Rapid Artificial Infestation Method for Assessing Fall Armyworm (Spodoptera frugiperda) Damage on Maize
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Maize Materials and S. frugiperda Colony
2.2. Laboratory Bioassays
2.3. Screenhouse Potted-Plant Infestation Trials
2.4. Field Infestation Trials
2.5. Data Analysis
3. Results
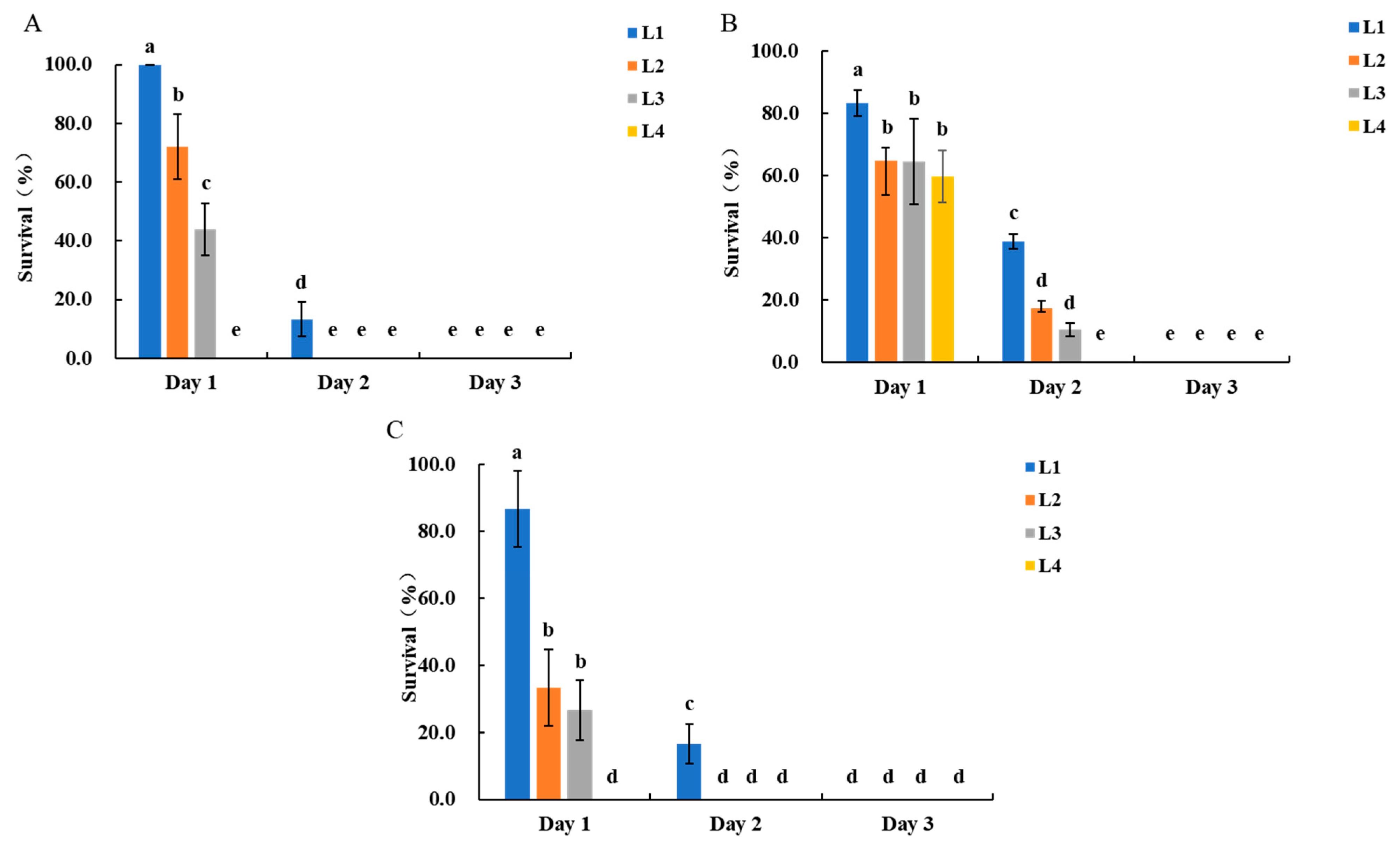
3.1. Laboratory Bioassays of Transgenic Maize Resistance to S. frugiperda
3.2. Potted-Plant Infestation Trials in Screenhouse for Assessing Transgenic Maize Resistance to S. frugiperda
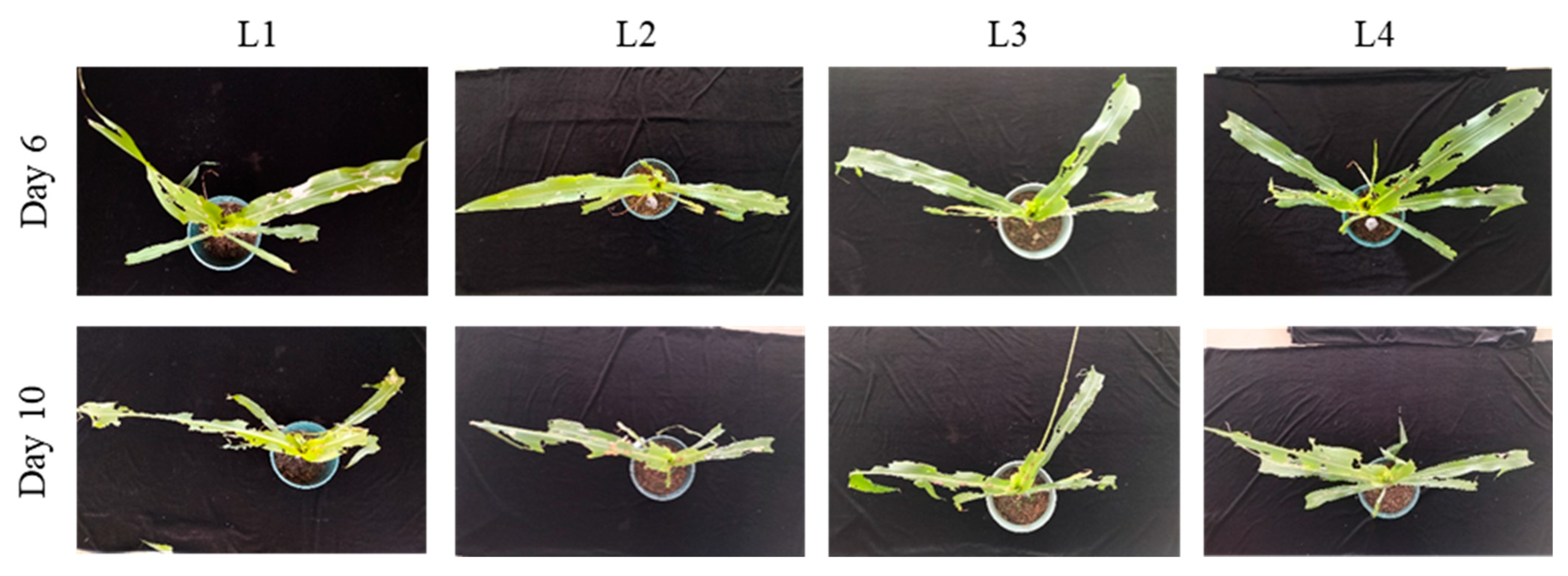
3.2.1. Optimization of Larval Age for Infestation Protocols
3.2.2. Optimization of Larval Density for Infestation Protocols in the Screenhouse
3.3. Field Infestation Trials for Assessing Transgenic Maize Resistance to S. frugiperda
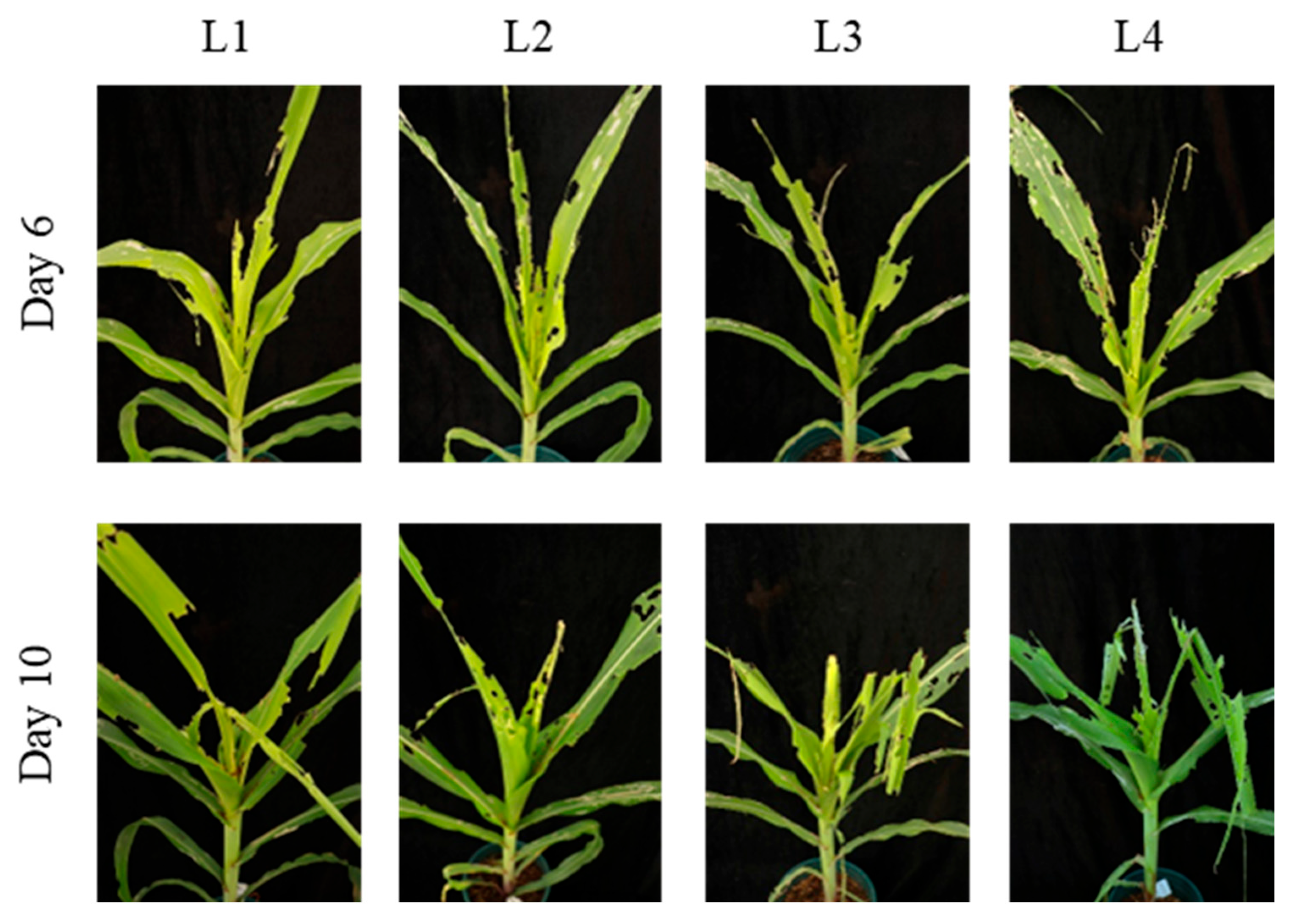
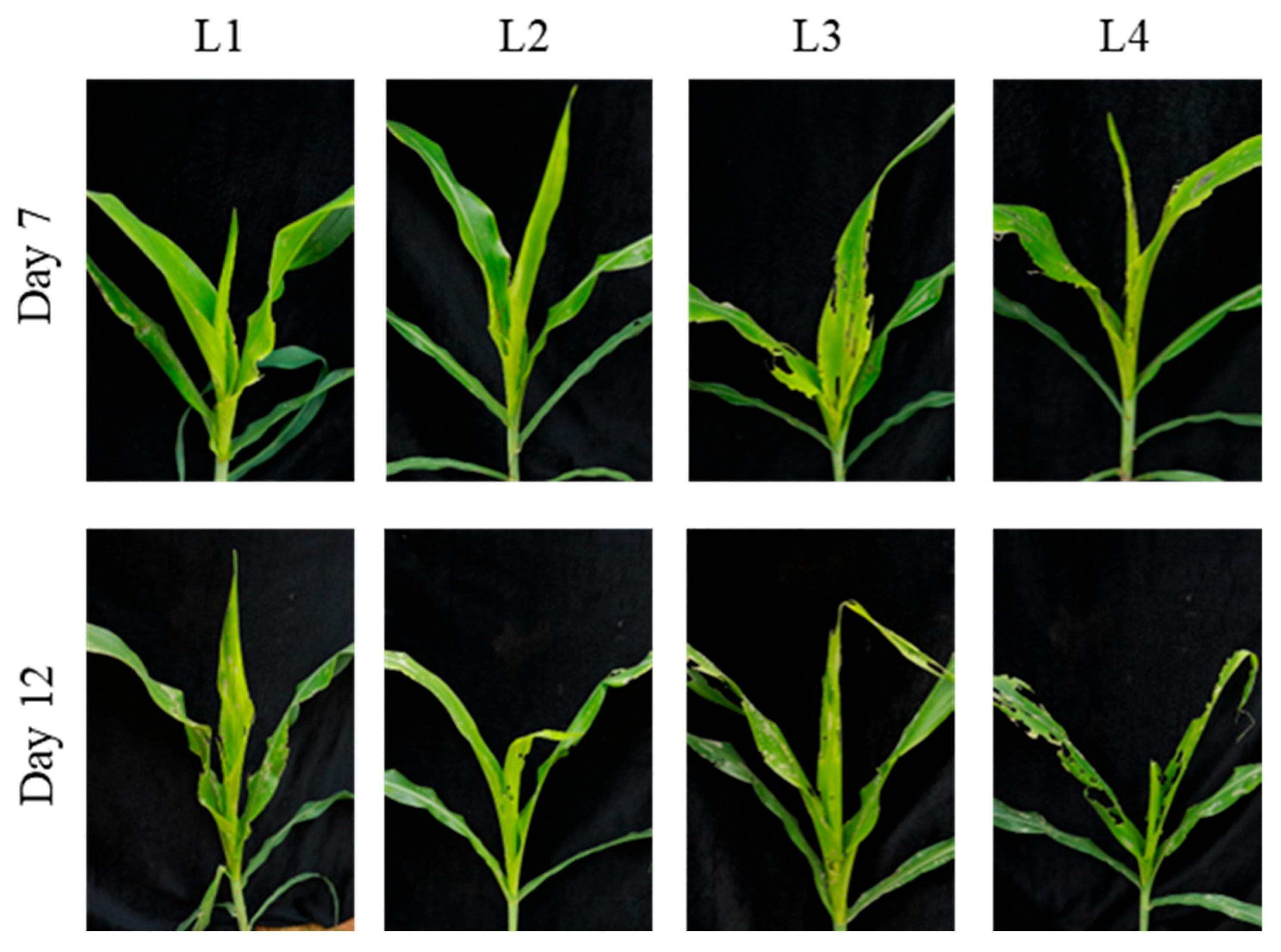
3.3.1. Comparison of Different Larval Ages for Infestation Protocols
3.3.2. Optimization of Larval Density for Infestation Protocols in the Field
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montezano, D.G.; Sosa-Gomez, D.R.; Specht, A.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.V.; Peterson, J.A.; Hunt, T.E. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef]
- Kenis, M. Prospects for classical biological control of Spodoptera frugiperda (Lepidoptera: Noctuidae) in invaded areas using parasitoids from the Americas. J. Econ. Entomol. 2023, 116, 331–341. [Google Scholar] [CrossRef]
- Sun, X.X.; Hu, C.X.; Jia, H.R.; Wu, Q.L.; Shen, X.J.; Zhao, S.Y.; Jiang, Y.Y.; Wu, K.M. Case study on the first immigration of fall armyworm, Spodoptera frugiperda invading into China. J. Integr. Agric. 2021, 20, 664–672. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, Q.L.; Zhang, H.W.; Wu, K.M. Spread of invasive migratory pest Spodoptera frugiperda and management practices throughout China. J. Integr. Agric. 2021, 20, 637–645. [Google Scholar] [CrossRef]
- Buntin, G.D.; All, J.N.; Lee, R.D.; Wilson, D.M. Plant-incorporated Bacillus thuringiensis resistance for control of fall armyworm and corn earworm (Lepidoptera: Noctuidae) in corn. J. Econ. Entomol. 2004, 97, 1603–1611. [Google Scholar] [CrossRef]
- Marques, L.H.; Santos, A.C.; Castro, B.A.; Moscardini, V.F.; Rosseto, J.; Silva, O.A.B.N.; Babcock, J.M. Assessing the Efficacy of Bacillus thuringiensis (Bt) Pyramided Proteins Cry1F, Cry1A.105, Cry2Ab2, and Vip3Aa20 Expressed in Bt Maize Against Lepidopteran Pests in Brazil. J. Econ. Entomol. 2019, 112, 803–811. [Google Scholar] [CrossRef]
- Buntin, G.D. Corn expressing Cry1Ab or Cry1F endotoxin for fall armyworm and corn earworm (Lepidoptera: Noctuidae) management in field corn for grain production. Fla. Entomol. 2008, 91, 523–530. [Google Scholar] [CrossRef]
- Siebert, M.W.; Nolting, S.P.; Hendrix, W.; Dhavala, S.; Craig, C.; Leonard, B.R.; Stewart, S.D.; All, J.; Musser, F.R.; Buntin, G.D.; et al. Evaluation of corn hybrids expressing Cry1F, Cry1A.105, Cry2Ab2, Cry34Ab1/Cry35Ab1, and Cry3Bb1 against southern United States insect pests. J. Econ. Entomol. 2012, 105, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Chandrasena, D.I.; Signorini, A.M.; Abratti, G.; Storer, N.P.; Olaciregui, M.L.; Alves, A.P.; Pilcher, C.D. Characterization of field-evolved resistance to Bacillus thuringiensis-derived Cry1F δ-endotoxin in Spodoptera frugiperda populations from Argentina. Pest Manag. Sci. 2018, 74, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.M.; Zhao, S.Y.; Liu, B.; Gao, Y.; Hu, C.X.; Li, W.J.; Yang, Y.Z.; Li, G.P.; Wang, L.L.; Yang, X.Q.; et al. Bt maize can provide non-chemical pest control and enhance food safety in China. Plant Biotechnol. J. 2023, 21, 391–404. [Google Scholar] [CrossRef]
- Rodriguez-Chalarca, J.; Valencia, S.J.; Rivas-Cano, A.; Santos-González, F.; Romero, D.P. Impact of Bt corn expressing Bacillus thuringiensis Berliner insecticidal proteins on the growth and survival of Spodoptera frugiperda larvae in Colombia. Front. Insect Sci. 2024, 4, 1268092. [Google Scholar] [CrossRef]
- Zhao, S.Y.; Yang, X.M.; Liu, D.Z.; Sun, X.X.; Li, G.P.; Wu, K.M. Performance of the domestic Bt corn event expressing pyramided Cry1Ab and Vip3Aa19 against the invasive Spodoptera frugiperda (J. E. Smith) in China. Pest Manag. Sci. 2023, 79, 1018–1029. [Google Scholar] [CrossRef]
- Gomez, V.A.; Villalba, G.E.; Arias, O.R.; Ramirez, M.B.; Gaona, E.F. Toxicity of the Bt protein expressed in leaves of different events of transgenic corn released in Paraguay against Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae). Rev. Soc. Entomol. Argent. 2017, 76, 1–10. [Google Scholar] [CrossRef]
- Botha, A.S.; Erasmus, A.; du Plessis, H.; Van den Berg, J. Efficacy of Bt maize for control of Spodoptera frugiperda (Lepidoptera: Noctuidae) in South Africa. J. Econ. Entomol. 2019, 112, 1260–1266. [Google Scholar] [CrossRef]
- Williams, W.P.; Buckley, P.M.; Davis, F.M. Combining ability for resistance in corn to fall armyworm and to southwestern corn-borer. Crop Sci. 1989, 29, 913–915. [Google Scholar] [CrossRef]
- Toepfer, S.; Fallet, P.; Kajuga, J.; Bazagwira, D.; Mukundwa, I.P.; Szalai, M.; Turlings, T.C.J. Streamlining leaf damage rating scales for the fall armyworm on maize. J. Pest. Sci. 2021, 94, 1075–1089. [Google Scholar] [CrossRef]
- Abel, C.A.; Coates, B.S. Evaluation of eight maize germplasms developed in Ecuador for resistance to leaf-feeding fall armyworm. Southwest. Entomol. 2020, 45, 75–84. [Google Scholar] [CrossRef]
- Moscardini, V.F.; Marques, L.H.; Santos, A.C.; Rossetto, J.; Silva, O.A.B.N.; Rampazzo, P.E.; Castro, B.A. Efficacy of Bacillus thuringiensis (Bt) maize expressing Cry1F, Cry1A.105, Cry2Ab2 and Vip3Aa20 proteins to manage the fall armyworm (Lepidoptera: Noctuidae) in Brazil. Crop Prot. 2020, 137, 105269. [Google Scholar] [CrossRef]
- Davis, F.M.; Ng, S.S.; Williams, W.P. Visual Rating Scales for Screening Whorl-Stage Corn for Resistance to Fall Armyworm; Technical Bulletin 186; Mississippi Agricultural and Forestry Research Experiment Station: Mississippi State, MS, USA, 1992. Available online: http://www.nal.usda.gov/ (accessed on 29 November 2025).
- Jiang, C.X.; Zhang, X.Y.; Xie, W.Q.; Wang, R.L.; Feng, C.H.; Ma, L.; Li, Q.; Yang, Q.F.; Wang, H.J. Predicting the potential distribution of the fall armyworm Spodoptera frugiperda (J. E. Smith) under climate change in China. Glob. Ecol. Conserv. 2022, 33, e01994. [Google Scholar] [CrossRef]
- Xu, Y.C.; Chi, H.P.; Shi, M.Y.; Lu, Z.Z.; Zalucki, M.P. Night warming has mixed effects on the development of the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), in Southern China. Insects 2024, 15, 180. [Google Scholar] [CrossRef]
- Kenis, M.; Benelli, G.; Biondi, A.; Calatayud, P.; Day, R.; Desneux, N.; Harrison, R.D.; Kriticos, D.; Rwomushana, I.; van den Berg, J.; et al. Invasiveness, biology, ecology, and management of the fall armyworm, Spodoptera frugiperda. Entomol. Gen. 2023, 43, 187–241. [Google Scholar] [CrossRef]
- Wyckhuys, K.A.G.; Akutse, K.S.; Amalin, D.M.; Araj, S.E.; Barrera, G.; Beltran, M.J.B.; Ben Fekih, I.; Calatayud, P.A.; Cicero, L.; Cokola, M.C.; et al. Global scientific progress and shortfalls in biological control of the fall armyworm Spodoptera frugiperda. Biol. Control 2024, 191, 105460. [Google Scholar] [CrossRef]
- Fan, Z.Y.; Kong, W.Z.; Ran, X.T.; Lv, X.L.; Ma, C.J.; Yan, H. Biological and physiological changes in Spodoptera frugiperda larvae induced by non-consumptive effects of the predator Harmonia axyridis. Agriculture 2024, 14, 1566. [Google Scholar] [CrossRef]
- Chen, Y.G.; Ni, X.Z.; Buntin, G.D. Physiological, nutritional, and biochemical bases of corn resistance to foliage-feeding fall armyworm. J. Chem. Ecol. 2009, 35, 297–306. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, L.F.C.; Ruiz-Sánchez, E.; Andueza-Noh, R.H.; Garruña-Hernández, R.; Latournerie-Moreno, L.; Mijangos-Cortés, J.O. Leaf damage by Spodoptera frugiperda J. E. Smith (Lepidoptera: Noctuidae) and its relation to leaf morphological traits in maize landraces and commercial cultivars. J. Plant Dis. Prot. 2020, 127, 103–109. [Google Scholar] [CrossRef]



| Larval Age | Day 6 | Day 10 | ||
|---|---|---|---|---|
| Number of Notched Leaves | Whorl Injury Type | Number of Notched Leaves | Whorl Injury Type | |
| L1 | 2.7 ± 0.6 b | Whorl leaf eaten | 3.7 ± 0.6 b | Whorl leaf eaten to breakage |
| L2 | 4.6 ± 0.6 a | Whorl leaf eaten to breakage | 5.6 ± 0.6 a | Whorl leaf eaten to breakage |
| L3 | 5.0 ± 1.0 a | Whorl leaf eaten to breakage | 6.3 ± 0.6 a | Whorl leaf eaten to breakage |
| L4 | 5.7 ± 0.6 a | Whorl leaf eaten to breakage | 6.6 ± 0.6 a | Whorl leaf eaten to breakage |
| Larval Age | Day 6 | Day 10 | ||
|---|---|---|---|---|
| Number of Notched Leaves | Whorl Injury Type | Number of Notched Leaves | Whorl Injury Type | |
| L1 | 3.3 ± 0.6 b | Whorl leaf eaten to breakage | 4.7 ± 0.6 b | Whorl leaf eaten to breakage |
| L2 | 5.3 ± 0.6 a | Whorl leaf eaten to breakage | 7.0 ± 1.0 a | Whorl leaf eaten to breakage |
| L3 | 6.3 ± 0.6 a | Whorl leaf eaten to breakage | 7.3 ± 0.6 a | Whorl leaf eaten to breakage |
| L4 | 6.7 ± 0.6 a | Whorl leaf eaten to breakage | 8.3 ± 0.6 a | Whorl leaf eaten to breakage |
| Number of Larvae | Day 6 | Day 10 | ||
|---|---|---|---|---|
| Number of Notched Leaves | Whorl Injury Type | Number of Notched Leaves | Whorl Injury Type | |
| 10 | 2.3 ± 0.6 b | Whorl leaf eaten | 3.3 ± 0.6 b | Whorl leaf eaten to breakage |
| 20 | 3.7 ± 0.6 a | Whorl leaf eaten | 5.3 ± 0.6 a | Whorl leaf eaten to breakage |
| 30 | 4.0 ± 0.0 a | Whorl leaf eaten | 5.3 ± 0.6 a | Whorl leaf eaten to breakage |
| 40 | 4.3 ± 0.6 a | Whorl leaf eaten to breakage | 6.0 ± 1.0 a | Whorl leaf eaten to breakage |
| Larval Age | Day 7 | Day 12 | ||
|---|---|---|---|---|
| Number of Notched Leaves | Whorl Injury Type | Number of Notched Leaves | Whorl Injury Type | |
| L1 | 0.3 ± 0.6 c | Windows/Notching | 2.3 ± 0.6 b | Whorl leaf eaten |
| L2 | 2.0 ± 0.0 b | Windows/Notching | 2.7 ± 0.6 b | Whorl leaf eaten to breakage |
| L3 | 3.0 ± 0.0 a | Whorl leaf eaten | 4.6 ± 0.6 a | Whorl leaf eaten to breakage |
| L4 | 3.3 ± 0.6 a | Whorl leaf eaten | 5.0 ± 1.0 a | Whorl leaf eaten to breakage |
| Larval Age | Day 7 | Day 12 | ||
|---|---|---|---|---|
| Number of Notched Leaves | Whorl Injury Type | Number of Notched Leaves | Whorl Injury Type | |
| L1 | 0.0 ± 0.0 d | Windows | 3.3 ± 0.6 b | Whorl leaf eaten |
| L2 | 1.6 ± 0.6 c | Windows/Notching | 4.0 ± 0.0 b | Whorl leaf eaten to breakage |
| L3 | 3.7 ± 0.6 b | Whorl leaf eaten | 5.3 ± 0.0 a | Whorl leaf eaten to breakage |
| L4 | 4.7 ± 0.6 a | Whorl leaf eaten to breakage | 5.6 ± 0.6 a | Whorl leaf eaten to breakage |
| Number of Larvae | Day 7 | Day 12 | ||
|---|---|---|---|---|
| Number of Notched Leaves | Whorl Injury Type | Number of Notched Leaves | Whorl Injury Type | |
| 10 | 1.7 ± 0.6 b | Whorl leaf eaten | 3.0 ± 0.0 b | Whorl leaf eaten to breakage |
| 20 | 2.0 ± 0.0 b | Whorl leaf eaten | 3.3 ± 0.6 b | Whorl leaf eaten to breakage |
| 30 | 3.7 ± 0.6 a | Whorl leaf eaten/eaten to breakage | 5.0 ± 0.0 a | Whorl leaf eaten to breakage |
| 40 | 3.7 ± 0.6 a | Whorl leaf eaten/eaten to breakage | 5.7 ± 0.6 a | Whorl leaf eaten to breakage |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Wu, C.; Chen, W.; Guo, X.; He, G.; Yang, G.; Zhu, L.; Yao, J.; Jiang, D. Rapid Artificial Infestation Method for Assessing Fall Armyworm (Spodoptera frugiperda) Damage on Maize. Insects 2026, 17, 136. https://doi.org/10.3390/insects17020136
Wu C, Chen W, Guo X, He G, Yang G, Zhu L, Yao J, Jiang D. Rapid Artificial Infestation Method for Assessing Fall Armyworm (Spodoptera frugiperda) Damage on Maize. Insects. 2026; 17(2):136. https://doi.org/10.3390/insects17020136
Chicago/Turabian StyleWu, Caiyao, Weiting Chen, Xinyu Guo, Gongwen He, Guiqin Yang, Lili Zhu, Juan Yao, and Dagang Jiang. 2026. "Rapid Artificial Infestation Method for Assessing Fall Armyworm (Spodoptera frugiperda) Damage on Maize" Insects 17, no. 2: 136. https://doi.org/10.3390/insects17020136
APA StyleWu, C., Chen, W., Guo, X., He, G., Yang, G., Zhu, L., Yao, J., & Jiang, D. (2026). Rapid Artificial Infestation Method for Assessing Fall Armyworm (Spodoptera frugiperda) Damage on Maize. Insects, 17(2), 136. https://doi.org/10.3390/insects17020136






