Pharmacogenetic Analysis Enables Optimization of Pain Therapy: A Case Report of Ineffective Oxycodone Therapy
Abstract
1. Introduction
2. Materials and Methods
3. Case Presentation
4. Discussion and Pharmaceutical Assessment
4.1. Assessment of Oxycodone
4.2. Assessment of Fentanyl and Morphine
4.3. Assessment of Non-Opioid Analgesics
4.4. Pharmaceutical Recommendation and Outcome
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meucci, R.D.; Fassa, A.G.; Faria, N.M.X. Prevalence of chronic low back pain: Systematic review. Rev. Saude Publica 2015, 49, 1. [Google Scholar] [CrossRef]
- Wu, P.H.; Kim, H.S.; Jang, I.-T. Intervertebral Disc Diseases PART 2: A Review of the Current Diagnostic and Treatment Strategies for Intervertebral Disc Disease. Int. J. Mol. Sci. 2020, 21, 2135. [Google Scholar] [CrossRef]
- Greitemann, B. S2k-Leitlinie zur Versorgung bei Bandscheibenvorfällen mit Radikulärer Symptomatik: Leitlinie zur Konservativen, Operativen und Rehabilitativen Versorgung bei Bandscheibenvorfällen mit Radikulärer Symptomatik. Available online: https://www.awmf.org/uploads/tx_szleitlinien/033-048l_S2k_Konservative-operative_rehabilitative-Versorgung-Bandscheibenvorfall-radikulae_2021-06_01.pdf (accessed on 1 April 2023).
- Schofferman, J.; Mazanec, D. Evidence-informed management of chronic low back pain with opioid analgesics. Spine J. 2008, 8, 185–194. [Google Scholar] [CrossRef]
- Deyo, A.R.; Von Korff, M.; Duhrkoop, D. Opioids for low back pain. BMJ 2015, 350, g6380. [Google Scholar] [CrossRef]
- Gouveia, N.; Rodrigues, A.; Ramiro, S.; Eusébio, M.; Machado, P.M.; Canhão, H.; Branco, J.C. The Use of Analgesic and Other Pain-Relief Drugs to Manage Chronic Low Back Pain: Results from a National Survey. Pain Pract. 2017, 17, 353–365. [Google Scholar] [CrossRef]
- Benjeddou, M.; Peiró, A.M. Pharmacogenomics and prescription opioid use. Pharmacogenomics 2021, 22, 235–245. [Google Scholar] [CrossRef]
- Crews, K.R.; Monte, A.A.; Huddart, R.; Caudle, K.E.; Kharasch, E.D.; Gaedigk, A.; Dunnenberger, H.M.; Leeder, J.S.; Callaghan, J.T.; Samer, C.F.; et al. Clinical Pharmacogenetics Implementation Consortium Guideline for CYP2D6, OPRM1, and COMT Genotypes and Select Opioid Therapy. Clin. Pharmacol. Ther. 2021, 110, 888–896. [Google Scholar] [CrossRef]
- Matic, M.; Nijenhuis, M.; Soree, B.; de Boer-Veger, N.J.; Buunk, A.-M.; Houwink, E.J.F.; Mulder, H.; Rongen, G.A.P.J.M.; van der Weide, J.; Wilffert, B.; et al. Dutch Pharmacogenetics Working Group (DPWG) guideline for the gene–drug interaction between CYP2D6 and opioids (codeine, tramadol and oxycodone). Eur. J. Hum. Genet. 2021, 30, 1105–1113. [Google Scholar] [CrossRef]
- Ko, T.-M.; Wong, C.-S.; Wu, J.-Y.; Chen, Y.-T. Pharmacogenomics for personalized pain medicine. Acta Anaesthesiol. Taiwanica 2016, 54, 24–30. [Google Scholar] [CrossRef]
- McDonnell, J.M.; Rigney, B.; Storme, J.; Ahern, D.P.; Cunniffe, G.; Butler, J.S. Pharmacogenetic profiling and individualised therapy in the treatment of degenerative spinal conditions. Ir. J. Med. Sci. 2022, in press. [Google Scholar] [CrossRef]
- Cairoli, F.R.; Appiani, F.; Sambade, J.M.; Comandé, D.; Arteaga, L.C.; Ciapponi, A. Efficacy and safety of opioid therapy guided by pharmacogenetics: A systematic review. Pharmacogenomics 2021, 22, 573–586. [Google Scholar] [CrossRef]
- Kaya-Akyüzlü, D.; Özkan-Kotiloğlu, S.; Bal, C.; Yalçın-Şahiner, Ş.; Avcıoğlu, G.; Danışman, M. Effects of UGT2B7 rs7662029 and rs7439366 polymorphisms on sublingual buprenorphine metabolism in heroin addicts: An improved PCR-RFLP assay for the detection of rs7662029 polymorphism. Environ. Toxicol. Pharmacol. 2022, 94, 103902. [Google Scholar] [CrossRef]
- PharmGKB. PGx Gene-Specific Information Tables. Available online: https://www.pharmgkb.org/page/pgxGeneRef (accessed on 28 April 2023).
- Ballester, P.; Muriel, J.; Peiró, A.M. CYP2D6 phenotypes and opioiD Metabolism: The panth to personalized analgesiaYYYY). Expert. Opin. Drug Metab. Toxicol. 2022, 18, 261–275. [Google Scholar] [CrossRef]
- Umukoro, N.N.; Aruldhas, B.W.; Rossos, R.; Pawale, D.; Renschler, J.S.; Sadhasivam, S. Pharmacogenomics of oxycodone: A narrative literature review. Pharmacogenomics 2021, 22, 275–290. [Google Scholar] [CrossRef]
- Lalovic, B.; Phillips, B.; Risler, L.L.; Howald, W.; Shen, D.D. Quantitative Contribution of CYP2D6 and CYP3A to oxycodone metabolism in human liver and intestinal microsomes. Drug Metab. Dispos. 2004, 32, 447–454. [Google Scholar] [CrossRef]
- Lalovic, B.; Kharasch, E.; Hoffer, C.; Risler, L.; Liu-Chen, L.; Shen, D.D. Pharmacokinetics and pharmacodynamics of oral oxycodone in healthy human subjects: Role of circulating active metabolites. Clin. Pharmacol. Ther. 2006, 79, 461–479. [Google Scholar] [CrossRef]
- Romand, S.; Spaggiari, D.; Marsousi, N.; Samer, C.; Desmeules, J.; Daali, Y.; Rudaz, S. Characterization of oxycodone in vitro metabolism by human cytochromes P450 and UDP-glucuronosyltransferases. J. Pharm. Biomed. Anal. 2017, 144, 129–137. [Google Scholar] [CrossRef]
- Coffman, B.L.; King, C.D.; Rios, G.R.; Tephly, T.R. The glucuronidation of opioids, other xenobiotics, and androgens by human UGT2B7Y(268) and UGT2B7H(268). Drug Metab. Dispos. 1998, 26, 73–77. [Google Scholar]
- Swissmedic. Product Information—Stocrin. Available online: http://www.swissmedicinfo.ch/ (accessed on 1 April 2023).
- Andreassen, T.N.; Eftedal, I.; Klepstad, P.; Davies, A.; Bjordal, K.; Lundström, S.; Kaasa, S.; Dale, O. Do CYP2D6 genotypes reflect oxycodone requirements for cancer patients treated for cancer pain? A cross-sectional multicentre study. Eur. J. Clin. Pharmacol. 2012, 68, 55–64. [Google Scholar] [CrossRef]
- Zwisler, S.T.; Enggaard, T.P.; Mikkelsen, S.; Brosen, K.; Sindrup, S.H. Impact of the CYP2D6 genotype on post-operative intravenous oxycodone analgesia. Acta Anaesthesiol. Scand. 2010, 54, 232–240. [Google Scholar] [CrossRef]
- Stamer, U.M.; Zhang, L.; Book, M.; Lehmann, L.E.; Stuber, F.; Musshoff, F. CYP2D6 Genotype Dependent Oxycodone Metabolism in Postoperative Patients. PLoS ONE 2013, 8, e60239. [Google Scholar] [CrossRef]
- Zwisler, S.T.; Enggaard, T.P.; Noehr-Jensen, L.; Pedersen, R.S.; Mikkelsen, S.; Nielsen, F.; Brosen, K.; Sindrup, S.H. The Hypoalgesic Effect of Oxycodone in Human Experimental Pain Models in Relation to the CYP2D6 Oxidation Polymorphism. Basic Clin. Pharmacol. Toxicol. 2009, 104, 335–344. [Google Scholar] [CrossRef]
- Samer, C.; Daali, Y.; Wagner, M.; Hopfgartner, G.; Eap, C.; Rebsamen, M.; Rossier, M.; Hochstrasser, D.; Dayer, P.; Desmeules, J. Genetic polymorphisms and drug interactions modulating CYP2D6 and CYP3A activities have a major effect on oxycodone analgesic efficacy and safety. Br. J. Pharmacol. 2010, 160, 919–930. [Google Scholar] [CrossRef]
- Susce, M.T.; Murray-Carmichael, E.; de Leon, J. Response to hydrocodone, codeine and oxycodone in a CYP2D6 poor metabolizer. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2006, 30, 1356–1358. [Google Scholar] [CrossRef]
- Foster, A.; Mobley, E.; Wang, Z. Complicated Pain Management in a CYP450 2D6 Poor Metabolizer. Pain Pract. 2007, 7, 352–356. [Google Scholar] [CrossRef]
- Maddocks, I.; Somogyi, A.; Abbott, F.; Hayball, P.; Parker, D. Attenuation of morphine-induced delirium in palliative care by substitution with infusion of oxycodone. J. Pain Symptom Manag. 1996, 12, 182–189. [Google Scholar] [CrossRef]
- Klimas, R.; Witticke, D.; El Fallah, S.; Mikus, G. Contribution of oxycodone and its metabolites to the overall analgesic effect after oxycodone administration. Expert Opin. Drug Metab. Toxicol. 2013, 9, 517–528. [Google Scholar] [CrossRef]
- Naito, T.; Takashina, Y.; Yamamoto, K.; Tashiro, M.; Ohnishi, K.; Kagawa, Y.; Kawakami, J. CYP3A5*3 Affects Plasma Disposition of Noroxycodone and Dose Escalation in Cancer Patients Receiving Oxycodone. J. Clin. Pharmacol. 2011, 51, 1529–1538. [Google Scholar] [CrossRef]
- Kuehl, P.; Zhang, J.; Lin, Y.; Lamba, J.; Assem, M.; Schuetz, J.; Watkins, P.B.; Daly, A.; Wrighton, S.A.; Hall, S.D.; et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat. Genet. 2001, 27, 383–391. [Google Scholar] [CrossRef]
- Hustert, E.; Haberl, M.; Burk, O.; Wolbold, R.; He, Y.-Q.; Klein, K.; Nuessler, A.C.; Neuhaus, P.; Klattig, J.; Eiselt, R.; et al. The genetic determinants of the CYP3A5 polymorphism. Pharmacogenetics 2001, 11, 773–779. [Google Scholar] [CrossRef]
- Bachmann, F.; Duthaler, U.; zu Schwabedissen, H.E.M.; Puchkov, M.; Huwyler, J.; Haschke, M.; Krähenbühl, S. Metamizole is a Moderate Cytochrome P450 Inducer Via the Constitutive Androstane Receptor and a Weak Inhibitor of CYP1A2. Clin. Pharmacol. Ther. 2021, 109, 1505–1516. [Google Scholar] [CrossRef]
- Burk, O.; Koch, I.; Raucy, J.; Hustert, E.; Eichelbaum, M.; Brockmöller, J.; Zanger, U.M.; Wojnowski, L. The Induction of Cytochrome P450 3A5 (CYP3A5) in the Human Liver and Intestine Is Mediated by the Xenobiotic Sensors Pregnane X Receptor (PXR) and Constitutively Activated Receptor (CAR). J. Biol. Chem. 2004, 279, 38379–38385. [Google Scholar] [CrossRef]
- Sawyer, M.B.; Innocenti, F.; Das, S.; Cheng, C.; Ramírez, J.; Pantle-Fisher, F.H.; Rn, C.W.; Badner, J.; Pei, D.; Boyett, J.M.; et al. A pharmacogenetic study of uridine diphosphate–glucuronosyltransferase 2B7 in patients receiving morphine. Clin. Pharmacol. Ther. 2003, 73, 566–574. [Google Scholar] [CrossRef]
- Trescot, A.M.; Datta, S.; Lee, M.; Hansen, H. Opioid pharmacology. Pain Physician 2008, 11, S133–S153. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Johnson, A.D.; Papp, A.C.; Sadée, W. Allelic Expression Imbalance of Human mu Opioid Receptor (OPRM1) Caused by Variant A118G. J. Biol. Chem. 2005, 280, 32618–32624. [Google Scholar] [CrossRef]
- Choi, S.-W.; Lam, D.M.; Wong, S.S.; Shiu, H.H.; Wang, A.X.; Cheung, C.-W. Effects of Single Nucleotide Polymorphisms on Surgical and Postsurgical Opioid Requirements: A Systematic Review and Meta-Analysis. Clin. J. Pain 2017, 33, 1117–1130. [Google Scholar] [CrossRef]
- Zwisler, S.T.; Enggaard, T.P.; Noehr-Jensen, L.; Mikkelsen, S.; Verstuyft, C.; Becquemont, L.; Sindrup, S.H.; Brosen, K. The antinociceptive effect and adverse drug reactions of oxycodone in human experimental pain in relation to genetic variations in the OPRM1 and ABCB1 genes. Fundam. Clin. Pharmacol. 2009, 24, 517–524. [Google Scholar] [CrossRef]
- Cajanus, K.; Kaunisto, M.A.; Tallgren, M.; Jokela, R.; Kalso, E. How Much Oxycodone Is Needed for Adequate Analgesia After Breast Cancer Surgery: Effect of the OPRM1 118A>G Polymorphism. J. Pain 2014, 15, 1248–1256. [Google Scholar] [CrossRef]
- Olsen, M.B.; Jacobsen, L.M.; Schistad, E.I.; Pedersen, L.M.; Rygh, L.J.; Røe, C.; Gjerstad, J. Pain Intensity the First Year after Lumbar Disc Herniation Is Associated with the A118G Polymorphism in the Opioid Receptor Mu 1 Gene: Evidence of a Sex and Genotype Interaction. J. Neurosci. 2012, 32, 9831–9834. [Google Scholar] [CrossRef]
- Boström, E.; Simonsson, U.S.; Hammarlund-Udenaes, M. Oxycodone Pharmacokinetics and Pharmacodynamics in the Rat in the Presence of the P-Glycoprotein Inhibitor PSC833. J. Pharm. Sci. 2005, 94, 1060–1066. [Google Scholar] [CrossRef]
- Metcalf, M.D.; Rosicky, A.D.; Hassan, H.E.; Eddington, N.D.; Coop, A.; Cunningham, C.W.; Mercer, S.L. Opioids and efflux transporters. Part 4: Influence of N-substitution on P-glycoprotein substrate activity of noroxymorphone analogues. Bioorganic Med. Chem. Lett. 2014, 24, 3592–3595. [Google Scholar] [CrossRef]
- Zwisler, S.T.; Enggaard, T.P.; Mikkelsen, S.; Verstuyft, C.; Becquemont, L.; Sindrup, S.H.; Brosen, K. Lack of Association of OPRM1 and ABCB1 Single-Nucleotide Polymorphisms to Oxycodone Response in Postoperative Pain. J. Clin. Pharmacol. 2012, 52, 234–242. [Google Scholar] [CrossRef]
- Zubieta, J.-K.; Heitzeg, M.M.; Smith, Y.R.; Bueller, J.A.; Xu, K.; Xu, Y.; Koeppe, R.A.; Stohler, C.S.; Goldman, D. COMT val 158 met Genotype Affects µ-Opioid Neurotransmitter Responses to a Pain Stressor. Science 2003, 299, 1240–1243. [Google Scholar] [CrossRef]
- Takashina, Y.; Naito, T.; Mino, Y.; Yagi, T.; Ohnishi, K.; Kawakami, J. Impact of CYP3A5 and ABCB1 Gene Polymorphisms on Fentanyl Pharmacokinetics and Clinical Responses in Cancer Patients Undergoing Conversion to a Transdermal System. Drug Metab. Pharmacokinet. 2012, 27, 414–421. [Google Scholar] [CrossRef]
- Wittwer, E.; Kern, S.E. Role of morphine’s metabolites in analgesia: Concepts and controversies. AAPS J. 2006, 8, E348–E352. [Google Scholar] [CrossRef]
- Ohno, S.; Kawana, K.; Nakajin, S. Contribution of UDP-Glucuronosyltransferase 1A1 and 1A8 to Morphine-6-Glucuronidation and Its Kinetic Properties. Drug Metab. Dispos. 2008, 36, 688–694. [Google Scholar] [CrossRef]
- Chen, Y.; Ning, M.; Tao, Y.; Hu, X.; Guo, L.; Ni, J.; Hu, J.; Shen, H. Roles of UGT2B7 C802T gene polymorphism on the efficacy of morphine treatment on cancer pain among the Chinese han population. Niger. J. Clin. Pract. 2019, 22, 1319–1323. [Google Scholar] [CrossRef]
- Holthe, M.; Klepstad, P.; Zahlsen, K.; Borchgrevink, P.; Hagen, L.; Dale, O.; Kaasa, S.; Krokan, H.; Skorpen, F. Morphine glucuronide-to-morphine plasma ratios are unaffected by the UGT2B7 H268Y and UGT1A1*28 polymorphisms in cancer patients on chronic morphine therapy: Clinical utility and future perspectives. Eur. J. Clin. Pharmacol. 2002, 58, 353–356. [Google Scholar] [CrossRef]
- Lötsch, J.; Skarke, C.; Liefhold, J.; Geisslinger, G. Genetic Predictors of the Clinical Response to Opioid Analgesics. Clin. Pharmacokinet. 2004, 43, 983–1013. [Google Scholar] [CrossRef]
- Hajj, A.; Halepian, L.; El Osta, N.; Chahine, G.; Kattan, J.; Khabbaz, L.R. OPRM1 c.118A>G Polymorphism and Duration of Morphine Treatment Associated with Morphine Doses and Quality-of-Life in Palliative Cancer Pain Settings. Int. J. Mol. Sci. 2017, 18, 669. [Google Scholar] [CrossRef]
- Chou, W.-Y.; Yang, L.-C.; Lu, H.-F.; Ko, J.-Y.; Wang, C.-H.; Lin, S.-H.; Lee, T.-H.; Concejero, A.; Hsu, C.-J. Association of μ-opioid receptor gene polymorphism (A118G) with variations in morphine consumption for analgesia after total knee arthroplasty. Acta Anaesthesiol. Scand. 2006, 50, 787–792. [Google Scholar] [CrossRef]
- Cheng, H.; Chu, X.; Yi, S. The Influence of OPRM1 A118G Polymorphism on the Dosage of Morphine in Patients with Advanced Liver Cancer. J. Coll. Physicians Surg. Pak. 2021, 31, 1375–1377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, L.; Zhao, X.; Shen, S.; Luo, X.; Zhang, Y. Association between MDR1/CYP3A4/OPRM1 gene polymorphisms and the post-caesarean fentanyl analgesic effect on Chinese women. Gene 2018, 661, 78–84. [Google Scholar] [CrossRef]
- Zhang, W.; Yuan, J.J.; Kan, Q.C.; Zhang, L.R.; Chang, Y.Z.; Wang, Z.Y. Study of the OPRM1 A118G genetic polymorphism associated with postoperative nausea and vomiting induced by fentanyl intravenous analgesia. Minerva Anestesiol. 2011, 77, 33–39. [Google Scholar] [PubMed]
- Zhang, W.; Chang, Y.Z.; Kan, Q.C.; Zhang, L.R.; Lu, H.; Chu, Q.J.; Wang, Z.Y.; Li, Z.S.; Zhang, J. Association of human μ-opioid receptor gene polymorphism A118G with fentanyl analgesia consumption in Chinese gynaecological patients. Anaesthesia 2010, 65, 130–135. [Google Scholar] [CrossRef]
- Theken, K.N.; Lee, C.R.; Gong, L.; Caudle, K.E.; Formea, C.M.; Gaedigk, A.; Klein, T.E.; Agúndez, J.A.; Grosser, T. Clinical Pharmacogenetics Implementation Consortium Guideline (CPIC) for CYP2C9 and Nonsteroidal Anti-Inflammatory Drugs. Clin. Pharmacol. Ther. 2020, 108, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Dean, L. Venlafaxine Therapy and CYP2D6 Genotype. In Medical Genetics Summaries; Pratt, V.M., Scott, S.A., Pirmohamed, M., Esquivel, B., Kattman, B.L., Malheiro, A.J., Eds.; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2012. [Google Scholar]
- Breitenstein, B.; Brückl, T.M.; Ising, M.; Müller-Myhsok, B.; Holsboer, F.; Czamara, D. ABCB1 gene variants and antidepressant treatment outcome: A meta-analysis. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2015, 168, 274–283. [Google Scholar] [CrossRef]
- Breitenstein, B.; Scheuer, S.; Pfister, H.; Uhr, M.; Lucae, S.; Holsboer, F.; Ising, M.; Brückl, T.M. The clinical application of ABCB1 genotyping in antidepressant treatment: A pilot study. CNS Spectrums 2014, 19, 165–175. [Google Scholar] [CrossRef]
- Zheng, M.; McErlane, K.M.; Ong, M.C. Hydromorphone metabolites: Isolation and identification from pooled urine samples of a cancer patient. Xenobiotica 2002, 32, 427–439. [Google Scholar] [CrossRef]
- Zheng, M.; McErlane, K.M.; Ong, M.C. Identification and synthesis of norhydromorphone, and determination of antinociceptive activities in the rat formalin test. Life Sci. 2004, 75, 3129–3146. [Google Scholar] [CrossRef]
- Vandenbossche, J.; Richards, H.; Francke, S.; Bergh, A.V.D.; Lu, C.C.; Franc, M.A. The effect of UGT2B7*2 polymorphism on the pharmacokinetics of OROS® hydromorphone in Taiwanese subjects. J. Clin. Pharmacol. 2014, 54, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.; Hagen, N.A. Hydromorphone. J. Pain Symptom Manag. 2005, 29, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.S. The Metabolism of Opioid Agents and the Clinical Impact of Their Active Metabolites. Clin. J. Pain 2011, 27, 824–838. [Google Scholar] [CrossRef] [PubMed]
- Freo, U.; Romualdi, P.; Kress, H.G. Tapentadol for neuropathic pain: A review of clinical studies. J. Pain Res. 2019, 12, 1537–1551. [Google Scholar] [CrossRef] [PubMed]

| Substance | Schedule |
|---|---|
| Oxycodone/Naloxone SR a 20/10 mg | 1-0-1-0 |
| Oxycodone oral Liq b 10 mg/mL | PRN c (max. 7 mg/day) |
| Ibuprofen 600 mg | 1-1-1-0 |
| Metamizol gtt d 0.5 mg/mL | 2-2-2-2 mL |
| Venlafaxine ER e 150 mg | 1-0-0-0 |
| Pantoprazole 40 mg | PRN c (max. 1 tablet/day) |
| various laxatives | different |
| Gene | Variant (Additionally Tested Variants in Gen Locus) | Genotype | Predicted Phenotype (Activity Score) |
|---|---|---|---|
| CYP2C9 | rs1057910 c.1075A > C (in *3) (rs1799853, rs9332131, rs7900194, rs28371685) | A/C | intermediate metabolizer (AS = 1, reduced function) |
| CYP2C8 | (rs10509681, rs11572080, rs1934951) | WT a, *1 | n.d. d (n.d. d) |
| CYP2C19 | (rs4244285, rs4986893, rs12248560, rs28399504) | WT a, *1 | normal metabolizer (n.d. d) |
| CYP2D6 | rs3892097 c.506-1G > A (in *4) rs1065852 c.100C > T (in *4 and *10) (CNV c, rs35742686, rs5030655, rs5030867, rs5030865, rs5030656, rs201377835, rs28371706, rs59421388, rs28371725) | G/A C/T | intermediate metabolizer (AS = 1, reduced function) |
| CYP3A5 | rs776746 c.219-237G > A (in *3) | A/G | intermediate metabolizer (n.d. d) |
| UGT2B7 | rs7439366 c.802C > T (in *2) | C/T | n.d.d (substance specific) |
| OPRM1 | rs1799971 c.118A > G | A/G | n.d. d (substance specific) |
| COMT | rs4680 c.472G > A (rs165599, rs4646316, rs9332377) | A/G | n.d. d (substance specific) |
| ABCB1 | rs2032583 c.2685 + 49T > C rs1045642 c.3435T > C rs2032582 c.2677G > A or c.2677G > T (rs1128503) | T/T C/T G/G | n.d. d (substance specific) |
| Substance | Clinical Effect | Pharmaceutical Assessment | Pharmaceutical Recommendation |
|---|---|---|---|
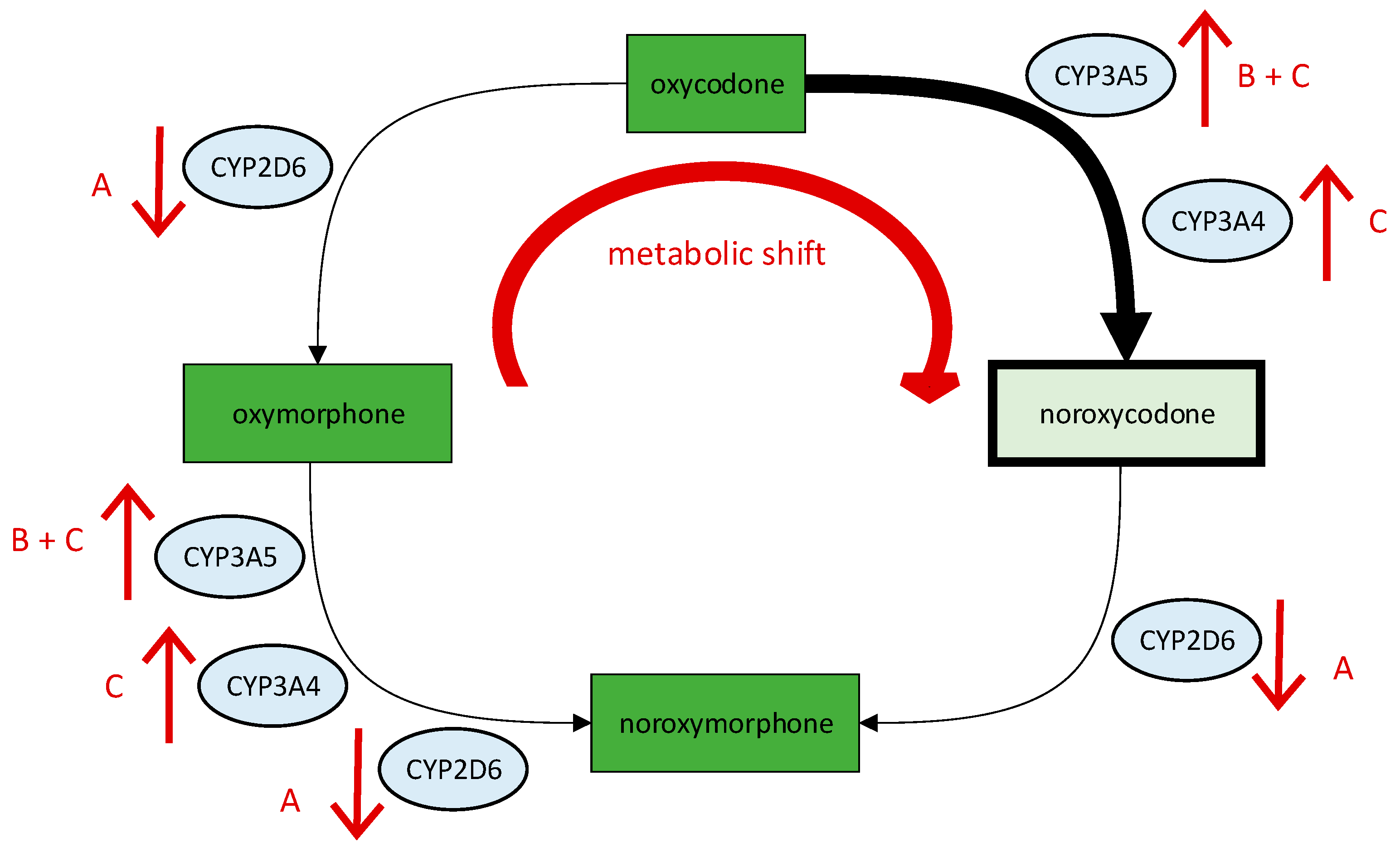
| Oxycodone | insufficient analgesic efficacy | Metabolic shift to inactive noroxycodone due to decreased activity of CYP2D6, increased activity of CYP3A5 and a drug interaction with metamizole. Impaired drug response at the µ-opioid receptor. | Avoid opiates that are bioactivated by CYP2D6 or inactivated by CYP3A5. Switch to hydromorphone or tapentadol. |
| Fentanyl | insufficient analgesic efficacy | Increased inactivation due to increased activity of CYP3A5 and a drug interaction with metamizole. Impaired drug response at the µ-opioid receptor. | |
| Morphine | insufficient analgesic efficacy | Impaired drug response at the µ-opioid receptor. | |
| Ibuprofen | gastrointestinal side effects | Decreased inactivation due to decreased activity of CYP2C9 and thus an increased risk of gastrointestinal side effects. | Avoid NSAIDs a that are inactivated by CYP2C9. Switch to paracetamol. Combine NSAIDs a with a PPI b. |
| Venlafaxine | good antidepressant but insufficient analgesic efficacy | Reduced metabolism to its active metabolite due to decreased activity of CYP2D6. Limited penetration of the blood–brain barrier due to the ABCB1 variant. | Continue venlafaxine therapy. Pregabalin as additional therapy option. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiss, F.M.; Stäuble, C.K.; Meyer zu Schwabedissen, H.E.; Allemann, S.S.; Lampert, M.L. Pharmacogenetic Analysis Enables Optimization of Pain Therapy: A Case Report of Ineffective Oxycodone Therapy. J. Pers. Med. 2023, 13, 829. https://doi.org/10.3390/jpm13050829
Wiss FM, Stäuble CK, Meyer zu Schwabedissen HE, Allemann SS, Lampert ML. Pharmacogenetic Analysis Enables Optimization of Pain Therapy: A Case Report of Ineffective Oxycodone Therapy. Journal of Personalized Medicine. 2023; 13(5):829. https://doi.org/10.3390/jpm13050829
Chicago/Turabian StyleWiss, Florine M., Céline K. Stäuble, Henriette E. Meyer zu Schwabedissen, Samuel S. Allemann, and Markus L. Lampert. 2023. "Pharmacogenetic Analysis Enables Optimization of Pain Therapy: A Case Report of Ineffective Oxycodone Therapy" Journal of Personalized Medicine 13, no. 5: 829. https://doi.org/10.3390/jpm13050829
APA StyleWiss, F. M., Stäuble, C. K., Meyer zu Schwabedissen, H. E., Allemann, S. S., & Lampert, M. L. (2023). Pharmacogenetic Analysis Enables Optimization of Pain Therapy: A Case Report of Ineffective Oxycodone Therapy. Journal of Personalized Medicine, 13(5), 829. https://doi.org/10.3390/jpm13050829








