Abstract
Skeletal alterations and their complications can significantly impact the quality of life and overall prognosis of patients living with HIV (PLWHIV). Considering skeletal alterations are often asymptomatic and unapparent during routine clinical evaluation, these conditions are frequently overlooked in the clinical management of PLWHIV. However, since the use of combined antiretroviral therapy (cART) has increased life expectancy in PLWHIV effectively, osteopenia, osteoporosis, and bone fragility are now considered to have a major health impact, with a substantial increase in healthcare costs. This narrative literature review aimed to provide a comprehensive overview of the contemporary literature related to bone changes in PLWHIV, focusing on the importance of taking a multi-scale approach in the assessment of bone hierarchical organization. Even though a low bone mineral density is frequently reported in PLWHIV, numerous ambiguities still remain to be solved. Recent data suggest that assessment of other bone properties (on various levels of the bone structure) could contribute to our understanding of bone fragility determinants in these individuals. Special attention is needed for women living with HIV/AIDS since a postmenopausal status was described as an important factor that contributes to skeletal alterations in this population. Further research on complex etiopathogenetic mechanisms underlying bone alterations in PLWHIV may lead to the development of new therapeutic approaches specifically designed to reduce the health burden associated with skeletal disorders in this population. A major challenge in the clinical management of PLWHIV lies in the adverse skeletal effects of some frequently prescribed cART regimens (e.g., regimens containing tenofovir disoproxil fumarate), which may require a switch to other pharmacological approaches for maintained HIV infection (e.g., regimens containing tenofovir alafenamide). Taken together, the findings are indicative that the HIV/AIDS status should be taken into consideration when designing new guidelines and strategies for individualized prevention, diagnosis, and treatment of increased bone fragility.
1. Introduction
Since the development of combined antiretroviral therapy (cART), there has been a tendency toward an increasing percentage of people living with HIV/AIDS (PLWHIV) over the age of 50, which is accompanied by an increased risk of bone fracture in this population [1,2,3]. Recent meta-analyses reported at least a twofold increase in fragility fracture risk in PLWHIV compared to the general population [2,4]. However, the risk of fragility fracture displays a certain level of site specificity in PLWHIV, with the most prominent susceptibility to vertebral, femoral, and wrist fractures noted in these individuals [2,3,4]. Particularly worrying is the report on a substantial increase in fracture risk among PLWHIV aged between 25 and 54 [5], revealing a fracture risk shift to the younger (working age) population [6]. Also, it is important to note that special considerations are needed for women living with HIV/AIDS (WLWHIV) [7] since the postmenopausal status was described as an important factor that contributes to an increased fracture risk in this population [2,8]. Moreover, the association between certain cART regimens and the fragility fracture risk is intensively debated in the contemporary literature [6,9], warranting further research.
Given that the occurrence of bone fractures and their complications are preventable [10,11], it is important to understand all factors that contribute to increased bone fragility in PLWHIV. Addressing the issue of an increased fracture risk will improve quality of life, promote healthy aging, and reduce healthcare costs for these individuals. Considering the clinical relevance of bone fragility, this article aimed to provide a comprehensive narrative overview of the contemporary literature related to skeletal alterations in PLWHIV, with a particular focus on the importance of a multi-scale approach in assessing the bone hierarchical organization.
2. Literature Search Strategy
An electronic search was conducted using the PubMed/Medline, Cochrane, Web of Science, and National Library of Medicine—National Institutes of Health databases on 3 June 2024. To identify published articles on skeletal alterations in PLWHIV, two authors independently obtained search results using the following search terms: “HIV” OR “AIDS” OR “PLWHIV” OR “WLWHIV” AND “osteopenia” OR “osteoporosis” OR “bone fracture” OR “bone mineral density” OR “bone micro-architecture” OR “bone quality”. Two authors independently reviewed the obtained search results. Only preclinical human studies and clinical studies written in English were considered eligible to be included in this review. Studies on animal models and studies written in other languages were excluded from this review. Discrepancies were resolved through joint discussion, and all authors agreed with the final pool of articles included in this review.
3. Osteodensitometry Findings in PLWHIV
Since it is not always possible to directly assess bone fracture occurrence and bone fracture risk (especially in an individualized manner), modern studies rely on the measurement of various clinical surrogate endpoints of increased bone fragility [12]. The “gold standard” in the clinical assessment of bone fragility is the bone mineral density (BMD) measurement using dual-energy X-ray absorptiometry (DXA) [10]. It is defined as the bone mineral content (BMC) per analyzed bone area (B.Ar). Still, BMD alterations are most commonly expressed as a T score (which refers to the number of standard deviations above or below the mean BMD of a population of healthy female adults at the age of their peak bone mass). Based on the most recent World Health Organization definition, osteoporosis is a systemic skeletal disease characterized by low bone mass and micro-architectural deterioration, causing increased susceptibility to fragility fracture, defined as a T score ≤ −2.5. Osteopenia is diagnosed if T score values are between −1 and −2.5 [10,13]. Alternatively, the Z score refers to the number of standard deviations above or below the mean BMD of a population that is of the same sex and age as the investigated patient [14], meaning that Z scores are preferably used in children, young male patients, premenopausal women, and for diagnosis of secondary osteoporosis. The prevalence of osteoporosis among PLWHIV is reported to be very variable (ranging between 0% and 34%) [13,15]. Still, numerous studies consistently report that a low lumbar spine and hip BMD are present both in men and women living with HIV/AIDS [8,15,16,17,18,19], which was confirmed in recent meta-analyses [4,20,21]. The discrepancy in previous reports about the skeletal alterations in PLWHIV could at least partially be explained by heterogeneity in the study design, number of participants, age, weight, and gender of the participants, and presence of accompanying comorbidities. These cofounding effects point towards the reasoning that the underlying factors causing low BMD in PLWHIV are multifactorial, warranting further clarification in future well-designed, large, prospective clinical studies. The most important obstacle in the clinical management of bone changes in PLWHIV is their asymptomatic nature [22], leaving them unapparent and frequently overlooked during routine clinical evaluations. Thus, it is of most importance to include data about the HIV/AIDS status when developing reliable guidelines for the diagnosis, treatment, and prevention of bone fragility. Based on heterogeneity and potential bias in the previous studies, an individualized approach in the clinical assessment of skeletal alteration in PLWHIV is mandatory to address covariant effects of other comorbidities effectively.
5. The Importance of Multi-Scale Bone Assessment in PLWHIV
Since bone fracture occurrence is primarily dependent on characteristics of the mechanical load (external factors), bone properties (intrinsic factors), and their mutual interaction (Figure 1), it is evident that increased susceptibility to bone fractures cannot be fully explained by alterations in DXA-assessed BMD values. It is known that a considerable number of individuals with bone fractures have physiological BMD values [49], meaning that other bone properties (on various levels of a hierarchical bone organization, Figure 1) should be investigated to fully understand all factors that contribute to increased bone fragility in PLWHIV [50]. Moreover, BMD measurement is of limited informative value in individualized fracture risk assessment [51], highlighting the need for research on other bone fragility determinants in PLWHIV. The importance of applying a hierarchical approach in assessing bone properties is highlighted by the fact that some therapy regimens improved bone strength and reduced the fracture risk without being accompanied by an adequately increasing BMD [52,53,54].
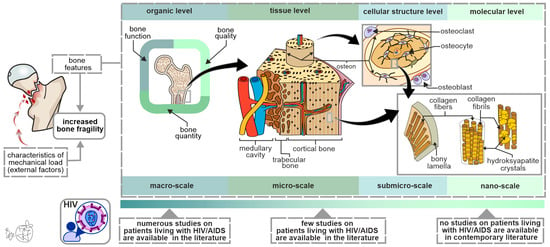
Figure 1.
Bone strength determinants in PLWHIV: the importance of the multi-scale approach in the assessment of bone hierarchical organization. Since low-intensity force (a fall from standing height) is not sufficient to fracture a healthy bone, this is indicative that the main cause of increased bone fragility originates from the structural bone features. It is important to note that the contemporary literature contains limited data about submicro- and nano-scale bone properties in PLWHIV, warranting further research.
Several attempts have been made to overcome the limitations of exclusively relying on DXA-generated BMD in clinical fracture risk assessment, one of which is known as the trabecular bone score (TBS) [55]. This gray-level textural measurement uses high-quality DXA images to indirectly estimate the lumbar micro-architecture (L1–L4). Recent studies revealed that PLWHIV are less likely to have normal TBS values [56]. Moreover, sub-clinical vertebral fracture occurrence was recently reported to be associated with TBS values in HIV-infected patients [57]. Also, loss of lean mass in PLWHIV was associated with lower TBS values, implying its potential as a therapeutic target for improving aging-associated bone strength decline [56]. Still, TBS has a major disadvantage due to its applicability to only one skeletal site and since it is an indirect measure of bone micro-architecture. To overcome these limitations, high-resolution peripheral quantitative computed tomography (HR-pQCT) was developed to allow a noninvasive 3D method for the clinical assessment of the bone micro-architecture at the distal radius and tibia. As shown in Table 2, the contemporary literature suggests that PLWHIV can display a range of micro-architectural alterations, which can contribute to site-specific bone strength decline in these individuals [55,58,59,60,61,62,63,64,65,66]. Namely, the most prominent alterations were noted in the distal tibia compared to the distal radius of PLWHIV. The differences in previous data about bone micro-architectural changes in PLWHIV may have originated from the study design (and associated bias of inclusion and exclusion criteria), the number of participants included in the study, the sex and age of the participants, the use of different cART regimes, and the presence of covariant bone-affecting comorbidities (Table 2). Also, the reliable applicability of HRpQCT is limited by its high costs and by the inability to access other clinically relevant fracture sites (e.g., proximal femora), indicating the need to use other state-of-the-art methodologies to further investigate other bone fragility determinants in PLWHIV [50]. Also, distinguishing the potential differences between trabecular and cortical bones and their contribution to the bone fragility of PLWHIV should be thoroughly pursued in the future using high-resolution imaging approaches [67].
Considering the lack of data in the contemporary literature (Figure 1), future studies should focus on morpho-structural and functional assessment of the osteocyte lacunar network, functional assessment of other bone cells, morpho-structural assessment of mineral and organic components of the bone extracellular matrix, and functional assessment of bone marrow adiposity to elucidate its role in increased bone fragility among PLWHIV. The informative value of these studies could be improved by utilizing multiple state-of-the-art methods to analyze various bone features within the same bone specimen from each individual patient [68]. Finally, by integrating clinical data, the hierarchical approach in bone assessment could lead to the development of patient-specific diagnostic algorithms for predicting bone strength in PLWHIV.

Table 2.
Contemporary studies on bone micro-architectural alterations in PLWHIV.
Table 2.
Contemporary studies on bone micro-architectural alterations in PLWHIV.
| Study (Reference) | Study Design | Number of Patients with HIV | Imaging Method | Assessed Skeletal Site | Main Results on Bone Micro-Architecture |
|---|---|---|---|---|---|
| Serrano S. et al. [58] | Case–control study | n = 22 male, n = 13 female, n = 9 | Optic microscopy | Iliac bone | No significant difference in BVTV, Tb.Th, or Tb.N; significantly reduced osteoid volume and mildly altered osteoblast activity in PLWHIV. |
| Yin M. et al. [59] | Case–control study | n = 46 female, n = 46 on ART, n = 37 | HRpQCT | DR, DT | No significant difference in trabecular or cortical micro-architecture in DR; reduced tibial Ct.Th was noted in postmenopausal WLWHIV; cART did not display a significant effect on bone micro-architecture. |
| Calmy A. et al. [60] | Case–control study | n = 22 female, n = 22 on ART, n = 22 | HRpQCT | DR, DT | No significant difference in radial micro-architecture; low tibial Tb.N and high tibial Tb.Sp were noted in premenopausal WLWHIV; cART did not display a significant effect on bone micro-architecture. |
| Biver E. et al. [61] | Case–control study | n = 28 male, n = 28 on ART, n = 28 | HRpQCT | DR, DT | Significantly low radial Tb.N and Ct.Th, coupled with high radial Tb.Sp, were noted in PLWHIV; reduced tibial Tb.Th was noted in men older than 60 years with long-term HIV infection. |
| Lo Re V. et al. [62] | Case–control study | n = 100 female, n = 100 HCV/HIV, n = 50 | HRpQCT | DT | Low tibial Ct.Th was noted in WLWHIV, while tibial trabecular density and Ct.Th were lower in individuals with HCV/HIV confection. |
| Sellier P et al. [63] | Case–control study | n = 100 male, n = 53 on TDF, n = 53 | HRpQCT | DR, DT | Trabecular micro-architecture deteriorated, while no significant changes were noted in the cortical compartment of PLWHIV treated with TDF. |
| Tan D. et al. [55] | Case–control study | n = 46 male, n = 36 with fracture, n = 23 | HRpQCT | DR, DT | PLWHIV with prior bone fracture had a lower tibial trabecular bone mass and Ct.Th, coupled with a mild trend toward higher radial cortical porosity. |
| Kazakia G. et al. [64] | Case–control study | n = 8 male, n = 8 on ART, n = 8 | MRI HRpQCT | PF, DR, DT | Lower Tb.Th and Tb.N of the femoral head, coupled with lower tibial Tb.N and higher tibial Tb.Sp in PLWHIV, compared to uninfected controls. |
| Foreman S. et al. [65] | Cross-sectional study | n = 43 male, n = 37 on ART, n = 43 | HRpQCT | UDR, UDT | Malnutrition, physical activity, longer duration of HIV infection, and use of the TDF/PI combination were associated with an altered bone micro-architecture in PLWHIV. |
| MacDonald H. et al. [66] | Case–control study | n = 50 female, n = 50 on ART, n = 50 | HRpQCT | DR, DT | Lower radial Tb.N and Tb.Th, coupled with lower tibial Tb.Th, were noted in WLWHIV. Tenofovir treatment may contribute to these bone deficits. |
| Shiau S. et al. [69] | Case–control study | n = 172 boys, n = 86 on ART, n = 172 | pQCT | DR, DT | Reduced trabecular area, Ct.Th, and periosteal cortical circumference were noted in children living with HIV compared to uninfected controls. |
Abbreviations: DR—distal radius; DT—distal tibia; PF—proximal femur; UDR—ultra-distal radius; UDT—ultra-distal tibia; BV/TV—bone volume/tissue volume; Tb.Th—trabecular thickness; Tb.N—trabecular number; Tb.Sp—trabecular separation; Ct.Th—cortical thickness; HRpQCT— high-resolution peripheral quantitative computed tomography; cART—combined antiretroviral therapy; TDF—tenofovir disoproxil fumarate; PI—protease inhibitor; PLWHIV—people living with HIV; WLWHIV—women living with HIV.
6. The Molecular Mechanisms Involved in Etiopathogenesis of Skeletal Alterations in PLWHIV
Etiopathogenetic mechanisms underlying bone alterations in PLWHIV are complex and not fully understood (Figure 2). It is also important to note that bone alterations could be associated with the direct effect of HIV infection per se, with the direct or indirect toxic effect of cART, and with the indirect effect of other well-known confounding bone-affecting factors (e.g., aging, postmenopausal hormonal changes, body weight) [70]. It is known that bone loss in PLWHIV results from the complex interplay between immunological disbalance, cytokine disruptions, nutritional deficiencies, low serum calcium and vitamin D levels, hypogonadism and other hormonal disturbances, liver and kidney dysfunction, and low levels of physical activity/immobilization (Figure 2) [10,13,24,71].
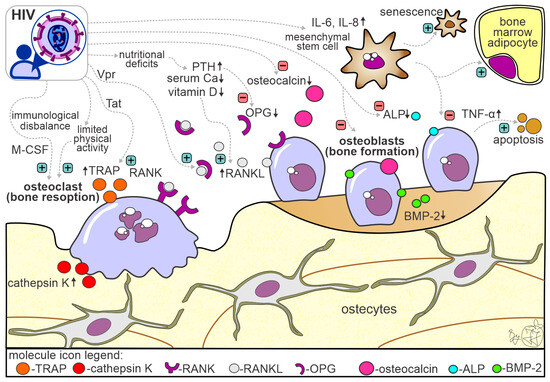
Figure 2.
Schematic representation of molecular mechanisms contributing to bone alterations in patients living with HIV/AIDS. The figure contains multiple factors leading to bone changes in PLWHIV, with an emphasis on the factors that cause reduced bone formation and factors that stimulate bone resorption (the activating effect is marked with a green sign, while the deactivating effect is marked with a red sign; upward arrow symbol indicate higher concentrations, while downward arrow symbol indicate lower concentration of the molecule). Abbreviations: PTH—parathyroid hormone; IL—interleukin; TNF-α—tumor necrosis factor-α; ALP—alkaline phosphatase; OC—osteocalcin; OPG—osteoprotegerin; Vpr—viral protein R; M-CSF—macrophage colony-stimulating factor; Tat—trans-activator of transcription; RANK—receptor activator for nuclear factor-kappa B; RANKL—receptor activator for nuclear factor kappa B ligand; BMP-2—bone morphogenic factor-2.
Initial pioneering studies reached contrary conclusions regarding the possibility that HIV could affect bone cells and display cytopathic effects [72,73]. However, more recent studies revealed that HIV trans-activator of transcription (Tat) and negative regulatory factor (Nef) are associated with reduced osteoblastic differentiation of bone marrow mesenchymal stem cells (Figure 2), causing a predominant differentiation to the adipocyte lineage [71,74]. Also, these HIV proteins are shown to induce senescence of bone marrow mesenchymal stem cells through nuclear factor-κB pathway activation and inhibition of autophagy [74,75]. Furthermore, HIV proteins can trigger in vitro osteoblast apoptosis mediated by the up-regulation of tumor necrosis factor-α (TNF-α), which may be associated with reduced bone formation [71,76,77]. The direct HIV interference with bone formation is heightened by altered calcium deposition and alkaline phosphatase activity and by reduced levels of bone morphogenetic protein-2 (BMP-2) [78,79]. Furthermore, altered osteocalcin and sclerostin levels were suggested to contribute to bone alterations noted in PLWHIV [80,81]. On the other hand, HIV proteins Tat and Vpr increase monocyte differentiation into osteoclasts [82,83], as well as boost bone resorption through increased expression of RANKL and lower expression of osteoprotegerin (OPG) [71,84]. A positive feedback loop exists between RANKL production and HIV replication, which may be relevant to bone loss in PLWHIV (Figure 2). Also, it has been noted that the altered serum RANKL/OPG ratio contributes to skeletal abnormalities in PLWHIV compared to non-infected individuals [85]. Persistent activation of pro-inflammatory cytokines (TNF-α, interleukin-1—IL-1, interleukin-6—IL-6,) has an activation effect predominantly on bone resorption in PLWHIV [86,87]. It is important to note that this hyperinflammatory effect on increased bone resorption is amplified in individuals with liver disease due to HCV coinfection [70,88]. Moreover, components of metabolic syndrome, dyslipidemia, metabolic-associated fatty liver disease, type 2 diabetes mellitus, and insulin resistance have negative effects on bone turnover, contributing to bone loss in individuals living with HIV/AIDS [89,90].
Other factors that may contribute to skeletal alterations in PLWHIV are associated with alcohol abuse, use of opioids, heroin, or other illicit drugs, corticosteroid use, and cART use [13,91,92]. The cART-associated effect on etiopathogenetic mechanisms of bone loss in PLWHIV was extensively elaborated elsewhere [13,79], but it is important to note that various cART regimens could display variable effects on bone health [93]. Recent studies suggested that PI has a predominant effect on an increased rate of bone remodeling [94]. Moreover, TDF-based cART regimens were reported to affect bone turnover through the reduction in extracellular adenosine levels, mediated by the inhibition of ATP release from bone cells, leading to predominant bone resorption [95]. In addition, TDF interferes with the binding of vitamin D with vitamin D-binding protein, reducing its availability for the production of the active form of vitamin D in the kidneys [95]. Lower vitamin D levels result in less calcium and phosphorus being absorbed in the intestines, which could be associated with higher levels of parathyroid hormone and subsequent bone resorption increase [95]. On the other hand, due to differences in pharmacokinetics, plasma concentrations of the active metabolite are lower in TAF-based cART regimens, meaning that TAF has been reported to have a better bone health safety profile [95]. There are a few data that suggest that tenofovir and indinavir have direct or indirect negative effects on osteoblast function (bone formation), while ritonavir has a negative effect on bone resorption via declining osteoclast differentiation [96,97,98], warranting further research.
Since our understanding of the multifactorial etiopathogenetic mechanisms responsible for declining bone health in PLWHIV is based on limited research data, future research should focus on using state-of-the-art methodologies to conduct human bone assessments that will fully resolve the bone fragility puzzle in these individuals. These new insights may lead to the development of new therapeutic modalities that will be specifically designed to mitigate the health burden associated with skeletal disorders in PLWHIV.
7. Conclusions
Skeletal alterations are common in PLWHIV. Numerous studies have contributed to our understanding of bone fragility determinants in PLWHIV, but countless ambiguities persist. More detailed research on bone properties (especially at the submicro- and nano-scale) is required to improve our understanding of bone fragility determinants in PLWHIV. Combined with the available clinical data, taking a hierarchical approach to evaluating structural bone properties could set the basis for developing a patient-specific diagnostic algorithm that will reliably predict the fracture risk in PLWHIV. Additionally, apart from general guidelines for good bone health, specific clinical guidelines for individualized prevention, diagnosis, and treatment of skeletal disorders in PLWHIV should be established and regularly implemented [43,99,100,101], especially in countries with resource-limited clinical settings.
Author Contributions
Conceptualization, J.J. and M.D.; investigation, J.J. and B.O.; writing—original draft preparation, J.J.; writing—review and editing, G.D., B.O., R.L. and M.D.; visualization, J.J.; supervision, G.D. and M.D.; project administration, J.J., B.O. and G.D.; funding acquisition, G.D. and M.D. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received financial support from the Science Fund of the Republic of Serbia [IDEAS program, grant number 7749444, BoFraM group] and the Ministry of Education and Science of the Republic of Serbia [grant numbers 451-03-1524/2023-04/18 and 451-03-66/2024-03/200110].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were generated during this narrative literature review. The obtained literature search results supporting the claims in this narrative review can be made available from the corresponding author upon a justified request.
Conflicts of Interest
The authors declare no conflicts of interest. The funding sources had no role in the study design, data collection, data analysis, data interpretation, manuscript writing, or decision to publish this manuscript.
References
- McGee, D.M.; Cotter, A.G. HIV and Fracture: Risk, Assessment and Intervention. HIV Med. 2024, 25, 511–528. [Google Scholar] [CrossRef]
- Pramukti, I.; Lindayani, L.; Chen, Y.C.; Yeh, C.Y.; Tai, T.W.; Fetzer, S.; Ko, N.Y. Bone Fracture among People Living with HIV: A Systematic Review and Meta-Regression of Prevalence, Incidence, and Risk Factors. PLoS ONE 2020, 15, e0233501. [Google Scholar] [CrossRef]
- Ilha, T.A.S.H.; Comim, F.V.; Copes, R.M.; Compston, J.E.; Premaor, M.O. HIV and Vertebral Fractures: A Systematic Review and Metanalysis. Sci. Rep. 2018, 8, 7838. [Google Scholar] [CrossRef]
- Chang, C.J.; Chan, Y.L.; Pramukti, I.; Ko, N.Y.; Tai, T.W. People with HIV Infection Had Lower Bone Mineral Density and Increased Fracture Risk: A Meta-Analysis. Arch. Osteoporos. 2021, 16, 47. [Google Scholar] [CrossRef]
- Young, B.; Dao, C.N.; Buchacz, K.; Baker, R.; Brooks, J.T. Increased Rates of Bone Fracture among HIV-Infected Persons in the HIV Outpatient Study (HOPS) Compared with the US General Population, 2000-2006. Clin. Infect. Dis. 2011, 52, 1061–1068. [Google Scholar] [CrossRef]
- Battalora, L.; Young, B.; Overton, E. Bones, Fractures, Antiretroviral Therapy and HIV Linda. Curr. Infect. Dis. Rep. 2014, 16, 393. [Google Scholar] [CrossRef]
- Caixas, U.; Tariq, S.; Morello, J.; Dragovic, G.; Lourida, G.; Hachfeld, A.; Nwokolo, N. Comorbidities and Menopause Assessment in Women Living with HIV: A Survey of Healthcare Providers across the WHO European Region. AIDS Care—Psychol. Socio-Med. Asp. AIDS/HIV 2024, 36, 107–114. [Google Scholar] [CrossRef]
- Cortes, Y.; Yin, M.T.; Reame, N.K. Bone Density and Fractures in HIV-Infected Postmenopausal Women: A Systematic Review. J. Assoc. Nurces AIDS Care 2015, 26, 387–398. [Google Scholar] [CrossRef]
- Costagliola, D.; Potard, V.; Lang, S.; Abgrall, S.; Duvivier, C.; Fischer, H.; Joly, V.; Lacombe, J.M.; Valantin, M.A.; Mary-Krause, M.; et al. Impact of Antiretroviral Drugs on Fracture Risk in HIV-Infected Individuals: A Case-Control Study Nested within the French Hospital Database on HIV (FHDH-ANRS CO4). J. Acquir. Immune Defic. Syndr. 2019, 80, 214–223. [Google Scholar] [CrossRef]
- Lima, A.L.L.M.; de Oliveira, P.R.; Plapler, P.G.; Marcolino, F.M.; Sugawara, A.; Gobbi, R.G.; dos Santos, A.L.G.; Camanho, G.L. Osteopenia and Osteoporosis in People Living with HIV: Multiprofessional Approach. HIV/AIDS—Res. Palliat. Care 2011, 1, 117–124. [Google Scholar] [CrossRef]
- Womack, J.A.; Murphy, T.E.; Leo-Summers, L.; Bates, J.; Jarad, S.; Gill, T.M.; Hsieh, E.; Rodriguez-Barradas, M.C.; Tien, P.C.; Yin, M.T.; et al. Assessing the Contributions of Modifiable Risk Factors to Serious Falls and Fragility Fractures among Older Persons Living with HIV. J. Am. Geriatr. Soc. 2023, 71, 1891–1901. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Delmas, P.D. Considerations for Development of Surrogate Endpoints for Antifracture Efficacy of New Treatments in Osteoporosis: A Perspective. J. Bone Miner. Res. 2008, 23, 1155–1167. [Google Scholar] [CrossRef]
- Venhoff, N.; Walker, U.A. Pathogenesis of Bone Disorders in HIV Infection. Int. J. Clin. Rheumtol. 2009, 4, 147–159. [Google Scholar] [CrossRef]
- Bloch, M.; Guaraldi, G. Bone Biomarkers in HIV. In Biomarkers in Bone Disease; Springer: Dordrecht, The Netherland, 2016; Volume 2016, pp. 1–27. [Google Scholar] [CrossRef]
- Cazanave, C.; Dupon, M.; Lavignolle-Aurillac, V.; Barthe, N.; Lawson-Ayayi, S.; Mehsen, N.; Mercie, P.; Morlat, P.; Thiebaut, R.; Dabis, F. Reduced Bone Mineral Density among HIV-Infected Patients in Taiwan: Prevalence and Associated Factors. AIDS 2008, 22, 395–402. [Google Scholar] [CrossRef]
- Bruera, D.; Luna, N.; David, D.O.; Bergoglio, L.M.; Zamudio, J. Decreased Bone Mineral Density in HIV-Infected Patients Is Independent of Antiretroviral Therapy. Aids 2003, 17, 1917–1923. [Google Scholar] [CrossRef]
- Mondy, K.; Yarasheski, K.; Powderly, W.G.; Whyte, M.; Claxton, S.; DeMarco, D.; Hoffmann, M.; Tebas, P. Longitudinal Evolution of Bone Mineral Density and Bone Markers in Human Immunodeficiency Virus-Infected Individuals. Clin. Infect. Dis. 2003, 36, 482–490. [Google Scholar] [CrossRef]
- Hileman, C.O.; Eckard, A.R.; McComsey, G.A. Bone Loss in HIV—A Contemporary Review. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 446–451. [Google Scholar] [CrossRef]
- Ahmed, M.; Mital, D.; Abubaker, N.E.; Panourgia, M.; Owles, H.; Papadaki, I.; Ahmed, M.H. Bone Health in People Living with HIV/AIDS: An Update of Where We Are and Potential Future Strategies. Microorganisms 2023, 11, 789. [Google Scholar] [CrossRef]
- Goh, S.S.L.; Lai, P.S.M.; Tan, A.T.B.; Ponnampalavanar, S. Reduced Bone Mineral Density in Human Immunodeficiency Virus-Infected Individuals: A Meta-Analysis of Its Prevalence and Risk Factors. Osteoporos. Int. 2018, 29, 595–613. [Google Scholar] [CrossRef]
- Starup-linde, J.; Rosendahl, B.; Storgaard, M. Management of Osteoporosis in Patients Living With HIV—A Systematic Review and Meta-Analysis. JAIDS J. Acquir. Immune Defic. Syndr. 2020, 83, 1–8. [Google Scholar] [CrossRef]
- Llop, M.; Sifuentes, W.A.; Bañón, S.; Macia-Villa, C.; Perez-Elías, M.J.; Rosillo, M.; Moreno, S.; Vázquez, M.; Casado, J.L. Increased Prevalence of Asymptomatic Vertebral Fractures in HIV-Infected Patients over 50 Years of Age. Arch. Osteoporos. 2018, 13, 56. [Google Scholar] [CrossRef]
- Brown, T.T.; Qaqish, R.B. Antiretroviral Therapy and the Prevalence of Osteopenia and Osteoporosis: A Meta-Analytic Review. Aids 2006, 20, 2165–2174. [Google Scholar] [CrossRef]
- Schinas, G.; Schinas, I.; Ntampanlis, G.; Polyzou, E.; Gogos, C.; Akinosoglou, K. Bone Disease in HIV: Need for Early Diagnosis and Prevention. Life 2024, 14, 522. [Google Scholar] [CrossRef]
- Moore, A.L.; Vashisht, A.; Sabin, C.A.; Mocroft, A.; Madge, S.; Phillips, A.N.; Studd, J.W.W.; Johnson, M.A. Reduced Bone Mineral Density in HIV-Positive Individuals. Aids 2001, 15, 1731–1733. [Google Scholar] [CrossRef]
- Carr, A.; Miller, J.; Eisman, J.A.; Cooper, D.A. Osteopenia in HIV-Infected Men: Association with Asymptomatic Lactic Acidemia and Lower Weight Pre-Antiretroviral Therapy. Aids 2001, 15, 703–709. [Google Scholar] [CrossRef]
- Knobel, H.; Guelar, A.; Vallecillo, G.; Nogués, X.; Díez, A. Osteopenia in HIV-Infected Patients: Is It the Disease or Is It the Treatment? Aids 2001, 15, 807–808. [Google Scholar] [CrossRef]
- Fernández-Rivera, J.; García, R.; Lozano, F.; Macías, J.; García-García, J.A.; Mira, J.A.; Corzo, J.E.; Gómez-Mateos, J.; Rueda, A.; Sánchez-Burson, J.; et al. Relationship between Low Bone Mineral Density and Highly Active Antiretroviral Therapy Including Protease Inhibitors in HIV-Infected Patients. HIV Clin. Trials 2003, 4, 337–346. [Google Scholar] [CrossRef]
- Amiel, C.; Ostertag, A.; Slama, L.; Baudoin, C.; N’Guyen, T.; Lajeunie, E.; Neit-Ngeilh, L.; Rozenbaum, W.; De Vernejoul, M.C. BMD Is Reduced in HIV-Infected Men Irrespective of Treatment. J. Bone Miner. Res. 2004, 19, 402–409. [Google Scholar] [CrossRef]
- García Aparicio, A.M.; Muñoz Fernández, S.; González, J.; Arribas, J.R.; Peña, J.M.; Vázquez, J.J.; Martínez, M.E.; Coya, J.; Martín Mola, E. Abnormalities in the Bone Mineral Metabolism in HIV-Infected Patients. Clin. Rheumatol. 2006, 25, 537–539. [Google Scholar] [CrossRef]
- Arnsten, J.; Freeman, R.; Howard, A.; Floris-Moore, M.; Lo, Y.; Klein, R. Decreased Bone Mineral Density and Increased Fracture Risk in Aging Men with or at Risk for HIV Infection. AIDS 2007, 21, 617–623. [Google Scholar] [CrossRef]
- Madeddu, G.; Spanu, A.; Solinas, P.; Babudieri, S.; Calia, G.M.; Lovigu, C.; Mannazzu, M.; Nuvoli, S.; Piras, B.; Bagella, P.; et al. Different Impact of NNRTI and PI-Including HAART on Bone Mineral Density Loss in HIV-Infected Patients. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4576–4589. [Google Scholar]
- Stellbrink, H.J.; Orkin, C.; Arribas, J.R.; Compston, J.; Gerstoft, J.; Van Wijngaerden, E.; Lazzarin, A.; Rizzardini, G.; Sprenger, H.G.; Lambert, J.; et al. Comparison of Changes in Bone Density and Turnover with Abacavir-Lamivudine versus Tenofovir-Emtricitabine in HIV-Infected Adults: 48-Week Results from the ASSERT Study. Clin. Infect. Dis. 2010, 51, 963–972. [Google Scholar] [CrossRef]
- McComsey, G.A.; Kitch, D.; Daar, E.S.; Tierney, C.; Jahed, N.C.; Tebas, P.; Myers, L.; Melbourne, K.; Ha, B.; Sax, P.E. Bone Mineral Density and Fractures in Antiretroviral-Naive Persons Randomized to Receive Abacavir-Lamivudine or Tenofovir Disoproxil Fumarate-Emtricitabine along with Efavirenz or Atazanavir-Ritonavir: AIDS Clinical Trials Group A5224s, a Substudy of ACTG. J. Infect. Dis. 2011, 203, 1791–1801. [Google Scholar] [CrossRef]
- Baranek, B.; Wang, S.; Cheung, A.M.; Mishra, S.; Tan, D.H.S. The Effect of Tenofovir Disoproxil Fumarate on Bone Mineral Density: A Systematic Review and Meta-analysis. Antivir. Ther. 2020, 25, 21–32. [Google Scholar] [CrossRef]
- Mills, A.; Arribas, J.R.; Andrade-Villanueva, J.; DiPerri, G.; Van Lunzen, J.; Koenig, E.; Elion, R.; Cavassini, M.; Madruga, J.V.; Brunetta, J.; et al. Switching from Tenofovir Disoproxil Fumarate to Tenofovir Alafenamide in Antiretroviral Regimens for Virologically Suppressed Adults with HIV-1 Infection: A Randomised, Active-Controlled, Multicentre, Open-Label, Phase 3, Non-Inferiority Study. Lancet Infect. Dis. 2016, 16, 43–52. [Google Scholar] [CrossRef]
- Pozniak, A.; Arribas, J.; Gathe, J.; Gupta, S.; Post, F.A.; Bloch, M.; Avihingsanon, A.; Crofoot, G.; Benson, P.; Lichtenstein, K.; et al. Switching to Tenofovir Alafenamide, Coformulated with Elvitegravir, Cobicistat, and Emtricitabine, in HIV-Infected Adults with Renal Impairment: 96-Week Results from a Single-Arm, Multicenter, Open-Label Phase 3 Study. J. Acquir. Immune Defic. Syndr. 2016, 71, 530–537. [Google Scholar] [CrossRef]
- Hill, A.; Hughes, S.L.; Gotham, D.; Pozniak, A.L. Tenofovir Alafenamide versus Tenofovir Disoproxil Fumarate: Is There a True Difference in Efficacy and Safety? J. Virus Erad. 2018, 4, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Walmsley, S.; Clarke, R.; Lee, T.; Singer, J.; Cheung, A.M.; Smaill, F. BEING: Bone Health in Aging Women with HIV: Impact of Switching Antiretroviral Therapy on Bone Mineral Density During the Perimenopausal Period. AIDS Res. Hum. Retroviruses 2023, 39, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Han, W.M.; Wattanachanya, L.; Apornpong, T.; Jantrapakde, J.; Avihingsanon, A.; Kerr, S.J.; Teeratakulpisarn, N.; Jadwattanakul, T.; Chaiwatanarat, T.; Buranasupkajorn, P.; et al. Bone Mineral Density Changes among People Living with HIV Who Have Started with TDF-Containing Regimen: A Five-Year Prospective Study. PLoS ONE 2020, 15, e0230368. [Google Scholar] [CrossRef] [PubMed]
- Casado, J.L.; Santiuste, C.; Vazquez, M.; Bañón, S.; Rosillo, M.; Gomez, A.; Perez-Eĺas, M.J.; Caballero, C.; Rey, J.M.; Moreno, S. Bone Mineral Density Decline According to Renal Tubular Dysfunction and Phosphaturia in Tenofovir-Exposed HIV-Infected Patients. Aids 2016, 30, 1423–1431. [Google Scholar] [CrossRef]
- Mothobi, N.Z.; Masters, J.; Marriott, D.J. Fanconi Syndrome Due to Tenofovir Disoproxil Fumarate Reversed by Switching to Tenofovir Alafenamide Fumarate in an HIV-Infected Patient. Ther. Adv. Infect. Dis. 2018, 5, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Ambrosioni, J.; Levi, L.; Alagaratnam, J.; Van Bremen, K.; Mastrangelo, A.; Waalewijn, H.; Molina, J.M.; Guaraldi, G.; Winston, A.; Boesecke, C.; et al. Major Revision Version 12.0 of the European AIDS Clinical Society Guidelines 2023. HIV Med. 2023, 24, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Soldado-Folgado, J.; Lerma-Chippirraz, E.; Arrieta-Aldea, I.; Bujosa, D.; García-Giralt, N.; Pineda-Moncusi, M.; Trenchs-Rodríguez, M.; Villar-García, J.; González-Mena, A.; Díez-Pérez, A.; et al. Bone Density, Microarchitecture and Tissue Quality after 1 Year of Treatment with Dolutegravir/Abacavir/Lamivudine. J. Antimicrob. Chemother. 2020, 75, 2998–3003. [Google Scholar] [CrossRef] [PubMed]
- Samet, J.H.; Cheng, D.M.; Libman, H.; Nunes, D.P.; Alperen, J.K.; Saitz, R. Alcohol Consumption and HIV Disease Progression. J. Acquir. Immune Defic. Syndr. 2007, 46, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Womack, J.A.; Goulet, J.L.; Gibert, C.; Brandt, C.; Chang, C.C.; Gulanski, B.; Fraenkel, L.; Mattocks, K.; Rimland, D.; Rodriguez-Barradas, M.C.; et al. Increased Risk of Fragility Fractures among HIV Infected Compared to Uninfected Male Veterans. PLoS ONE 2011, 6, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Saitz, R.; Mesic, A.; Ventura, A.S.; Winter, M.R.; Heeren, T.C.; Sullivan, M.M.; Walley, A.Y.; Patts, G.J.; Meli, S.M.; Holick, M.F.; et al. Alcohol Consumption and Bone Mineral Density in People with HIV and Substance Use Disorder: A Prospective Cohort Study. Alcohol. Clin. Exp. Res. 2018, 42, 1518–1529. [Google Scholar] [CrossRef] [PubMed]
- Jadzic, J.; Milovanovic, P.; Cvetkovic, D.; Ivovic, M.; Tomanovic, N.; Bracanovic, M.; Zivkovic, V.; Nikolic, S.; Djuric, M.; Djonic, D. Mechano-Structural Alteration in Proximal Femora of Individuals with Alcoholic Liver Disease: Implications for Increased Bone Fragility. Bone 2021, 150, 116020. [Google Scholar] [CrossRef] [PubMed]
- Binkley, N.; Morin, S.N.; Martineau, P.; Lix, L.M.; Hans, D.; Leslie, W.D. Frequency of Normal Bone Measurement in Postmenopausal Women with Fracture: A Registry-Based Cohort Study. Osteoporos. Int. 2020, 31, 2337–2344. [Google Scholar] [CrossRef] [PubMed]
- Olali, A.Z.; Carpenter, K.A.; Myers, M.; Sharma, A.; Yin, M.T.; Al-Harthi, L.; Ross, R.D. Bone Quality in Relation to HIV and Antiretroviral Drugs. Curr. HIV/AIDS Rep. 2022, 19, 312–327. [Google Scholar] [CrossRef]
- Marshall, D.; Johnell, O.; Wedel, H. Meta-Analysis of How Well Measures of Bone Mineral Density Predict Occurrence of Osteoporotic Fractures. Br. Med. J. 1996, 312, 1254–1259. [Google Scholar] [CrossRef]
- Sarkar, S.; Mitlak, B.H.; Wong, M.; Stock, J.L.; Black, D.M.; Harper, K.D. Relationships between Bone Mineral Density and Incident Vertebral Fracture Risk with Raloxifene Therapy. J. Bone Miner. Res. 2002, 17, 1–10. [Google Scholar] [CrossRef]
- Bjarnason, N.H.; Sarkar, S.; Duong, T.; Mitlak, B.; Delmas, P.D.; Christiansen, C. Six and Twelve Month Changes in Bone Turnover Are Related to Reduction in Vertebral Fracture Risk during 3 Years of Raloxifene Treatment in Postmenopausal Osteoporosis. Osteoporos. Int. 2001, 12, 922–930. [Google Scholar] [CrossRef]
- Schini, M.; Vilaca, T.; Lui, L.-Y.; Ewing, S.K.; Thompson, A.; Vittinghoff, E.; Bauer, D.C.; Bouxsein, M.L.; Black, D.M.; Eastell, R. Pre-Treatment Bone Mineral Density (BMD) and the Benefit of Pharmacologic Treatment on Fracture Risk and BMD Change: Analysis from the FNIH-ASBMR SABRE Project. J. Bone Miner. Res. 2024, zjae068. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.H.S.; Raboud, J.; Szadkowski, L.; Szabo, E.; Hu, H.; Wong, Q.; Cheung, A.M.; Walmsley, S.L. Novel Imaging Modalities for the Comparison of Bone Microarchitecture among HIV+ Patients with and without Fractures: A Pilot Study. HIV Clin. Trials 2017, 18, 28–38. [Google Scholar] [CrossRef]
- Sharma, A.; Ma, Y.; Tien, P.; Scherzer, R.; Anastos, K.; Cohen, M.; Hans, D.; Yin, M. HIV Infection Is Associated with Abnormal Bone Microarchitecture: Measurement of Trabecular Bone Score in the Women’s Interagency HIV Study. J. Acuir Immune Defic. Syndr. 2018, 78, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Ciullini, L.; Pennica, A.; Argento, G.; Novarini, D.; Teti, E.; Pugliese, G.; Aceti, A.; Conti, F.G. Trabecular Bone Score (TBS) Is Associated with Sub-Clinical Vertebral Fractures in HIV-Infected Patients. J. Bone Miner. Metab. 2018, 36, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Serrano, S.; Mariñoso, M.L.; Soriano, J.C.; Rubiés-Prat, J.; Aubia, J.; Coll, J.; Bosch, J.; Del Rio, L.; Vila, J.; Goday, A.; et al. Bone Remodelling in Human Immunodeficiency Virus-1-Infected Patients. A Histomorphometric Study. Bone 1995, 16, 185–191. [Google Scholar] [CrossRef]
- Yin, M.T.; Shu, A.; Zhang, C.A.; Boutroy, S.; McMahon, D.J.; Ferris, D.C.; Colon, I.; Shane, E. Trabecular and Cortical Microarchitecture in Postmenopausal HIV-Infected Women. Calcif. Tissue Int. 2013, 92, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Calmy, A.; Chevalley, T.; Delhumeau, C.; Toutous-Trellu, L.; Spycher-Elbes, R.; Ratib, O.; Zawadynski, S.; Rizzoli, R. Long-Term HIV Infection and Antiretroviral Therapy Are Associated with Bone Microstructure Alterations in Premenopausal Women. Osteoporos. Int. 2013, 24, 1843–1852. [Google Scholar] [CrossRef]
- Biver, E.; Calmy, A.; Delhumeau, C.; Durosier, C.; Zawadynski, S.; Rizzoli, R. Microstructural Alterations of Trabecular and Cortical Bone in Long-Term HIV-Infected Elderly Men on Successful Antiretroviral Therapy. Aids 2014, 28, 2417–2427. [Google Scholar] [CrossRef]
- Lo Re, V.; Lynn, K.; Stumm, E.R.; Long, J.; Nezamzadeh, M.S.; Baker, J.F.; Hoofnagle, A.N.; Kapalko, A.J.; Mounzer, K.; Zemel, B.S.; et al. Structural Bone Deficits in HIV/HCV-Coinfected, HCV-Monoinfected, and HIV-Monoinfected Women. J. Infect. Dis. 2015, 212, 924–933. [Google Scholar] [CrossRef]
- Sellier, P.; Ostertag, A.; Collet, C.; Trout, H.; Champion, K.; Fernandez, S.; Lopes, A.; Morgand, M.; Clevenbergh, P.; Evans, J.; et al. Disrupted Trabecular Bone Micro-Architecture in Middle-Aged Male HIV-Infected Treated Patients. HIV Med. 2016, 17, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Kazakia, G.J.; Carballido-Gamio, J.; Lai, A.; Nardo, L.; Facchetti, L.; Pasco, C.; Zhang, C.A.; Han, M.; Parrott, A.H.; Tien, P.; et al. Trabecular Bone Microstructure Is Impaired in the Proximal Femur of Human Immunodeficiency Virus-Infected Men with Normal Bone Mineral Density. Quant. Imaging Med. Surg. 2018, 8, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Foreman, S.C.; Wu, P.H.; Kuang, R.; John, M.D.; Tien, P.C.; Link, T.M.; Krug, R.; Kazakia, G.J. Factors Associated with Bone Microstructural Alterations Assessed by HR-PQCT in Long-Term HIV-Infected Individuals. Bone 2020, 133, 115210. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, H.M.; Maan, E.J.; Berger, C.; Dunn, R.A.; Côté, H.C.F.; Murray, M.C.M.; Pick, N.; Prior, J.C. Deficits in Bone Strength, Density and Microarchitecture in Women Living with HIV: A Cross-Sectional HR-PQCT Study. Bone 2020, 138, 115509. [Google Scholar] [CrossRef]
- Ito, M.; Nishida, A.; Koga, A.; Ikeda, S.; Shiraishi, A.; Uetani, M.; Hayashi, K.; Nakamura, T. Contribution of Trabecular and Cortical Components to the Mechanical Properties of Bone and Their Regulating Parameters. Bone 2002, 31, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Jadzic, J.; Djuric, M. Structural Basis of Increased Bone Fragility in Aged Individuals: Multi-Scale Perspective. Med. Res. 2024, 57, 67–74. [Google Scholar] [CrossRef]
- Shiau, S.; Yin, M.T.; Strehlau, R.; Burke, M.; Patel, F.; Kuhn, L.; Coovadia, A.; Norris, S.A.; Arpadi, S.M. Deficits in Bone Architecture and Strength in Children Living with HIV on Antiretroviral Therapy. J. Acquir. Immune Defic. Syndr. 2020, 84, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Biver, E. Osteoporosis and HIV Infection. Calcif. Tissue Int. 2022, 110, 624–640. [Google Scholar] [CrossRef]
- Delpino, M.V.; Quarleri, J. Influence of HIV Infection and Antiretroviral Therapy on Bone Homeostasis. Front. Endocrinol. 2020, 11, 502. [Google Scholar] [CrossRef]
- Mellert, W.; Kleinschmidt, A.; Schmidt, J.; Festl, H.; Emler, S.; Roth, W.K.; Erfle, V. Infection of Human Fibroblast and Osteoblast-like Cells with HIV-1. AIDS 1990, 4, 527–536. [Google Scholar] [CrossRef]
- Nacher, M.; Serrano, S.; González, A.; Hernández, A.; Mariñoso, M.L.; Vilella, R.; Hinarejos, P.; Díez, A.; Aubia, J. Osteoblasts in HIV-Infected Patients: HIV-1 Infection and Cell Function. Aids 2001, 15, 2239–2243. [Google Scholar] [CrossRef] [PubMed]
- Beaupere, C.; Garcia, M.; Larghero, J.; Fève, B.; Capeau, J.; Lagathu, C. The HIV Proteins Tat and Nef Promote Human Bone Marrow Mesenchymal Stem Cell Senescence and Alter Osteoblastic Differentiation. Aging Cell 2015, 14, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Cotter, E.J.; Chew, N.; Powderly, W.G.; Doran, P.P. HIV Type 1 Alters Mesenchymal Stem Cell Differentiation Potential and Cell Phenotype Ex Vivo. AIDS Res. Hum. Retroviruses 2011, 27, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Gibellini, D.; De Crigins, E.; Ponti, C.; Cimatti, L.; Borderi, M.; Tschon, M.; Giardino, R.; Re, M.C. HIV-1 Triggers Apoptosis in Primary Osteoblasts and HOBIT Cells Through TNFa Activation. J. Med. Virol. 2008, 80, 1507–1514. [Google Scholar] [CrossRef]
- Cotter, E.J.; Malizia, A.P.; Chew, N.; Powderly, W.G.; Doran, P.P. HIV Proteins Regulate Bone Marker Secretion and Transcription Factor Activity in Cultured Human Osteoblasts with Consequent Potential Implications for Osteoblast Function and Development. AIDS Res. Hum. Retroviruses 2007, 23, 1521–1529. [Google Scholar] [CrossRef]
- Caldwell, R.L.; Gadipatti, R.; Lane, K.B.; Shepherd, V.L. HIV-1 TAT Represses Transcription of the Bone Morphogenic Protein Receptor-2 in U937 Monocytic Cells. J. Leukoc. Biol. 2005, 79, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Borderi, M.; Gibellini, D.; Vescini, F.; De Crignis, E.; Cimatti, L.; Biagetti, C.; Tampellini, L.; Re, M.C. Metabolic Bone Disease in HIV Infection. Aids 2009, 23, 1297–1310. [Google Scholar] [CrossRef] [PubMed]
- Watt, J.; Schuon, J.; Davis, J.; Ferguson, T.F.; Welsh, D.A.; Molina, P.E.; Ronis, M.J.J. Reduced Serum Osteocalcin in High-Risk Alcohol Using People Living With HIV Does Not Correlate With Systemic Oxidative Stress or Inflammation: Data From the New Orleans Alcohol Use in HIV Study. Alcohol. Clin. Exp. Res. 2019, 43, 2374–2383. [Google Scholar] [CrossRef]
- Slama, L.; Reddy, S.; Phair, J.; Palella, F.J.; Brown, T.T.; Margolick, J.B.; Crain, B.; Dobs, A.; Farzadegan, H.; Gallant, J.; et al. Changes in Bone Turnover Markers with HIV Seroconversion and ART Initiation. J. Antimicrob. Chemother. 2017, 72, 1456–1461. [Google Scholar] [CrossRef]
- Gibellini, D.; De Crignis, E.; Ponti, C.; Borderi, M.; Clò, A.; Miserocchi, A.; Viale, P.; Carla Re, M. HIV-1 Tat Protein Enhances RANKL/M-CSF-Mediated Osteoclast Differentiation. Biochem. Biophys. Res. Commun. 2010, 401, 429–434. [Google Scholar] [CrossRef]
- Raynaud-Messina, B.; Bracq, L.; Dupont, M.; Souriant, S.; Usmani, S.M.; Proag, A.; Pingris, K.; Soldan, V.; Thibault, C.; Capilla, F.; et al. Bone Degradation Machinery of Osteoclasts: An HIV-1 Target That Contributes to Bone Loss. Proc. Natl. Acad. Sci. USA 2018, 115, E2556–E2565. [Google Scholar] [CrossRef]
- Fakruddin, J.M.; Laurence, J. HIV Envelope Gp120-Mediated Regulation of Osteoclastogenesis via Receptor Activator of Nuclear Factor ΚB Ligand (RANKL) Secretion and Its Modulation by Certain HIV Protease Inhibitors through Interferon-γ/RANKL Cross-Talk. J. Biol. Chem. 2003, 278, 48251–48258. [Google Scholar] [CrossRef] [PubMed]
- Titanji, K.; Vunnava, A.; Sheth, A.N.; Delille, C.; Lennox, J.L.; Sanford, S.E.; Foster, A.; Knezevic, A.; Easley, K.A.; Weitzmann, M.N.; et al. Dysregulated B Cell Expression of RANKL and OPG Correlates with Loss of Bone Mineral Density in HIV Infection. PLoS Pathog. 2014, 10, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Hileman, C.O.; Labbato, D.E.; Storer, N.J.; Tangpricha, V.; McComsey, G.A. Is Bone Loss Linked to Chronic Inflammation in Antiretroviral-Naive HIV-Infected Adults? A 48-Week Matched Cohort Study. Aids 2014, 28, 1759–1767. [Google Scholar] [CrossRef] [PubMed]
- McGinty, T.; Mirmonsef, P.; Mallon, P.W.G.; Landay, A.L. Does Systemic Inflammation and Immune Activation Contribute to Fracture Risk in HIV? Curr. Opin. HIV AIDS 2016, 11, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Carrero, A.; Berenguer, J.; Hontañón, V.; Guardiola, J.M.; Navarro, J.; von Wichmann, M.A.; Téllez, M.J.; Quereda, C.; Santos, I.; Sanz, J.; et al. Effects of Hepatitis C Virus (HCV) Eradication on Bone Mineral Density in Human Immunodeficiency Virus/ HCV-Coinfected Patients. Clin. Infect. Dis. 2021, 73, E2026–E2033. [Google Scholar] [CrossRef]
- Husain, N.E.; Noor, S.K.; Elmadhoun, W.M.; Almobarak, A.O.; Awadalla, H.; Woodward, C.L.; Mital, D.; Ahmed, M.H. Diabetes, Metabolic Syndrome and Dyslipidemia in People Living with HIV in Africa: Re-Emerging Challenges Not to Be Forgotten. HIV/AIDS—Res. Palliat. Care 2017, 9, 193–202. [Google Scholar] [CrossRef]
- Caeran, G.; De Almeida, L.L.; Ilha, T.A.S.H.; De Carvalho, J.A.M.; Stein, C.; Moresco, R.N.; Haygert, C.J.P.; Comim, F.V.; Premaor, M.O. Insulin Resistance and Its Association with Osteoporosis in People Living with HIV. J. Endocr. Soc. 2022, 6, bvac148. [Google Scholar] [CrossRef]
- Kim, T.W.; Ventura, A.S.; Winter, M.R.; Heeren, T.C.; Holick, M.F.; Walley, A.Y.; Bryant, K.J.; Saitz, R. Alcohol and Bone Turnover Markers among People Living with HIV and Substance Use Disorder. Alcohol. Clin. Exp. Res. 2020, 44, 992–1000. [Google Scholar] [CrossRef]
- Liang, J.; Nosova, E.; Reddon, H.; Nolan, S.; Socías, E.; Barrios, R.; Milloy, M.J. Longitudinal Patterns of Illicit Drug Use, Antiretroviral Therapy Exposure and Plasma HIV-1 RNA Viral Load among HIV-Positive People Who Use Illicit Drugs. Aids 2020, 34, 1389–1396. [Google Scholar] [CrossRef]
- Harris, V.W.; Brown, T.T. Bone Loss in the HIV-Infected Patient: Evidence, Clinical Implications, and Treatment Strategies. J. Infect. Dis. 2012, 205, 391–398. [Google Scholar] [CrossRef]
- Duvivier, C.; Kolta, S.; Assoumou, L.; Ghosn, J.; Rozenberg, S.; Murphy, R.L.; Katlama, C.; Costagliola, D. Greater Decrease in Bone Mineral Density with Protease Inhibitor Regimens Compared with Nonnucleoside Reverse Transcriptase Inhibitor Regimens in HIV-1 Infected Naive Patients. Aids 2009, 23, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Soldado-Folgado, J.; Rins-Lozano, O.; Arrieta-Aldea, I.; Gonzále-Mena, A.; Cañas-Ruano, E.; Knobel, H.; Garcia-Giralt, N.; Güerri-Fernández, R. Changes in Bone Quality after Switching from a TDF to a TAF Based ART: A Pilot Randomized Study. Front. Endocrinol. 2023, 14, 1076739. [Google Scholar] [CrossRef]
- Malizia, A.P.; Cotter, E.; Chew, N.; Powderly, W.G.; Doran, P.P. HIV Protease Inhibitors Selectively Induce Gene Expression Alterations Associated with Reduced Calcium Deposition in Primary Human Osteoblasts. AIDS Res. Hum. Retroviruses 2007, 23, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.W.H.; Wei, S.; Faccio, R.; Takeshita, S.; Tebas, P.; Powderly, W.G.; Teitelbaum, S.L.; Ross, F.P. The HIV Protease Inhibitor Ritonavir Blocks Osteoclastogenesis and Function by Impairing RANKL-Induced Signaling. J. Clin. Investig. 2004, 114, 206–213. [Google Scholar] [CrossRef]
- Karras, A.; Lafaurie, M.; Bourgarit, A.; Droz, D.; Sereni, D.; Legendre, C.; Martinez, F.; Molina, J. Tenofovir-Related Nephrotoxicity in Human Immunodeficiency Virus—Infected Patients: Three Cases of Renal Failure, Fanconi Syndrome, and Nephrogenic Diabetes Insipidus. Clin. Infect. Dis. 2003, 36, 1070–1074. [Google Scholar] [CrossRef]
- McComsey, G.A.; Tebas, P.; Shane, E.; Yin, M.T.; Overton, E.T.; Huang, J.S.; Aldrovandi, G.M.; Cardoso, S.W.; Santana, J.L.; Brown, T.T. Bone Disease in HIV Infection: A Practical Review and Recommendations for HIV Care Providers. Clin. Infect. Dis. 2010, 51, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Aberg, J.A.; Gallant, J.E.; Ghanem, K.G.; Emmanuel, P.; Zingman, B.S.; Horberg, M.A. Primary Care Guidelines for the Management of Persons Infected with HIV: 2013 Update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin. Infect. Dis. 2014, 58, e1–e34. [Google Scholar] [CrossRef]
- Biver, E.; Calmy, A.; Aubry-Rozier, B.; Birkhauser, M.; Bischoff-Ferrari, H.; Ferrari, S.; Frey, D.; Kressig, R.; Lamy, O.; Suhm, N.; et al. Diagnosis, Prevention, and Treatment of Bone Fragility in People Living with HIV: A Position Statement from the Swiss Association against Osteoporosis. Osteoporos. Int. 2019, 30, 1125–1135. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).