The lncRNA41584-miR3047-z-BmCDK20 ceRNA Regulatory Network Influences Reproductive Development in Male Silkworms (Bombyx mori)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Silkworm Breeding and BmN/HEK293T Cell Culture
2.2. Total RNA Extraction and Quantitative Real-Time RT–PCR (RT–qPCR)
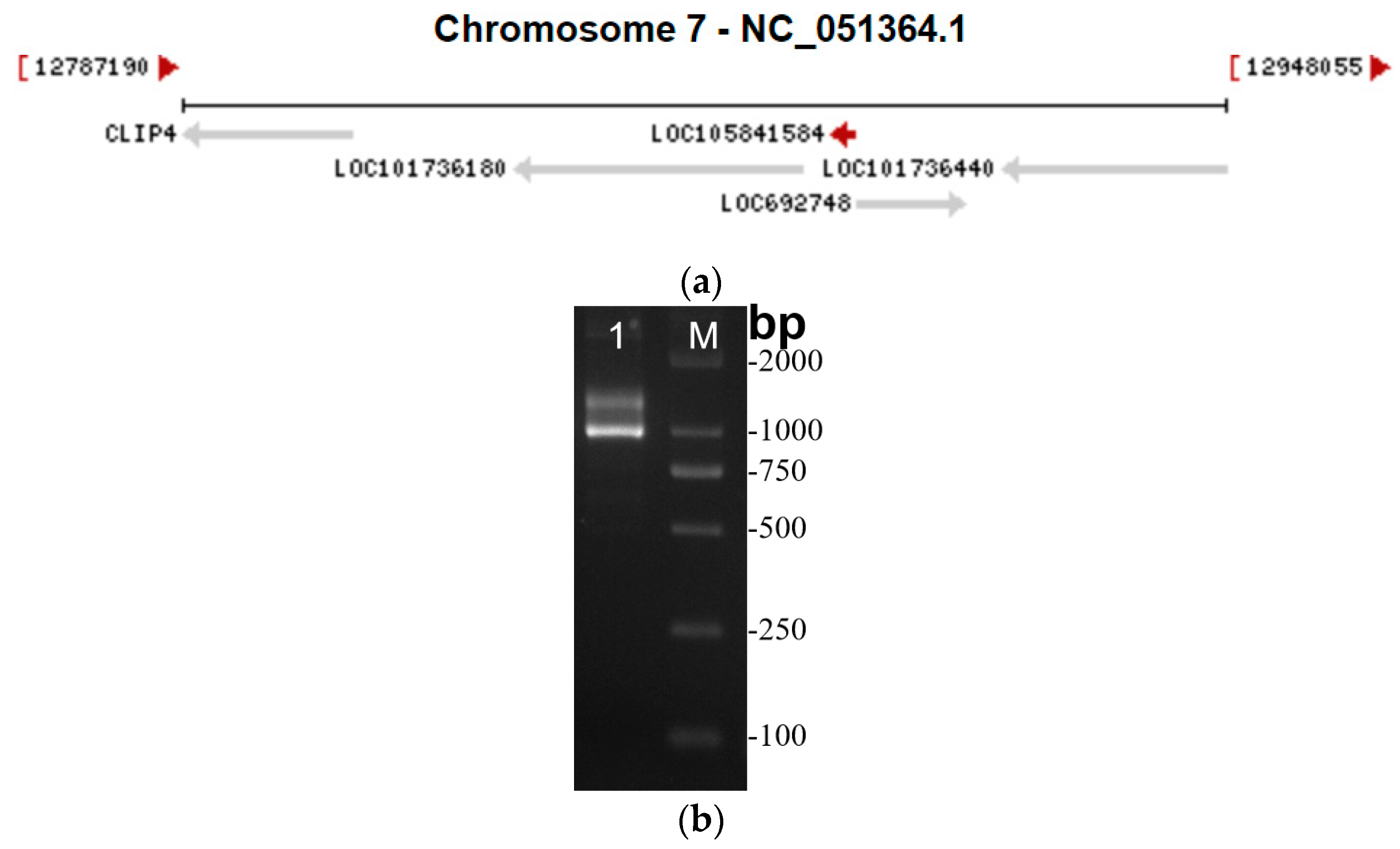
2.3. Full-Length Amplification of lncRNA41584
2.4. Subcellular Localization of lncRNA41584 in Testicular Cells
2.4.1. Fluorescence In Situ Hybridization (FISH)
2.4.2. Nuclear–Cytoplasmic RNA Separation
2.5. Tissue and Temporal Expression Profiles of lncRNA41584
2.6. RNAi and Overexpression of lncRNA41584, miR-3047-z, and BmCDK20 in the BmN Cell Line
2.7. MTT Assay for Detecting Cell Proliferation
2.8. Flow Cytometry Analysis of the Cell Cycle at 48 h After Knockdown of lncRNA41584 and BmCDK20
2.9. Dual-Luciferase Reporter Assay
2.10. Knockdown of lncRNA41584 and Overexpression of miR-3047-z in B. mori
2.11. Tissue Paraffin Sectioning
3. Results
3.1. Full-Length Amplification of lncRNA41584
3.2. Temporal and Spatial Expression Profiles of lncRNA41584
3.3. Subcellular Localization of lncRNA41584
3.4. Prediction of the ceRNA Regulatory Network of lncRNA41584 and Its Transcriptional Level in JMS
3.5. Impact of lncRNA41584/miR-3047-z/BmCDK20 on BmN Cell Proliferation
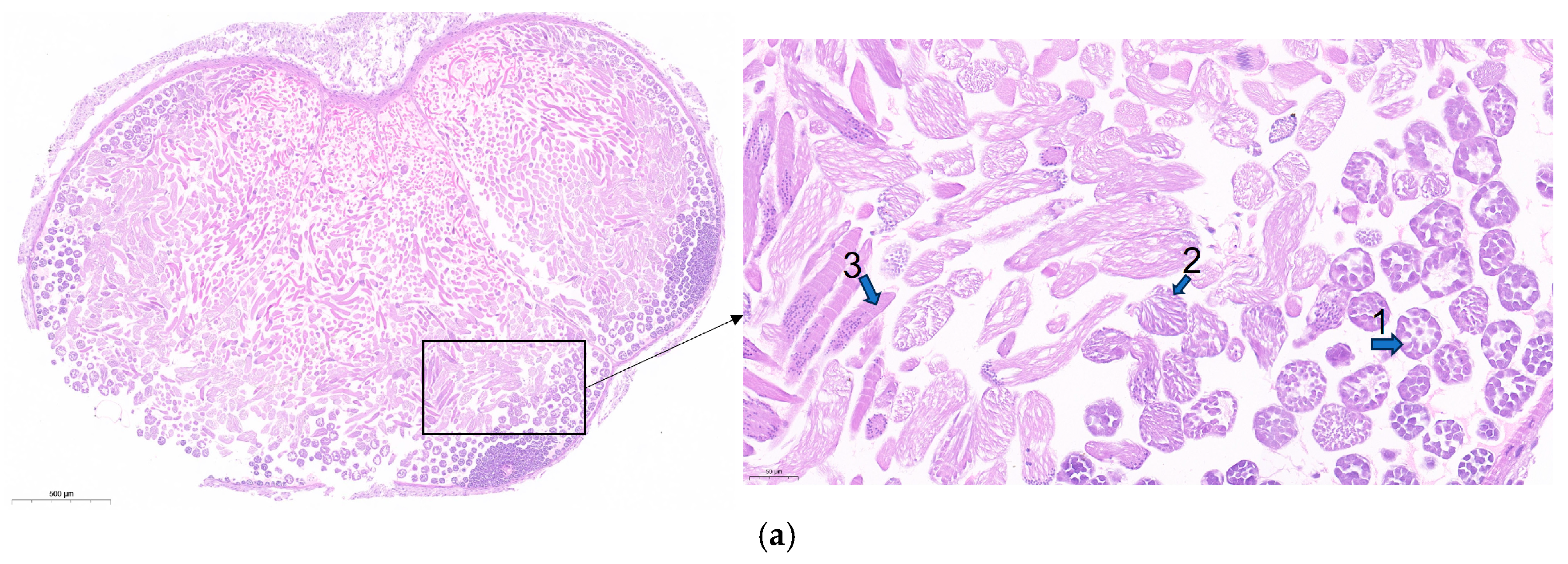
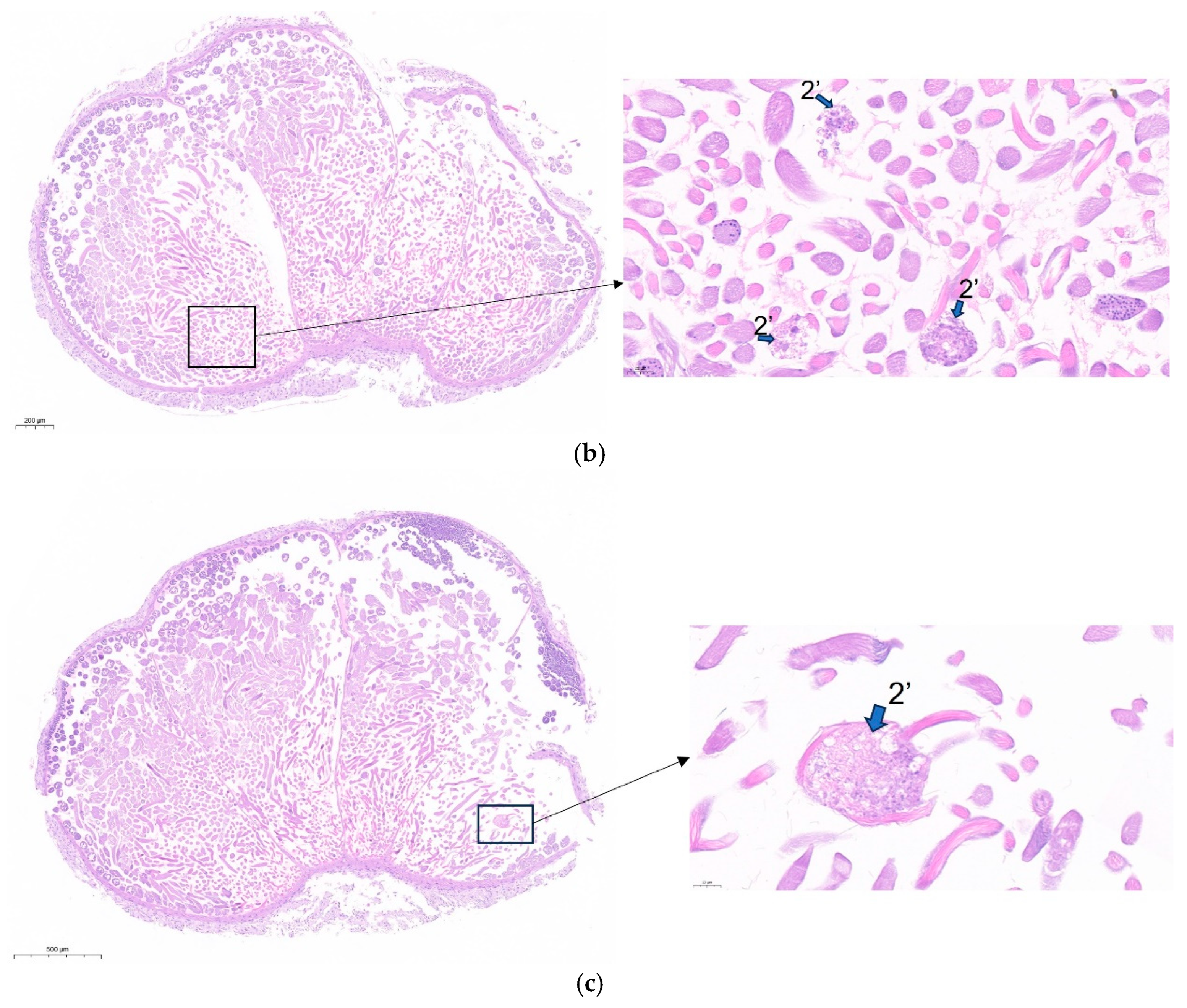
3.6. Knockdown of lncRNA41584 or Overexpression of miR-3047-z Leads to Abnormal Spermatogenesis and Reduced Male Fertility in B. mori
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Osanai, M.; Kasuga, H.; Aigaki, T. Physiological role of apyrene spermatozoa of Bombyx mori. Cell. Mol. Life Sci. CMLS 1987, 43, 593–596. [Google Scholar] [CrossRef]
- Chen, K.; Chen, S.; Xu, J.; Yu, Y.; Liu, Z.; Tan, A.; Huang, Y. Maelstrom regulates spermatogenesis of the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 2019, 109, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, D.; Zheng, S.; Yi, M.; Wang, S.; Liu, Y.; Jing, L.; Liu, Z.; Yang, D.; Liu, Y.; et al. The Prmt5-Vasa module is essential for spermatogenesis in Bombyx mori. PLoS Genet. 2023, 19, e1010600. [Google Scholar] [CrossRef]
- Joshi, M.; Rajender, S. Long non-coding RNAs (lncRNAs) in spermatogenesis and male infertility. Reprod. Biol. Endocrinol. 2020, 18, 103. [Google Scholar] [CrossRef]
- Hu, K.; Li, L.; Liao, Y.; Liang, M. LncRNA Gm2044 highly expresses in spermatocyte and inhibits Utf1 translation by interacting with Utf1 mRNA. Genes Genom. 2018, 40, 781–787. [Google Scholar] [CrossRef]
- Chan, W.Y.; Lee, T.L.; Wu, S.M.; Ruszczyk, L.; Rennert, O.M. Transcriptome analyses of male germ cells with serial analysis of gene expression (SAGE). Mol. Cell. Endocrinol. 2006, 250, 8–19. [Google Scholar] [CrossRef]
- Li, L.; Wang, M.; Wu, X.; Geng, L.; Xue, Y.; Wei, X.; Jia, Y. A long non-coding RNA interacts with Gfra1 and maintains survival of mouse spermatogonial stem cells. Cell Death Dis. 2016, 7, e2140. [Google Scholar] [CrossRef]
- Kataruka, S.; Akhade, V.S.; Kayyar, B.; Rao, M.R.S. Mrhl Long Noncoding RNA Mediates Meiotic Commitment of Mouse Spermatogonial Cells by Regulating Sox8 Expression. Mol. Cell. Biol. 2017, 37, 18. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Tian, H.; Cao, Y.X.; He, X.; Chen, L.; Song, X.; Ping, P.; Huang, H.; Sun, F. Downregulation of miR-320a/383-sponge-like long non-coding RNA NLC1-C (narcolepsy candidate-region 1 genes) is associated with male infertility and promotes testicular embryonal carcinoma cell proliferation. Cell Death Dis. 2015, 6, e1960. [Google Scholar] [CrossRef]
- Hu, K.; Zhang, J.; Liang, M. LncRNA AK015322 promotes proliferation of spermatogonial stem cell C18-4 by acting as a decoy for microRNA-19b-3p. Vitr. Cell. Dev. Biol.-Anim. 2017, 53, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Qijing, L.; Qin, P.; Na, L.; Zhe, Z.; Juqing, Z.; Xin, H.; Sha, P.; Guangpeng, L.; Kuldip, S.; Shulin, C. H19 regulates the proliferation of bovine male germline stem cells via IGF-1 signaling pathway. J. Cell. Physiol. 2019, 234, 915–926. [Google Scholar]
- Rashid, F.; Shah, A.; Shan, G. Long Non-coding RNAs in the Cytoplasm. Genom. Proteom. Bioinform. 2016, 14, 8. [Google Scholar] [CrossRef]
- Salmena, L.; Poliseno, L.; Tay, Y.; Kats, L.; Pandolfi, P.P. A ceRNA Hypothesis: The Rosetta Stone of a Hidden RNA Language? Cell 2011, 146, 353–358. [Google Scholar] [CrossRef]
- Fabian, M.R.; Sonenberg, N.; Filipowicz, W. Regulation of mRNA translation and stability by microRNAs. Annu. Rev. Biochem. 2010, 79, 351–379. [Google Scholar] [CrossRef]
- Zhou, L.; Li, Y.Y.; Li, J.R.; Yao, H.Y.; Huang, J.; Li, C.; Wang, L. Multi-omics analysis to identify CBR3-AS1-hsa-miR-145-5p-MAP3K5 pathway as a ferroptosis-related ceRNA network in benign prostatic hyperplasia. Genes Dis. 2024, 11, 101184. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, G.; Xu, M.; Ying, X.; Li, B.; Cao, F.; Cheng, S.; Xiao, B.; Cheng, M.; Liang, L. Comprehensive analysis of PTEN-related ceRNA network revealing the key pathways WDFY3-AS2-miR-21-5p/miR-221-3p/miR-222-3p-TIMP3 as potential biomarker in tumorigenesis and prognosis of kidney renal clear cell carcinoma. Mol. Carcinog. 2022, 61, 508–523. [Google Scholar] [CrossRef]
- Yu, X.; Huang, J.; Liu, X.; Li, J.; Yu, M.; Li, M.; Xie, Y.; Li, Y.; Qiu, J.; Xu, Z. LncRNAH19 acts as a ceRNA of let-7g to facilitate endothelial-to-mesenchymal transition in hypoxic pulmonary hypertension via regulating TGF-β signalling pathway. Respir. Res. 2024, 25, 270. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.P.; Lu, L.Z.; Li, H.Y.; Chen, L.; Gu, T.T.; Zhao, X.Y.; Yao, Y.Y.; Li, J.H. The effect of LINC9137 targeting miR-140-3p-NKAIN3 signal axis on the development of goose testis sertoli cells. Poult. Sci. 2024, 103, 11. [Google Scholar] [CrossRef]
- Patterson, J.O.; Basu, S.; Rees, P.; Nurse, P. CDK control pathways integrate cell size and ploidy information to control cell division. eLife 2021, 10, 17. [Google Scholar] [CrossRef]
- Cao, Y.M.; Sun, Q.; Chen, Z.L.; Lu, J.; Geng, T.; Ma, L.; Zhang, Y.Z. CDKN2AIP is critical for spermiogenesis and germ cell development. Cell Biosci. 2022, 12, 18. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, J.; Lu, Y.; Zhang, S.; Huang, J.; Chen, J.; Bei, J.X.; Yang, K.; Wu, G.; Huang, K.; et al. CDK20 interacts with KEAP1 to activate NRF2 and promotes radiochemoresistance in lung cancer cells. Oncogene 2017, 36, 5321–5330. [Google Scholar] [CrossRef]
- Bai, J.H.; Guo, D.Z.; Li, J.; Wang, H.F.; Wang, C.; Liu, Z.G.; Guo, X.Q.; Wang, Y.; Xu, B.H. The role of AccCDK20 and AccCDKN1 from Apis cerana cerana in development and response to pesticide and heavy metal toxicity. Pest. Biochem. Physiol. 2023, 190, 10. [Google Scholar] [CrossRef]
- Noguchi, T.; Nakamura, K.; Satoda, Y.; Katoh, Y.; Nakayama, K. CCRK/CDK20 regulates ciliary retrograde protein trafficking via interacting with BROMI/TBC1D32. PLoS ONE 2021, 16, e0258497. [Google Scholar] [CrossRef]
- Huang, T.C.; Zhong, S.S.; Sun, J.; Shen, D.X.; Zhang, X.L.; Zhao, Q.L. Whole transcriptome analysis identifies differentially expressed mRNA, miRNA and lncRNA associated with male sterility in the silkworm, Bombyx mori. Comp. Biochem. Physiol. D-Genom. Proteom. 2024, 52, 13. [Google Scholar] [CrossRef]
- Pruitt, K.D.; Brown, G.R.; Hiatt, S.M.; Thibaud-Nissen, F.; Astashyn, A.; Ermolaeva, O.; Farrell, C.M.; Hart, J.; Landrum, M.J.; Mcgarvey, K.M. RefSeq: An update on mammalian reference sequences. Nucleic Acids Res. 2013, 42, D756–D763. [Google Scholar] [CrossRef] [PubMed]
- Min-Jie, N.; Zhi-Hong, H.; Qiang, L.; Mo-Fang, L.; Min-Hua, L.; Jin-Song, Z.; Li, Z.; Yong-Lian, Z.; Juan, M. Identification and Characterization of a Novel Non-Coding RNA Involved in Sperm Maturation. PLoS ONE 2011, 6, e26053. [Google Scholar]
- Anguera, M.C.; Ma, W.; Clift, D.; Namekawa, S.; Lee, J.T. Tsx Produces a Long Noncoding RNA and Has General Functions in the Germline, Stem Cells, and Brain. PLoS Genet. 2011, 7, e1002248. [Google Scholar] [CrossRef]
- Matson, C.K.; Murphy, M.W.; Griswold, M.D.; Yoshida, S.; Bardwell, V.J.; Zarkower, D. The mammalian doublesex homolog DMRT1 is a transcriptional gatekeeper that controls the mitosis versus meiosis decision in male germ cells. Dev. Cell 2010, 19, 612–624. [Google Scholar] [CrossRef] [PubMed]
- Misuzu, K.; Kai, O.; Shin, M.; Akira, S.; Honoo, S.; Kimura, A.P. A Testis-Specific Long Non-Coding RNA, lncRNA-Tcam1, Regulates Immune-Related Genes in Mouse Male Germ Cells. Front. Endocrinol. 2017, 8, 299. [Google Scholar]
- Satoh, Y.; Takei, N.; Kawamura, S.; Takahash, N.; Kotani, T.; Kimura, A.P. A novel testis-specific long noncoding RNA, Tesra, activates the Prss42/Tessp-2 gene during mouse spermatogenesis. Biol. Reprod. 2019, 100, 833–848. [Google Scholar] [CrossRef]
- Molyneaux, K.A.; Zinszner, H.; Kunwar, P.S.; Schaible, K.; Lehmann, R. The chemokine SDF1/CXCL12 and its receptor CXCR4 regulate mouse germ cell migration and survival. Development 2003, 130, 4279–4286. [Google Scholar] [CrossRef] [PubMed]
- Maatouk, D.M.; Loveland, K.L.; Mcmanus, M.T.; Moore, K.; Harfe, B.D. Dicer1 Is Required for Differentiation of the Mouse Male Germline. Biol. Reprod. 2008, 79, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Anders, L.; Ke, N.; Hydbring, P.; Choi, Y.J.; Widlund, H.R.; Chick, J.M.; Zhai, H.; Vidal, M.; Gygi, S.P.; Braun, P. A Systematic Screen for CDK4/6 Substrates Links FOXM1 Phosphorylation to Senescence Suppression in Cancer Cells. Cancer Cell 2011, 20, 620–634. [Google Scholar] [CrossRef] [PubMed]
- Bramson, H.N.; Corona, J.; Davis, S.T.; Dickerson, S.H.; Edelstein, M.; Frye, S.V.; Gampe, R.T.; Harris, P.A.; Hassell, A.; Holmes, W.D. Oxindole-based inhibitors of cyclin-dependent kinase 2 (CDK2): Design, synthesis, enzymatic activities, and X-ray crystallographic analysis. J. Med. Chem. 2001, 44, 4339. [Google Scholar] [CrossRef]










| Primer | Sequence 5′-3′ |
|---|---|
| BmCDK20-gF | CGTTGGTCGAATCGGTGAAGGA |
| BmCDK20-gR | GTCAGTTCATGCTCGCATTGGT |
| lncRNA41584-gF | TTGTGACGGTGGCGTTATG |
| lncRNA41584-gR | TTCCCGCTTTGTCGCTTGT |
| Long Primer | CTAATACGACTCACTATAGGGCAAGCAGTGGTATCAACGCAGAGT |
| Short Primer | CTAATACGACTCACTATAGGGC |
| lncRNA41584-5race | GACTGAAGCCGTGCCCGTCTATCCAATCTG |
| lncRNA41584-F | GTAAAGACAATTTGATGTTATCATCTAAAAAAATC |
| lncRNA41584-R | CGTGGGTTGAATCTGTTTTTAATTATTAC |
| 41584-Cy3 | CAAUCUGGUGUAAUGUUAAAU |
| NC-Cy3 | UGCUUUGCACGGUAACGCCUGUUUU |
| 18S-Cy3 | CUUCCUUGGAUGUGGUAGCCGUUUC |
| lncRNA41584-siRNA | CGAUUUCGUUCCGAAAUUATT |
| miR-3047-z | uACAAAACuuuAAAAACu |
| BmCDK20-siRNA | GAGAAGAUCCAGCGAGAAATT |
| BmGAPDH-gF | GTGTCCTCAGACTTCATTGG |
| BmGAPDH-gR | AATGACTCTGCTGGAATAACC |
| BmU6-F | CGTATACTAAAATTGGAACGATACAG |
| BmU6-R | ATTTTGCGTGTCATCCTTGC |
| BmActin-F | CCGTATGCGAAAGGAAATCA |
| BmActin-R | TTGGAAGGTAGAGAGGGAGG |
| 41584-GSP-1 | GTGTCCCGATGTTTTTGTGACG |
| 41584-GSP-2 | TTATATTAGTATTTTATCCGTTTTTAGATC |
| miR-3047-z RT | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACGTTTTT |
| miR-3047-z F | GCGCGCGCGACAAAAC |
| miRNA reverse primer | AGTGCAGGGTCCGAGGTATT |
| lncRNA41584-Xho1 F | ccgctcgagGTAAAGACAATTTGATG |
| lncRNA41584-Not1 R | ATTTGCGGCCGCTTTTCGTGGGTTGAATCTG |
| BmCDK20-xho1 F | CCGCTCGAGCTAAATTCAACTTGCTACAAAAATAATTATG |
| BmCDK20-Not1 R | ATTTGCGGCCGCACACAAAACTTATAGCCTACTCAG |
| BmPCNA-gF | TGAATCTAGGCAGCATGTCAA |
| BmPCNA-gR | TCCTGTGCTTTTATTGTGGCTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, T.; Sun, J.; Zhong, S.; Shen, D.; Qian, H.; Zhao, Q. The lncRNA41584-miR3047-z-BmCDK20 ceRNA Regulatory Network Influences Reproductive Development in Male Silkworms (Bombyx mori). Insects 2025, 16, 1120. https://doi.org/10.3390/insects16111120
Huang T, Sun J, Zhong S, Shen D, Qian H, Zhao Q. The lncRNA41584-miR3047-z-BmCDK20 ceRNA Regulatory Network Influences Reproductive Development in Male Silkworms (Bombyx mori). Insects. 2025; 16(11):1120. https://doi.org/10.3390/insects16111120
Chicago/Turabian StyleHuang, Tianchen, Juan Sun, Shanshan Zhong, Dongxu Shen, Heying Qian, and Qiaoling Zhao. 2025. "The lncRNA41584-miR3047-z-BmCDK20 ceRNA Regulatory Network Influences Reproductive Development in Male Silkworms (Bombyx mori)" Insects 16, no. 11: 1120. https://doi.org/10.3390/insects16111120
APA StyleHuang, T., Sun, J., Zhong, S., Shen, D., Qian, H., & Zhao, Q. (2025). The lncRNA41584-miR3047-z-BmCDK20 ceRNA Regulatory Network Influences Reproductive Development in Male Silkworms (Bombyx mori). Insects, 16(11), 1120. https://doi.org/10.3390/insects16111120





