Dispersal Ecology of the Beet Armyworm in the Florida Panhandle: Implications for Outbreaks and Insecticide Resistance Spread
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Larval and Moth Sampling
2.2. Stable Hydrogen Isotopes Analysis
2.3. Inferences on the Probability of Origin
3. Results
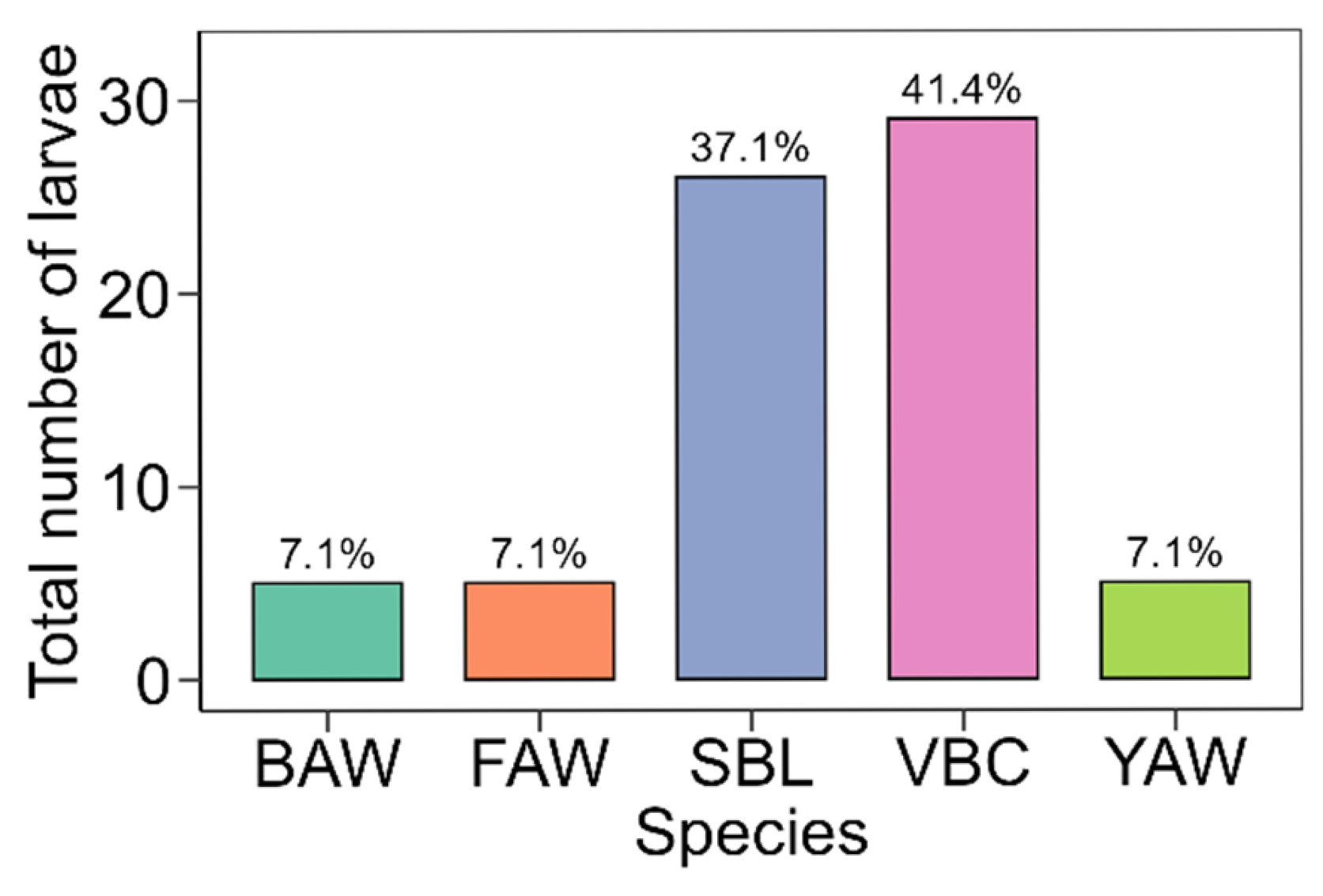
3.1. Larvae Recovered
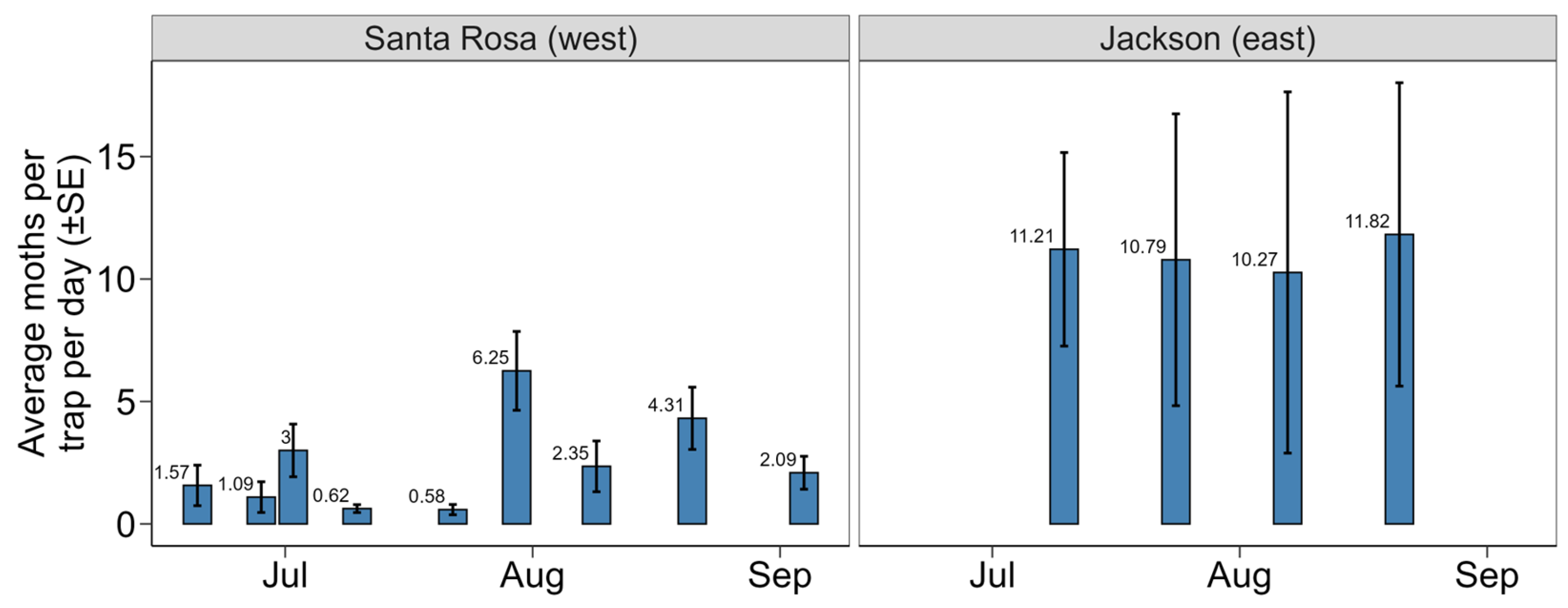
3.2. Pheromone Trapping
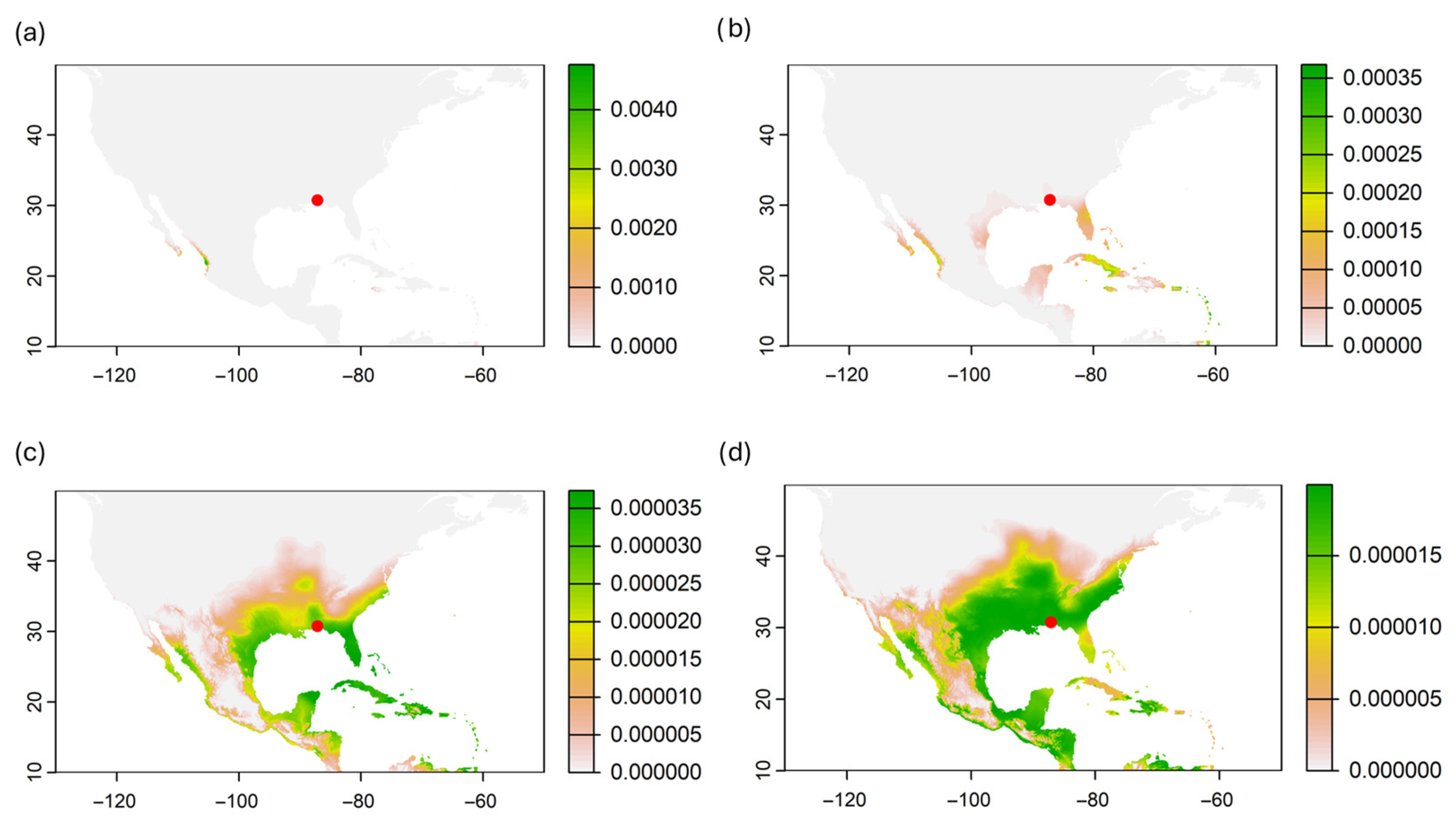
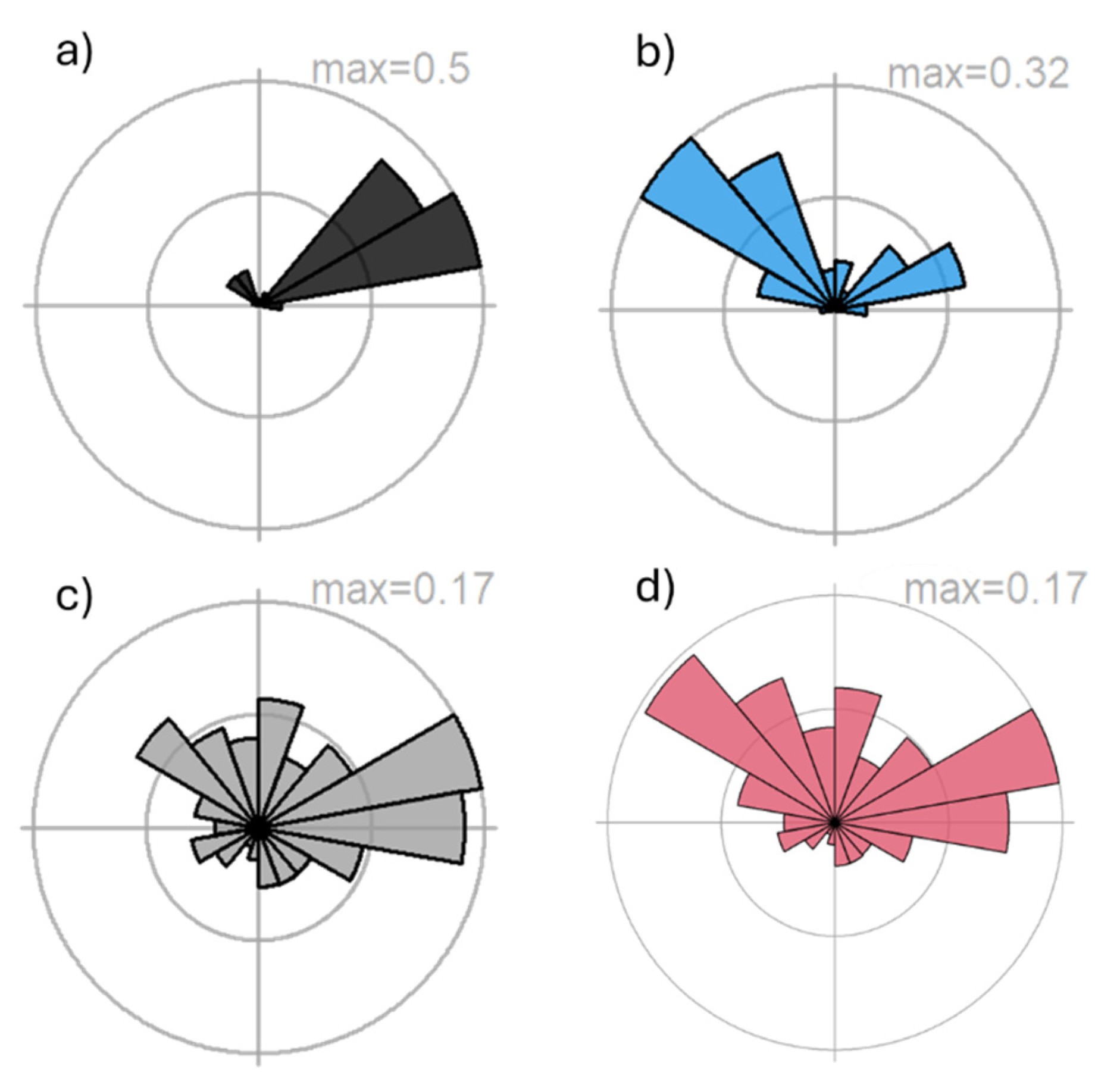
3.3. Probability of Origin
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BAW | Beet armyworm |
| Bt | Bacillus thuringiensis |
| FAW | Fall armyworm |
| IPM | Integrated Pest Management |
| IRM | Insect Resistance Management |
| SBL | Soybean looper |
| VBC | Velvet bean caterpillar |
| YAW | Yellow-striped armyworm |
References
- Capinera, J.L. Handbook of Vegetable Pests; Elsevier: Amsterdam, The Netherlands, 2001; 816p, ISBN 978-0-12-158861-8. [Google Scholar]
- Greenberg, S.M.; Sappington, T.W.; Legaspi, B.C.; Liu, T.-X.; Sétamou, M. Feeding and Life History of Spodoptera exigua (Lepidoptera: Noctuidae) on Different Host Plants. Ann. Entomol. Soc. Am. 2001, 94, 566–575. [Google Scholar] [CrossRef]
- CABI. Spodoptera exigua (Beet Armyworm). 2019, 29808. Available online: https://www.cabidigitallibrary.org/doi/abs/10.1079/cabicompendium.29808 (accessed on 6 August 2025).
- Pearson, A.C. Biology, Population Dynamics, and Pest Status of the Beet Armyworm (Spodoptera exigua) in the Imperial Valley of California. Ph.D. Thesis, University of California, Riverside, CA, USA, 1982. [Google Scholar]
- Capinera, J.L. Beet Armyworm, Spodoptera exigua (Hübner) (Insecta: Lepidoptera: Noctuidae); Entomology and Nematology Department, UF/IFAS Extension, University of Florida: Gainesville, FL, USA, 1999. [Google Scholar]
- Ruberson, J. Environmental Conditions and Biological Control of the Beet Armyworm. In Proceedings of the Beltwide Cotton Conference, Nashville, TN, USA, 9–12 January 1996; Volume 1, pp. 116–118. [Google Scholar]
- Williams, M.R. Cotton Insect Loss Estimates—1998. In Proceedings of the Beltwide Cotton Conference, Orlando, FL, USA, 3–7 January 1999; Volume 2, pp. 807–809. [Google Scholar]
- Ruberson, J.; Herzog, G.; Lambert, W.; Lewis, W. Management of the Beet Armyworm: Integration of Control Approaches. In Proceedings of the Beltwide Cotton Conference, San Diego, CA, USA, 5–8 January 1994; National Cotton Council: Memphis, TN, USA, 1994; pp. 857–859. [Google Scholar]
- Rabelo, M.M.; Santos, I.B.; Paula-Moraes, S.V. Spodoptera exigua (Hubner) (Lepidoptera: Noctuidae) Fitness and Resistance Stability to Diamide and Pyrethroid Insecticides in the United States. Insects 2022, 13, 365. [Google Scholar] [CrossRef] [PubMed]
- Brewer, M.J.; Trumble, J.T. Field Monitoring for Insecticide Resistance in Beet Armyworm (Lepidoptera: Noctuidae). J. Econ. Entomol. 1989, 82, 1520–1526. [Google Scholar] [CrossRef]
- Kerns, D.L.; Palumbo, J.C.; Tellez, T. Resistance of Field Strains of Beet Armyworm (Lepidoptera: Noctuidae) from Arizona and California to Carbamate Insecticides. J. Econ. Entomol. 1998, 91, 1038–1043. [Google Scholar] [CrossRef]
- Rabelo, M.M.; Paula-Moraes, S.V.; Pereira, E.J.G.; Siegfried, B.D. Contrasting Susceptibility of Lepidopteran Pests to Diamide and Pyrethroid Insecticides in a Region of Overwintering and Migratory Intersection. Pest Manag. Sci. 2020, 76, 4240–4247. [Google Scholar] [CrossRef]
- Che, W.; Shi, T.; Wu, Y.; Yang, Y. Insecticide Resistance Status of Field Populations of Spodoptera exigua (Lepidoptera: Noctuidae) from China. J. Econ. Entom. 2013, 106, 1855–1862. [Google Scholar] [CrossRef]
- Zuo, Y.; Ma, H.; Lu, W.; Wang, X.; Wu, S.; Nauen, R.; Wu, Y.; Yang, Y. Identification of the Ryanodine Receptor Mutation I4743M and Its Contribution to Diamide Insecticide Resistance in Spodoptera exigua (Lepidoptera: Noctuidae). Insect Sci. 2020, 27, 791–800. [Google Scholar] [CrossRef]
- Wang, P.; Yang, F.; Wang, Y.; Zhou, L.-L.; Luo, H.-B.; Zhang, S.; Si, S.-Y. Monitoring the Resistance of the Beet Armyworm (Lepidoptera: Noctuidae) to Four Insecticides in Southern China from 2014 to 2018. J. Econ. Entomol. 2021, 114, 332–338. [Google Scholar] [CrossRef]
- Hafeez, M.; Ullah, F.; Khan, M.M.; Li, X.; Zhang, Z.; Shah, S.; Imran, M.; Assiri, M.A.; Fernández-Grandon, G.M.; Desneux, N.; et al. Metabolic-Based Insecticide Resistance Mechanism and Ecofriendly Approaches for Controlling of Beet Armyworm Spodoptera exigua: A Review. Environ. Sci. Pollut. Res. 2022, 29, 1746–1762. [Google Scholar] [CrossRef]
- Moraes, J.G.T.; Pereira, E.J.G.; Esquivel, I.L.; Paula-Moraes, S.V. Performance of Chlorantraniliprole against Beet Armyworm Populations from the Florida Panhandle, 2024. Arthropod Manag. Tests 2025, 50, tsaf031. [Google Scholar] [CrossRef]
- Mikkola, K.; Salmensuu, P. Migration of Laphygma exigua HB. (Lep., Noctuidae) in Northwestern Europe in 1964. Ann. Zool. Fenn. 1965, 2, 124–139. [Google Scholar]
- Wang, X.; Feng, Q.; Zhou, X.; Zhang, H.; Wu, S.; Wu, K. Seasonal Migratory Activity of the Beet Armyworm Spodoptera exigua (Hübner) in the Tropical Area of China. Insects 2024, 15, 986. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, E.R. Migration by Spodoptera exigua and S. frugiperda, North American Style. In Movement of Highly Mobile Insects: Concepts and Methodology in Research; North Carolina State University: Raleigh, NC, USA, 1979; pp. 386–393. [Google Scholar]
- Hobson, K.A. Tracing Origins and Migration of Wildlife Using Stable Isotopes: A Review. Oecologia 1999, 120, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Paula-Moraes, S.V.; Calixto, E.S.; Santos, A.A.; Reay-Jones, F.P.F.; Reisig, D.D.; Farhan, Y.; Smith, J.L.; Hutchison, W.D. Continental-Scale Migration Patterns and Origin of Helicoverpa zea (Lepidoptera: Noctuidae) Based on a Biogeochemical Marker. Environ. Entomol. 2024, 53, nvae034. [Google Scholar] [CrossRef] [PubMed]
- Calixto, E.S.; Paula-Moraes, S.V. Hydrogen Stable Isotopes Indicate Reverse Migration of Fall Armyworm in North America. Insects 2025, 16, 471. [Google Scholar] [CrossRef]
- Nagoshi, R.N.; Meagher, R.L. Review of Fall Armyworm (Lepidoptera: Noctuidae) Genetic Complexity and Migration. Fla. Entomol. 2008, 91, 546–554. [Google Scholar] [CrossRef]
- Tessnow, A.E.; Nagoshi, R.N.; Meagher, R.L.; Fleischer, S.J. Revisiting Fall Armyworm Population Movement in the United States and Canada. Front. Insect Sci. 2023, 3, 1104793. [Google Scholar] [CrossRef]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Mächler, M.; Bolker, B.M. glmmTMB Balances Speed and Flexibility among Packages for Zero-Inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression; Sage Publications: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Lenth, R. Emmeans: Estimated Marginal Means, Aka Least-Squares Means, R package version 1.3.0; R Foundation for Statistical Computing: Vienna, Austria, 2020.
- Hobson, K.A.; Wassenaar, L.I. Stable Isotope Ecology: An Introduction. Oecologia 1999, 120, 312–313. [Google Scholar] [CrossRef]
- Wassenaar, L.; Hobson, K. Comparative Equilibration and Online Technique for Determination of Non-Exchangeable Hydrogen of Keratins for Use in Animal Migration Studies. Isot. Environ. Health Stud. 2003, 39, 211–217. [Google Scholar] [CrossRef]
- Ma, C.; Vander Zanden, H.B.; Wunder, M.B.; Bowen, G.J. assignR: An R Package for Isotope-Based Geographic Assignment. Methods Ecol. Evol. 2020, 11, 996–1001. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing: Vienna, Austria, 2024.
- Hendricks, D.E.; Hubbard, D.W.; Hardee, D.D. Occurrence of Beet Armyworm Moths in the Lower Mississippi River Delta as Indicated by Numbers Caught in Traps in 1994. Southwest. Entomol. 1995, 20, 157–164. [Google Scholar]
- Zhang, H.; Xu, D.; Deng, X.; Liu, Z.; He, Z.; Wu, J.; Zhuo, Z. Impact of Temperature Variation on the Biological Traits and Lifecycle of Spodoptera exigua (Lepidoptera: Noctuidae): A Meta-Analysis Approach. Insects 2025, 16, 155. [Google Scholar] [CrossRef]
- Williams, M. Cotton Insect Loss Estimates—1993. In Proceedings of the Beltwide Cotton Conference, San Diego, CA, USA, 5–8 January 1994; pp. 743–763. [Google Scholar]
- Burris, E.; Graves, J.B.; Leonard, B.R.; White, C.A. Beet Armyworms (Lepidoptera: Noctuidae) in Northeast Louisiana: Observations on an Uncommon Insect Pest. Fla. Entomol. 1994, 77, 454. [Google Scholar] [CrossRef]
- Johnson, S.J. Migration and the Life History Strategy of the Fall Armyworm, Spodoptera frugiperda in the Western Hemisphere. Int. J. Trop. Insect. Sci. 1987, 8, 543–549. [Google Scholar] [CrossRef]
- Westbrook, J.; Fleischer, S.; Jairam, S.; Meagher, R.; Nagoshi, R. Multigenerational Migration of Fall Armyworm, a Pest Insect. Ecosphere 2019, 10, e02919. [Google Scholar] [CrossRef]
- Gould, F.; Blair, N.; Reid, M.; Rennie, T.; Lopez, J.; Micinski, S. Bacillus Thuringiensis—Toxin Resistance Management: Stable Isotope Assessment of Alternate Host Use by Helicoverpa zea. Proc. Natl. Acad. Sci. USA 2002, 99, 16581–16586. [Google Scholar] [CrossRef]
- Westbrook, J.K. Noctuid Migration in Texas within the Nocturnal Aeroecological Boundary Layer. Integr. Comp. Biol. 2008, 48, 99–106. [Google Scholar] [CrossRef]
- Westbrook, J.K.; López, J.D. Long-Distance Migration in Helicoverpa zea: What We Know and Need to Know. Southwest. Entomol. 2010, 35, 355–360. [Google Scholar] [CrossRef]
- Gutiérrez-Moreno, R.; Mota-Sanchez, D.; Blanco, C.A.; Whalon, M.E.; Terán-Santofimio, H.; Rodriguez-Maciel, J.C.; DiFonzo, C. Field-Evolved Resistance of the Fall Armyworm (Lepidoptera: Noctuidae) to Synthetic Insecticides in Puerto Rico and Mexico. J. Econ. Entomol. 2019, 112, 792–802. [Google Scholar] [CrossRef]
- Beuzelin, J.M.; Larsen, D.J.; Roldán, E.L.; Schwan Resende, E. Susceptibility to Chlorantraniliprole in Fall Armyworm (Lepidoptera: Noctuidae) Populations Infesting Sweet Corn in Southern Florida. J. Econ. Entomol. 2022, 115, 224–232. [Google Scholar] [CrossRef]
- Reisig, D.; Goldsworthy, E.; Kerns, D.D.; Paula-Moraes, S.V.; Jurat-Fuentes, J.L. First Characterization of Vip3Aa Resistance in Beet Armyworm, Spodoptera exigua (Hübner), Collected from Bollgard 3 Cotton. In Proceedings of the Beltwide Cotton Conferences, New Orleans, LA, USA, 14–16 January 2025. [Google Scholar]

| Sample ID | δ2H | Date Collection | Florida County |
|---|---|---|---|
| 1 | −28.22 | 20 June 2024 | Santa Rosa |
| 2 | −21.85 | 20 June 2024 | Santa Rosa |
| 3 | −18.19 | 20 June 2024 | Santa Rosa |
| 4 | −44.59 | 20 June 2024 | Santa Rosa |
| 5 | −46.32 | 22 July 2024 | Santa Rosa |
| 6 | −59.11 | 22 July 2024 | Santa Rosa |
| 7 | −61.52 | 22 July 2024 | Santa Rosa |
| 8 | −63.84 | 22 July 2024 | Santa Rosa |
| 9 | −63.93 | 21 August 2024 | Santa Rosa |
| 10 | −59.62 | 21 August 2024 | Santa Rosa |
| 11 | −63.56 | 21 August 2024 | Santa Rosa |
| 12 | −33.45 | 21 August 2024 | Santa Rosa |
| 13 | −44.12 | 21 August 2024 | Santa Rosa |
| 14 | −40.56 | 10 July 2024 | Jackson |
| 15 | −33.38 | 10 July 2024 | Jackson |
| 16 | −39.04 | 10 July 2024 | Jackson |
| 17 | −44.41 | 10 July 2024 | Jackson |
| 18 | −71.02 | 7 August 2024 | Jackson |
| 19 | −47.52 | 7 August 2024 | Jackson |
| 20 | −65.97 | 7 August 2024 | Jackson |
| 21 | −32.70 | 7 August 2024 | Jackson |
| 22 | −65.41 | 7 August 2024 | Jackson |
| 23 | −62.40 | 21 August 2024 | Jackson |
| 24 | −59.18 | 21 August 2024 | Jackson |
| 25 | −60.17 | 21 August 2024 | Jackson |
| 26 | −59.84 | 21 August 2024 | Jackson |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calixto, E.S.; Moraes, J.G.T.d.; Carter, E.; Esquivel, I.L.; Paula-Moraes, S.V. Dispersal Ecology of the Beet Armyworm in the Florida Panhandle: Implications for Outbreaks and Insecticide Resistance Spread. Insects 2025, 16, 1131. https://doi.org/10.3390/insects16111131
Calixto ES, Moraes JGTd, Carter E, Esquivel IL, Paula-Moraes SV. Dispersal Ecology of the Beet Armyworm in the Florida Panhandle: Implications for Outbreaks and Insecticide Resistance Spread. Insects. 2025; 16(11):1131. https://doi.org/10.3390/insects16111131
Chicago/Turabian StyleCalixto, Eduardo Soares, João Gabriel Tardin de Moraes, Ethan Carter, Isaac L. Esquivel, and Silvana V. Paula-Moraes. 2025. "Dispersal Ecology of the Beet Armyworm in the Florida Panhandle: Implications for Outbreaks and Insecticide Resistance Spread" Insects 16, no. 11: 1131. https://doi.org/10.3390/insects16111131
APA StyleCalixto, E. S., Moraes, J. G. T. d., Carter, E., Esquivel, I. L., & Paula-Moraes, S. V. (2025). Dispersal Ecology of the Beet Armyworm in the Florida Panhandle: Implications for Outbreaks and Insecticide Resistance Spread. Insects, 16(11), 1131. https://doi.org/10.3390/insects16111131








