Abstract
Background/Objectives: Precision medicine is transforming care for children with cancer, but raises new challenges. We explored parents’, oncologists’ and scientists’ perspectives on three aspects of a precision medicine trial for poor prognosis childhood cancer: data sharing, requests for additional tumor biopsies, and confidentiality. Methods: Data were collected through PRISM-Impact, a psychosocial sub-study within the Zero Childhood Cancer Program’s PRISM trial. Parents completed questionnaires at enrolment and one year later, and an optional interview after receiving their child’s trial results. Bereaved parents completed a questionnaire six months after bereavement (T1B). Oncologists and scientists were interviewed one year following trial commencement. Quantitative data were analyzed descriptively, and qualitative data thematically. Results: Parents (n = 126) considered additional tumor biopsies acceptable when risks were low and their child or oncologist supported the request. Oncologists (n = 26) emphasized weighing risk–benefit, ensuring parents felt fully informed, and research value. Most parents supported data sharing (≥89–96%), including after bereavement, despite potential privacy concerns. Parents supported overseas and interstate testing, and scientists having access to identifiable health information. Scientists (n = 10) found working with identifiable data emotionally challenging. Conclusions: Parents, oncologists, and scientists showed high acceptance of procedural aspects of precision medicine. Future trials should address privacy concerns and ensure informed consent recognizes that parents’ high acceptability of procedures may be linked to their hopes for benefit, reinforcing the need for informed consent.
Keywords:
precision medicine; pediatric oncology; scientists; parents; data sharing; biopsy; confidentiality 1. Introduction
Childhood cancer remains a leading cause of disease-related death amongst children and adolescents worldwide [1,2]. Despite improved survival rates, children with rare, relapsed, or refractory cancers often still have a poor prognosis [3] with new treatment approaches urgently needed [4]. Precision medicine uses comprehensive molecular profiling to understand a patient’s cancer and tailor treatments [5,6], aiming for more effective and less toxic therapies [7]. Several pediatric precision medicine programs are underway [8] requiring parents, oncologists, and scientists to consider procedural factors, such as additional tumor biopsies, sharing patient data, and confidentiality in a clinical research setting.
Participation in precision medicine programs relies on the availability of tissue samples for molecular profiling [9]. Samples from standard procedures are usually sufficient after routine clinical testing [10]; however, if unavailable or poor in quality or quantity, an additional biopsy may be required [9,11,12] along with blood specimens [13]. Parents must weigh the risks of such procedures against potential benefits. Parents have reported that factors such as quality of life, survival, and cost are more influential than their child requiring a biopsy when deciding on trial enrolment [14]. However, some parents hesitate to join future trials if biopsies are required [15,16]. Willingness tends to increase when oncologists recommend the procedure [15], underscoring the need to understand oncologists’ perspectives. Clinicians, including oncologists and surgeons, face challenges weighing up biopsy risks for precision medicine trial enrolment [17,18]. Oncologists are more likely to recommend a biopsy if the likelihood of finding a molecular target is high, invasiveness and morbidity rate are low, and results could influence clinical management [19]. Since molecular analysis may not directly benefit patients [20] and the value of research biopsies remains debated [21], further exploration of oncologists’ perspectives is warranted.
In the context of a pediatric precision medicine research trial, parents may also be asked to consent to share their child’s data. For the purposes of this study, “data” refers to de-identified genetic information (germline and tumor).
Clinical data sharing is essential to advance research output and address knowledge gaps in pediatric oncology [22,23,24]. As childhood cancers are rare and histologically diverse [25], and with precision medicine becoming standard care, large datasets are crucial to maximize benefits [26]. Parents may need to consider the new challenges posed by genomic analysis in precision medicine. Despite efforts to protect privacy during data sharing, risks persist, including the risks to patients’ personal information [27]. Sharing pediatric data is further complicated due to ethical concerns surrounding the use of data from participants who are unable to provide consent themselves (i.e., children < 18 years) [28,29].
There is currently no literature on parents’ attitudes toward data sharing within a precision medicine trial. Existing studies show both adult patients and parents of pediatric patients are largely willing to share genomic data [30,31], but parents generally prioritize privacy more [30]. Parents also expressed concerns about unspecified future risks for their children with evolving technologies or laws [30,31]. Since parents’ willingness to share data can depend on anticipated benefit [32], it would be valuable to understand parents’ attitudes following the return of trial results, including bereaved parents. Precision medicine trials are bringing non-patient-facing professionals, such as scientists, closer to clinical care than ever before. Scientists involved in precision medicine trials have reported benefits such as seeing how their contributions directly influence patient outcomes through exposure to clinical decision-making during molecular tumor board (MTB) meetings [33]. This involvement means scientists now have greater access to identifiable patient information pertaining to the samples they are analyzing. Currently, our understanding of scientists’ experiences with managing confidentiality in this setting is lacking.
Given that the procedural aspects and confidentiality issues described can raise new ethical considerations [34,35,36], it is important to understand parents’, oncologists’, and scientists’ attitudes toward these matters to inform the development of future trials. Therefore, we aimed to answer the following research questions:
- What are parents’ attitudes toward sharing their child’s anonymous trial data and sending samples interstate and overseas?
- How do parents and oncologists view the potential need for additional tumor biopsies and blood samples in a pediatric precision medicine trial?
- What are parents’ and scientists’ perspectives on confidentiality in a pediatric precision medicine trial?
2. Materials and Methods
2.1. PRISM
The PRecISion Medicine for Children with Cancer (PRISM) trial is embedded in the Zero Childhood Cancer Program (ZERO), Australia’s national precision medicine program for children (≤21 years) with poor prognosis cancer (expected survival <30%; Australian and New Zealand Clinical Trials Registry, NCT03336931, 8 November 2017) [37]. ZERO’s PRISM trial utilizes comprehensive genomic profiling of the tumor, germline, and, where possible, targeted drug screening and/or drug efficacy testing, for identification of potentially more likely effective personalized treatment recommendations [37]. The results of this testing are brought to an MTB consisting of clinicians and scientists, which reviews each patient’s results and delivers treatment recommendations to the patient’s oncologist [33]. PRISM’s information sheet and consent form inform parents that the trial may require their child to undergo an additional tumor biopsy. It also states that PRISM testing is conducted in laboratories both in Australia and overseas, and that de-identified germline and tumor genetic information will also contribute to research databases or registries in Australia or overseas. All staff in the ZERO program are required to undertake patient privacy training.
2.2. PRISM- Impact
PRISM-Impact is a psychosocial substudy that runs alongside ZERO’s PRISM trial (Figure 1). Parents opted in to contact from the PRISM-Impact team through the PRISM trial consent form. PRISM and PRISM-Impact were conducted in accordance with the Declaration of Helsinki and received institutional board approval of Hunter New England Local Health District (ethical and governance approval number: HREC/17/HNE/29, 2 May 2017). PRISM-Impact uses a prospective mixed-methods design to explore families’, healthcare professionals’, and scientists’ experiences of participating in PRISM. The current study presents a subset of data collected as part of PRISM-Impact (between September 2017 and September 2021). Due to different consent processes with adult patients, we present data only from parents of patients aged 0–17 years. PRISM is now closed to recruitment, but an ongoing follow-up of several remaining PRISM-Impact families is still underway.
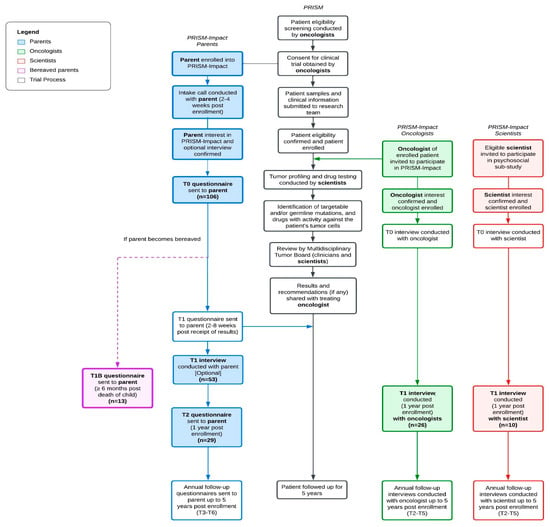
Figure 1.
PRISM and PRISM-Impact study design and procedures in parallel. Note: Words in bold indicate the participant groups relevant to this study. Boxes shaded indicate the procedures and timepoints relevant to this study.
2.3. Procedure
Shortly after enrolling in PRISM, we contacted parents to confirm their consent to participate in PRISM-Impact. We sent parents questionnaires at enrolment (T0) and one year later (T2). We invited bereaved parents to complete an adapted questionnaire six months after their child’s death (T1B). A subset of parents also took part in an optional semi-structured interview after receiving PRISM results (T1). We invited oncologists and scientists involved in the trial to participate in a semi-structured interview one year following trial commencement (T1) via a personalized email from the PRISM investigator (DZ). Interview topics varied by professional group and aligned with each participant’s expertise. We collected clinical information (e.g., diagnosis) from the PRISM study central database. The PRISM-Impact questionnaires and interviews included validated measures (adapted from refs. [15,19,38]) and open-ended interview questions with probes to elicit detailed responses. The questionnaires and interviews were developed and pilot tested with input from a multidisciplinary expert panel. We added measures assessing parents’ attitudes towards data sharing into the PRISM-Impact study via an ethics amendment approved on 1 April 2019, and so were completed by only a subset of the PRISM-Impact sample. Supplementary Table S1 summarizes the parent questionnaires and semi-structured interviews, which cover topics including demographics, views on data sharing, and perspectives on additional biopsies. Seven psychosocial researchers (JY, FL, EB, CR, JH, RD, and NH) conducted in-depth semi-structured interviews, which lasted 28.35 min on average (range: 15–76 min). Interviews were audio-recorded and transcribed verbatim with identifiable information (e.g., participant names) removed prior to analysis.
2.4. Data Analysis
We used a convergent parallel design for this mixed-method study [39]. We analyzed quantitative and qualitative data independently and integrated findings afterwards.
2.5. Quantitative Analyses
We used the Statistical Package for the Social Sciences (v28.0) to perform quantitative analyses [40]. We used descriptive statistics to report on participants’ demographics, parents’ attitudes toward data sharing according to recipient and purpose, and parents’ perceptions of the benefits and negatives of data sharing. We collapsed the latter responses into three categories (positive = ‘benefits outweighing negatives’, negative = ‘negatives outweighing benefits’, neutral = ‘benefits and negatives are equal’) to simplify the presentation and interpretation and descriptively examined each parent’s change in response between enrolment (T0) and one year post enrolment (T2) and change in perceptions from enrolment (T0) to post bereavement (T1B).
2.6. Qualitative Analyses
We used a thematic analysis with an inductive approach [41] with the support of coding software NVivo [42] to analyze qualitative data. One researcher (Y.L.) became familiar with the transcripts and developed an initial coding framework guided by the study aims [41]. The initial coding framework was discussed and revised (Y.L. and K.H.) to reach consensus. Interviews were then coded line by line (Y.L.) to develop the themes and subthemes, which were critically appraised via ongoing discussion (Y.L and K.H). We then aligned these themes with the quantitative dataset and extracted illustrative quotes to create a cohesive overview.
3. Results
3.1. Participants
3.1.1. Parents
Data sharing measures were completed by 106 parents at T0, 29 parents at T2, and 13 parents at T1B. In total, we included data of 126 parents from 110 families in this manuscript (see supplementary material note; for information on factors associated with participation and attrition). During intake, 63 parents opted into a T1 interview, and 53 parents completed the interview.
Supplementary Table S2 summarizes parents’ baseline characteristics (n = 126, 61.1% mothers). Table 1 summarizes their children’s (n = 110) characteristics which included an equal number of girls (50%) and boys, had an average age of 7.8 (±5.5) years at cancer diagnosis, and 8.9 (±5.6) years at PRISM enrolment. Like the overall PRISM trial cohort, patients had been diagnosed with a central nervous system tumor (36.4%), sarcoma (31.8%), hematologic malignancy (16.4%), neuroblastoma (7.3%), or other tumor (8.2%). Around half (50.9%) had experienced at least one relapse prior to enrolment in PRISM.

Table 1.
Demographics of children of participating parents in PRISM-Impact.
3.1.2. Oncologists and Scientists
We invited 57 oncologists to participate, 30 of whom opted in (response rate 52.6%) and 26 completed interviews (participation rate 86.6%). We invited 24 scientists to participate, 11 of whom opted in (response rate 45%) and 10 completed interviews (participation rate 90.9%). Table 2 summarizes the characteristics of participating oncologists and scientists.

Table 2.
Demographics of scientists and oncologists.
3.2. Research Question 1: Attitudes Toward Sharing of the Child’s Anonymous, Individual Trial Data
Figure 2 summarizes parents’ attitudes toward data sharing at each time point (T0, T2, and T1B). Across all time points, most parents (≥89–96%) reported that they were very or somewhat likely to share their child’s data with all recipients and for all purposes mentioned except in cases involving litigation related to unsafe medical products. In contrast, bereaved parents (T1B) did not express opposition to sharing data for this purpose. One bereaved parent indicated they would be very unlikely to share data for the purpose of performing research on health problems that might affect their family or child.
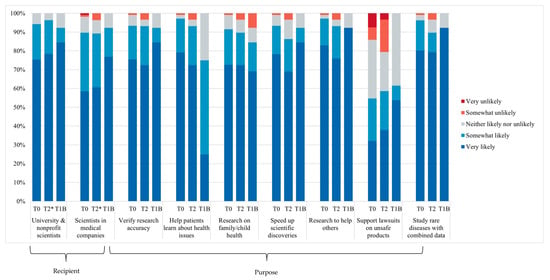
Figure 2.
Parents’ attitudes toward data sharing, according to recipient and purpose at T0 (n = 106), T2 (n = 29), and T1B (n = 13). T2* missing data n = 1.
At all time points, most parents perceived that the potential benefits of data sharing outweighed the potential negative consequences (86–93%) (Figure S1). Amongst parents who responded at both T0 and T2 (n = 14), seven parents’ perceptions remained the same, four parents perceived data sharing more positively over time, and three parents perceived data sharing more negatively—although for two parents this was a positive to neutral change. Amongst parents who responded at both T0 and T1B (n = 8), six parents’ perceptions remained unchanged, and two parents perceived data sharing more negatively (Figure S2).
Table 3 summarizes the themes and quotes representing parents’ attitudes toward sending and testing their child’s sample interstate and overseas. We identified three themes: parents’ support and motivations for sharing; parents’ preferences for sharing; and parents’ attitudes toward privacy protection. All parents agreed to their child’s tissue samples being sent interstate and overseas for PRISM analysis. Their support for sharing was motivated by an anticipated benefit for their child or other children with cancer, research advancement, and positive experiences with data sharing. One parent expressed concern over the potential for medical insurance discrimination if their child’s genetic data was exposed, but still believed the outcomes of sharing outweighed any concerns (Parent 12179).

Table 3.
Themes and quotes representing parents’ attitudes toward their child’s tissue sample being sent and tested overseas and interstate.
3.3. Research Question 2: Perspectives on Additional Tumor Biopsy Requests
Table 4 summarizes the themes and quotes representing parents’ and oncologists’ attitudes toward tumor biopsies. Parents (n = 5) whose child had undergone an additional biopsy for PRISM shared that they felt comfortable with the procedure as they believed it was necessary to achieve the best outcome for their child. Amongst parents whose child did not (n = 36) or parents who were unsure (n = 3; due to child undergoing numerous procedures) if their child required an additional biopsy, some expressed hesitancy when asked to consider the possibility of another operation. When deciding whether to pursue an additional biopsy, parents described weighing up the risks and benefits, as well as considering their child’s and experts’ opinions. Nine parents reported that their child had to provide extra blood samples for participation in the trial. All parents expressed comfort with this and praised the PRISM team for incorporating the blood collection into routine blood draws, as it reduced burden and discomfort on their family. ‘It didn’t seem like a particularly disruptive involvement on our part; it was just something that was a part of his care’ (Parent 11147). Several parents noted that their child was accustomed to blood sampling as part of their oncological care, particularly when a central line was in place, and therefore, it resulted in minimal impact. However, one parent shared that their child ‘wasn’t particularly happy’ (Parent 11006) about the additional blood collection but attributed this to a general sense of distress associated with attending the clinic. Another parent stated they would have been more hesitant to allow for PRISM samples if a separate needle procedure had been required.

Table 4.
Themes and quotes representing parents’ and oncologists’ attitudes toward additional tumor biopsies.
3.4. Research Question 3: Perspectives on Confidentiality
Table 5 summarizes the themes and quotes representing parents’ and scientists’ attitudes toward confidentiality within the trial. We identified three themes from parent interviews: support for identification of the child’s sample, influence of family preferences on privacy, and confidence in scientists’ professionalism. Parents in our sample were either ambivalent or supportive of scientists seeing their child’s name on samples. Parents believed linking a name to the sample humanizes the data and gives recognition to their child as “more than a number” (Parent 12238). Parents also felt comfortable with scientists seeing their child’s name, as they felt it would highlight the significance of the sample being worked on. This perspective contrasts with the conventional view of samples as anonymized data and raises questions about whether, in some circumstances, identification may be beneficial. Parents’ privacy preferences tended to align with family characteristics. For example, the tendency to be more outspoken or more private about their child’s cancer. Additionally, some parents were amenable to the use of their child’s name, provided their child was also in agreement. Parents also expressed confidence in the professionalism of scientists, trusting them to uphold confidentiality requirements

Table 5.
Themes and quotes representing parents’ and scientists’ attitudes toward confidentiality.
4. Discussion
This is the first study to explore key stakeholders’ attitudes toward data sharing, additional biopsy requests, and patient confidentiality in a precision medicine trial for poor-prognosis childhood cancer.
Parents’ positive attitudes toward sharing their child’s clinical data aligned with previous research [30,31] and revealed parents were driven by altruism and a desire to advance research. These motivations were also consistent with parents’ initial reasons for participating in the PRISM trial [43]. Our results showed that most parents’ views remained stable over time, even after receiving trial results or experiencing bereavement, suggesting parents’ support was not contingent on treatment outcomes. Previously reported data sharing concerns, such as potential discrimination by insurance companies [44,45,46,47,48,49,50,51,52], also emerged in our study. In September 2024, Australia introduced a legislative ban on such discrimination [53], which may help address long-term concerns; however, uncertainty about its implementation persists [54]. These findings highlight the need for clear, up-to-date information resources for enrolling families.
Parents showed less support towards sharing data for use in medical lawsuits, possibly reflecting discomfort with sharing data for commercial purposes [49,51,52]. Parents were most willing to share data with scientists from universities and non-profits, but also expressed support for sharing with medical companies. This may reflect recognition of their role in drug development [44] and the need for therapeutic research [46] to address the lack of targeted therapy for children, which is often a barrier in pediatric precision medicine trials [55,56].
All parents agreed to send their child’s tissue samples interstate and overseas for analysis. One possible reason for such high acceptance could be that parents were interviewed after they had received their child’s precision medicine trial results, and therefore perceived tissue sharing as having benefited their child and favored research advancement [57,58,59,60,61]. Only one parent expressed concern regarding potential insurance discrimination in the case of a privacy breach. Parents’ general lack of concern could be attributed to a sense of trust. In another study of pediatric data sharing, parents’ lack of privacy concerns reflected their trust in the primary researcher, with this trust extending to any decisions that the primary researcher made [62]. Response bias may also be a factor as parents who chose to participate in PRISM-Impact may have held more favorable views about research compared to the broader population of families undergoing precision medicine.
Overall, parents and oncologists supported additional biopsies, prioritizing safety, informed consent and potential patient or research benefit. Considering parents often defer complex decisions to oncologists [63,64], clinicians play a key role in ensuring families are informed and their expectations are managed [17,65]. Oncologists in our study also weighed population-level benefits (i.e., research value) in requesting biopsies for molecular profiling in children [66]. These findings underscore the need to balance research value with ethical principles, including beneficence, non-maleficence and respect for the child’s assent/dissent, particularly in poor prognosis settings where equipoise must be considered.
Parents and scientists in our study provided novel insights into patient confidentiality in a precision medicine trial. Parents and scientists value identifiable information in humanizing data. Parents appreciated their child being acknowledged as ‘more than a number’, reflecting a growing sentiment that patients want to be treated as people and a belief that they will receive better care if healthcare professionals know more about them [67]. While scientists felt a stronger emotional connection at the research clinical interface [17,33]. Precision medicine trials encourage closer collaboration between patient-facing and non-patient-facing professionals [33]. Though traditionally working with de-identified data, research scientists in precision medicine trials are increasingly exposed to individual cases, particularly during MTB meetings and show heightened care in managing confidentiality.
Strengths and Limitations
A key strength of our study was the inclusion of multiple stakeholders in a national trial, allowing us to capture diverse views, including those of bereaved parents. While the absolute number of bereaved parents was small, their participation rate was high. Our large parent sample at enrolment (T0) was also a strength, though attrition over time was likely due to health deterioration or escalating demands of care for their child, thus limiting our ability to assess changes in perceptions over time. This sample was likely not representative of all families undergoing PM; those who opted to participate may have had particularly positive or negative experiences. We were unable to explore reasons for non-participation due to ethical restrictions. Future studies should examine families’ reasons for declining participation to explore further. Not all domains of interest were explored using a mixed method approach, i.e., confidentiality and attitudes towards biopsies were assessed solely through interviews (T1). Lastly, our study was limited by the exclusion of non-English-speaking parents. Given that culturally and linguistically diverse participants could have additional concerns regarding trial procedures or informed consent [68], further insight into these participants’ perspectives is necessary.
5. Conclusions
This study demonstrates that parents, oncologists, and research scientists show high levels of acceptance towards key procedural aspects of precision medicine trials, including additional biopsies, data sharing, and patient confidentiality, which, for the most part, outweigh the concerns expressed. Parents enduring support for data sharing, even after bereavement, suggest that their motivations extend beyond a hope for therapeutic outcomes from the trial for the benefit of their child. Parents’ willingness to accept additional biopsy procedures highlights the importance of informed consent and careful management of expectations in precision medicine trials. For oncologists, the findings point to an ongoing ethical responsibility to balance patient risk–benefit with research value, while scientists reflected on their responsibilities of working with identifiable patient information and maintaining confidentiality.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jpm15110531/s1, Supplementary note: Factors associated with participation and attrition (parents); Table S1. Questionnaire and semi-structured interview questions; Table S2. Baseline characteristics of participating parents; Figure S1. Parents’ perceptions of the benefits and negatives of data sharing; Figure S2. Parents perceptions toward potential benefits of sharing anonymous, individual clinical trial data weighed against the potential negative consequences.
Author Contributions
Conceptualization, Y.L., K.H. and C.E.W.; methodology, Y.L., K.H. and C.E.W.; formal analysis, Y.L., K.H., N.R. and R.D., investigation, Y.L., and K.H.; data curation, Y.L. and R.D.; writing—original draft preparation, Y.L., K.H. and R.D.; writing—review and editing, Y.L., K.H., N.R., R.D., C.E.W., B.C.M., D.S.Z., L.M.S.L., V.T., J.K., M.J.C., M.H., K.M.T. and P.B.; visualization, Y.L. and R.D.; supervision, K.H.; project administration, R.D.; funding acquisition, D.S.Z., V.T., K.H. and C.E.W. All authors have read and agreed to the published version of the manuscript.
Funding
K.H. is supported by the Cancer Institute Translational Program Grant (2021/TPG2112). K.H., L.L, V.T., and M.J.C. are supported by the Medical Research Future Fund through the Emerging Priorities and Consumer Driven Research scheme, co-funding the Zero Childhood Cancer National Personalised Medicine Program for children with high-risk cancer, a joint initiative of the Children’s Cancer Institute and the Kids Cancer Centre, Sydney Children’s Hospital, Randwick. C.E.W. is supported by the NHMRC of Australia (APP2008300). K.H., R.D., and M.J.C are supported by Luminesce Alliance—Innovation for Children’s Health. Luminesce Alliance—Innovation for Children’s Health is a not-for-profit cooperative joint venture between the Sydney Children’s Hospitals Network, the Children’s Medical Research Institute, and the Children’s Cancer Institute. It has been established with the support of the NSW government to coordinate and integrate pediatric research. Luminesce Alliance is also affiliated with the University of Sydney and UNSW Sydney.
Institutional Review Board Statement
PRISM and PRISM-Impact was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review of Hunter New England Local Health District (HREC/17/HNE/29, 2 May 2017).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy/ethical reasons.
Acknowledgments
The authors would like to thank all the families, clinicians and scientists who participated in this study. We would also like to acknowledge the following people who contributed to PRISM-Impact: Janine Vetsch, Emily Duve, Nicholas Handelsman, Jacqueline Hunter, Yasmin Christensen, Gabrielle Lambert-Bridges, Lachlan Munro, and Piratat Techekesari.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PRISM | PRecISion Medicine for Children with Cancer |
| MTB | Molecular Tumor Board |
| ZERO | Zero Childhood Cancer National Precision Medicine Program |
References
- Bhakta, N.; Force, L.M.; Allemani, C.; Atun, R.; Bray, F.; Coleman, M.P.; Steliarova-Foucher, E.; Frazier, A.L.; Robison, L.L.; Rodriguez-Galindo, C.; et al. Childhood cancer burden: A review of global estimates. Lancet Oncol. 2019, 20, e42–e53. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare (AIHW). Australia’s Children; AIHW: Canberra, Australia, 2022. [Google Scholar]
- De La Rocha, A.M.A.; Berlow, N.E.; Fader, M.; Coats, E.R.; Saghira, C.; Espinal, P.S.; Galano, J.; Khatib, Z.; Abdella, H.; Maher, O.M.; et al. Feasibility of functional precision medicine for guiding treatment of relapsed or refractory pediatric cancers. Nat. Med. 2024, 30, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Ko, R.H.; Ji, L.; Barnette, P.; Bostrom, B.; Hutchinson, R.; Raetz, E.; Seibel, N.L.; Twist, C.J.; Eckroth, E.; Sposto, R.; et al. Outcome of patients treated for relapsed or refractory acute lymphoblastic leukemia: A Therapeutic Advances in Childhood Leukemia Consortium study. J. Clin. Oncol. 2010, 28, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Cahaney, C.; Dhir, A.; Ghosh, T. Role of precision medicine in pediatric oncology. Pediatr. Ann. 2022, 51, e8–e14. [Google Scholar] [CrossRef] [PubMed]
- Langenberg, K.P.; Looze, E.J.; Molenaar, J.J. The landscape of pediatric precision oncology: Program design, actionable alterations, and clinical trial development. Cancers 2021, 13, 4324. [Google Scholar] [CrossRef]
- Vo, K.T.; Parsons, D.W.; Seibel, N.L. Precision medicine in pediatric oncology. Surg. Oncol. Clin. N. Am. 2019, 29, 63. [Google Scholar] [CrossRef]
- McCabe, M.G.; Geoerger, B.; Chesler, L.; Hargrave, D.; Parsons, D.W.; van Tilburg, C.M.; Schleiermacher, G.; Hickman, J.A.; George, S.L. Precision medicine for childhood cancer: Current limitations and future perspectives. JCO Precis. Oncol. 2024, 8, e2300117. [Google Scholar] [CrossRef]
- Mody, R.J.; Prensner, J.R.; Everett, J.; Parsons, D.W.; Chinnaiyan, A.M. Precision medicine in pediatric oncology: Lessons learned and next steps. Pediatr. Blood Cancer 2017, 64, e26288. [Google Scholar] [CrossRef]
- Chang, W.; Brohl, A.S.; Patidar, R.; Sindiri, S.; Shern, J.F.; Wei, J.S.; Song, Y.K.; Yohe, M.E.; Gryder, B.; Zhang, S.; et al. MultiDimensional ClinOmics for precision therapy of children and adolescent young adults with relapsed and refractory cancer: A report from the center for cancer research. Clin. Cancer Res. 2016, 22, 3810–3820. [Google Scholar] [CrossRef] [PubMed]
- Brisson, A.R.; Matsui, D.; Rieder, M.J.; Fraser, D.D. Translational research in pediatrics: Tissue sampling and biobanking. Pediatrics 2012, 129, 153–162. [Google Scholar] [CrossRef]
- Friedman, A.A.; Letai, A.; Fisher, D.E.; Flaherty, K.T. Precision medicine for cancer with next-generation functional diagnostics. Nat. Rev. Cancer 2015, 15, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Compton, C.C.; Robb, J.A.; Anderson, M.W.; Berry, A.B.; Birdsong, G.G.; Bloom, K.J.; Branton, P.A.; Crothers, J.W.; Cushman-Vokoun, A.M.; Hicks, D.G.; et al. Preanalytics and precision pathology: Pathology practices to ensure molecular integrity of cancer patient biospecimens for precision medicine. Arch. Pathol. Lab. Med. 2019, 143, 1346–1363. [Google Scholar] [CrossRef] [PubMed]
- De Abreu Lourenco, R.; McCarthy, M.C.; McMillan, L.J.; Sullivan, M.; Gillam, L. Understanding decisions to participate in genomic medicine in children’s cancer care: A comparison of what influences parents, health care providers, and the general community. Pediatr. Blood Cancer 2021, 68, e29101. [Google Scholar] [CrossRef]
- Marron, J.M.; DuBois, S.G.; Bender, J.G.; Kim, A.; Crompton, B.D.; Meyer, S.C.; Janeway, K.A.; Mack, J.W. Patient/parent perspectives on genomic tumor profiling of pediatric solid tumors: The Individualized Cancer Therapy (iCat) experience. Pediatr. Blood Cancer 2016, 63, 1974–1982. [Google Scholar] [CrossRef]
- McCarthy, M.C.; De Abreu Lourenco, R.; McMillan, L.J.; Meshcheriakova, E.; Cao, A.; Gillam, L. Finding Out What Matters in Decision-Making Related to Genomics and Personalized Medicine in Pediatric Oncology: Developing Attributes to Include in a Discrete Choice Experiment. Patient 2020, 13, 347–361. [Google Scholar] [CrossRef]
- McGill, B.C.; Wakefield, C.E.; Hetherington, K.; Munro, L.J.; Warby, M.; Lau, L.; Tyrrell, V.; Ziegler, D.S.; O’brien, T.A.; Marshall, G.M.; et al. “Balancing Expectations with Actual Realities”: Conversations with Clinicians and Scientists in the First Year of a High-Risk Childhood Cancer Precision Medicine Trial. J. Pers. Med. 2020, 10, 9. [Google Scholar] [CrossRef]
- Daly, R.; Hetherington, K.; Wadling, B.R.; Jacobs, C.; Karpelowsky, J.; Wakefield, C.E. It provides families with other avenues for treatment when there are no other options Surgeons’ perspectives of being part of a precision medicine trial for poor prognosis paediatric cancer patients: A short report. Cancer Med. 2024, 13, e7209. [Google Scholar] [CrossRef]
- Cohen, B.; Roth, M.; Marron, J.M.; Gray, S.W.; Geller, D.S.; Hoang, B.; Gorlick, R.; Janeway, K.A.; Gill, J. Pediatric Oncology Provider Views on Performing a Biopsy of Solid Tumors in Children with Relapsed or Refractory Disease for the Purpose of Genomic Profiling. Ann. Surg. Oncol. 2016, 23 (Suppl. S5), 990–997. [Google Scholar] [CrossRef] [PubMed]
- Overman, M.J.; Modak, J.; Kopetz, S.; Murthy, R.; Yao, J.C.; Hicks, M.E.; Abbruzzese, J.L.; Tam, A.L. Use of research biopsies in clinical trials: Are risks and benefits adequately discussed? J. Clin. Oncol. 2013, 31, 17–22. [Google Scholar] [CrossRef]
- Peppercorn, J. Toward improved understanding of the ethical and clinical issues surrounding mandatory research biopsies. Am. Soc. Clin. Oncol. 2013, 31, 1–2. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Vundamati, D.S.; Farooqi, M.S.; Guest, E. Precision Medicine in Pediatric Cancer: Current Applications and Future Prospects. High Throughput 2018, 7, 39. [Google Scholar] [CrossRef]
- Forrest, S.J.; Geoerger, B.; Janeway, K.A. Precision medicine in pediatric oncology. Curr. Opin. Pediatr. 2018, 30, 17–24. [Google Scholar] [CrossRef]
- Hadjadj, D.; Deshmukh, S.; Jabado, N. Entering the era of precision medicine in pediatric oncology. Nat. Med. 2020, 26, 1684–1685. [Google Scholar] [CrossRef] [PubMed]
- Stiller, C.A. Epidemiology and genetics of childhood cancer. Oncogene 2004, 23, 6429–6444. [Google Scholar] [CrossRef] [PubMed]
- Elhussein, A.; Baymuradov, U.; Elhadad, N.; Natarajan, K.; Gürsoy, G. A framework for sharing of clinical and genetic data for precision medicine applications. Nat. Med. 2024, 30, 3578–3589. [Google Scholar] [CrossRef] [PubMed]
- Bonomi, L.; Huang, Y.; Ohno-Machado, L. Privacy challenges and research opportunities for genomic data sharing. Nat. Genet. 2020, 52, 646–654. [Google Scholar] [CrossRef]
- Rahimzadeh, V.; Schickhardt, C.; Knoppers, B.M.; Sénécal, K.; Vears, D.F.; Fernandez, C.V.; Pfister, S.; Plon, S.; Terry, S.; Williams, J.; et al. Key implications of data sharing in pediatric genomics. JAMA Pediatr. 2018, 172, 476–481. [Google Scholar] [CrossRef]
- Ali, J.B.; Holman, R.; Goodwin, A.L.; Heraty, S.; Jones, E.J. Parent attitudes towards data sharing in developmental science. Open Res. Eur. 2024, 3, 182. [Google Scholar] [CrossRef]
- Burstein, M.D.; Robinson, J.O.; Hilsenbeck, S.G.; McGuire, A.L.; Lau, C.C. Pediatric Data Sharing in Genomic Research: Attitudes and Preferences of Parents. Pediatrics 2014, 133, 690–697. [Google Scholar] [CrossRef]
- Oliver, J.M.; Slashinski, M.J.; Wang, T.; Kelly, P.A.; Hilsenbeck, S.G.; McGuire, A.L. Balancing the risks and benefits of genomic data sharing: Genome research participants’ perspectives. Public Health Genom. 2012, 15, 106–114. [Google Scholar] [CrossRef]
- Courbier, S.; Dimond, R.; Bros-Facer, V. Share and protect our health data: An evidence based approach to rare disease patients’ perspectives on data sharing and data protection—Quantitative survey and recommendations. Orphanet J. Rare Dis. 2019, 14, 175. [Google Scholar] [CrossRef]
- Daly, R.; Hetherington, K.; Hazell, E.; Wadling, B.R.; Tyrrell, V.; Tucker, K.M.; Marshall, G.M.; Ziegler, D.S.; Lau, L.M.S.; Trahair, T.N.; et al. Precision medicine is changing the roles of healthcare professionals, scientists, and research staff: Learnings from a childhood cancer precision medicine trial. J. Pers. Med. 2023, 13, 1033. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, A.; Rehmann-Sutter, C.; Bozzaro, C. Patients’ and professionals’ views related to ethical issues in precision medicine: A mixed research synthesis. BMC Med. Ethics 2021, 22, 116. [Google Scholar] [CrossRef]
- Matrana, M.R.; Campbell, B. Precision Medicine and the Institutional Review Board: Ethics and the Genome. Ochsner J. 2020, 20, 98–103. [Google Scholar] [CrossRef]
- Olympios, N.; Collet, L.; Paesmans, M.; Jungels, C.; Kotecki, N.; Awada, A.; Aftimos, P. Analyses of the Rationale and Implementation of Research Biopsies in Oncology Clinical Trials at a Tertiary Cancer Center. Oncology 2021, 26, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.; Mayoh, C.; Lau, L.M.S.; Khuong-Quang, D.-A.; Pinese, M.; Kumar, A.; Barahona, P.; Wilkie, E.E.; Sullivan, P.; Bowen-James, R.; et al. Whole genome, transcriptome and methylome profiling enhances actionable target discovery in high-risk pediatric cancer. Nat. Med. 2020, 26, 1742–1753. [Google Scholar] [CrossRef] [PubMed]
- Mello, M.M.; Lieou, V.; Goodman, S.N. Clinical Trial Participants’ Views of the Risks and Benefits of Data Sharing. N. Engl. J. Med. 2018, 378, 2202–2211. [Google Scholar] [CrossRef]
- Creswell, J.W.; Creswell, J.D. Research Design: Qualitative, Quantitative, and Mixed Methods Approaches; SAGE Publications: Thousand Oaks, CA, USA, 2017. [Google Scholar]
- IBM Corp. Statistical Package for the Social Sciences (SPSS), version 28.0; IBM Corp.: Armonk, NY, USA, 2021. [Google Scholar]
- Braun, V.; Clarke, V. Using thematic analysis in psychology. Qual. Res. Psychol. 2006, 3, 77–101. [Google Scholar] [CrossRef]
- Ltd. QIP. NVivo. 12 ed2018. Lumivero (2023) NVivo (Version 14), Released 2023. Available online: www.lumivero.com (accessed on 1 October 2025).
- Wakefield, C.E.; Hetherington, K.; Robertson, E.G.; Donoghoe, M.W.; Hunter, J.D.; Vetsch, J.; Marron, J.M.; Tucker, K.M.; Marshall, G.M.; Broom, A.; et al. Hopes, concerns, satisfaction and regret in a precision medicine trial for childhood cancer: A mixed-methods study of parent and patient perspectives. Br. J. Cancer 2023, 129, 1634–1644. [Google Scholar] [CrossRef]
- Broes, S.; Verbaanderd, C.; Casteels, M.; Lacombe, D.; Huys, I. Sharing of Clinical Trial Data and Samples: The Cancer Patient Perspective. Front. Med. 2020, 7, 33. [Google Scholar] [CrossRef]
- Franklin, E.F.; Nichols, H.M.; House, L.; Buzaglo, J.; Thiboldeaux, K. Cancer Patient Perspectives on Sharing of Medical Records and Mobile Device Data for Research Purposes. J. Patient Exp. 2020, 7, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Goodman, D.; Johnson, C.O.; Bowen, D.; Smith, M.; Wenzel, L.; Edwards, K. De-identified genomic data sharing: The research participant perspective. J. Community Genet. 2017, 8, 173–181. [Google Scholar] [CrossRef]
- Hutchings, E.; Loomes, M.; Butow, P.; Boyle, F.M. A systematic literature review of health consumer attitudes towards secondary use and sharing of health administrative and clinical trial data: A focus on privacy, trust, and transparency. Syst. Rev. 2020, 9, 235. [Google Scholar] [CrossRef] [PubMed]
- Jamal, L.; Sapp, J.C.; Lewis, K.; Yanes, T.; Facio, F.M.; Biesecker, L.G.; Biesecker, B.B. Research participants’ attitudes towards the confidentiality of genomic sequence information. Eur. J. Hum. Genet. 2014, 22, 964–968. [Google Scholar] [CrossRef]
- Rogith, D.; Yusuf, R.A.; Hovick, S.R.; Peterson, S.K.; Burton-Chase, A.M.; Li, Y.; Meric-Bernstam, F.; Bernstam, E.V. Attitudes regarding privacy of genomic information in personalized cancer therapy. J. Am. Med. Inform. Assoc. 2014, 21, e320–e325. [Google Scholar] [CrossRef]
- Trinidad, M.G.; Platt, J.; Kardia, S.L.R. The public’s comfort with sharing health data with third-party commercial companies. Humanit. Soc. Sci. Commun. 2020, 7, 149. [Google Scholar] [CrossRef] [PubMed]
- Trinidad, S.B.; Fullerton, S.M.; Bares, J.M.; Jarvik, G.P.; Larson, E.B.; Burke, W. Genomic research and wide data sharing: Views of prospective participants. Genet. Med. 2010, 12, 486–495. [Google Scholar] [CrossRef]
- Vidgen, M.E.; Kaladharan, S.; Malacova, E.; Hurst, C.; Waddell, N. Sharing genomic data from clinical testing with researchers: Public survey of expectations of clinical genomic data management in Queensland, Australia. BMC Med. Ethics 2020, 21, 119. [Google Scholar] [CrossRef]
- Commonwealth of Australia. Total Ban on the Use of Adverse Genetic Testing Results in Life Insurance [Press Release]; Commonwealth of Australia: Canberra, Australia, 2024. [Google Scholar]
- Tiller, J.; Lacaze, P.; Otlowski, M. The Australian moratorium on genetics and life insurance: Evaluating policy compared to Parliamentary recommendations regarding genetic discrimination. Public Health Genom. 2022, 32, 3242235. [Google Scholar] [CrossRef]
- Nishiwaki, S.; Ando, Y. Gap between pediatric and adult approvals of molecular targeted drugs. Sci. Rep. 2020, 10, 17145. [Google Scholar] [CrossRef]
- Salzer, E.; Hutter, C. Therapy concepts in the context of precision medicine for pediatric malignancies—Children are not adults. Memo Mag. Eur. Med. Oncol. 2021, 14, 273–277. [Google Scholar] [CrossRef]
- Antommaria, A.H.M.; Brothers, K.B.; Myers, J.A.; Feygin, Y.B.; Aufox, S.A.; Brilliant, M.H.; Conway, P.; Fullerton, S.M.; Garrison, N.A.; Horowitz, C.R.; et al. Parents’ attitudes toward consent and data sharing in biobanks: A multisite experimental survey. AJOB Empir. Bioeth. 2018, 9, 128–142. [Google Scholar] [CrossRef]
- McMurter, B.; Parker, L.; Fraser, R.B.; Magee, J.F.; Kozancyzn, C.; Fernandez, C.V. Parental views on tissue banking in pediatric oncology patients. Pediatr. Blood Cancer 2011, 57, 1217–1221. [Google Scholar] [CrossRef] [PubMed]
- Neidich, A.B.; Joseph, J.W.; Ober, C.; Ross, L.F. Empirical data about women’s attitudes towards a hypothetical pediatric biobank. Am. J. Med. Genet. Part A 2008, 146, 297–304. [Google Scholar] [CrossRef]
- Salvaterra, E.; Locatelli, F.; Strazzer, S.; Borgatti, R.; D”Angelo, G.; Lenzi, L. Paediatric Biobanks: Opinions, Feelings and Attitudes of Parents towards the Specimen Donation of Their Sick Children to a Hypothetical Biobank. Pathobiology 2014, 81, 304–308. [Google Scholar] [CrossRef]
- Mazariego, C.; Daly, R.; McGill, B.; Kelada, L.; McKay, S.; Hetherington, K.; Ziegler, D.S.; Wakefield, C.E.; Taylor, N. Barriers to access of precision guided therapies for children with high-risk cancer. Pediatr. Blood Cancer 2024, 71, e31147. [Google Scholar] [CrossRef]
- Manhas, K.P.; Page, S.; Dodd, S.X.; Letourneau, N.; Ambrose, A.; Cui, X.; Tough, S.C. Parent Perspectives on Privacy and Governance for a Pediatric Repository of Non-Biological, Research Data. J. Empir. Res. Hum. Res. Ethics 2015, 10, 88–99. [Google Scholar] [CrossRef]
- Kilicarslan-Toruner, E.; Akgun-Citak, E. Information-seeking behaviours and decision-making process of parents of children with cancer. Eur. J. Oncol. Nurs. 2013, 17, 176–183. [Google Scholar] [CrossRef] [PubMed]
- McKenna, K.; Collier, J.; Hewitt, M.; Blake, H. Parental involvement in paediatric cancer treatment decisions. Eur. J. Cancer Care 2010, 19, 621–630. [Google Scholar] [CrossRef]
- Vetsch, J.; Wakefield, C.E.; Duve, E.; McGill, B.C.; Warby, M.; Tucker, K.M.; Malkin, D.; Lau, L.; Ziegler, D.S. Parents’, Health Care Professionals’, and Scientists’ Experiences of a Precision Medicine Pilot Trial for Patients With High-Risk Childhood Cancer: A Qualitative Study. JCO Precis. Oncol. 2019, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.E.; Pui, C.-H.; Yang, J.J. The Promise and the Reality of Genomics to Guide Precision Medicine in Pediatric Oncology: The Decade Ahead. Clin. Pharmacol. Ther. 2020, 107, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, D.L.; Min, D.-J.; Davis-Collins, A.; DeBlieux, P. Treating Patients As People: What Do Hospital Patients Want Clinicians to Know About Them As a Person? J. Patient Exp. 2020, 7, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Woodward-Kron, R.; Hughson, J.-A.; Parker, A.; Bresin, A.; Hajek, J.; Knoch, U.; Phan, T.D.; Story, D. Culturally and Linguistically Diverse Populations in Medical Research: Perceptions and Experiences of Older Italians, Their Families, Ethics Administrators and Researchers. J. Public Health Res. 2016, 5, 667. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).