Efficacy of the InvictDetectTM Immunostrip® to Taxonomically Identify the Red Imported Fire Ant, Solenopsis invicta, Using A Single Worker Ant
Abstract
1. Introduction
2. Materials and Methods
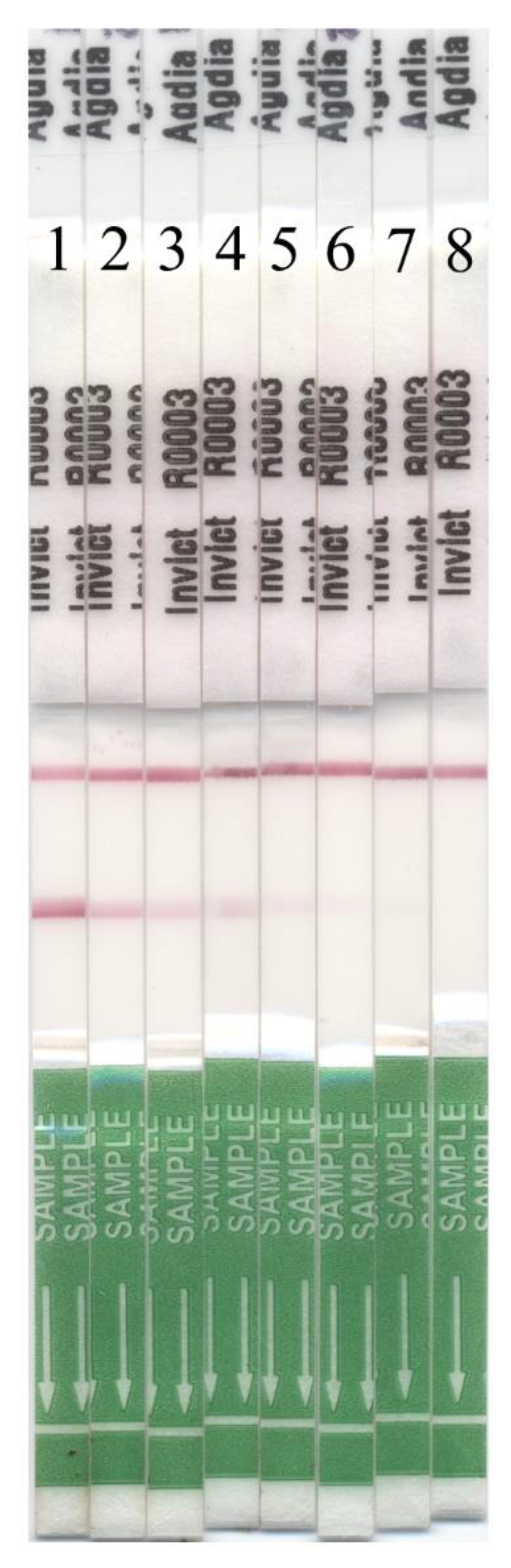
2.1. InvictDetectTM Field Tests
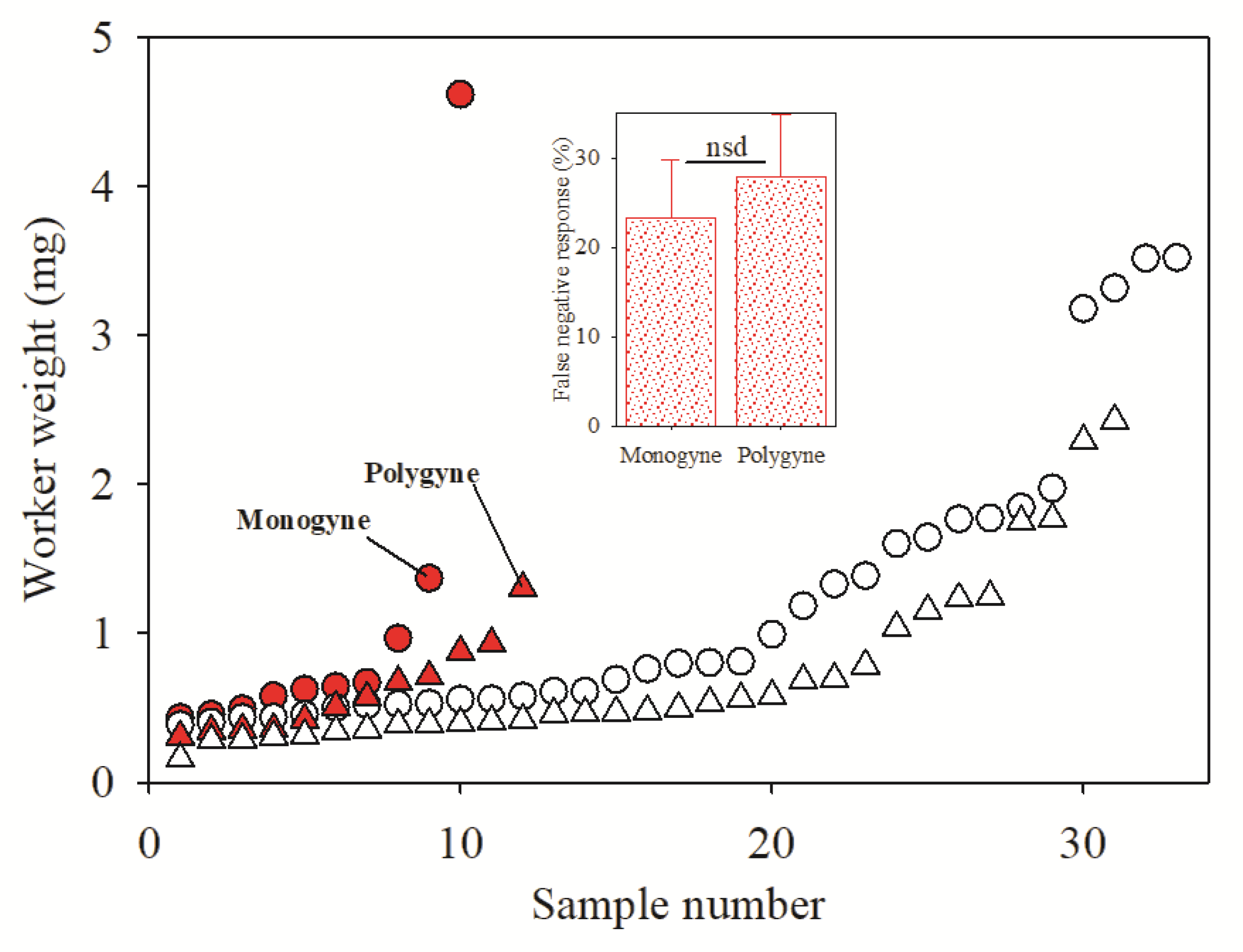
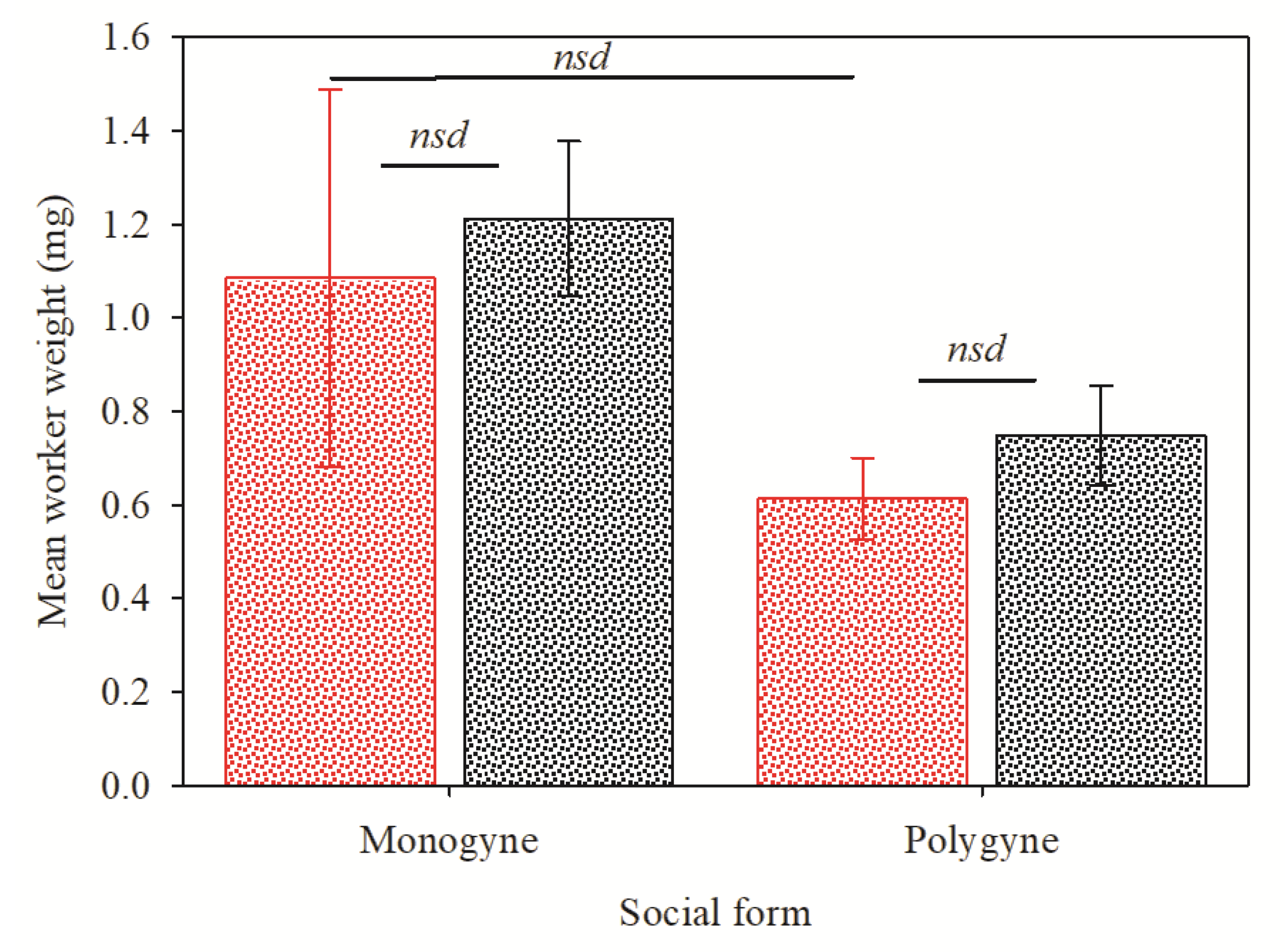
2.2. Influence of Worker Weight on InvictDetectTM Response
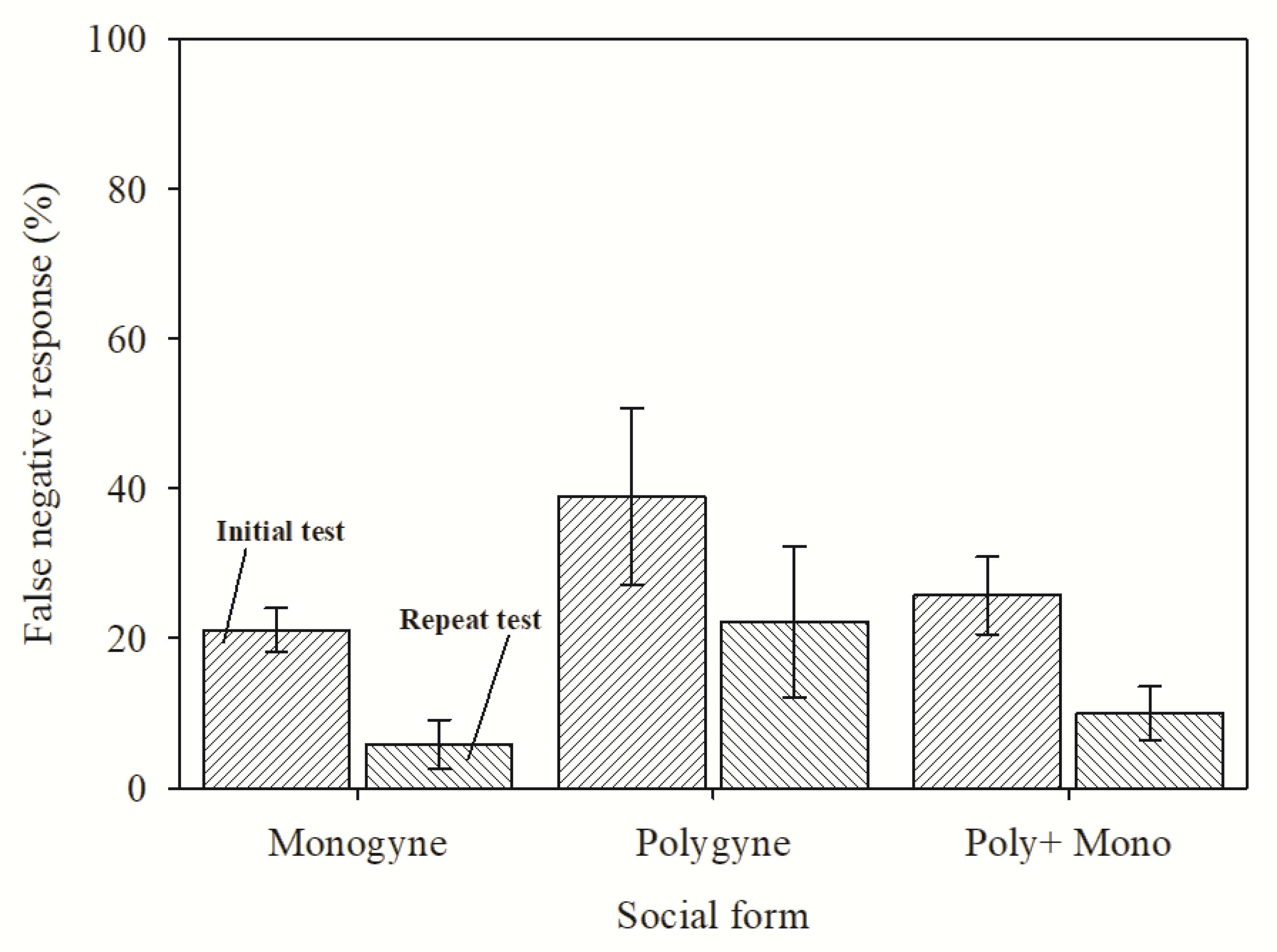
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tschinkel, W.R. The Fire Ants; The Belknap Press of Harvard University Press: Cambridge, UK, 2006; p. 723. [Google Scholar]
- GISD. Global Invasive Species Database: 100 of the World’s Worst Invasive Alien Species. Available online: http://www.iucngisd.org/gisd/100_worst.php (accessed on 15 July 2019).
- Buren, W.F.; Allen, G.E.; Whitcomb, W.H.; Lennartz, F.E.; Williams, R.N. Zoogeography of imported fire ants. J. N. Y. Entomol. Soc. 1974, 82, 113–124. [Google Scholar]
- Wetterer, J.; Davis, L., Jr. Solenopsis invicta (Hymenoptera: Formicidae) in the Lesser Antilles. Fla. Entomol. 2010, 93, 128–129. [Google Scholar] [CrossRef]
- McCubbin, K.I.; Weiner, J.M. Fire ants in Australia: A new medical and ecological hazard. Med. J. Aust. 2002, 176, 518–519. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.S.Y.; Yuen, K.Y. Red imported fire ants in Hong Kong. Hong Kong Med. J. 2005, 11, 131–132. [Google Scholar] [PubMed]
- CABI. Solenopsis invicta (Red Imported Fire Ant) In: Invasive Species Compendium. Available online: www.cabi.org/isc (accessed on 15 July 2019).
- Callcott, A.M.; Collins, H.L. Invasion and range expansion of imported fire ants (Hymenoptera: Formicidae) in North America from 1918–1995. Fla. Entomol. 1996, 79, 240–251. [Google Scholar] [CrossRef]
- Pereira, R.M. Areawide suppression of fire ant populations in pastures: Project update. J. Agric. Urban Entomol. 2003, 20, 123–130. [Google Scholar]
- Valles, S.M.; Strong, C.A.; Callcott, A.-M.A. Development of a lateral flow immunoassay for rapid field detection of the red imported fire ant, Solenopsis invicta (Hymenoptera: Formicidae). Anal. Bioanal. Chem. 2016, 408, 4693–4703. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.R. Ant venoms. Curr. Opin. Allergy Clin. Immunol. 2010, 10, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Valles, S.M.; Strong, C.A.; Callcott, A.M.A. Multiplexed lateral flow immunoassay to discriminate Solenopsis invicta, Solenopsis richteri, and Solenopsis invicta x richteri hybrids. Insectes Sociaux 2018, 65, 493–501. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, Y.; Kim, W.J.; Kim, A.Y.; Nguyen, P.; Lyu, D.P.; Lee, H.S.; Koh, Y.H. Developing molecular diagnostics for detection of red imported fire ants using two genes, Sinv11108 and Sinv11977. Arch. Insect Biochem. Physiol. 2019, 102, e21610. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, N.; Sakamoto, Y.; Goka, K. Rapid detection of the red fire ant Solenopsis invicta (Hymenoptera: Formicidae) by loop-mediated isothermal amplification. Appl. Entomol. Zool. 2019, 54, 319–322. [Google Scholar] [CrossRef]
- Buren, W.F. Revisionary studies on the taxonomy of the imported fire ants. J. Ga. Entomol. Soc. 1972, 7, 26. [Google Scholar]
- Valles, S.M.; Porter, S.D. Identification of polygyne and monogyne fire ant colonies (Solenopsis invicta) by multiplex PCR of Gp-9 alleles. Insectes Soc. 2003, 50, 199–200. [Google Scholar] [CrossRef]
- Porter, S.D.; Tschinkel, W.R. Fire ant polymorphism (Hymenoptera, Formicidae)—Factors affecting worker size. Ann. Entomol. Soc. Am. 1985, 78, 381–386. [Google Scholar] [CrossRef]
- SAS. SAS Procedures Guide for Personal Computers; SAS Institute: Cary, NC, USA, 1988. [Google Scholar]
- Haight, K.L.; Tschinkel, W.R. Patterns of venom synthesis and use in the fire ant, Solenopsis invicta. Toxicon 2003, 42, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Goodisman, M.A.D.; Ross, K.G. Relationship of queen number and worker size in polygyne colonies of the fire ant Solenopsis invicta. Insectes Soc. 1996, 43, 303–307. [Google Scholar] [CrossRef]
- Eliyahu, D.; Ross, K.G.; Haight, K.L.; Keller, L.; Liebig, J. Venom alkaloid and cuticular hydrocarbon profiles are associated with social organization, queen fertility status, and queen genotype in the fire ant Solenopsis invicta. J. Chem. Ecol. 2011, 37, 1242–1254. [Google Scholar] [CrossRef] [PubMed]
- Deslippe, R.J.; Guo, Y.J. Venom alkaloids of fire ants in relation to worker size and age. Toxicon 2000, 38, 223–232. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valles, S.M.; Strong, C.A.; Emmitt, R.S.; Culkin, C.T.; Weeks, R.D., Jr. Efficacy of the InvictDetectTM Immunostrip® to Taxonomically Identify the Red Imported Fire Ant, Solenopsis invicta, Using A Single Worker Ant. Insects 2020, 11, 37. https://doi.org/10.3390/insects11010037
Valles SM, Strong CA, Emmitt RS, Culkin CT, Weeks RD Jr. Efficacy of the InvictDetectTM Immunostrip® to Taxonomically Identify the Red Imported Fire Ant, Solenopsis invicta, Using A Single Worker Ant. Insects. 2020; 11(1):37. https://doi.org/10.3390/insects11010037
Chicago/Turabian StyleValles, Steven M., Charles A. Strong, Robert S. Emmitt, Christopher T. Culkin, and Ronald D. Weeks, Jr. 2020. "Efficacy of the InvictDetectTM Immunostrip® to Taxonomically Identify the Red Imported Fire Ant, Solenopsis invicta, Using A Single Worker Ant" Insects 11, no. 1: 37. https://doi.org/10.3390/insects11010037
APA StyleValles, S. M., Strong, C. A., Emmitt, R. S., Culkin, C. T., & Weeks, R. D., Jr. (2020). Efficacy of the InvictDetectTM Immunostrip® to Taxonomically Identify the Red Imported Fire Ant, Solenopsis invicta, Using A Single Worker Ant. Insects, 11(1), 37. https://doi.org/10.3390/insects11010037



