Simple Summary
Flavonoids are plant phenolic compounds whose biological activities include participation in plant responses to various stresses of biological and environmental origins, including protection against insect herbivore attack. We were interested in whether the specific flavonoids detected in soybean leaves have the potential to discourage the pea aphid from infesting other leguminous plants, peas in particular. We immersed the pea leaves in ethanolic solutions of the flavonoids apigenin, daidzein, genistein and kaempferol, offered them to the pea aphids and observed their behavior when they probed plant tissues with their piercing–sucking mouthparts. We discovered that aphids readily probed the pea leaves whether they were treated with flavonoids or not. However, later on, the behavior of the aphids changed depending on the flavonoid applied. Apigenin, daidzein and kaempferol caused a decrease in the intensity of plant sap ingestion. In addition, daidzein and kaempferol made the finding of sap-transporting vessels more difficult for aphids. In contrast, genistein did not affect the pea aphids’ feeding activity. Our findings provide the plant breeders and plant protection services with information on what direction their efforts should take to protect leguminous plants against aphids in a sustainable and environmentally friendly way.
Abstract
Flavonoids detected in soybean Glycine max (L.) Merr. (Fabaceae) cause various alterations in the metabolism, behavior, and development of insect herbivores. The pea aphid Acyrthosiphon pisum (Harris) (Hemiptera: Aphididae) poses potential threat to soybeans, but the effect of individual flavonoids on its feeding-associated behavior is relatively unknown. We monitored probing behavior (stylet penetration activities) of A. pisum on its preferred host plant, Pisum sativum L. untreated (control) and treated with 0.1% ethanolic solutions of flavonoids apigenin, daidzein, genistein, and kaempferol. We applied the electrical penetration graph (electropenetrography, EPG) technique, which visualizes the movements of aphid stylets within plant tissues. None of the applied flavonoids affected the propensity to probe the plants by A. pisum. However, apigenin enhanced the duration of probes in non-phloem tissues, which caused an increase in the frequency and duration of stylet mechanics derailment and xylem sap ingestion but limited the ingestion of phloem sap. Daidzein caused a delay in reaching phloem vessels and limited sap ingestion. Kaempferol caused a reduction in the frequency and duration of the phloem phase. Genistein did not affect aphid probing behavior. Our findings provide information for selective breeding programs of resistant plant cultivars to A. pisum.
1. Introduction
Soybean Glycine max (L.) Merr. (Fabaceae) flavonoids have been broadly studied for their importance in plant metabolism, the establishment of symbiotic relationships, response to abiotic and biotic stresses and human health-promoting effects [1,2,3,4,5,6,7]. Soybean is attacked by a number of insect pests, and some of them can cause severe economic damage. Soybean flavonoids have been identified as compounds that might negatively affect the behavior and development of herbivores. Specifically, a disruption in the metamorphosis of the redbanded stink bug Piezodorus guildinii (Westwood) (Hemiptera: Pentatomidae) occurred on certain genistein- and rutin-rich soybean cultivars [8]. Rutin reduced growth and deterred feeding of the cabbage looper Trichoplusia ni (Hübner) (Lepidoptera: Noctuidae) larvae and velvet bean Anticarsia gemmatalis Hübner (Lepidoptera: Noctuidae) caterpillars [9,10], and inhibited the early larval growth of the fruit-worm Helicoverpa zea (Boddie) (Lepidoptera: Noctuidae) [11]. Moreover, soybean flavonoids are induced by herbivory. Daidzein and formononetin in soybean leaves were induced by Spodoptera litura (F.) (Lepidoptera: Noctuidae) [3]. The infestation by soybean aphids Aphis glycines Matsumura (Hemiptera: Aphididae) caused the accumulation of daidzein, formononetin, and genistein [12]. Flavonoids were strongly induced in soybean leaves infested by the cowpea aphid Aphis craccivora Koch [13,14]. Likewise, an increase in flavonoid contents was observed in Pisum sativum L. (Fabaceae) infested by the pea aphid Acyrthosiphon pisum (Harris) (Hemiptera: Aphididae) [15,16,17]. The infestation of A. pisum induced the accumulation of genistein in alfalfa Medicago sativa L., which, in turn, reduced the survival rate of the aphids [18].
The natural induction of flavonoids in plants in response to insect feeding serves as a biomarker for the selection of resistant lines in breeding strategies [14]. The development of plant resistance has been considered as an alternative to neurotoxic insecticides for the purpose of preventing insect feeding [19]. Of the three plant resistance mechanisms, antixenosis, antibiosis, and tolerance, antixenosis and antibiosis are based on effects of plant traits on insect behavior and development. Tolerance is the ability of plants to survive insect damage [20]. Efforts, both conventional and using genetic engineering, have been made to obtain soybean lines and cultivars with at least moderate levels of antixenosis or antibiosis resistance to insects, including aphids [7,19,20,21]. The enhanced synthesis of flavonoids or the modification of their profiles in plants to make them less acceptable to herbivores are the suggested mechanisms for a biotechnological approach, as well as selective breeding programs in Fabaceae in general and in G. max in particular [14,22,23]. Interestingly, biopesticide synergy was observed when plant flavonoids were combined with entomopathogenic baculovirus: the soybean flavonoid compounds daidzein, genistein and kaempferol were found to synergistically improve biopesticide AfMNPV baculovirus activity against T. ni [24].
Flavonoids occur in many plant tissues, where they are present inside the cells or on the surfaces of different plant organs [22]. Herbivores such as Coleoptera or caterpillars of Lepidoptera and Hymenoptera (Symphyta) that possess chewing–biting mouthparts come into contact with flavonoids when they crush plant tissues for consumption. Following the damage, the plant structure is destroyed and allelochemicals are released from their storage spaces. The discharged flavonoids make contact with taste receptors on insect mouthparts and, depending on individual sensitivity and plant–herbivore compatibility, affect the herbivore behavior: they attract it to the plant or deter its feeding [25]. In contrast, herbivores such as Hemiptera or Thysanoptera that possess sucking–piercing mouthparts feed upon sap from various plant tissues without breaking plant integrity [26]. Among those herbivores, aphids (Hemiptera: Aphididae) exhibit the most sophisticated way of feeding: their mouthparts’ stylets penetrate plant tissues intercellularly to reach phloem sieve elements, from which they acquire nutrients [27]. Before they reach the phloem, aphids taste the contents of cells that are adjacent to the stylets’ route; stylets make short punctures into cells without destroying them and then aphids suck up samples of cell fluids [28]. At this stage, aphids may come into contact with plant allelochemicals, including flavonoids that are stored in epidermis and mesophyll cells [27,29]. When its stylets reach sieve elements, the aphid will possibly start feeding, i.e., it will uptake the phloem sap [27,30,31]. Along with nutrients, flavonoids transported through phloem vessels may be ingested [32,33,34]. Thus, plant flavonoids may affect aphid probing behavior when taken up from cells of peripheral tissues and/or sieve elements. In other words, the pre-ingestive and/or ingestive phases of probing, respectively, may be modified depending on the amount and/or the composition of flavonoid mixture in plant tissues.
The effect of individual flavonoids on the pea aphid probing behavior has rarely been investigated. Usually, flavonoids were added to sucrose–agarose diets [35,36,37]. The results of those experiments showed that high luteolin and genistein concentration reduced the ingestion of the diet by A. pisum [35]. At the same time, an increase in the developmental time, the pre-reproductive period, and mortality and a decrease in the fecundity and the intrinsic rate of natural increase in A. pisum occurred [35]. Naringenin reduced ingestion when applied in a low concentration and stimulated ingestion when applied in a high concentration [36]. Mixtures of saponins with apigenin incorporated into gels resulted in a reduction in the number of aphid probes and their duration [37]. A high concentration of genistein in artificial diets reduced the survival rate of the Pisum host race of A. pisum on Medicago sativa [18]. Under semi-natural conditions, when flavonoids were applied to the plant surface, rutin hindered the time it took to reach sieve elements and to accept phloem sap for continuous feeding by A. pisum on peas Pisum sativum L. (Fabaceae), while quercetin promoted probing activities of A. pisum within non-phloem and phloem tissues [38].
In our previous studies on antixenosis in eight soybean cultivars against the pea aphid, we discovered that A. pisum readily probed into leaf tissues of all cultivars, but the probes were usually terminated before they reached vascular tissues [39]. Nevertheless, the analyzed cultivars represented a spectrum of susceptibility to A. pisum, from relatively susceptible to highly resistant. We deduced that antixenosis mechanisms were active in peripheral tissues’ epidermis and/or mesophyll in soybean leaves, and we hypothesized that the varied response of A. pisum to individual cultivars was probably associated with the variation in soybean leaf flavonoid composition among these cultivars [39]. Apigenin and genistein occurred in all soybean cultivars, while the relatively susceptible or highly resistant cultivars also contained daidzein or kaempferol, respectively [39]. The content of apigenin ranged from 1.05 to 5.38 μg/g dry weight and genistein—from 0.61 to 3.05 μg/g dry weight, and the differences were not related to cultivar susceptibility levels [39]. We speculated at the time that the content of apigenin and genistein in all soybean cultivars studied probably made all of them relatively unacceptable to A. pisum, but the variation in susceptibility could have been caused by the presence of other flavonoids. The present study was designed to verify that idea.
The aim of the present work was to investigate the direct impact of flavonoids detected in soybean leaf tissues, specifically apigenin, daidzein, genistein and kaempferol, on pre-ingestive and ingestive phases of A. pisum probing on its host plant P. sativum. The effects of apigenin and genistein on A. pisum probing behavior were previously studied using artificial diets [35,37]. To our knowledge, the influence of daidzein and kaempferol on A. pisum probing behavior remains unknown. We decided to carry out our study under semi-natural conditions. For this purpose, we applied flavonoids to pea plants and monitored aphid probing using the electrical penetration graph (EPG) technique. EPG, known also as electropenetrography, visualizes the activities of aphid stylets in specific plant tissues. The values of parameters derived from EPG recordings are reliable and precise indicators of aphid behavioral responses to plant resistance mechanisms or modification in plant suitability due to the exogenous application of allelochemicals [40,41,42,43].
2. Materials and Methods
2.1. Plant and Aphid Cultures
The laboratory culture of Pisum sativum-derived Acyrthosiphon pisum was maintained as a multiclonal colony on P. sativum cv. “Milwa” in the laboratory at 20 °C, 65% r.h., and a L16:D8 photoperiod in a growing chamber, Sanyo MLR-351H (Sanyo Electronics Co. Ltd., Osaka, Japan). Two-to-three-day-old adult apterous females of A. pisum and three-week-old plants with two to three fully developed leaves were used for experiments. The plants used for experiments were the same plant species and cultivar that was used for the rearing of aphids. All experiments were carried out under the same conditions of temperature, r.h., and photoperiod as used for the rearing of plants and aphids.
2.2. Application of Flavonoids
Flavonoids apigenin, daidzein, genistein and kaempferol (Figure 1) were purchased from Sigma Aldrich, Poland. To mimic the natural environment under laboratory conditions, the flavonoids were offered to aphids by application through their host plants. The preparation and application of the compounds followed the procedure originally described by [44] with later modifications [38]. Briefly, each compound was dissolved in 70% ethanol to obtain the 0.1% solution. One leaf of an intact plant was dipped in the ethanolic solution of a given compound for 30 s, so all compounds were applied on the adaxial and abaxial leaf surfaces. Leaves of a similar size to the control plants were immersed in 70% ethanol, which was used as a solvent for the studied flavonoids. No negative effects of ethanol on plants or aphids were observed after it had been used according to the described procedure [42]. Our previous studies and works by other authors that used aphids as sensors demonstrated that exogenously applied compounds of various chemical groups, including flavonoids, penetrated the cuticle and epidermis and passed into deeper plant tissue layers. The transcuticular application of some of those compounds caused considerable disturbances in plant recognition and acceptance by aphids, which were reflected in the alterations of EPG-monitored aphid probing behavior [38,42,43,44]. The treated and control leaves were allowed to dry for 1 h before the start of the experiment to permit the evaporation of the solvent [38]. Every plant and aphid was used only once.
Figure 1.
Basic skeleton structure of flavonoids and structures of flavonoids applied in the present study: apigenin, daidzein, genistein, and kaempferol.
2.3. Monitoring of Aphid Probing Behavior (EPG No-Choice Test)
EPG requires the attachment of the plant and the aphid to their respective electrodes to make them parts of an electrical circuit. The circuit is completed when the aphid inserts its stylets into the plant. Weak voltage is supplied in the circuit, and all changing electric properties are recorded as EPG waveforms that can be correlated with aphid activities and the stylet position in plant tissues [27,40,45,46]. In the present study, aphids were connected to a golden wire electrode with conductive silver paint and starved for 1 h prior to the experiment. The probing behavior of A. pisum on peas untreated and treated with flavonoids was monitored for 8 h continuously using 4- and 8-channel DC EPG recording equipment (available at www.epgsystems.eu; Dillenburg 12, 6703 CJ Wageningen, the Netherlands). Signals were saved on the computer and analyzed with PROBE 3.1 software provided by dr. W. F. Tjallingii (available at www.epgsystems.eu); Dillenburg 12, 6703 CJ Wageningen, the Netherlands). The following aphid behaviors were distinguished: no penetration (waveform ‘np’—aphid stylets outside the plant), pathway phase penetration of non-phloem tissues (waveforms ‘A, B, and C’), derailed stylet movements (waveform ‘F’), salivation into sieve elements (waveform ‘E1’), ingestion of phloem sap (waveform ‘E2’), and ingestion of xylem sap (waveform ‘G’) [47]. Aphid activities visualized as EPG waveforms were analyzed according to their frequency and duration and presented in a configuration related to activities in peripheral and vascular tissues. The interpretation of EPG variables in terms of plant suitability to the aphids follows the interpretation provided by detailed studies on aphid–plant interactions [27,28,30,31,40,41,45,46,48,49].
Aphids for EPG experiments were 2–3-day-old (2–3 days after the final molt) viviparous apterous females selected randomly from the stock culture. According to [50], the use of aphids of random ages gives a clear view of the behavior of adult aphids within a population. The plants used in the bioassays were at growth stages 12–13 (two to three leaves unfolded) according to the BBCH scale (Biologische Bundesanstalt, Bundessortenamt und CHemische Industrie) [51]. Each aphid was given access to a freshly prepared plant. Each plant–aphid set was considered as a replication and was tested only once. The number of replications (=8 h EPG recordings) for each plant–flavonoid combination was 24 (i.e., two rounds of 12 simultaneous recordings). All experiments were carried out under the same conditions of temperature, relative humidity (r.h.), and photoperiod as those used for the rearing of plants and aphids. All bioassays started at 10:00–11:00 h MEST (Middle European Summer Time) and were carried out within five consecutive days for each round of EPG recordings (one day—12 replications of a given flavonoid treatment). Aphids show distinct diurnal feeding activity, with peak activity during daytime, independently of host plants [27,52].
2.4. Statistical Analysis
EPG variables describing aphid probing behavior were calculated using the Excel-VBA Macro [53], accessed from www.epgsystems.eu (accessed on 1 June 2021) and the means and standard deviations were subsequently calculated using the EPG analysis Excel worksheet created for this study [48,49]. In non-sequential variables (such as total durations of specific EPG waveforms and the number of EPG-recorded aphid activities), when a given waveform had not been recorded for an individual, the duration of that waveform was given the value of 0. In sequential variables (such as the time until specific EPG waveforms appeared in the recording), when variables related to the phloem phase (E1 or E2) were involved, only aphids that reached phloem phase were included in statistical analysis. The E1/E2 transition patterns were included in E2. The waveform patterns that were not terminated before the end of the experimental period (8 h) were included in the calculations. Recordings that terminated due to the aphid falling from the plant or where the EPG signal was unclear were discarded from analysis. Only the replications that included a complete 8 h recording were kept for analysis, which were: control (n = 23), apigenin-treated (n = 17), daidzein-treated (n = 18), genistein-treated (n = 16), kaempferol-treated (n = 15). Due to failure to meet the assumptions of analysis of variance, the obtained data were analyzed by the Kruskal–Wallis test and post hoc multiple comparisons of mean ranks for all groups (Dunn’s test). The Kruskal–Wallis test is a non-parametric alternative to the one-factor ANOVA test for independent measures and it is commonly used to analyze data deriving from EPG recordings of aphid probing. The mean and SD values given in Table 1 is a representation of non-Gaussian data, but the statistical analysis was carried out by non-parametric tests in which all individual data were included. All statistical calculations were performed using StatSoft (Statistica 13.3 package).

Table 1.
EPG-recorded stylet penetration activities of Acyrthosiphon pisum on Pisum sativum treated with 0.1% ethanolic solutions of apigenin, daidzein, genistein and kaempferol. All values represent means ± SD of cumulative time or number of events within an 8 h period. Different letters in rows denote statistically significant differences among treatments (Kruskal–Wallis test, p < 0.05).
3. Results
EPG waveforms generated at the aphid–plant interface visualized three major aphid activities, irrespective of treatment: non-probing (=aphid stylets outside the plant), probing in non-vascular tissues epidermis and mesophyll, and probing in vascular tissues xylem and phloem (Table 1, Figure 2a,b).
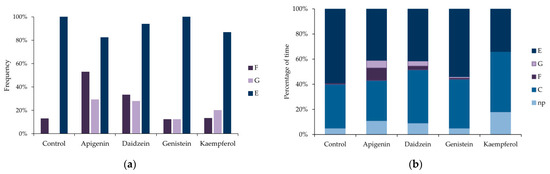
Figure 2.
Occurrence of waveform patterns demonstrating EPG-recorded stylet penetration activities of Acyrthosiphon pisum on Pisum sativum non-treated (n = 23) and treated with 0.1% apigenin (n = 17), daidzein (n = 18), genistein (n = 16) and kaempferol (n = 15): (a) frequency of occurrence of E (phloem phase), G (xylem phase), and F (derailed stylet activities) expressed as percentage of aphids that showed given activity; (b) percentage of time assigned to non-probing ‘np’ and probing activities (pathway C, activity F, xylem phase G and phloem phase E = phloem salivation E1 + phloem sap ingestion E2) Figure 2b is based on data presented in Table 1.
Probing in non-vascular tissues included typical pathway activity that represents the progressive movement of stylets within the apoplast accompanied by short punctures into cells adjacent to the stylet track (EPG waveform C) and derailed stylet activities (EPG waveform F). Probing in vascular tissues embraced the ingestion of xylem sap (EPG waveform G), salivation into sieve elements (EPG waveform E1), and ingestion of phloem sap from sieve elements (EPG waveform E2) (Table 1, Figure 2a,b).
On the control plants, aphid stylet penetration within plant tissues (= total duration of probing) occupied 95% of the 8 h aphid access time to plants (Table 1). All aphids showed pathway activity C and phloem phase E, while derailed stylet activities F occurred in 13% of aphids and no aphid showed xylem phase G (Figure 2a). Aphids devoted 5% of the experimental time to non-probing activities, 35% to typical pathway probing C, derailed stylet activities F occupied 1% of time on average, and phloem phase E, 59% (Figure 2b). Phloem phases E1 and E2 engaged 62% of total probing time (phloem phase index = 0.62 ± 0.04) (Table 1). Probing was divided into 16.5 ± 2.0 probes of 0.7 h duration on average. Brief, shorter than 3 min probes amounted to 51% of all probes. The first probe was usually made 1.3 min after aphids gained access to the plant and was 30.6 ± 12.2 min long on average. Nearly 30% of all probes and 38% of brief probes occurred before the first phloem phase. The first phloem phase appeared 1.0 ± 0.2 h after aphids started probing and it usually comprised sustained sap ingestion (Table 1). All aphids reached sieve elements (= started phloem phase) within three hours from the onset of the experiment (Figure 3a) and activities within the phloem were the main aphid occupation from the second hour of the experiment onwards (53–73% of all activities) (Figure 3b). Sap ingestion occurred in 4.6 periods on average, and each was approximately 1.4 h long. The participation of salivation in phloem phase activities was relatively low (phloem salivation index = 0.04 ± 0.01) (Table 1).
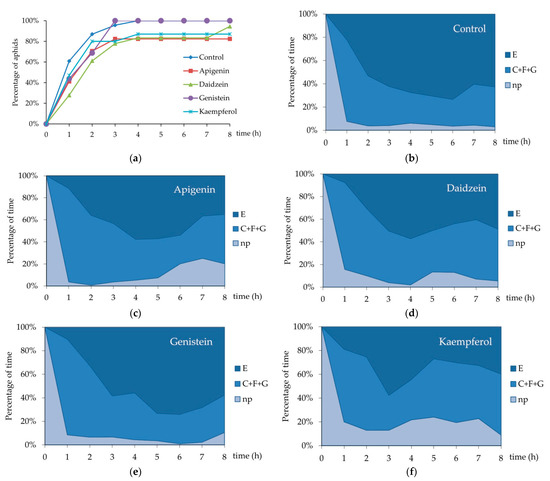
Figure 3.
Sequential changes in EPG-recorded stylet penetration activities of Acyrthosiphon pisum on Pisum sativum: (a) Cumulative percentage of aphids that attained phloem phase during the 8 h EPG monitoring on P. sativum treated with 0.1% apigenin, daidzein, genistein and kaempferol; (b) sequential changes in penetration activities of A. pisum on control untreated P. sativum; (c) sequential changes in penetration activities of A. pisum on P. sativum treated with 0.1% apigenin; (d) sequential changes in penetration activities of A. pisum on P. sativum treated with 0.1% daidzein; (e) sequential changes in penetration activities of A. pisum on P. sativum treated with 0.1% genistein; (f) sequential changes in penetration activities of A. pisum on P. sativum treated with 0.1% kaempferol. Panels (b–f) represent the proportion of time (average percentage of cumulative time for aphids in the group) devoted to np—non-probing, C + F + G—pathway + derailed stylet activities + xylem phase, and E—phloem phase E1 (salivation) + E2 (sap ingestion) activities of A. pisum during the consecutive hours of 8 h EPG recording (control n = 23; apigenin-treated n = 17; daidzein-treated n = 18; genistein-treated n = 16; kaempferol-treated n = 15).
On peas treated with 0.1% apigenin, 11% of the time was assigned to non-probing, 32% to pathway C, and 41% to the phloem phase. Activities F and G did occur in aphids on apigenin-treated plants and their frequencies and durations were relatively high; activity F occurred in 53% of aphids and occupied 10% of the time, while G occurred in 29% of aphids and engaged 6% of the time (Table 1, Figure 2a,b). Statistically significant differences with respect to the control were detected in the number and the mean duration of all probes and probes before the first phloem phase (Table 1; Kruskal–Wallis test, p < 0.05). There were 2.5 times fewer probes in general and 2.8 times fewer probes before the first phloem phase on apigenin-treated peas than on the control. Accordingly, the probes were 2.3 times longer than on the control peas (Table 1). The proportion of phloem phase during probing was similar to the control, but only 80% of aphids reached the phloem phase within 8 h of access to plants (Figure 3a). In addition, the phloem phase never exceeded 60% of aphid activities on plants and the proportion of sap ingestion activity decreased over time to reach 35% of all activities at the end of the monitoring period (Figure 3c).
On peas treated with 0.1% daidzein, 9% of the time was assigned to non-probing, 42% to pathway C, and 41% to the phloem phase. Activity F occurred in 33% of aphids and occupied 3% of the time, while G occurred in 28% of aphids and engaged 4% of the time (Table 1, Figure 2a,b). Aphids made significantly fewer brief probes than on the control: there were 1.5 fewer brief probes in general (Table 1; Kruskal–Wallis test; p < 0.05), but the number of brief probes before the first phloem phase was similar as compared to the control (Table 1). Although nearly all aphids reached the phloem phase (Figure 3a), they needed almost three times more time to reach the sieve elements than on the control. At the same time, the number of phloem phases was significantly lower in aphids on daidzein-treated plants than in aphids on the control peas (Table 1). Generally, the proportion of time assigned to sap ingestion was slightly lower than in the control and never exceeded 60% of all aphid activities on plants (49% at the end of the monitoring period) (Figure 3d).
On peas treated with 0.1% genistein, 11% of the time was assigned to non-probing, 32% to pathway C, and 41% to the phloem phase. The frequency and duration of F and G were relatively low: F and G occurred in 13% of aphids and each engaged 1% of the time (Table 1, Figure 2a,b). Aphid behavior was generally similar to that on the control. However, the number of phloem sap ingestion phases was 2.4 times lower than on the control (Table 1). All aphids reached the sieve elements within three hours after access to the plants (Figure 3a) and the proportion of time assigned to sap ingestion was relatively high (within the range of 10–75% of all activities) and comparable to the control during the 8 h of monitoring (58% at the end of monitoring period) (Figure 3e).
On peas treated with 0.1% kaempferol, 18% of the time was assigned to non-probing, 48% to pathway C, and 34% to the phloem phase. Activities F and G did not occur (Table 1, Figure 2a,b). The total time of non-probing was 3.5 times longer than on the control; non-probing intervals between probes were significantly longer than on control (Table 1; Kruskal–Wallis test, p < 0.05). Non-probing occupied 10% to 25% of all aphid activities during the monitoring period (Figure 3f). Within eight hours after access to plants, 87% of aphids managed to reach the sieve elements (Figure 3a) but the proportion of time assigned to sap ingestion ranged from 19% to 58% of all activities (40% at the end of monitoring period) (Figure 3f). The phloem phase index was relatively low (0.37 ± 0.08 as compared to 0.62 ± 0.04 on control) (Table 1).
4. Discussion
The specificity of aphid response to non-volatile plant allelochemicals derives from the lack of contact chemoreceptors on their mouthparts. In contrast to biting–chewing insects that possess chemosensitive sensilla on various elements of the feeding apparatus and in the wall of cibarium, the fluid-feeding herbivores, including aphids, have no chemoreceptors on the stylets that enter the tissues of the host [54]. The only taste organ is located in the hypopharynx [27,55,56]. The existence and activity of external contact chemoreceptors on the aphid body, such as sensilla at the tibial–tarsal junction, have not been confirmed unambiguously, although it was shown that aphids could detect non-volatile chemicals on plant surface using the hairs on the tips of antennae [27]. Thus, once an aphid finds itself on a plant, it must insert its stylets into plant tissues to gather and evaluate information on the suitability of the substrate [56]. Aphids usually start probing immediately after they are allowed to, both under natural and laboratory conditions [57]. The collection of chemical information by the probing aphid is a two-stage process. First, during the pre-ingestive stage, the aphid probes within peripheral tissues and tests small cytoplasm samples from cells adhering to stylet tracks [28]. Then, if the probing continues and the stylets reach phloem tissues, the aphid switches to the ingestive stage and starts the uptake of sap from sieve elements [58]. The continuation of probing both in peripheral and phloem tissues depends on the composition of allelochemicals and the allelochemical/nutrient proportion in these tissues, respectively [41,56,59,60]. The termination of probing during the pre-ingestive stage may indicate the presence of feeding deterrents in peripheral tissues, while the termination of sap ingestion soon after the onset of feeding may indicate either the presence of feeding deterrents or low nutritional value of sap, or both [56,59,61].
We discovered several important differences in the pea aphid behavior on flavonoid-treated peas in relation to the control. These differences occurred in diverse probing phases, depending on the flavonoid treatment. The behavior of aphids at the plant surface, before they inserted stylets in experimental plants for the first time, was rather consistent. There was no delay in the onset of probing on plants treated with flavonoids in relation to the control. This means that aphids did not respond to deterrent factors, if they existed, at this stage of the probing process. However, the time to initiate a probe was the shortest on apigenin-treated plants in relation to other flavonoid treatments. Nevertheless, it is typical for aphids to start probing in a few minutes’ time after access to their host plants if no repellent factors are present [62,63,64,65]. The initiation of stylet penetration by aphids in EPG experiments may be affected by a variety of internal and external factors, such as plant surface features including color, texture and phytochemicals (volatile and non-volatile) [62]. In addition to wax and cutin, the plant cuticle contains secondary metabolites, including flavonoids [66,67]. Aphids can detect non-volatile chemicals on the plant surface by using contact chemoreceptors on the tips of antennae [68]. Aphids touch plants with antennae before probing and when they insert stylets, they position their antennae along the body [27,68,69]. We cannot exclude the fact that a certain amount of exogenously applied flavonoids in the present study remained on the plant surface, but apparently it did not affect aphid behavior significantly. A similar lack of effect on the time taken to initiate the first penetration was observed in the EPG-recorded behavior of the peach potato aphid Myzus persicae (Sulz.) on plants treated with deterrent polygodial [70], nerolidol and farnesol [71], or other compounds [38,42,43,63,68,69]. In summary, we determined that none of the applied flavonoids affected the propensity to probe the plants by A. pisum, as all aphids on all plants started the first probe almost immediately after having access to the leaves.
The insertion of stylets in plant tissues begins the pre-ingestive stage in aphid probing. The period before the first period of activity in phloem tissues from the onset of the experiment (time to first E1 in experiment from first probe) depends on epidermal, mesophyll, general vascular, and early phloem factors. The first cells that are punctured for gustatory purposes are epidermal and outer mesophyll cells [28]. The time needed by stylets to cross one layer of cells is approximately 2–3 min [72]; therefore, a high proportion of probes shorter than that time, especially during the phase before phloem elements are reached, suggests the presence of deterrent factors in outer plant tissues, mainly the epidermis [58,59,73,74]. On non-host plants, resistant plant cultivars or plants treated with feeding deterrents, the total duration of probing, the duration of the first probe, the duration of all probes and especially probes that precede the first phloem phase are good indicators of plant suitability and the values of these parameters are usually lower than on suitable hosts [27,45,58,75]. In the present study, the total duration of probing was similar on all plants, except kaempferol-treated plants, on which an increase in the total duration of non-probing and the mean duration of non-probing intervals between probes occurred. During the pre-ingestive stage, when aphid stylets probed within non-vascular tissues, all aphids spent similar time on typical pathway activity C, which was the progressive movement of stylets towards the phloem. At the same time, the total number of probes and the number of brief probes shorter than 3 min was lowest on apigenin-treated plants and highest on kaempferol-treated plants. On apigenin-treated plants, aphids needed fewer but longer probes before the phloem phase than on other plants, including the control. However, an increase in the frequency and duration of derailed stylet mechanics visualized as waveform F occurred on apigenin- and daidzein-treated plants. The occurrence of waveform F in EPG recordings is difficult to interpret. It is usually attributed to mechanical stylet difficulties when for some reason stylets lose their proper position in the stylet bundle and therefore are unable to penetrate normally [76]. The increased frequency and duration of F was observed in aphids on resistant plants [77], ageing plants [73], insecticide-treated plants [78], on virus-infected plants (M. persicae on cabbage) [79], in aposymbiotic aphids [73,80] and in parasitized aphids [81]. On the other hand, fewer instances of F occurred in aphids whose structural sheath protein was silenced [82] or on plants with Potato Leafroll Virus (PLRV) infection symptoms (M. persicae on potato) [73].
The time taken to reach the first sieve element phase is a good indicator of deterrent factors that prevent the aphid from the continuation of pathway probing towards vascular tissues [83]. Indeed, the time taken to reach the first sieve element phase on resistant plant genotypes was significantly greater than in susceptible genotypes [84,85,86]. Similar delays before the first phloem phase were observed in the pea aphid after the exogenous application of rutin [38], and in peach potato aphid after the application of naringenin derivatives [87], citral and its derivatives [88], β-thujone oxime [71], cis-jasmone [43], or nerolidol [83]. In the present study, considerable delay in finding phloem tissues and commencing sap ingestion occurred on daidzein-treated plants in comparison to control and other flavonoid-treated plants. The highest success rate in reaching phloem vessels occurred on genistein-treated plants and the lowest on apigenin and kaempferol-treated plants.
The ingestive stage in pea aphid probing on flavonoid-treated plants in the present study consisted of bouts of xylem and phloem sap ingestion. On apigenin- and daidzein-treated plants, xylem sap ingestion occurred relatively more frequently and lasted longer than on other flavonoid-treated plants. On the control plants, xylem sap ingestion did not occur. Xylem sap ingestion is generally considered an aphid strategy for maintaining or restoring water (osmotic) balance [89,90,91]. However, the incidence of this activity may also be related to aphid age, plant genotype, and their interaction [59] or the abundance of obligatory symbionts [90]. Nevertheless, phloem sap is a basic food source of aphids [61]. In the present study, the highest proportion of phloem phase and phloem sap ingestion phase in all probing activities occurred on genistein-treated plants. Additionally, on genistein-treated plants, the individual bouts of sap ingestion activity were longest. In contrast, a visible trend towards the reduction in phloem phase duration occurred on kaempferol-treated plants. The shorter duration of sap ingestion in resistant genotypes or non-hosts shows that resistance factors are present in phloem tissues [58,84,85,86,92,93,94]. The reduction in the duration of sap ingestion bouts may also be caused by the exogenous application of xenobiotics, such as halogenated piperitone derivatives [95], farnesol and nerolidol [83] or camphene and ionone [88]. Sap ingestion activity is always preceded by a short phase of salivation to prevent the phloem wound response [96,97]. Repeated and prolonged phloem salivation is common on resistant plants or non-hosts and indicates the presence of antixenosis factors in phloem vessels [92,96,98,99,100]. The proportion of salivation during the phloem phase was similar in all aphids in the present study, irrespective of treatment.
The results of EPG experiments demonstrated a wide spectrum of changes in the pea aphid probing behavior induced by the exogenous application of selected flavonoids to pea plants. None of the flavonoids prevented A. pisum from probing. The activity of apigenin was expressed in non-vascular and vascular tissues: apigenin enhanced the duration of probes in non-phloem tissues and caused an increase in the frequency and duration of stylet mechanics derailment and xylem sap ingestion but deterred the ingestion of phloem sap. The activity of daidzein was detectable in non-vascular and vascular tissues: daidzein caused a delay in reaching phloem vessels and moderately limited sap ingestion. Genistein did not affect aphid probing behavior. Kaempferol caused a reduction in the frequency and duration of the phloem phase.
In our former study, we hypothesized that the content of apigenin and genistein in the eight soybean cultivars examined probably made them relatively unacceptable to the pea biotype of A. pisum [39]. The outcomes of the present work partly support our deductions. Apparently, genistein is very well tolerated by the pea aphid, which we demonstrated in our study, and which is in accordance with data derived in experiments with the use of artificial diets: genistein caused negative effects on the probing behavior and life parameters of A. pisum only at high concentrations [18,35]. At the same time, soybean cultivar ‘Aldana’, which had the lowest content of genistein, was the most resistant cultivar [39]. Therefore, we are of the opinion that an increase in genistein in plant tissues would not necessarily make them more resistant to A. pisum. However, it is likely that the enrichment of plants with apigenin may be the right direction for breeding resistant cultivars. The long duration of non-probing intervals between individual probes may suggest that aphids might have probably walked out from the plants, had they been free to move. At the same time, apigenin restricted sap ingestion on the treated plants, which confirms results of studies that applied artificial diets [37]. Our current findings also seem to explain the riddle of two soybean cultivars ‘Madlen’ and ‘Violetta’ which had similar flavonoid composition: both contained genistein, apigenin and daidzein but ‘Madlen’ was relatively susceptible, while ‘Violetta’ was relatively resistant [39]. The content of genistein was three times higher in ‘Violetta’ than in ‘Madlen’ but, as stated earlier, it is unlikely that genistein was of major importance. The content of daidzein was 2.3 times higher in relatively susceptible ‘Madlen’ than in ‘Violetta’ while the content of apigenin was 4.7 times lower in ‘Madlen’. Both daidzein and apigenin appear to restrict the pea aphid probing activities, but the relatively higher content of apigenin probably makes ‘Violetta’ less palatable to A. pisum than ‘Madlen’. Finally, it can be presumed that the total unacceptability of cultivar ‘Aldana’ to A. pisum is caused by apigenin, genistein, kaempferol and rutin. However, the content of apigenin was comparable to the contents of this flavonoid in relatively acceptable soybean cultivars and the content of genistein was one of the lowest. In another study, we determined that rutin caused a delay in reaching phloem vessels and a reduction in the duration of sap ingestion [38]. However, the content of rutin in this cultivar was 4.7 times lower than in the relatively susceptible cultivar ‘Augusta’. We suggested at the time that kaempferol was probably responsible for the rejection of ‘Aldana’ by A. pisum. The present study confirmed that idea.
In summary, the application of apigenin and daidzein generates modifications in the pre-ingestive and ingestive stages of probing of A. pisum and the application of kaempferol affects the ingestive stage of the pea aphid probing activities. However, additional experiments will be needed to establish the optimal dose and the concentration and persistence of exogenously applied flavonoids in plant tissues if these compounds were considered for practical use, as the effects of flavonoid treatments are clearly dose-dependent [36,37,38].
Author Contributions
Conceptualization, K.S., B.K. and B.G.; methodology, B.G. and B.K.; validation, K.S., B.G.; formal analysis, K.S.; investigation, K.S.; resources, B.K. and B.G.; data curation, K.S. and B.G.; writing—original draft preparation, K.S., B.K. and B.G.; writing—review and editing, K.S. and B.G.; visualization, K.S.; supervision, B.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All data are contained in the present article.
Acknowledgments
The authors express their sincere thanks to the external anonymous reviewers for their valuable comments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Agati, G.; Azzarellob, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Nakata, R.; Aboshi, T.; Yoshinaga, N.; Teraishi, M.; Okumoto, Y.; Ishihara, A.; Morisaka, H.; Huffaker, A.; Schmelz, E.A.; et al. Insect-induced daidzein, formononetin and their conjugates in soybean leaves. Metabolites 2014, 4, 532–546. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Stacey, G.; Yu, O. Endogenous isoflavones are essential for the establishment of symbiosis between soybean and Bradyrhizobium japonicum. Plant J. 2006, 48, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Pabich, M.; Materska, M. Biological effect of soy isoflavones in the prevention of civilization diseases. Nutrients 2019, 11, 1660. [Google Scholar] [CrossRef]
- Peiretti, P.G.; Karamać, M.; Janiak, M.; Longato, E.; Meineri, G.; Amarowicz, R.; Gai, F. Phenolic composition and antioxidant activities of soybean (Glycine max (L.) Merr.) plant during growth cycle. Agronomy 2019, 9, 153. [Google Scholar] [CrossRef]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef] [PubMed]
- Bentivenha, J.P.F.; Canassa, V.F.; Baldin, E.L.L.; Borguini, M.L.; Lima, G.P.P.; Lourenção, A.L. Role of the rutin and genistein flavonoids in soybean resistance to Piezodorus guildinii (Hemiptera: Pentatomidae). Arthropod Plant Interact. 2018, 12, 311–320. [Google Scholar] [CrossRef]
- Hoffmann-Campo, C.B.; Harborne, J.B.; McCaffery, A.R. Pre-ingestive and post-ingestive effects of soya bean extracts and rutin on Trichoplusia ni growth. Entomol. Exp. Appl. 2001, 98, 181–194. [Google Scholar] [CrossRef]
- Piubelli, G.C.; Hoffmann-Campo, C.B.; Moscardi, F.; Miyakubo, S.H.; de Oliveira, M.C. Are chemical compounds important for soybean resistance to Anticarsia gemmatalis? J. Chem. Ecol. 2005, 31, 1509–1524. [Google Scholar] [CrossRef]
- Isman, M.B.; Duffey, S.S. Toxicity of tomato phenolic compounds to the fruitworm, Heliothis zea. Entomol. Exp. Appl. 1982, 31, 370–3761. [Google Scholar] [CrossRef]
- Hohenstein, J.D.; Studham, M.E.; Klein, A.; Kovinich, N.; Barry, K.; Lee, Y.-J.; MacIntosh, G.C. Transcriptional and chemical changes in soybean leaves in response to long-term aphid colonization. Front. Plant Sci. 2019, 10, 310. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.V.; Dung, D.M.; Dai, D.N. Accumulation of flavonoids in soybean under effects of cyanobacterial crude extract and aphid infestation. Vietnam. J. Sci. Technol. 2016, 54, 271–277. [Google Scholar] [CrossRef]
- War, A.R.; Sharma, S.P.; Sharma, H.C. Differential induction of flavonoids in groundnut in response to Helicoverpa armigera and Aphis craccivora infestation. Int. J. Insect Sci. 2016, 8, 55–64. [Google Scholar] [CrossRef]
- Mai, V.C.; Drzewiecka, K.; Jeleń, H.; Narożna, D.; Rucińska-Sobkowiak, R.; Kęsy, J.; Floryszak-Wieczorek, J.; Gabryś, B.; Morkunas, I. Differential induction of Pisum sativum defense signaling molecules in response to pea aphid infestation. Plant Sci. 2014, 221–222, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Morkunas, I.; Woźniak, A.; Formela, M.; Mai, V.C.; Marczak, Ł.; Narożna, D.; Borowiak-Sobkowiak, B.; Kühn, C.; Grimm, B. Pea aphid infestation induces changes in flavonoids, antioxidative defence, soluble sugars and sugar transporter expression in leaves of pea seedlings. Protoplasma 2016, 253, 1063–1079. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, A.; Formela, M.; Bilman, P.; Grześkiewicz, K.; Bednarski, W.; Marczak, Ł.; Narożna, D.; Dancewicz, K.; Mai, V.C.; Borowiak-Sobkowiak, B.; et al. The dynamics of the defense strategy of pea induced by exogenous nitric oxide in response to aphid infestation. Int. J. Mol. Sci. 2017, 18, 329. [Google Scholar] [CrossRef] [PubMed]
- Yuan, E.; Yan, H.; Gao, J.; Guo, H.; Ge, F.; Sun, Y. Increases in genistein in Medicago sativa confer resistance against the Pisum host race of Acyrthosiphon pisum. Insects 2019, 10, 97. [Google Scholar] [CrossRef]
- Parrott, W.; Walker, D.; Zhu, S.; Boerma, H.R.; All, J. Genomics of insect-soybean interactions. In Genetics and Genomics of Soybean. Plant Genetics and Genomics: Crops and Models; Stacey, G., Ed.; Springer: New York, NY, USA, 2008; Volume 2, pp. 269–292. [Google Scholar]
- Chang, H.-X.; Hartman, G.L. Characterization of insect resistance loci in the USDA soybean germplasm collection using genome-wide association studies. Front. Plant Sci. 2017, 8, 670. [Google Scholar] [CrossRef]
- Lattanzio, V.; Arpaia, S.; Cardinali, A.; Di Venere, D.; Linsalata, V. Role of endogenous flavonoids in resistance mechanism of Vigna to aphids. J. Agric. Food Chem. 2000, 48, 5316–5320. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V. Phenolic compounds: Introduction. In Natural Products; Ramawat, K.G., Merillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1544–1580. [Google Scholar]
- Simmonds, M.S.J. Flavonoid–insect interactions: Recent advances in our knowledge. Phytochemistry 2003, 64, 21–30. [Google Scholar] [CrossRef]
- Hay, W.T.; Behle, R.W.; Berhow, M.A. Biopesticide synergy when combining plant flavonoids and entomopathogenic baculovirus. Sci. Rep. 2020, 10, 6806. [Google Scholar] [CrossRef] [PubMed]
- Frazier, J.L.; Chyb, S. Use of feeding inhibitors in insect control. In Regulatory Mechanisms in Insect Feeding; Chapman, R.F., de Boer, G., Eds.; Springer: Boston, MA, USA, 1995; pp. 364–377. [Google Scholar]
- Grimaldi, D.; Engel, M.S. Evolution of the Insects; Cambridge University Press: New York, NY, USA, 2005; p. 770. [Google Scholar]
- Pettersson, J.; Tjallingii, W.F.; Hardie, J. Host-plant selection and feeding. In Aphids as Crop Pests; van Emden, H.F., Harrington, R., Eds.; CABI: Wallingford, UK, 2017; pp. 173–195. [Google Scholar]
- Tjallingii, W.F.; Hogen Esch, T.H. Fine-structure of aphid stylet routes in plant-tissues in correlation with EPG signals. Physiol. Entomol. 1993, 18, 317–328. [Google Scholar] [CrossRef]
- Agati, G.; Stefano, G.; Biricolti, S.; Tattini, M. Mesophyll distribution of antioxidant flavonoid glycosides in Ligustrum vulgare leaves under contrasting sunlight irradiance. Ann. Bot. 2009, 104, 853–861. [Google Scholar] [CrossRef]
- Tjallingii, W.F. Sieve element acceptance by aphids. Eur. J. Entomol. 1994, 91, 47–52. [Google Scholar]
- Prado, E.; Tjallingii, W.F. Aphid activities during sieve element punctures. Entomol. Exp. Appl. 1994, 72, 157–165. [Google Scholar] [CrossRef]
- Petrussa, E.; Braidot, E.; Zancani, M.; Peresson, C.; Bertolini, A.; Patui, S.; Vianello, A. Plant flavonoids—Biosynthesis, transport and involvement in stress responses. Int. J. Mol. Sci. 2013, 14, 14950–14973. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, B.F.; Zangerl, A.R.; Dermody, O.; Bilgin, D.D.; Casteel, C.L.; Zavala, J.A.; DeLucia, E.H.; Berenbaum, M.R. Impact of elevated levels of atmospheric CO2 and herbivory on flavonoids of soybean (Glycine max Linnaeus). J. Chem. Ecol. 2010, 36, 35–45. [Google Scholar] [CrossRef]
- Zhao, J. Flavonoid transport mechanisms: How to go, and with whom. Trends Plant Sci. 2015, 20, 576–585. [Google Scholar] [CrossRef]
- Goławska, S.; Łukasik, I. Antifeedant activity of luteolin and genistein against the pea aphid, Acyrthosiphon pisum. J. Pest Sci. 2012, 85, 443–450. [Google Scholar] [CrossRef]
- Goławska, S.; Sprawka, I.; Łukasik, I.; Goławski, A. Are naringenin and quercetin useful chemicals in pest-management strategies? J. Pest Sci. 2014, 87, 173–180. [Google Scholar] [CrossRef]
- Goławska, S.; Sprawka, I.; Łukasik, I. Effect of saponins and apigenin mixtures on feeding behavior of the pea aphid, Acyrthosiphon pisum Harris. Biochem. Syst. Ecol. 2014, 55, 137–144. [Google Scholar] [CrossRef]
- Stec, K.; Kordan, B.; Gabrys, B. Quercetin and rutin as modifiers of aphid probing behavior. Molecules 2021, 26, 3622. [Google Scholar] [CrossRef]
- Stec, K.; Kordan, B.; Sergiel, I.; Biesaga, M.; Gasik, J.; Bocianowski, J.; Gabryś, B. Antixenosis in Glycine max (L.) Merr against Acyrthosiphon pisum (Harris). Sci. Rep. 2021, 11, 15289. [Google Scholar] [CrossRef]
- Martin, B.; Collar, L.; Tjallingii, W.F.; Fereres, A. Intracellular ingestion and salivation by aphids may cause the acquisition and inoculation of non-persistently transmitted plant viruses. J. Gen. Virol. 1997, 78, 2701–2705. [Google Scholar] [CrossRef] [PubMed]
- Tjallingii, W.F.; Mayoral, A. Criteria for host-plant acceptance by aphids. In Proceedings of the 8th International Symposium on Insect-Plant Relationships, Wageningen, The Netherlands, 9–13 March 1992; Menken, S.B.J., Visser, J.H., Harrewijn, P., Eds.; Springer, Series Entomologica: Dordrecht, The Netherlands, 1992; Volume 49, pp. 280–282. [Google Scholar]
- Halarewicz-Pacan, A.; Gabryś, B.; Dancewicz, K.; Wawrzeńczyk, C. Enantiospecific effect of limonene and limonene-derived bicyclic lactones on settling and probing behaviour of the peach-potato aphid Myzus persicae (Sulz.). J. Plant Prot. Res. 2003, 43, 133–142. [Google Scholar]
- Paprocka, M.; Gliszczyńska, A.; Dancewicz, K.; Gabryś, B. Novel hydroxy- and epoxy-cis-jasmone and dihydrojasmone derivatives affect the foraging of the peach potato aphid Myzus persicae (Sulzer) (Homoptera: Aphididae). Molecules 2018, 23, 2362. [Google Scholar] [CrossRef] [PubMed]
- Polonsky, J.; Bhatnagar, S.C.; Griffiths, D.C.; Pickett, J.A.; Woodcock, C.M. Activity of qassinoids as antifeedants against aphids. J. Chem. Ecol. 1989, 15, 993–998. [Google Scholar] [CrossRef]
- Tjallingii, W.F. Aphid-plant interactions: What goes on in the depth of the tissue? Proc. Exp. Appl. Entomol. 1995, 6, 163–169. [Google Scholar]
- Backus, E.A.; Cervantes, F.A.; Guedes, R.N.C.; Li, A.Y.; Wayadande, A.C. AC-DC electropenetrography for in-depth studies of feeding and oviposition behaviors. Ann. Entomol. Soc. Am. 2019, 112, 236–248. [Google Scholar] [CrossRef]
- Huang, F.; Tjallingii, W.F.; Zhang, P.; Zhang, J.; Lu, Y.; Lin, J. EPG waveform characteristics of solenopsis mealybug stylet penetration on cotton. Entomol. Exp. Appl. 2012, 143, 47–54. [Google Scholar] [CrossRef]
- van Helden, M.; Tjallingii, W.F. Tissue localization of lettuce resistance to the aphid Nasonovia ribisnigri using electrical penetration graphs. Entomol. Exp. Appl. 1993, 68, 269–278. [Google Scholar] [CrossRef]
- Garzo, E.; Rizzo, E.; Fereres, A.; Gomez, S.K. High levels of arbuscular mycorrhizal fungus colonization on Medicago truncatula reduces plant suitability as a host for pea aphids (Acyrthosiphon pisum). Insect Sci. 2020, 27, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Ten Broeke, C.J.M.; Diecke, M.; van Loon, J. Feeding behaviour and performance of different populations of the black currant-lettuce aphid, Nasonovia ribisnigri, on resistant and susceptible lettuce. Entomol. Exp. Appl. 2013, 148, 130–171. [Google Scholar] [CrossRef]
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants: BBCH; Monograph; Julius Kühn-Institut: Quedlinburg, Germany, 2018. [Google Scholar]
- Joschinski, J.; Beer, K.; Helfrich-Forster, C.; Krauss, J. Pea aphids (Hemiptera: Aphididae) have diurnal rhythms when raised independently of a host plant. J. Insect Sci. 2016, 16, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Schliephake, E.; Habekuss, A.; Scholz, M.; Ordon, F. Barley yellow dwarf virus transmission and feeding behaviour of Rhopalosiphum padi on Hordeum bulbosum clones. Entomol. Exp. Appl. 2013, 146, 347–356. [Google Scholar] [CrossRef]
- Chapman, R.F. Chemosensory regulation of feeding. In Regulatory Mechanisms in Insect Feeding, 1st ed.; Chapman, R.F., de Boer, G., Eds.; Chapman & Hall: New York, NY, USA, 1995; pp. 364–381. [Google Scholar]
- Wensler, R.J.D.; Filshie, B.K. Gustatory sense organs in the food canal of aphids. J. Morphol. 1969, 129, 473–492. [Google Scholar] [CrossRef]
- Powell, G.; Tosh, C.R.; Hardie, J. Host plant selection by aphids: Behavioral, evolutionary, and applied perspectives. Annu. Rev. Entomol. 2006, 51, 309–330. [Google Scholar] [CrossRef]
- Powell, G.; Hardie, J. The chemical ecology of aphid host alternation: How do return migrants find the primary host plant? Appl. Entomol. Zool. 2001, 36, 259–267. [Google Scholar] [CrossRef]
- Prado, E.; Tjallingii, W.F. Behavioral evidence for local reduction of aphid-induced resistance. J. Insect Sci. 2007, 7, 48. [Google Scholar] [CrossRef]
- Pompon, J.; Pelletier, Y. Changes in aphid probing behaviour as a function of insect age and plant resistance level. Bull. Entomol. Res. 2012, 102, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.E. Symbiotic microorganisms: Untapped resources for insect pest control. Trends Biotechnol. 2007, 25, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.E. The nutritional physiology of aphids. Adv. Insect Physiol. 2003, 31, 73–140. [Google Scholar]
- Dancewicz, K.; Sznajder, K.; Załuski, D.; Kordan, B.; Gabryś, B. Behavioral sensitivity of Myzus persicae to volatile isporenoids in plant tissues. Entomol. Exp. Appl. 2016, 160, 229–240. [Google Scholar] [CrossRef]
- Powell, G.; Maniar, S.P.; Pickett, J.A.; Hardie, J. Aphid responses to non-host epicuticular lipids. In Proceedings of the 10th International Symposium on Insect-Plant Relationships, Oxford, UK, 4–10 July 1998; Simpson, S.J., Mordue, A.J., Hardie, J., Eds.; Springer, Series Entomologica: Dordrecht, The Netherlands, 1999; Volume 56, pp. 115–123. [Google Scholar]
- Todd, J.C.; Rouf Mian, M.A.; Backus, E.A.; Finer, J.J.; Redinbaugh, M.G. Feeding Behavior of Soybean Aphid (Hemiptera: Aphididae) Biotype 2 on Resistant and Susceptible Soybean. J. Econ. Entomol. 2016, 109, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Baudry, X.; Doury, G.; Couty, A. Antagonist effects of the leek Allium porrum as a companion plant on aphid host plant colonization. Sci. Rep. 2021, 11, 4032. [Google Scholar] [CrossRef] [PubMed]
- Nalam, V.J.; Han, J.; Pitt, W.J.; Acharya, S.R.; Nachappa, P. Location, location, location: Feeding site affects aphid performance by altering access and quality of nutrients. PLoS ONE 2021, 16, 0245380. [Google Scholar] [CrossRef]
- Serrano, M.; Coluccia, F.; Torres, M.; L’Haridon, F.; Métraux, J.P. The cuticle and plant defense to pathogens. Front. Plant Sci. 2014, 5, 274. [Google Scholar] [CrossRef]
- Ziv, C.; Zhao, Z.; Gao, Y.G.; Xia, Y. Multifunctional roles of plant cuticle during plant-pathogen interactions. Front. Plant Sci. 2018, 9, 1088. [Google Scholar] [CrossRef]
- Powell, G.; Hardie, J.; Pickett, J.A. Behavioural evidence for detection of the repellent polygodial by aphid antennal tip sensilla. Physiol. Entomol. 1995, 20, 141–146. [Google Scholar] [CrossRef]
- Hardie, J.; Holyoak, M.; Taylor, N.J.; Griffiths, D.C. The combination of electronic monitoring and video-assisted observations of plant penetration by aphids and behavioural effects of polygodial. Entomol. Exp. Appl. 1992, 62, 233–239. [Google Scholar] [CrossRef]
- Powell, G.; Hardie, J.; Pickett, J.A. Effects of the antifeedant polygodial on plant penetration by aphids, assessed by video and electrical recording. Entomol. Exp. Appl. 1993, 68, 193–200. [Google Scholar] [CrossRef]
- Wróblewska-Kurdyk, A.; Gniłka, R.; Dancewicz, K.; Grudniewska, A.; Wawrzeńczyk, C.; Gabryś, B. β-thujone and its derivatives modify the probing behavior of the peach potato aphid. Molecules 2019, 24, 1847. [Google Scholar] [CrossRef] [PubMed]
- van Hoof, H.A. An Investigation of the Biological Transmission of a Non-Persistent Virus; The Agricultural University: Wageningen, The Netherlands, 1958. [Google Scholar]
- Alvarez, A.E.; Garzo, E.; Verbeek, M.; Vosman, B.; Dicke, M.; Tjallingii, W.F. Infection of potato plants with potato leafroll virus changes attraction and feeding behaviour of Myzus persicae. Entomol. Exp. Appl. 2007, 125, 135–144. [Google Scholar] [CrossRef]
- Gabryś, B.; Tjallingii, W.F.; van Beek, T.A. Analysis of EPG recorded probing by cabbage aphid on host plant parts with different glucosinolate contents. J. Chem. Ecol. 1997, 23, 1661–1673. [Google Scholar] [CrossRef]
- Kordan, B.; Dancewicz, K.; Wróblewska, A.; Gabryś, B. Intraspecific variation in alkaloid profile of four lupine species with implications for the pea aphid probing behaviour. Phytochem. Lett. 2012, 5, 71–77. [Google Scholar] [CrossRef]
- Tjallingii, W.F. Electrical recording of stylet penetration activities. In Aphids, Their Biology, Natural Enemies and Control; Minks, A.K., Harrewijn, P., Eds.; Elsevier: Amsterdam, The Netherlands, 1988; Volume 2B, pp. 95–108. [Google Scholar]
- Marchetti, E.; Civolani, S.; Leis, M.; Chicca, M.; Tjallingii, W.F.; Pasqualini, E.; Baronio, P. Tissue location of resistance in apple to the rosy apple aphid established by electrical penetration graphs. Bull. Insectology 2009, 62, 203–208. [Google Scholar]
- Garzo, E.; Moreno, A.; Plaza, M.; Fereres, A. feeding behavior and virus-transmission ability of insect vectors exposed to systemic insecticides. Plants 2020, 9, 895. [Google Scholar] [CrossRef] [PubMed]
- Wamonje, F.O.; Donnelly, R.; Tungadi, T.D.; Murphy, A.M.; Pate, A.E.; Woodcock, C.; Caulfield, J.; Mutuku, J.M.; Bruce, T.J.A.; Gilligan, C.A.; et al. Different plant viruses induce changes in feeding behavior of specialist and generalist aphids on common bean that are likely to enhance virus transmission. Front. Plant Sci. 2020, 10, 1811. [Google Scholar] [CrossRef]
- Machado-Assefh, C.R.; Alvarez, A.E. Probing behavior of aposymbiotic green peach aphid (Myzus persicae) on susceptible Solanum tuberosum and resistant Solanum stoloniferum plants. Insect Sci. 2018, 25, 127–136. [Google Scholar] [CrossRef]
- He, Y.-Q.; Zhang, Y.-Q.; Chen, J.-N.; Chen, W.-L.; Zeng, X.-Y.; Chen, H.-T.; Ding, W. Effects of Aphidius gifuensis on the feeding behavior and potato virus Y transmission ability of Myzus persicae. Insect Sci. 2018, 25, 1025–1034. [Google Scholar] [CrossRef]
- Will, T.; Vilcinskas, A. The structural sheath protein of aphids is required for phloem feeding. Insect Bioch. Mol. Biol. 2015, 57, 34–40. [Google Scholar] [CrossRef]
- Wróblewska-Kurdyk, A.; Dancewicz, K.; Gliszczyńska, A.; Gabryś, B. New insight into the behaviour modifying activity of two natural sesquiterpenoids farnesol and nerolidol towards Myzus persicae (Sulzer) (Homoptera: Aphididae). Bull. Entomol. Res. 2020, 110, 249–258. [Google Scholar] [CrossRef]
- Chandran, P.; Reese, J.C.; Khan, S.A.; Wang, D.; Schapaugh, W.; Campbell, L.R. Feeding behavior comparison of soybean aphid (Hemiptera: Aphididae) biotypes on different soybean genotypes. J. Econ. Entomol. 2013, 106, 2234–2240. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kordan, B.; Wróblewska-Kurdyk, A.; Bocianowski, J. Variation in susceptibility of rapeseed cultivars to the peach potato aphid. J. Pest Sci. 2021, 94, 435–449. [Google Scholar] [CrossRef]
- Kordan, B.; Stec, K.; Słomiński, P.; Laszczak-Dawid, A.; Wróblewska-Kurdyk, A.; Gabryś, B. Antixenosis potential in pulses against the pea aphid (Hemiptera: Aphididae). J. Econ. Entomol. 2019, 112, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Stec, K.; Kozłowska, J.; Wróblewska-Kurdyk, A.; Kordan, B.; Anioł, M.; Gabryś, B. Effect of naringenin and its derivatives on the probing behavior of Myzus persicae (Sulz.). Molecules 2020, 25, 3185. [Google Scholar] [CrossRef] [PubMed]
- Dancewicz, K.; Szumny, A.; Wawrzeńczyk, C.; Gabryś, B. Repellent and antifeedant activities of citral-derived lactones against the peach potato aphid. Int. J. Mol. Sci. 2020, 21, 8029. [Google Scholar] [CrossRef]
- Spiller, N.J.; Koenders, L.; Tjallingii, W.F. Xylem ingestion by aphids—A strategy for maintaining water balance. Entomol. Exp. Appl. 1990, 55, 101–104. [Google Scholar] [CrossRef]
- Pompon, J.; Quiring, D.; Goyer, C.; Giordanengo, P.; Pelletier, Y. A phloem-sap feeder mixes phloem and xylem sap to regulate osmotic potential. J. Insect Physiol. 2011, 57, 1317–1322. [Google Scholar] [CrossRef]
- Will, T.; van Bel, A.J.E. Physical and chemical interactions between aphids and plants. J. Exp. Bot. 2006, 57, 729–737. [Google Scholar] [CrossRef]
- Gabryś, B.; Pawluk, M. Acceptability of different species of Brassicaceae as hosts for the cabbage aphid. In Proceedings of the 10th International Symposium on Insect-Plant Relationships, Oxford, UK, 4–10 July 1998; Simpson, S.J., Mordue, A.J., Hardie, J., Eds.; Springer, Series Entomologica: Dordrecht, The Netherlands, 1999; Volume 56, pp. 105–109. [Google Scholar]
- Kordan, B.; Stec, K.; Słomiński, P.; Giertych, M.J.; Wróblewska-Kurdyk, A.; Gabryś, B. Susceptibility of forage legumes to infestation by the pea aphid Acyrthosiphon pisum (Harris) (Hemiptera: Aphididae). Crop. Pasture Sci. 2018, 69, 775–784. [Google Scholar] [CrossRef]
- Halarewicz, A.; Gabryś, B. Probing behavior of bird cherry-oat aphid Rhopalosiphum padi (L.) on native bird cherry Prunus padus L. and alien invasive black cherry Prunus serotina Erhr. in Europe and the role of cyanogenic glycosides. Arthropod Plant Interact. 2012, 6, 497–505. [Google Scholar] [CrossRef]
- Grudniewska, A.; Dancewicz, K.; Białońska, A.; Ciunik, Z.; Gabryś, B.; Wawrzeńczyk, C. Synthesis of piperitone-derived halogenated lactones and their effect on aphid probing, feeding, and settling behavior. RSC Adv. 2011, 1, 498–510. [Google Scholar] [CrossRef]
- Tjallingii, W.F. Salivary secretions by aphids interacting with proteins of phloem wound responses. J. Exp. Bot. 2006, 57, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Will, T.; Tjallingii, W.F.; Thonnessen, A.; van Bel, A.J.E. Molecular sabotage of plant defense by aphid saliva. Proc. Natl. Acad. Sci. USA 2007, 104, 10536–10541. [Google Scholar] [CrossRef] [PubMed]
- Schliephake, E. Aphid resistance in raspberry and feeding behaviour of Amphorophora idaei. J. Plant Dis. Prot. 2010, 117, 60–66. [Google Scholar] [CrossRef]
- Ramírez, C.C.; Niemeyer, H.M. Salivation into sieve elements in relation to plant chemistry: The case of the aphid Sitobion fragariae and the wheat, Triticum aestivum. In Proceedings of the 10th International Symposium on Insect-Plant Relationships, Oxford, UK, 4–10 July 1998; Simpson, S.J., Mordue, A.J., Hardie, J., Eds.; Springer, Series Entomologica: Dordrecht, The Netherlands, 1999; Volume 56, pp. 111–114. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).