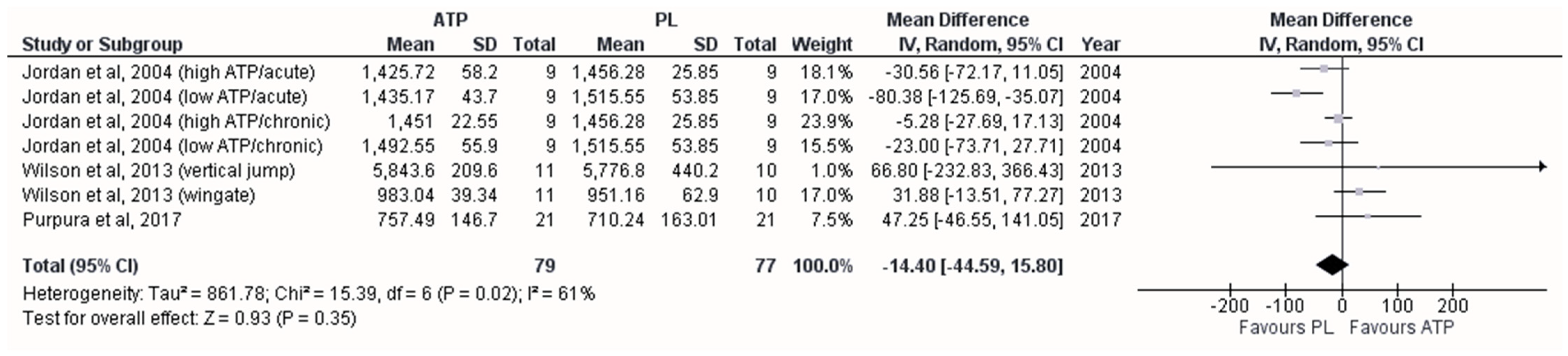
3.5. Effect on Maximum Anaerobic Power
3.6. Safety
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Porter, C.; Wall, B.T. Skeletal muscle mitochondrial function: Is it quality or quantity that makes the difference in insulin resistance? J. Physiol. 2012, 590, 5935–5936. [Google Scholar] [CrossRef]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef]
- Squire, J. Special Issue: The Actin-Myosin Interaction in Muscle: Background and Overview. Int. J. Mol. Sci. 2019, 20, 5715. [Google Scholar] [CrossRef] [PubMed]
- Dirksen, R.T. Sarcoplasmic reticulum-mitochondrial through-space coupling in skeletal muscle. Appl. Physiol. Nutr. Metab. 2009, 34, 389–395. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, Y.; Kerrick, W.G. The off rate of Ca(2+) from troponin C is regulated by force-generating cross bridges in skeletal muscle. J. Appl. Physiol. (1985) 2002, 92, 2409–2418. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G.; Kennedy, C. P2X receptors in health and disease. Adv. Pharmacol. 2011, 61, 333–372. [Google Scholar] [CrossRef]
- Ellsworth, M.L. Red blood cell-derived ATP as a regulator of skeletal muscle perfusion. Med. Sci. Sports Exerc. 2004, 36, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Alonso, J. ATP as a mediator of erythrocyte-dependent regulation of skeletal muscle blood flow and oxygen delivery in humans. J. Physiol. 2012, 590, 5001–5013. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, M.; Al-Khazraji, B.K.; Mortensen, S.P.; Jackson, D.N.; Ellis, C.G.; Hellsten, Y. Effect of extraluminal ATP application on vascular tone and blood flow in skeletal muscle: Implications for exercise hyperemia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R281–R290. [Google Scholar] [CrossRef]
- Sarelius, I.; Pohl, U. Control of muscle blood flow during exercise: Local factors and integrative mechanisms. Acta Physiol. 2010, 199, 349–365. [Google Scholar] [CrossRef]
- Kichenin, K.; Seman, M. Chronic oral administration of ATP modulates nucleoside transport and purine metabolism in rats. J. Pharmacol. Exp. Ther. 2000, 294, 126–133. [Google Scholar] [PubMed]
- Purpura, M.; Rathmacher, J.A.; Sharp, M.H.; Lowery, R.P.; Shields, K.A.; Partl, J.M.; Wilson, J.M.; Jäger, R. Oral adenosine-5′-triphosphate (atp) administration increases postexercise atp levels, muscle excitability, and athletic performance following a repeated sprint bout. JANA 2017, 36, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Allado, E.; Poussel, M.; Albuisson, E.; Paysant, J.; Temperelli, M.; Hily, O.; Moussu, A.; Benhajji, N.; Gauchard, G.C.; Chenuel, B. Physical Activity Capacity Assessment of Patients with Chronic Disease and the Six-Minute Walk Test: A Cross-Sectional Study. Healthcare 2022, 10, 758. [Google Scholar] [CrossRef] [PubMed]
- Kalsi, K.K.; Chiesa, S.T.; Trangmar, S.J.; Ali, L.; Lotlikar, M.D.; Gonzalez-Alonso, J. Mechanisms for the control of local tissue blood flow during thermal interventions: Influence of temperature-dependent ATP release from human blood and endothelial cells. Exp. Physiol. 2017, 102, 228–244. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J. Potential mechanisms for a role of metabolic stress in hypertrophic adaptations to resistance training. Sports Med. 2013, 43, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Nunes de Moura, H.P.; Jager, R.; Purpura, M.; Rathmacher, J.A.; Fuller, J.C., Jr.; Rossi, F.E. Dose Response of Acute ATP Supplementation on Strength Training Performance. Front. Sports Act. Living 2021, 3, 780459. [Google Scholar] [CrossRef]
- Jordan, A.N.; Jurca, R.; Abraham, E.H.; Salikhova, A.; Mann, J.K.; Morss, G.M.; Church, T.S.; Lucia, A.; Earnest, C.P. Effects of oral ATP supplementation on anaerobic power and muscular strength. Med. Sci. Sports Exerc. 2004, 36, 983–990. [Google Scholar] [CrossRef]
- Jäger, R.; Roberts, M.D.; Lowery, R.P.; Joy, J.M.; Cruthirds, C.L.; Lockwood, C.M.; Rathmacher, J.A.; Purpura, M.; Wilson, J.M. Oral adenosine-5′-triphosphate (ATP) administration increases blood flow following exercise in animals and humans. J. Int. Soc. Sports Nutr. 2014, 11, 28. [Google Scholar] [CrossRef]
- Lowery, R.P.; Joy, J.M.; Rathmacher, J.A.; Baier, S.M.; Fuller, J.C., Jr.; Shelley, M.C., 2nd; Jager, R.; Purpura, M.; Wilson, S.M.; Wilson, J.M. Interaction of Beta-Hydroxy-Beta-Methylbutyrate Free Acid and Adenosine Triphosphate on Muscle Mass, Strength, and Power in Resistance Trained Individuals. J. Strength. Cond. Res. 2016, 30, 1843–1854. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef]
- Higgins, J.P. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1; Collaboration TC: London, UK, 2008. [Google Scholar]
- Blobaum, P. Physiotherapy evidence database (PEDro). J. Med. Libr. Assoc. 2006, 94, 477–478. [Google Scholar]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Wilson, J.M.; Joy, J.M.; Lowery, R.P.; Roberts, M.D.; Lockwood, C.M.; Manninen, A.H.; Fuller, J.C.; De Souza, E.O.; Baier, S.M.; Wilson, S.M.; et al. Effects of oral adenosine-5′-triphosphate supplementation on athletic performance, skeletal muscle hypertrophy and recovery in resistance-trained men. Nutr. Metab. 2013, 10, 57. [Google Scholar] [CrossRef]
- Freitas, M.C.; Cholewa, J.M.; Gerosa-Neto, J.; Goncalves, D.C.; Caperuto, E.C.; Lira, F.S.; Rossi, F.E. A Single Dose of Oral ATP Supplementation Improves Performance and Physiological Response During Lower Body Resistance Exercise in Recreational Resistance-Trained Males. J. Strength. Cond. Res. 2019, 33, 3345–3352. [Google Scholar] [CrossRef]
- AIS. Australian Sports Commission. Benefits and Risks of Using Supplements and Sports Foods. 2021. Available online: https://www.ais.gov.au/nutrition/supplements (accessed on 6 May 2022).
- AIS. Australian Sports Commission. Supplements and Sports Foods in High Performance Sport. 2022. Available online: https://www.ais.gov.au/__data/assets/pdf_file/0014/1000841/Position-Statement-Supplements-and-Sports-Foods.pdf (accessed on 10 July 2022).
- Jäger, R.; Purpura, M.; Rathmacher, J.A.; Fuller, J.C., Jr.; Pitchford, L.M.; Rossi, F.E.; Kerksick, C.M. Health and ergogenic potential of oral adenosine-5′-triphosphate (ATP) supplementation. J. Funct. Foods 2021, 78, 104357. [Google Scholar] [CrossRef]
- Moynes, J.; Bentley, R.F.; Bravo, M.; Kellawan, J.M.; Tschakovsky, M.E. Persistence of functional sympatholysis post-exercise in human skeletal muscle. Front. Physiol. 2013, 4, 131. [Google Scholar] [CrossRef]
- Gajecki, D.; Gawrys, J.; Szahidewicz-Krupska, E.; Doroszko, A. Role of Erythrocytes in Nitric Oxide Metabolism and Paracrine Regulation of Endothelial Function. Antioxidants 2022, 11, 943. [Google Scholar] [CrossRef] [PubMed]
- Gliemann, L.; Hansen, C.V.; Rytter, N.; Hellsten, Y. Regulation of skeletal muscle blood flow during exercise. Curr. Opin. Physiol. 2019, 10, 146–155. [Google Scholar] [CrossRef]
- Joyner, M.J.; Casey, D.P. Regulation of increased blood flow (hyperemia) to muscles during exercise: A hierarchy of competing physiological needs. Physiol. Rev. 2015, 95, 549–601. [Google Scholar] [CrossRef] [PubMed]
- Clausen, T. Excitation of skeletal muscle is a self-limiting process, due to run-down of Na+, K+ gradients, recoverable by stimulation of the Na+, K+ pumps. Physiol. Rep. 2015, 3, e12373. [Google Scholar] [CrossRef] [PubMed]
- Leermakers, P.A.; Dybdahl, K.L.T.; Husted, K.S.; Riisager, A.; de Paoli, F.V.; Pinos, T.; Vissing, J.; Krag, T.O.B.; Pedersen, T.H. Depletion of ATP Limits Membrane Excitability of Skeletal Muscle by Increasing Both ClC1-Open Probability and Membrane Conductance. Front. Neurol. 2020, 11, 541. [Google Scholar] [CrossRef]
- Chaudry, I.H. Does ATP cross the cell plasma membrane. Yale J. Biol. Med. 1982, 55, 1–10. [Google Scholar]
- Chaudry, I.H.; Baue, A.E. Further evidence for ATP uptake by rat tissues. Biochim. Biophys. Acta 1980, 628, 336–342. [Google Scholar] [CrossRef]
- Ito, N.; Ruegg, U.T.; Takeda, S. ATP-Induced Increase in Intracellular Calcium Levels and Subsequent Activation of mTOR as Regulators of Skeletal Muscle Hypertrophy. Int. J. Mol. Sci. 2018, 19, 2804. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, Z.; Hogan, B.; Barakat, A.I.; Misbah, C. ATP Release by Red Blood Cells under Flow: Model and Simulations. Biophys. J. 2018, 115, 2218–2229. [Google Scholar] [CrossRef]
- Forsyth, A.M.; Wan, J.; Owrutsky, P.D.; Abkarian, M.; Stone, H.A. Multiscale approach to link red blood cell dynamics, shear viscosity, and ATP release. Proc. Natl. Acad. Sci. USA 2011, 108, 10986–10991. [Google Scholar] [CrossRef]
- Gov, N.S.; Safran, S.A. Red blood cell membrane fluctuations and shape controlled by ATP-induced cytoskeletal defects. Biophys. J. 2005, 88, 1859–1874. [Google Scholar] [CrossRef]
- Sprague, R.S.; Ellsworth, M.L.; Stephenson, A.H.; Kleinhenz, M.E.; Lonigro, A.J. Deformation-induced ATP release from red blood cells requires CFTR activity. Am. J. Physiol. 1998, 275, H1726–H1732. [Google Scholar] [CrossRef]
- Driss, T.; Vandewalle, H. The measurement of maximal (anaerobic) power output on a cycle ergometer: A critical review. Biomed. Res. Int. 2013, 2013, 589361. [Google Scholar] [CrossRef]
- Pereira, R.; Machado, M.; Miragaya dos Santos, M.; Pereira, L.N.; Sampaio-Jorge, F. Muscle activation sequence compromises vertical jump performance. Serb. J. Sports Sci. 2008, 2, 85–90. [Google Scholar]
- Kirby, B.S.; Voyles, W.F.; Carlson, R.E.; Dinenno, F.A. Graded sympatholytic effect of exogenous ATP on postjunctional alpha-adrenergic vasoconstriction in the human forearm: Implications for vascular control in contracting muscle. J. Physiol. 2008, 586, 4305–4316. [Google Scholar] [CrossRef] [PubMed]
- Rosenmeier, J.B.; Hansen, J.; Gonzalez-Alonso, J. Circulating ATP-induced vasodilatation overrides sympathetic vasoconstrictor activity in human skeletal muscle. J. Physiol. 2004, 558, 351–365. [Google Scholar] [CrossRef]
- Coolen, E.J.; Arts, I.C.; Bekers, O.; Vervaet, C.; Bast, A.; Dagnelie, P.C. Oral bioavailability of ATP after prolonged administration. Br. J. Nutr. 2011, 105, 357–366. [Google Scholar] [CrossRef]
| Author/Year/Country | Study Design | Sample | Duration of the Study | Test of Performance | |
|---|---|---|---|---|---|
| ATP Doses | PL | ||||
| Jordan et al. (2004). USA [17] | RCT Double-blind | High (225 mg, n = 9) Low (150 mg, n = 9) | n = 9 | 21 days (14 days with supplementation) | Two tests of 30 s on a cycle ergometer at maximum intensity. 1RM bench press. Three sets of bench press (70% 1RM) to exhaustion. |
| Wilson et al. (2013). USA [24] | RCT Double-blind and parallel | 400 mg, n = 11 | n = 10 | 12 weeks | 1RM bench press, back squat, and deadlift. Cycling test at maximum intensity for 10 s. Vertical jump Body composition by DXA and ultrasonography. |
| Purpura et al. (2017). USA [12] | RCT Double-blind | 400 mg, n = 21 | n = 21 | 15 days | Ten series of 6 secs on a cycle ergometer at maximum intensity. Three vertical jumps before and after the last set of cycling. |
| Freitas et al. (2019). Brazil [25] | RCT Double-blind, crossover | 400 mg, n = 11 | n = 11 | One day | Four sets of half squats (80% 1RM) to failure. |
| Dos Santos Nunes de Moura et al. (2021). Brazil [16] | RCT Double-blind, crossover | 400 mg, n = 20 | n = 20 | One day | Four sets of half squats (80% 1RM) to failure |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Marenco, R.; Estrada-Sánchez, I.A.; Medina-Escobedo, M.; Chim-Aké, R.; Lugo, R. The Effect of Oral Adenosine Triphosphate (ATP) Supplementation on Anaerobic Exercise in Healthy Resistance-Trained Individuals: A Systematic Review and Meta-Analysis. Sports 2024, 12, 82. https://doi.org/10.3390/sports12030082
González-Marenco R, Estrada-Sánchez IA, Medina-Escobedo M, Chim-Aké R, Lugo R. The Effect of Oral Adenosine Triphosphate (ATP) Supplementation on Anaerobic Exercise in Healthy Resistance-Trained Individuals: A Systematic Review and Meta-Analysis. Sports. 2024; 12(3):82. https://doi.org/10.3390/sports12030082
Chicago/Turabian StyleGonzález-Marenco, Roberto, Ivonne Azeret Estrada-Sánchez, Martha Medina-Escobedo, Rodolfo Chim-Aké, and Roberto Lugo. 2024. "The Effect of Oral Adenosine Triphosphate (ATP) Supplementation on Anaerobic Exercise in Healthy Resistance-Trained Individuals: A Systematic Review and Meta-Analysis" Sports 12, no. 3: 82. https://doi.org/10.3390/sports12030082
APA StyleGonzález-Marenco, R., Estrada-Sánchez, I. A., Medina-Escobedo, M., Chim-Aké, R., & Lugo, R. (2024). The Effect of Oral Adenosine Triphosphate (ATP) Supplementation on Anaerobic Exercise in Healthy Resistance-Trained Individuals: A Systematic Review and Meta-Analysis. Sports, 12(3), 82. https://doi.org/10.3390/sports12030082







