Urban Areas Create Refugia for Odonates in a Semi-Arid Region
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
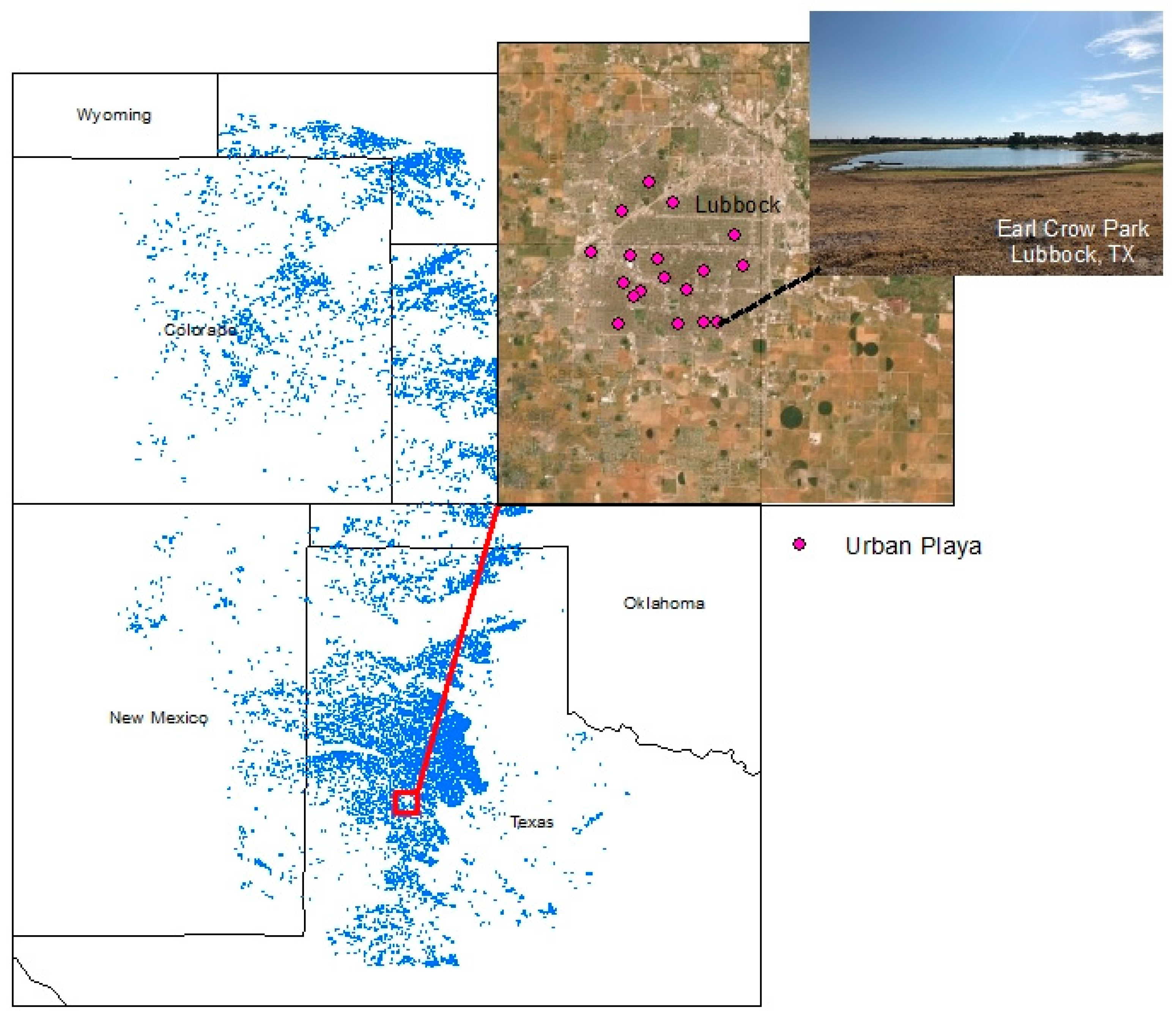
2.1. Study System
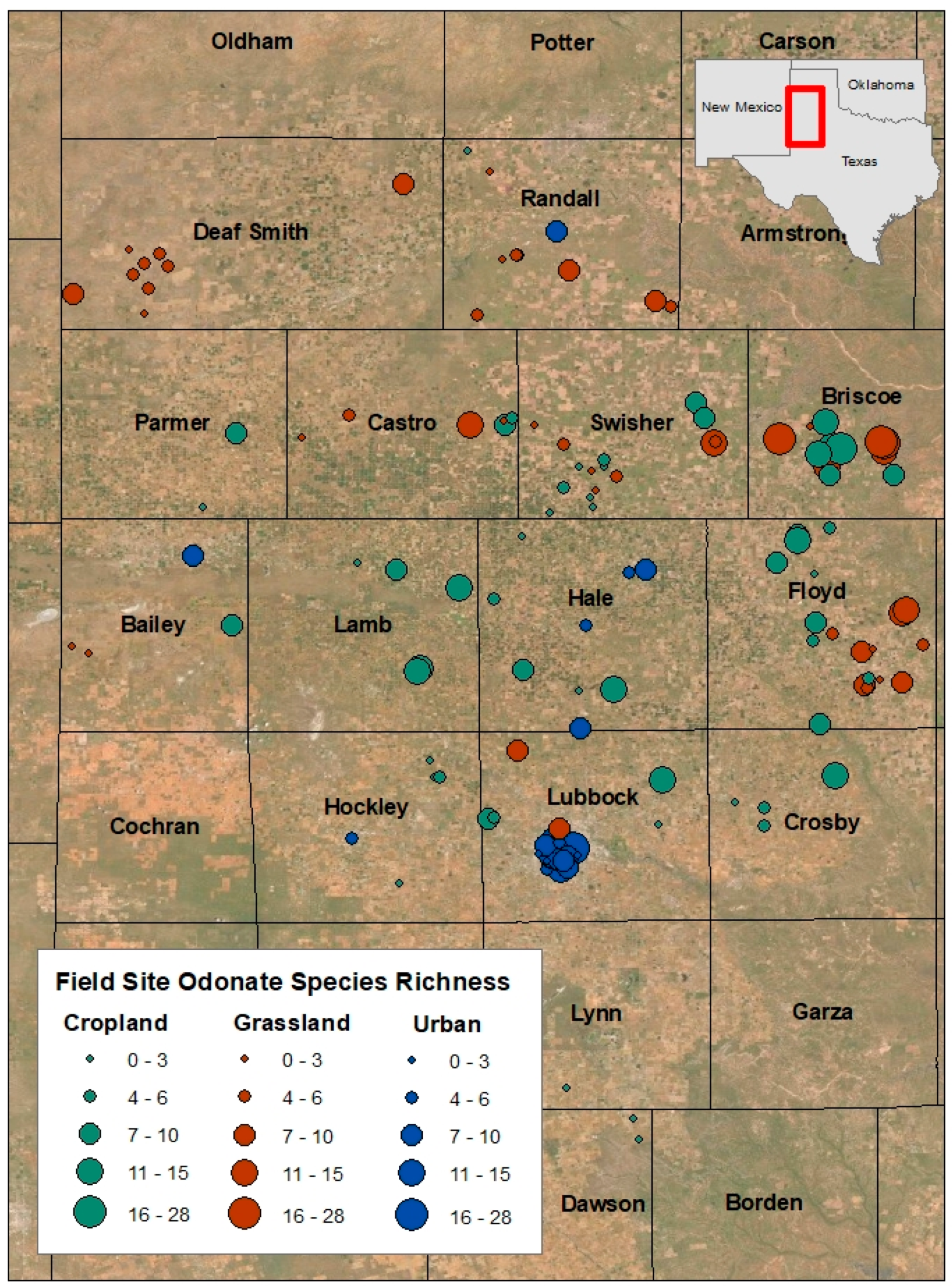
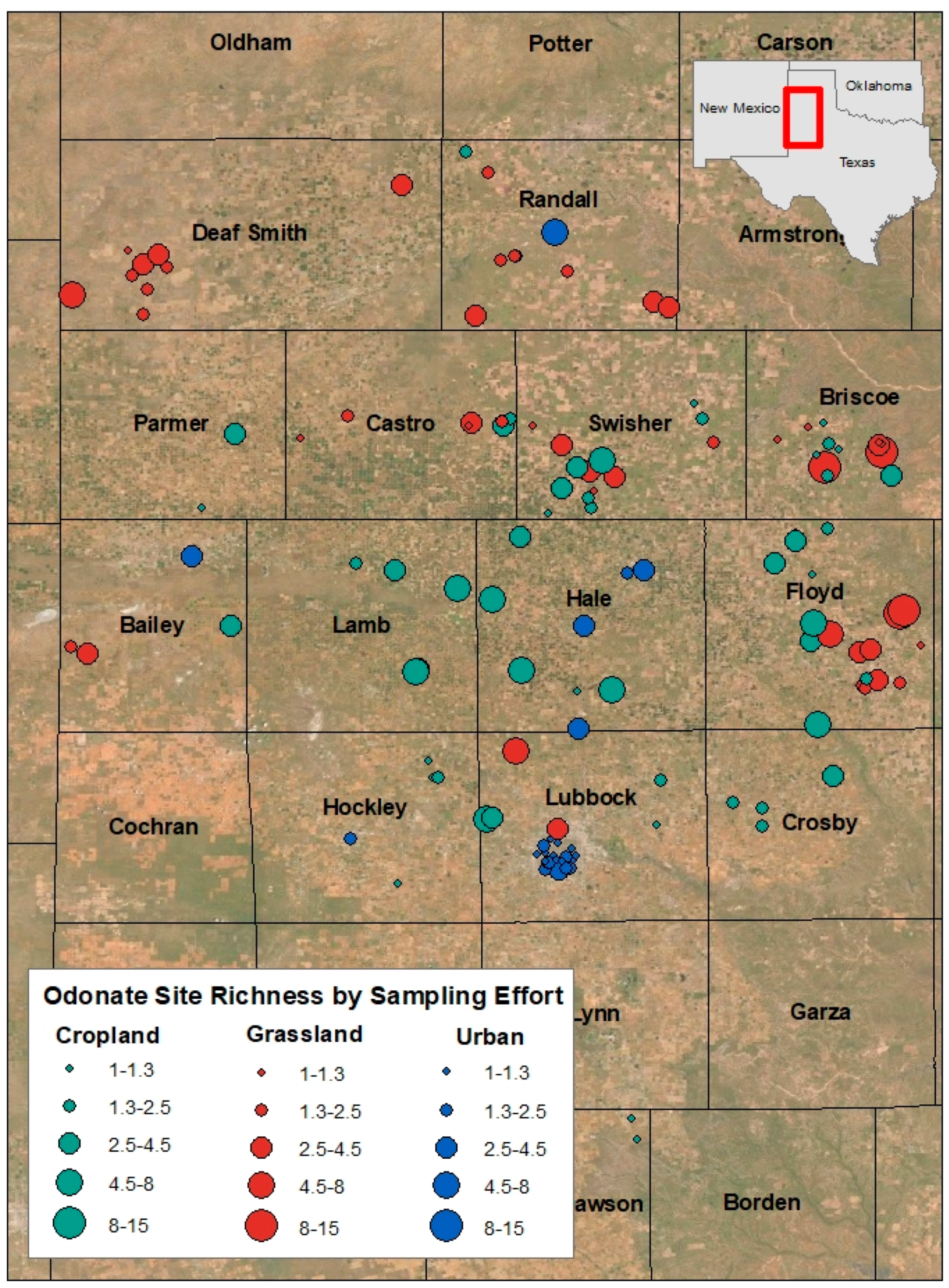
2.2. Data and Collection Methods
2.3. Odonate Assemblage Characterization and Analysis
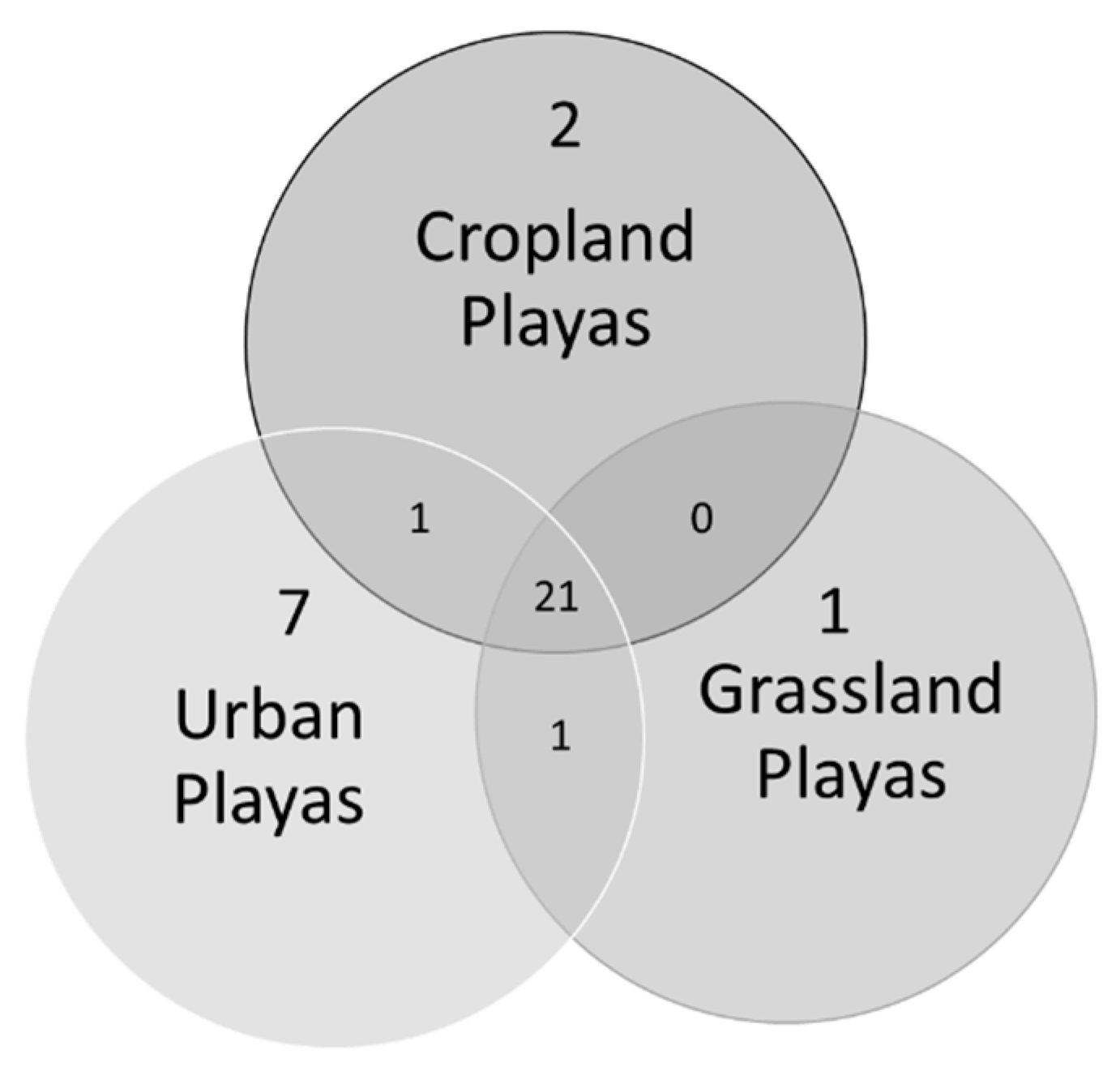
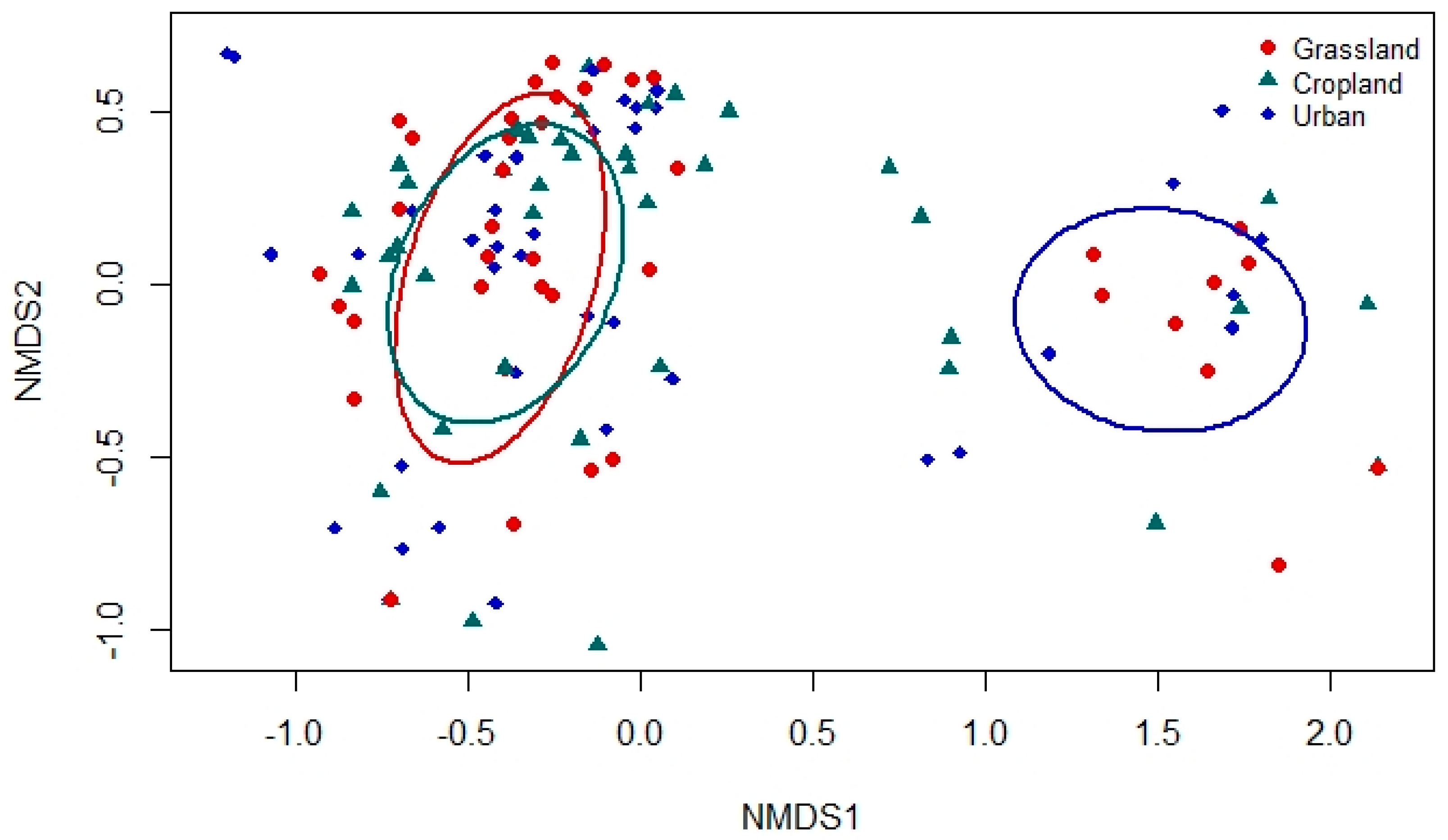
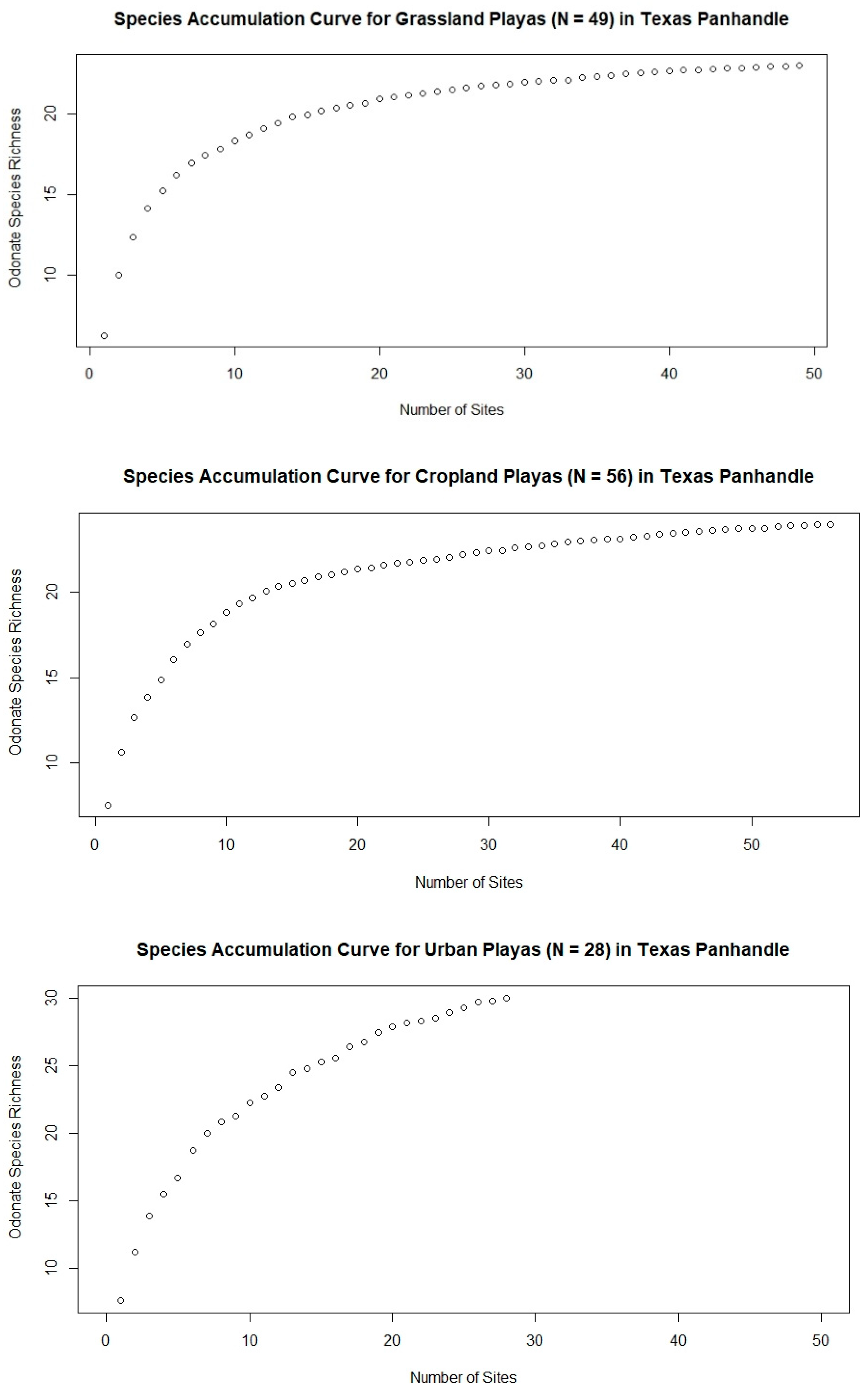
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, S.; Niu, Z.; Chen, Y.; Li, L.; Zhang, H. Global wetlands: Potential distribution, wetland loss, and status. Sci. Total Environ. 2017, 586, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Strayer, D.L.; Dudgeon, D. Freshwater biodiversity conservation: Recent progress and future challenges. J. N. Am. Benthol. Soc. 2010, 29, 344–358. [Google Scholar] [CrossRef]
- Dixon, M.J.R.; Loh, J.; Davidson, N.C.; Beltrame, C.; Freeman, R.; Walpole, M. Tracking global change in ecosystem area: The Wetland Extent Trends Index. Biol. Conserv. 2016, 193, 27–35. [Google Scholar] [CrossRef]
- Minckley, T.A.; Turner, D.S.; Weinstein, S.R. The relevance of wetland conservation in arid regions: A re-examination of vanishing communities in the American Southwest. J. Arid Environ. 2013, 88, 213–221. [Google Scholar] [CrossRef]
- Maleki, S.; Soffianian, A.R.; Koupaei, S.S.; Saatchi, S.; Pourmanafi, S.; Shiekholeslam, F. Habitat mapping as a tool for water bird conservation planning in an arid zone wetland: The case study Hamun wetland. Ecol. Eng. 2016, 95, 594–603. [Google Scholar] [CrossRef]
- Tickner, D.; Opperman, J.J.; Abell, R.; Acreman, M.; Arthington, A.H.; Bunn, S.E.; Cooke, S.J.; Dalton, J.; Darwall, W.; Edwards, G.; et al. Bending the curve of global freshwater biodiversity loss: An emergency recovery plan. BioScience 2020, 40, 330–342. [Google Scholar] [CrossRef]
- Renner, S.; Périco, E.; Sahlén, G. Man-made lakes form species-rich dragonfly communities in the Brazilian Atlantic forest (Odonata). Odonatologica 2016, 45, 135–154. [Google Scholar] [CrossRef]
- Holtmann, L.; Brüggeshemke, J.; Juvchem, M.; Fartmann, T. Odonate assemblages of urban stormwater ponds: The conservation value depends on pond type. J. Insect Conserv. 2019, 23, 123–132. [Google Scholar] [CrossRef]
- Perron, M.A.C.; Pick, F.R. Stormwater ponds as habitat for Odonata in urban areas: The importance of obligate wetlands plant species. Biodiv. Conserv. 2020, 29, 913–931. [Google Scholar] [CrossRef]
- Vilenica, M.; Pozojević, I.; Vučkovic, N.; Mihaljević, Z. How suitable are man-made water bodies as habitats for Odonata? Knowl. Manage. Aquat. Ecosyst. 2020, 421, 1–10. [Google Scholar] [CrossRef]
- Taylor, J.L.; Acevedo, W.; Auch, R.F.; Drummond, M.A. Status and Trends of Land Change in the Great Plains of the United States—1973 to 2000; U.S. Geological Survey Professional Paper; U.S. Department of the Interior, U.S. Geological Survey: Liston, VA, USA, 2015; Volume 1794-B, p. vi-179. [CrossRef]
- Johnson, L.A.; Haukos, D.A.; Smith, L.M.; McMurry, S.T. Physical loss and modification of Southern Great Plains playas. J. Environ. Manag. 2012, 112, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Bolen, E.G.; Smith, L.M.; Schramm, H.L., Jr. Playa lakes: Prairie wetlands of the Southern high plains. BioScience 1989, 39, 615–623. [Google Scholar] [CrossRef]
- Smith, L.M.; Haukos, D.A.; McMurry, S.T.; LaGrange, T.; Willis, D. Ecosystem services provided by playas in the High Plains: Potential influences of USDA conservation programs. Ecol. Appl. 2011, 21, S82–S92. [Google Scholar] [CrossRef]
- Smith, L. Playas of the Great Plains; University of Texas Press: Austin, TX, USA, 2003. [Google Scholar]
- Johnson, W.P.; Rice, M.B.; Haukos, D.A.; Thorpe, P.P. Factors influencing the occurrence of inundated playas wetlands during winter on the Texas High Plains. Wetlands 2011, 31, 1287–1296. [Google Scholar] [CrossRef]
- McIntyre, N.E.; Collins, S.D.; Heintzman, L.J.; Starr, S.M.; van Gestel, N. The challenge of assaying landscape connectivity in a changing world: A 27-year case study in the southern Great Plains (USA) playa network. Ecol. Indic. 2018, 91, 607–616. [Google Scholar] [CrossRef]
- Cariveau, A.B.; Pavlacky, D.C., Jr.; Bishop, A.A.; LaGrange, T.G. Effects of surrounding land use on playa inundation following intense rainfall. Wetlands 2011, 31, 65–73. [Google Scholar] [CrossRef]
- Bartuszevige, A.M.; Pavlacky, D.C., Jr.; Burris, L.; Herbener, K. Inundation of playa wetlands in the western Great Plains relative to landcover context. Wetlands 2012, 32, 1103–1113. [Google Scholar] [CrossRef]
- Collins, S.D.; Heintzman, L.J.; Starr, S.M.; Wright, C.K.; Henebry, G.M.; McIntyre, N.E. Hydrological dynamics of temporary wetlands in the southern Great Plains as a function of surrounding land use. J. Arid Environ. 2014, 109, 6–14. [Google Scholar] [CrossRef]
- Raebel, E.M.; Merckx, T.; Feber, R.E.; Riordan, P.; MacDonald, D.W.; Thompson, D.J. Identifying high-quality pond habitats for Odonata in lowland England: Implications for agri-environment schemes. Insect Conserv. Diver. 2012, 5, 422–432. [Google Scholar] [CrossRef]
- Starr, S.M.; Heintzman, L.J.; Mulligan, K.R.; Barbato, L.S.; McIntyre, N.E. Using remotely sensed imagery to document how land use drives turbidity of playa waters in Texas. Remote Sens. 2016, 8, 192. [Google Scholar] [CrossRef]
- Jeanmougin, M.; Leprieur, F.; Loïs, G.; Clergeau, P. Fine-scale urbanization affects Odonata species diversity in ponds of a megacity (Paris, France). Acta Oecol. 2014, 59, 26–34. [Google Scholar] [CrossRef]
- Fish, E.B.; Atkinson, E.L.; Mollhagen, T.R.; Shanks, C.H.; Brenton, C.M. Playa Lakes Database for the Texas Portion of the Playa Lakes Joint Venture Region; Technical publication #T-9-813; Department of Natural Resources Management, Texas Tech University: Lubbock, TX, USA, 1998. [Google Scholar]
- Haukos, D.A.; Smith, L.M. The importance of playa wetlands to biodiversity of the Southern high plains. Landsc. Urban Plann. 1994, 28, 83–98. [Google Scholar] [CrossRef]
- Ruiz, L.; Parikh, N.; Heintzman, L.J.; Collins, S.D.; Starr, S.M.; Wright, C.K.; Henebry, G.M.; van Gestel, N.; McIntyre, N.E. Dynamic connectivity of temporary wetlands in the southern Great Plains. Landsc. Ecol. 2014, 23, 1–11. [Google Scholar] [CrossRef]
- Heintzman, L.J.; McIntyre, N.E. Quantifying the effects of projected urban growth on connectivity among wetlands in the Great Plains (USA). Landsc. Urban Plann. 2019, 186, 1–12. [Google Scholar] [CrossRef]
- Starr, S.M.; McIntyre, N.E. Land-cover changes and influences on playa wetland inundation on the Southern High Plains. J. Arid Environ. 2020, 175, 1–11. [Google Scholar] [CrossRef]
- Corbet, P.S. A Biology of Dragonflies, 1st ed.; H.F. & G. Witherby LTD: London, UK, 1962; pp. 31–40. [Google Scholar]
- Tsai, J.-S.; Venne, L.S.; McMurry, S.T.; Smith, L.M. Influences of land use and wetland characteristics on water loss rates and hydroperiods of playas in the Southern High Plains, USA. Wetlands 2007, 27, 683–692. [Google Scholar] [CrossRef]
- Venne, L.S.; Anderson, T.A.; Zhang, B.; Smith, L.M.; McMurry, S.T. Organochlorine pesticide concentrations in sediment and amphibian tissue in playa wetlands in the Southern high plains, USA. Bull. Environ. Contam. Toxicol. 2008, 80, 497–501. [Google Scholar] [CrossRef]
- Hernandez, K.M.; Reece, B.A.; McIntyre, N.E. Effects of anthropogenic land use on Odonata in playas of the Southern High Plains. West. N. Am. Nat. 2006, 66, 273–278. [Google Scholar] [CrossRef]
- Reece, B.A.; McIntyre, N.E. Odonata of playas in the Southern High Plains, Texas. Southwest. Nat. 2009, 54, 96–99. [Google Scholar] [CrossRef]
- Heintzman, L.J.; Anderson, T.A.; Carr, D.L.; McIntyre, N.E. Local and landscape influences on PAH contamination in urban stormwater. Landsc. Urban Plann. 2015, 142, 29–37. [Google Scholar] [CrossRef]
- Anderson, T.A.; Salice, C.J.; Erickson, R.A.; McMurry, S.T.; Cox, S.B.; Smith, L.M. Effects of landuse and precipitation on pesticides and water quality in playa lakes of the Southern high plains. Chemosphere 2013, 92, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Faust, D.R.; Knowles, N.; Magruder, E.; Haukos, D.A.; Cobb, G.P.; Maul, J.D.; Anderson, T.A.; Smith, P.M. Inorganic and organic contaminants in sediments from an urban playa and associated toxicity among Hyallella azteca. Toxicology 2012, 94, 1746–1757. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Monitoring Guidance for Determining the Effectiveness of Nonpoint Source Controls. Chapter 3, Biological Monitoring of Aquatic Communities. 1997. Available online: https://www.epa.gov/sites/production/files/2015-10/documents/monitoring_chap3_1997.pdf (accessed on 9 February 2021).
- Patten, M.A.; Bried, J.T.; Smith-Patten, B.D. Survey data matter: Predicted niche of adults vs breeding Odonata. Freshw. Sci. 2015, 34, 1114–1122. [Google Scholar] [CrossRef]
- Foster, S.E.; Soluk, D.A. Protecting more than the wetland: The importance of biased sex ratios and habitat segregation for conservation of the Hine’s emerald dragonfly, Somatochlora hineana Williamson. Biol. Conserv. 2006, 127, 158–166. [Google Scholar] [CrossRef]
- Bried, J.T.; Hager, B.J.; Hunt, P.D.; Fox, J.N.; Jensen, H.J.; Vowels, K.M. Bias of reduced-effort community surveys for adult Odonata of lentic waters. Insect Conserv. Diver. 2012, 5, 213–222. [Google Scholar] [CrossRef]
- Oksanen, J.F.; Blanchet, G.F.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. vegan: Community Ecology Package. Accessed from R package version 2.5-7. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 9 May 2021).
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2021; Available online: http://www.rstudio.com/ (accessed on 9 May 2021).
- Gardener, M. Community Ecology: Analytical Methods Using R and Excel®; Pelagic Publishing: Exeter, UK, 2014; pp. 460–466. [Google Scholar]
- Kietzka, G.J.; Pryke, J.S.; Samways, M.J. Comparative effects of urban and agricultural land transformation on Odonata assemblages in a biodiversity hotspot. Basic Appl. Ecol. 2018, 33, 89–98. [Google Scholar] [CrossRef]
- Chovanec, A. Man-made wetlands in urban recreational areas—a habitat for endangered species? Landsc. Urban Plann. 1994, 29, 43–54. [Google Scholar] [CrossRef]
- Gaston, K.J.; Blackburn, T.M.; Greenwood, J.J.D.; Gregory, R.D.; Quinn, R.M.; Lawton, J.H. Abundance-occupancy relationships. J. Appl. Ecol. 2000, 37, 39–59. [Google Scholar] [CrossRef]
- Bried, J.T.; Dillon, A.M.; Hager, B.J.; Patten, M.A.; Luttbeg, B. Criteria to infer local species residency in standardized adult dragonfly surveys. Freshw. Sci. 2015, 34, 1105–1113. [Google Scholar] [CrossRef]
- Bried, J.T.; Hinchliffe, R.P. Improving taxonomic resolution in large-scale freshwater biodiversity monitoring: An example using wetlands and Odonata. Insect Conserv. Div. 2019, 12, 9–17. [Google Scholar] [CrossRef]
- Bried, J.T.; Ries, L.; Smith, B.; Patten, M.; Abbott, J.; Ball-Damerow, J.; Cannings, R.; Cordero-Rivera, A.; Córdoba-Aguilar, A.; De Marco, P., Jr.; et al. Towards global volunteer monitoring of odonate abundance. BioScience 2020, 70, 914–923. [Google Scholar] [CrossRef]
- Corbet, P.S. Dragonflies: Behavior and Ecology of Odonata; Cornell University Press: Ithaca, NY, USA, 1999; pp. 386–387. [Google Scholar]
- Córdoba-Aguilar, A.; Rocha-Ortega, M. Damselfly (Odonata: Calopterygidae) population decline in an urbanizing watershed. J. Insect Sci. 2019, 19, 1–6. [Google Scholar] [CrossRef] [PubMed]
- United States Census Bureau. 2019 Census Lubbock Texas Quickfacts. Available online: https://www.census.gov/quickfacts/fact/table/lubbockcitytexas,lubbockcountytexas/.PST045219 (accessed on 24 January 2021).
- McIntyre, N.E.; Knowles-Yanez, K.; Hope, D. Urban ecology as an interdisciplinary field: Differences in the use of “urban” between the social and natural sciences. Urban Ecosyst. 2000, 4, 5–24. [Google Scholar] [CrossRef]
- McIntyre, N.E. Urban areas and urban ecology. In The Routledge Handbook of Urban Ecology, 2nd ed.; Douglas, I., Anderson, P.M.L., Goode, D., Houck, M.C., Maddox, D., Nagendra, H., Yok, T.P., Eds.; Routledge: Abingdon, UK, 2021; pp. 5–12. [Google Scholar]
- Goertzen, D.; Suhling, F. Promoting dragonfly diversity in cities: Major determinants and implications for urban pond design. J. Insect. Conserv. 2013, 17, 399–409. [Google Scholar] [CrossRef]
- Jere, A.; Darshetkar, A.; Patwardhan, A.; Koparde, P. Assessing the response of odonates (dragonflies and damselflies) to a tropical urbanization gradient. J. Urban Ecol. 2020, 6, 1–7. [Google Scholar] [CrossRef]
- Janssen, A.; Hunger, H.; Konold, W.; Pufal, G.; Staab, M. Simple pond restoration measures increase dragonfly (Insecta: Odonata) diversity. Biodivers. Conserv. 2018, 27, 2311–2328. [Google Scholar] [CrossRef]
- Hassall, C. The ecology of urban ponds. WIREs Water 2014, 1, 187–206. [Google Scholar] [CrossRef]

| Species | Grassland | Cropland | Urban |
|---|---|---|---|
| (n = 49) | (n = 56) | (n = 28) | |
| Zygoptera | |||
| Lestes alacer | 1 | 1 | 1 |
| Lestes australis | 1 | 1 | 1 |
| Enallagma civile | 1 | 1 | 1 |
| Enallagma basidens | 1 | ||
| Ischnura ramburii | 1 | ||
| Ischnura barberi | 1 | ||
| Ischnura damula | 1 | 1 | |
| Ischnura demorsa | 1 | 1 | 1 |
| Ischnura denticollis | 1 | 1 | 1 |
| Ischnura posita | 1 | ||
| Ischnura hastata | 1 | 1 | 1 |
| Telebasis salva | 1 | ||
| Argia apicalis | 1 | ||
| Anisoptera | |||
| Rhionaeschna multicolor | 1 | 1 | 1 |
| Anax junius | 1 | 1 | 1 |
| Plathemis lydia | 1 | 1 | 1 |
| Libellula saturata | 1 | 1 | 1 |
| Libellula pulchella | 1 | 1 | 1 |
| Libellula luctuosa | 1 | 1 | 1 |
| Orthemis ferruginea | 1 | 1 | 1 |
| Perithemis tenera | 1 | 1 | 1 |
| Brachymesia gravida | 1 | ||
| Celithemis eponina | 1 | ||
| Erythemis vesiculosa | 1 | 1 | |
| Erythemis simplicicollis | 1 | 1 | 1 |
| Erythrodiplax umbrata | 1 | ||
| Sympetrum corruptum | 1 | 1 | 1 |
| Pachydiplax longipennis | 1 | 1 | 1 |
| Dythemis fugax | 1 | ||
| Tramea onusta | 1 | 1 | 1 |
| Tramea lacerata | 1 | 1 | 1 |
| Pantala flavescens | 1 | 1 | 1 |
| Pantala hymenaea | 1 | 1 | 1 |
| Playa Type (n) | % Wet | % Dry | Total Site Visits |
|---|---|---|---|
| Grassland (51) | 52 | 48 | 88 |
| Cropland (56) | 53 | 47 | 49 |
| Urban (28) | 98 | 2 | 28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Husband, D.M.; McIntyre, N.E. Urban Areas Create Refugia for Odonates in a Semi-Arid Region. Insects 2021, 12, 431. https://doi.org/10.3390/insects12050431
Husband DM, McIntyre NE. Urban Areas Create Refugia for Odonates in a Semi-Arid Region. Insects. 2021; 12(5):431. https://doi.org/10.3390/insects12050431
Chicago/Turabian StyleHusband, Danielle M., and Nancy E. McIntyre. 2021. "Urban Areas Create Refugia for Odonates in a Semi-Arid Region" Insects 12, no. 5: 431. https://doi.org/10.3390/insects12050431
APA StyleHusband, D. M., & McIntyre, N. E. (2021). Urban Areas Create Refugia for Odonates in a Semi-Arid Region. Insects, 12(5), 431. https://doi.org/10.3390/insects12050431






