Simple Summary
Life tables are crucial for unraveling the complex dynamics of pest populations, providing insights into demographic characteristics across different developmental stages and sexes. While numerous studies have highlighted the potential of life table data, such as mortality and emergence rates, in formulating effective IPM strategies, reports of successful implementation in real-world scenarios are scarce. A significant issue is the disconnect between theory and practical application, likely due to a lack of comprehensive research on utilizing the data generated by this tool. This review summarizes the research progress on life tables, particularly their applications in pest control, with the aim of providing a reference for the implementation of effective pest management strategies.
Abstract
Life tables are indispensable in IPM, offering an analysis of insect population dynamics. These tables record survival rates, fecundity, and other parameters at various developmental stages, enabling the identification of key factors that affect population numbers and the prediction of growth trajectories. This review discusses the application of life tables in agricultural pest management, including the assessment of the pest control capacity of natural enemies, the evaluation of biological agents, and the screening of insect-resistant plant species. In vector insect control, life tables are used to evaluate the transmission risks, model the population dynamics, and interfere with the life cycles of vector insects. For invasive pests, life tables help us to monitor population dynamics and predict future population sizes. In chemical pest control, life tables assist in evaluating the fitness costs of pesticide resistance, guiding insecticide selection, and optimizing application timing. In the final section, we explore future research directions, emphasizing the potential of integrating new technologies such as genomics, ethology, and satellite remote sensing to enhance life table analysis and improve IPM strategies.
1. Introduction
Insect life tables are essential tools in the systematic study of insect populations. These techniques primarily employ parameters such as survival rates, instantaneous mortality rates, mortality distributions, and life expectancies to characterize the life history of insects. Their applications include the fields of insect development, biological control, evolution, and invasion biology. In both ecology and entomology, life tables are utilized to analyze the life cycles and population dynamics of diverse biological populations [1].
Life tables originated in the fields of human sociology and demography, where they were employed to describe and predict changes in population mortality, birth rates, and age distributions [2]. In ecology, population life tables serve a similar purpose, focusing on the dynamics and survival of biological populations. However, traditional life tables have typically focused on a single sex, usually females, which limits their ability to capture the full dynamics of the entire population [3]. Advancements in entomology and the development of new research techniques have led to continuous improvements in classical insect life table methods [4]. The emergence of life tables reflects a deeper exploration of biological traits and population heterogeneity. These life tables analyze the survival rates and reproductive capacities of both sexes across various developmental stages, offering a more comprehensive model of population dynamics. By considering the biological characteristics and behavioral differences between sexes, these tables facilitate a more accurate assessment of the population growth potential and the effectiveness of control strategies [5].
For life tables with raw data on development, the TWOSEX-MSChart program [6] is a versatile tool that can be used to analyze the raw life table data of related insects, providing detailed insights into their development, survival, and daily reproductive capacity. The age-stage-specific survival rate (sxj: the probability that a newborn egg will survive to age x and stage j); the age-specific survival rate (lx: the probability that a newborn egg will survive to age x); the female fecundity (F: eggs/female); the age-stage-specific fecundity (fxj: the number of hatched eggs produced by a female adult at age x); the age-specific fecundity (mx: the number of eggs per individual at age x); and the age-specific maternity (lxmx: the product of lx and mx) were calculated. All the population parameters, including the intrinsic rate of increase (rm), were calculated using the Lotka–Euler equation with the age indexed from zero; the finite rate of increase (λ) was calculated as λ = er; the net reproductive rate (R0) is the sum of all lxmx (age-specific maternity), which considers the survival rate; and the mean generation time (T) is the length of time required by a population to increase to R0-fold of its size as time approaches infinity and the population settles down to a stable age-stage distribution. Key indicators, such as rm, λ, R0, and T, are essential for evaluating the biological characteristics of insect populations [7]. By recording these parameters, we can infer valuable insights into the structure of the insect population, its growth and decline patterns, and other ecological aspects. Life tables serve as an important tool for studying population ecology and for summarizing the survival and reproductive potential of insect populations on different hosts and under various environmental conditions [8].
Insect life tables are pivotal instruments within the realm of insect population ecology and pest management. Their utility extends across a spectrum of research domains, including, but not limited to, pest control, resistance dynamics, extensive insect breeding, the harnessing of biological control agents, and the enhancement of plant resistance [6]. The formulation of a life table encapsulates key statistical metrics of an insect population, thereby offering a structured framework for the documentation and computation of all demographic shifts throughout the insect’s life cycle. A thorough examination of life tables stands as a cornerstone for the management of insects, resistance profiling, the interplay of predators and prey, biological control strategies, and the logistics of large-scale insect cultivation [9].
This article delves into the application of life table methodologies in the realm of integrated pest management, with a pronounced emphasis on biological and chemical control measures. It endeavors to provide robust methods and advanced technologies that enable a scientifically rigorous assessment of pest control efficacy. The ultimate goal is to identify and select the most efficacious control strategies for prospective implementation.
2. Application of Life Tables in Biological Control of Pests
2.1. Application in Agricultural Pest Control
Agricultural pests pose a significant threat to crops and agricultural production, impacting biodiversity and causing substantial economic losses. In response to these challenges, modern agriculture increasingly advocates for IPM strategies, which rely heavily on the construction and analysis of pest life table data. These data provide critical insights into how different environmental variables, control strategies, and biological interactions influence pest population growth rates, mortality risks, and reproductive success [10]. Life table technology is indispensable for optimizing pest control measures, ensuring crop safety, and maintaining a healthy ecosystem [11]. By serving as a scientific bridge between theoretical research and practical applications, life tables contribute to the sustainable development of pest management programs (Figure 1 and Table S1).
2.1.1. Evaluation of the Pest Control Capacity of Natural Enemies of Pests
In agricultural ecosystems, biological control methods that leverage the relationships between organisms and their environment, as well as among different species, are gaining increased attention for protecting ecosystems and ensuring environmental safety [12]. Given that agricultural pests pose a significant threat to global biodiversity and agriculture, natural enemies play a crucial role in managing pest populations [13]. The most traditional method for evaluating the pest control ability of natural enemies is to construct insect life tables. By documenting the growth and development of pests under the pressure of natural enemies, this method assesses the pest control ability of natural enemies from the perspective of pest population dynamics. Natural enemies are usually classified as predators, parasitoids, and pathogens [14].
Predatory natural enemies are potential biological control agents for rapidly reproducing pests, such as mites [15], whiteflies [16], and thrips [17]. The intrinsic natural growth rate rm of a population is of great interest as a key parameter in entomology and is considered particularly important in the study of predators [18]. Among the common predatory natural enemies, Coccinellidae stands out due to being known for preying on aphids, scale insects, whiteflies, and spider mites [19]. The Chrysopidae, commonly known as lacewings, are renowned for their formidable predatory capabilities against a broad spectrum of agricultural and forestry pests, occupying a crucial position in biological control strategies [20]. Both the larval and adult stages of these insects actively prey upon a diverse range of pests, such as aphids, whiteflies, mites, and cotton bollworms. Moreover, Pentatomidae, which includes predatory stink bugs, contributes significantly to natural pest management by feeding on a diverse range of hosts, such as moth larvae, various beetles, and leaf beetles [21].
By utilizing a two-sex life table for analysis, the feeding potential and efficacy of Orius strigicollis Poppius (Heteroptera: Anthocoridae) were quantified under the selection pressures of different prey densities [22]. It was found that, when feeding on 10 Pectinophora gossypiella Saunders (Lepidoptera: Gelechiidae) eggs, the females showed significantly increased feeding capabilities and the highest R0 and gross reproductive rates (GRR) were achieved; in addition, O. strigicollis showed a higher and similar r (faster development and highest survival rates) when fed with 10 or 15 eggs of P. gossypiella, owing to a higher fecundity and shorter or faster development times. This revealed the potential of O. strigicollis as an effective predator of P. gossypiella [22]. By combining the Holling disc equation with the age-stage, two-sex life table technique, it was demonstrated that each life stage of O. strigicollis exhibited a type II functional response when presented with third-instar nymphs of Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae) and Trialeurodes vaporariorum Westwood (Hemiptera: Aleyrodidae) [23]. The calculated prey handling time for O. strigicollis was shorter when fed T. vaporariorum; additionally, the nymphal development and the total pre-oviposition period of adult females of O. strigicollis were significantly shorter when fed B. tabaci, confirming its potential application as a biocontrol agent in integrated pest management, especially for controlling B. tabaci efficiently [23]. The consumption, development, and reproduction of three native Chinese phytase enzymes—Neoseiulus californicus McGregor (Mesostigmata: Phytoseiidae), Neoseiulus barkeri Hughes (Mesostigmata: Phytoseiidae), and Amblyseius orientalis Ehara (Mesostigmata: Phytoseiidae)—were comprehensively evaluated using life table parameters, with Amblyseius swirskii Athias-Henriot (Mesostigmata: Phytoseiidae) serving as a control. When fed Polyphagotarsonemus latus Banks (Acari: Tarsonemidae), A. orientalis exhibited the highest consumption rate, the shortest development time, and the highest cumulative fecundity. Notably, its rm was 0.12, and A. orientalis was the sole species among the three that was anticipated to experience population growth when fed P. latus. It is recommended that A. orientalis be considered as a potential biological control agent for this pest [24].
Parasitic natural enemies are also crucial in biological control. By examining the morphological features, parasitism rates, and developmental history of key parasitic natural enemies, valuable insights can be gained. These insights are vital for conserving the biodiversity of these natural enemies and utilizing local species for pest control [25]. In the realm of ecology, the parasitic wasp families Trichogrammatidae [26], Ichneumonidae [27], Braconidae [28], and Pteromalidae [29] have emerged as crucial biocontrol agents. They are proficient at managing the populations of harmful insects by strategically employing parasitic tactics. Through the analysis of two-sex life tables, it was found that Sitotroga cerealella Olivier (Lepidoptera: Gelechiidae) reared on maize exhibited significantly higher values of λ, r, and R0. Additionally, the mean parasitism, mean adult emergence, longevity of adults, and total adult longevity of Trichogramma chilonis Ishii (Hymenoptera: Trichogrammatidae) were recorded as being the highest on S. cerealella eggs reared on maize. According to evolutionary models, more females will oviposit in large hosts than in small hosts, which is consistent with the finding that maize, being a larger host, supports a higher proportion of female offspring in T. chilonis. Identifying the most susceptible and favorite host (maize) will help us improve the large-scale production of T. chilonis under laboratory conditions [30]. Temperature significantly influenced the biological parameters of Trichogramma euproctidis Girault (Hymenoptera: Trichogrammatidae); at 32.5 °C, it achieved peak parasitism rates on Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) eggs, produced the highest number of female offspring, and attained the greatest survival rate [31]. This strain of T. euproctidis is adapted to high temperatures and harsh environmental conditions, showing potential for use in integrated management programs in Southwest Iran [31]. The fertility life table parameters of Tetrastichus howardi Olliff (Hymenoptera: Eulophidae) parasitizing Plutella xylostella Linnaeus (Lepidoptera: Plutellidae) and studies on the effects of the natal host on the behavior of T. howardi towards host volatiles and the parasitism rate have shown that the Ro and rm of T. howardi are 13.6 and 0.124, respectively, while the mean generation time is 20.9 days. Furthermore, the natal host Tenebrio molitor Linnaeus (Coleoptera: Tenebrionidae) or P. xylostella does not affect the fitness or parasitism rate of T. howardi. Therefore, T. howardi reared on the artificial host maintains its attraction to and potential to parasitize P. xylostella [32].
As the demand for sustainable agricultural development grows, insect pathogens are gaining increasing attention for pest control [33]. Nonetheless, systematic research methods are required to evaluate their efficacy and optimize strategies. The age-stage, two-sex life table technique is a powerful tool that can comprehensively assess pest survival, reproduction, and population growth and quantify the impacts of insect pathogens on these parameters [34]. Constructing these life tables allows for the precise evaluation of the long-term effects of insect pathogens and the refinement of field strategies. Using an age-stage, two-sex life table technique to study the role of insect pathogens in pest control can provide a robust scientific basis for biological control and integrated pest management, promoting sustainable agricultural development. The impacts of two entomopathogenic fungi, Beauveria bassiana Vuill (Hypocreales: Cordycipitaceae) and Metarhizium anisopliae Sorokin (Hypocreales: Clavicipitaceae), on the life history parameters of Coccinella septempunctata Linnaeus (Coleoptera: Coccinellidae), a significant generalist predator, were examined. The findings indicated that entomopathogenic fungi do not have any significant side effects on the performance or biology of C. septempunctata, revealing the nonpersistent impact of biocontrol agents on pest control [35]. An investigation into the biological and biochemical impacts of M. anisopliae, B. bassiana, and Purpureocillium lilacinum Thom (Hypocreales: Ophiocordycipitaceae) on the third-instar larvae of Culex pipiens Linnaeus (Diptera: Culicidae) laboratory colony revealed that M. anisopliae demonstrated a superior efficacy. It displayed the highest larval mortality (88%) and the shortest LT50 (22.6 h), indicating its potential as the most effective biological control agent among the fungi assessed. In addition, the study revealed a reduction in female fecundity, the number of hatched eggs, the pupation percentage, and the adult emergence percentage, as well as changes in biochemical indicators. Thus, M. anisopliae has proven to be an effective biological control agent for C. pipiens [36]. Exposure to sublethal (LC20) and lethal concentrations (LC50) of B. bassiana significantly impacted the parental generation (F0) of Spodoptera exigua Hubner (Lepidoptera: Noctuidae), and these effects cascaded to the demographic parameters of the first filial generation (F1). The infected F1 offspring exhibited a decreased r, an extended T, and a reduced R0. Furthermore, the fecundity of the B. bassiana-infected groups was notably lower than that of the control. These findings highlight the enduring effects of B. bassiana on the biological parameters and population dynamics of S. exigua, underscoring its potential as an eco-friendly biopesticide for pest management [37].
2.1.2. Evaluation of the Pest Control Capacity of Bio-Pesticides
Pest outbreaks and resilience pose significant threats to food security, highlighting the need for effective pest control measures. Traditional chemical pesticides have limitations, including environmental pollution and the development of pest resistance. Therefore, new pest control technologies, such as biopesticides, are essential to address these challenges and provide sustainable solutions for crop protection [38]. Biopesticides, including microbial biopesticides and other biocontrol agents, offer a promising alternative to traditional chemical pesticides. These biologics are derived from various sources, such as microorganisms (e.g., metabolites) and plants (e.g., secretions; essential oils; and extracts of bark, roots, and leaves) [39]. They encompass a diverse array of microbial pesticides [40], including bacteria, fungi, and viruses, as well as biochemical pesticides [41], such as pheromones and natural plant inducers, and plant-derived pesticides [42], such as plant extracts. These components leverage the natural antagonistic properties of these organisms and compounds to disrupt pest life cycles, deter feeding, or induce resistance in host plants, thereby providing sustainable solutions for agriculture and environmental protection [40,41,42]. To better understand the factors and effects of biopesticides on pest control, it is essential to evaluate their long-term impacts on pest population sizes and dynamics, as well as their potential effects on pests and other beneficial insects. Life table evaluation techniques can be employed to assess these impacts, providing valuable insights for the effective use of biopesticides in IPM strategies [43]. This evaluation will help optimize the application of biopesticides and enhance their role in sustainable agriculture. Research has shown that treating second-instar larvae of Helicoverpa armigera Hubner (Lepidoptera: Noctuidae) with HearNPV, a nucleopolyhedrovirus to H. armigera, and then parasitizing them with Habrobracon hebetor Say (Hymneoptera: Braconidae) results in sublethal effects on H. hebetor, including reduced longevity and fecundity at sublethal concentrations (e.g., LC30) and decreased population growth parameters (e.g., R0 and rm). However, under field conditions, H. hebetor can still effectively control H. armigera when released 2 days after HearNPV application, suggesting that their combined use in pest management is feasible [44].
2.1.3. Screening of Insect-Resistant Plant Species
Host resistance plays a vital role in integrated pest management. Host plant resistance is a key component of pest management and one of the most appreciated control strategies in advanced agriculture [45]. This phenomenon is the result of heritable plant traits that cause plants to suffer less damage compared to plants lacking these qualities. Insect-resistant crop varieties, such as rice, maize, and cotton [46,47], reduce pest populations by enhancing their tolerance to insect damage. Three types of resistance determine the relationship between insects and plants: antibiosis, antixenosis, and tolerance [48]. In recent years, the field of biotechnology has witnessed remarkable advancements, including gene-editing technologies, the interspecies transfer of resistance genes, and the enhancement of systemic acquired resistance. These innovations hold great promise for the development of pest-resistant crops. Life tables have emerged as a powerful tool to effectively evaluate the potential of these novel biotechnologies, by integrating pest severity data across diverse plant species, combining the extent of pest infestations on different plants, comparing the degree of variation in damage between varieties, and providing a qualitative evaluation of plant resistance to pests, particularly the screening of resistant and susceptible varieties. For example, the biological parameters and fecundity life table of Melanaphis sorghi Theobald (Hemiptera: Aphididae), a pest that infests sorghum crops, were estimated on 15 sorghum hybrids. The results identified specific sorghum varieties that were less suitable for the pest and showed resistance to M. sorghi [49]. The r, R0, and T of Toxoptera aurantii Boyer de Fonscolombe (Hemiptera: Aphididae) on six different tea tree varieties were analyzed to determine their population dynamics and host adaptation, which were used to guide integrated pest management and the screening of T. aurantii-resistant host varieties [50].
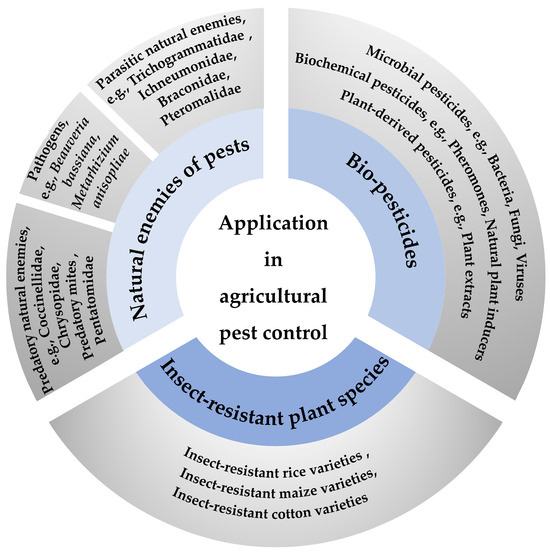
Figure 1.
Summary of application of life table in agricultural pest control. Three primary strategies—natural enemies of pests, biopesticides, and insect-resistant plant species—were used for agricultural pest control. Natural enemies of pests: predatory natural enemies [19,20,21], pathogens [35,36], and parasitic natural enemies [26,27,28,29]. Biopesticides: microbial [40], biochemical [41], and plant-derived pesticides [42]. Insect-resistant plant species: rice [46], maize, and cotton [47]. Specific examples are provided in the outer circle.
2.2. Application in Vector Insect Control
The effective management of vector insects is of paramount importance in curbing the dissemination of infectious diseases. Vector insects serve as critical conduits for the transmission of pathogens to both human and animal hosts, thus occupying a central position in public health and disease prevention initiatives [51]. Numerous infectious diseases rely heavily on vector insects for their transmission. For instance, mosquitoes, which are among the most prevalent vectors, are primarily responsible for the propagation of illnesses such as malaria, dengue fever, yellow fever, and Zika virus disease. Their broad distribution and biting behaviors significantly contribute to their role as vectors [52]. In Diptera, certain species have been shown to transmit intestinal diseases such as cholera and typhoid fever [53]. These insects facilitate pathogen transmission through direct contact with the hosts, thereby necessitating robust control measures to prevent disease outbreaks. Life tables offer a valuable tool for understanding the life cycle and population dynamics of vector insects. By providing structured and data-driven insights, life tables enable the formulation of targeted and effective control strategies. These approaches can be customized to target the unique traits and weaknesses of vector insect populations, thus optimizing the effectiveness of disease control initiatives. Evaluating the life history characteristics of vector insects using life tables can help us understand their survival rate, longevity, and fecundity at different stages; this approach aids in the development of a better understanding of the population dynamics of vector insects. Additionally, life table parameters can be used to predict the population growth rate and trends in the changes in vectors. Ultimately, this information can be used to formulate targeted strategies, providing a scientific basis for the implementation of effective vector control measures [54] (Figure 2 and Table S1).
2.2.1. Assessing the Risk of Transmission by Vector Insects
Life tables hold crucial significance in assessing the transmission risk associated with vector insects. They serve as an indispensable tool, providing essential indicators for evaluating the transmission potential of these vector pests and the corresponding disease transmission risk. By analyzing life table parameters, such as the longevity, survival rates, and reproduction rates, we can gain a comprehensive understanding of the population growth dynamics and disease transmission capacity of the insects under various environmental conditions. With these insights, we can then develop targeted interventions aimed at reducing the population size and mitigating the disease transmission risk posed by vector pests. By analyzing the life table of Anopheles balabacensis Baisas (Diptera: Culicidae), the main vector of Plasmodium knowlesi Sinton and Mulligan (Haemosporida: Plasmodiidae), to assess the potential for the nonzoonotic transmission of P. knowlesi, it is possible to estimate the survival rate of mosquitoes better. These estimates of mosquito survival rates enable an assessment of the duration and likelihood of parasite development in a mosquito and its transmission to give rise to a secondary case [55]. By studying the population growth and survival rates of the malaria vector Anopheles stephensi Liston (Diptera: Culicidae) in different water pollutants and analyzing the impact of changes in water quality on this particular mosquito, the risk of transmission of vector-borne diseases can be assessed, which is an important guide for the development of effective public health strategies and policies [56].
2.2.2. Modeling the Dynamics of Vector Insects
Accurately modeling the population dynamics of vector insects is essential for understanding and controlling the spread of the diseases they transmit. Life table data form the cornerstone of such modeling efforts. By integrating environmental factors, climatic data, and disease transmission parameters, life table data can be used to develop mathematical models that simulate and predict the population changes and disease transmission dynamics of vector pests [57]. These models incorporate various factors, such as environmental conditions, climatic variables, and disease-transmission parameters [57]. The information generated by these models is extremely valuable for guiding the development and refinement of vector-pest-control strategies [58]. By leveraging this information, we can design more targeted and effective interventions to combat the threat posed by vector insects and the diseases they carry. By employing a pseudostage-structured population dynamics model, environmental dependencies were deduced from life cycle observations for Culex quinquefasciatus Say (Diptera: Culicidae) and C. pipiens. Photoperiodicity and temperature emerged as critical factors influencing the duration of the larval stage. It was discovered that meticulously timed life history observations under natural field settings can accurately predict insect development across the annual cycle [59]. A temperature-dependent phenology model for the whitefly vector has also been developed using the Insect Life Cycle Modeling (ILCYM) software [60]. The impact of temperature on the whitefly’s virus transmission efficiency was assessed via controlled lab experiments at eight constant temperatures (10–25 °C). The vector’s transmission capacity was the strongest at 15 °C (about 70% infection probability) but dropped sharply to <10% at 10 and 20 °C. A nonlinear function describing the temperature-dependent transmission probability of a single adult whitefly was validated using transmission frequencies under fluctuating temperatures. This function, along with life table parameters from the temperature-dependent phenology model, formed a comprehensive temperature-responsive model for predicting PYVV’s spread potential and transmission probabilities. The best-performing risk index was used to create risk maps. These maps not only accurately reflected the virus’s actual occurrence but also predicted high-risk areas where it had not been reported before. Surveillance in western Panama, a predicted high-risk area, led to the virus’s identification there, where it was previously unknown [60].
2.2.3. Interference with the Life Cycle of Vector Insects
The application of life table data is crucial for understanding the life cycle of vector insects and pinpointing key stages for intervention. These data reveal the various developmental stages of vector pests and their associated vulnerabilities. By targeting these stages, such as disrupting reproduction, larval hatching, or adult longevity, we can effectively reduce the population size and disease transmissibility of vector pests. This life-table-based approach provides a strategic framework for developing interventions that disrupt the life cycle of vector insects and mitigate the risk of disease transmission [61]. The entomopathogenic bacterium Chromobacterium anophelis Frankland (Flavobacteriales: Flavobacteriaceae) sp. nov. IRSSSOUMB001 was utilized to assess its pathogenic potential against the larval stage of Anopheles coluzzii Coetzee and Wilkerson (Diptera: Culicidae), as well as its influence on the reproductive capabilities and transgenerational consequences of mosquitoes. This research confirmed the efficacy of the bacterium in infecting A. coluzzii, which serves as a vector for malaria transmission. Furthermore, this study highlighted the pronounced virulence of the strain IRSSSOUMB001 against insecticide-resistant A. coluzzii larvae and its capacity to decrease mosquito fecundity and the fitness of its progeny [62].
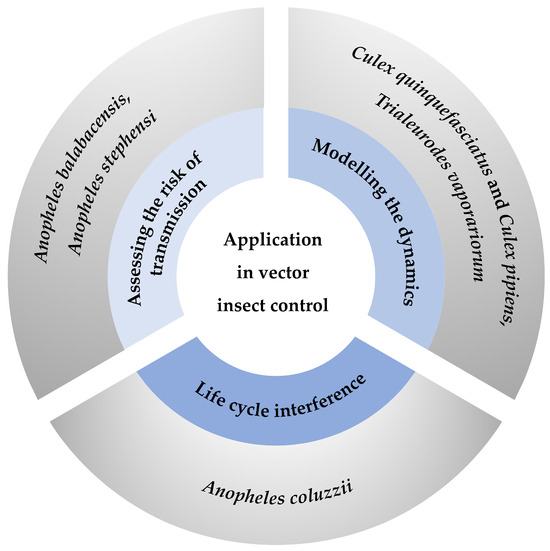
Figure 2.
Summary of application of life tables in vector insect control. Three primary strategies for controlling vector insects include assessing the risk of transmission [55,56], modeling the dynamics [59,60], and performing life cycle interference [62]. Each strategy is represented by a segment of the circle, with specific examples provided in the outer circle.
2.3. Application in Invasive Pest Control
Biological invasions are increasingly recognized as important spatial processes that drive global change and threaten biodiversity, regional economies, and ecosystem function [63]. The application of life tables to invasive pests is an important tool for the study and management of invasive pest population dynamics, life cycles, reproductive potential, and impacts on ecosystems. Life table analyses can help monitor the population sizes, growth rates, and trends of invasive pests. By collecting data on the life cycle, survival rates, and reproduction rates of the population, life table models can be constructed to predict future population sizes. Life tables provide important information about the life cycle characteristics of invasive pests, such as the hatching rate, development time, sex ratio, and longevity [64]. These data can be used to understand the life history and life history parameters of a pest.
Investigations have employed life table analyses to evaluate the suitability of three plant species—tomatoes, potatoes, and eggplants—as hosts for the invasive mealybug, Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae). The results revealed that all the tested host plants were indeed suitable for P. solenopsis. Notably, the eggplant host plant exhibited the highest fecundity, R0, and λ, as well as the longest adult longevity (males: 6.50 ± 0.34 days; females: 24.15 ± 0.50 days). These findings provide critical insights that will significantly aid in the development of targeted and effective management strategies for successfully controlling this invasive pest in major Mediterranean crop systems [65]. The study constructed a life table for Acanthococcus lagerstroemiae Kuwana (Hemiptera: Eriococcidae) to analyze the impact of plant nutrient conditions on its population. The results indicated that, under nutrient-deficient conditions (cultivated with water only), A. lagerstroemiae had a higher intrinsic rate of increase, finite rate of increase, and net reproductive rate. In contrast, under nutrient-rich conditions (0.1 MS nutrient solution), the mean generation time of A. lagerstroemiae was longer. This suggests that A. lagerstroemiae performs better on plants under nutrient-deficient conditions. These findings provide a basis for developing environmentally friendly pest management strategies [66].
3. Application of Life Tables in Chemical Control of Pests
Chemical application is considered one of the most critical methods of pest control, especially in intensive agricultural practices [67]. This is because the effectiveness of chemical control is significantly influenced by the morphological and developmental stage structure of pest populations, which is crucial for determining the susceptibility of pests to insecticides and ensuring the success of pest management strategies [68] (Figure 3 and Table S1).
3.1. Evaluating the Fitness Costs of Pesticide Resistance in Pests
The frequent use of insecticides has led to the development of resistance in many insects [69]. As pest resistance to insecticides gradually increases, the efficacy of these chemicals diminishes, and in some cases, they may even fail to control the pests, which in turn impacts agricultural production. Therefore, evaluating the fitness costs associated with pesticide resistance in pests is crucial for understanding the dynamics of resistance and developing effective control measures. An investigation into the correlation between imidacloprid resistance and the fitness of the melon aphid Aphis gossypii Glover (Hemiptera: Aphididae) was undertaken, offering valuable insights into the potential fitness trade-offs related to resistance against this neonicotinoid insecticide. The imidacloprid-resistant lines (ImR) presented prolonged developmental stages, shortened longevity, and decreased fecundity. Key demographic parameters were significantly reduced in ImR, indicating that resistance has a fitness cost. At the molecular level, the expression of genes related to development and reproduction changed, and some of these genes were downregulated. These findings have important implications for understanding the development and spread of resistance, and they provide a scientific basis for field management, helping to delay the development and spread of resistance [70]. A comprehensive study on the meadow nightshade moth Spodoptera frugiperda Smith and Abbot (Lepidoptera: Noctuidae) and its ability to adapt to chlorpyrifos on various host plants has been conducted. The life table parameters of the moths on these different hosts indicated the presence of a fitness cost associated with resistance to chlorpyrifos at both the individual and population levels. This finding suggests that the withdrawal of selective agents from the environment could result in a decrease in resistance levels, presenting an opportunity to restore susceptibility to these agents [71].
3.2. Guiding the Selection of Insecticides
Synthetic insecticides have become an integral component of global plant protection strategies [72]. Given their widespread use, selecting the appropriate insecticide has emerged as a pivotal step in effective pest management. This decision-making process necessitates a thorough understanding of both the target pest and its environmental context [73]. Life tables, which offer detailed insights into the life cycle and population dynamics of pests, are instrumental in guiding the selection of insecticides. The choice of insecticide is a complex and multifaceted decision that demands careful consideration of the pest’s biology and the broader ecological implications. Life tables provide essential data on pest life cycles and population dynamics, enabling more informed and targeted insecticide choices. By integrating these data with an understanding of insecticide mechanisms and types, pest management strategies can be optimized to achieve effective control while minimizing their environmental impact. Using an age-stage life table methodology, the sublethal concentration (LC50) of triflumizole-pyrimidines against Laodelphax striatellus Fallén (Hemiptera: Delphacidae) was evaluated. Compared with those of the F0 generation, the r, λ, and R0 of the F5 generation were significantly lower, suggesting that the LC50 of triflumezopyrim may impede the generational growth and reproduction of L. striatellus. This evaluation is intended to provide a foundation for future research attempting to elucidate the adaptability and resistance mechanisms of L. striatellus in response to sublethal doses of triflumizole pyrimidine [74]. A thorough assessment of alternative pesticide efficacy was carried out by testing the susceptibility of Bradysia odoriphaga Yang and Zhang (Diptera: Sciaridae) and Bradysia difformis Johannsen (Diptera: Sciaridae) to multiple insecticides. The toxicity of eight pesticides was evaluated against several parasitic species, with a focus on how sublethal doses of dinotefuran and lufenuron affected their life history traits and detoxification enzyme functions. The results indicated that dinotefuran and lufenuron were particularly toxic to B. odoriphaga and B. difformis out of the tested compounds. Moreover, sublethal concentrations of these pesticides significantly impaired the life history parameters across both generations of the species. These findings offer valuable insights for targeted pest control, underscoring the efficacy of dinotefuran and lufenuron as effective management tools [75].
3.3. Guiding the Application of Insecticides
The rational application of insecticides is essential for effective pest control while minimizing the environmental impact. Life table data play a pivotal role in guiding the timing and dosage of insecticide applications, ensuring that interventions are both effective and efficient. A simulation using a sex-based life table can predict the optimal period for pest control and accurately determine the optimal timing and number of chemical control applications [76]. Following the application of pesticides, the population size and age structure of pests undergo significant changes. By analyzing the population structure and considering factors such as the lethality of insecticides for different insect states and age classes, the persistence period of insecticides, and the economic threshold (ET), we can utilize the amphoteric life table and the TIMING—MSChart to predict the effects of insecticide use on the growth and reproduction of pests and their progeny [77]. This approach enables us to simulate the appropriate timing of control measures, thereby optimizing the use of insecticides and enhancing the effectiveness of pest management strategies. A detailed life table was constructed for the coffee berry borer Hypothenemus hampei Ferrari (Coleoptera: Curculionidae) by meticulously calculating its survival and reproduction rates across various developmental stages. This comprehensive analysis allowed for the determination of R0 for each population, thereby pinpointing the optimal timing for the most effective pest control interventions [78]. A comprehensive study was conducted to elucidate the life cycle characteristics of the brown marmorated stink bug Halyomorpha halys Stål (Hemiptera: Pentatomidae) under various temperature conditions. Through a comprehensive approach involving both laboratory and field experiments, this study examined the influence of temperature fluctuations on the diverse life stages of H. halys in diverse geographical regions within the United States. Furthermore, it quantified the varying patterns of these pivotal parameters at distinct temperature levels, thereby providing a solid scientific foundation for predicting and managing the population dynamics of this insect pest [79].
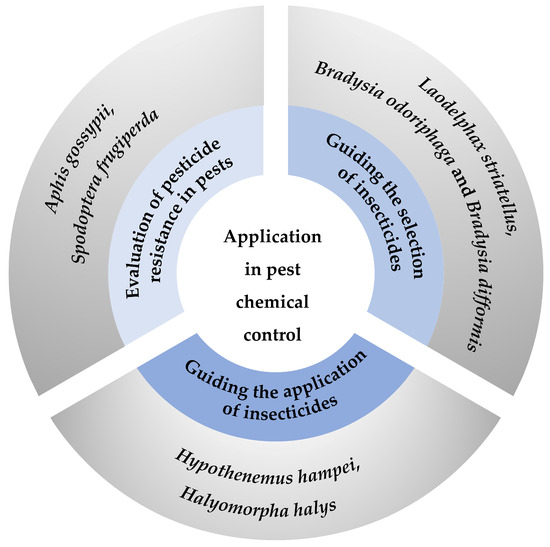
Figure 3.
Summary of application of life tables in pest chemical control. Three primary strategies for pest chemical control include evaluating pesticide resistance [70,71] and guiding both the selection [74,75] and the application [78,79] of insecticides. Each strategy is represented by a segment of the circle, with specific examples provided in the outer circle.
4. Discussion and Future Perspectives
Life tables have significantly advanced pest management by quantifying critical demographic parameters such as survival rates, developmental timelines, and reproductive output. Studies on predators such as O. strigicollis and parasitoids such as Trichogramma species have demonstrated their efficacy under controlled conditions, with peak performance linked to intermediate prey densities and host-specific adaptations [22,23,30,80]. Similarly, entomopathogenic fungi (EPFs) show compatibility with predators such as C. septempunctata, suggesting potential synergies in multi-agent biocontrol systems [35]. However, these findings remain largely confined to laboratory settings, and the field-scale validation of ecological interactions—such as cascading trophic effects or multi-predator dynamics—is still limited. This gap underscores the need to reconcile controlled-environment insights with the complexity of natural agroecosystems, where biotic factors (e.g., natural enemies) and abiotic stressors (e.g., temperature fluctuations) interact unpredictably [36,71].
A critical limitation of current life table applications lies in their narrow focus on single-species or single-factor interactions. For instance, while fitness costs associated with pesticide resistance—such as a reduced fecundity in imidacloprid-resistant A. gossypii [70] or chlorpyrifos-resistant S. frugiperda [71]—highlight trade-offs exploitable for resistance management, most studies have neglected population-level evolutionary drivers such as gene flow and selection pressures [81]. Similarly, invasive pests such as S. frugiperda exhibit diet-dependent population growth in artificial rearing systems, yet these outcomes often diverge from field realities, where the host plant quality and climate variability modulate invasion success [66,71]. Cross-species comparisons and multi-trophic analyses (e.g., pest–plant–microbe interactions) remain understudied, limiting the generalizability of life table-derived strategies.
Emerging technologies offer promising solutions to scalability and contextual limitations. Automated video tracking, remote sensing, and machine learning algorithms enable the real-time, large-scale monitoring of pest behavior and population trends [62,82], while genomic tools elucidate resistance mechanisms in pests such as A. gossypii [70]. Climate-driven models, such as temperature-sensitive phenological frameworks for T. vaporariorum [83], integrate environmental variables to predict pest outbreaks with increasing precision. However, the effectiveness of these tools hinges on their validation across diverse agroecosystems and their integration with traditional life table data. Bridging this gap requires adaptive “smart IPM” systems that dynamically synthesize laboratory-derived parameters, field observations, and environmental datasets to optimize intervention timing and spatial targeting.
The context-specific nature of life table outcomes further complicates broad applications. For example, the efficacy of M. anisopliae against C. pipiens larvae varies markedly with the instar stage and microhabitat conditions [36], while the fitness costs in resistant pests often depend on the host plant chemistry or regional climate patterns [70,71]. Such variability necessitates localized validation and regionally tailored management strategies. Longitudinal studies across multiple generations and agroecological zones are critical to deciphering resistance evolution, predator–prey coadaptation, and the long-term stability of biocontrol agents. Additionally, expanding life table frameworks to assess indirect ecological impacts—such as the effects of insect-resistant crops on pollinators or soil microbiota—would enhance the sustainability of IPM programs [54]. To fully realize the potential of life tables in IPM, future research must prioritize cross-disciplinary integration. Combining population models with food web analytics, genomic insights, and climate resilience frameworks will enable a holistic understanding of pest dynamics. Concurrently, establishing open-access databases of regionally calibrated life table parameters could guide context-specific interventions. By addressing these challenges, life table analyses can transition from a descriptive tool to a predictive engine for designing adaptive, ecologically informed pest management strategies that balance efficacy with environmental stewardship.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/insects16030261/s1. Table S1: Summary of research on the application of life tables in integrated pest control.
Author Contributions
Conceptualization, Z.C., Y.L., R.Y., B.L., J.Z., H.L. and L.W. (Lingjun Wang); software, Y.L. and D.S.; methodology, L.W. (Lingjun Wang) and B.L.; validation, Y.W., J.Z. and H.L.; formal analysis, R.Y.; investigation, Z.C.; data curation, Z.C.; writing—original draft preparation, Z.C., Y.L., Y.W., D.S. and L.W. (Liang Wang); writing—review and editing, J.Z., B.L., H.L., R.Y. and L.W. (Lingjun Wang); visualization, Z.C.; supervision, H.L., B.L. and J.Z.; project administration, R.Y. and L.W. (Lingjun Wang); funding acquisition, L.W. (Lingjun Wang) and R.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation Project of China, grant numbers 82060374 and 82360401; the Science and Technology Foundation of Guizhou Province, grant number QKHJC–ZK-[2023]-YB517; the Open Project Fund of Key Laboratory of Parasite and Vector Biology, grant number NHCKFKT2023-10; the Youth Science and Technology Talent Growth Project of Education Department of Guizhou Province, grant number [2024]137; and the Science and Technology Foundation of Zunyi City, grant number ZSKH-HZ (2024)336.
Data Availability Statement
Data sharing is not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| IPM | integrated pest management |
| PYVV | potato yellow vein virus |
| ILCYM | insect life cycle modeling |
| GIS | geographic information system |
References
- Jia, X.; Wang, Y.; Zhao, Z. Estimation of the invasiveness of alien fruit flies with life table. J. Plant Prot. 2023, 50, 839–840. [Google Scholar]
- Schumacher, A.E.; Kyu, H.H.; Aali, A.; Abbafati, C.; Abbas, J.; Abbasgholizadeh, R.; Abbasi, M.A.; Abbasian, M.; Abd ElHafeez, S.; Abdelmasseh, M.; et al. Global age-sex-specific mortality, life expectancy, and population estimates in 204 countries and territories and 811 subnational locations, 1950–2021, and the impact of the COVID-19 pandemic: A comprehensive demographic analysis for the global burden of disease study 2021. Lancet 2024, 403, 1989–2056. [Google Scholar]
- Forchibe, E.E.; Fening, K.O.; Vershiyi, D.T.; Cobblah, A.M.; Afreh-Nuamah, K. Comparative bionomics and life table studies of Lipaphis erysimi pseudobrassicae (Davis) and Myzus persicae (Sulzer) (Hemiptera: Aphididae) on three cabbage varieties. Bull. Entomol. Res. 2023, 113, 380–388. [Google Scholar] [CrossRef]
- van Klink, R.; August, T.; Bas, Y. Emerging technologies revolutionize insect ecology and monitoring. Trends Ecol. Evol. 2022, 37, 872–885. [Google Scholar] [CrossRef]
- Carey, J.R. Insect biodemography. Annu. Rev. Entomol. 2001, 46, 79–110. [Google Scholar] [CrossRef]
- Chi, H.; You, M.; Atlıhan, R.; Smith, C.L.; Kavousi, A.; Özgökçe, M.S.; Güncan, A.; Tuan, S.-J.; Fu, J.-W.; Xu, Y.-Y.; et al. Age-Stage, two-sex life table: An introduction to theory, data analysis, and application. Entomol. Gen. 2020, 40, 103–124. [Google Scholar] [CrossRef]
- Hsin, C.; Jianwei, F.; Minsheng, Y. Age-stage, two-sex life table and its application in population ecology and integrated pest management. J. Plant Prot. 2019, 62, 255–262. [Google Scholar]
- Ning, S.; Zhang, W.; Sun, Y. Development of insect life tables: Comparison of two demographic methods of Delia antiqua (Diptera: Anthomyiidae) on different hosts. Sci. Rep. 2017, 7, 4821. [Google Scholar] [CrossRef] [PubMed]
- Novianto, D.; Hadi, U.K.; Soviana, S.; Darusman, H.S. Comparison of diurnal biting activity, life table, and demographic attributes of Aedes albopictus (Asian tiger mosquito) from different urbanized settings in West Java, Indonesia. Acta Trop. 2023, 241, 106771. [Google Scholar] [CrossRef]
- Zhang, K.X.; Ma, Y.; Li, C.C. Population growth of Tetranychus truncatus (Acari: Tetranychidae) on different drought-tolerant potato cultivars. J. Econ. Entomol. 2023, 116, 405–415. [Google Scholar] [CrossRef]
- Zhu, Y.; Qi, F.; Tan, X. Use of age-stage, two-sex life table to compare the fitness of Bactrocera dorsalis (Diptera: Tephritidae) on Northern and Southern Host Fruits in China. Insects 2022, 13, 258. [Google Scholar] [CrossRef]
- Van den Berg, J.; du Plessis, H. Chemical control and insecticide resistance in Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Econ. Entomol. 2022, 115, 1761–1771. [Google Scholar] [CrossRef] [PubMed]
- Withers, A.J.; Rice, A.; de Boer, J.; Wilson, K. The distribution of covert microbial natural enemies of a globally invasive crop pest, Fall Armyworm, in Africa: Enemy release and spillover events. J. Anim. Ecol. 2022, 91, 1826–1841. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Cong, L.; Zhang, Y. Advances in biological control of leguminous insect pests. Chin. Agric. Sci. Bull. 2021, 37, 113–120. [Google Scholar]
- Zhang, Q.; Lu, Y.W.; Liu, X.Y.; Li, Y. Phylogenomics resolves the higher-level phylogeny of herbivorous eriophyoid mites (Acariformes: Eriophyoidea). BMC Biol. 2024, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Saurabh, S.; Mishra, M.; Rai, P.; Pandey, R. Tiny flies: A mighty pest that threatens agricultural productivity-a case for next-generation control strategies of Whiteflies. Insects 2021, 12, 585. [Google Scholar] [CrossRef]
- Mound, L.A.; Wang, Z.; Lima, É.F.B.; Marullo, R. Problems with the concept of "pest" among the diversity of pestiferous thrips. Insects 2022, 13, 61. [Google Scholar] [CrossRef]
- Janssen, A.; Fonseca, M.M.; Marcossi, I.; Kalile, M.O. Estimating intrinsic growth rates of arthropods from partial life tables using predatory mites as examples. Exp. Appl. Acarol. 2022, 86, 327–342. [Google Scholar] [CrossRef]
- Li, H.; Li, B.; Lövei, G.L.; Kring, T.J.; Obrycki, J.J. Interactions among native and non-native predatory coccinellidae influence biological control and biodiversity. Ann. Entomol. Soc. Am. 2021, 114, 119–136. [Google Scholar] [CrossRef]
- Hassan, M.A.; Liu, X. The green lacewings of Pakistan (Neuroptera: Chrysopidae): A faunal review with new records of genera and species. Zootaxa 2022, 5180, 1–83. [Google Scholar] [CrossRef]
- Li, J.; Tian, X.; Hsiang, T.; Yang, Y.; Shi, C. Microbial community structure and metabolic function in the venom glands of the predatory stink bug, Picromerus lewisi (Hemiptera: Pentatomidae). Insects 2024, 15, 727. [Google Scholar] [CrossRef]
- Ali, S.; Li, S.; Jaleel, W.; Khan, M.M.; Wang, J.; Zhou, X. Using a two-sex life table tool to calculate the fitness of Orius strigicollis as a predator of Pectinophora gossypiella. Insects 2020, 11, 275. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.U.; Zhou, X.; Ali, S. Predatory functional response and fitness parameters of Orius strigicollis Poppius when fed Bemisia tabaci and Trialeurodes vaporariorum as determined by age-stage, two-sex life table. PeerJ 2020, 8, e9540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sheng, F.; Wang, E.; Lv, J.; Xu, X. Amblyseius orientalis shows high consumption and reproduction on Polyphagotarsonemus latus in China. Exp. Appl. Acarol. 2023, 91, 561–569. [Google Scholar] [CrossRef]
- Xi, O.; Zhu, D.; Zhong, W. Investigation on parasitic natural enemy resources of Yponomeuta padella (Lepidoptera: Yponomeutidae) in wild fruit forest of western Tianshan Mountains, Xinjiang. Sci. Silvae Sin. 2022, 58, 131–139. [Google Scholar]
- Sousa, T.C.D.S.; Leite, N.A.; Sant Ana, J. Responses of Trichogramma pretiosum (Hymenoptera: Trichogrammatidae) to rice and corn plants, fed and oviposited by Spodoptera frugiperda (Lepidoptera: Noctuidae). Neotrop. Entomol. 2021, 50, 697–705. [Google Scholar] [CrossRef]
- Gaione-Costa, A.; DE Pdua, D.G.; Delazari, T.M.; Santos, A.R.S.; Kloss, T.G. Redescription and oviposition behavior of an orb-weaver spider parasitoid Hymenoepimecis cameroni Townes, 1966 (Hymenoptera: Ichneumonidae). Zootaxa 2022, 5134, 415–425. [Google Scholar] [CrossRef]
- Tomanović, Ž.; Kavallieratos, N.G.; Ye, Z.; Nika, E.P. Cereal aphid parasitoids in Europe (Hymenoptera: Braconidae: Aphidiinae): Taxonomy, biodiversity, and ecology. Insects 2022, 13, 1142. [Google Scholar] [CrossRef]
- Pagac, A.A.; Geden, C.J.; Burgess, E.R.; Riggs, M.R.; Machtinger, E.T. Filth Fly Parasitoid (Hymenoptera: Pteromalidae) monitoring techniques and species composition in Poultry Layer Facilities. J. Med. Entomol. 2022, 59, 2006–2012. [Google Scholar] [CrossRef]
- Salim, M.; Ullah, I.; Saljoqi, A.U.R. Life table study of Sitotroga cerealella on different cereals and its implications on the performance of the egg parasitoid (Trichogramma chilonis) under laboratory conditions. Sci. Rep. 2023, 13, 10961. [Google Scholar] [CrossRef]
- Tabebordbar, F.; Shishehbor, P.; Ebrahimi, E.; Polaszek, A.; Ugine, T.A. Effect of different constant temperatures on life history and life table parameters of Trichogramma euproctidis (Hymenoptera: Trichogrammatidae). J. Econ. Entomol. 2022, 115, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Moraes, R.J.S.S.; Silva-Torres, C.S.A.; Barbosa, P.R.R. Olfaction Response and Fertility Life Table Parameters of Tetrastichus howardi (Hymenoptera: Eulophidae) Parasitizing Plutella xylostella (Lepidoptera: Plutellidae) and the factitious host Tenebrio molitor (Coleoptera: Tenebrionidae). Neotrop. Entomol. 2023, 52, 921–931. [Google Scholar] [CrossRef]
- Islam, W.; Adnan, M.; Shabbir, A.; Naveed, H. Insect-fungal-interactions: A detailed review on entomopathogenic fungi pathogenicity to combat insect pests. Microb. Pathog. 2021, 159, 105122. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, R.F.; Freed, S.; Ahmed, R.; Raza, M.; Naeem, A. Virulence and transgenerational effects of Metarhizium anisopliae on Oxycarenus hyalinipennis. Pest Manag. Sci. 2023, 79, 3843–3851. [Google Scholar] [CrossRef]
- Rizwan, M.; Atta, B.; Arshad, M. Nondetrimental impact of two concomitant entomopathogenic fungi on life history parameters of a generalist predator, Coccinella septempunctata (Coleoptera: Coccinellidae). Sci. Rep. 2021, 11, 20699. [Google Scholar] [CrossRef]
- Hamama, H.M.; Zyaan, O.H.; Abu Ali, O.A. Virulence of entomopathogenic fungi against Culex pipiens: Impact on biomolecules availability and life table parameters. Saudi J. Biol. Sci. 2022, 29, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Zafar, J.; Shoukat, R.F.; Zhu, Z.; Fu, D.; Xu, X. Two-Sex Life Table Analysis for Optimizing Beauveria bassiana Application against Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae). J. Fungi 2024, 10, 469. [Google Scholar] [CrossRef]
- Mangan, R.; Bussière, L.F.; Polanczyk, R.A.; Tinsley, M.C. Increasing ecological heterogeneity can constrain biopesticide resistance evolution. Trends Ecol. Evol. 2023, 38, 605–614. [Google Scholar] [CrossRef]
- Ayilara, M.S.; Adeleke, B.S.; Akinola, S.A. Biopesticides as a promising alternative to synthetic pesticides: A case for microbial pesticides, phytopesticides, and nanobiopesticides. Front. Microbiol. 2023, 14, 1040901. [Google Scholar] [CrossRef]
- Bravo, A.; Soberón, M. Can microbial-based insecticides replace chemical pesticides in agricultural production? Microb. Biotechnol. 2023, 16, 2011–2014. [Google Scholar] [CrossRef]
- Francis, F.; Jacquemyn, H.; Delvigne, F.; Lievens, B. From diverse origins to specific targets: Role of microorganisms in indirect pest biological control. Insects 2020, 11, 533. [Google Scholar] [CrossRef]
- Mitsumoto, A.; Yamazaki, T. A pilot test of olive weevil repellents in an olive orchard. Yakugaku Zasshi 2024, 144, 675–683. [Google Scholar] [CrossRef]
- Naranjo, S.E.; Cañas, L.; Ellsworth, P.C. Mortality dynamics of a polyphagous invasive herbivore reveal clues in its agroecosystem success. Pest Manag. Sci. 2022, 78, 3988–4005. [Google Scholar] [CrossRef]
- Allahyari, R.; Aramideh, S.; Michaud, J.P.; Safaralizadeh, M.H.; Rezapanah, M.R. Negative Life History Impacts for Habrobracon hebetor (Hymneoptera: Braconidae) that Develop in Bollworm Larvae Inoculated with Helicoverpa armigera Nucleopolyhedrovirus. J. Econ. Entomol. 2020, 113, 1638–1655. [Google Scholar] [CrossRef]
- Mansour, M.R.; Eryan, N.L. Effects of certain weather, biotic factors and chemical components on the population of aphids in egyptian wheat fields. Egypt. Acad. J. Biol. Sciences. A Entomol. 2022, 15, 1–13. [Google Scholar]
- Li, C.; Xiong, Z.; Fang, C.; Liu, K. Transcriptome and metabolome analyses reveal the responses of brown planthoppers to RH resistant rice cultivar. Front. Physiol. 2022, 13, 1018470. [Google Scholar] [CrossRef] [PubMed]
- Machado, E.P.; dos SRodrigues Junior, G.L.; Führ, F.M.; Zago, S.L.; Marques, L.H.; Santos, A.C.; Nowatzki, T.; Dahmer, M.L.; Omoto, C.; Bernardi, O. Cross-crop resistance of Spodoptera frugiperda selected on Bt maize to genetically modified soybean expressing Cry1Ac and Cry1F proteins in Brazil. Sci. Rep. 2020, 10, 10080. [Google Scholar] [CrossRef]
- Kumari, P.; Jasrotia, P.; Kumar, D. Biotechnological approaches for host plant resistance to insect pests. Front. Genet. 2022, 13, 914029. [Google Scholar] [CrossRef]
- Avellar, G.S.; Mendes, S.M.; Marriel, I.E. Resistance of sorghum hybrids to Sorghum Aphid. Braz. J. Biol. 2022, 82, e264139. [Google Scholar] [CrossRef]
- Lu, C.; Shen, N.; Jiang, W. Different tea germplasms distinctly influence the adaptability of Toxoptera aurantii (Hemiptera: Aphididae). Insects 2023, 14, 695. [Google Scholar] [CrossRef]
- Haridas, C.V.; Tenhumberg, B. Modeling effects of ecological factors on evolution of polygenic pesticide resistance. J. Theor. Biol. 2018, 456, 224–232. [Google Scholar] [CrossRef]
- Jones, R.T.; Ant, T.H.; Cameron, M.M.; Logan, J.G. Novel control strategies for mosquito-borne diseases. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2021, 376, 20190802. [Google Scholar] [CrossRef]
- Harnish, J.M.; Link, N.; Yamamoto, S. Drosophila as a model for infectious diseases. Int. J. Mol. Sci. 2021, 22, 2724. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Sharma, G.; Bhattacherjee, R. Life table, survival and fecundity parameters of Aedes albopictus (Diptera: Culicidae) strains from desert and coastal regions of India. Acta Trop. 2022, 235, 106625. [Google Scholar] [CrossRef] [PubMed]
- Chua, T.H.; Manin, B.O.; Fornace, K. Life table analysis of Anopheles balabacensis, the primary vector of Plasmodium knowlesi in Sabah, Malaysia. Parasites Vectors 2022, 15, 442. [Google Scholar] [CrossRef]
- Fazeli-Dinan, M.; Azarnoosh, M.; Özgökçe, M.S. Global water quality changes posing threat of increasing infectious diseases, a case study on malaria vector Anopheles stephensi coping with the water pollutants using age-stage, two-sex life table method. Malar. J. 2022, 21, 178. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.F.; Meyers, A.C.; Hodo, C.L. Trypanosoma cruzi infection in dogs along the US-Mexico border: R0 changes with vector species composition. Epidemics 2023, 45, 100723. [Google Scholar] [CrossRef]
- Sporleder, M.; Gamarra, H.; Carhuapoma, P.; Goicochea, L.; Kroschel, J.; Kreuze, J. A temperature-dependent phenology model for Bemisia tabaci MEAM1 (Hemiptera: Aleyrodidae). Environ. Entomol. 2023, 52, 832–846. [Google Scholar] [CrossRef]
- Erguler, K.; Mendel, J.; Petrić, D.V.; Petrić, M.; Kavran, M. A dynamically structured matrix population model for insect life histories observed under variable environmental conditions. Sci. Rep. 2022, 12, 11587. [Google Scholar] [CrossRef]
- Gamarra, H.; Carhuapoma, P.; Cumapa, L. A temperature-driven model for potato yellow vein virus transmission efficacy by Trialeurodes vaporariorum (Hemiptera: Aleyrodidae). Virus Res. 2020, 289, 198109. [Google Scholar] [CrossRef]
- Hameed, A.; Rosa, C.; Rajotte, E.G. The effect of species Soybean Vein Necrosis Orthotospovirus (SVNV) on life table parameters of its vector, Soybean Thrips (Neohydatothrips variabilis Thysanoptera: Thripidae). Insects 2022, 13, 632. [Google Scholar] [CrossRef]
- Gnambani, E.J.; Bilgo, E.; Dabiré, R.K.; Belem, A.M.G.; Diabaté, A. Infection of the malaria vector Anopheles coluzzii with the entomopathogenic bacteria Chromobacterium anophelis sp. nov. IRSSSOUMB001 reduces larval survival and adult reproductive potential. Malar. J. 2023, 22, 122. [Google Scholar] [CrossRef] [PubMed]
- Acharya, R.; Malekera, M.J.; Dhungana, S.K.; Sharma, S.R.; Lee, K.Y. Impact of rice and potato host plants is higher on the reproduction than growth of corn strain fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2022, 13, 256. [Google Scholar] [CrossRef]
- Bălăcenoiu, F.; Toma, D.; Nețoiu, C. From field data to practical knowledge: Investigating the bioecology of the Oak Lace Bug-an invasive insect species in Europe. Insects 2023, 14, 882. [Google Scholar] [CrossRef] [PubMed]
- Abbes, K.; Harbi, A.; Guerrieri, E.; Chermiti, B. Using Age-Stage Two-Sex Life Tables to Assess the Suitability of Three Solanaceous Host Plants for the Invasive Cotton Mealybug Phenacoccus solenopsis Tinsley. Plants 2024, 13, 1381. [Google Scholar] [CrossRef]
- Xie, R.; Wu, B.; Gu, M.; Qin, H. Life table construction for crapemyrtle bark scale (Acanthococcus lagerstroemiae): The effect of different plant nutrient conditions on insect performance. Sci. Rep. 2022, 12, 11472. [Google Scholar] [CrossRef] [PubMed]
- Gul, H.; Gadratagi, B.G.; Güncan, A. Fitness costs of resistance to insecticides in insects. Front. Physiol. 2023, 14, 1238111. [Google Scholar] [CrossRef]
- Skouras, P.J.; Karanastasi, E.; Demopoulos, V. Toxicity and influence of sublethal exposure to sulfoxaflor on the aphidophagous predator Hippodamia variegata (Coleoptera: Coccinellidae). Toxics 2023, 11, 533. [Google Scholar] [CrossRef]
- Wang, W.; Huang, Q.; Liu, X.; Liang, G. Differences in the sublethal effects of sulfoxaflor and acetamiprid on the Aphis gossypii Glover (Homoptera: Aphididae) are related to its basic sensitivity level. Insects 2022, 13, 498. [Google Scholar] [CrossRef]
- Ullah, F.; Xu, X.; Gul, H.; Güncan, A.; Hafeez, M. Impact of imidacloprid resistance on the demographic traits and expressions of associated genes in Aphis gossypii Glover. Toxics 2022, 10, 658. [Google Scholar] [CrossRef]
- Garlet, C.G.; Moreira, R.P.; Gubiani, P.D.S. Fitness cost of chlorpyrifos resistance in Spodoptera frugiperda (Lepidoptera: Noctuidae) on different host plants. Environ. Entomol. 2021, 50, 898–908. [Google Scholar] [CrossRef]
- Afza, R.; Afzal, A.; Riaz, M.A. Sublethal and transgenerational effects of synthetic insecticides on the biological parameters and functional response of Coccinella septempunctata (Coleoptera: Coccinellidae) under laboratory conditions. Front. Physiol. 2023, 14, 1088712. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Valbon, W.; Qiu, M.; Hu, C.T.; Yang, J. Insecticidal and repellent properties of rapid-acting fluorine-containing compounds against Aedes aegypti mosquitoes. ACS Infect. Dis. 2023, 9, 1396–1407. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, X.; Gu, F. Sublethal effects of triflumezopyrim on biological traits and detoxification enzyme activities in the small brown planthopper Laodelphax striatellus (Hemiptera: Delphacidae). Front. Physiol. 2020, 11, 261. [Google Scholar] [CrossRef]
- Zhu, G.; Ding, W.; Zhao, Y.; Xue, M.; Zhao, H. Biological and physiological responses of two bradysia pests, Bradysia odoriphaga and Bradysia difformis, to dinotefuran and lufenuron. Pestic. Biochem. Physiol. 2023, 190, 105338. [Google Scholar] [CrossRef] [PubMed]
- Naeem, A.; Hafeez, F.; Iftikhar, A. Laboratory induced selection of pyriproxyfen resistance in Oxycarenus hyalinipennis Costa (Hemiptera: Lygaeidae): Cross-resistance potential, realized heritability, and fitness costs determination using age-stage, two-sex life table. Chemosphere 2021, 269, 129367. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Fu, J.; Li, J. Projection of insect population dynamics with age-stage, two-sex life table and its application in pest management. Acta Entomol. Sin. 2023, 66, 255–266. [Google Scholar]
- Mariño, Y.A.; Bayman, P.; Sabat, A.M. Demography and perturbation analyses of the coffee berry borer Hypothenemus hampei (Coleoptera: Curculionidae): Implications for management. PLoS ONE 2021, 16, e0260499. [Google Scholar] [CrossRef]
- Mermer, S.; Maslen, E.A.; Dalton, D.T. Temperature-dependent life table parameters of brown marmorated stink bug, Halyomorpha halys (Stål) (Hemiptera: Pentatomidae) in the United States. Insects 2023, 14, 248. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, Q.; Keyhani, N.O. Biocontrol performance and mass production potential of the larval endoparasitoid Campoletis chlorideae Uchida (Hymenoptera: Ichneumonidae) against the fall armyworm, Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae). Egypt. J. Biol. Pest Control 2024, 34, 44. [Google Scholar] [CrossRef]
- Zhou, D.-H.; Zhang, Q.-G. Fast drug rotation reduces bacterial resistance evolution in a microcosm experiment. J. Evol. Biol. 2023, 36, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Sharada, K.; Choudhary, S.L.; Harikrishna, T. GeoAgriGuard: AI-Driven Pest and Disease Management with Remote Sensing for Global Food Security. Remote Sens. Earth Syst. Sci. 2025. [Google Scholar] [CrossRef]
- Burc, E.; Girard-Tercieux, C.; Metz, M. Life-history adaptation under climate warming magnifies the agricultural footprint of a cosmopolitan insect pest. Nat. Commun. 2025, 16, 827. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).