Simple Summary
The ladybug Henosepilachna vigintioctomaculata is a widely distributed leaf-eating pest. Lambda-cyhalothrin, a synthetic pyrethroid insecticide, is widely used to control leaf-eating pests. However, the effects of this insecticide on biological activity, cross-generations, and detoxification enzyme activity of this ladybug are imperfectly known. We reared H. vigintioctomaculata for at least three generations under experimental conditions to determine the effects of lambda-cyhalothrin on biological activity. We evaluated the sublethal effects on F0 generation adults, and cross-generational effects on F1 adults, using age–stage bisexual life tables, and examined the detoxification enzyme activity of F0 adults. Sublethal concentrations of lambda-cyhalothrin significantly reduced F0 adult longevity and average fecundity, and inhibited various life table parameters in the F1 population; there was also a cross-generational genetic effect, with population growth being inhibited. Low concentrations of lambda-cyhalothrin significantly inhibit H. vigintioctomaculata population growth. Multifunctional oxidase, carboxylesterase, and glutathione S-transferase play important roles in H. vigintioctomaculata resistance to lambda-cyhalothrin.
Abstract
Lambda-cyhalothrin is a synthetic pyrethroid insecticide that is widely used to control leaf-eating pests. Because of increased insecticide resistance, an understanding of sublethal cross-generational effects of insecticides is important. We examine the effects of sublethal concentrations (SLCs) (LC10, LC20, and LC40) of lambda-cyhalothrin on the growth, reproduction, and detoxification enzyme activities of F0 and F1 generation Henosepilachna vigintioctomaculata. Lambda-cyhalothrin is toxic to adult H. vigintioctomaculata, with an LC40 at 48 h of 0.355 mg L−1. At SLCs, lambda-cyhalothrin significantly reduces the longevity and average fecundity of F0 and F1 adults, and prolongs the durations of the egg, larval, and pupal stages and adult preoviposition period. Additionally, an increased lambda-cyhalothrin concentration significantly decreases net reproductive rates, and both finite and intrinsic rates of increase in the F1 generation, and significantly increases the average generation cycle. The detoxification enzyme activity of F1 adults treated with SLCs of lambda-cyhalothrin for 48 h trends upwards. Results indicate that low concentrations of lambda-cyhalothrin induce glutathione S-transferase and carboxylesterase activities and inhibit multifunctional oxidase activity. The growth, development, and reproduction of the H. viltioctomaculata F1 population remain inhibited by lambda-cyhalothrin treatment in the adult stage, and inhibitory effects increase with increased lambda-cyhalothrin concentration. The control efficacy of lambda-cyhalothrin against H. viltioctomaculata shows cross-generational effects.
1. Introduction
The 28-spotted ladybug Henosepilachna vigintioctomaculata (Coleoptera: Coccinellidae) is a crop pest that occurs widely throughout China, and elsewhere in countries such as Korea, Japan, Russia, and Australia. This ladybug is a phytophagous pest that feeds mainly on crops such as Solanaceae, Leguminosae, Cruciferae, and Cucurbitaceae, with adults and larvae eating the tender leaves and stems [1,2]. When leaves are eaten, a semi-transparent parallel-shaped depression is formed, and in severe cases, only leaf veins and the epidermis remain, causing the leaves to wilt, yellow, and die. China is an important producer of potato—a globally important food crop—and H. vigintioctomaculata seriously affects potato agriculture [2,3,4]. Changes in microclimates and promotion of intercropping of corn, vegetables, and potatoes have also led to H. vigintioctomaculata becoming both an increasingly serious pest in potato plantations in the Yunnan, Guizhou, and Sichuan regions, and a main spreader of potato brown spot disease, which also poses a threat to local potato production. In severe cases, yields can be reduced by 50% [5,6,7].
The insecticide lambda-cyhalothrin is highly efficient at controlling leaf-eating pests [8]. After spraying, insecticide residue, in general, gradually decreases over time, and with crop growth [9]. The physiology and behavior of some pests exposed to insecticides at sublethal doses may change [10,11]. Most sublethal insecticide doses have inhibitory or delayed effects on pest populations, but some can also stimulate growth and development, and promote pest proliferation [12]. These sublethal effects on pests can lead to physiological and biochemical changes and insecticide resistance. Therefore, understanding the sublethal effects of insecticides on pests is important to evaluate their efficacy and assess the risks associated with their use [13]. Pests can reduce the effects of insecticides by regulating detoxification enzyme activities [14]. Among enzymes, glutathione S-transferase (GST), carboxylesterase (CarE), and multifunctional oxidase (MFO) are the most important. Low concentrations of insecticides or plant secondary metabolites can induce or inhibit the activity of various enzymes, thereby affecting pest metabolic processes, promoting resistance and providing continuous selection pressure, reducing the effectiveness of insecticides for pest control [15,16].
While the median lethal concentration (LC50) or median lethal dose (LD50) have been commonly used to evaluate the effects of insecticides on pests, they only report the response to insecticides at certain developmental stages [17]. Life tables can be used to more comprehensively analyze the effects of pesticides on pests at the population level. Compared with traditional life tables, an age–age bisexual life table increases statistics for males, accurately describes age differentiation of insects, differentiates between the pre- and total-oviposition periods of adults, and can comprehensively describe changes in the entire population [18,19,20]. Beta-cypermethrin, phoxim, and abamectin pesticides are mostly used to control potato ladybird adults [4].While the sublethal effects of insecticides on target species are being increasingly explored, the effects of the lethal concentration (LC) of lambda-cyhalothrin on the growth, development, and fecundity of H. vigintioctomaculata are imperfectly known. We first determine the sublethal concentration (SLC) of lambda-cyhalothrin on the F0 generation, and its effect on the F1 generation at SLCs. We construct an age–instar bisexual life table for the F1 population and compare changes in parameters such as developmental duration, survival rate, fecundity, and longevity at different lambda-cyhalothrin concentrations. We also report the detoxifying enzyme activities after SLC treatment of F0 adults. The results provide a theoretical basis for a comprehensive assessment of the potential of lambda-cyhalothrin to control H. vigintioctomaculata, and for more informed applications of this insecticide.
2. Materials and Methods
2.1. Insect Rearing
Henosepilachna vigintioctomaculata were collected from Luliang County, Qujing City potato fields, Yunnan Province (103°48′52.02″ E, 25°17′20.74″ N, altitude 1878 m) in September 2023. Insects were placed in an insect feeding cage (50 × 50 × 50 cm) at room temperature. Potted potatoes (Yunshu 108) grown at (25 ± 1) °C, relative humidity 70% ± 5%, and a photoperiod 16:8 h L/D were cultivated as a host; fresh leaves were cut to feed the ladybugs when the plants had grown to 15–20 cm. Before experimentation, adult ladybugs were reared for at least three generations with no exposure to insecticide. Three-day-old adults were used as the initial insect source. Insect feeding conditions were consistent with potato cultivation conditions.
2.2. Insecticide and Chemicals and Enzyme Assay Kits
The stock insecticide solution was 2.5% lambda-cyhalothrin WG (Henan Yongguan Qiaodi Agricultural Science and Technology Co., Ltd, (Zhengzhou, China). Multifunctional oxidase (MFO) activity detection kits (A162-1-1), Carboxylesterase (CarE) activity detection kits (A133-1-1), and Glutathione S-transferase (GSH-ST) activity detection kits (A004-1-1) were purchased from Nanjing Jiancheng Bioengineering Institute Co., Ltd (Nanjing, China).
2.3. Determining Biological Activity
The sensitivity of H. vigintioctomaculata to lambda-cyhalothrin was determined using a leaf film method [21]. Five lambda-cyhalothrin treatments (1.25, 1.00, 0.75, 0.50, and 0.25 mg L−1) were initially established; water was used as a control. Each treatment contained 30 × 3 d old similarly sized adult ladybugs, and was replicated three times. Before experimentation, adult ladybugs were starved for 48 h. Potato leaves were immersed in each treatment concentration for 20 s, then removed, placed in an insect box (15 × 12 × 5 cm), dried, and their petiole wrapped with water-soaked cotton wool. Ladybugs were then added. Ladybug mortality was recorded after 48 h of treatment. Feeding conditions were the same as in Section 2.1. Ladybugs were pronounced dead if touched by a brush and no reaction occurred. A virulence regression curve, 95% confidence intervals, chi-square values (χ2), and degrees of freedom (df) were calculated for LC10, LC20, and LC40 concentrations.
2.4. Effects of Lambda-Cyhalothrin Sublethal Treatment on F0 and F1 H. vigintioctomaculata
Potato leaves were immersed in LC10, LC20, and LC40 concentrations for 20 s, then removed and dried; water was used as a control. Sixty healthy 3 d old similarly sized male and female adults (1:1, morphological identification) were fed potato leaves and reared in an artificial climate box [4]. After 48 h of exposure to leaves immersed in different concentrations of insecticide (without changing leaves), surviving H. vigintioctomaculata were removed, and males and females were paired, placed in a Petri dish, and fed fresh, untreated potato leaves. The initial stage of the F0 generation experiment involved approximately 30 individuals, with three biological replicates. At least 15 pairs of ladybugs from each insecticide treatment were paired; the fresh potato leaves in the insect box were replaced daily. The oviposition date, oviposition amount, and adult longevity of single females were observed regularly (8:00 and 18:00 h). If a male died before a female, it was replaced. We refer to adult contemporary H. vigintioctomaculata treated with SLCs of lambda-cyhalothrin as the parent generation (F0), and offspring produced by natural mating of these parents as the filial generation (F1) [2].
Eggs (90, oviposition < 5 h) laid on the same day by an F0 female in each lambda-cyhalothrin treatment and the control group were used as the F1 generation. These eggs were placed into circular plastic, numbered Petri dishes (diameter 60 mm) with moist filter paper, and then moved to an artificial climate box for hatching, where the hatching rate was determined. The feeding conditions were the same as in Section 2.1. Individual newly hatched F1-generation larvae treated with different insecticide concentrations were inoculated separately into Petri dishes for feeding. Fresh potato leaves were placed into Petri dishes, and ladybug development was monitored every 24 h. After 24 h of pupation, pupae were weighed, then transferred into individual centrifuge tubes sealed with absorbent cotton, and returned to the climate chamber. After adult eclosion, pupal duration was determined. For each insecticide concentration treatment, female and male adults that emerged on the same day were randomly selected and paired 1:1 and placed into new culture dishes. A wet cotton ball was placed in the dish to provide moisture, and fresh leaves were replaced the next day. Test insects ate and deposited eggs. The number of eggs laid by a single female and the longevity of adult females and males were observed daily (8:00 and 18:00 h) until all adults had died. The number of eggs hatched/the total number of eggs laid by females represents the egg-hatching rate. The time from newly hatched larvae to pupation represents the larval development period, and the time from pupation to adult eclosion represents the pupal development period. The percentage of the number of pupae that could break the shell and become adults divided by the total number of pupae × 100 represents the eclosion rate.
2.5. Determining Detoxifying Enzyme Activities
Detoxifying enzyme activities were determined using the method of Jiang et al. [22]. F0-generation adult ladybugs were fed fresh leaves treated with LC10, LC20, and LC40 concentrations of lambda-cyhalothrin, with four replicates per treatment. After 48 h, surviving adults were collected and placed into centrifuge tubes. Each replicate contained 10 individuals, frozen in liquid nitrogen and stored at −80 °C for enzyme activity determination. Ten similarly sized adults were selected from each concentration treatment. PBS (pH 7.4) was added to an ice bath homogenate at a weight (g)–volume (mL) ratio of 1:9; the homogenate was centrifuged at 4 °C and 12,000 r/min for 30 min. The supernatant was recovered to determine GST, CarE, and MFO enzyme activities, and protein concentration, following kit instructions.
2.6. Data Analysis
A probability unit analysis was used to analyze the toxicity regression equation of lambda-cyhalothrin using SPSS 26.0 (IBM Co., Ltd., Armonk, NY, USA); SLCs were determined. One way ANOVA was used on data related to detoxification enzymes. Tukey’s multiple range test was used for multiple comparisons, and Student’s t tests were used to identify significant differences between treatments in pairwise comparisons. Original growth and development, survival rate, and fecundity data for the F1 generation were collated and imported into Twosex-MSChart software (v 5/7/2024) to calculate developmental duration, adult longevity, adult preoviposition period (APOP), total preoviposition period (TPOP), fecundity, net reproductive rate (R0), finite rate of increase (λ), intrinsic rate of increase (r), and average generation cycle (T) for each stage [23,24,25,26]. All variances and standard errors were obtained by using 100,000 random sampling bootstraps, and the differences for each parameter of the insecticide treatments were evaluated by paired bootstrap tests [27,28]. Plots were generated using SigmaPlot 14.0. The specific calculation formulas are as follows:
λ = er
3. Results
3.1. Toxicity of Lambda-Cyhalothrin to Henosepilachna vigintioctomaculata Adults
After 48 h treatment, the LC10, LC20, and LC40 values for lambda-cyhalothrin exposure to H. vigintioctomaculata adults were 0.193, 0.251, and 0.355 mg L−1, respectively. Three concentration values overlapped with other 95% CL values for subsequent experiments (Table 1).

Table 1.
Sublethal concentrations of lambda-cyhalothrin for newly hatched adult H. vigintioctomaculata (F0) 48 h after treatment.
3.2. Effect of Sublethal Concentration of Lambda-Cyhalothrin on Longevity and Fecundity of F0 H. vigintioctomaculata
LC10, LC20, and LC40 concentrations of lambda-cyhalothrin significantly affected the longevity and average fecundity of F0 adults (Table 2). Compared with the control group, the higher the concentration of lambda-cyhalothrin, the shorter the adult life span (female and male), with the life span of females being slightly longer. After exposure to lambda-cyhalothrin concentrations (LC10, LC20, and LC40), individual egg production and F1 egg hatching rates also significantly decreased compared with the control group.

Table 2.
Impact of sublethal concentrations of lambda-cyhalothrin on F0 generation adult H. vigintioctomaculata longevity and fecundity.
3.3. Effect of Sublethal Concentration of Lambda-Cyhalothrin on Growth, Development, Fecundity, and Pupal Weight of F1 H. vigintioctomaculata
LC10, LC20, and LC40 concentrations of lambda-cyhalothrin affected each F1 generation developmental stage (Table 3). Compared with the control, the duration of the egg, larval, and pupal stages in the LC10 and LC20 treatment groups differed significantly (p < 0.05); the lifespan of male and female adults in the LC40 concentration treatment group was 16.57% and 19.76%, significantly shorter than control group adults. APOP and TPOP were prolonged with increased lambda-cyhalothrin concentrations; the control group’s APOP and TPOP durations were the shortest (6.20 d and 23.53 d), and those in the LC40 treatment were the longest (7.10 d and 30.45 d, respectively). Compared with other treatments, the numbers of eggs laid by females exposed to the LC40 concentration was lowest (13.40 eggs/female); the number of eggs laid by females in the control group (55.59 eggs/female) was 1.13×, 1.70×, and 4.15× that of the LC10, LC20, and LC40 treatments, respectively. Additionally, compared with controls, the pupal weight and adult emergence rate of the F1 generation were also significantly lower in the LC10, LC20, and LC40 treatments (p < 0.05), with the effect stronger with increased insecticide concentration (Figure 1, Table 3).

Table 3.
Fecundity and duration of various life-history parameters of progeny of lambda-cyhalothrin-treated H. vigintioctomaculata.
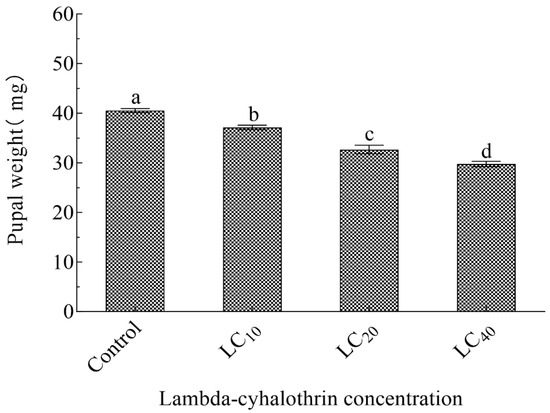
Figure 1.
Pupal weight of progeny of lambda-cyhalothrin-treated H. vigintioctomaculata. Note: Data are means ± SE; different lowercase letters above whiskers indicate significant differences (p < 0.05, Tukey’s multiple range test).
3.4. Population Parameters
Compared with the controls, the LC10, LC20, and LC40 treatments significantly affected the F1 generation’s life table parameters. The values for the r, λ, and R0 of the F1 population decreased with increased lambda-cyhalothrin concentration, and T increased (Table 4).

Table 4.
Population parameters of F1 H. vigintioctomaculata descended from the F0 generation exposed to lambda-cyhalothrin.
3.5. Age–Stage Specific Maternity
At each concentration, the lx curves follow a similar trend: there is a period of stability, and then a gradual and then steep decline (Figure 2), indicating that the death of individuals in the H. vigintioctomaculata F1 population mainly occurred in the later stages. The fx curves are more variable, indicating greater variation in the emergence and oviposition of female adults, resulting in the curve appearing high and low. For the LC10, LC20, and LC40 lambda-cyhalothrin concentrations, peak adult female fx values were 13.77 (37 d), 14.11 (55 d), and 8.46 (58 d) (Figure 2). The peak number of eggs of fx in the control group was greatest (15.62 eggs d−1). Over time, the age-specific survival rate lx of the control group trended downwards. The mx curve indicated that the control group began reproducing at 33 d, whereas in other concentrations reproduction began 2–11 d later. Multiple peaks appear in the mx curve, indicating changes in individual spawning periods. The age-specific oviposition rate lxmx in the LC40-treated individuals decreased sharply, from 5.00 individuals d−1 in the control group to 1.70 individuals d−1 (Figure 2).

Figure 2.
lx, fx, mx, and lxmx values of F1 H. vigintioctomaculata descended from F0 ladybugs treated with lambda-cyhalothrin and control values.
3.6. Effect of Lambda-Cyhalothrin Exposure on Detoxifying Enzyme Activity
The detoxifying enzyme activities in the adult H. vigintioctomaculata treated with SLCs of lambda-cyhalothrin trended upwards (Figure 3). After 48 h exposure at LC20 and LC40 concentrations, female GST activities decreased significantly by 28.88% and 44.88% compared with control values, and male activities decreased significantly by 39.39% and 44.95%, respectively (p < 0.05); at LC10 there was an increase in GST activity. CarE activity first increased, then decreased with increased lambda-cyhalothrin concentration; the enzyme activities in the LC40 treatment were significantly lower than those in either of the other treatments (p < 0.05). With an increased treatment concentration, MFO activity was inhibited. Additionally, the GST activity of adults in the LC20 treatment and CarE activity in the LC10 and LC40 treatments differed significantly (p < 0.05).

Figure 3.
(A) GST, (B) CarE, and (C) MFO activities in H. vigintioctomaculata adults treated with lambda-cyhalothrin. Note: Data are means ±SE; different uppercase and lowercase letters indicate significant differences between males and females, respectively (p < 0.05, Tukey’s multiple range test). Asterisks indicate significant differences in detoxifying enzyme activities between females and males (* p < 0.05, independent samples t-test).
4. Discussion
Insecticides are used extensively in agriculture, and they contribute to the development of pest resistance [17]. Integrated pest-management programs underscore the importance and necessity of moderate insecticide use [29]. Lambda-cyhalothrin is an efficient, broad-spectrum and quick-acting pyrethroid insecticide developed by ICI, UK. It has contact and stomach poisoning effects, and has no internal absorption effect. It is mainly used to control pests with chewing or piercing and sucking mouthparts [8,9,14]. Using a leaf-dip method, we evaluated the bioactivity of lambda-cyhalothrin against adult H. vigintioctomaculata [4]. Our LC40 value of 0.355 mg L−1 indicates that this insecticide exhibits potent toxicity toward H. vigintioctomaculata. Chemical control is the main strategy to manage H. vigintioctomaculata. However, post-application in the field, insecticide toxicity generally decreases because of various abiotic environmental factors, producing SLCs [30]. This reduced toxicity can induce sublethal effects in certain pest individuals that may be passed on to subsequent generations. Sublethal effects may influence the growth, development, behavior, and reproductive capacity of target pests and their offspring, and change population dynamics [30,31,32].
Many studies have reported that sublethal insecticide concentrations exert significant inhibitory effects. For instance, treatment with chlorantraniliprole at LC10 concentrations significantly reduces the survival rate and longevity of F1 generations of Sogatella furcifera [33]. Studies on the development of Cydia pomonella under lambda-cyhalothrin stress have yielded similar results [34]. We report SLCs of lambda-cyhalothrin (LC10, LC20, and LC40) to significantly reduce the lifespan and average oviposition of the F0 generation H. vigintioctomaculata compared with the control group ladybugs (Table 2). Furthermore, exposure to LC40 concentrations significantly reduced the F1 population size, with delays observed in egg, larval, and pupal developmental duration, further indicative of sublethal effects (Table 3). These findings are consistent with those for exposure of Paracoccus marginatus to SLCs of spirotetramat, with extended nymphal periods from the F0 to F2 generations and pre-adult delays [35]. We also report the pupal weight—an important indicator of insect stress resistance and environmental adaptability—of the F1 generation of H. vigintioctomaculata to decrease with increased SLC of lambda-cyhalothrin [36]. Leaves treated with LC40 concentrations also showed negligible feeding damage, likely attributable to the antifeedant properties of lambda-cyhalothrin [37]. Reduced feeding reduced the growth and development of H. vigintioctomaculata, and reduced body weight.
The impact of insecticides on pest fecundity manifests as sublethal effects [31]. The toxic excitatory response is influenced by various factors, with exposure duration (or generational exposure) being a key determinant [38]. Low doses of imidacloprid stimulate peach aphids, leading to increased methylation levels in the F2 generation compared with the F1 generation. This phenomenon may be linked to genetic adaptability induced by low-dose insecticide stress [39]. We report that SLCs of lambda-cyhalothrin also induce transgenerational effects in H. vigintioctomaculata. Specifically, the average oviposition, r, λ, and R0 values of both the F0 and F1 generations decreased with increased lambda-cyhalothrin concentration, limiting F1 population growth (Table 2 and Table 3). A similar phenomenon is reported for pests such as Paracoccus marginatus, Aphis gossypii, and Rhizoglyphus robini [9,14,40]. These findings suggest that insecticide treatments can effectively slow transgenerational population growth of a variety of species. This inhibitory effect may be attributed to the downregulation of gene expression associated with vitellogenin (Vg) and its receptor synthesis in females exposed to sublethal doses of insecticide. For example, the reduced expression of Vg and vitellogenin receptor (VgR) genes leads to decreases in their respective contents [40,41,42]. Furthermore, age–stage-specific maternity curves (Figure 2) indicate that lambda-cyhalothrin stress significantly suppresses the F1 generation’s reproductive capacity, with the effect increasing at higher concentrations. Interestingly, many insecticides (e.g., triazophos and thiamethoxam) regulate pest fecundity by modulating the expression of Vg and VgR through the TOR protein kinase and juvenile hormone signaling pathways [43,44]. Whether lambda-cyhalothrin similarly regulates the reproductive capacity of H. vigintioctomaculata adults via these pathways is unknown.
The reduced sensitivity of insects to insecticides typically involves multiple mechanisms, with the most prominent being the regulation of insecticide metabolism through detoxifying enzymes [15]. These enzymes play important roles in the metabolic processing of chemicals in insects. Insecticides can induce or inhibit their activity, and by exerting continuous selective pressure, drive the evolution of insecticide resistance [45]. Insecticides can affect various population parameters in insects such as developmental periods, reproductive capacity, and adult longevity. Additionally, they can influence the activity of detoxifying enzymes [46]. Different insecticides or plant secondary metabolites have distinct inhibitory or inducing effects on detoxifying enzyme activities in the same pest species [31]. For example, after 24 h of spirotetramat exposure, low concentrations induced a significant increase in GST and CarE activity in Bradysia odoriphaga [47]. Similarly, treatment with lambda-cyhalothrin significantly increased GST activity in Cydia pomonella, but suppressed CarE activity [48]. We report that, after 48 h of exposure to lambda-cyhalothrin, LC10 concentrations induced an increase in both GST and CarE activities in adult H. vigintioctomaculata. At LC20, the CarE activity in adult males and females was significantly higher than in the control group, but lower than in the LC10 treatment group, possibly because of the lower insecticide dose (where CarE was activated to participate in insecticide metabolism). Additionally, the GST activity of adults in the LC20 treatment and CarE activity in the LC10 and LC40 treatments differed significantly (p < 0.05). Similar GST and CarE activity results were reported by Kinareikina [49]. At increased dosage, enzyme activity was progressively suppressed. The degree of MFO activity correlated positively with insecticide dosage. At low lambda-cyhalothrin concentrations, the activities of CarE and GST in H. vigintioctomaculata were induced, but MFO activity was significantly reduced. Further molecular-level studies on higher resistance strains are required to elucidate the underlying mechanisms of resistance to lambda-cyhalothrin.
After the exposure of adult H. vigintioctomaculata in the F0 generation to SLCs of lambda-cyhalothrin, F1 population growth was significantly reduced, and the degree of reduction increased with increased insecticide concentration. This suggests that field application of lambda-cyhalothrin exerts transgenerational effects on H. vigintioctomaculata. In agricultural practice, the rational use of lambda-cyhalothrin to mitigate damage caused by H. vigintioctomaculata to crops is viable. However, the dosage and frequency of application must be managed to avoid enhancing pest fitness or promoting resistance through prolonged use of a single class of insecticide. Furthermore, the implementation of insecticide rotation—using two or more insecticides with different modes of action—could delay the development of resistance and support sustainable pest management strategies.
5. Conclusions
As a new insecticide with a high efficiency, low toxicity, and low residue, lambda-cyhalothrin has high insecticidal activity against Coleoptera pests. We report the effects of SLCs of this insecticide on the growth and development, longevity, and fecundity of H. vigintioctomaculata using an age–age bisexual life table. Compared with the control group, male and female longevity and fecundity all decreased, and other life table parameters deceased with increased SLCs. At low concentrations, this insecticide also increases the activity of detoxifying metabolic enzymes. Field application of lambda-cyhalothrin has a cross-generational effect on H. vigintioctomaculata that may affect F1 generations. This provides a reference for how to improve the evaluation of the effects of insecticides.
Author Contributions
H.C. conceived and designed the experiments. W.L. and J.C. conducted the experiments. M.N. and G.D. analyzed the data. W.L. and H.C. obtained the funding for the study. All authors contributed to writing and editing of the manuscript. Writing—original draft, W.L. and M.N.; Writing—review and editing, J.C.; Supervision, H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by Yunnan Fundamental Research Projects Association (grant no. 202301AU070005), the Special Basic Cooperative Research Programs of Yunnan Provincial Undergraduate Universities (202301BA070001-076), the Special Basic Cooperative Research Innovation Programs of Qujing Science and Technology Bureau & Qujing Normal University (KJLH2024ZD05), and Innovative Research Team in Qujing Normal University.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Matsishina, N.V.; Ermak, M.V.; Fisenko, P.V.; Sobko, O.A. Henosepilachna vigintioctomaculata Motschulsky (Coleoptera: Coccinellidae): Morphotypes in an East Asian population. J. Insect Biodivers. 2023, 38, 15–23. [Google Scholar] [CrossRef]
- Li, W.; Bashir, N.H.; Chen, H.; Li, X.; Wang, Z.; Du, L.; Tian, R. Effect of different host plants on the growth, development, and reproduction of Henosepilachna vigintioctomaculata (Coleoptera: Coccinellidae). Chin. J. Appl. Entomol. 2024, 61, 287–294. [Google Scholar]
- Ze, L.J.; Jin, L.; Li, G.Q. The compatible effects of RNA interference targeting laccase2 with biocontrol in Henosepilachna vigintioctopunctata. Entomol. Gen. 2023, 43, 117–126. [Google Scholar] [CrossRef]
- Ding, X.; Aerziguli, R.; Ji, L.; Liu, S.; Fu, K.; Tursun, A.; Guo, W. The occurrence and harm of Henosepilachna vigintioctomaculata. Xinjiang Agric. Sci. 2022, 59, 983–989. [Google Scholar] [CrossRef]
- Tamang, S.; Alam, T.; Rana, L. Biointensive integrated pest management of potato. In Biointensive Integrated Pest Management for Horticultural Crops, 1st ed.; Kumar, A., Saha, S., Choudhary, J.S., Eds.; CRC Press: London, UK, 2021; pp. 207–240. [Google Scholar]
- Nithish, A. Study on management of damage caused by Henosepilachna vigintioctopunctata in brinjal. J. Pharmacog. Phytochem. 2020, 9, 2874–2881. [Google Scholar]
- Zhou, J.; Xu, R.; Chen, Z.; Jia, Y.; Xu, K. Phototactic behavior of Henosepilachna vigintioctomaculata motschulsky (Coleoptera: Coccinellidae). Coleopts. Bull. 2015, 69, 806–812. [Google Scholar] [CrossRef]
- Keyhanian, A.A.; Barari, H.; Mobasheri, M.T. Comparison of the efficacy of insecticides, alphacypermethrin and lambda-cyhalothrin, against canola flea beetles. Appl. Entomol. Phytopathol. 2021, 44, 113–122. [Google Scholar]
- Qiu, Y.; Chen, Z. Intergenerational effects of sublethal lambda-cyhalothrin exposure on Aphis gossypii glover (Hemiptera: Aphididae) reproduction and development. Insects 2024, 15, 173. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.M.; Liu, H.Y.; Xin, Z.; Xue, M. Lethal and sublethal effects of spinosad on Spodoptera exigua (Lepidoptera: Noctuidae). J. Econ. Entomol. 2013, 106, 1825–1831. [Google Scholar] [CrossRef]
- Boina, D.R.; Onagbola, E.O.; Salyani, M.; Stelinski, L.L. Antifeedant and sublethal effects of imidacloprid on Asian citrus psyllid, Diaphorina citri. Pest Manag. Sci. 2009, 65, 870–877. [Google Scholar] [CrossRef]
- Cutler, G.C.; Amichot, M.; Benelli, G.; Guedes, R.N.C.; Qu, Y.; Rix, R.R.; Ullah, F.; Desneux, N. Hormesis and insects: Effects and interactions in agroecosystems. Sci. Total Environ. 2022, 825, 153899. [Google Scholar] [CrossRef] [PubMed]
- Gul, H.; Güncan, A.; Ullah, F.; Desneux, N.; Liu, X. Intergenerational sublethal effects of flonicamid on cotton aphid, Aphis gossypii: An age-stage, two-sex life table study. Insects 2024, 15, 529. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Chen, Y.T.; Chen, Y.; Zhao, J.W.; Fu, J.W.; Shi, M.Z. Sublethal effects of lambda-cyhalothrin on the biological characteristics, detoxification enzymes, and genes of the papaya mealybug, Paracoccus marginatus. J. Pest Sci. 2024, 7, 1–15. [Google Scholar] [CrossRef]
- van Leeuwen, T.; Dermauw, W. The molecular evolution of xenobiotic metabolism and resistance in chelicerate mites. Ann. Rev. Entomol. 2016, 61, 475–498. [Google Scholar] [CrossRef]
- Guo, W.; Yang, H.; Duan, M.; Xu, H.; Zhao, W.; Wang, C. Sublethal effects of tolfenpyrad and its impact on detoxifying enzymes to Spodoptera exigua. Plant Protect. 2024, 50, 168–175. [Google Scholar] [CrossRef]
- Batool, N.; Abubakar, M.; Noureldeen, A.; Naqqash, M.N.; Alghamdi, A.; Al Dhafar, Z.M.; Baakdah, F.; Mozūratis, R. Toxicity and sublethal effect of chlorantraniliprole on multiple generations of Aedes aegypti L. (Diptera: Culicidae). Insects 2024, 15, 851. [Google Scholar] [CrossRef]
- Li, W.; Bashir, N.H.; Naeem, M.; Tian, R.; Tian, X.; Chen, H. Age-stage, two-sex life table of Atractomorpha lata (Orthoptera: Pyrgomorphidae) at different temperatures. Insects 2024, 15, 493. [Google Scholar] [CrossRef]
- Borges, I.; Dury, G.J.; Soares, A.O. Population growth parameters of Scymnus nubilus fed single-aphid diets of Aphis fabae or Myzus persicae. Insects 2024, 15, 486. [Google Scholar] [CrossRef]
- Ahn, J.J.; Choi, K.S. Population parameters and growth of Riptortus pedestris (Fabricius) (Hemiptera: Alydidae) under fluctuating temperature. Insects 2022, 13, 113. [Google Scholar] [CrossRef]
- Guo, L.; Bian, Q.; Zhang, H.; Gao, X.; Liang, P. Bioassay technique for Plutella xylostella: Leaf-dip method. Chin. J. Appl. Entomol. 2013, 50, 556–560. [Google Scholar]
- Jiang, M.; Qian, X.; Zhou, Z.; Liu, Y.; Zhang, M.; Yang, Y. Impacts of sublethal doses of spinetoram on the biological traits and detoxifying enzymes of the tomato leaf miner, Tuta absoluta (Lepidoptera: Gelechiidae). Insects 2024, 15, 990. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Liu, H.S.I. Two new methods for study of insect population ecology. Bull. Inst. Zool Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H.; Su, H.Y. Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead) (Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer) (Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ. Entomol. 2006, 35, 10–21. [Google Scholar] [CrossRef]
- Tuan, S.J.; Lee, C.C.; Chi, H. Population and damage projection of Spodoptera litura (F.) on peanuts (Arachis hypogaea L.) under different conditions using the age-stage, two-sex life table. Pest Manag. Sci. 2014, 70, 805–813. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX-MSChart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis; National Chung Hsing University in Taiwan: Taichung City, Taiwan, 2023. [Google Scholar]
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap; Chapman and Hall: New York, NY, USA, 1993. [Google Scholar]
- Dean, A.N.; Niemi, J.B.; Tyndall, J.C.; Hodgson, E.W.; O’Neal, M.E. Developing a decision-making framework for insect pest management: A case study using Aphis glycines (Hemiptera: Aphididae). Pest Manag. Sci. 2021, 77, 886–894. [Google Scholar] [CrossRef]
- Havasi, M.; Zahedi Golpayegani, A.; Bandani, A. The sublethal concentration of Cyflumetofen adversely affect demographic parameters of Tetranychus urticae (Acari: Tetranychidae): Using age-stage, two-sex life tables. Int. J. Acarol. 2022, 48, 331–337. [Google Scholar] [CrossRef]
- Fan, R.; Fan, Z.; Sun, Z.; Chen, Y.; Gui, F. Insecticide susceptibility and detoxification enzyme activity of frankliniella occidentalis under three habitat conditions. Insects 2023, 14, 643. [Google Scholar] [CrossRef]
- Wang, D.; Gong, P.; Li, M.; Qiu, X.; Wang, K. Sublethal effects of spinosad on survival, growth and reproduction of Helicoverpa armigera (Lepidoptera: Noctuidae). Pest Manag. Sci. 2009, 65, 223–227. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Z.; Jin, D. Sublethal effects of chlorantraniliprole on the experimental populations of the white-backed planthopper, Sogatella furcifera (Hemiptera: Delphacidae). Acta Entomol. Sin. 2012, 55, 1161–1167. [Google Scholar] [CrossRef]
- Ju, D.; Liu, Y.X.; Liu, X.; Dewer, Y.; Mota-Sanchez, D.; Yang, X.Q. Exposure to lambda-cyhalothrin and abamectin drives sublethal and transgenerational effects on the development and reproduction of Cydia pomonella. Ecotoxicol. Environ. Saf. 2023, 252, 114581. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Chen, Y.T.; Wang, Q.Y.; Zheng, L.Z.; Fu, J.W.; Shi, M.Z. Sublethal and transgenerational toxicities of chlorfenapyr on biological traits and enzyme activities of Paracoccus marginatus (Hemiptera: Pseudococcidae). Insects 2022, 13, 874. [Google Scholar] [CrossRef]
- Li, D.; Peng, C.; Ma, R.; Chen, Y.; Gui, F.; Sun, Z. Effects of sublethal concentrations chlorfenapyr on growth, development and enzyme activity of Tuta absoluta. J. Environ. Entomol. 2024, 46, 1349–1357. [Google Scholar] [CrossRef]
- Bian, D.; Ren, Y.; Ye, W.; Dai, M.; Li, F.; Wei, J.; Sun, H.; Li, B. Evaluation of tolerance to λ-cyhalothrin and response of detoxification enzymes in silkworms reared on artificial diet. Ecotoxicol. Environ. Saf. 2022, 232, 113232. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E.; Brown, P.H.; Calabrese, E.J. A gift from parent to offspring: Transgenerational hormesis. Trends Plant Sci. 2021, 26, 1098–1100. [Google Scholar] [CrossRef]
- Ayyanath, M.-M.; Cutler, G.C.; Scott-Dupree, C.D.; Prithiviraj, B.; Kandasamy, S.; Prithiviraj, K. Gene expression during imidacloprid-induced hormesis in green peach aphid. Dose Response 2014, 12, 480–497. [Google Scholar] [CrossRef]
- Alimirzaee, S.; Khajehali, J.; Van Leeuwen, T. Hormetic effects of neonicotinoid insecticides on Rhizoglyphus robini (Acari: Acaridae). Pestic. Biochem. Physiol. 2023, 192, 105396. [Google Scholar] [CrossRef]
- Ullah, F.; Gul, H.; Tariq, K.; Desneux, N.; Gao, X.; Song, D. Thiamethoxam induces transgenerational hormesis effects and alteration of genes expression in Aphis gossypii. Pestic. Biochem. Physiol. 2020, 165, 104557. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, J.; Wang, Q.; Ji, X.; Wang, W.; Huang, W.; Rui, C.; Cui, L. Hormesis effects of sulfoxaflor on Aphis gossypii feeding, growth, reproduction behaviour and the related mechanisms. Sci. Total Environ. 2023, 872, 162240. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, X.; Yang, H.; Gong, M.; Long, G.; Jin, D. Role of SfJHAMT and SfFAMeT in the reproductive regulation of Sogatella furcifera and its expression under insecticide stress. Pestic. Biochem. Physiol. 2021, 173, 104779. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, X.; Yang, H.; Gong, M.; Long, G.; Jin, D. Role of insecticide-mediated transcription of the TOR and JH signaling pathway-related genesin the regulation ofreproduction in Sogatella furcifera. Entomol. Gen. 2022, 42, 771–779. [Google Scholar] [CrossRef]
- Hafeez, M.; Liu, S.; Jan, S.; Ali, B.; Shahid, M.; Fernández-Grandon, G.M.; Nawaz, M.; Ahmad, A.; Wang, M. Gossypol-induced fitness gain and increased resistance to deltamethrin in beet armyworm, Spodoptera exigua (Hübner). Pest Manag. Sci. 2019, 75, 683–693. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, T.; van Pottelberge, S.; Tirry, L. Biochemical analysis of a chlorfenapyr-selected resistant strain of Tetranychus urticae Koch. Pest Manag. Sci. 2006, 62, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Q.; Ding, J.; Wang, Y.; Zhang, Z.; Liu, F.; Mu, W. Sublethal effects of chlorfenapyr on the life table parameters, nutritional physiology and enzymatic properties of Bradysia odoriphaga (Diptera: Sciaridae). Pestic. Biochem. Physiol. 2018, 148, 93–102. [Google Scholar] [CrossRef]
- Li, P.R.; Shi, Y.; Ju, D.; Liu, Y.X.; Wang, W.; He, Y.S.; Zhang, Y.Y.; Yang, X.Q. Metabolic functional redundancy of the CYP9A subfamily members leads to P450-mediated lambda-cyhalothrin resistance in Cydia pomonella. Pest Manag. Sci. 2023, 79, 1452–1466. [Google Scholar] [CrossRef]
- Kinareikina, A.; Silivanova, E. Impact of insecticides at sublethal concentrations on the enzyme activities in adult Musca domestica L. Toxics 2023, 11, 47. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).