Abstract
Lithiasis is a known side effect of ceftriaxone administration in children. Sex, age, weight, dosage, and duration of intake have been reported as risk factors for the formation of calcification or stones in the bile and urine excretory systems of children who received ceftriaxone. The purpose of this systematic review is to investigate the reported effects of ceftriaxone administration in pediatric patients who were admitted to a hospital due to infection, the likelihood of gallstones, nephroliths, or precipitations in both the biliary and urinary systems, as well as investigate the relationship with their mother’s history during pregnancy. Original studies and literature reviews from the PubMed database were included in the study. No time limit related to research or publication was set for the articles. The results were evaluated, aiming to understand the outcomes and identify any predisposing factors relevant to this side effect. Of the 181 found articles, 33 were appropriate for inclusion in the systematic review. The administered dose of ceftriaxone presented variability. Symptoms, such as abdominal pain and vomiting, were associated with ceftriaxone-related lithiasis in many cases. It was noted that most of the results were the outcomes of retrospective observation and not of prospective randomized research. Definitively, more randomized control studies with long-term outcomes are needed to identify the exact association between ceftriaxone and lithiasis in children.
1. Introduction
Ceftriaxone, introduced in 1984, is a third-generation cephalosporin that is effective against a broad range of micro-organisms with favorable tissue penetration and is widely used for a variety of serious infections [1]. It is administered parenterally, binds to serum proteins, and has a long plasma half-life, often permitting a single daily dose. It exhibits minimal dose-dependent pharmacokinetics since its free fraction in the serum is concentration-dependent [2]. It is mainly excreted via urine, but a significant amount is secreted (unmetabolized) through the biliary system [3].
Biliary pseudolithiasis is a well-documented side effect of ceftriaxone, first described in 1986 [4]. It occurs in 15–46% of children treated with ceftriaxone, and it is resolved after interruption of administration, justifying the term pseudolithiasis [5,6]. The high calcium-binding affinity of ceftriaxone has been suggested as the main pathogenetic factor of precipitation and formation of calcification and stones in the biliary tracts [7]. Occasionally, ceftriaxone-induced pseudolithiasis may cause severe complications, such as obstruction and infection [8,9]. Therefore, more interventions for the resolution of biliary lithiasis have occasionally been applied, such as the use of ursodeoxycholic acid [10]. The predisposing factors for calcification or gallstone formation have been investigated according to the characteristics of children with biliary pseudolithiasis, such as age, gender, body weight, dose, fasting, and bed rest [6,11]. The method of administration, i.e., bolus injection versus 30 min of drip infusion, and its role in the occurrence of cholelithiasis has also been studied [12]. Prenatal diagnosis of ceftriaxone-associated cholelithiasis has been reported, even in pregnancy [13].
Apart from biliary pseudolithiasis, a strong association between nephrolithiasis and ceftriaxone therapy in children has been reported, with an incidence of 1.4% [14,15]. This type of nephrolithiasis may rarely evolve into acute urinary retention, acute renal injury, and hydronephrosis [16]. Although ceftriaxone-related urolithiasis or calcification in the urinary tract is believed to be rare, the increased use of ultrasonography has led to increased detection rates [17]. Urine pH seems to play a role in the increased urinary concentration of calcium and is related to ceftriaxone-associated calcification and lithiasis [17,18]. Obstruction, infection, genetic polymorphisms, immunodeficiencies, hemolytic anemia, and anomalies of the urogenital tract may favor nephrolithiasis [19,20]. Ceftriaxone-related nephrolithiasis has been associated with alterations to the intestinal microbiome [21]. Finally, duration of therapy is a known risk factor for both biliary pseudolithiasis and nephrolithiasis [22,23].
The aim of this systematic review is to investigate what is known and what is new regarding the association between ceftriaxone and biliary and urinary tract lithiasis in children and to outline possible prevention strategies.
2. Methods
We performed a literature search with the following inclusion criteria for article types: original articles, reviews, meta-analyses, case reports, articles obtained in full text, articles in English, and articles on humans. No time limit was set for articles related to their research or publication.
The database used was PubMed/Medline. The keyword combinations were ceftriaxone, children, and lithiasis (n = 20 articles), ceftriaxone, children, and pseudocholelithiasis (n = 4), ceftriaxone, children, and stones (n = 56), ceftriaxone, children, and urolithiasis (n = 27), ceftriaxone, pediatric patients, and stones (n = 18), ceftriaxone, pediatric patients, and lithiasis (n = 7), ceftriaxone, children, and urolithiasis (n = 27), ceftriaxone, children, and nephrolithiasis (n = 22). In total, 181 articles were extracted. The principles of the preferred reporting items for systematic reviews and meta-analyses (PRISMA) methodology were used for the analysis of the retrieved information [24]. The review has been registered in the international prospective register of systematic reviews.
3. Results
Of the 181 articles, 52 were not included because their title and content did not refer to the development of lithiasis or pseudolithiasis. Of the remaining 129 articles, some reappeared multiple times and, therefore, were removed, resulting in a total of 72. Of the remaining articles, 19 were not written in English and were excluded. Eventually, 53 articles were considered suitable for inclusion in the systematic review; however, 20 of these were eliminated due to their lack of relevant data and were, therefore, rejected (Figure 1). Eventually, 33 studies were included. Of those, 12 were case reports, 11 were prospective studies, nine were retrospective studies, and only one was a randomized controlled trial. Twenty-one studies reported evidence of biliary calcification, biliary pseudolithiasis, or gallstones in patients who had received or were receiving ceftriaxone during the time of the detection of lithiasis. Seven studies regarded lithiasis of the urinary tract, and five studies presented data combining both systems. The outcomes for ceftriaxone administration and the clinical presentation of lithiasis, as well as the clinical and laboratory findings and outcomes, are outlined for biliary lithiasis (Table 1), urinary lithiasis (Table 2), and combined biliary and urinary lithiasis presentation (Table 3).
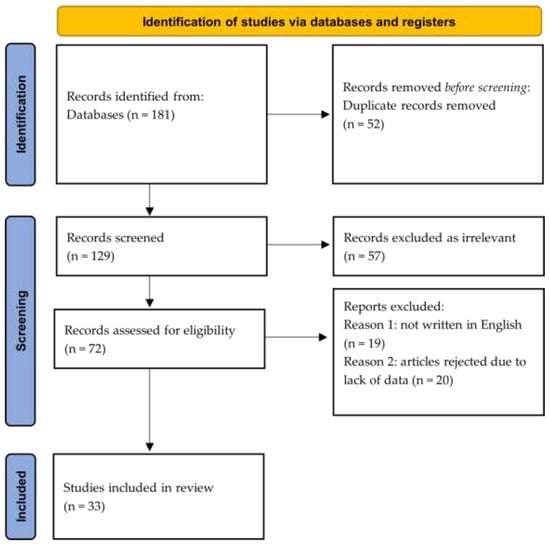
Figure 1.
Flow diagram of literature search and article selection process according to the PRISMA guidelines [24].

Table 1.
Summary of the main findings from the articles included in the study for exclusively biliary ceftriaxone-related complications.

Table 2.
Summary of the main findings from the articles included in the study for exclusively urinary tract ceftriaxone-related complications.

Table 3.
Summary of the main findings from the articles included in the study regarding combined biliary and urinary lithiasis outcomes.
4. Discussion
A systematic review was performed to assess the association between ceftriaxone administration and lithiasis in children. The results of the identified studies were analyzed, aiming to understand the side effects and detect any predisposing factors. In this section, the clinical and laboratory expressions of crystal formation regarding distinct biliary, urinary, and combined lithiasis are discussed in terms of pathophysiology, clinical presentation, diagnosis, and outcomes.
4.1. Biliary Tract Lithiasis
Ceftriaxone is the most commonly associated risk factor for pediatric cholelithiasis. The incidence of ceftriaxone-associated cholelithiasis has been defined as 15–46% [5,27]. In a large retrospective study, it was associated with 20% of all children with crystal formation [20]. The drug was considered a lithiasis risk factor within the infancy age group [20].
Ceftriaxone is excreted unmetabolized into the bile, resulting in a concentration 20–150 times greater than in the serum [7]. The relocation of the anion-charged drug through hepatocytes is mediated by transporting peptides [47]. Furthermore, its high calcium-binding affinity results in the formation of insoluble salts with calcium deposited into the bile. The biochemical process of pH-influenced binding of ceftriaxone with calcium and salt formation [18] is of clinical interest. The aggregation of ceftriaxone-calcium salts into the bile may result in sedimentation and the formation of calcification or even lithiasis.
Palanduz et al. considered age not to be associated with biliary lithiasis [35]. Gender was considered irrelevant with lithiasis as well [26,28,35]. The mean age of the patients reported in the case reports with lithiasis of the biliary tract in this study was 6 years (Table 1).
Lithiasis was associated with excessive daily doses [6,18]. The majority of studies reported a high dose of at least 100 mg/kg/d [11,12,23,28,30,31,34,35,38]. Four studies reported a daily dose of 50–100 mg/kg [18,34,35,36]. Pseudolithiasis occurred even in daily doses under 60 mg/kg/d [35] (Table 1).
The administration period until the diagnosis of lithiasis was under 10 days [25,28,37] and from 1 to 3 weeks [30,35,38]. In one study, it was 50 days [23]. Duration of administration was not associated with pseudolithiasis by all researchers [25]. A finding of interest from the only randomized controlled study was that the combination of an age of over 12 months, a daily dose of more than 2 g, and a duration of therapy longer than 5 days were associated with biliary precipitations [37]. Bacterial meningitis was associated with a higher presentation of gallstones, requiring higher doses and a prolonged treatment period, which reached even 60 days [12] (Table 1).
Abdominal pain, fever, a positive Murphy’s sign, and vomiting frequently occurred between the fourth and seventh day after administration [30,32,33,43]. Abdominal pain was reported to initiate even after the discontinuation of ceftriaxone [38].
Tuna Kirsaclioglu et al. observed that ceftriaxone could lead to stable gallstones and complicating diseases, such as cholecystitis [20]. Fasting (with lithiasis reported in the first week of administration), bed rest (lithiasis reported during the first 5 days), feeding habits, and activity patterns are the factors considered to affect lithiasis [11,36]. A family history of lithiasis has been reported as an important risk factor for the formation of gallstones [36]. Although ceftriaxone is often used with other drugs, it has been exclusively associated with lithiasis [23,26,33] (Table 1).
The detection time of lithiasis using ultrasound is reported to be between 10–20 days [12,25,27]. However, there are studies that report a diagnosis time of less than a week [26,31,32,34,35,37]. Cholelithiasis was detected even after 35 h [18]. Biliary calcification and gallstones were the main findings using ultrasonography [29,48] (Table 1).
Bor et al. determined that ceftriaxone-related cholelithiasis was most likely to dissolve in up to a maximum period of 90 days [27]. Most cases of pseudolithiasis were resolved during the first week after ceftriaxone discontinuation [26,27,28,43,45]. Resolution occurred after up to 3 months as well [6,23]. Lithiasis persisted in one patient for 7 months [12]. It was reported that patients whose gallstones dissolved were significantly younger (mean age: 8 years) compared to other patients with cholelithiasis of different etiology (mean age: 9.5 years) [20].
4.2. Urinary Tract Lithiasis
Nephrolithiasis and crystalline nephropathy are induced by numerous drugs, with 1–2% of all urinary stones occurring due to drug administration. Two pathogenetic mechanisms have been described. The first includes poorly soluble drugs with high urine excretion, which favors crystallization [49]. Antiseptic molecules, such as sulfonamides, the first historically implicated drugs in the formation of renal calculi [50], and ceftriaxone are included in this process, and their molecules have been identified in the crystals. The drugs that activate the formation of urinary calculi as a metabolic effect on urinary pH or the excretion of calcium, phosphate, oxalate, citrate, uric acid, or other purines are included in the second mechanism [51]. In this case, the drug molecules are not contained in the crystals, rendering their identification difficult in terms of causative factors [52].
Kimata et al. demonstrated that ceftriaxone could significantly increase the urinary excretion of calcium in children, inferring that this mechanism may be associated with lithiasis or calcification formation [17]. Transient hypercalciuria and the elevated excretion of oxalic and uric acid have been identified as well [53]. Nevertheless, there are studies on crystal formation with normal urine calcium or other salt levels [22,46] (Table 2 and Table 3).
The formation of urine crystals after ceftriaxone administration is affected by a variety of risk factors, such as urinary stasis, family history, metabolic abnormalities (i.e., hypercalciuria), abnormal urine pH, infection, environmental factors (i.e., high temperature), low urine output, high drug dosage, long treatment, high urinary drug excretion, and concomitant therapies [48,54].
Urinary tract lithiasis presents in a wide age range from infancy to puberty. Age has been considered as a possible associated factor [6,22]. Half of the children with urinary lithiasis included in Table 2 were under the age of 3 years, while four children were younger than one year (Table 2). In their prospective study, Fesharakinia et al. correlated male gender with nephrolithiasis [44]. It is of interest that only one study reported a low dose of 50 mg/kg/d, which was combined with hypercalciuria [40]. Doses of 100 mg/kg/d [19,44] and 50–100 mg/kg/d [17,22] were reported in patients with urolithiasis. In two studies, administration lasted less than 10 days [22,42]. Shen et al. treated patients with acute kidney injuries induced by ceftriaxone, which had been referred to after ceftriaxone had been administered in extremely high doses. In these overdosed patients, cystoscopy and ureteral catheterization were performed. All patients recovered except for one, who underwent surgical excision for the nephrolith [42].
The detection of lithiasis occurred within 10–20 days [18,41]. Kidney stone formation was the most common finding. Urinary bladder calcification subsided when ceftriaxone was discontinued [43]. Smaller calculi were excreted more easily in the first weeks after the discontinuation of administration, while larger stones lasted for months [22]. Although resolution occurred early (until the end of the first week) [29,43], there were cases of resolution that occurred after 7 months [39]. In a study with a follow-up period of 3 years, no recurrent urolithiasis was reported [42].
4.3. Combined Biliary and Urinary Tract Lithiasis
Lithiasis of both excretory systems was reported in patients from 1 month to 18 years in the articles of this study (Table 3). Two prospective studies reported an association with age, particularly in patients that were older than 5 years [6,46]. Fesharakinia et al. reported a presentation of 6.3% urolithiasis and 1% cholelithiasis [44].
Acun et al. attributed the lithiasis of the biliary and urinary bladder calculi to a combination of high dosage (100 kg/d) and low infusion flow (20 min) [43]. High doses of 100 mg/kg/d and patients with bacterial meningitis were considered risk factors [6]. As in the Acun et al. study, in which the administration time was short, in the Biner et al. study, the drug was administered in bolus doses [6,43]. The doses implicated in this group were 100 mg/kg/d [43,45] and 50–100 mg/kg/d [6,44] (Table 3). The duration of ceftriaxone administration lasted for 10 days [6] and from 1 to 3 days [44,46] until lithiasis was diagnosed. There was no association between lithiasis and the season of hospitalization [44].
Ustyol et al. reported behavior that was analogous to the ceftriaxone behavior of cefotaxime in a third prospective study, with 40% of the drug binding to serum proteins and 60% being excreted through the urinary tract [46], inferring a wider third-generation cephalosporin association with lithiasis. However, ceftriaxone-associated lithiasis prevailed over that of cefotaxime.
Lithiasis resolution occurred in all the articles of this study. In the prospective study of Biner et al., in which 17% of patients who received ceftriaxone presented biliary and 0.6% urinary lithiasis, all the patients recovered completely after the discontinuation of the antibiotic [6]. In all patients who co-operated with the follow-up of the research, no crystals were identified after the discontinuation of treatment [44].
4.4. Limitations
This review has certain limitations. Although being the most thoroughly used in medicine, the database that we used does not completely cover all available information. With the inclusion of articles only written in the English language, we may have excluded some interesting articles in other languages. Most of the articles included in the final database were of a retrospective nature or case reports, implying consistency biases in data collection and validation.
5. Conclusions
Although any form of ceftriaxone-related lithiasis consists of one of the known etiologies of pediatric stone formation, the final number of research articles justifying the inclusion criteria was small; most importantly, they included only one randomized controlled study. Thus, the information recorded was a result of observation rather than scientific documentation at a higher level. Definitively, more randomized control studies with long-term outcomes are needed.
It is of note that the clinical presentation and diagnosis of lithiasis are related to the dose and duration of administration. However, more parameters should be questioned as potential risk factors, such as dietary habits, fasting, and bed resting, especially when an antibiotic is mandatory due to severe infectious diseases, such as bacterial meningitis, or longstanding conditions, such as bed rest in hospitals. It is noteworthy that a combination of many factors apart from the dose of the antibiotic may contribute to a lithiasis outcome.
As a clinical implication in the future for the prevention and management of ceftriaxone-induced lithiasis, the clinician should anticipate crystal formation in the biliary and urinary systems when ceftriaxone is administered, especially in high doses with a fast administration rate and for a prolonged time. There should be an intense focus on clinical signs and serum and urine calcium levels, as well as routine liver and urine tests and renal ultrasounds should be performed at the end of the first week from the initiation of ceftriaxone administration. Thus, if crystal formation occurs, the discontinuation of an antibiotic may result in resolution because of a timely reaction.
In order to provide an answer to the title of the study, the drug is not guilty. Under wise usage and thorough consideration of its possible side effects, crystal formation may be anticipated and prevented. It is a valuable tool in the hands of the clinician, who must use it with prudence, taking into consideration the presence of the predisposing lithiasis parameters and taking timely measures for the screening and diagnosis of the side effects.
Author Contributions
Conceptualization, A.L. and X.S.; methodology, A.L., A.V. (Antonios Vezakis), A.P. and X.S., Software, A.L., A.K. (Ageliki Karatza), L.A.P., M.F., F.F.T., A.V. (Athanasios Vlachodimitropoulos) and I.G.; validation, A.L., A.V. (Antonios Vezakis), A.P., D.G., I.S., A.K. (Aimilia Kanellopoulou) and X.S.; formal analysis, A.L., A.K. (Ageliki Karatza), I.G., D.G., I.S., A.K. (Aimilia Kanellopoulou) and X.S.; investigation, A.L., A.K. (Ageliki Karatza), L.A.P., M.F., F.F.T., A.V. (Athanasios Vlachodimitropoulos) and I.G.; resources, A.V. (Antonios Vezakis), A.P. and I.G.; data curation, A.L., A.K. (Ageliki Karatza), L.A.P., F.F.T., A.V. (Athanasios Vlachodimitropoulos), D.G., I.S., A.K. (Aimilia Kanellopoulou) and X.S., writing–original draft preparation, A.L. and X.S.; writing–review and editing, A.L., A.K. (Ageliki Karatza), L.A.P., M.F., F.F.T., A.V. (Athanasios Vlachodimitropoulos), A.V. (Antonios Vezakis), A.P., I.G., D.G., I.S., A.K. (Aimilia Kanellopoulou) and X.S.; visualization, A.L., A.K. (Ageliki Karatza), L.A.P., M.F., F.F.T., A.V. (Athanasios Vlachodimitropoulos), A.V. (Antonios Vezakis), A.P., I.G., D.G., I.S., A.K. (Aimilia Kanellopoulou) and X.S.; supervision, X.S.; project administration, A.L. and X.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. The Institutional Review Board is not applicable considering the literature review nature of the study.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- McCracken, G.H., Jr.; Siegel, J.D.; Threlkeld, N.; Thomas, M. Ceftriaxone pharmacokinetics in newborn infants. Antimicrob. Agents Chemother. 1983, 23, 341–343. [Google Scholar] [CrossRef]
- Patel, I.H.; Chen, S.; Parsonnet, M.; Hackman, M.R.; Brooks, M.A.; Konikoff, J.; Kaplan, S.A. Pharmacokinetics of ceftriaxone in humans. Antimicrob. Agents Chemother. 1981, 20, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Shiffman, M.L.; Keith, F.B.; Moore, E.W. Pathogenesis of ceftriaxone-associated biliary sludge. In vitro studies of calcium-ceftriaxone binding and solubility. Gastroenterology 1990, 99, 1772–1778. [Google Scholar] [CrossRef] [PubMed]
- Herek, O.; Pakdemirli, E.; Koçer, N. Ceftriaxone-associated biliary pseudolithiasis in children. Eur. Radiol. 2001, 11, 902. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Cao, Y.; Fu, J.; Chen, R.; Lu, L.; Tu, Y. Sonographic assessment of ceftriaxone-associated biliary pseudolithiasis in Chinese children. J. Int. Med. Res. 2010, 38, 2004–2010. [Google Scholar] [CrossRef] [PubMed]
- Biner, B.; Oner, N.; Celtik, C.; Bostancioglu, M.; Tuncbilek, N.; Guzel, A.; Karasalihoglu, S. Ceftriaxone-associated biliary pseudolithiasis in children. J. Clin. Ultrasound. 2006, 34, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Cuzzolin, L.; Oggiano, A.M.; Clemente, M.G.; Locci, C.; Antonucci, L.; Antonucci, R. Ceftriaxone-associated biliary pseudolithiasis in children: Do we know enough? Fundam. Clin. Pharmacol. 2021, 35, 40–52. [Google Scholar] [CrossRef]
- Doi, Y.; Takii, Y.; Ito, H.; Jingu, N.; To, K.; Kimura, S.; Kimura, K.; Sanefuji, K.; Ikeda, H.; Tachibana, S.; et al. Usefulness of endoscopic managements in patients with ceftriaxone-induced pseudolithiasis causing biliary obstruction. Case Rep. Med. 2017, 2017, 3835825. [Google Scholar] [CrossRef]
- Kim, S.; Gura, K.M.; Puder, M. Acute necrotizing cholecystitis: A rare complication of ceftriaxone-associated pseudo-lithiasis. Pediatr. Surg. Int. 2006, 22, 562–564. [Google Scholar] [CrossRef]
- Gokce, S.; Yıldırım, M.; Erdogan, D. A retrospective review of children with gallstone: Single-center experience from Central Anatolia. Turk. J. Gastroenterol. 2014, 25, 46–53. [Google Scholar] [CrossRef]
- Murata, S.; Aomatsu, T.; Yoden, A.; Tamai, H. Fasting and bed rest, even for a relatively short period, are risk factors for ceftriaxone-associated pseudolithiasis. Pediatr. Int. 2015, 57, 942–946. [Google Scholar] [CrossRef]
- Dinleyici, E.C.; Bor, O.; Kebapci, M.; Aydogdu, S.D. Ceftriaxone-associated cholelithiasis: 30 min drip infusion versus bolus injection. Pediatr. Int. 2010, 52, 890. [Google Scholar] [CrossRef]
- Troyano-Luque, J.; Padilla-Perez, A.; Martinez-Wallin, I.; Alvarez de la Rosa, M.; Mastrolia, S.A.; Trujillo, J.L.; Perez-Medina, T. Short and long term outcomes associated with fetal cholelithiasis: A report of two cases with antenatal diagnosis and postnatal follow-up. Case Rep. Obstet. Gynecol. 2014, 2014, 714271. [Google Scholar] [CrossRef] [PubMed]
- de Moor, R.A.; Egberts, A.C.; Schröder, C.H. Ceftriaxone-associated nephrolithiasis and biliary pseudolithiasis. Eur. J. Pediatr. 1999, 158, 975–977. [Google Scholar] [CrossRef]
- Tasic, V.; Sofijanova, A.; Avramoski, V. Nephrolithiasis in a child with acute pyelonephritis. Ceftriaxone-induced nephrolithiasis and biliary pseudolithiasis. Pediatr. Nephrol. 2005, 20, 1510–1513. [Google Scholar] [CrossRef] [PubMed]
- Akl, K.F.; Masri, A.T.; Hjazeen, M.M. Acute urine retention induced by ceftriaxone. Saudi J. Kidney Dis. Transplant. 2011, 22, 1226–1228. [Google Scholar]
- Kimata, T.; Kaneko, K.; Takahashi, M.; Hirabayashi, M.; Shimo, T.; Kino, M. Increased urinary calcium excretion caused by ceftriaxone: Possible association with urolithiasis. Pediatr. Nephrol. 2012, 27, 605–609. [Google Scholar] [CrossRef]
- Ito, R.; Yoshida, A.; Taguchi, K.; Enoki, Y.; Yokoyama, Y.; Matsumoto, K. Experimental verification of factors influencing calcium salt formation based on a survey of the development of ceftriaxone-induced gallstone-related disorder. J. Infect. Chemother. 2019, 25, 972–978. [Google Scholar] [CrossRef]
- Stojanovic, V.; Djuric Vijatov, G. Nephrolithiasis caused by ceftriaxone in a 3-year-old child with ureteropelvic junction obstruction. Case Rep. Med. 2009, 2009, 365962. [Google Scholar] [CrossRef] [PubMed]
- Tuna Kirsaclioglu, C.; Çuhacı Çakır, B.; Bayram, G.; Akbıyık, F.; Işık, P.; Tunç, B. Risk factors, complications and out-come of cholelithiasis in children: A retrospective, single-centre review. J. Paediatr. Child Health. 2016, 52, 944–949. [Google Scholar] [CrossRef]
- Joshi, S.; Goldfarb, D.S. The use of antibiotics and risk of kidney stones. Curr. Opin. Nephrol. Hypertens. 2019, 28, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Youssef, D.M.; Sherief, L.M.; Sherbiny, H.S.; El Attar, M.Y.; El Sheikh, A.R.M.; Fawzy, F.M.; Adham, T. Prospective study of nephrolithiasis occurrence in children receiving ceftriaxone. Nephrology 2016, 21, 432–437. [Google Scholar] [CrossRef]
- Du, Y.; Wang, Y.; Xie, J.; Ma, R.; Gao, M.; Xue, J.; Li, X. Ceftriaxone associated biliary pseudolithiasis in a child: A case report and review of the literature. Int. J. Clin. Exp. Med. 2018, 11, 7502–7509. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef]
- Alemayehu, H.; Desai, A.A.; Thomas, P.; Sharp, S.W.; St Peter, S.D. Ceftriaxone-induced pseudolithiasis in children treated for perforated appendicitis. Pediatr. Surg. Int. 2014, 30, 323–326. [Google Scholar] [CrossRef]
- Araz, N.; Okan, V.; Demirci, M.; Araz, M. Pseudolithiasis due to ceftriaxone treatment for meningitis in children: Report of 8 cases. Tohoku J. Exp. Med. 2007, 211, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Bor, O.; Dinleyici, E.C.; Kebapci, M.; Aydogdu, S.D. Ceftriaxone-associated biliary sludge and pseudocholelithiasis during childhood: A prospective study. Pediatr. Int. 2004, 46, 322–324. [Google Scholar] [CrossRef] [PubMed]
- Ceran, C.; Oztoprak, I.; Cankorkmaz, L.; Gumus, C.; Yildiz, T.; Koyluoglu, G. Ceftriaxone-associated biliary pseudolithiasis in paediatric surgical patients. Int. J. Antimicrob. Agents 2005, 25, 256–259. [Google Scholar] [CrossRef]
- Dooki, M.R.; Norouzi, A. Cholelithiasis in childhood: A cohort study in north of Iran. Iran J. Pediatr. 2013, 23, 588–592. [Google Scholar]
- Fretzayas, A.; Liapi, O.; Papadopoulou, A.; Nicolaidou, P.; Stamoulakatou, A. Is Ceftriaxone-induced biliary pseudolithiasis influenced by UDP-Glucuronosyltransferase 1A1 gene polymorphisms? Case Rep. Med. 2011, 2011, 730250. [Google Scholar] [CrossRef]
- Krzemien, G.; Ksiazczyk, T.; Szmigielska, A.; Bombinski, P.; Roszkowska-Blaim, M.; Werner, B.; Brzewski, M. Ceftriaxone-associated acute gallbladder enlargement—An unexpected diagnosis in the child with urinary tract infection. Dev. Period. Med. 2015, 19, 182–185. [Google Scholar]
- Kutuya, N.; Ozaki, Y.; Okazaki, T. A symptomatic child with ceftriaxone-associated biliary pseudolithiasis. J. Med. Ultrason. 2008, 35, 125–128. [Google Scholar] [CrossRef]
- Lemberg, D.; Day, A.S.; Wyeth, B. Biliary colic: Is it gallstones? J. Paediatr. Child Health 2005, 41, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, A.; Kaya, M.; Zeyrek, D.; Ozturk, E.; Kat, N.; Ziylan, S.Z. Ultrasonographic findings in ceftriaxone: Associated biliary sludge and pseudolithiasis in children. Acta Radiol. 2005, 46, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Palanduz, A.; Yalçin, I.; Tonguç, E.; Guler, N.; Ones, U.; Salman, N.; Somer, A. Sonographic assessment of ceftriaxone-associated biliary pseudolithiasis in children. J. Clin. Ultrasound. 2000, 28, 166–168. [Google Scholar] [CrossRef]
- Rozmanic, V.; Banac, S.; Ivosevic, D.; Cace, N. Biliary colic and sonographic evidence of pseudocholelithiasis 36 h after treatment with ceftriaxone. J. Paediatr. Child Health 2006, 42, 658–659. [Google Scholar] [CrossRef]
- Soysal, A.; Eraşov, K.; Akpinar, I.; Bakir, M. Biliary precipitation during ceftriaxone therapy: Frequency and risk factors. Turk. J. Pediatr. 2007, 49, 404–407. [Google Scholar]
- von Martels, J.Z.H.; Van de Meeberg, E.K.; Holman, M.; Ligtenberg, J.J.; Ter Maaten, J.C. Pseudolithiasis after recent use of ceftriaxone: An unexpected diagnosis in a child with abdominal pain. Am. J. Emerg. Med. 2013, 31, 1294.e5–1294.e6. [Google Scholar] [CrossRef] [PubMed]
- Avci, Z.; Koktener, A.; Uras, N.; Catal, F.; Karadag, A.; Tekin, O.; Degirmencioglu, H.; Baskin, E. Nephrolithiasis associated with ceftriaxone therapy: A prospective study in 51 children. Arch. Dis. Child. 2004, 89, 1069–1072. [Google Scholar] [CrossRef]
- Lozanovski, V.J.; Gucev, Z.; Avramoski, V.J.; Kirovski, I.; Makreski, P.; Tasic, V. Ceftriaxone associated urolithiasis in a child with hypercalciuria. Hippokratia 2011, 15, 181–183. [Google Scholar]
- Mohkam, M.; Karimi, A.; Gharib, A.; Daneshmand, H.; Khatami, A.; Ghojevand, N.; Sharifian, M. Ceftriaxone associated nephrolithiasis: A prospective study in 284 children. Pediatr. Nephrol. 2007, 22, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Liu, W.; Fang, X.; Jia, J.; Lin, H.; Xu, M.; Geng, H. Acute kidney injury caused by ceftriaxone-induced urolithiasis in children: A single-institutional experience in diagnosis, treatment, and follow-up. Int. Urol. Nephrol. 2014, 46, 1909–1914. [Google Scholar] [CrossRef] [PubMed]
- Acun, C.; Erdem, L.O.; Sogut, A.; Erdem, C.Z.; Tomac, N.; Gundogdu, S. Ceftriaxone-induced biliary pseudolithiasis, and urinary bladder sludge. Pediatr. Int. 2004, 46, 368–370. [Google Scholar] [CrossRef] [PubMed]
- Fesharakinia, A.; Ehsanbakhsh, A.R.; Ghorashadizadeh, N. Ceftriaxone-associated nephrolithiasis in children. Iran J. Pediatr. 2013, 23, 643–647. [Google Scholar] [PubMed]
- Prince, J.S.; Senac, M.O., Jr. Ceftriaxone-associated nephrolithiasis, and biliary pseudolithiasis in a child. Pediatr. Radiol. 2003, 33, 648–651. [Google Scholar] [CrossRef]
- Ustyol, L.; Bulut, M.D.; Agengin, K.; Bala, K.A.; Yavuz, A.; Bora, A.; Demiroren, K.; Dogan, M. Comparative evaluation of ceftriaxone-and cefotaxime-induced biliary pseudolithiasis or nephrolithiasis: A prospective study in 154 children. Hum. Exp. Toxicol. 2017, 36, 547–553. [Google Scholar] [CrossRef]
- Chandra, P.; Brouwer, K.L. The complexities of hepatic drug transport: Current knowledge and emerging concepts. Pharm. Res. 2004, 21, 719–735. [Google Scholar] [CrossRef]
- Bogue, C.O.; Murphy, A.J.; Gerstle, J.T.; Moineddin, R.; Daneman, A. Risk factors, complications, and outcomes of gallstones in children: A single-center review. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 303–308. [Google Scholar] [CrossRef]
- Daudon, M.; Frochot, V.; Bazin, D.; Paul Jungers, P. Drug-Induced kidney stones and crystalline nephropathy: Pathophysiology, prevention, and treatment. Drugs 2018, 78, 163–201. [Google Scholar] [CrossRef]
- Lehr, D. Clinical toxicity of sulfonamides. Ann. N. Y. Acad. Sci. 1957, 69, 417–447. [Google Scholar] [CrossRef]
- Daudon, M.; Jungers, P. Drug-induced renal calculi: Epidemiology, prevention, and management. Drugs 2004, 64, 245–275. [Google Scholar] [CrossRef]
- Matlaga, B.R.; Shah, O.D.; Assimos, D.G. Drug-induced urinary calculi. Rev. Urol. 2003, 5, 227–231. [Google Scholar] [PubMed]
- Karliczek, S.B.; Döring, S.; Vogt, S.; Beintker, M.; Berg, W.; Misselwitz, J. Ceftriaxone-associated nephrolithiasis: Two case reports. Monatsschr. Kinderheilkd. 1996, 144, 702–706. [Google Scholar]
- Rapado, A.; Traba, M.L.; Caycho, C.; Cifuentes-Delatte, L. Drug-induced renal stones: Incidence, clinical expression and stone analysis. Contrib. Nephrol. 1987, 58, 25–29. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).