Effects of Multi-Generational Rearing on Job’s Tears on the Performance and Host Plant Preference of Spodoptera frugiperda (Lepidoptera: Noctuidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants
2.2. Insects
2.3. Effects of Multi-Generational Rearing on Job’s Tears Leaves on the Performance of FAW
2.4. Effects of Multi-Generational Rearing on Job’s Tears Leaves on the Host Plant Preference of FAW
2.5. Life Table Data Analysis
2.6. Population Projection
2.7. Statistical Analysis
3. Results
3.1. Effects of Multi-Generational Rearing on the Growth and Reproductive Performance of FAW
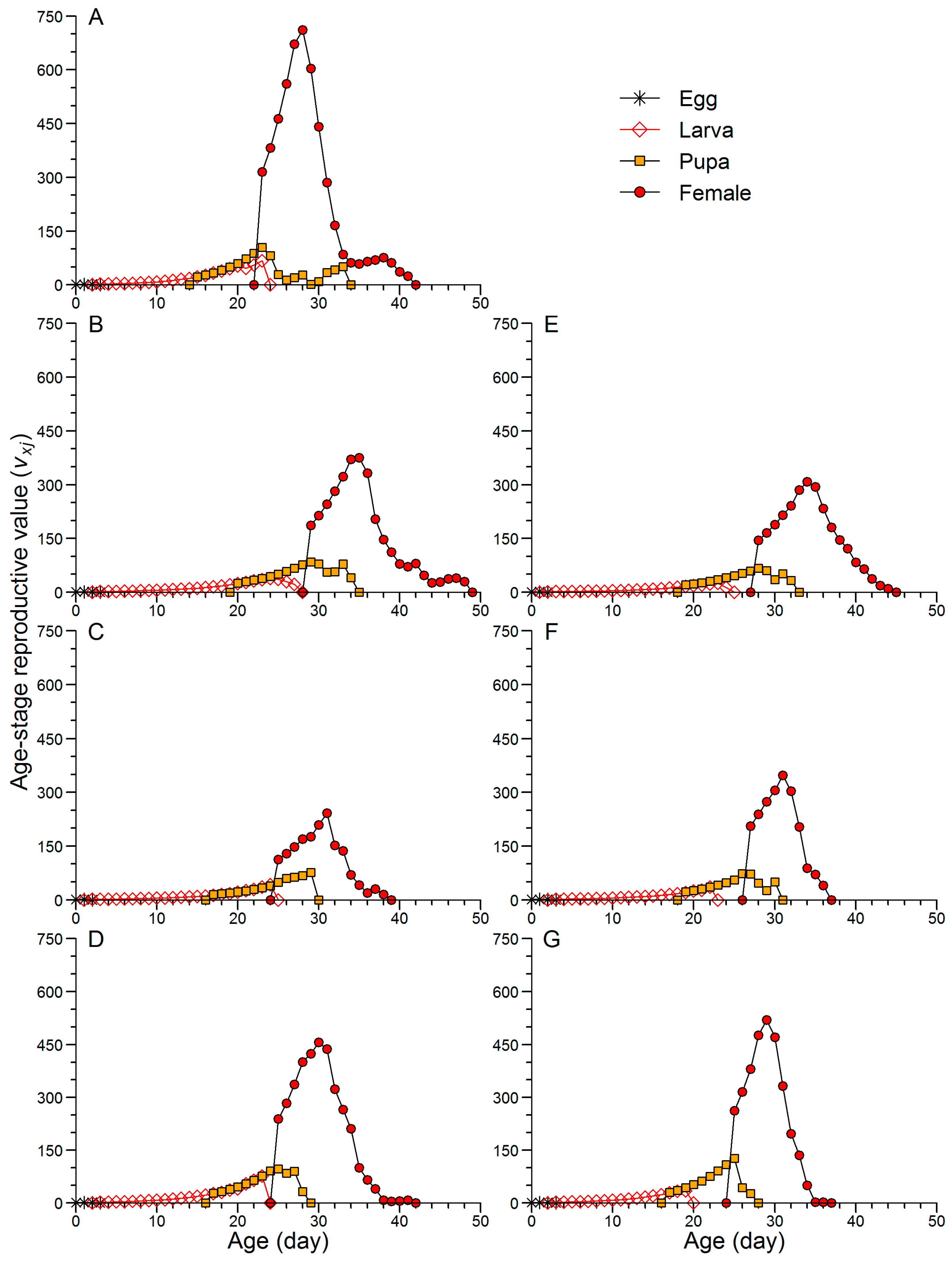
3.2. Effects of Continuous Rearing on the Life Table Performance of FAW
3.3. Effects of Continuous Rearing on the Population Parameters of FAW
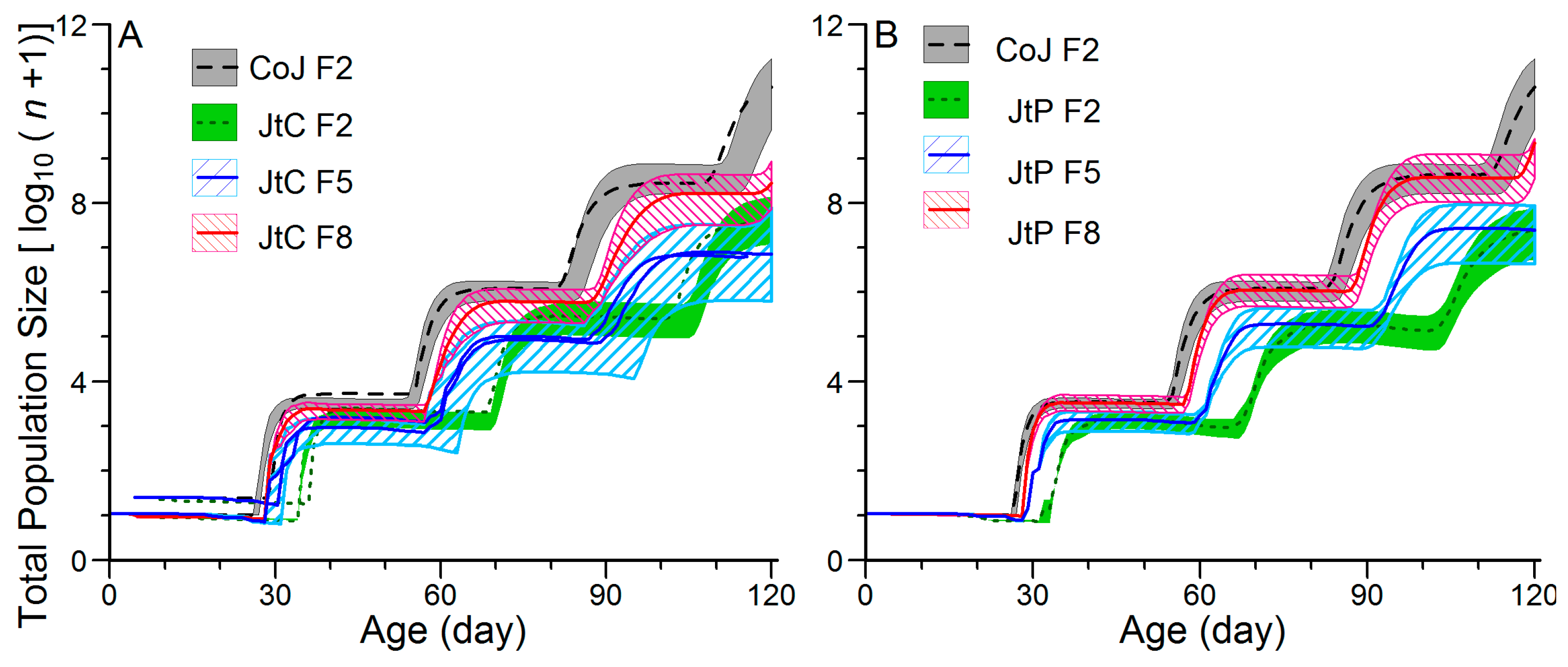
3.4. Effects of Continuous Rearing on the Population Projection of FAW
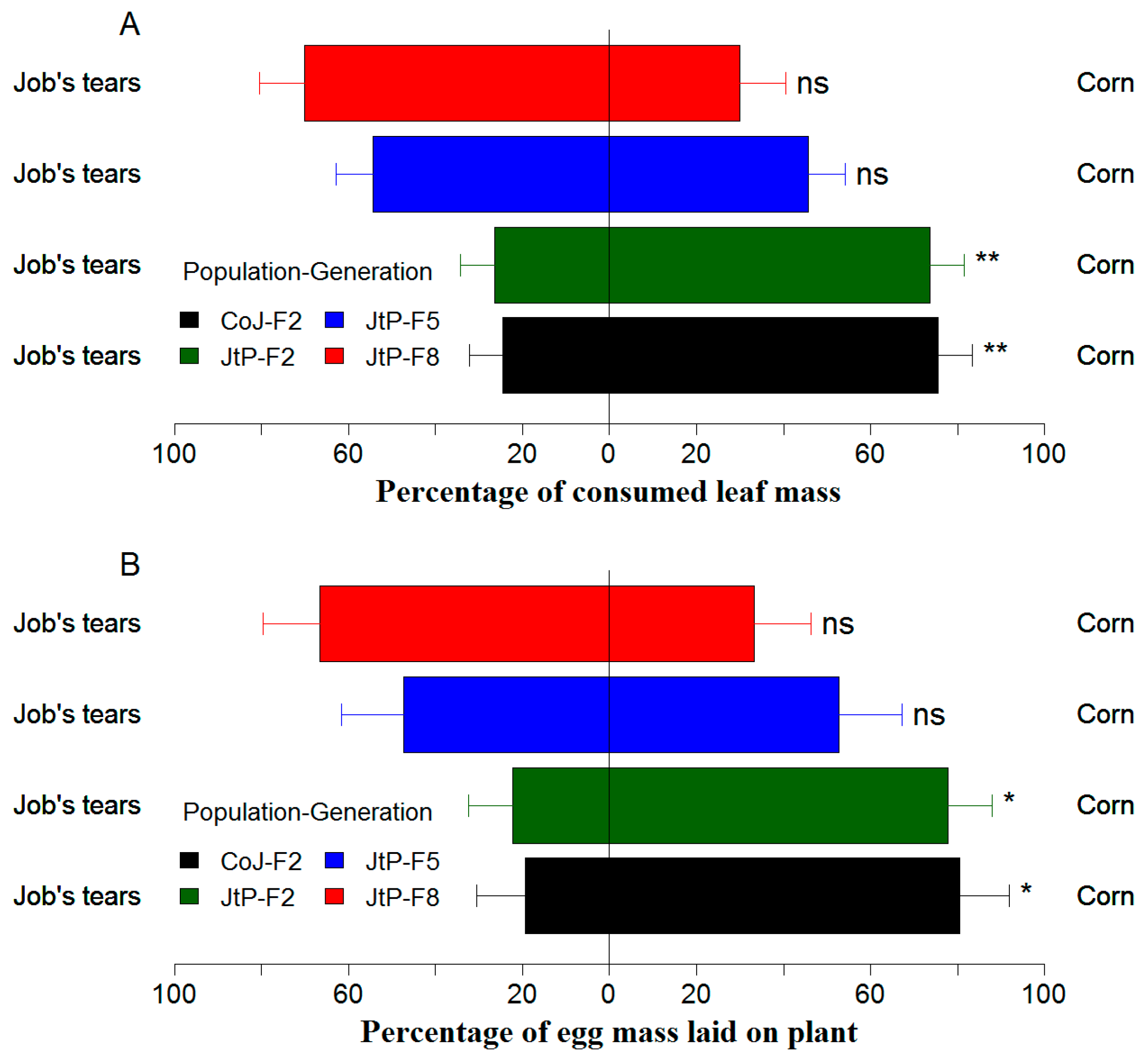
3.5. Effects of Continuous Rearing on Feeding and Oviposition Preference of FAW
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kenis, M.; Benelli, G.; Biondi, A.; Calatayud, P.-A.; Day, R.; Desneux, N.; Harrison, R.D.; Kriticos, D.; Rwomushana, I.; van den Berg, J.; et al. Invasiveness, biology, ecology, and management of the fall armyworm, Spodoptera frugiperda. Entomol. Gen. 2023, 43, 187–241. [Google Scholar] [CrossRef]
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamò, M. First report of outbreaks of the fall armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in West and Central Africa. PLoS ONE 2016, 11, e0165632. [Google Scholar] [CrossRef]
- Maruthadurai, R.; Ramesh, R. Occurrence, damage pattern and biology of fall armyworm, Spodoptera frugiperda (J.E. smith) (Lepidoptera: Noctuidae) on fodder crops and green amaranth in Goa, India. Phytoparasitica 2020, 48, 15–23. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Liu, J.; Xie, M.C.; Li, Y.H.; Yang, J.J.; Zhang, M.L.; Qiu, K. Observation on law of diffusion damage of Spodoptera frugiperda in China in 2019. Plant Prot. 2019, 45, 10–19. (In Chinese) [Google Scholar] [CrossRef]
- Sun, X.-X.; Hu, C.-X.; Jia, H.-R.; Wu, Q.-L.; Shen, X.-J.; Zhao, S.-Y.; Jiang, Y.-Y.; Wu, K.-M. Case study on the first immigration of fall armyworm, Spodoptera frugiperda invading into China. J. Integr. Agric. 2021, 20, 664–672. [Google Scholar] [CrossRef]
- Montezano, D.G.; Sosa-Gómez, D.R.; Specht, A.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.V.; Peterson, J.A.; Hunt, T.E. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef]
- Sparks, A.N. Fall Armyworm Symposium: A Review of the Biology of the Fall Armyworm. Fla. Entomol. 1979, 62, 82–87. [Google Scholar] [CrossRef]
- Nagoshi, R.N.; Koffi, D.; Agboka, K.; Adjevi, A.K.M.; Meagher, R.L.; Goergen, G. The fall armyworm strain associated with most rice, millet, and pasture infestations in the Western Hemisphere is rare or absent in Ghana and Togo. PLoS ONE 2021, 16, e0253528. [Google Scholar] [CrossRef]
- Nam, K.; Nègre, N.; Saldamando Benjumea, C.I. Two host-plant strains in the fall armyworm. Insect Sci. 2024, 31, 1675–1683. [Google Scholar] [CrossRef]
- Durand, K.; An, H.; Nam, K. Invasive fall armyworms are corn strain. Sci. Rep. 2024, 14, 5696. [Google Scholar] [CrossRef]
- Acharya, R.; Akintola, A.A.; Malekera, M.J.; Kamulegeya, P.; Nyakunga, K.B.; Mutimbu, M.K.; Shrestha, Y.K.; Hemayet, J.S.M.; Hoat, T.X.; Dao, H.T.; et al. Genetic relationship of fall armyworm (Spodoptera frugiperda) populations that invaded Africa and Asia. Insects 2021, 12, 439. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, B.; Jiang, Y.Y.; Liu, J.; Wu, K.M.; Xiao, Y.T. Molecular characterization analysis of fall armyworm populations in China. Plant Prot. 2019, 45, 20–27. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, Q.-L.; Zhang, H.-W.; Wu, K.-M. Spread of invasive migratory pest Spodoptera frugiperda and management practices throughout China. J. Integr. Agric. 2021, 20, 637–645. [Google Scholar] [CrossRef]
- Yao, L.; Fang, M.; Li, X.; Li, G.; Tang, Q. Oviposition and feeding selectivity of Spodoptera frugiperda to three weeds. Plant Prot. 2020, 46, 181–184. [Google Scholar] [CrossRef]
- Liu, B.; Huang, B.; Zhao, J.; Huang, J.; Zhao, W.; Lv, G.; Li, G.; Feng, H. Occurrence of fall armyworm Spodoptera frugiperda in Xinxiang city, Henan province in autumn 2019. Plant Prot. 2020, 46, 181–185. (In Chinese) [Google Scholar] [CrossRef]
- Fang, M.; Yao, L.; Tang, Q.-F.; Li, G.-T.; Jiang, X.-C. Feeding adaptability of fall armyworm Spodoptera frugiperda to several weeds. Journal of Plant Protection 2020, 47, 1055–1061. (In Chinese) [Google Scholar]
- Zhang, Y.; Zhang, Z.; Liu, J.; Jiang, Y.; Li, X.; Ba, T.; Chen, Z.; Lin, P.; Huang, H. Oviposition and feeding preference of Spodoptera frugiperda to gramineous weeds. Plant Prot. 2021, 47, 117–122. (In Chinese) [Google Scholar]
- Su, X.-N.; Li, C.-Y.; Xu, Y.-J.; Huang, S.-H.; Liu, W.-L.; Liao, Z.-X.; Zhang, Y.-P. Feeding preference and adaptability of fall armyworm Spodoptera frugiperda on five species of host plants and six weeds. J. Environ. Entomol. 2022, 44, 263–272. (In Chinese) [Google Scholar]
- Xi, X.-J.; Zhu, Y.-G.; Tong, Y.-P.; Yang, X.-L.; Tang, N.-N.; Ma, S.-M.; Li, S.; Cheng, Z. Assessment of the genetic diversity of different Job’s tears (Coix lacryma-jobi L.) accessions and the active composition and anticancer effect of its seed oil. PLoS ONE 2016, 11, e0153269. [Google Scholar] [CrossRef]
- Li, H.; Peng, L.; Yin, F.; Fang, J.; Cai, L.; Zhang, C.; Xiang, Z.; Zhao, Y.; Zhang, S.; Sheng, H.; et al. Research on Coix seed as a food and medicinal resource, it’s chemical components and their pharmacological activities: A review. J. Ethnopharmacol. 2024, 319, 117309. [Google Scholar] [CrossRef]
- Feng, L.; Zhao, Y.; Zhang, Z.; Zhang, S.; Zhang, H.; Yu, M.; Ma, Y. The edible and medicinal value of Coix lacryma-jobi and key cultivation techniques for high and stable yield. Nat. Resour. 2020, 11, 569–575. [Google Scholar] [CrossRef]
- Huang, H.; Lu, P.; Zhu, Y.; Li, Y. Ecological types, diversity and utilization values of Coix lacryma-jobi in China. China Seed Ind. 1995, 14, 4–8. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, Y.; Wu, Z.; Cheng, Z. High-quality development and regionalization of medicinal and edible coix. Guizhou Agric. Sci. 2019, 47, 129–134. [Google Scholar]
- Fujian Province Released Three Group Standards for Coix Seeds. Available online: https://www.fujian.gov.cn/zwgk/ztzl/sxzygwzxsgzx/zx/202412/t20241228_6599141.htm (accessed on 22 May 2025). (In Chinese)
- Chen, Y.-C.; Chen, D.-F.; Yang, M.-F.; Liu, J.-F. The effect of temperatures and hosts on the life cycle of Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2022, 13, 211. [Google Scholar] [CrossRef]
- Li, D.; Zhi, J.; Zhang, T.; Ye, J.; Yu, Y.; Hu, C. Preference of Spodoptera frugiperda to four plants. Plant Prot. 2019, 45, 50–54. (In Chinese) [Google Scholar] [CrossRef]
- Kühnle, A.; Müller, C. Responses of an oligophagous beetle species to rearing for several generations on alternative host-plant species. Ecol. Entomol. 2011, 36, 125–134. [Google Scholar] [CrossRef]
- Wang, P.; He, P.-c.; Hu, L.; Chi, X.-l.; Keller, M.A.; Chu, D. Host selection and adaptation of the invasive pest Spodoptera frugiperda to indica and japonica rice cultivars. Entomol. Gen. 2022, 42, 403–411. [Google Scholar] [CrossRef]
- Yao, F.-L.; Wu, Y.-Y.; Zhou, S.-J.; Ding, X.-L.; Guan, Z.-X.; Lu, X.-S.; Zheng, Y.; Ramirez-Romero, R.; Desneux, N.; Weng, Q.-Y.; et al. Effects of continuous and transgenerational rearing in peanut leaves on the performance and enzyme activity of Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Econ. Entomol. 2024, 117, 2259–2268. [Google Scholar] [CrossRef]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H.; You, M.; Atlıhan, R.; Smith, C.L.; Kavousi, A.; Özgökçe, M.S.; Güncan, A.; Tuan, S.-J.; Fu, J.-W.; Xu, Y.-Y. Age-Stage, two-sex life table: An introduction to theory, data analysis, and application. Entomol. Gen. 2020, 40, 103–124. [Google Scholar] [CrossRef]
- Amir-Maafi, M.; Chi, H.; Chen, Z.-Z.; Xu, Y.-Y. Innovative bootstrap-match technique for life table set up. Entomol. Gen. 2022, 42, 597–609. [Google Scholar] [CrossRef]
- Chi, H.; Kara, H.; Özgökçe, M.S.; Atlihan, R.; Güncan, A.; Rişvanlı, M.R. Innovative application of set theory, Cartesian product, and multinomial theorem in demographic research. Entomol. Gen. 2022, 42, 863–874. [Google Scholar] [CrossRef]
- Chi, H.; Kavousi, A.; Gharekhani, G.; Atlihan, R.; Özgökçe, M.S.; Güncan, A.; Gökçe, A.; Smith, C.L.; Benelli, G.; Guedes, R.N.C. Advances in theory, data analysis, and application of the age-stage, two-sex life table for demographic research, biological control, and pest management. Entomol. Gen. 2023, 43, 705–735. [Google Scholar] [CrossRef]
- Chi, H.; Güncan, A.; Kavousi, A.; Gharakhani, G.; Atlihan, R.; Özgökçe, M.S.; Shirazi, J.; Amir-Maafi, M.; Maroufpoor, M.; Taghizadeh, R. TWOSEX-MSChart: The key tool for life table research and education. Entomol. Gen. 2022, 42, 845–849. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX-MSChart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis. 2023. Available online: https://zenodo.org/records/7484085 (accessed on 23 July 2025).
- Huang, H.-W.; Chi, H.; Smith, C.L. Linking demography and consumption of Henosepilachna vigintioctopunctata (Coleoptera: Coccinellidae) fed on Solanum photeinocarpum (Solanales: Solanaceae): With a new method to project the uncertainty of population growth and consumption. J. Econ. Entomol. 2017, 111, 1–9. [Google Scholar] [CrossRef]
- Chi, H. Timing of control based on the stage structure of pest populations: A simulation approach. J. Econ. Entomol. 1990, 83, 1143–1150. [Google Scholar] [CrossRef]
- Wei, M.; Chi, H.; Guo, Y.; Li, X.; Zhao, L.; Ma, R. Demography of Cacopsylla chinensis (Hemiptera: Psyllidae) reared on four cultivars of Pyrus bretschneideri (Rosales: Rosaceae) and P. communis pears with estimations of confidence intervals of specific life table statistics. J. Econ. Entomol. 2020, 113, 2343–2353. [Google Scholar] [CrossRef]
- Lockwood, J.R. On the statistical analysis of multiple-choice feeding preference experiments. Oecologia 1998, 116, 475–481. [Google Scholar] [CrossRef]
- Roa, R. Design and analysis of multiple-choice feeding-preference experiments. Oecologia 1992, 89, 509–515. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Wu, Z.; Shi, P.; Zeng, Y.; Huang, W.; Huang, Q.; Ma, X.; Guo, L. Population life tables of Spodoptera frugiperda (Lepidoptera: Noctuidae) fed on three host plants. Plant Prot. 2019, 45, 59–64. (In Chinese) [Google Scholar]
- Ramasubramanian, T.; Yogambal, C.; Singaravelu, B. Bio-ecology of fall armyworm, Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) in sugarcane. Sugar Tech. 2025, 27, 252–261. [Google Scholar] [CrossRef]
- Lv, L.; Xia, H.; Guo, L.; Chang, X.; Wang, P.; Zhang, S. Effects of corn and sorghum on oviposition site selection and fitness of Spodoptera frugiperda. Chin. J. of Appl. Entomol. 2022, 59, 542–550. (In Chinese) [Google Scholar]
- Lu, J.; Zhang, B.; Zhuang, M.; Ren, M.; Li, D.; Yan, H.; Long, J.; Jiang, X. Preference and performance of the fall armyworm, Spodoptera frugiperda, on six cereal crop species. Entomol. Exp. Appl. 2023, 171, 492–501. [Google Scholar] [CrossRef]
- Gong, Z.; Dong, J.; Li, Y.; Zhang, Z.; Duan, Y.; Jiang, Y.; Miao, J.; Li, T.; Zhang, J.; Li, H.; et al. Life table study of Spodoptera frugiperda at different wheat stages and the effect of larval population density on wheat yield. Pest Manag. Sci. 2023, 79, 4057–4065. [Google Scholar] [CrossRef]
- Lu, H.; Yang, P.; Xu, Y.; Luo, L.; Zhu, J.; Cui, N.; Kang, L.; Cui, F. Performances of survival, feeding behavior and gene expression in aphids reveal their different fitness to host alteration. Sci. Rep. 2016, 6, 19344. [Google Scholar] [CrossRef]
- Hafeez, M.; Li, X.; Ullah, F.; Zhang, Z.; Zhang, J.; Huang, J.; Khan, M.M.; Chen, L.; Ren, X.; Zhou, S.; et al. Behavioral and physiological plasticity provides insights into molecular based adaptation mechanism to strain shift in Spodoptera frugiperda. Int. J. Mol. Sci. 2021, 22, 10284. [Google Scholar] [CrossRef]
- Coudron, T.A.; Wittmeyer, J.; Kim, Y. Life history and cost analysis for continuous rearing of Podisus maculiventris (Say) (Heteroptera: Pentatomidae) on a zoophytophagous artificial diet. J. Econ. Entomol. 2002, 95, 1159–1168. [Google Scholar] [CrossRef]
- Ghaemmaghami, E.; Fathipour, Y.; Bagheri, A.; Talebi, A.A.; Reddy, G.V.P. Continuous rearing on Ephestia kuehniella reshaped quality of the parasitoid wasp Trichogramma brassicae (Hymenoptera: Trichogrammatidae). J. Asia-Pac. Entomol. 2021, 24, 166–174. [Google Scholar] [CrossRef]
- Momenian, G.; Sarayloo, H.M.; Afshari, A. Effect of continuous rearing generations on some biological parameters of Habrobracon hebetor (Hymenoptera: Braconidae) under insectarium conditions. Arthropods 2022, 11, 1–17. [Google Scholar]
- Wang, W.; He, P.; Zhang, Y.; Liu, T.; Jing, X.; Zhang, S. The population growth of Spodoptera frugiperda on six cash crop species and implications for its occurrence and damage potential in China. Insects 2020, 11, 639. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, J.; Huang, D.-L.; Shi, M.-Z.; Chi, H.; Rostami, E.; Kavousi, A.; Fu, J.-W. Comparative demography of group-and individually-reared life tables of papaya mealybug with an innovative life table analysis for species in which females and males have a different number of stages. Entomol. Gen. 2024, 44, 727–735. [Google Scholar] [CrossRef]
- Zhao, J.; Hoffmann, A.; Jiang, Y.; Xiao, L.; Tan, Y.; Zhou, C.; Bai, L. Competitive interactions of a new invader (Spodoptera frugiperda) and indigenous species (Ostrinia furnacalis) on maize in China. J. Pest. Sci. 2021, 95, 159–168. [Google Scholar] [CrossRef]
- Price, T.D.; Qvarnström, A.; Irwin, D.E. The role of phenotypic plasticity in driving genetic evolution. Proc. Roy. Soc. London Ser. B Biol. Sci. 2003, 270, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Gripenberg, S.; Mayhew, P.J.; Parnell, M.; Roslin, T. A meta-analysis of preference–performance relationships in phytophagous insects. Ecol. Lett. 2010, 13, 383–393. [Google Scholar] [CrossRef]
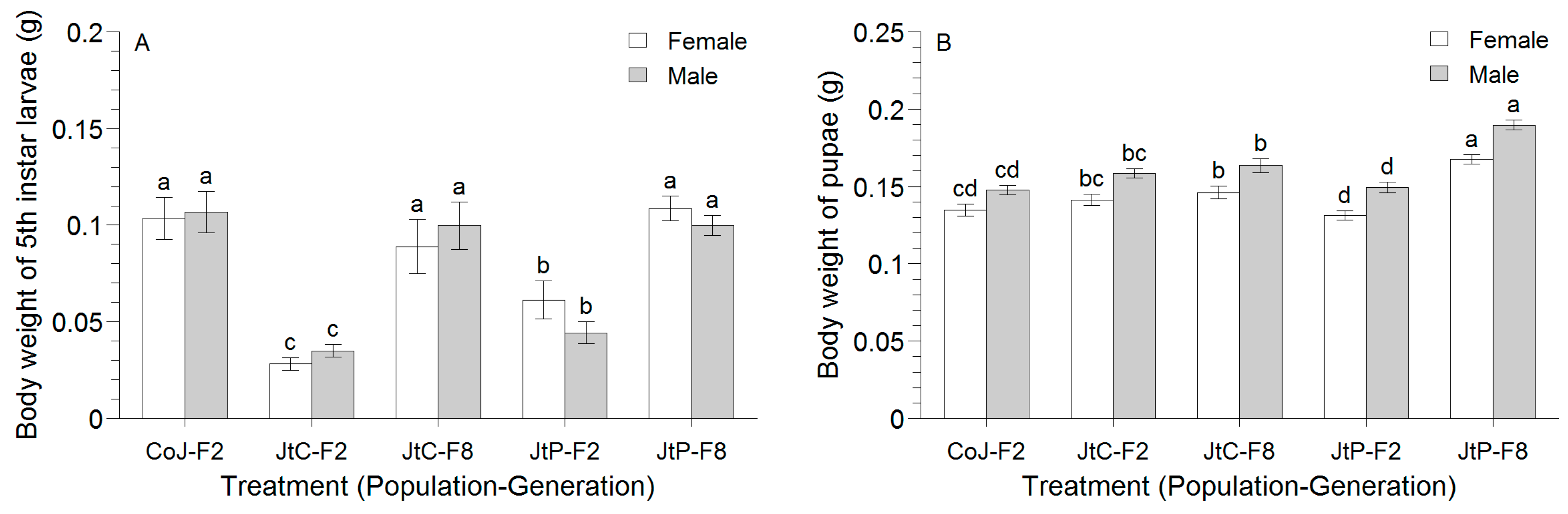
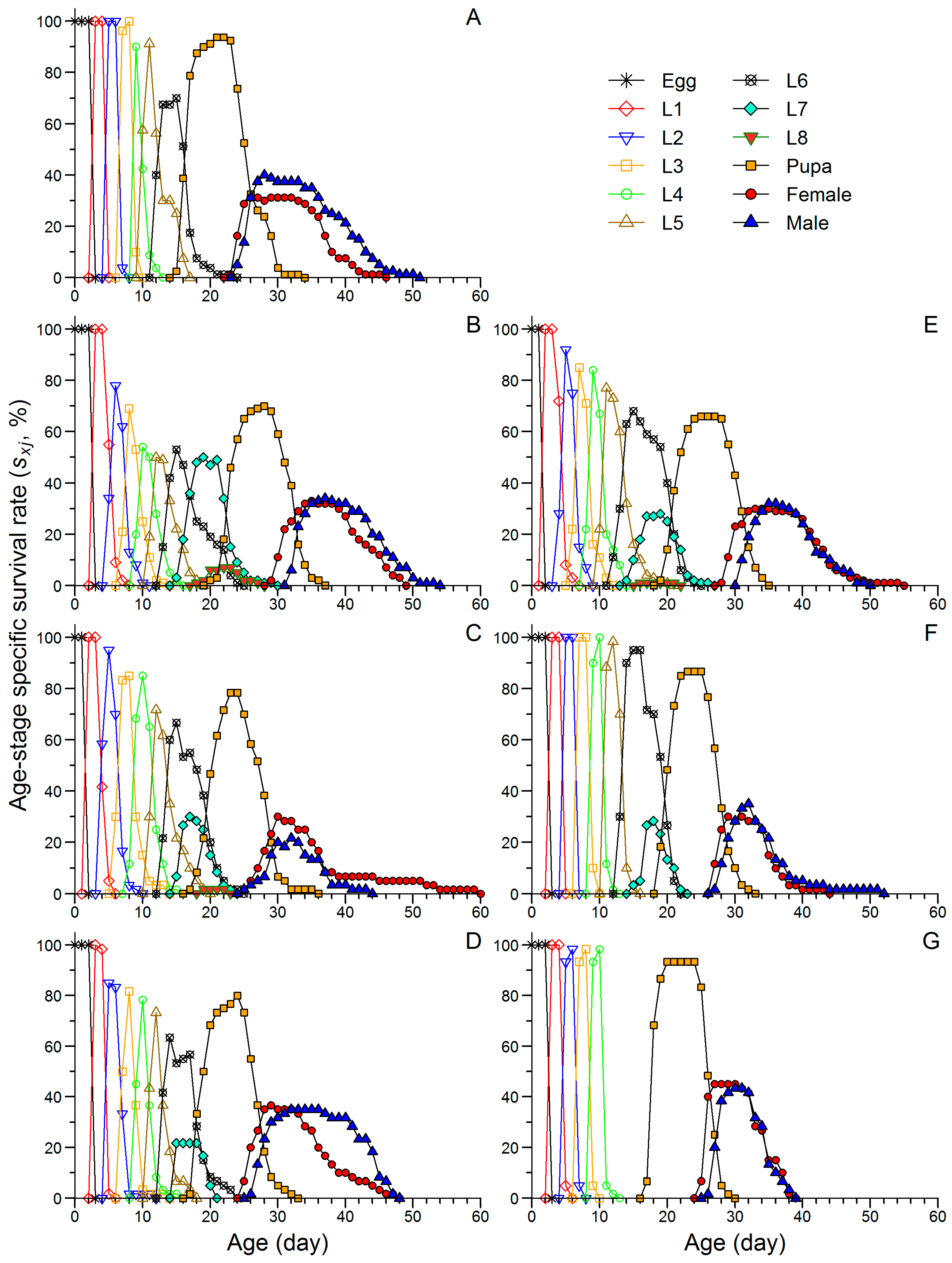
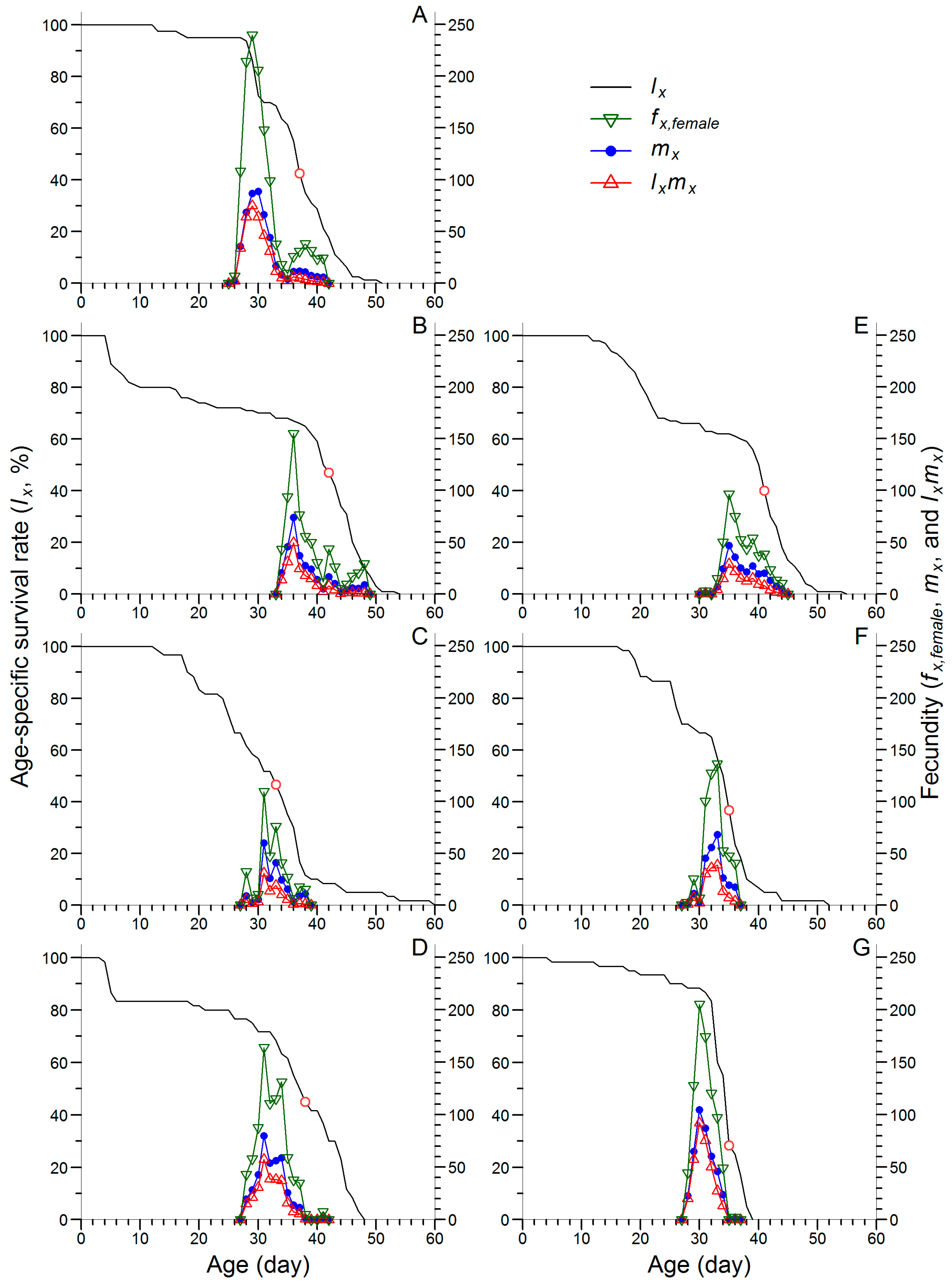
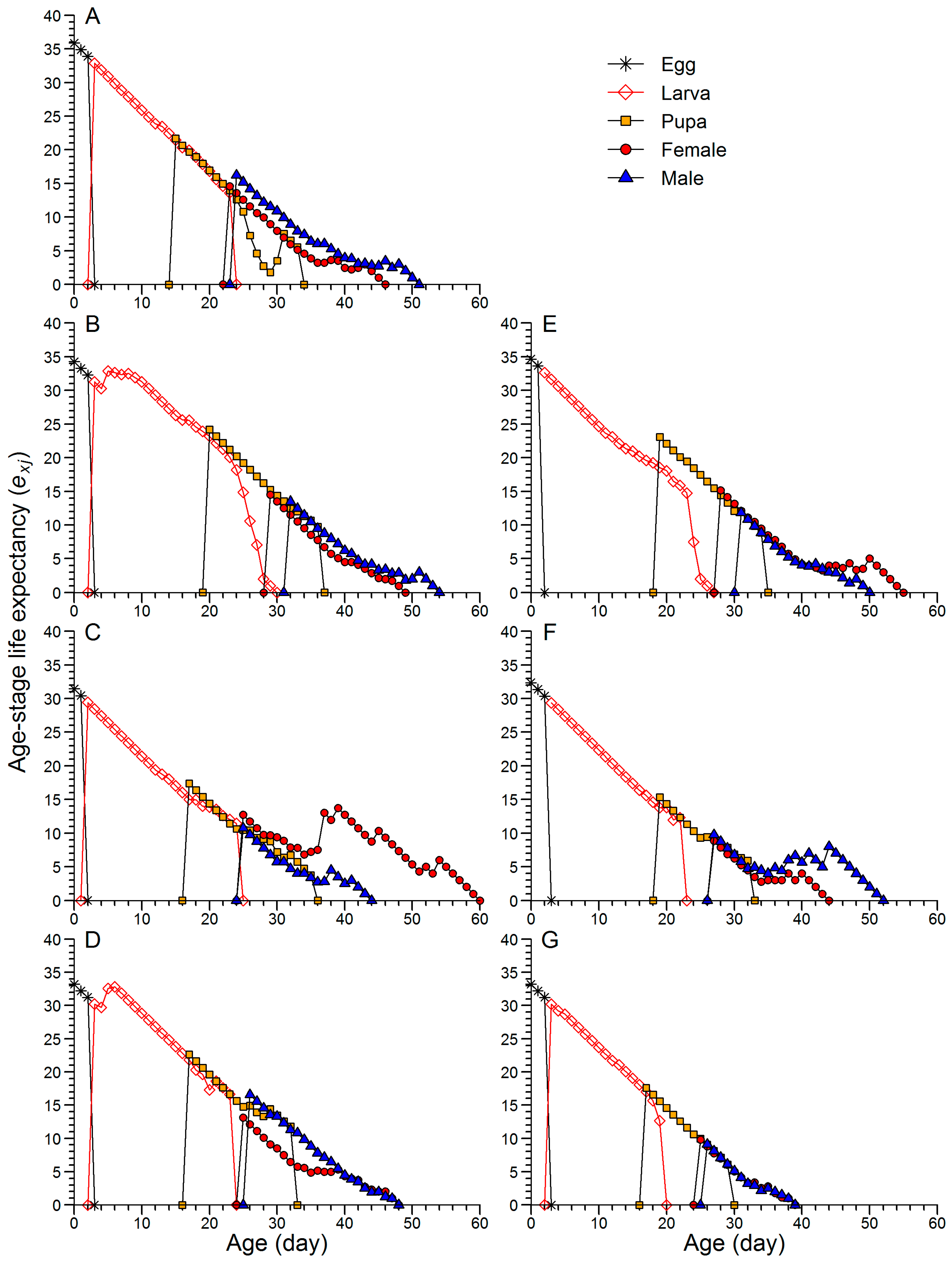
| Parameters | Corn | Job’s Tears Cultivar ‘Cuiyi 1’ | ||||||
|---|---|---|---|---|---|---|---|---|
| n | F2 Generation | n | F2 Generation | n | F5 Generation | n | F8 Generation | |
| Development time (d) | ||||||||
| Pre-adult | 59 | 25.5 ± 0.2 d | 68 | 32.5 ± 0.2 a | 36 | 28.8 ± 0.4 b | 45 | 27.6 ± 0.3 c |
| Eggs | 80 | 3.0 ± 0.0 a | 100 | 3.0 ± 0.0 a | 60 | 2.0 ± 0.0 b | 60 | 3.0 ± 0.0 a |
| Larvae | 77 | 14.0 ± 0.2 d | 70 | 20.3 ± 0.2 a | 49 | 18.5 ± 0.2 b | 48 | 16.3 ± 0.2 c |
| 1st instar | 80 | 2.0 ± 0.0 c | 85 | 2.7 ± 0.1 a | 60 | 2.5 ± 0.1 b | 51 | 2.0 ± 0.0 c |
| 2nd instar | 80 | 2.0 ± 0.0 b | 82 | 2.3 ± 0.1 a | 60 | 2.5 ± 0.1 a | 50 | 2.5 ± 0.1 a |
| 3rd instar | 80 | 2.1 ± 0.0 c | 81 | 2.2 ± 0.1 b | 59 | 2.5 ± 0.1 a | 50 | 2.1 ± 0.1 bc |
| 4th instar | 80 | 1.5 ± 0.1 c | 80 | 2.2 ± 0.1 b | 58 | 2.7 ± 0.1 a | 50 | 2.1 ± 0.1 b |
| 5th instar | 78 | 3.0 ± 0.2 a | 80 | 2.4 ± 0.1 b | 57 | 2.5 ± 0.1 b | 50 | 2.3 ± 0.1 b |
| 6th instar | 57 | 4.6 ± 0.1 a | 78 | 3.6 ± 0.2 b | 51 | 3.9 ± 0.2 b | 49 | 4.0 ± 0.2 b |
| 7th instar | / | / | 54 | 5.4 ± 0.2 a | 17 | 4.7 ± 0.4 a | 12 | 4.9 ± 0.2 a |
| 8th instar | / | / | 7 | 5.6 ± 0.4 a | 1 | 4.0 b | / | / |
| Pupae | 59 | 8.6 ± 0.1 b | 68 | 9.1 ± 0.1 a | 36 | 8.6 ± 0.2 b | 45 | 8.4 ± 0.1 b |
| Female adult longevity (d) | 27 | 12.6 ± 0.7 a | 33 | 12.2 ± 0.6 a | 20 | 10.2 ± 1.6 a | 22 | 11.5 ± 0.9 a |
| Male adult longevity (d) | 32 | 14.4 ± 0.9 a | 35 | 12.0 ± 0.7 b | 16 | 6.6 ± 0.9 c | 23 | 14.3 ± 0.9 ab |
| Total longevity (d) | 80 | 35.9 ± 0.8 a | 100 | 34.3 ± 1.6 ab | 60 | 31.4 ± 1.2 b | 60 | 33.2 ± 1.8 ab |
| Proportion of female adults (Nf/N) (%) | 80 | 33.8 ± 5.3 a | 100 | 33.0 ±4.7 a | 60 | 33.3 ± 6.1 a | 60 | 36.7 ± 6.2 a |
| Proportion of fertile female adults (Nfr/Nf) (%) | 80 | 96.3 ± 3.7 a | 100 | 84.8 ± 6.3 a | 60 | 50.0 ± 11.4 b | 60 | 90.9 ± 6.2 a |
| Proportion of male adults (Nm/N) (%) | 80 | 40.0 ± 5.5 a | 100 | 35.0 ± 4.8 a | 60 | 26.7 ± 5.7 a | 60 | 38.3 ± 6.3 a |
| APOP (d) | 26 | 3.4 ± 0.2 b | 28 | 5.2 ± 0.5 a | 10 | 3.9 ± 0.5 b | 20 | 5.1 ± 0.7 a |
| TPOP (d) | 26 | 28.4 ± 0.4 c | 28 | 36.6 ± 0.5 a | 10 | 31.7 ± 0.7 b | 20 | 31.6 ± 0.7 b |
| Oviposition day (Od) (d) | 26 | 5.5 ± 0.2 a | 28 | 4.2 ± 0.4 b | 10 | 3.3 ± 0.3 c | 20 | 4.1 ± 0.4 bc |
| Fecundity (eggs/female) | 27 | 1054.6 ± 66.6 a | 33 | 542.4 ± 69.8 b | 20 | 278.1 ± 76.8 c | 22 | 734.4 ± 109.0 b |
| Parameters | Corn | Job’s Tears Cultivar ‘Puiyi 6’ | ||||||
|---|---|---|---|---|---|---|---|---|
| n | F2 Generation | n | F2 Generation | n | F5 Generation | n | F8 Generation | |
| Development time (d) | ||||||||
| Pre-adult | 59 | 25.5 ± 0.2 d | 63 | 31.3 ± 0.2 a | 41 | 28.7 ± 0.2 b | 54 | 26.6 ± 0.1 c |
| Eggs | 80 | 3.0 ± 0.0 a | 100 | 2.0 ± 0.0 b | 60 | 3.0 ± 0.0 a | 60 | 3.0 ± 0.0 a |
| Larvae | 77 | 14.0 ± 0.2 d | 67 | 19. 5 ± 0.2 a | 52 | 17.4 ± 0.1 b | 56 | 15.3 ± 0.1 c |
| 1st instar | 80 | 2.0 ± 0.0 b | 100 | 2.8 ± 0.1 a | 60 | 2.0 ± 0.0 b | 59 | 2.0 ± 0.0 b |
| 2nd instar | 80 | 2.0 ± 0.0 b | 100 | 2.2 ± 0.0 a | 60 | 2.0 ± 0.0 b | 59 | 2.0 ± 0.0 b |
| 3rd instar | 80 | 2.0 ± 0.0 b | 100 | 2.1 ± 0.0 a | 60 | 2.1 ± 0.0 a | 59 | 2.0 ± 0.0 b |
| 4th instar | 80 | 1.5 ± 0.1 c | 100 | 2.2 ± 0.0 a | 60 | 2.0 ± 0.0 b | 59 | 2.0 ± 0.0 b |
| 5th instar | 78 | 3.0 ± 0.2 a | 95 | 3.0 ± 0.1 a | 60 | 2.7 ± 0.1 b | 58 | 2.1 ± 0.0 c |
| 6th instar | 57 | 4.6 ± 0.1 bc | 80 | 5.1 ± 0.2 ab | 56 | 5.3 ± 0.2 a | 56 | 4.5 ± 0.2 c |
| 7th instar | / | / | 18 | 5.9 ± 0.3 a | 13 | 4.4 ± 0.3 b | 10 | 3.9 ± 0.3 b |
| 8th instar | / | / | 1 | 6.0 | / | / | / | / |
| Pupae | 59 | 8.6 ± 0.1 b | 63 | 9.7 ± 0.1 a | 41 | 8.6 ± 0.1 b | 54 | 8.6 ± 0.1 b |
| Female adult longevity (d) | 27 | 12.6 ± 0.7 a | 31 | 13.0 ± 0.8 a | 19 | 7.9 ± 0.7 b | 28 | 8.8 ± 0.5 b |
| Male adult longevity (d) | 32 | 14.4 ± 0.9 a | 32 | 10.4 ± 0.6 b | 22 | 7.4 ± 0.9 c | 26 | 7.5 ± 0.4 c |
| Total longevity (d) | 80 | 35.9 ± 0.8 a | 100 | 34.7 ± 1.2 ab | 60 | 32.4 ± 0.9 b | 60 | 33.2 ± 0.8 b |
| Proportion of female adults (Nf/N) (%) | 80 | 33.8 ± 5.3 b | 100 | 31.0 ± 4.6 b | 60 | 31.7 ± 6.0 b | 60 | 47.7 ± 6.4 a |
| Proportion of fertile female adults (Nfr/Nf) (%) | 80 | 96.3 ± 3.7 a | 100 | 87.1 ± 6.1 ab | 60 | 84.2 ± 8.5 ab | 60 | 78.6 ± 7.8 b |
| Proportion of male adults (Nm/N) (%) | 80 | 40.0 ± 5.5 a | 100 | 32.0 ± 4.7 a | 60 | 36.7 ± 6.2 a | 60 | 43.3 ± 6.4 a |
| APOP (d) | 26 | 3.4 ± 0.2 b | 27 | 5.7 ± 0.5 a | 16 | 3.6 ± 0.3 b | 22 | 3.7 ± 0.3 b |
| TPOP (d) | 26 | 28.4 ± 0.4 d | 27 | 35.9 ± 0.5 a | 16 | 31.5 ± 0.3 b | 22 | 29.7 ± 0.3 c |
| Oviposition day (Od) (d) | 26 | 5.5 ± 0.2 a | 27 | 4.4 ± 0.4 b | 16 | 3.3 ± 0.2 c | 22 | 3.7 ± 0.2 bc |
| Fecundity (eggs/female) | 27 | 1054.6 ± 66.6 a | 31 | 445.8 ± 62.4 c | 19 | 439.7 ± 56.6 c | 28 | 721.1 ± 84.1 b |
| Population Parameter | Corn | Job’s Tears Cultivar ‘Cuiyi 1’ | ||
|---|---|---|---|---|
| F2 Generation | F2 Generation | F5 Generation | F8 Generation | |
| Intrinsic rate of increase, r (d−1) | 0.193 ± 0.006 a | 0.137 ± 0.006 c | 0.138 ± 0.012 c | 0.172 ± 0.008 b |
| Finite rate of increase, λ (d−1) | 1.212 ± 0.007 a | 1.147 ± 0.006 c | 1.148 ± 0.014 c | 1.188 ± 0.009 b |
| Net reproductive rate, R0 | 355.9 ± 60.0 a | 179.0 ± 34.3 bc | 92.7 ± 30.2 c | 269.3 ± 60.5 ab |
| Mean generation time, T (d) | 30.6 ± 0.3 c | 37.8 ± 0.3 a | 32.9 ± 0.6 b | 32.6 ± 0.4 b |
| Population Parameter | Corn | Job’s Tears Cultivar ‘Puyi 6’ | ||
|---|---|---|---|---|
| F2 Generation | F2 Generation | F5 Generation | F8 Generation | |
| Intrinsic rate of increase, r (d−1) | 0.193 ± 0.006 a | 0.131 ± 0.006 b | 0.148 ± 0.007 b | 0.185 ± 0.006 a |
| Finite rate of increase, λ (d−1) | 1.212 ± 0.007 a | 1.109 ± 0.020 b | 1.165 ± 0.010 b | 1.185 ± 0.008 a |
| Net reproductive rate, R0 | 355.9 ± 60.0 a | 138.2 ± 28.2 b | 139.2 ± 31.7 b | 336.5 ± 60.5 a |
| Mean generation time, T (d) | 30.6 ± 0.3 d | 37.6 ± 0.3 a | 33.1 ± 0.4 b | 31.4 ± 0.2 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, F.-L.; Wu, Y.-Y.; Lei, G.-K.; Huang, X.-Y.; Ding, X.-L.; Lu, X.-S.; Zheng, Y.; He, Y.-X. Effects of Multi-Generational Rearing on Job’s Tears on the Performance and Host Plant Preference of Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2025, 16, 773. https://doi.org/10.3390/insects16080773
Yao F-L, Wu Y-Y, Lei G-K, Huang X-Y, Ding X-L, Lu X-S, Zheng Y, He Y-X. Effects of Multi-Generational Rearing on Job’s Tears on the Performance and Host Plant Preference of Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects. 2025; 16(8):773. https://doi.org/10.3390/insects16080773
Chicago/Turabian StyleYao, Feng-Luan, Yao-Yao Wu, Gao-Ke Lei, Xiao-Yan Huang, Xue-Ling Ding, Xue-Song Lu, Yu Zheng, and Yu-Xian He. 2025. "Effects of Multi-Generational Rearing on Job’s Tears on the Performance and Host Plant Preference of Spodoptera frugiperda (Lepidoptera: Noctuidae)" Insects 16, no. 8: 773. https://doi.org/10.3390/insects16080773
APA StyleYao, F.-L., Wu, Y.-Y., Lei, G.-K., Huang, X.-Y., Ding, X.-L., Lu, X.-S., Zheng, Y., & He, Y.-X. (2025). Effects of Multi-Generational Rearing on Job’s Tears on the Performance and Host Plant Preference of Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects, 16(8), 773. https://doi.org/10.3390/insects16080773







