Nest Architecture Drives Sex-Specific Emergence Success in a Predator Wasp (Hymenoptera, Vespidae, Discoelius wangi)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Sampling
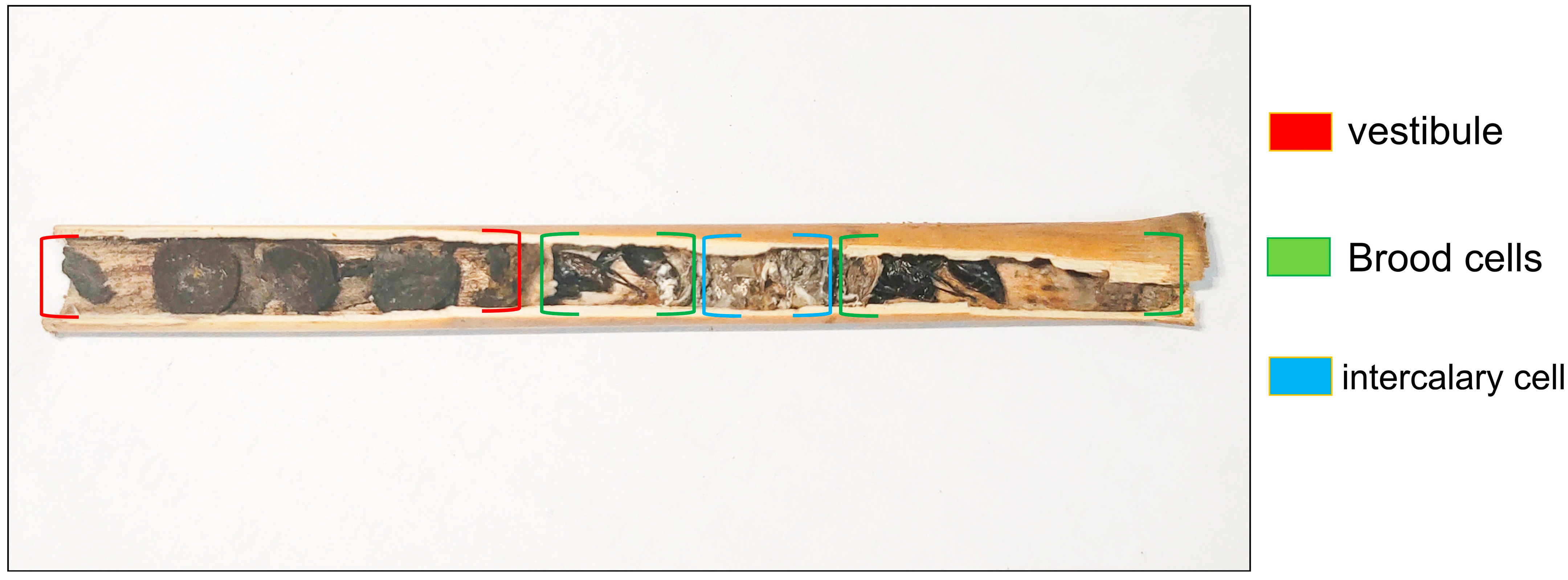
2.3. Data Collection and Processing
2.4. Statistical Analyses
3. Results
3.1. Nest-Building Status of Discoelius wangi in Trap Nests
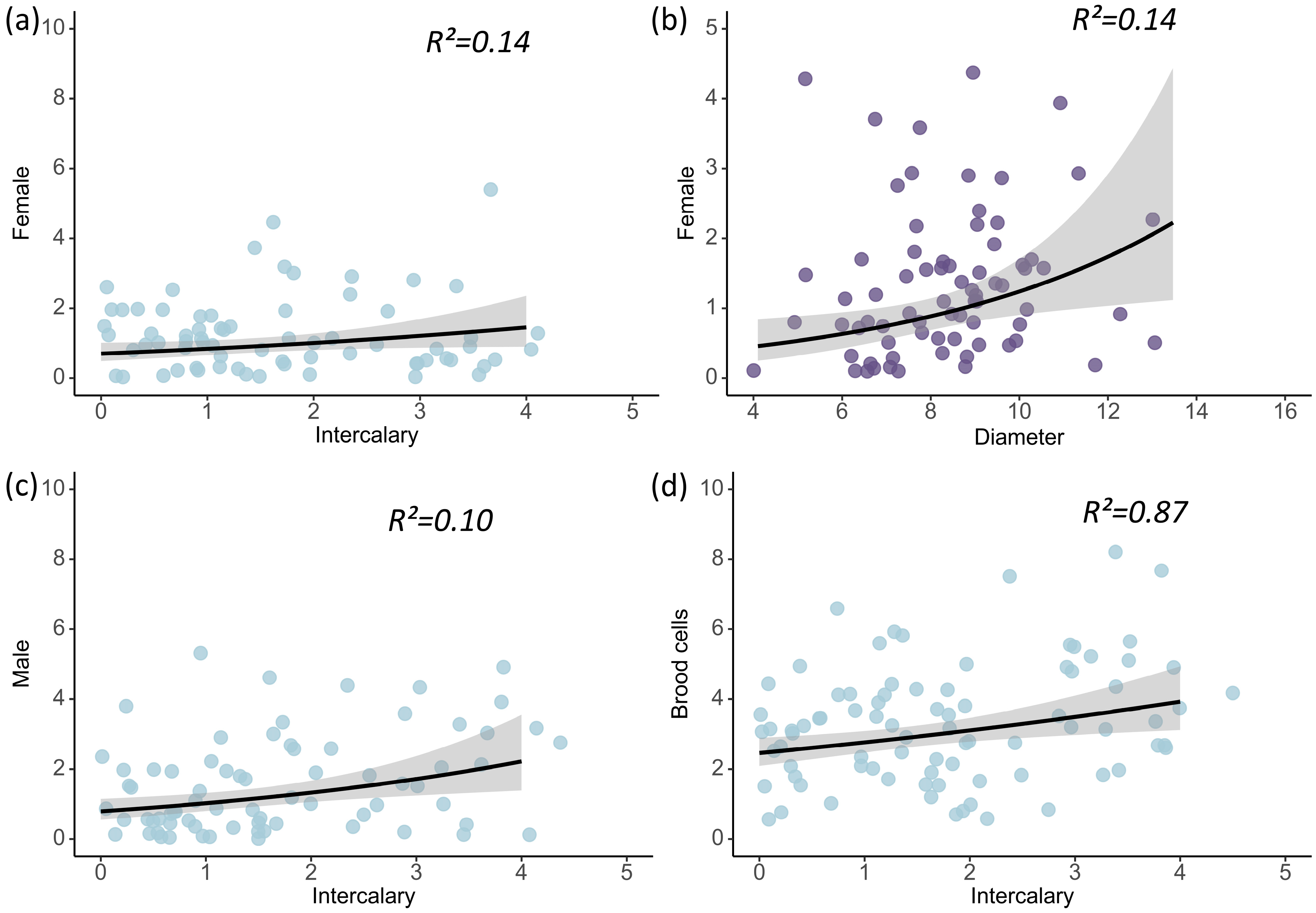
3.2. Impacts of Nest Architecture on the Number of Brood Cells of D. wangi
3.3. Effects of Nest Architecture on the Offspring Quantity of D. wangi
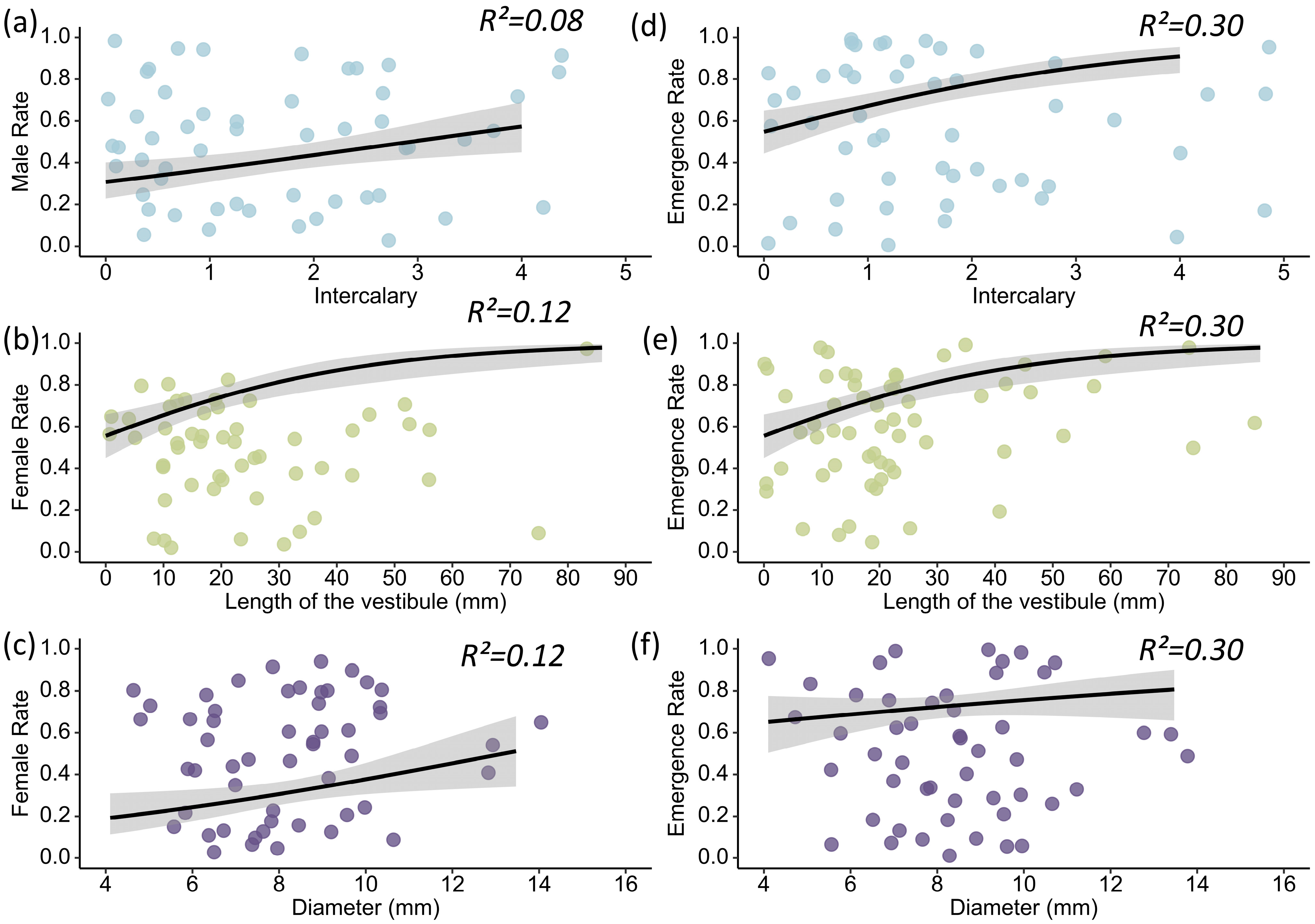
3.4. Effects of Nest Architecture on the Offspring Emergence Rate of Discoelius wangi
4. Discussion
4.1. Biological Significance of the Nest-Building Characteristics of Discoelius wangi
4.2. Effects of Nest Architecture on the Number of Brood Cells of Discoelius wangi
4.3. Differential Effects of Nest Architecture on the Offspring Quantity of Discoelius wangi
4.4. The Complex Regulation of Nest Architecture on the Emergence Rate of Offspring in D. wangi
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Schmid, B.; Schuldt, A.; Li, S.; Wang, M.-Q.; Fornoff, F.; Staab, M.; Guo, P.-F.; Anttonen, P.; Chesters, D.; et al. Multitrophic arthropod diversity mediates tree diversity effects on primary productivity. Nat. Ecol. Evol. 2023, 7, 832–840. [Google Scholar] [CrossRef]
- Li, Y.; Wang, M.-Q.; Chesters, D.; Anttonen, P.; Guo, S.-K.; Guo, P.-F.; Chen, J.-T.; Ma, K.; Bruelheide, H.; Schuldt, A.; et al. Differential impacts on herbivore diversity and scale dependence of tree diversity in subtropical forests. J. Ecol. 2023, 111, 666–675. [Google Scholar] [CrossRef]
- Benda, D.; Pohl, H.; Beutel, R.G.; Straka, J. Solitary folded-winged wasps of the genus Zethus Fabricius (Vespidae, Zethinae) parasitised by two new species of Strepsiptera on different continents. J. Hymenopt. Res. 2024, 97, 721–739. [Google Scholar] [CrossRef]
- Budrienė, A.; Budrys, E.; Orlovskyte, S. A bilateral gynandromorph of Discoelius dufourii (Hymenoptera, Vespidae, Zethinae): Morphology and mating behaviour. J. Hymenopt. Res. 2021, 81, 23–41. [Google Scholar] [CrossRef]
- Wilson, T.; Looney, C.; Tembrock, L.R.; Dickerson, S.; Orr, J.; Gilligan, T.M.; Wildung, M. Insights into the prey of Vespa mandarinia (Hymenoptera: Vespidae) in Washington state, obtained from metabarcoding of larval feces. Front. Insect Sci. 2023, 3, 1134781. [Google Scholar] [CrossRef]
- Tsujii, M.; Endo, T.; Matsui, Y.; Sugiura, S. Indirect interactions between a native and a supposedly non-native wasp species (Hymenoptera: Vespidae: Eumeninae: Anterhynchium). Eur. J. Entomol. 2022, 119, 122–132. [Google Scholar] [CrossRef]
- Du, T.-T.; Lu, H.-X.; Wang, M.-Q.; Li, Y.; Shi, X.-Y.; Orr, M.; Li, J.; Luo, A.; Klein, A.-M.; Zhu, C.-D.; et al. A solitary wasp boosts nesting success through nest architecture (Hymenoptera, Vespidae, Anterhynchium flavomarginatum). J. Hymenopt. Res. 2025, 98, 709–719. [Google Scholar] [CrossRef]
- Fateryga, A.V. The nest structure in four species of solitary wasps of the subfamily Eumeninae (Hymenoptera, Vespidae). Entomol. Rev. 2013, 93, 281–292. [Google Scholar] [CrossRef]
- Ribeiro, M.D.F.; Taura, T.A. Presence of Plebeia aff. flavocincta Nests in Urban Areas. Sociobiology 2019, 66, 66–74. [Google Scholar] [CrossRef]
- Rinehart, J.D.; Grula, C.C.; Rinehart, J.P.; Bowsher, J.H. Nesting cavity diameter has implications for management of the alfalfa leafcutting bee (Hymenoptera: Megachilidae). J. Econ. Entomol. 2023, 117, 127–135. [Google Scholar] [CrossRef]
- Akram, W.; Sajjad, A.; Ghramh, H.A.; Ali, M.; Khan, K.A. Nesting Biology and Ecology of a Resin Bee, Megachile Cephalotes (Megachilidae: Hymenoptera). Insects 2022, 13, 1058. [Google Scholar] [CrossRef]
- Costa, C.C.F.d.; Buschini, M.L.T. Biology of a trap-nesting wasp of one species the ground-nesting Liris (Hymenoptera: Crabronidae) from the Atlantic Forest of southern Brazil. Zoológico 2016, 33, e20150185. [Google Scholar] [CrossRef]
- Kunjwal, N.; Khan, M.S.J.A.B. Trap nesting bees of genus Megachile and nesting biology of three species of subgenus Callomegachile (Hymenoptera; Megachilidae) from North India. Anim. Biol. 2023, 73, 171–194. [Google Scholar] [CrossRef]
- Müller, A. Nest architecture and pollen hosts of the boreoalpine osmiine bee species Hoplitis (Alcidamea) tuberculata (Hymenoptera, Megachilidae). J. Hymenopt. Res. 2015, 47, 53–64. [Google Scholar] [CrossRef]
- Vieira, G.C.; Cristaldo, P.F.; Parizotto, D.R. Management of Centris analis (Hymenoptera: Apidae): Investigating the Ideal Dimensions of Trap Nests for Use in Crop Pollination. Neotrop. Entomol. 2022, 51, 397–403. [Google Scholar] [CrossRef]
- Yuan, D.; Yang, M.; Yu, L.; An, M.; He, Q.; Mu, J.; Yan, L. Population Structure, Distribution, and Spatial Characteristics of Alsophila spinulosa in Chishui, China. Diversity 2023, 15, 1200. [Google Scholar] [CrossRef]
- Guo, P.-F.; Wang, M.-Q.; Orr, M.; Li, Y.; Chen, J.-T.; Zhou, Q.-S.; Staab, M.; Fornoff, F.; Chen, G.-H.; Zhang, N.-L.; et al. Tree diversity promotes predatory wasps and parasitoids but not pollinator bees in a subtropical experimental forest. Basic Appl. Ecol. 2021, 53, 134–142. [Google Scholar] [CrossRef]
- Lebreton, S.; Chevrier, C.; Darrouzet, E. Sex allocation strategies in response to conspecifics’ offspring sex ratio in solitary parasitoids. Behav. Ecol. 2010, 21, 107–112. [Google Scholar] [CrossRef]
- Mignini, M.; Lorenzi, M.C. Vibratory signals predict rank and offspring caste ratio in a social insect. Behav. Ecol. Sociobiol. 2015, 69, 1739–1748. [Google Scholar] [CrossRef]
- Masciocchi, M.; Martinez, A.S.; Pereira, A.J.; Villacide, J.M.; Corley, J.C. Dispersal behavior of yellowjacket (Vespula germanica) queens. Insect Sci. 2018, 25, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Brozoski, F.; de Lima, V.A.; Ferrari, R.R.; Buschini, M.L.T. Nesting Biology of the Potter Wasp Ancistrocerus flavomarginatus (Hymenoptera, Vespidae, Eumeninae) Revealed by Trap-Nest Experiments in Southern Brazil. Neotropical entomology 2023, 52, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Gathmann, A.; Tscharntke, T. Foraging ranges of solitary bees. J. Anim. Ecol. 2002, 71, 757–764. [Google Scholar] [CrossRef]
- Münster-Swendsen, M.; Calabuig, I. Interaction between the solitary bee Chelostoma florisomne and its nest parasite Sapyga clavicornis—Empty cells reduce the impact of parasites. Ecol. Entomol. 2000, 25, 63–70. [Google Scholar] [CrossRef]
- Seidelmann, K.; Bienasch, A.; Pröhl, F. The impact of nest tube dimensions on reproduction parameters in a cavity nesting solitary bee, Osmia bicornis (Hymenoptera: Megachilidae). Apidologie 2016, 47, 114–122. [Google Scholar] [CrossRef]
- Scobie, A.A.; Starr, C.K.J.S. Nest Structure of the Neotropical Social Wasp Mischocyttarus baconi (Hymenoptera: Vespidae). Apidologie 2014, 59, 235–239. [Google Scholar] [CrossRef]
- Boomsma, J.J.; Eickwort, G.C. Colony structure, provisioning and sex allocation in the sweat bee Halictus ligatus (Hymenoptera: Halictidae). Biol. J. Linn. Soc. 1993, 48, 355–377. [Google Scholar] [CrossRef]
- Dang, H.T.; Nguyen, L.T.P. Nesting biology of the potter wasp Rhynchium brunneum brunneum (Fabricius, 1793) (Hymenoptera: Vespidae: Eumeninae) in North Vietnam. J. Asia-Pac. Entomol. 2019, 22, 427–436. [Google Scholar] [CrossRef]
- Liu, W.; Li, Y.; Lu, H.; Hao, Y.; Zhang, K.; Dang, X.; Fan, X.; Zhang, H.; Zhou, Z.; Zhu, C.; et al. Diversity of Bacterial Communities Associated with Solitary Bee Osmia excavata Alfken (Hymenoptera: Megachilidae). Eur. J. Entomol. 2023, 13, 1524. [Google Scholar] [CrossRef]
- Ronald, C.S.; Kevin, M.O.N.; Casey, M.D. Nesting Biology and Offspring Development of the Cavity-Nesting Solitary Wasp Isodontia elegans (F. Smith) from Trap-Nests in Oregon. West. North Am. Nat. 2021, 81, 558–570. [Google Scholar] [CrossRef]
- Ali, H.; Iqbal, J.; Ali, M.; Said, F.; Abbas, H.M.K.; Rasool, K.G.; Husain, M.; Aldawood, A.S. Bionomical observations of Small Carpenter Bee, Ceratina smaragdula Fibricius (Hymenoptera: Apidae): Bionomics of small carpenter bee, Ceratina smaragdula. Sociobiology 2024, 71, e9505. [Google Scholar] [CrossRef]
- Mikát, M.; Rehan, S.M. Sociality in the North African small carpenter bee, Ceratina albosticta. Insectes Sociaux 2022, 69, 315–324. [Google Scholar] [CrossRef]
- Mikát, M.; Benda, D.; Waldhauser, V.; Maxerová, T.; Fraňková, T.; Smyčková, J.; Mrozek, J.; Straka, J. Nesting biology, phenology and sociality in a small carpenter bee Ceratina teunisseni a species endemic to Crete. Insectes Sociaux 2025, 72, 463–473. [Google Scholar] [CrossRef]
- Grillo, A.C.; Longo, G.O. Physical contact stress can trigger larval release in the brooding coral Siderastrea stellata. Mar. Biodivers. 2024, 54, 48. [Google Scholar] [CrossRef]
- Mduda, C.A.; Hussein, J.M.; Muruke, M.H. Discrimination of Tanzanian stingless bee species (Hymenoptera, Apidae, Meliponini) based on nest characteristics. Biologia 2024, 79, 465–481. [Google Scholar] [CrossRef]

| Male | Estimate | Std. Error | Z-Value | p |
|---|---|---|---|---|
| (Intercept) | 0.163 | 0.112 | 1.449 | 0.147 |
| Intercalary | 0.291 | 0.114 | 2.552 | 0.011 |
| Female | Estimate | Std. Error | Z-Value | p |
| (Intercept) | −0.128 | 0.119 | −1.072 | 0.274 |
| Diameter | 0.275 | 0.116 | 2.373 | 0.018 |
| Intercalary | 0.233 | 0.117 | 1.999 | 0.046 |
| Brood Cells | Estimate | Std. Error | Z-Value | p |
| (Intercept) | 1.071 | 0.060 | 17.937 | <0.001 |
| Intercalary | 0.141 | 0.061 | 2.324 | 0.020 |
| Male Rate | Estimate | Std. Error | Z-Value | p |
| (Intercept) | −0.386 | 0.124 | −3.121 | 0.002 |
| Intercalary | 0.276 | 0.124 | 2.224 | 0.026 |
| Female Rate | Estimate | Std. Error | Z-Value | p |
| (Intercept) | −0.804 | 0.132 | −6.105 | <0.001 |
| Diameter | 0.371 | 0.143 | 2.601 | 0.009 |
| Length of the vestibule | 0.234 | 0.142 | 1.653 | 0.098 |
| Emergence Rate | Estimate | Std. Error | Z-Value | p |
| (Intercept) | 1.007 | 0.142 | 7.072 | <0.001 |
| Diameter | 0.318 | 0.144 | 2.200 | 0.028 |
| Length of the vestibule | 0.395 | 0.140 | 2.815 | 0.005 |
| Intercalary | 0.494 | 0.141 | 3.492 | 0.0005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, X.-L.; Lu, H.-X.; Orr, M.; Du, T.-T.; Chen, J.-T.; Shi, X.-Y.; Cheng, R.; Zhou, Q.-S.; Luo, A.; Zhu, C.-D.; et al. Nest Architecture Drives Sex-Specific Emergence Success in a Predator Wasp (Hymenoptera, Vespidae, Discoelius wangi). Insects 2025, 16, 1197. https://doi.org/10.3390/insects16121197
Xie X-L, Lu H-X, Orr M, Du T-T, Chen J-T, Shi X-Y, Cheng R, Zhou Q-S, Luo A, Zhu C-D, et al. Nest Architecture Drives Sex-Specific Emergence Success in a Predator Wasp (Hymenoptera, Vespidae, Discoelius wangi). Insects. 2025; 16(12):1197. https://doi.org/10.3390/insects16121197
Chicago/Turabian StyleXie, Xue-Li, Hai-Xia Lu, Michael Orr, Ting-Ting Du, Jing-Ting Chen, Xiao-Yu Shi, Rui Cheng, Qing-Song Zhou, Arong Luo, Chao-Dong Zhu, and et al. 2025. "Nest Architecture Drives Sex-Specific Emergence Success in a Predator Wasp (Hymenoptera, Vespidae, Discoelius wangi)" Insects 16, no. 12: 1197. https://doi.org/10.3390/insects16121197
APA StyleXie, X.-L., Lu, H.-X., Orr, M., Du, T.-T., Chen, J.-T., Shi, X.-Y., Cheng, R., Zhou, Q.-S., Luo, A., Zhu, C.-D., & Guo, P.-F. (2025). Nest Architecture Drives Sex-Specific Emergence Success in a Predator Wasp (Hymenoptera, Vespidae, Discoelius wangi). Insects, 16(12), 1197. https://doi.org/10.3390/insects16121197






