Simple Summary
Episyrphus balteatus is the only one hoverfly species among the top 25 biological control agents by adoption across countries. This study systematically evaluated the predatory functional responses, control efficacy, and oviposition and predatory preferences of E. balteatus against Aphis craccivora Koch, Myzus persicae Sulzer, and Megoura crassicauda Mordvilko under laboratory conditions. The results showed that the best functional response model of both second- and third-instar E. balteatus larvae to these aphid species was the Holling type III model, except for the third instar to A. craccivora, for which the Holling type II model was superior. E. balteatus had good biocontrol efficiency, and the oviposition and predatory preferences of E. balteatus were consistent with preferring M. crassicauda. These results strengthened and optimized the application of E. balteatus as BCAs for these three aphid species.
Abstract
Larvae of Episyrphus balteatus De Geer (Diptera: Syrphidae) are important natural enemies of common agricultural pests such as aphids (Hemiptera: Aphididae). This well-known aphidophagous flower fly is used as a biological control agent. The predatory functional responses, control efficacy, and oviposition and predatory preferences of E. balteatus against Aphis craccivora Koch, Myzus persicae Sulzer, and Megoura crassicauda Mordvilko were systematically determined through controlled laboratory experiments. The best functional response model of both second- and third-instar E. balteatus larvae to these three aphid species was the Holling type III model, except for the third-instar larvae to A. craccivora, for which the Holling type II model was superior. The A. craccivora population decline rates for ratios of 1:500 and 1:1000 were 94.67% and 100.00% on day 12 after inoculation; the M. persicae population decline rates for ratios of 1:2000 and 1:4000 reached 96.67% and 95.42% by day 12, and the M. crassicauda population at a ratio of 1:250 was completely eliminated by day 9, achieving a 100.00% population decline rate. The oviposition and predatory preferences of E. balteatus were consistent, in that it preferred M. crassicauda for oviposition and had a positive predatory preference for this aphid species. These results provide scientific evidence for the biological control strategy of E. balteatus against these aphids.
1. Introduction
Hoverflies (Syrphidae) constitute a major group within the order Diptera, encompassing approximately 200 genera and nearly 6000 species. They exhibit a global distribution and are found on all continents, except some remote islands [1,2]. The family Syrphidae comprises three subfamilies: Syrphinae, Eristalinae, and Microdontinae. Among these, only species within the subfamily Syrphinae (representing approximately one-third of hoverfly diversity) are predatory. Their larvae feed on pests such as whiteflies, thrips, and scale insects, with a particularly significant role in suppressing aphid populations [3]. For instance, a single third-instar larva can consume up to 60 cabbage aphids (Brevicoryne brassicae) per day, with lifetime consumption exceeding 300 aphids [4]. And adult hoverflies preferentially oviposit within aphid colonies [5], ensuring that emerging larvae can promptly regulate aphid numbers [6]. Episyrphus balteatus De Geer (Diptera: Syrphidae), commonly known as the marmalade hoverfly, is a holometabolous insect that undergoes four developmental stages of ontogeny: egg, larva, pupa, and adult [7]. Its distribution ranges from Asia and Europe to Africa and Australia, with a particularly dominant presence in China [8]. The larvae of E. balteatus are predators with a broad diet, feeding on over 100 species of aphids and some young lepidopteran larvae (such as those of Spodoptera frugiperda Smith) worldwide [9,10], performing an effective biological control function against pests [11]. Biological control efficacy studies demonstrate that releasing third-instar E. balteatus larvae against soybean aphids at a predator/prey ratio of 1:150 achieved a pest suppression rate of 59.87% after 72 h [12]. Similarly, releasing newly hatched E. balteatus larvae for aphid control in greenhouses on vegetables and chrysanthemums resulted in aphid population reduction rates exceeding 80% after 72 h [13]. As an important migratory hoverfly species, it can seasonally migrate over long distances, consuming trillions of aphids, due to which it represents the only syrphid species among the 25 most important biological control agents (BCAs) used in augmentative biological control [14], thereby playing a crucial role in maintaining the stability of global agricultural ecosystems [3,15]. Aphids (Hemiptera: Aphididae) are some of the most economically significant agricultural pests globally. They cause direct damage by feeding on the plant phloem and indirect damage by transmitting plant viruses and producing honeydew, which hinders photosynthesis [16]. Aphis craccivora Koch, Myzus persicae Sulzer, and Megoura crassicauda Matsumura are three common aphid species in global agricultural ecosystems within the Hemiptera order and Aphididae family. A. craccivora is an economically important pest that affects legumes (such as peas, cowpeas, and peanuts) worldwide. Notably, A. craccivora can transmit two major plant viruses, bean leaf roll virus and faba bean necrotic yellows virus, which severely impact the quality and yield of legumes [17]. M. crassicauda is another globally significant pest that primarily harms leguminous plants such as broad beans, peas, and soybeans, among which broad beans are the most severely affected [18]. M. persicae, a cosmopolitan pest, has a broad host range covering more than 30 families and over 100 plant species. More critically, it transmits more than 100 plant viruses, such as beet yellows virus and potato viruses [19]. Additionally, the strong reproductive capacity, ecological adaptability, and pesticide resistance of these aphid species have led to increasingly severe damage to crops, posing a great threat to the balance of the ecosystem. In aphid control, the use of sustainable methods such as biological control can help address the environmental risks and the ‘3R’ problems (resistance, resurgence, and residue) associated with reliance on insecticides [20]. Among these methods, utilizing natural enemy insects to enhance biological control services has emerged as a promising alternative.
The natural enemies of aphids include parasitic natural enemies, predatory natural enemies, pathogenic microorganisms, etc. [21]. Predatory natural enemies such as E. balteatus are generalist species, targeting a wide host range, which gives them great potential as BCAs against insect pests compared to specialist species like parasitoids, which exhibit a narrow host range [22]. However, parasitoids still dominate approximately 80% of biocontrol programs due to their lower food requirement and ability to maintain balance at lower host densities [23]. To enhance the use of predators as generalist BCAs, a deeper understanding of their predatory capabilities, control efficacy, and preferences for prey is one of the approaches that can promote their use in applied biological control practices.
This study aims to systematically investigate the predatory ability, control efficacy, and oviposition and predatory preferences of E. balteatus against A. craccivora, M. persicae, and M. crassicauda through controlled laboratory experiments. The specific objectives include (1) evaluating the predatory functional response of E. balteatus toward the three aphid species; (2) assessing the control efficacy of E. balteatus against the three aphid species under caged conditions; and (3) exploring its oviposition and predatory preferences for aphids. Through this research, we hope to strengthen and optimize the application of E. balteatus to these three aphid species as a BCA.
2. Materials and Methods
2.1. Test Aphid and Hoverfly
Aphids (A. craccivora, M. persicae, and M. crassicauda) and E. balteatus were collected from an experimental field at the Langfang Experimental Station, Chinese Academy of Agricultural Sciences (Langfang, China; 39°30′29′′ N, 116°36′8′′ E). They were reared in a greenhouse at 23 ± 1 °C, 50 ± 5% RH, and 16:8 (L:D) h. The three aphid species were reared on broad bean plantlets. The broad bean plantlets were planted in plastic pots (12 cm upper diameter × 9 cm lower diameter × 10 cm height) filled with a substrate of nutrient soil and vermiculite. The captured E. balteatus adults (17 females, 20 males) were placed separately in nylon mesh cages (80 cm × 80 cm × 80 cm). Inside the cages, they were provided with a mixture of pollen (rape/corn = 3:1) and 10% v/v honey solution for feeding, as well as broad bean plantlets infested with aphids for oviposition. The honey solution was replaced once a day, and the pollen and broad bean plantlets were replaced every four days. The larvae hatched from the eggs were reared on a mixed diet of aphids in plastic containers (50 cm × 40 cm × 15 cm) until they pupated.
2.2. Predatory Functional Response of E. balteatus to Aphids
One E. balteatus larva that was starved for 24 h was introduced into a Petri dish (with small ventilation holes in the lid, 9 cm diameter × 1.5 cm height) that contained broad bean leaves (6 pcs/dish). Mature female aphids of similar body sizes (A. craccivora, M. persicae, or M. crassicauda) were placed into the Petri dishes. And the number of consumed aphids, determined by counting either the aphid exoskeletons or the remaining live aphids over a 24 h period, was recorded. Each treatment of predator–prey combination (a second or third instar E. balteatus larva to each density of each aphid species) was repeated five times. The aphid density settings are shown in Table 1.

Table 1.
Species and density of aphids preyed on by E. balteatus larvae.
2.3. Control Efficacy of E. balteatus to Aphids Under Caged Conditions
One ten-day-old female adult of E. balteatus that was starting to lay eggs was reared in a cage (50 cm × 35 cm × 45 cm, 200-mesh nylon) with broad bean plantlets (3 pots per cage, 20 plants per pot) infested with aphids (A. craccivora, M. persicae, or M. crassicauda), mixture of pollen (rape/corn = 3:1), and 10% v/v honey water. The initial aphid density was set at five gradients (Table 2); each treatment was repeated 3 times. The number of aphids and larvae in the cages was recorded on the 3rd, 6th, 9th, and 12th days, and the aphid population decline rate was calculated using the following equation: Population decline rate (%) = [(No. of pre-treatment aphid population − No. of post-treatment aphid population)/No. of pre-treatment aphid population] × 100.

Table 2.
The initial E. balteatus/aphid release ratios.
2.4. Preference of E. balteatus for A. craccivora, M. persicae, and M. crassicauda
2.4.1. Oviposition Preference
Ten-day-old adult E. balteatus individuals (5 females and 5 males) that had already begun laying eggs were placed in a nylon mesh cage (80 cm × 80 cm × 80 cm). Three pots of broad bean plantlets, each infested with A. craccivora, M. persicae, or M. crassicauda (200 aphids per plantlet, 5 plantlets per pot), were arranged at equal intervals along the diagonal of the cage for oviposition. Additionally, a mixture of pollen (rape/corn = 3:1) and 10% v/v honey water was provided for feeding. The number of E. balteatus eggs on the broad bean plantlets with different aphid species was recorded daily. The experiment was repeated five times. The oviposition preference rate (%) = (number of eggs on a type of aphid plantlet/total number of eggs) × 100.
2.4.2. Predatory Preference
The method was the same as described in Section 2.2. Adult aphids (A. craccivora:M. persicae:M. crassicauda = 1:1:1) were attached to the Petri dish; for 2nd-instar larvae, 10 individuals of each aphid species were added, and for 3rd-instar larvae, 30 individuals of each aphid species were added. After 24 h, the number of each aphid species consumed was recorded. Each treatment was repeated three times. The predatory preference (Ci) is calculated using the following equation [24]:
where Fi represents the proportion of the i-th prey in the environment (the Fi value is constant at 1/3 in this experiment), and Qi represents the proportion of the i-th prey consumed by the predator. When Ci = 0, it indicates that the predator has no preference for the i-th prey species; when 0 < Ci < 1, it indicates a positive preference for the i-th prey species; and when −1 < Ci < 0, it indicates a negative preference for the i-th prey species.
Ci = (Qi − Fi)/(Qi + Fi),
2.5. Statistical Methodology
According to Okuyama and Ruyle [25], different functional response models can be described in general form using the equations in Table 3.

Table 3.
Different functional response models.
3. Results
3.1. Predatory Functional Response of E. balteatus to Aphids
The functional response of the second- and third-instar E. balteatus larvae to A. craccivora, M. persicae, and M. crassicauda is depicted in Figure 1. In the case of the second-instar E. balteatus larvae to these three aphid species, the predatory response curves generated by the Holling type II and Beddington–DeAngelis models and by the Holling type III and the θ-sigmoid models closely resemble each other. The third-instar larvae to A. craccivora, however, showed a similar fit between the θ-sigmoid and Beddington–DeAngelis functional response models (Figure 1; Table 4).
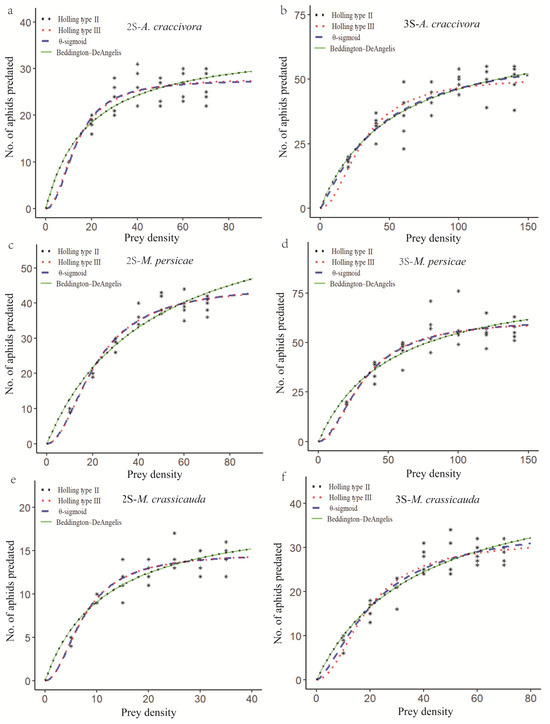
Figure 1.
Functional response of 2nd- or 3rd-instar E. balteatus larvae to different densities of A. craccivora (a,b), M. persicae (c,d), or M. crassicauda (e,f). The symbols (*) in the figure shows the number of aphids predated by E. balteatus larvae.

Table 4.
Parameters for the fits of four functional response models of 2nd- and 3rd-instar E. balteatus larvae to A. craccivora, M. persicae, and M. crassicauda.
The estimated values for the functional response model parameters of the second- and third-instar E. balteatus larvae to A. craccivora, M. persicae, and M. crassicauda are presented in Table 4. According to the model selection method described by Hilborn and Mangel [27], which states that a smaller AIC value indicates a better-fitting model, the best functional response models of both second- and third-instar E. balteatus larvae to these three aphid species were the Holling type III model, except for the third-instar larvae to A. craccivora, for which the Holling type II model was better (Table 4).
3.2. Control Efficacy of E. balteatus to Aphids Under Caged Conditions
For the ratios of 1:500, 1:1000, 1:2000, and 1:4000, the A. craccivora population peaked on day 6 after inoculation, reaching 1553.33, 3033.33, 14,193.33, and 9396.67 individuals, respectively (except for the 1:6000 ratio, which peaked on day 9 at 11,500 individuals). As the larvae emerged and their numbers increased, the A. craccivora population decline rates for ratios of 1:500, 1:1000, 1:2000, 1:4000, and 1:6000 were 94.67%, 100.00%, 77.50%, 35.58%, and 24.72% on day 12, respectively, showing significant differences between the treatment groups (F5,12 = 213.431, p < 0.001) (Figure 2a,b, Table 5).
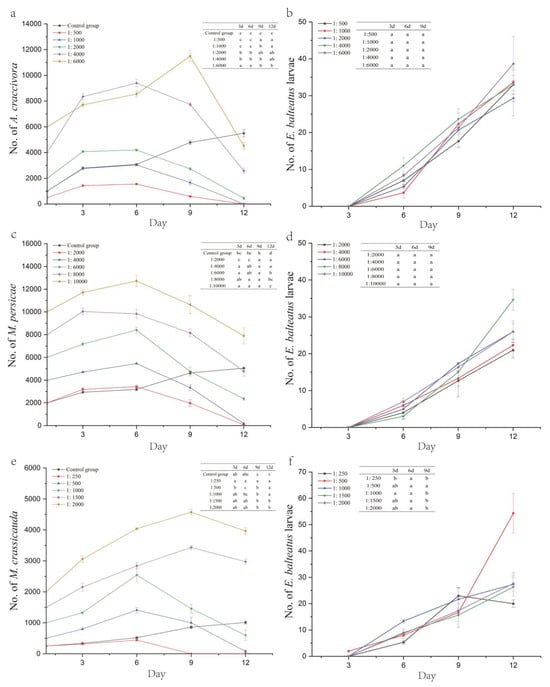
Figure 2.
Number of aphids and E. balteatus larvae under different hoverfly/aphid release ratios (E. balteatus–A. craccivora (a,b), E. balteatus–M. persicae (c,d), and E. balteatus–M. crassicauda (e,f)). Different lowercase letters indicate statistically significant differences in no. of E. balteatus larvae (b,d,f) between treatment groups.

Table 5.
The population decline rate of A. craccivora, M. persicae, and M. crassicauda due to E. balteatus under different hoverfly/aphid ratios.
At hoverfly/aphid ratios of 1:2000, 1:4000, 1:6000, and 1:10,000, the M. persicae population peaked on day 6, reaching 3426.67, 5460, 8400, and 12,723.33 individuals, respectively (except for the 1:8000 ratio, which peaked earlier on day 3 at 10,043.33 individuals). Following larval emergence, the M. persicae population decline rates for ratios of 1:2000, 1:4000, 1:6000, 1:8000, and 1:10,000 reached 96.67%, 95.42%, 60.72%, 40.25%, and 21.10% by day 12, with significant differences between the treatment groups (F5,12 = 384.062, p < 0.001) (Figure 2c,d, Table 5).
At hoverfly/aphid ratios of 1:250, 1:500, and 1:1000, the M. crassicauda population exhibited continuous growth in the early stage, peaking on day 6 at 443.33, 1410, and 2543.33 individuals, respectively. In contrast, populations with ratios of 1:1500 and 1:2000 peaked later, on day 9, reaching 3433.33 and 4573.33 individuals. The aphid population at a ratio of 1:250 was completely eliminated by day 9, achieving a 100.00% population decline rate, a significantly higher efficacy than other ratios (F5,12 = 28,334, p < 0.001). At ratios of 1:500, 1:1000, 1:1500, and 1:2000, the population decline rates by day 12 were 84.67%, 40.33%, −98.22%, and −98.17%, respectively, with significant differences observed (F5,12 = 107.871, p < 0.001) (Figure 2e,f, Table 5).
3.3. Preference of E. balteatus for A. craccivora, M. persicae, and M. crassicauda
3.3.1. Oviposition Preference
There were significant differences in the oviposition preference of E. balteatus adults among the three aphid species (F2,12 = 67.768, p < 0.001) (Figure 3). The oviposition preference rate was ranked as M. crassicauda (51.92%) > A. craccivora (28.32%) > M. persicae (19.76%).
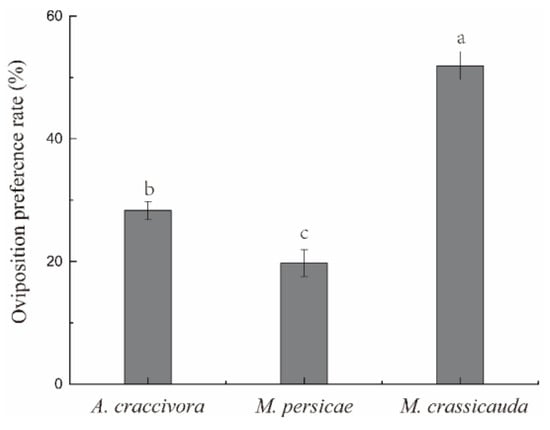
Figure 3.
The oviposition preference rate of E. balteatus adults. Different lowercase letters in the figure reflect significant differences (p < 0.05).
3.3.2. Predatory Preference
There were significant differences in the Ci of second-instar (F2,6 = 29.749, p = 0.001) and third-instar (F2,6 = 297.473, p < 0.001) E. balteatus larvae among the three aphid species (Table 6). The Ci was ranked as A. craccivora (Ci > 0) > M. crassicauda (Ci > 0) > M. persicae (Ci < 0).

Table 6.
The predatory preference of E. balteatus larvae among different aphid species.
4. Discussion
Numerous studies have documented the functional response of the cosmopolitan hoverfly E. balteatus [28,29]. However, data remain scarce regarding its predation on A. craccivora, M. persicae, and M. crassicauda. In this study, E. balteatus larvae demonstrated effective predation on all three aphid species, with third-instar larvae exhibiting greater voracity than second instars (under the highest prey density set in this experiment, the feeding amounts of third-instar larvae on A. craccivora, M. persicae, and M. crassicauda were 49.4, 56, and 28.8, respectively; for the second instar, they were 26, 39.4, and 13.8 (Figure 1)). These findings were also supported by Baskaran et al. [30], who documented the highest predation rates in third-instar larvae of four syrphid species on Aphis gossypii. Similar patterns were observed for Pseudodoros clavatus on Aphis craccivora [31] and for E. balteatus upon Aphis fabae [32]. The high predation of the final instar stage represents a logical consequence of both its larger body size and the additional nutritional demands required for subsequent pupal development. In our study, both second- and third-instar larvae of E. balteatus exhibited a Holling type III functional response to Myzus persicae, while it was Holling type II in Jalilian et al.’s study [33], which is likely attributable to geographic population differences. It is worth noting that the second- and third-instar E. balteatus to A. craccivora exhibited Holling type III and II functional responses, respectively, supporting the view that a single predator species can display variable responses depending on predator size/age and prey species/size [29]. The comparison of the a or h values obtained from different studies conducted and analyzed using a similar approach may be relevant [34]. According to the Holling type II model, the h values obtained for second-instar and third-instar E. balteatus larvae feeding on the three aphid species were consistently higher than those for E. corollae larvae (A. craccivora: L2 = 0.012 h, L3 = 0.007 h; M. persicae: L2 = 0.006 h, L3 = 0.005 h; M. crassicauda: L2 = 0.021 h, L3 = 0.011 h) [35]. Notably, second-instar E. balteatus larvae displayed higher a values on A. craccivora and M. crassicauda than third instars, a pattern consistent with observations by Evelin et al. [36] for Allograpta exotica preying on A. craccivora, where first-instar larvae exhibited higher attack rates than second instars.
The predator/prey release ratio serves as a crucial parameter in biological control applications. Our study demonstrated that the control efficacy of E. balteatus on these three aphid species showed a positive correlation with release ratios, consistent with Li et al.’s findings using E. corollae against Aphis gossypii [37]. While higher predator/prey ratios generally enhance control effectiveness under certain conditions, the recommended release ratios for A. craccivora, M. persicae, and M. crassicauda—considering cost-effectiveness—are 1:2000, 1:4000, and 1:500, respectively. However, E. balteatus exhibited significantly reduced or even ceased oviposition during later experimental stages, attributable to increasing aphid population density and aphid-induced wilting of broad bean plants, reflecting the mutual constraints of natural enemies and pest populations [38]. In this experiment, a 1:250 ratio of adult hoverflies to M. crassicauda achieved a 100.00% population decline rate by day 9, though no control effect was observed by day 3. The population decline rate of 1:150 ratio of third-instar E. balteatus larvae to soybean aphids was 59.87% by day 3 in Lan et al.’s study [12]. This demonstrates that adults (producing multiple larvae after about 3 days of egg hatching) offers broader and more effective control than larvae alone, albeit with a slower onset of action. In some scenarios, a combined application of both adults and larvae may be advisable for optimal results. Additionally, planting diverse flowering species or intercropping nectar-rich plants (such as rape, chrysanthemum, fatsia japonica, etc.) [39] near crops can enhance attraction of wild hoverfly adults for more sustainable biological control.
The ‘preference–performance’ hypothesis (also known as optimal oviposition theory) posits that female insects preferentially lay eggs on substrates that maximize larval survival and growth, which has been empirically supported by numerous studies [40,41]. Episyrphus balteatus adults demonstrated a significant oviposition preference for M. crassicauda in this study, and our previous studies showed that the developmental duration of E. balteatus larvae on M. crassicauda was significantly shorter than that on A. craccivora and M. persicae, and the larvae survival rate of E. balteatus on M. crassicauda was the highest [7], which echoes the above ‘preference–performance’ hypothesis.
The predatory preferences of hoverflies can significantly influence their effectiveness as BCAs against target pests [42]. Predatory preferences are influenced by multiple factors, including prey density, mobility, and the nutritional composition of prey (the primary determinant) [43]. According to the optimal foraging theory, predators generally select prey that best satisfies their survival and reproductive requirements [44]. This study revealed that both second- and third-instar E. balteatus larvae exhibited positive predatory preferences (Ci > 0) for A. craccivora and M. crassicauda under equal prey density conditions. This preference likely stems from the superior energy intake and enhanced developmental benefits provided by these two aphid species, as evidenced by significantly higher life-table parameters (net reproductive rate (R0), intrinsic rate of natural increase (r), and finite rate of increase (λ) compared to those fed on M. persicae) [7]. The oviposition preference and predatory preference (Ci) of E. balteatus for the three aphid species exhibited different ranking orders. This discrepancy can likely be attributed to the larger body size of adult M. crassicauda compared to A. craccivora, which could render them more difficult to prey upon.
Hoverflies are well known in the biological control of invertebrate pests, with studies confirming their efficacy in suppressing pest populations [45]. Currently, the most widely utilized and researched BCAs in commercial applications globally are parasitoid wasps, predatory flower bugs, and lacewings, etc., with only one hoverfly species—E. balteatus—ranking among the top 25 BCAs by adoption across countries [14]. This study systematically evaluates E. balteatus’s biocontrol efficacy against A. craccivora, M. persicae, and M. crassicauda under laboratory conditions, assessing its feasibility as a BCA for these aphid species. The complex and changeable natural environment, interspecific competition, and interference within hoverfly colonies merit attention. We advocate for expanded research on hoverfly biology, anticipating that such efforts could unlock their broader application in conservation and augmentative biological pest control.
5. Conclusions
The results showed that the best functional response model of both second- and third-instar E. balteatus larvae to these aphid species was the Holling type III model, except for the response of the third instar to A. craccivora, for which the Holling type II model was best. E. balteatus had good biocontrol efficiency, and the oviposition and predatory preferences of E. balteatus were consistent, with a preference for M. crassicauda. These results strengthen and optimize the application of E. balteatus as BCAs for these three aphid species.
Author Contributions
Conceptualization, S.J., H.L. and K.W.; methodology, S.J., H.L. and K.W.; software, S.J. and H.L.; validation, H.L. and K.W.; resources, K.W.; writing—original draft preparation, S.J.; writing, all authors; visualization, all authors; supervision, K.W.; project administration, K.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Academician Workstation of the Agricultural High-Tech Industrial Area of the Yellow River Delta, National Center of Technology Innovation for Comprehensive Utilization of Saline-Alkali Land (2022SZX13).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We are very grateful to Haoyu Tang, Xinhang Wang, and Shijiao Chu for their help with data collation.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BCAs | biological control agents |
| AIC | Akaike information criterion |
References
- Mengual, X.; Ståhls, G.; Rojo, S. Molecular phylogeny of Allograpta (Diptera, Syrphidae) reveals diversity of lineages and non-monophyly of phytophagous taxa. Mol. Phylogenet. Evol. 2008, 49, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Mengual, X.; Ståhls, G.; Rojo, S. Phylogenetic relationships and taxonomic ranking of pipizine flower flies (Diptera: Syrphidae) with implications for the evolution of aphidophagy. Cladistics 2015, 31, 491–508. [Google Scholar] [CrossRef]
- Wotton, K.R.; Gao, B.; Menz, M.H.M.; Morris, R.K.A.; Ball, S.G.; Lim, K.S.; Reynolds, D.R.; Hu, G.; Chapman, J.W. Mass seasonal migrations of hoverflies provide extensive pollination and crop protection services. Curr. Biol. 2019, 29, 2167–2173. [Google Scholar] [CrossRef]
- Li, X.; Luo, Y. Study on the predation effect of Eupeodes corollae Fabricius on three species of aphids. J. Yunnan Agric. Univ. 2001, 2, 102–104+110. [Google Scholar] [CrossRef]
- Eiko, K.; Mitsuhiro, S. Assessment of the maple aphid colony by the hover fly, Episyrphus balteatus (de Geer) (Diptera: Syrphidae) I. J. Ethol. 1986, 4, 121–127. [Google Scholar] [CrossRef]
- Michaud, J.; Belliure, B. Impact of syrphid predation on production of migrants in colonies of the brown citrus aphid, Toxoptera citricida (Homoptera: Aphididae). Biol. Control 2001, 21, 91–95. [Google Scholar] [CrossRef]
- Jiang, S.; Li, H.; He, L.; Wu, K. Population fitness of the hoverfly, Episyrphus balteatus (De Geer) (Diptera: Syrphidae) fed on different aphid species. Chin. J. Biol. Control 2023, 39, 254–263. [Google Scholar]
- Yang, Y.; Wang, H.; Wang, Q.; Cao, L.; Liang, D.; Zhang, Z. Control aphids in vegetable fields with Syrphid flies. Chin. J. Biol. Control 2002, 3, 124–127. [Google Scholar]
- Li, H.; Wu, K. Bidirectional predation between larvae of the hoverfly Episyrphus balteatus (Diptera: Syrphidae) and the fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Econ. Entomol. 2022, 115, 545–555. [Google Scholar] [CrossRef]
- Brigitie, T. Estimating predatory efficiency of Episyrphus balteatus (Diptera: Syrphidae) in cereal fields. Environ. Entomol. 1995, 24, 687–691. [Google Scholar]
- Luo, Y.; Li, X. Predatory functional response of Episyrphus balteatus to Myzus persicae. J. Yunnan Agric. Univ. 2000, 109–111. [Google Scholar]
- Lan, X.; Luo, J.; Cheng, X. Analysis of predatory hoverfly species and their damage control effects on soybean aphids in the northeast soybean ecological region. J. Appl. Entomol. 2011, 48, 1625–1630. [Google Scholar]
- Sun, Y.; Wu, K.; Zhang, Y.; Lu, Y.; Yu, H. Episyrphus balteaus (De Geer) and Metasyrphus corollae (Fabricius) attractants. CN103355293B, 18 March 2015. [Google Scholar]
- Cock, M.J.W.; Lenteren, V.; Brodeur, J.C.; Barratt, J.; Bigler, B.I.P.; Bolckmans, F.; Cônsoli, K.; Haas, F.L.; Mason, F.; Parra, P.G.; et al. Do new access and benefit sharing procedures under the convention on biological diversity threaten the future of biological control? BioControl 2010, 55, 199–218. [Google Scholar] [CrossRef]
- Clem, C.S.; Hobson, K.A.; Harmon, T.; Alexandra, N. Insights into natal origins of migratory Nearctic hoverflies (Diptera: Syrphidae): New evidence from stable isotope (δ2H) assignment analyses. Ecography 2022, 2023, 06465. [Google Scholar]
- Dedryver, C.A.; Ralec, A.L.; Fabre, F. The conflicting relationships between aphids and men: A review of aphid damage and control strategies. Comptes Rendus Biol. 2010, 333, 539–553. [Google Scholar] [CrossRef]
- Li, X.; Ju, Q.; Zhao, Z.; Xie, H.; Wang, J.; Qu, M. Control effects and safety assessment of four insecticideson Aphis craccivora. Shandong Agric. Sci. 2013, 45, 93–95. [Google Scholar]
- Takemura, M.; Nishida, R.; Mori, N.; Kuwahara, Y. Acylated flavonol glycosides as probing stimulants of a bean aphid, Megoura crassicauda, from Vicia angustifolia. Phytochemistry 2002, 61, 135–140. [Google Scholar] [CrossRef]
- Emden, H.F.v.; Eastop, V.F.; Hughes, R.D.; Way, M.J. The ecology of myzus persicae. Annu. Rev. Entomol. 1969, 14, 197–270. [Google Scholar] [CrossRef]
- Barratt, B.I.P.; Moran, V.C.; Bigler, F.; Lenteren, J.C.V. The status of biological control and recommendations for improving uptake for the future. BioControl 2018, 63, 155–167. [Google Scholar] [CrossRef]
- Pan, M.; Zhang, Y.; Cao, H.; Wang, X.; Liu, T. Research progress, application and prospect of biological control of aphids in major crops in China. J. Plant Prot. 2022, 49, 146–172. [Google Scholar]
- Stiling, P.; Cornelissen, T. What makes a successful biocontrol agent? A meta-analysis of biological control agent performance. Biol. Control 2005, 34, 236–246. [Google Scholar] [CrossRef]
- Singh, R.; Singh, G. Aphids and their biocontrol. In Ecofriendly Pest Management for Food Security; Omkar, Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 63–108. [Google Scholar]
- Zhou, J.; Chen, C. Methods for determining the quantity of predator selectivity for prey. Acta Ecol. Sinica. 1987, 50–56. [Google Scholar]
- Okuyama, T.; Ruyle, R.L. Solutions for functional response experiments. Acta Oecologica 2011, 37, 512–516. [Google Scholar] [CrossRef]
- Okuyama, T. On selection of functional response models: Holling’s models and more. BioControl 2013, 58, 293–298. [Google Scholar] [CrossRef]
- Hilborn, R.; Mangel, M. The Ecological Detective: Confronting Models with Data; Princeton University Press: Princeton, NJ, USA, 1997; Volume 3. [Google Scholar]
- Putra, N.S.; Yasuda, H. Effects of prey species and its density on larval performance of two species of hoverfly larvae, Episyrphus balteatus de Geer and Eupeodes corollae Fabricius (Diptera: Syrphidae). Appl. Entomol. Zool. 2006, 41, 389–397. [Google Scholar] [CrossRef]
- Maryam, S.; Hossein, M.; Babak, G. Host plant effect on functional response and consumption rate of Episyrphus balteatus (Diptera: Syrphidae) feeding on different densities of Aphis gossypii (Hemiptera: Aphididae). J. Crop. Prot. 2013, 2, 375–385. [Google Scholar]
- Baskaran, R.; Sasikumar, S.; SRajavel, D.; Suresh, K. Influence of semi-synthetic diet on fecundity of Paragus serratus. Ann. Plant Prot. Sci. 2009, 17, 235–236. [Google Scholar]
- Torrealba, J.; Arcaya, E. Respuesta funcional de la larva de Pseudodoros clavatus (Fabricius, 1794) (Diptera: Syrphidae) al áfido negro del matarratón Aphis craccivora Koch, 1854 (Hemiptera: Aphididae). Entomotropica 2014, 29, 9–16. [Google Scholar]
- Ali, R.A.J.; Hussein, S.N. Responses of Episyrphus balteatus DeGeer (Diptera: Syrphidae) in relation to prey density and predator size. J. Asia-Pac. Entomol. 2014, 17, 207–211. [Google Scholar]
- Jalilian, F.; Fathipour, Y.; Talebi, A.A.; Sedaratian, A. Functional response and mutual interference of Episyrphus balteatus and Scaeva albomaculata (Dip.: Syrphidae) fed on Myzus persicae (Hom.: Aphididae). Appl. Entomol. Phytopathol. 2011, 78, 257–274. [Google Scholar]
- Enkegaard, A.; Brødsgaard, H.F.; Hansen, D.L. Macrolophus caliginosus: Functional response to whiteflies and preference and switching capacity between whiteflies and spider mites. Entomol. Exp. Appl. 2003, 101, 81–88. [Google Scholar] [CrossRef]
- Jiang, S.; Li, H.; Wu, K. Predation and control effect of Eupeodes corollae Fabricius (Diptera: Syrphidae) on leguminous plant aphids. Agronomy 2023, 13, 1739. [Google Scholar] [CrossRef]
- Evelin, A.; Celeste, P.-B.; Ximo, M.; Jacobo, Z.-V.J.; Santos, R. Life table and predation rates of the syrphid fly Allograpta exotica, a control agent of the cowpea aphid Aphis craccivora. Biol. Control 2017, 115, 74–84. [Google Scholar]
- Li, H.; Wyckhuys, K.A.G.; Wu, K. Hoverflies provide pollination and biological pest control in greenhouse-grown horticultural crops. Front. Plant Sci. 2023, 14, 1118388. [Google Scholar] [CrossRef]
- Kelton, D.W.; James, D.H. Temporal dynamics of natural enemy–pest interactions in a changing environment. Biol. Control 2014, 75, 18–27. [Google Scholar]
- Jia, H.; Liu, Y.; Li, X.; Li, H.; Pan, Y.; Hu, C.; Zhou, X.; Wyckhuys, K.A.G.; Wu, K. Windborne migration amplifies insect-mediated pollination services. Elife 2022, 11, e76230. [Google Scholar] [CrossRef]
- John, J. On optimal oviposition behavior in phytophagous insects. Theor. Popul. Biol. 1978, 14, 350–356. [Google Scholar] [CrossRef]
- Bonebrake, T.C.; Boggs, C.L.; McNally, J.M.; Ranganathan, J.; Ehrlich, P.R. Oviposition behavior and offspring performance in herbivorous insects: Consequences of climatic and habitat heterogeneity. Oikos 2010, 119, 927–934. [Google Scholar] [CrossRef]
- Donatti-Ricalde, M.G.; de Carvalho Silva, A.; Ricalde, M.P.; Rouws, J.R.C.; Mayhe-Nunes, A.J.; de Souza Abboud, A.C. Abundance of natural enemies and aphids in okra crops (Abelmoschus esculentus—Malvaceae) diversified with Tithonia rotundifolia (Asteraceae). Biol. Control 2023, 187, 105399. [Google Scholar] [CrossRef]
- Zhang, L.; Li, T.; Qin, Z.; Cao, K.; Gao, Y.; Wang, J.; Ge, Y.; Shi, W. Predation preference and nutritional values of four different aphid species for Orius sauteri (Hemiptera: Anthocoridae). Egypt. J. Biol. Pest Control 2022, 32, 23. [Google Scholar] [CrossRef]
- Pyke, G.H.; Pulliam, H.R.; Charnov, E.L. Optimal foraging:a selective review of theory and tests. Q. Rev. Biol. 2025, 52, 137–154. [Google Scholar] [CrossRef]
- Nelson, E.H.; Hogg, B.N.; Mills, N.J.; Daane, K.M. Syrphid flies suppress lettuce aphids. BioControl 2012, 57, 819–826. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).