Relative Effectiveness of Amorphous Silica, Malathion, and Pirimiphos Methyl in Controlling Sitophilus oryzae and Tribolium castaneum and Their Long-Term Effects on Stored Wheat Under Laboratory Conditions
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Insects and Cultures
2.1.2. Chemicals
2.1.3. Insecticides
2.2. Methods
2.2.1. Insecticides Formulated on Dusts
2.2.2. Admixing Dusts with Grain
2.2.3. Method of Bioassays
2.2.4. Adsorption Isotherm of Silica Used for Bioassays
2.2.5. Residual Efficacy of Pirimiphos Methyl, Malathion, and Amorphous Silica Against T. castaneum and S. oryzae
2.3. Statistical Analysis
3. Results and Discussion
3.1. Physical Properties of Different Dusts and Their Effects on S. oryzae
3.2. Effect of Different Dusts on S. oryzae at 12% Grain Moisture Content and 25 °C
3.3. Effect of Different Dusts on S. oryzae at 15% Grain Moisture Content and 25 °C
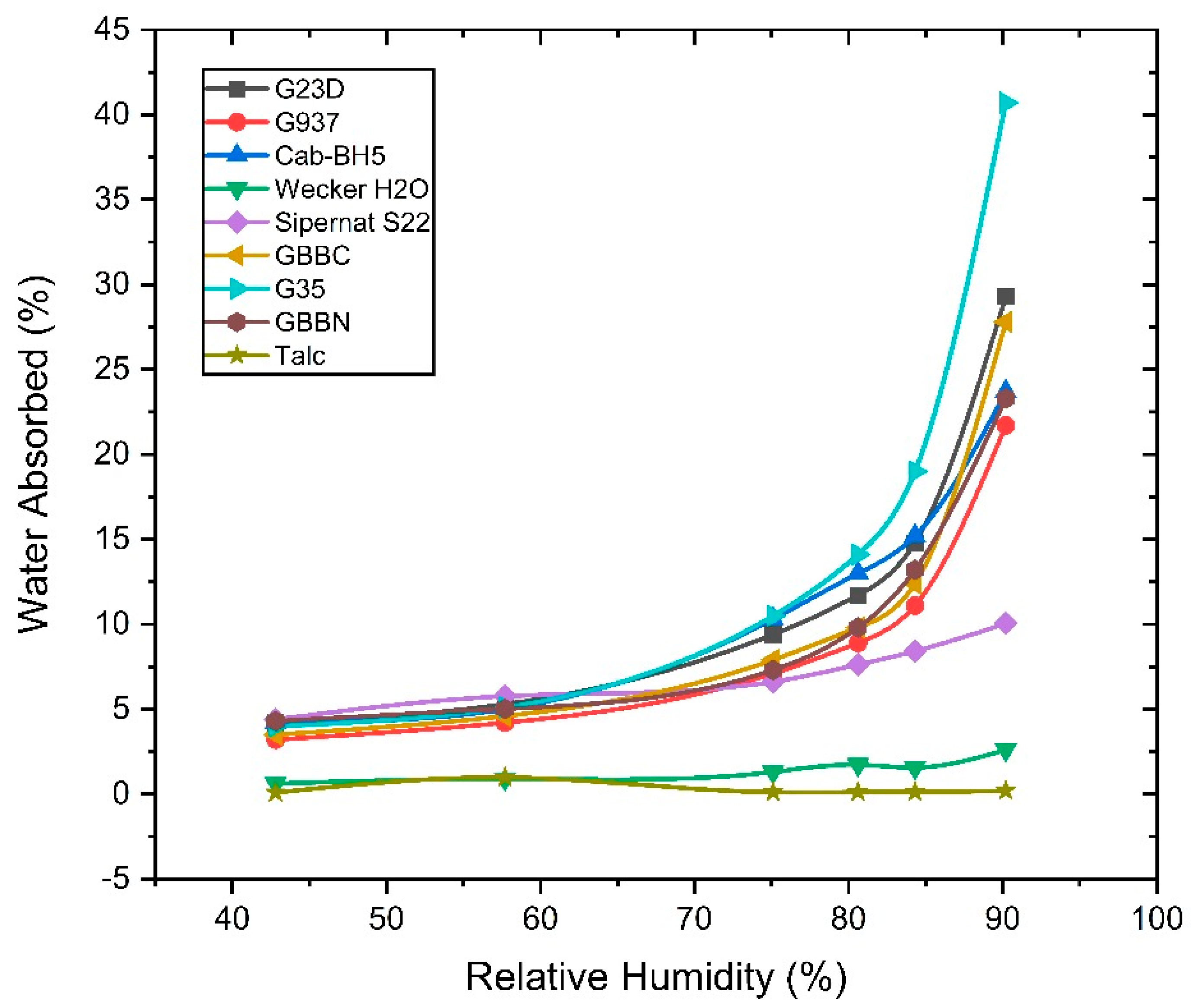
3.4. Water Adsorption Isotherm of Various Amorphous Silica Used for Bioassays
3.5. Effects of Sipernat 22 on Sitophilus oryzae and Tribolium castaneum at Two Grain Moisture Contents and 25 °C
3.6. Joint Action of Insecticides and Silica on T. castaneum and S. oryzae
3.7. Residual Efficacy of Pirimiphos Methyl, Malathion, and Amorphous Silica Against T. castaneum and S. oryzae
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A.O.A.C | Association of Official Analytical Chemists |
| FAO | Food and Agriculture Organization |
| IPM | Integrated pest management |
References
- Food and Agriculture Organization [FAO]. FAOSTAT; FAO: Atltanta, GA, USA, 2023. [Google Scholar]
- Kahraman, R.; Kahraman, A. Sustainable agricultural production: Perspective on wheat and dry bean–world and turkey. In Advanced Strategies for Agriculture—II; Eren, A., Ed.; IKSAD Publishing House: Ankara, Türkiye, 2023; pp. 3–42. [Google Scholar]
- Abdalla, A.; Stellmacher, T.; Becker, M. Trends and prospects of change in wheat self-sufficiency in Egypt. Agriculture 2022, 13, 7. [Google Scholar] [CrossRef]
- Tadesse, M. Post-harvest loss of stored grain, its causes and reduction strategies. Food Sci. Qual. Manag. 2020, 96, 26–35. [Google Scholar]
- Srivastava, C.; Subramanian, S. Storage insect pests and their damage symptoms: An overview. Indian J. Entomol. 2016, 78, 53–58. [Google Scholar] [CrossRef]
- Alnaji, L.K. Effectiveness of Methyl Phoxim and Malathion on Stored Grain Insects and the Fate of the Insecticide Residues on the Properties of the Grain Products. Master’s Thesis, Kansas State University, Manhattan, KS, USA, 1978. [Google Scholar]
- Baliota, G.V.; Lampiri, E.; Batzogianni, E.N.; Athanassiou, C.G. Insecticidal effect of four insecticides for the control of different populations of three stored-product beetle species. Insects 2022, 13, 325. [Google Scholar] [CrossRef] [PubMed]
- Rumbos, C.I.; Dutton, A.C.; Tsiropoulos, N.G.; Athanassiou, C.G. Persistence and residual toxicity of two pirimiphos-methyl formulations on wheat against three stored-product pests. J. Stored Prod. Res. 2018, 76, 14–21. [Google Scholar] [CrossRef]
- Pathak, V.M.; Verma, V.K.; Rawat, B.S.; Kaur, B.; Babu, N.; Sharma, A.; Dewali, S.; Yadav, M.; Kumari, R.; Singh, S. Current status of pesticide effects on environment, human health and it’s eco-friendly management as bioremediation: A comprehensive review. Front. Microbiol. 2022, 13, 962619. [Google Scholar] [CrossRef]
- Shawir, M.; le Patourel, G.N.J.; Moustafa, F.I. Amorphous silica as an additive to dust formulations of insecticides for stored grain pest control. J. Stored Prod. Res. 1988, 24, 123–130. [Google Scholar] [CrossRef]
- Agarwal, M. Contact Insecticides: Chemicals, Diatomaceous Earth, and Amorphous Silica for Stored-Grain Protection. In Control and Management of Pests in Stored Products; Jayas, D.S., Ed.; CRC Press: Boca Raton, FL, USA, 2024; pp. 459–491. [Google Scholar]
- Gentz, M.C.; Murdoch, G.; King, G.F. Tandem use of selective insecticides and natural enemies for effective, reduced-risk pest management. Biol. Control 2010, 52, 208–215. [Google Scholar] [CrossRef]
- Oxley, T.; Pixton, S. Determination of moisture content in cereals. II.—Errors in the determination by oven drying of known changes in moisture content. J. Sci. Food Agric. 1960, 11, 315–319. [Google Scholar] [CrossRef]
- le Patourel, G.N.J. The effect of grain moisture content on the toxicity of a sorptive silica dust to four species of grain beetle. J. Stored Prod. Res. 1986, 22, 63–69. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis, 3rd ed.; Cambridge University Press: London, UK, 1971. [Google Scholar]
- Young, J.F. Humidity control in the laboratory using salt solutions—A review. J. Appl. Chem. 1967, 17, 241–245. [Google Scholar] [CrossRef]
- Kirkpatrick, L.A.; Feeney, B.C. A Simple Guide to IBM SPSS: For Version 23.0; Delmar Learning: Boston, MA, USA, 2015. [Google Scholar]
- Ebeling, W. Sorptive dusts for pest control. Annu. Rev. Entomol. 1971, 16, 123–158. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ning, C.; Pan, T.; Cai, K. Role of silica nanoparticles in abiotic and biotic stress tolerance in plants: A review. Int. J. Mol. Sci. 2022, 23, 1947. [Google Scholar] [CrossRef] [PubMed]
- Zing Zing, B.; Messi Ambassa, L.M.; Ehabe Ejolle, E.; Belibi Belibi, D.P.; Kede, C.M. Protection of Stored Common Bean and Maize Grains Using Optimally Synthesized Biosilica from Rice Husk Ash. J. Food Biochem. 2024, 2024, 3741615. [Google Scholar] [CrossRef]
- Superfine, K. Effect of grain moisture content and storage time on efficacy of inert and botanical dusts for the control of Sitophilus zeamais in stored maize. J. Stored Prod. Postharvest Res. 2012, 3, 145–151. [Google Scholar] [CrossRef]
- El-Sayed, F.; El-Zun, H.; Abd El-latif, A.; Nasr, M.E. Insecticidal effect of some inert dusts against three of stored grain insects at Kafr El-Sheikh Governorate. J. Plant Prot. Pathol. 2010, 1, 959–972. [Google Scholar] [CrossRef]
- Batistič, L.; Bohinc, T.; Novljan, M.; Indihar, E.; Košir, I.J.; Šilc, U.; Horvat, A.; Trdan, S. Cabbage pest control with local inert dusts: Do diatomaceous earth and wood ash have the highest potential? Acta Agric. Scand. B Soil Plant Sci. 2025, 75, 2466448. [Google Scholar] [CrossRef]
- Ng, E.-P.; Mintova, S. Nanoporous materials with enhanced hydrophilicity and high water sorption capacity. Microporous Mesoporous Mater. 2008, 114, 1–26. [Google Scholar] [CrossRef]
- Chen, Z.; He, X.; Xiao, C.; Kim, S.H. Effect of humidity on friction and wear—A critical review. Lubricants 2018, 6, 74. [Google Scholar] [CrossRef]
- Dobrijevic, D. Decoupling the Effects of Atmospheric Humidity and Soil Moisture on Cereal Physiology. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 2021. [Google Scholar]
- Stejskal, V.; Vendl, T.; Aulicky, R.; Athanassiou, C. Synthetic and natural insecticides: Gas, liquid, gel and solid formulations for stored-product and food-industry pest control. Insects 2021, 12, 590. [Google Scholar] [CrossRef]
- Du, X. The Physiochemical Responses of Stored Grain Insect Pests to Synthetic Amorphous Silica (SAS) Powders. Ph.D. Thesis, Murdoch University, Murdoch, WA, Australia, 2021. [Google Scholar]
- Rimola, A.; Costa, D.; Sodupe, M.; Lambert, J.-F.; Ugliengo, P. Silica Surface Features and Their Role in the Adsorption of Biomolecules: Computational Modeling and Experiments. Chem. Rev. 2013, 113, 4216–4313. [Google Scholar] [CrossRef]
- Zornoza-Indart, A.; Lopez-Arce, P. Silica nanoparticles (SiO2): Influence of relative humidity in stone consolidation. J. Cult. Herit. 2016, 18, 258–270. [Google Scholar] [CrossRef]
- Abd El-Aziz, S.E. Control strategies of stored product pests. J. Entomol. 2011, 8, 101–122. [Google Scholar] [CrossRef]
- Meharg, C.; Meharg, A.A. Silicon, the silver bullet for mitigating biotic and abiotic stress, and improving grain quality, in rice? Environ. Exp. Bot. 2015, 120, 8–17. [Google Scholar] [CrossRef]
- Claverie, M.; Dumas, A.; Carême, C.; Poirier, M.; Le Roux, C.; Micoud, P.; Martin, F.; Aymonier, C. Synthetic talc and talc-like structures: Preparation, features and applications. Chem. A Eur. J. 2018, 24, 519–542. [Google Scholar] [CrossRef]
- Ziaee, M.; Ebadollahi, A.; Wakil, W. Integrating inert dusts with other technologies in stored products protection. Toxin Rev. 2021, 40, 404–419. [Google Scholar] [CrossRef]
- Abo-Elghar, G.E.; El-Sheikh, A.E.; El-Sayed, F.M.; El-Maghraby, H.M.; El-Zun, H.M. Persistence and residual activity of an organophosphate, pirimiphos-methyl, and three IGRs, hexaflumuron, teflubenzuron and pyriproxyfen, against the cowpea weevil, Callosobruchus maculatus (Coleoptera: Bruchidae). Pest Manag. Sci. 2004, 60, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Foong, S.Y.; Ma, N.L.; Lam, S.S.; Peng, W.; Low, F.; Lee, B.H.K.; Alstrup, A.K.O.; Sonne, C. A recent global review of hazardous chlorpyrifos pesticide in fruit and vegetables: Prevalence, remediation and actions needed. J. Hazard. Mater. 2020, 400, 123006. [Google Scholar] [CrossRef]
- Manivannan, S.; Subramanyam, B.; Siliveru, K. Efficacy of two amorphous silica powders applied to soft red winter wheat against the lesser grain borer, Rhyzopertha dominica (F.) (Coleoptera: Bostrichidae). J. Stored Prod. Res. 2024, 106, 102264. [Google Scholar] [CrossRef]
- Twaibu, A. Toxicity of Different Insecticides to Sitophilus oryzae Tribolium castaneum and Tribolium confusum Infesting Corn. Master’s Thesis, University of Arkansas, Fayetteville, AR, USA, 2023. [Google Scholar]
- White, N.D.G.; Jayas, D.S.; Demianyk, C.J. Degradation and biological impact of chlorpyrifos-methyl on stored wheat and pirimiphos-methyl on stored maize in western Canada. J. Stored Prod. Res. 1997, 33, 125–135. [Google Scholar] [CrossRef]
- Gao, N. Management of Indian Meal Moth and Maize Weevil in Stored Popcorn Using Approved Grain Protectants. Master’s Thesis, Kansas State University, Manhattan, KS, USA, 2018. [Google Scholar]
- Li, Y.; Agarwal, M.; Cao, Y.; Ren, Y. Effect of synthetic amorphous silica powder on the cuticle of Tribolium castaneum and Sitophilus oryzae using hyperspectral imaging technique. Pest Manag. Sci. 2020, 76, 314–323. [Google Scholar] [CrossRef] [PubMed]
| Dust Type | Commercial Name | pH 5% Aqueous Suspension | Bulk Density g/100 mL | Specific Surface Area m2/g * | Oil Adsorption Capacity g/100 g ** | Particle Size Range (µm Primary Particle) *** | Supplier |
|---|---|---|---|---|---|---|---|
| Fumed silica | Cab-O-Sil EH5 | 4.2 | 3.7 | 390 | 732 | 0.007 | A |
| Fumed silica | Wacker HDK H20 | 4.5 | 3.5 | 170 | 347 | 0.005 | B |
| Precipitated s. | G35 | 7.0 | 15 | 320 | 200 | 2 | C |
| Precipitated s. | GBBN | 7.0 | 15 | 240 | 200 | 8 | C |
| Precipitated s. | G937 | 7.0 | 18 | 320 | 170 | 4 | C |
| Precipitated s. | GBBC | 7.0 | 16 | 240 | 260 | 2 | C |
| Precipitated s. | G23D | 7.0 | 31 | 850 | 90 | 5 | D |
| Precipitated s. | Sipernat 22 | - | 9 | 190 | 335 | 0.2 | D |
| Minerals | Talc | 7.4 | 96 | NA | 40 | ~30.4 | B |
| Name of Dust | Regression of N.E.D. Response (y) on Log Dose (x) | LC50 (95% Fid. Limits) mg/100 g Wheat | p Value |
|---|---|---|---|
| Cab-O-Sil EH5 | Y = −5.9 ± 3.7 x | 41.0 (36.8, 45.7) | 0.11 |
| Wacker HDK H20 | Y = −6.1 ± 4.8 x | 19.4 (17.2, 21.5) | 0.17 |
| G35 | Y = −3.3 ± 2.1 x | 34.2 (27.5, 43.6) | 0.12 |
| GBBN | Y = −4.9 ± 3.1 x | 37.1 (29.2, 43.7) | 0.09 |
| G937 | Y = −6.9 ± 4.4 x | 34.6 (32.2, 37.3) | 0.09 |
| GBBC | Y = −7.3 ± 4.9 x | 31.3 (27.1, 34.9) | 0.10 |
| G23D | Y = −6.1 ± 3.8 x | 38.3 (35.2, 41.6) | 0.51 |
| Sipernat S22 | Y = −9.9 ± 5.9 x | 46.6 (44.1, 49.2) | 0.45 |
| Talc | — | — | — |
| Name of Dust | Regression of N.R.D. Response (y) on Log Dose(x) | LC50 (95% Fid. Limits) (mg/100 g Wheat) | p Value |
|---|---|---|---|
| Cab-O-Sil EH5 | Y = −3.0 + 1.6 x | 75.5 (62.1, 91.8) | 0.09 |
| Wacker HDK H20 | Y = −5.8 + 3.5 x | 47.1 (40.5, 54.7) | 0.19 |
| G35 | Y = −4.0 + 2.4 x | 45.8 (38.4, 53.4) | 0.64 |
| GBBN | Y = −5.4 + 2.9 x | 70.2 (59.8, 82.5) | 0.07 |
| G937 | Y = −6.6 + 3.5 x | 73.1 (66.7, 80.1) | 0.77 |
| GBBC | Y = −7.7 + 4.0 x | 82.5 (75.7, 89.8) | 0.76 |
| G23D | Y = −3.7 + 2.2 x | 46.7 (21.0, 75.2) | 0.01 |
| Sipernat S22 | Y = −8.0 + 4.3 x | 73.1 (68.1, 79.7) | 0.94 |
| Talc | -- | -- | -- |
| Grain Moisture Content (%) | Name of Insect | Regression of N.B.D. Response (y) on Log Dose (x) | LC50 (95% Fid. Limits) mg/100 g Wheat | p Value |
|---|---|---|---|---|
| 12 | T. castaneum | Y = −7.5 ± 6.8 x | 12.5 (11.5, 13.7) | 0.00 |
| S. oryzae | Y = −9.9 ± 4.9 x | 46.6 (44.1, 49.2) | 0.45 | |
| 15 | T. castaneum | Y = −8.7 ± 7.1 x | 16.8 (16.1, 17.6) | 0.54 |
| S. oryzae | Y = −7.8 ± 4.2 x | 68.7 (63.1, 74.7) | 0.11 |
| Name of Insect | Formulation | Regression of N.B.D. Response (y) on Log Dose (x) | LC50 (95% Fid. Limits) µg a.i./100 g Wheat | p Value |
|---|---|---|---|---|
| T. castaneum | Malathion on talc (4%) | Y = −0.8 ± 7.4 x | 52.3 (45.2, 59.4) | 0.29 |
| Malathion on silica (0.2%) | Y = −10.2 ± 9.8 x | 21.5 (20.3, 22.6) | 0.45 | |
| Pirimiphos methyl on talc (2%) | Y = −0.0 ± 13.8 x | 20.1 (18.9, 21.9) | 0.22 | |
| Pirimiphos methyl on silica (0.1%) | Y = −6.3 ± 5.7 x | 13.4 (12.2, 14.3) | 0.45 | |
| S. oryzae | Malathion on talc (4%) | Y = −2.9 ± 9.3 x | 84.7 (80.5, 88.2) | 0.23 |
| Malathion on silica (0.2%) | Y = −3.6 ± 3.4x | 23.3(21.4, 25.5) | 0.20 | |
| Pirimiphos methyl on talc (2%) | Y = −1.3 ± 5.7 x | 32.1 (30.2, 34.7) | 0.35 | |
| Pirimiphos methyl on silica (0.1%) | Y = −4.1 ± 3.4 x | 15.5 (14.3, 16.6) | 0.22 |
| Grain Moisture Content | Formulation | Mean Percent of Kill at Time Intervals from Application (Week) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 WK | 6 WK | 10 WK | 12 WK | 14 WK | 17 WK | 19 WK | 22 WK | 25 WK | ||
| 12% | Malathion (8 ppm) on talc | 100 a ± 0.0 | 100 a ± 0.0 | 94.7 b ± 2.1 | 66 b ± 1.21 | 16.3 b ± 1.09 | 11.3 b ± 1.13 | 10.7 b ± 0.51 | 7.3 b ± 0.45 | 0.0 b ± 0.0 |
| Pirimiphos methyl (4 ppm) on talc | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | |
| Malathion on silica | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | |
| Pirimiphos methyl on silica | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | |
| Silica (0.47 g/kg) | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | |
| LSD 5% | - | - | 1.777 | 0.985 | 0.918 | 0.951 | 0.429 | 0.379 | - | |
| 15% | Malathion (8 ppm) | 100 a ± 0.0 | 100 a ± 0.0 | 9.3 c ± 0.44 | 10.7 c ± 0.57 | 13.3 d ± 0.22 | 1.3 d ± 0.06 | 0.7 d ± 0.05 | 0.0 d ± 0.0 | 0.0 d ± 0.0 |
| Pirimiphos methyl (4 ppm) | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | 94.7 b ± 2.41 | 92.7 b ± 4.92 | 92 b ± 5.21 | |
| Malathion on silica | 100 a ± 0.0 | 100 a ± 0.0 | 95.3 a ± 3.71 | 100 a ± 0.0 | 94 b ± 3.31 | 94 b ± 3.83 | 94 b ± 4.66 | 96.7 ab ± 1.49 | 96.7 ab ± 1.68 | |
| Pirimiphos methyl on silica | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | |
| Silica (0.47 g/kg) | 39.3 b ± 2.70 | 36.6 b ± 2.10 | 60.7 b ± 5.40 | 53.3 b ± 3.70 | 46 c ± 2.90 | 38 c ± 2.29 | 50 c ± 3.17 | 56.7 c ± 4.36 | 58.6 c ± 4.29 | |
| LSD 5% | 2.273 | 1.768 | 5.529 | 3.152 | 3.710 | 3.758 | 5.162 | 5.676 | 5.856 | |
| Grain Moisture Content | Formulation | Mean Percent of Kill at Time Intervals from Application (Week) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 WK | 6 WK | 10 WK | 12 WK | 14 WK | 17 WK | 19 WK | 22 WK | 25 WK | ||
| 12% | Malathion (8 ppm) | 100 a ± 0.0 | 100 a ± 0.0 | 74.7 b ± 3.33 | 32.7 b ± 1.94 | 16.7 c ± 1.58 | 0.0 e ± 0.0 | 0.0 d ± 0.0 | 0.0 e ± 0.0 | 0.0 e ± 0.0 |
| Pirimiphos methyl (4 ppm) | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | 99.3 a ± 5.67 | 100 a ± 0.0 | 65.3 c ± 3.26 | 63.3 b ± 5.83 | 33.3 d ± 2.75 | 30.7 d ± 1.57 | |
| Malathion on silica | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | 96 a ± 4.49 | 98 a ± 4.21 | 84.7 b ± 3.48 | 57.3 bc ± 2.49 | 70.7 b ± 3.25 | 72.7 b ± 3.19 | |
| Pirimiphos methyl on silica | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | 98.7 a ± 5.92 | 100 a ± 0.0 | 100 a ± 0.0 | 94 a ± 4.37 | 93.3 a ± 6.18 | 92.7 a ± 5.99 | |
| Silica (0.47 g/kg) | 57 b ± 2.48 | 58 b ± 1.94 | 60.7 c ± 3.42 | 53.3 c ± 3.71 | 57.3 b ± 3.48 | 59.3 d ± 3.13 | 56 c ± 2.66 | 59.3 c ± 4.68 | 60.7 c ± 4.19 | |
| LSD 5% | 2.088 | 1.634 | 4.019 | 8.623 | 4.787 | 4.803 | 6.859 | 7.447 | 6.843 | |
| 15% | Malathion (8 ppm) | 100 a ± 0.0 | 64.7 b ± 3.53 | 31.3 b ± 2.47 | 4 d ± 0.30 | 0.0 d ± 0.0 | 0.0 e ± 0.0 | 0.0 d ± 0.0 | 0.0 e ± 0.0 | 0.0 ± 0.0 |
| Pirimiphos methyl (4 ppm) | 100 a ± 0.0 | 100 a ± 0.0 | 98.7 a ± 4.92 | 96.7 a ± 3.60 | 99.3 a ± 4.40 | 27.3 c ± 1.70 | 25.3 b ± 0.60 | 4 d ± 0.79 | 10.6 d ± 1.85 | |
| Malathion on silica | 100 a ± 0.0 | 100 a ± 0.0 | 97.3 a ± 2.99 | 99.3 a ± 4.93 | 82 b ± 3.94 | 46.7 b ± 2.16 | 42 a ± 3.54 | 21.3 b ± 1.27 | 26.7 b ± 2.02 | |
| Pirimiphos methyl on silica | 100 a ± 0.0 | 100 a ± 0.0 | 100 a ± 0.0 | 83.3 b ± 2.50 | 99.3 a ± 4.62 | 55.3 a ± 3.13 | 45.7 a ± 3.93 | 40.7 a ± 2.98 | 47.3 a ± 3.48 | |
| Silica (0.47 g/kg) | 16.7 b ± 2.09 | 15.3 c ± 1.11 | 18 c ± 2.18 | 15.3 c ± 3.09 | 18.7 c ± 1.78 | 16.7 d ± 1.39 | 15.3 c ± 2.44 | 16 c ± 1.73 | 18.7 c ± 2.18 | |
| LSD 5% | 1.757 | 3.116 | 5.588 | 6.134 | 6.489 | 3.693 | 4.936 | 3.167 | 4.154 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfuhaid, N.A.; Shawir, M.S. Relative Effectiveness of Amorphous Silica, Malathion, and Pirimiphos Methyl in Controlling Sitophilus oryzae and Tribolium castaneum and Their Long-Term Effects on Stored Wheat Under Laboratory Conditions. Insects 2025, 16, 981. https://doi.org/10.3390/insects16090981
Alfuhaid NA, Shawir MS. Relative Effectiveness of Amorphous Silica, Malathion, and Pirimiphos Methyl in Controlling Sitophilus oryzae and Tribolium castaneum and Their Long-Term Effects on Stored Wheat Under Laboratory Conditions. Insects. 2025; 16(9):981. https://doi.org/10.3390/insects16090981
Chicago/Turabian StyleAlfuhaid, Nawal Abdulaziz, and Mohamed S. Shawir. 2025. "Relative Effectiveness of Amorphous Silica, Malathion, and Pirimiphos Methyl in Controlling Sitophilus oryzae and Tribolium castaneum and Their Long-Term Effects on Stored Wheat Under Laboratory Conditions" Insects 16, no. 9: 981. https://doi.org/10.3390/insects16090981
APA StyleAlfuhaid, N. A., & Shawir, M. S. (2025). Relative Effectiveness of Amorphous Silica, Malathion, and Pirimiphos Methyl in Controlling Sitophilus oryzae and Tribolium castaneum and Their Long-Term Effects on Stored Wheat Under Laboratory Conditions. Insects, 16(9), 981. https://doi.org/10.3390/insects16090981





