Abstract
Introduction: Anaemia and bone metabolism alterations are common in inflammatory bowel disease (IBD), which is a heterogeneous group of diseases that include Crohn’s disease (CD) and ulcerative colitis (UC) with a rich intestinal and extraintestinal symptomatology. All these make the diagnostic procedures complicated and difficult. Purpose and scope: The aim of this study was to assess the effect of parenteral iron administration on biomarkers of mineral and bone homeostasis over time. Materials and methods: The study was a single-centre non-randomised prospective study. It was carried out between 2016 and 2020 in a group of patients in the Department of Internal Medicine and Gastroenterology Subunit of Inflammatory Bowel Diseases at the National Institute of Medicine of the Ministry of the Interior and Administration in Warsaw. At the first examination, the baseline disease severity, initial evaluation of anaemia (morphology, iron (Fe), total iron binding capacity (TIBC), ferritin, vitamin B12, folic acid) and bone mineral metabolism including C-reactive protein (CRP), albumins, alkaline phosphatase (ALP), Calcium, osteocalcin, phosphate in serum and in urine, parathyroid hormone (PTH), vitamin D3, fibroblast growth factor (iFGF23) and procollagen type 1N propeptide (P1NP) C-terminal telopeptide (CTX), was initially assessed. On the basis of peripheral blood counts, an appropriate dose of iron (iron derisomaltose or caboxymaltose) was administered. During the subsequent appointments on week 1, 4, and 12 morphology, iron (Fe), total iron binding capacity (TIBC), ferritin, vitamin B12, folic acid, C-reactive protein (CRP), albumins, alkaline phosphatase (ALP), Calcium, osteocalcin, phosphate in serum and in urine, parathyroid hormone (PTH), vitamin D3, fibroblast growth factor (iFGF23) and procollagen type 1N propeptide (P1NP) C-terminal telopeptide (CTX), were evaluated. Results: A total of 56 patients were enrolled into the study: 24 women and 32 men. In the group, 32 patients had Crohn’s disease (CD) and 24 had ulcerative colitis (UC). We found a statistically significant increase in the concentration of albumin (p = 0.031), haemoglobin (p < 0.001), haematocrit (p < 0.001), MCV (p < 0.001), MCHC (p = 0.001), iron (p < 0.001) and ferritin (p < 0.001) after the administration of parenteral iron. The influence of individual iron formulations on the analysed parameters (phosphate concentration in serum and in the urine, iFGF23, P1NP, PTH, vitamin D, haemoglobin and ferritin) was similar. Interestingly, an inverse correlation was found between the concentration of phosphorus in the blood and iFGF23 at certain time-points; however, in the study group they did not significantly affect the disturbances of calcium and phosphate metabolism. Conclusions: In the study group, transient and non-significant disorders of phosphate metabolism were found, which does not constitute a contraindication to treatment with parenteral iron in inflammatory bowel disease patients, which was safe and efficient.
1. Introduction
Inflammatory bowel disease (IBD) is a heterogeneous group of diseases that includes Crohn’s disease (CD) and ulcerative colitis (UC) and it is characterized by a rich intestinal and extraintestinal symptomatology, including iron deficiency anaemia and bone metabolic disease [1,2,3,4]. Anaemia is a common complication of IBD; in adults with IBD, its prevalence may affect up to almost half of the IBD population [5] and as much as three fourths of those who are hospitalised [6,7,8]. It is considered an inevitable complication of the disease and iron deficiency anaemia (IDA) was identified as the most common type but still it is an underestimated problem [9]. IDA has a significant negative impact on patients’ quality of life and it was observed that iron repletion may improve it [6,7]. Typical symptoms of IDA are fatigue, headache, vertigo, or tachycardia and they may not be easily distinguished from symptoms of IBD. Less common symptoms include restless leg syndrome or reduced cognitive and physical performance.
Given the fact that oral iron formulations may be badly tolerated by IBD patients, the European Crohn’s and Colitis Organisation (ECCO) guidelines state that iron deficiency and IDA should be treated with high-dose IV iron [8]. Even though there is some data showing that in mild or moderate anaemia in IBD oral formulations may be useful, in general administered intravenously iron is more effective, delivers a faster response and is better tolerated than oral iron [10,11]. Despite its effectiveness, the anaemia may recur in IBD patients (median: 19 months for iron deficiency and 10 months for IDA), which makes repeated infusions necessary. The most widely used IV iron formulations in Europe are ferric carboxymaltose (Ferinject®) and iron derisomaltoside (Monover®). These medications are safe, though hypophosphatemia is a well-known side effect of both intravenous preparations [12,13]. Data from clinical trials and from literature indicate that some of these medications may be more likely to cause hypophosphatemia than others and may be related with elevation of intact fibroblast growth factor -23 (iFGF-23).
Phosphate is essential in human physiology [14,15] and a deficiency (0.65 mmol/L) may cause symptoms like fatigue, proximal muscle weakness and bone pain [11], being difficult to distinguish from IBD symptoms. Furthermore, prolonged hypophosphatemia can result in osteomalacia. There is little data on the clinical manifestations of IV iron in IBD patients and its influence on bone mineral homeostasis.
The aim of this study was to investigate changes in calcium and phosphate levels in blood and urine, iFGF 23 after iron infusions of carboxymaltose and derisomaltose in adults with IBD.
2. Materials and Methods
2.1. Study Design and Patient Population
Study Population
We conducted a non-randomised single-centre prospective study. Patients with IBD were prospectively recruited in the Department of Internal Medicine and Gastroenterology’s IBD Subunit at the Central Clinical Hospital in Warsaw, Poland. The inclusion criteria were an age of 18 years or more, a verified diagnosis of IBD based on clinical, endoscopic, biochemical and histological findings, a minimum of 6 months from diagnosis and patients who needed iron supplementation according to ECCO guidelines and were able to read and understand Polish and give written consent. Disease activity was evaluated by the Crohn’s Disease Activity Index (CDAI) in patients with Crohn’s disease and the Truelove Score in those with UC. A CDAI score of less than 150 points was defined as remission; between 150 and 220 was low disease activity; between 220 and 450 points moderate disease activity; and more than 450 high. We excluded from the study patients who had received a blood transfusion in the last 2 months, those who had received oral supplementation in the last 3 months, those with a severe comorbidity such as chronic kidney disease, thyroid, or parathyroid gland disease, those with osteoporosis or new calcium or vitamin supplementation, those with a severe infection such as Clostridioides difficile and those who did not cooperate with the clinician. The inclusion period lasted from 2016 until 2020. On the basis of peripheral blood counts, an appropriate dose of iron (ferric derisomaltoside- Monover, FDI) or in case of intolerance or unresponsive to FDI, iron carboxymaltose (Ferinject, FCM) was administered. Patients who during the iron infusion presented serious adverse events were excluded from the study. During the subsequent appointments on day 7, in week 4, and week 12, the following parameters were measured in the patients: in the peripheral blood serum – blood count, phosphate in serum, calcium, ALP, iFGF23, vitamin D3, PTH, CRP, Cr, albumin, iron, TIBC, ferritin, osteocalcin, P1NP and C-telopeptide; in the urine – phosphate; and in the stool – calprotectin. Disease activity was evaluated by the disease activity form.
2.2. Clinical, Sociodemographic and Laboratory Variables
Data such as gender, type of disease, previous operations and current medications were collected by interviews and from medical records at enrolment. The demographic and clinical data are shown in Table 1.

Table 1.
Patient demographics at baseline.
All laboratory data analysis was performed at the local laboratory. A C-protein (CRP) level of 5 or higher was chosen to indicate active inflammation. Samples were collected in the morning between 8.00 and 11.00 am after an overnight fast. A urine sample was obtained in the same day. The blood was allowed to clot, then separated and centrifuged at 4 °C; it was finally frozen at −40 °C before processing. Laboratory investigations were measured by routine hospital laboratory methods and included serum albumin, alkaline phosphatase, calcium, phosphate, CRP, serum 25-(OH)-D and bone markers and phosphate in urine.
2.3. Statistical Analysis
BM SPSS Statistics 25 was used to answer the research question. Descriptive statistics, together with Kolmogorov-Smirnov tests, were calculated. The U Mann–Whitney test, Friedman test, chi-square test of independence and Pearson’r correlation was performed. p < 0.05 was considered statistically significant.
2.4. Ethical Considerations
The Ethical Committee of the National Institute of Medicine (previously Central Clinical Hospital) of the Ministry of the Interior and Administration approved this study (28/2016).
3. Results
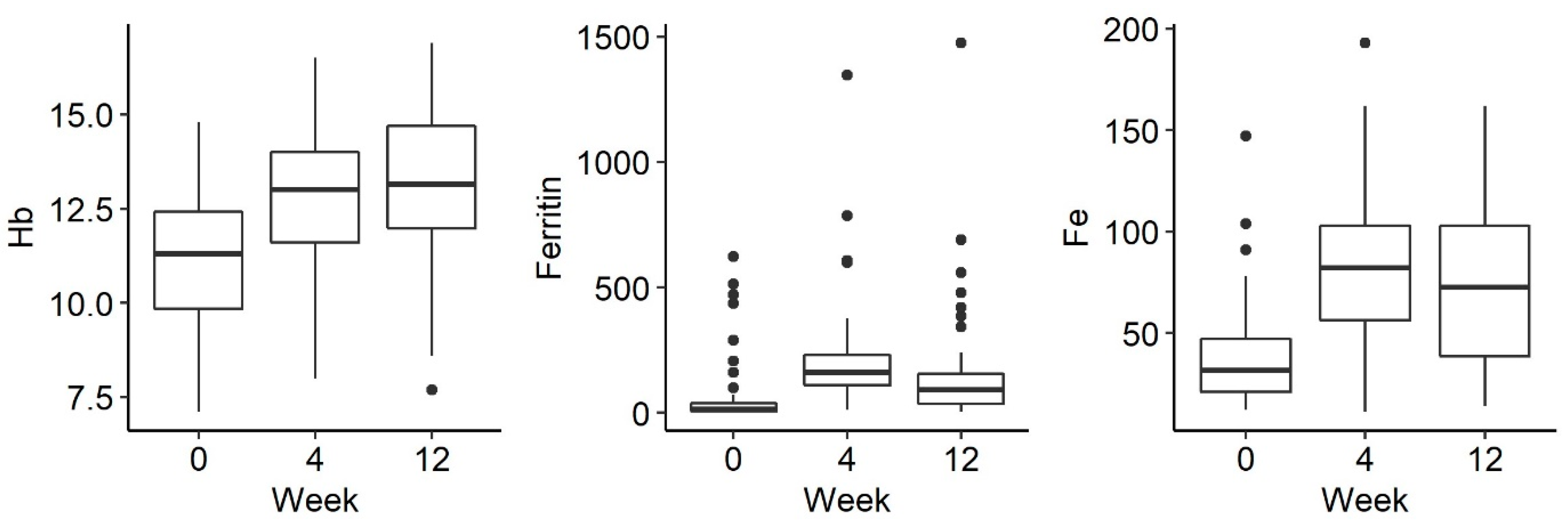
Of the 56 patients, 44 received ferric derisomaltoside and 12 received ferric carboxymaltose. The changes in biological indicators were analysed between the 3 measurements: before drug administration and 1 week, 4 weeks and 12 weeks after administration. There was a statistically significant increase in the concentration of haemoglobin (p < 0.001), iron (p < 0.001) and ferritin (p < 0.001) after the administration of parenteral iron (Figure 1).
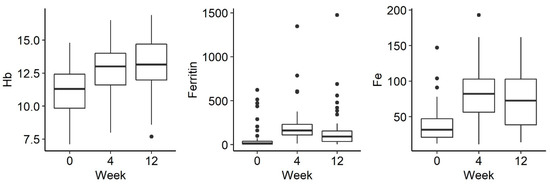
Figure 1.
Changes in Hg (hemoglobin), ferritin and Fe (iron) levels over time: before iron administration and 4 and 12 weeks after administration.
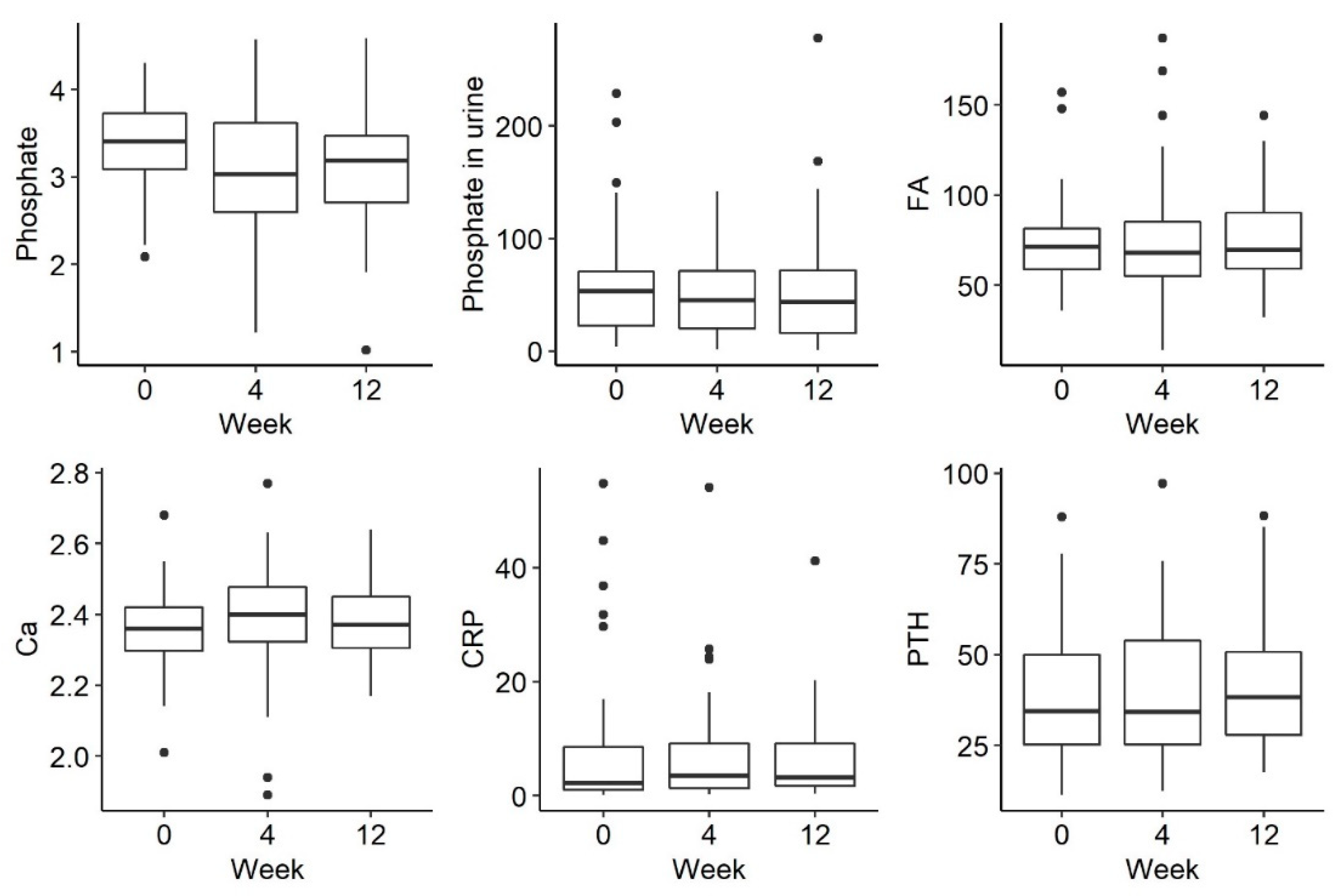
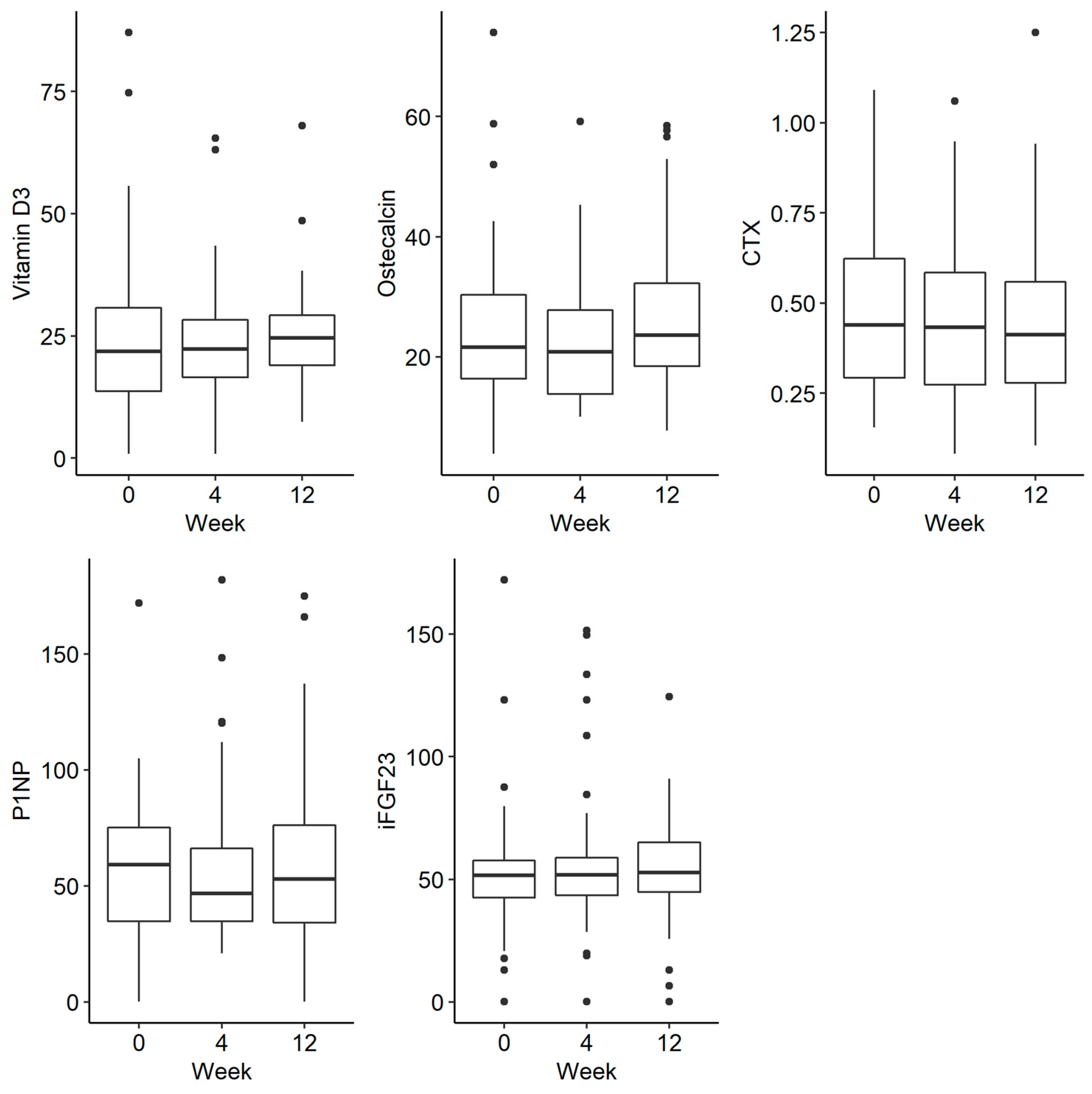
There was a trend towards a decreasing phosphate concentration at week 4, followed by an increase at week 12, but this was not statistically significant (p = 0.054). There were no statistically significant differences in the remaining parameters tested, such as phosphorus in urine and iFGF23, at the selected time-points There were no statistically significant differences in the examined disorders of calcium and phosphate metabolism and bone turnover markers (Figure 2 and Figure 3).
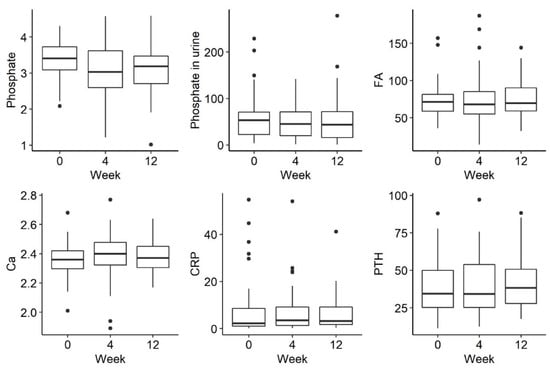
Figure 2.
Changes in the concentration of phosphate, phosphate in urine, FA (alkaline phosphatase), Ca (calcium), CRP (C-reactive protein) and PTH (parathyroid hormone) before iron administration and 4 weeks and 12 weeks after administration.
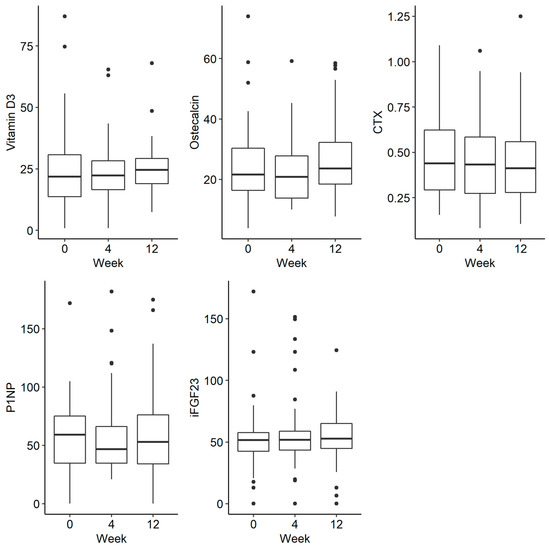
Figure 3.
Changes in the concentration of vitamin D, osteocalcin, CTX (C-terminal telopeptide), P1NP (procollagen type 1N propeptide) and iFGF23 (intact fibroblast growth factor) before iron administration and 4 and 12 weeks after administration.
The change in biological markers between the 1st measurement and the measurement on day 7 after drug administration was assessed, and also the differences in the biological markers 7 days after drug administration. Detailed data are shown in Table 2.

Table 2.
The influence of iron formulations on the tested parameters in 3 measurements, including post hoc tests.
There were no significant differences in measurements in patients receiving iron formulations (Table 3).

Table 3.
Comparison of the biological indicators between patients using ferric derisomaltose and ferric carboxymaltose.
The analysis did not reveal any significant relationships between changes in biological indicators and the type of drug taken. The changes in individual biological indices were similar in subjects taking derisomaltose or ferric carboxymaltose (Table 3).
Relationship of Phosphate and iFGF23 Levels in Individual Measurements
The relationship of phosphate level and iFGF23 in each of the 3 measurements was analysed. Detailed data are presented in Table 4.

Table 4.
Comparison of changes in biological markers in patients receiving ferric derisomaltose and ferric carboxymaltose.
A statistically significant correlation was demonstrated in weeks 4 and 12 between the decrease in phosphate concentration and the increase in iFGF23. The correlation was clearly visible in the ferric carboxymaltose group (Table 5). Although there was a relationship between these parameters, in the study group they did not significantly affect the calcium and phosphate metabolism disturbances (Table 6).

Table 5.
Relationship of phosphate and iFGF23 levels in individual measurements.

Table 6.
The relationship of phosphorus and iFGF23 levels in individual measurements, broken down by drug groups.
4. Discussion
In this non-randomised single-centre prospective study on anaemic patients with IBD, we found that both iron formulations were safe and effective in treating anaemia in IBD patients.
Our study has many strengths. Firstly, it evaluated the efficacy and security of both intravenous iron formulas in IBD patients, which is an important issue given the fact that up to 95% of patients may be at risk of bone mineral alterations [16,17,18,19,20,21]. Jahnsen et al. have shown that hypovitaminosis in IBD patients is common and that patients with CD and after small-bowel resections are especially at risk of developing secondary hyperparathyroidism and low BMD [20]. Previous clinical trials’ data suggest higher risk for the development of hypophosphatemia associated with FCM [13], however higher risk of mild hypersensitivity reactions were found in FDI group [20]. Moreover, as reported in recent review and metanalysis hypophosphatemia may persist at the end of the study periods (maximum 3 months) in up to 45% of patients treated with FCM [22]. According to our study, iron formulas were safe. Even though after iron repletion in the 4th week trend towards a decrease of phosphate was observed in our patients it was transient and not statistically significant. Looking more closely, increase and normalization of phosphate levels was observed in the 12th week of observation. As it was previously shown in population of patients with IBD, both formulations are safe and effective in this group of patients [23,24,25].
Secondly, in order to check the possible mechanism of hypophosphatemia and its relationship with particular iron formulations, we analysed iFGF23, which is a hormone produced by the osteocytes which increases the rate of urinary excretion of phosphate and inhibits 1,25-dihydroxyvitamin D production [26]. We found a statistically significant correlation in weeks 4 and 12 between the decrease in phosphate concentration and the increase in iFGF23, especially in the FCM group. Although there was a relationship between these parameters, they did not significantly affect the calcium and phosphate metabolism disturbances. This is consistent with the data from Dahlerup et al., who also did not find any severe disturbances regarding iFGF2 in patients who received iron derisomaltose [25]. In the clinical trial performed by Wolf et al. comparing influence of intravenous ferric derisomaltose vs ferric carboxymaltose on hypophosphatemia and its effect on biomarkers of mineral and bone homeostasis, lower incidence of hypophosphatemia in the FDI group was observed [27]. Also Detlie et al. among IBD patients showed that ferric carboxymaltose was associated with a higher incidence of hypophosphatemia compared with iron derisomaltose [28]. Our results demonstrate and prove that both iron formulations are safe and effective in the treatment of anaemia in IBD patients.
Thirdly, in our study we have observed a statistically significant increase of haemoglobin, MCHC, iron and ferritin levels (p < 0.001) after iron repletion. That observation was related to the intended effect of the therapy as previously had been described in other studies [26].
This trial has its limitations: first of all, even though groups of patients were sufficient to evaluate effect of iron formulas on bone mineral alterations in IBD patients, may not be sufficient to compare effect of each formula on bone mineral alteration (FDI was applied in majority of patients, whereas when patients were allergic to it or did not respond to it, FCM was administered). This way most of patients received FDI and the group who received FCM was smaller. Secondly, this study was a real world study and patients were not randomly assigned to two groups, what may constitute a limitation.
5. Conclusions
This study indicates that transient and non-significant disorders of phosphate metabolism were found after parenteral iron infusions in the studied group, which do not constitute a contraindication to treatment with parenteral iron preparations. We consider iron iv administration safe and efficient in IBD patients.
Author Contributions
Conceptualization, G.R. and E.T.-M.; methodology E.T.-M. and K.L.; software, E.T.-M.; validation, G.R. and E.T.-M.; formal analysis, E.T.-M., T.K. and M.C.; investigation, E.T.-M., K.L., P.S. and M.W.; resources, E.T.-M.; data curation, E.T.-M., K.L., P.S. and M.W.; writing—original draft preparation, E.T.-M.; writing—review and editing, G.R. and E.T.-M.; visualization, E.T.-M.; supervision, E.T.-M.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the The Ethical Committee of the National Institute of Medicine (previously Central Clinical Hospital) of the Ministry of the Interior and Administration approved this study (28/2016).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Harbord, M.; Annese, V.; Vavricka, S.R.; Allez, M.; Barreiro-de Acosta, M.; Boberg, K.M.; Burisch, J.; De Vos, M.; De Vries, A.M.; Dick, A.D.; et al. The First European Evidence-based Consensus on Extra-intestinal Manifestations in Inflammatory Bowel Disease. J. Crohns. Colitis. 2016, 10, 239–254. [Google Scholar] [CrossRef]
- Uździcki, A.; Wawrzynowicz-Syczewska, M. Characteristic features of ulcerative colitis withconcomitant primary sclerosing cholangitis. Gastroenterol. Rev. Przegląd Gastroenterol. 2021, 16, 184–187. [Google Scholar]
- Łodyga, M.; Eder, P.; Gawron-Kiszka, M.; Dobrowolska, A.; Gonciarz, M.; Hartleb, M.; Kłopocka, M.; Małecka-Wojciesko, E.; Radwan, P.; Reguła, J.; et al. Guidelines for the management of patients with Crohn’s disease. Recommendations of the Polish Society of Gastroenterology and the Polish National Consultant in Gastroenterology. Gastroenterol. Rev. Przegląd Gastroenterol. 2021, 16, 257–296. [Google Scholar] [CrossRef]
- Tulewicz-Marti, E.; Moniuszko, A.; Rydzewska, G. Management of anemia in inflammatory bowel disease: A challenge in everyday clinical practice. Prz. Gastroenterol. 2017, 12, 239–243. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Gomollón, F. Common misconceptions in the diagnosis and management of anemia in inflammatory bowel disease. Am. J. Gastroenterol. 2008, 103, 1299–1307. [Google Scholar] [CrossRef]
- König, P.; Jimenez, K.; Saletu-Zyhlarz, G.; Mittlböck, M.; Gasche, C. Iron deficiency, depression, and fatigue in inflammatory bowel diseases. Eisenmangel, Depression und Erschöpfung bei CED. Z Gastroenterol. 2020, 58, 1191–1200. [Google Scholar]
- Wells, C.W.; Lewis, S.; Barton, J.R.; Corbett, S. Effects of changes in hemoglobin level on quality of life and cognitive function in inflammatory bowel disease patients. Inflamm. Bowel Dis. 2006, 12, 123–130. [Google Scholar] [CrossRef]
- Dignass, A.U.; Gasche, C.; Bettenworth, D.; Birgegård, G.; Danese, S.; Gisbert, J.P.; Gomollon, F.; Iqbal, T.; Katsanos, K.; Koutroubakis, I.; et al. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J. Crohns. Colitis. 2015, 9, 211–222. [Google Scholar] [CrossRef]
- Shah, Y.; Patel, D.; Khan, N. Iron deficiency anemia in IBD: An overlooked comorbidity. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 771–781. [Google Scholar] [CrossRef]
- Vernero, M.; Boano, V.; Ribaldone, D.G.; Pellicano, R.; Astegiano, M. Oral iron supplementation with Feralgine® in inflammatory bowel disease: A retrospective observational study. Minerva Gastroenterol. Dietol. 2019, 65, 200–203. [Google Scholar] [CrossRef]
- Abbati, G.; Incerti, F.; Boarini, C.; Pileri, F.; Bocchi, D.; Ventura, P.; Buzzetti, E.; Pietrangelo, A. Safety and efficacy of sucrosomial iron in inflammatory bowel disease patients with iron deficiency anemia. Intern. Emerg. Med. 2019, 14, 423–431. [Google Scholar] [CrossRef]
- Maas, L.A.; Krishna, M.; Parian, A.M. Ironing It All Out: A Comprehensive Review of Iron Deficiency Anemia in Inflammatory Bowel Disease. Dig. Dis. Sci. 2022, 68, 357–369. [Google Scholar] [CrossRef]
- Zoller, H.; Schaefer, B.; Glodny, B. Iron-induced hypophosphatemia: An emerging complication. Curr. Opin. Nephrol. Hypertens. 2017, 26, 266–275. [Google Scholar] [CrossRef]
- Geerse, D.A.; Bindels, A.J.; Kuiper, M.A.; Roos, A.N.; Spronk, P.E.; Schultz, M.J. Treatment of hypophosphatemia in the intensive care unit: A review. Crit. Care 2010, 14, R147. [Google Scholar] [CrossRef]
- Goretti Penido, M.; Alon, U.S. Phosphate homeostasis and its role in bone. Pediatr. Nephrol. 2012, 27, 2039–2048. [Google Scholar] [CrossRef]
- Siffledeen, J.S.; Siminoski, K.; Steinhart, H.; Greenberg, G.; Fedorak, R.N. The frequency of vitamin D deficiency in adults with Crohn’s disease. Can. J. Gastroenterol. 2003, 17, 473–478. [Google Scholar] [CrossRef]
- Siffledeen, J.S.; Fedorak, R.N.; Siminoski, K.; Jen, H.; Vaudan, E.; Abraham, N.; Seinhart, H.; Greenberg, G. Bones and Crohn’s: Risk factors associated with low bone mineral density in patients with Crohn’s disease. Inflamm. Bowel. Dis. 2004, 10, 220–228. [Google Scholar] [CrossRef]
- Castro, F.D.; Magalhães, J.; Carvalho, P.B.; Moreira, M.J.; Mota, P.; Cotter, J. Lower levels of vitamin D correlate with clinical disease activity and quality of life in Inflammatory Bowel Disease. Arq. Gastroenterol. 2015, 52, 260–265. [Google Scholar] [CrossRef]
- Frigstad, S.O.; Høivik, M.L.; Jahnsen, J.; Cvancarova, M.; Grimstad, T.; Berset, I.P.; Huppertz-Hauss, G.; Hovde, Ø.; Bernklev, T.; Moum, B.; et al. Fatigue is not associated with vitamin D deficiency in inflammatory bowel disease patients. World J. Gastroenterol. 2018, 24, 3293–3301. [Google Scholar] [CrossRef]
- Jahnsen, J.; Falch, J.A.; Mowinckel, P.; Aadland, E. Vitamin D status, parathyroid hormone and bone mineral density in patients with inflammatory bowel disease. Scand. J. Gastroenterol. 2021, 37, 192–199. [Google Scholar] [CrossRef]
- Schaefer, B.; Tobiasch, M.; Viveiros, A.; Tilg, H.; Kennedy, N.A.; Wolf, M.; Zoller, H. Hypophosphataemia after treatment of iron deficiency with intravenous ferric carboxymaltose or iron isomaltoside-a systematic review and meta-analysis. Br. J. Clin. Pharmacol. 2021, 87, 2256–2273. [Google Scholar] [CrossRef]
- Bager, P.; Hvas, C.L.; Dahlerup, J.F. Drug-specific hypophosphatemia and hypersensitivity reactions following different intravenous iron infusions. Br. J. Clin. Pharmacol. 2017, 83, 1118–1125. [Google Scholar] [CrossRef]
- Stein, J.; Walper, A.; Klemm, W.; Farrag, K.; Aksan, A.; Dignass, A. Safety and efficacy of intravenous iron isomaltoside for correction of anaemia in patients with inflammatory bowel disease in everyday clinical practice. Scand. J. Gastroenterol. 2018, 53, 1059–1065. [Google Scholar] [CrossRef]
- Stein, J.; Aksan, A.; Klemm, W.; Nip, K.; Weber-Mangal, S.; Dignass, A. Safety and Efficacy of Ferric Carboxymaltose in the Treatment of Iron Deficiency Anaemia in Patients with Inflammatory Bowel Disease, in Routine Daily Practice. J. Crohns. Colitis. 2018, 12, 826–834. [Google Scholar] [CrossRef]
- Dahlerup, J.F.; Jacobsen, B.A.; van der Woude, J.; Bark, L.Å.; Thomsen, L.L.; Lindgren, S. High-dose fast infusion of parenteral iron isomaltoside is efficacious in inflammatory bowel disease patients with iron-deficiency anaemia without profound changes in phosphate or fibroblast growth factor 23. Scand. J. Gastroenterol. 2016, 51, 1332–1338. [Google Scholar] [CrossRef]
- Shimada, T.; Hasegawa, H.; Yamazaki, Y.; Muto, T.; Hino, R.; Takeuchi, Y.; Fujita, T.; Nakahara, K.; Fukumoto, S.; Yamashita, T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J. Bone Miner. Res. 2004, 19, 429–435. [Google Scholar] [CrossRef]
- Wolf, M.; Rubin, J.; Achebe, M.; Econs, M.J.; Peacock, M.; Imel, E.A.; Thomsen, L.L.; Carpenter, T.O.; Weber, T.; Brandenburg, V.; et al. Effects of Iron Isomaltoside vs Ferric Carboxymaltose on Hypophosphatemia in Iron-Deficiency Anemia: Two Randomized Clinical Trials. JAMA 2020, 323, 432–443. [Google Scholar] [CrossRef]
- Detlie, T.E.; Lindstrøm, J.C.; Jahnsen, M.E.; Finnes, E.; Zoller, H.; Moum, B.; Jahnsen, J. Hypophosphatemia after high-dose intravenous iron treatment in patients with inflammatory bowel disease: Mechanisms and possible clinical impact. World J. Gastroenterol. 2021, 27, 2039–2053. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).