Multiple Metastases of Parathyroid and Papillary Thyroid Carcinoma in a Female Patient Treated with Long-Term Hemodialysis
Abstract
1. Introduction
2. Material and Methods
2.1. Patients and Sample Collection
2.2. Imaging and Intraoperation Navigation Methods
- -
- Single-channel gamma probe Gamma Finder2. The navigational activity of 99mTc-MIBI (150–300 MBq) is administered intravenously 60–90 min before surgery. The detection parathyroid tissue was performed, focusing on the data of preoperative topical diagnostics (ultrasound, CT, planar scintigraphy, SPECT/CT). The gamma probe is used for differential diagnosis of the neck tumors and confirmation of the complete tumor removal.
- -
- Fluorescent angiography system SPY 3000, Novodaq. Intraoperatively, 3–4 mL of the vial with ICG was administered to the patient intravenously, followed by the injection of 10 mL 0.9% NaCl 30–60. ICG appears directly in the tissues; the detecting device must be kept at a distance of 5–20 cm from the area of interest.
2.3. Morphological Examination and Immunohistochemical (IHC) Staining
2.4. Gene Sequencing
3. Case Report
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hundahl, S.A.; Fleming, I.D.; Fremgen, A.M.; Menck, H.R. Two hundred eighty-six cases of parathyroid carcinoma treated in the U.S. between 1985-1995: A National Cancer Data Base Report. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer 1999, 86, 538–544. [Google Scholar] [CrossRef]
- Lee, P.K.; Jarosek, S.L.; Virnig, B.A.; Evasovich, M.; Tuttle, T.M. Trends in the incidence and treatment of parathyroid cancer in the United States. Cancer 2007, 109, 1736–1741. [Google Scholar] [CrossRef]
- Wei, C.H.; Harari, A. Parathyroid carcinoma: Update and guidelines for management. Curr. Treat. Options Oncol. 2012, 13, 11–23. [Google Scholar] [CrossRef]
- Mokrysheva, N.; Mirnaya, S.; Dobreva, E.; Maganeva, I.; Kovaleva, E.V.; Krupinova, J.A.; Kryukova, I.V.; Tevosyan, L.K.; Lukyanov, S.V.; Markina, N.V.; et al. Primary hyperparathyroidism in Russia according to the registry. Probl. Endocrinol. 2019, 65, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Villar-del-Moral, J.; Jiménez-García, A.; Salvador-Egea, P.; Martos-Martínez, J.M.; Nuño-Vázquez-Garza, J.M.; Serradilla-Martín, M.; Gómez-Palacios, A.; Moreno-Llorente, P.; Ortega-Serrano, J.; de la Quintana-Basarrate, A. Prognostic factors and staging systems in parathyroid cancer: A multicenter cohort study. Surgery 2014, 156, 1132–1144. [Google Scholar] [CrossRef]
- Talat, N.; Schulte, K.-M. Clinical presentation, staging and long-term evolution of parathyroid cancer. Ann. Surg. Oncol. 2010, 17, 2156–2174. [Google Scholar] [CrossRef]
- Hundley, J.C.; Albertson, D.A.; Bradley, R.F.; Levine, E.A. Resection of Pulmonary Metastasis From Parathyroid Carcinoma. Am. Surg. 2009, 69, 779–783. [Google Scholar] [CrossRef]
- Bollerslev, J.; Schalin-Jäntti, C.; Rejnmark, L.; Siggelkow, H.; Morreau, H.; Thakker, R.; Sitges-Serra, A.; Cetani, F.; Marcocci, C.; Guistina, A.; et al. Unmet therapeutic, educational and scientific needs in parathyroid disorders: Consensus Statement from the first European Society of Endocrinology Workshop (PARAT). Eur. J. Endocrinol. 2019, 181, P1–P19. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.-T.; Sippel, R.S.; Chen, H.; Schneider, D.F. Is central lymph node dissection necessary for parathyroid carcinoma? Surgery 2014, 156, 1336–1341. [Google Scholar] [CrossRef] [PubMed]
- Machado, N.N.; Wilhelm, S.M. Parathyroid Cancer: A Review. Cancers 2019, 11, 1676. [Google Scholar] [CrossRef]
- Di Meo, G.; Sgaramella, L.I.; Ferraro, V.; Prete, F.P.; Gurrado, A.; Testini, M. Parathyroid carcinoma in multiple endocrine neoplasm type 1 syndrome: Case report and systematic literature review. Clin. Exp. Med. 2018, 18, 585–593. [Google Scholar] [CrossRef]
- Sharretts, J.M.; Kebebew, E.; Simonds, W.F. Parathyroid cancer. Semin. Oncol. 2010, 37, 580–590. [Google Scholar] [CrossRef]
- Ullah, A.; Khan, J.; Waheed, A.; Sharma, N.; Pryor, E.K.; Stumpe, T.R.; Velasquez Zarate, L.; Cason, F.D.; Kumar, S.; Misra, S.; et al. Parathyroid Carcinoma: Incidence, Survival Analysis, and Management: A Study from the SEER Database and Insights into Future Therapeutic Perspectives. Cancers 2022, 14, 1426. [Google Scholar] [CrossRef]
- Asare, E.A.; Sturgeon, C.; Winchester, D.J.; Liu, L.; Palis, B.; Perrier, N.D.; Evans, D.B.; Wang, T.S. Parathyroid Carcinoma: An Update on Treatment Outcomes and Prognostic Factors from the National Cancer Data Base (NCDB). Ann. Surg. Oncol. 2015, 22, 3990–3995. [Google Scholar] [CrossRef]
- Lo, W.M.; Good, M.L.; Nilubol, N.; Perrier, N.D.; Patel, D.T. Tumor Size and Presence of Metastatic Disease at Diagnosis are Associated with Disease-Specific Survival in Parathyroid Carcinoma. Ann. Surg. Oncol. 2018, 25, 2535–2540. [Google Scholar] [CrossRef]
- Shane, E. Parathyroid carcinoma. J. Clin. Endocrinol. Metab. 2001, 86, 485–493. [Google Scholar] [CrossRef]
- Flye, M.W.; Brennan, M.F. Surgical resection of metastatic parathyroid carcinoma. Ann. Surg. 1981, 193, 425–435. [Google Scholar] [CrossRef]
- Sandelin, K.; Tullgren, O.; Farnebo, L.O. Clinical course of metastatic parathyroid cancer. World J. Surg. 1994, 18, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Obara, T.; Okamoto, T.; Ito, Y.; Yamashita, T.; Kawano, M.; Nishi, T.; Tani, M.; Sato, K.; Demura, H.; Fujimoto, Y. Surgical and medical management of patients with pulmonary metastasis from parathyroid carcinoma. Surgery 1993, 114, 1040–1048. [Google Scholar] [PubMed]
- Harari, A.; Waring, A.; Fernandez-Ranvier, G.; Hwang, J.; Suh, I.; Nishi, T.; Tani, M.; Sato, K.; Demura, H.; Fujimoto, Y. Parathyroid Carcinoma: A 43-Year Outcome and Survival Analysis. J. Clin. Endocrinol. Metab. 2011, 96, 3679–3686. [Google Scholar] [CrossRef] [PubMed]
- Akirov, A.; Asa, S.L.; Larouche, V.; Mete, O.; Sawka, A.M.; Jang, R.; Ezzat, S. The Clinicopathological Spectrum of Parathyroid Carcinoma. Front. Endocrinol. 2019, 10, 731. [Google Scholar] [CrossRef]
- Beus, K.S.; Stack, B.C. Synchronous thyroid pathology in patients presenting with primary hyperparathyroidism. Am. J. Otolaryngol. 2004, 25, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb, M.; O’Neal, P.; Shih, J.L.; Hartzband, P.; Connolly, J.; Hasselgren, P.-O. Synchronous parathyroid carcinoma, parathyroid adenoma, and papillary thyroid carcinoma in a patient with severe and long-standing hyperparathyroidism. Endocr. Pract. 2009, 15, 463–468. [Google Scholar] [CrossRef]
- Edafe, O.; Debono, M.; Tahir, F. Balasubramanian SP Simultaneous presentation of parathyroid carcinoma, papillary thyroid cancer and ACTH-independent hypercortisolism due to benign cortical adenoma. BMJ Case Rep. 2019, 12, e230438. [Google Scholar] [CrossRef]
- Song, C.; Wang, J.; Cai, X.; Gao, L. Synchronous parathyroid carcinoma and papillary thyroid carcinoma: A case study and review of literature. Int. J. Clin. Exp. Pathol. 2016, 9, 302–309. [Google Scholar]
- Seib, C.D.; Sosa, J.A. Evolving Understanding of the Epidemiology of Thyroid Cancer. Endocrinol. Metab. Clin. N. Am. 2019, 48, 23–35. [Google Scholar] [CrossRef]
- Ruiz, E.M.L.; Niu, T.; Zerfaoui, M.; Kunnimalaiyaan, M.; Friedlander, P.L.; Abdel-Mageed, A.B.; Kandil, E. A novel gene panel for prediction of lymph-node metastasis and recurrence in patients with thyroid cancer. Surgery 2020, 167, 73–79. [Google Scholar] [CrossRef]
- Rodrigo, J.P.; Hernandez-Prera, J.C.; Randolph, G.W.; Zafereo, M.E.; Hartl, D.M.; Silver, C.E.; Suárez, C.; Owen, R.P.; Bradford, C.R.; Mäkitie, A.A.; et al. Parathyroid cancer: An update. Cancer Treat. Rev. 2020, 86, 102012. [Google Scholar] [CrossRef]
- Mamedova, E.O.; Mokrysheva, N.G.; Pigarova, E.A.; Voronkova, I.A.; Kuznetsov, S.N.; Vasilyev, E.V.; Petrov, V.M.; Kuznetsov, N.S.; Rozhinskaya, L.Y.; Tiulpakov, A.N. Molecular and genetic features of primary hyperparathyroidism in young patients. Probl. Endocrinol. 2016, 62, 4–11. [Google Scholar] [CrossRef][Green Version]
- Starker, L.F.; Åkerström, T.; Long, W.D.; Delgado-Verdugo, A.; Donovan, P.; Udelsman, R.; Lifton, R.P.; Carling, T. Frequent Germ-Line Mutations of the MEN1, CASR, and HRPT2/CDC73 Genes in Young Patients with Clinically Non-familial Primary Hyperparathyroidism. Horm. Cancer 2012, 3, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Goudet, P.; Murat, A.; Binquet, C.; Cardot-Bauters, C.; Costa, A.; Ruszniewski, P.; Niccoli, P.; Ménégaux, F.; Chabrier, G.; Borson-Chazot, F.; et al. Risk Factors and Causes of Death in MEN1 Disease. A GTE (Groupe d’Etude des Tumeurs Endocrines) Cohort Study Among 758 Patients. World J. Surg. 2010, 34, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, P.J.; Satta, M.A.; Simmgen, M.; Drake, W.M.; Williamson, C.; Lowe, D.G.; Britton, K.; Chew, S.L.; Thakker, R.; Besser, G.M. Metastatic parathyroid carcinoma in the MEN2A syndrome. Clin. Endocrinol. 1997, 47, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, J.J.; Lamas, C.; Estrada, J.; Lucas, T. MEN-2A syndrome and pulmonary metastasis. Postgrad. Med. J. 2002, 78, 51–52. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Posada-González, M.; Gómez-Ramírez, J.; Luque-Ramírez, M.; Guijarro, M.; Martín-Pérez, E.; Rodríguez-Sánchez, A.; García-Sanz, I.; Larrañaga, E. Nonfunctional Metastatic Parathyroid Carcinoma in the Setting of Multiple Endocrine Neoplasia Type 2A Syndrome. Surg. Res. Pract. 2014, 2014, 731481. [Google Scholar] [CrossRef]
- Wassif, W.S.; Moniz, C.F.; Friedman, E.; Wong, S.; Weber, G.; Nordenskjöld, M.; Peters, T.J.; Larsson, C. Familial isolated hyperparathyroidism: A distinct genetic entity with an increased risk of parathyroid cancer. J. Clin. Endocrinol. Metab. 1993, 77, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- Simonds, W.F.; Robbins, C.M.; Agarwal, S.K.; Hendy, G.N.; Carpten, J.D.; Marx, S.J. Familial Isolated Hyperparathyroidism Is Rarely Caused by Germline Mutation in HRPT2, the Gene for the Hyperparathyroidism-Jaw Tumor Syndrome. J. Clin. Endocrinol. Metab. 2004, 89, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Singh Ospina, N.; Sebo, T.J.; Thompson, G.B.; Clarke, B.L.; Young, W.F. Prevalence of parathyroid carcinoma in 348 patients with multiple endocrine neoplasia type 1—Case report and review of the literature. Clin. Endocrinol. 2016, 84, 244–249. [Google Scholar] [CrossRef]
- Dong, Q.; Debelenko, L.V.; Chandrasekharappa, S.C.; Emmert-Buck, M.R.; Zhuang, Z.; Guru, S.C.; Manickam, P.; Skarulis, M.; Lubensky, I.A.; Liotta, L.A.; et al. Loss of Heterozygosity at 11q13: Analysis of Pituitary Tumors, Lung Carcinoids, Lipomas, and Other Uncommon Tumors in Subjects with Familial Multiple Endocrine Neoplasia Type. J. Clin. Endocrinol. Metab. 1997, 82, 1416–1420. [Google Scholar] [CrossRef][Green Version]
- Desai, D.; McPherson, L.A.; Higgins, J.P.T.; Weigel, R.J. Genetic Analysis of a Papillary Thyroid Carcinoma in a Patient with MEN1. Ann. Surg. Oncol. 2001, 8, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Park, J.-S.; Kim, C.-S.; Kang, E.-S.; Cha, B.-S.; Lim, S.-K.; Kim, K.-R.; Lee, H.-C.; Ahn, C.-W. A Case of Multiple Endocrine Neoplasia Type 1 Combined with Papillary Thyroid Carcinoma. Yonsei Med. J. 2008, 49, 503–506. [Google Scholar] [CrossRef][Green Version]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–423. [Google Scholar] [CrossRef]
- Hannan, F.M.; Nesbit, M.A.; Christie, P.T.; Fratter, C.; Dudley, N.E.; Sadler, G.P.; Thakker, R.V. Familial isolated primary hyperparathyroidism caused by mutations of the MEN1 gene. Nat. Clin. Pract. Endocrinol. Metab. 2008, 4, 53–58. [Google Scholar] [CrossRef]
- Ellard, S.; Hattersley, A.T.; Brewer, C.M.; Vaidya, B. Detection of an MEN1 gene mutation depends on clinical features and supports current referral criteria for diagnostic molecular genetic testing. Clin. Endocrinol. 2005, 62, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Brandi, M.L.; Agarwal, S.K.; Perrier, N.D.; Lines, K.E.; Valk, G.D.; Thakker, R.V. Multiple Endocrine Neoplasia Type 1: Latest Insights. Endocr. Rev. 2021, 42, 133–170. [Google Scholar] [CrossRef] [PubMed]
- Caswell, R.C.; Owens, M.M.; Gunning, A.C.; Ellard, S.; Wright, C.F. Using Structural Analysis In Silico to Assess the Impact of Missense Variants in MEN1. J. Endocr. Soc. 2019, 3, 2258–2275. [Google Scholar] [CrossRef] [PubMed]
- Miki, H.; Sumitomo, M.; Inoue, H.; Kita, S.; Monden, Y. Parathyroid carcinoma in patients with chronic renal failure on maintenance hemodialysis. Surgery 1996, 120, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Tomasetti, C.; Vogelstein, B. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science 2015, 347, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, S.E.; Perrier, N.D. Parathyroid carcinoma. Curr. Opin. Oncol. 2006, 18, 16–22. [Google Scholar] [CrossRef]
- Erickson, L.A.; Mete, O.; Juhlin, C.C.; Perren, A.; Gill, A.J. Overview of the 2022 WHO Classification of Parathyroid Tumors. Endocr. Pathol. 2022, 33, 64–89. [Google Scholar] [CrossRef]
- Voronkova, I.A.; Mokrysheva, N.G.; Kazantseva, I.A.; Gurevich, L.E. Clinical and morphological characteristics of parathyroid carcinoma. Arkhiv Patol. 2018, 80, 65–72. [Google Scholar] [CrossRef]
- Falchetti, A. Genetics of multiple endocrine neoplasia type 1 syndrome: What’s new and what’s old. F1000Research 2017, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- Busaidy, N.L.; Jimenez, C.; Habra, M.A.; Schultz, P.N.; El-Naggar, A.K.; Clayman, G.L.; Asper, J.A.; Diaz, E.M.; Evans, D.B.; Gagel, R.F.; et al. Parathyroid carcinoma: A 22-year experience. Head Neck 2004, 26, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Bradwell, A.R.; Harvey, T.C. Control of hypercalcaemia of parathyroid carcinoma by immunisation. Lancet 1991, 353, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Horie, I.; Ando, T.; Inokuchi, N.; Mihara, Y.; Miura, S.; Imaizumi, M.; Usa, T.; Kinoshita, N.; Sekine, I.; Kamihara, S.; et al. First Japanese Patient Treated with Parathyroid Hormone Peptide Immunization for Refractory Hypercalcemia Caused by Metastatic Parathyroid Carcinoma. Endocr. J. 2010, 57, 287–292. [Google Scholar] [CrossRef]
- Betea, D.; Bradwell, A.R.; Harvey, T.C.; Mead, G.P.; Schmidt-Gayk, H.; Ghaye, B.; Daly, A.F.; Beckers, A. Hormonal and biochemical normalization and tumor shrinkage induced by anti-parathyroid hormone immunotherapy in a patient with metastatic parathyroid carcinoma. J. Clin. Endocrinol. Metab. 2004, 89, 3413–3420. [Google Scholar] [CrossRef]
- Rozhinskaya, L.; Pigarova, E.; Sabanova, E.; Mamedova, E.; Voronkova, I.; Krupinova, J.; Dzeranova, L.; Tiulpakov, A.; Gorbunova, V.; Orel, N.; et al. Diagnosis and treatment challenges of parathyroid carcinoma in a 27-year-old woman with multiple lung metastases. Endocrinol. Diabetes Metab. Case Rep. 2017, 2017, 16-0113. [Google Scholar] [CrossRef]

| Imaging | Type of Surgery | Histopathological Results | |
|---|---|---|---|
| 2004 | Ultrasound scan—20 mm node of right thyroid lobe | Surgery 1— right hemithyroidectomy and isthmectomy | Follicular adenoma of the right lobe of the thyroid with secondary sclerosis and calcification |
| 2010 | Ultrasound: ‘a mass in the left lower parathyroid gland, 68 × 32 × 26 mm’. 99mTc-MIBI scintigraphy: ‘a high uptake of 99mTc-MIBI in the left lower parathyroid gland. Neck CT: ‘polycyclic focus of tissue with round curves of size of 59 × 35 × 30 mm between thyroid and esophagus’. Chest CT: ‘lesions 5.5 and 7.5 mm in the middle and lower lung fields on the left and the middle lung field on the right (the largest in S4). Differential diagnosis between post-inflammatory changes and metastases was not possible’ | Surgery 2—selective parathyroidectomy (left lower parathyroid gland) and total thyroidectomy | Parathyroid cancer (pT2Nx)
|
| 2012 | Chest CT: ‘lesions with a diameter of 2–5 mm in all fields of both lungs; no change compared to the previous examinations’ | - | - |
| 2016 | Ultrasound: ‘multiple hypoechoic lesions measuring up to 4–8 mm around left lobe and in the thyroid bed on both sides; altered lymph nodes suspicious for metastases’ | - | - |
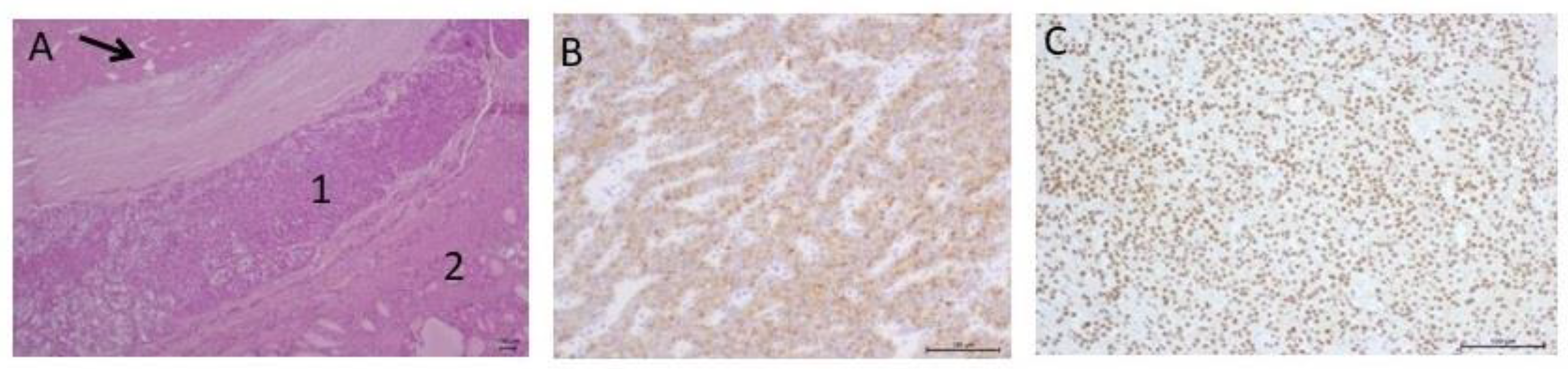
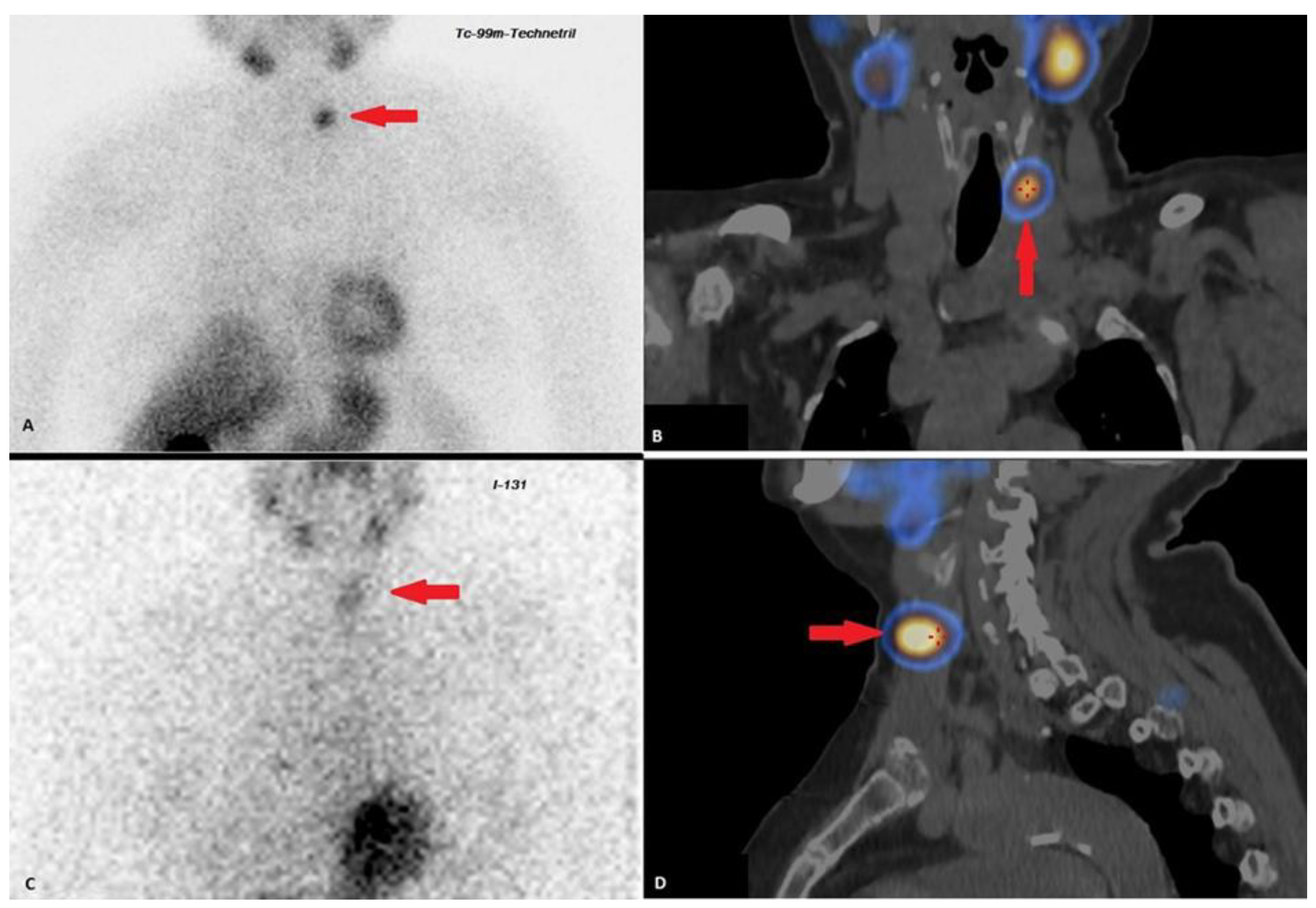
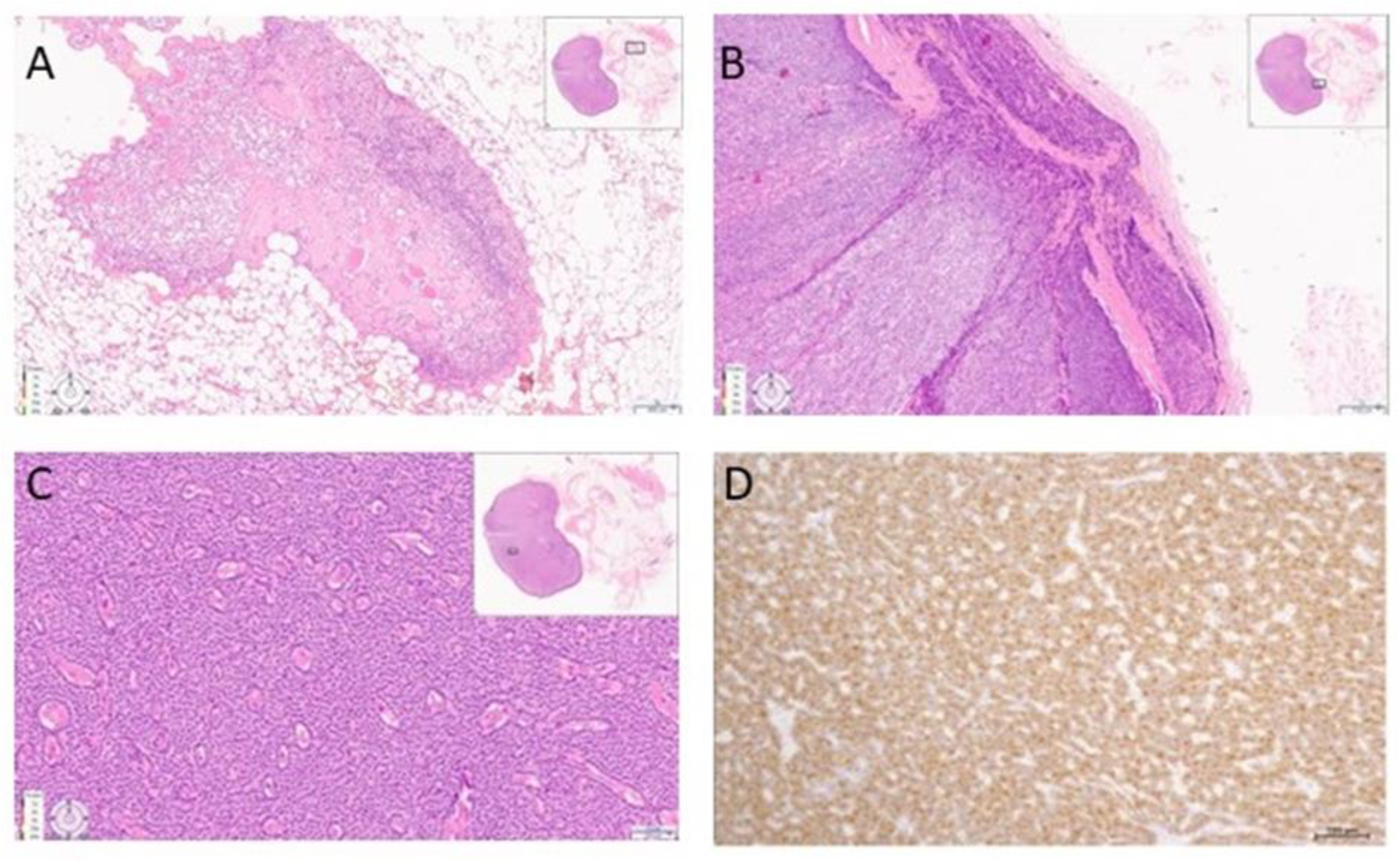
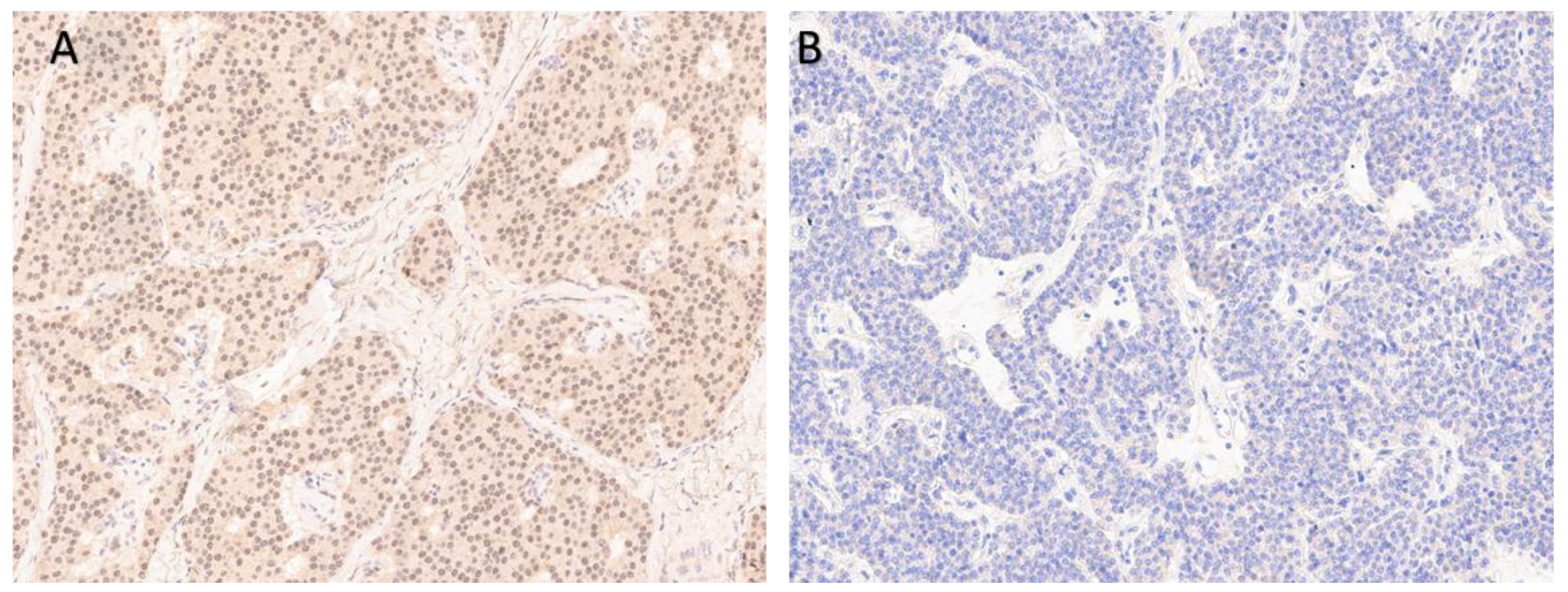
| 2018–2019 | Ultrasound: ‘multiple hypoechoic lesions with dimensions of 12 × 7 mm, 9 × 6 mm, 11 × 6 mm and 9 × 5 mm of the left lobe; 1.4 cm lower a new lesion 0.6 cm in diameter’ 99mTc-MIBI scintigraphy with SPECT/CT: ‘a round lesion with clear contours and an inhomogeneous structure, measuring 14 × 10 × 15 mm and with significant radiopharmaceutical uptake, above the jugular notch, anteriorly to the trachea, slightly to the left of the midline’. Planar whole-body scintigraphy with I-131: tissue accumulating 131I is visualized in the projection of the thyroid bed, on the left. | Surgery 3—total parathyroidectomy with the adjacent soft tissues and central lymph node dissection using intraoperative navigation methods | Metastases of parathyroid cancer (with a diameter of about 15 mm) and papillary thyroid cancer (follicular variant) to the lymph nodes with total and subtotal replacement of node tissue IHC: diffuse expression of PTH and parafibromin (Figure 1B,C), Ki-67—7%. |
| Time\ Parameter | PTH, pg/mL (15–65) | Albumin-Corrected Calcium, mmol/L (2.15–2.55) | Ionized Calcium, mmol/L (1.03–1.29) | Phosphorus, mmol/L (0.74–1.52) | Creatinine, μmol/L | eGFR (CKD-EPI), mL/min/1.73 m2 | ||
|---|---|---|---|---|---|---|---|---|
| 2009 | 2500 | 3.36 | - | - | 110 | 51 | ||
| october 2010 | 3910 | 3.26 | 1.67 | 1.5 | 287 | 15 | ||
| december 2010, after surgery 2 | 11.7 | - | 1.24 | - | - | 14 | ||
| 2012 | 17.9 | - | 1.25 | - | 429.0 | 10 | ||
| 2013 | 423 | 2.26 | 1.05 | 1.65 | 471.1 | 9 | ||
| 2016 | 1713 | 2.47 | - | 3.2 | Since 2015 renal replacement therapy (hemodialysis) | |||
| 2017 | 679 | 2.49 | 2.28 | |||||
| 2019, after surgery 3 | 388 | 1.99 | 1.3 | 1.0 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krupinova, J.; Kim, E.; Eremkina, A.; Urusova, L.; Voronkova, I.; Slaschuk, K.; Dobreva, E.; Mokrysheva, N. Multiple Metastases of Parathyroid and Papillary Thyroid Carcinoma in a Female Patient Treated with Long-Term Hemodialysis. J. Pers. Med. 2023, 13, 548. https://doi.org/10.3390/jpm13030548
Krupinova J, Kim E, Eremkina A, Urusova L, Voronkova I, Slaschuk K, Dobreva E, Mokrysheva N. Multiple Metastases of Parathyroid and Papillary Thyroid Carcinoma in a Female Patient Treated with Long-Term Hemodialysis. Journal of Personalized Medicine. 2023; 13(3):548. https://doi.org/10.3390/jpm13030548
Chicago/Turabian StyleKrupinova, Julia, Ekaterina Kim, Anna Eremkina, Lilia Urusova, Iya Voronkova, Konstantin Slaschuk, Ekaterina Dobreva, and Natalia Mokrysheva. 2023. "Multiple Metastases of Parathyroid and Papillary Thyroid Carcinoma in a Female Patient Treated with Long-Term Hemodialysis" Journal of Personalized Medicine 13, no. 3: 548. https://doi.org/10.3390/jpm13030548
APA StyleKrupinova, J., Kim, E., Eremkina, A., Urusova, L., Voronkova, I., Slaschuk, K., Dobreva, E., & Mokrysheva, N. (2023). Multiple Metastases of Parathyroid and Papillary Thyroid Carcinoma in a Female Patient Treated with Long-Term Hemodialysis. Journal of Personalized Medicine, 13(3), 548. https://doi.org/10.3390/jpm13030548







