Effect of Intravenous Iron Administration on Bone Mineral and Iron Homeostasis in Patients with Inflammatory Bowel Disease—Results of a Prospective Single-Centre Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
Study Population
2.2. Clinical, Sociodemographic and Laboratory Variables
2.3. Statistical Analysis
2.4. Ethical Considerations
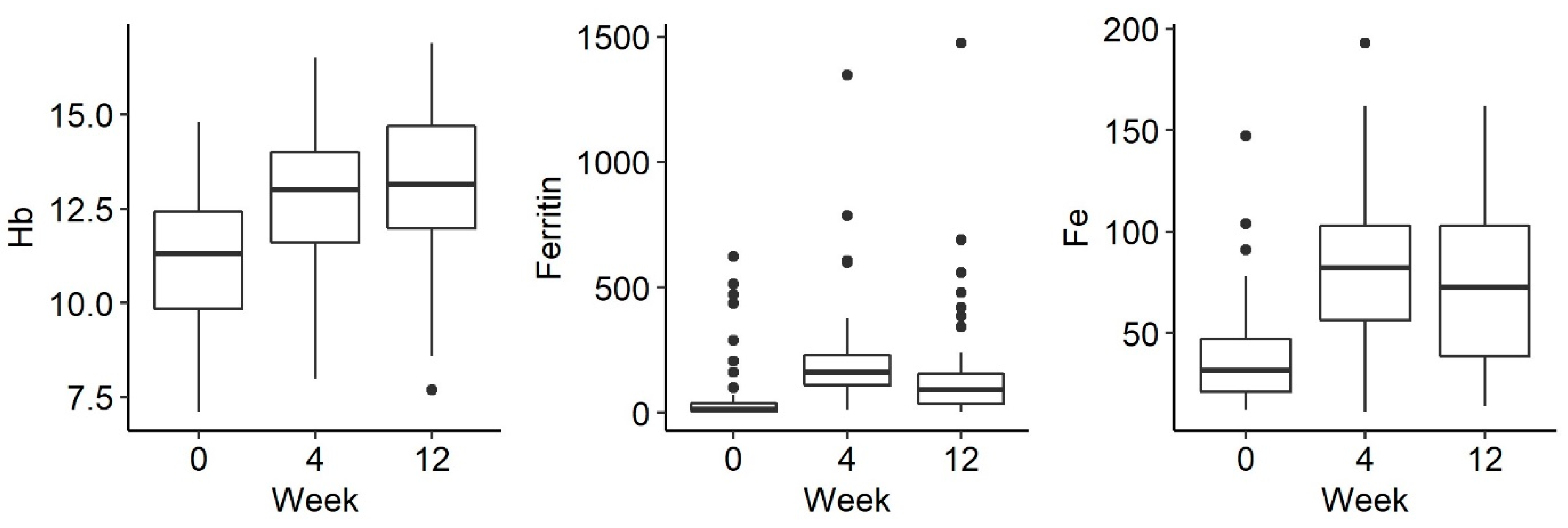
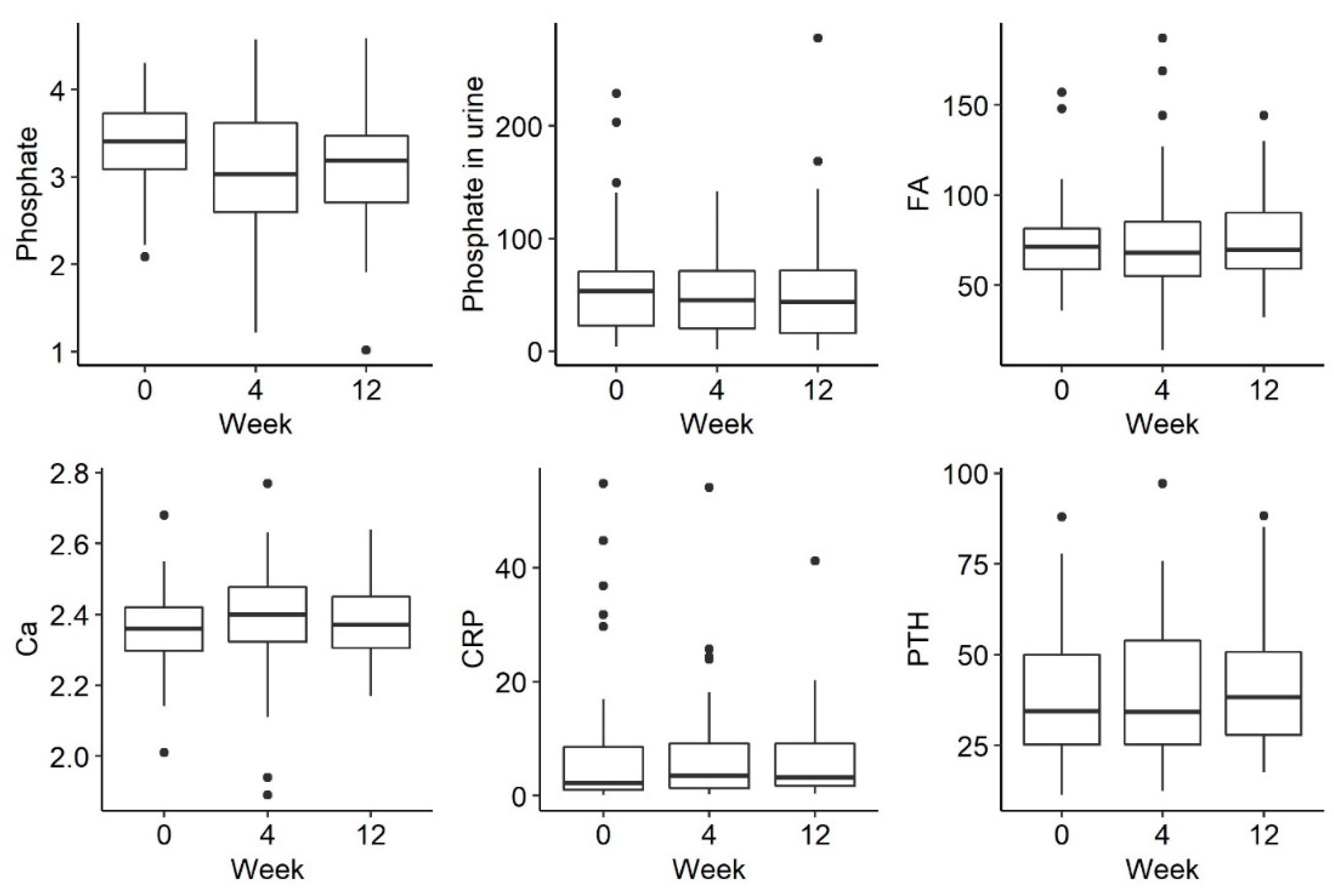
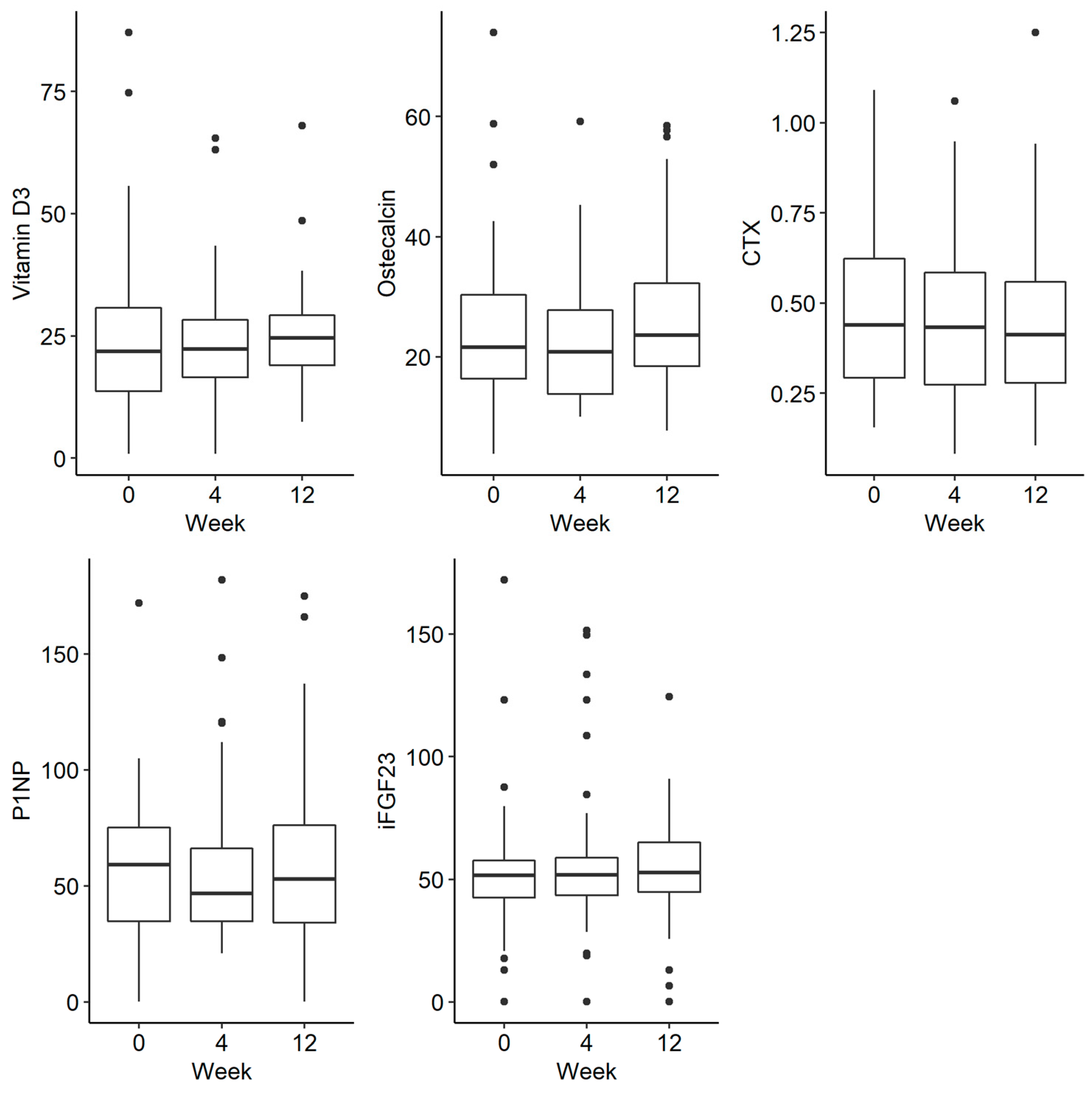
3. Results
Relationship of Phosphate and iFGF23 Levels in Individual Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Harbord, M.; Annese, V.; Vavricka, S.R.; Allez, M.; Barreiro-de Acosta, M.; Boberg, K.M.; Burisch, J.; De Vos, M.; De Vries, A.M.; Dick, A.D.; et al. The First European Evidence-based Consensus on Extra-intestinal Manifestations in Inflammatory Bowel Disease. J. Crohns. Colitis. 2016, 10, 239–254. [Google Scholar] [CrossRef]
- Uździcki, A.; Wawrzynowicz-Syczewska, M. Characteristic features of ulcerative colitis withconcomitant primary sclerosing cholangitis. Gastroenterol. Rev. Przegląd Gastroenterol. 2021, 16, 184–187. [Google Scholar]
- Łodyga, M.; Eder, P.; Gawron-Kiszka, M.; Dobrowolska, A.; Gonciarz, M.; Hartleb, M.; Kłopocka, M.; Małecka-Wojciesko, E.; Radwan, P.; Reguła, J.; et al. Guidelines for the management of patients with Crohn’s disease. Recommendations of the Polish Society of Gastroenterology and the Polish National Consultant in Gastroenterology. Gastroenterol. Rev. Przegląd Gastroenterol. 2021, 16, 257–296. [Google Scholar] [CrossRef]
- Tulewicz-Marti, E.; Moniuszko, A.; Rydzewska, G. Management of anemia in inflammatory bowel disease: A challenge in everyday clinical practice. Prz. Gastroenterol. 2017, 12, 239–243. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Gomollón, F. Common misconceptions in the diagnosis and management of anemia in inflammatory bowel disease. Am. J. Gastroenterol. 2008, 103, 1299–1307. [Google Scholar] [CrossRef]
- König, P.; Jimenez, K.; Saletu-Zyhlarz, G.; Mittlböck, M.; Gasche, C. Iron deficiency, depression, and fatigue in inflammatory bowel diseases. Eisenmangel, Depression und Erschöpfung bei CED. Z Gastroenterol. 2020, 58, 1191–1200. [Google Scholar]
- Wells, C.W.; Lewis, S.; Barton, J.R.; Corbett, S. Effects of changes in hemoglobin level on quality of life and cognitive function in inflammatory bowel disease patients. Inflamm. Bowel Dis. 2006, 12, 123–130. [Google Scholar] [CrossRef]
- Dignass, A.U.; Gasche, C.; Bettenworth, D.; Birgegård, G.; Danese, S.; Gisbert, J.P.; Gomollon, F.; Iqbal, T.; Katsanos, K.; Koutroubakis, I.; et al. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J. Crohns. Colitis. 2015, 9, 211–222. [Google Scholar] [CrossRef]
- Shah, Y.; Patel, D.; Khan, N. Iron deficiency anemia in IBD: An overlooked comorbidity. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 771–781. [Google Scholar] [CrossRef]
- Vernero, M.; Boano, V.; Ribaldone, D.G.; Pellicano, R.; Astegiano, M. Oral iron supplementation with Feralgine® in inflammatory bowel disease: A retrospective observational study. Minerva Gastroenterol. Dietol. 2019, 65, 200–203. [Google Scholar] [CrossRef]
- Abbati, G.; Incerti, F.; Boarini, C.; Pileri, F.; Bocchi, D.; Ventura, P.; Buzzetti, E.; Pietrangelo, A. Safety and efficacy of sucrosomial iron in inflammatory bowel disease patients with iron deficiency anemia. Intern. Emerg. Med. 2019, 14, 423–431. [Google Scholar] [CrossRef]
- Maas, L.A.; Krishna, M.; Parian, A.M. Ironing It All Out: A Comprehensive Review of Iron Deficiency Anemia in Inflammatory Bowel Disease. Dig. Dis. Sci. 2022, 68, 357–369. [Google Scholar] [CrossRef]
- Zoller, H.; Schaefer, B.; Glodny, B. Iron-induced hypophosphatemia: An emerging complication. Curr. Opin. Nephrol. Hypertens. 2017, 26, 266–275. [Google Scholar] [CrossRef]
- Geerse, D.A.; Bindels, A.J.; Kuiper, M.A.; Roos, A.N.; Spronk, P.E.; Schultz, M.J. Treatment of hypophosphatemia in the intensive care unit: A review. Crit. Care 2010, 14, R147. [Google Scholar] [CrossRef]
- Goretti Penido, M.; Alon, U.S. Phosphate homeostasis and its role in bone. Pediatr. Nephrol. 2012, 27, 2039–2048. [Google Scholar] [CrossRef]
- Siffledeen, J.S.; Siminoski, K.; Steinhart, H.; Greenberg, G.; Fedorak, R.N. The frequency of vitamin D deficiency in adults with Crohn’s disease. Can. J. Gastroenterol. 2003, 17, 473–478. [Google Scholar] [CrossRef]
- Siffledeen, J.S.; Fedorak, R.N.; Siminoski, K.; Jen, H.; Vaudan, E.; Abraham, N.; Seinhart, H.; Greenberg, G. Bones and Crohn’s: Risk factors associated with low bone mineral density in patients with Crohn’s disease. Inflamm. Bowel. Dis. 2004, 10, 220–228. [Google Scholar] [CrossRef]
- Castro, F.D.; Magalhães, J.; Carvalho, P.B.; Moreira, M.J.; Mota, P.; Cotter, J. Lower levels of vitamin D correlate with clinical disease activity and quality of life in Inflammatory Bowel Disease. Arq. Gastroenterol. 2015, 52, 260–265. [Google Scholar] [CrossRef]
- Frigstad, S.O.; Høivik, M.L.; Jahnsen, J.; Cvancarova, M.; Grimstad, T.; Berset, I.P.; Huppertz-Hauss, G.; Hovde, Ø.; Bernklev, T.; Moum, B.; et al. Fatigue is not associated with vitamin D deficiency in inflammatory bowel disease patients. World J. Gastroenterol. 2018, 24, 3293–3301. [Google Scholar] [CrossRef]
- Jahnsen, J.; Falch, J.A.; Mowinckel, P.; Aadland, E. Vitamin D status, parathyroid hormone and bone mineral density in patients with inflammatory bowel disease. Scand. J. Gastroenterol. 2021, 37, 192–199. [Google Scholar] [CrossRef]
- Schaefer, B.; Tobiasch, M.; Viveiros, A.; Tilg, H.; Kennedy, N.A.; Wolf, M.; Zoller, H. Hypophosphataemia after treatment of iron deficiency with intravenous ferric carboxymaltose or iron isomaltoside-a systematic review and meta-analysis. Br. J. Clin. Pharmacol. 2021, 87, 2256–2273. [Google Scholar] [CrossRef]
- Bager, P.; Hvas, C.L.; Dahlerup, J.F. Drug-specific hypophosphatemia and hypersensitivity reactions following different intravenous iron infusions. Br. J. Clin. Pharmacol. 2017, 83, 1118–1125. [Google Scholar] [CrossRef]
- Stein, J.; Walper, A.; Klemm, W.; Farrag, K.; Aksan, A.; Dignass, A. Safety and efficacy of intravenous iron isomaltoside for correction of anaemia in patients with inflammatory bowel disease in everyday clinical practice. Scand. J. Gastroenterol. 2018, 53, 1059–1065. [Google Scholar] [CrossRef]
- Stein, J.; Aksan, A.; Klemm, W.; Nip, K.; Weber-Mangal, S.; Dignass, A. Safety and Efficacy of Ferric Carboxymaltose in the Treatment of Iron Deficiency Anaemia in Patients with Inflammatory Bowel Disease, in Routine Daily Practice. J. Crohns. Colitis. 2018, 12, 826–834. [Google Scholar] [CrossRef]
- Dahlerup, J.F.; Jacobsen, B.A.; van der Woude, J.; Bark, L.Å.; Thomsen, L.L.; Lindgren, S. High-dose fast infusion of parenteral iron isomaltoside is efficacious in inflammatory bowel disease patients with iron-deficiency anaemia without profound changes in phosphate or fibroblast growth factor 23. Scand. J. Gastroenterol. 2016, 51, 1332–1338. [Google Scholar] [CrossRef]
- Shimada, T.; Hasegawa, H.; Yamazaki, Y.; Muto, T.; Hino, R.; Takeuchi, Y.; Fujita, T.; Nakahara, K.; Fukumoto, S.; Yamashita, T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J. Bone Miner. Res. 2004, 19, 429–435. [Google Scholar] [CrossRef]
- Wolf, M.; Rubin, J.; Achebe, M.; Econs, M.J.; Peacock, M.; Imel, E.A.; Thomsen, L.L.; Carpenter, T.O.; Weber, T.; Brandenburg, V.; et al. Effects of Iron Isomaltoside vs Ferric Carboxymaltose on Hypophosphatemia in Iron-Deficiency Anemia: Two Randomized Clinical Trials. JAMA 2020, 323, 432–443. [Google Scholar] [CrossRef]
- Detlie, T.E.; Lindstrøm, J.C.; Jahnsen, M.E.; Finnes, E.; Zoller, H.; Moum, B.; Jahnsen, J. Hypophosphatemia after high-dose intravenous iron treatment in patients with inflammatory bowel disease: Mechanisms and possible clinical impact. World J. Gastroenterol. 2021, 27, 2039–2053. [Google Scholar] [CrossRef]
| CD n = 32 | UC n = 24 | |
|---|---|---|
| Female, n (%) Male, n (%) | 12 (37.5%) 20 (62.5%) | 12 (50%) 12 (50%) |
| Median age (years) (SD) | 38 ± 14.99 | 35.35 ± 13.51 |
| Disease activity | CDAI < 150: 14 patients CDAI 151–220: 12 patients CDAI 221–450: 6 patients CDAI > 450: 0 patient | Truelove Witts scale remission: 2 mild:12 moderate: 9 severe: 1 |
| Current use of medications | ||
| 5-ASA (mesalasine or suphasalasine) | 30 (93.8%) | 23 (96%) |
| Corticosteroids Prednisone or methylprednisolone | 4 (12.5%) | 5 (20.8%) |
| Budesonide | 3 (9.4%) | 6 (25%) |
| Immunosuppressants | ||
| Azathioprine | 16 (50%) | 9 (37.5%) |
| Mercaptopurine | 2 (6.25%) | 2 (8.3%) |
| Methotrexate | 2 (6.25%) | |
| Biologic agents | ||
| Infliximab | 4 (12.5%) | |
| Adalimumab | 4 (12.5%) | |
| Vedolizumab | 1 (3.1%) | 3 (1.25%) |
| Ustekinumab Etrolizumab | 1(4.1%) |
| N | DAY “0” | 4 WEEKS AFTER IV Fe | 12 WEEKS AFTER IV Fe | p | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean Rank | Me | IQR | Mean Rank | Me | IQR | Mean Rank | Me | IQR | |||
| Albumins | 34 | 1.63 a | 4.29 | 0.49 | 2.18 b | 4.36 | 0.47 | 2.19 b | 4.40 | 0.50 | 0.031 |
| ALP | 36 | 1.79 a | 71.50 | 23.50 | 2.24 a | 68.00 | 32.50 | 1.97 a | 69.50 | 32.50 | 0.155 |
| VITAMIN B12 | 21 | 2.14 a | 397.00 | 237.50 | 1.81 a | 329.00 | 232.50 | 2.05 a | 360.00 | 268.00 | 0.526 |
| CTX | 41 | 2.29 a | 0.44 | 0.33 | 1.91 a | 0.43 | 0.31 | 1.79 a | 0.41 | 0.28 | 0.060 |
| CA | 42 | 1.77 a | 2.36 | 0.11 | 2.00 a | 2.38 | 0.15 | 2.23 a | 2.38 | 0.14 | 0.106 |
| CRP | 30 | 1.72 a | 4.00 | 9.53 | 2.18 a | 3.65 | 7.83 | 2.10 a | 2.80 | 893 | 0.151 |
| Ferritin | 37 | 1.09 a | 1.00 | 22.50 | 2.78 b | 155.00 | 121.00 | 2.12 c | 91.00 | 14050 | <0.001 |
| Folic acid | 20 | 2.25 a | 7.20 | 9.05 | 2.03 a | 9.55 | 9.28 | 1.73 a | 6.70 | 9.63 | 0.245 |
| HB | 38 | 1.16 a | 11.30 | 2.80 | 2.38 b | 13.05 | 2.50 | 2.46 b | 13.10 | 2.68 | <0.001 |
| HCT | 38 | 1.22 a | 36.90 | 7.80 | 2.43 b | 41.70 | 5.55 | 2.34 b | 40.65 | 8.43 | <0.001 |
| MCV | 38 | 1.34 a | 85.45 | 10.03 | 2.00 b | 88.05 | 9.82 | 2.66 c | 89.70 | 7.88 | <0.001 |
| MCHC | 38 | 1.55 a | 29.70 | 2.30 | 2.01 b | 30.75 | 2.13 | 2.43 b | 31.95 | 2.60 | 0.001 |
| Osteocalcin | 32 | 1.84 a | 17.90 | 15.73 | 1.86 a | 19.90 | 14.25 | 2.30 a | 23.55 | 15.00 | 0.116 |
| PHOSPHATE IN SERUM | 41 | 2.30 a | 3.38 | 0.65 | 1.88 a | 3.00 | 0.99 | 1.82 a | 3.19 | 0.76 | 0.054 |
| Phosphate in urine | 36 | 2.14 a | 58.45 | 57.78 | 1.88 a | 52.85 | 62.35 | 1.99 a | 53.60 | 63.45 | 0.527 |
| PTH | 37 | 1.92 a | 38.30 | 29.10 | 2.14 a | 33.60 | 27.90 | 1.95 a | 37.20 | 23.30 | 0.594 |
| FE | 38 | 1.17 a | 29.00 | 22.00 | 2.58 b | 81.50 | 50.50 | 2.25 b | 73.00 | 65.25 | <0.001 |
| TIBIC | 35 | 2.69 a | 361.00 | 62.00 | 1.57 b | 285.00 | 70.00 | 1.74 b | 280.00 | 103.00 | <0.001 |
| VIT D3 | 33 | 1.94 a | 19.20 | 18.35 | 1.86 a | 21.90 | 12.60 | 2.20 a | 24.60 | 14.50 | 0.359 |
| IFGF23 | 41 | 1.88 a | 51.53 | 17.05 | 1.98 a | 49.64 | 18.63 | 2.15 a | 54.49 | 22.13 | 0.461 |
| P1NP | 40 | 2.05 a | 53.80 | 38.29 | 1.88 a | 45.05 | 32.95 | 2.08 a | 54.98 | 41.86 | 0.622 |
| Ferric Derisomaltose | Ferric Carboxymaltose | Z | p | |||||
|---|---|---|---|---|---|---|---|---|
| Mean Rank | Me | IQR | Mean Rank | Me | IQR | |||
| Change between day 7 and baseline measurement | ||||||||
| Albumins | 9.15 | 0.11 | 0.57 | 8.50 | −0.14 | 0.57 | −0.23 | 0.821 |
| ALP | 8.75 | 3.00 | 17.75 | 10.17 | 5.00 | 6.00 | −0.44 | 0.659 |
| Vitamin B12 | 3.00 | −32.00 | 106.00 | 3.00 | 6.00 | 21.00 | 0.00 | 1.000 |
| CTX | 11.75 | −0.05 | 0.21 | 16.21 | 0.04 | 0.18 | −1.36 | 0.173 |
| Ca | 12.94 | 0.03 | 0.17 | 11.43 | 0.05 | 0.19 | −0.48 | 0.633 |
| CRP | 10.67 | 0.40 | 5.80 | 11.83 | 3.76 | 47.75 | −0.39 | 0.697 |
| Ferritin | 12.13 | 459.00 | 276.00 | 8.17 | 93.00 | 732.00 | −1.32 | 0.186 |
| Folate acid | 1.50 | −2.80 | 4.50 | 4.00 | 1.00 | 0.00 | −1.73 | 0.083 |
| Hb | 12.35 | 0.70 | 1.65 | 12.86 | 1.00 | 1.50 | −0.16 | 0.873 |
| Hct | 12.15 | 2.30 | 4.25 | 13.36 | 3.50 | 5.40 | −0.38 | 0.703 |
| MCV | 12.85 | 1.60 | 3.15 | 11.64 | 2.20 | 5.20 | −0.38 | 0.703 |
| MCHC | 12.12 | −0.10 | 1.00 | 13.43 | 0.20 | 0.80 | −0.41 | 0.679 |
| Osteocalcin | 12.33 | −4.85 | 10.00 | 13.00 | −5.75 | 14.85 | −0.20 | 0.841 |
| Phosphate | 14.18 | −0.32 | 1.21 | 8.43 | −0.99 | 0.75 | −1.81 | 0.070 |
| Phosphate in urine | 12.27 | −4.10 | 33.80 | 9.86 | −12.70 | 177.30 | −0.81 | 0.418 |
| PTH | 14.56 | −1.85 | 23.25 | 9.00 | −22.40 | 44.50 | −1.69 | 0.090 |
| Fe | 9.13 | 53.00 | 54.00 | 13.25 | 117.00 | 149.50 | −1.30 | 0.193 |
| TIBIC | 9.67 | −32.00 | 101.00 | 11.25 | −3.50 | 103.00 | −0.50 | 0.617 |
| Vitamin D3 | 6.00 | −0.75 | 2.40 | 8.60 | −0.20 | 1.80 | −1.17 | 0.241 |
| iFGF23 | 11.59 | 12.01 | 40.60 | 13.17 | 21.74 | 114.16 | −0.49 | 0.624 |
| P1NP | 10.40 | −9.90 | 26.37 | 12.50 | −5.20 | 23.69 | −0.70 | 0.484 |
| Measurement 7th day | ||||||||
| Albumins | 9.23 | 4.35 | 0.37 | 8.25 | 4.19 | 1.02 | −0.34 | 0.734 |
| ALP | 8.50 | 68.00 | 36.00 | 11.33 | 70.00 | −66.00 | −0.88 | 0.377 |
| Vitamin B12 | 4.50 | 548.50 | -475.00 | 2.00 | 338.00 | −291.00 | −1.73 | 0.083 |
| CTX | 12.17 | 0.27 | 0.44 | 15.14 | 0.35 | 0.25 | −0.91 | 0.364 |
| Ca | 13.74 | 2.41 | 0.18 | 9.50 | 2.23 | 0.27 | −1.34 | 0.182 |
| CRP | 10.40 | 3.30 | 9.20 | 12.50 | 6.75 | 5.48 | −0.70 | 0.483 |
| Ferritin | 12.13 | 482.00 | 238.00 | 8.17 | 145.50 | 840.50 | −1.32 | 0.186 |
| Folate acid | 1.50 | 2.90 | −2.80 | 4.00 | 3.90 | −380 | −1.73 | 0.083 |
| Hb | 12.38 | 11.70 | 2.95 | 12.79 | 11.60 | 2.90 | −0.13 | 0.899 |
| Hct | 12.38 | 38.80 | 8.05 | 12.79 | 36.70 | 8.80 | −0.13 | 0.899 |
| MCV | 13.06 | 89.50 | 10.65 | 11.14 | 85.60 | 7.90 | −0.60 | 0.546 |
| MCHC | 12.76 | 30.40 | 1.35 | 11.86 | 30.70 | 2.40 | −0.29 | 0.775 |
| Osteocalcin | 11.53 | 17.60 | 10.73 | 15.42 | 22.25 | 19.58 | −1.17 | 0.243 |
| Phosphate | 13.65 | 2.95 | 1.25 | 9.71 | 2.65 | 1.63 | −1.24 | 0.216 |
| Phosphate in urine | 11.19 | 23.10 | 33.80 | 13.86 | 54.20 | 77.40 | −0.87 | 0.385 |
| PTH | 14.39 | 36.50 | 29.15 | 9.43 | 29.10 | 26.00 | −1.51 | 0.130 |
| Fe | 9.53 | 110.00 | 63.00 | 11.75 | 162.50 | 183.50 | −0.70 | 0.484 |
| TIBIC | 10.03 | 306.00 | 50.00 | 9.88 | 308.00 | 100.50 | −0.05 | 0.960 |
| Vitamin D3 | 7.25 | 19.40 | 24.80 | 6.60 | 14.20 | 20.55 | −0.29 | 0.770 |
| iFGF23 | 12.44 | 64.40 | 38.52 | 12.67 | 61.98 | 118.35 | −0.07 | 0.947 |
| P1NP | 11.67 | 44.65 | 27.50 | 15.00 | 55.75 | 59.98 | −1.00 | 0.317 |
| Change * | Ferric Derisomaltose (n = 39) | Ferric Carboxymaltose (n = 11) | Z | p | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean Rank | Me | IQR | Mean Rank | Me | IQR | ||||
| Albumins | 1 | 21.18 | 0.17 | 0.34 | 25.11 | 0.17 | 0.62 | −0.84 | 0.03 |
| 2 | 21.04 | 0.07 | 0.40 | 27.45 | 0.31 | 0.56 | −1.39 | 0.166 | |
| ALP | 1 | 23.28 | 1.00 | 14.50 | 21.69 | 0.50 | 23.00 | −0.31 | 0.755 |
| 2 | 23.71 | 3.50 | 22.50 | 22.75 | 1.50 | 25.00 | −0.20 | 0.842 | |
| Vitamin B12 | 1 | 17.54 | −17.00 | 162.00 | 17.36 | −60.00 | 167.00 | −0.04 | 0.966 |
| 2 | 19.98 | 5.00 | 111.00 | 14.06 | −95.00 | 166.50 | −1.46 | 0.144 | |
| CTX | 1 | 23.79 | −0.02 | 0.25 | 29.70 | 0.04 | 0.32 | −1.17 | 0.244 |
| 2 | 21.47 | −0.04 | 0.18 | 24.00 | −0.04 | 0.45 | −0.54 | 0.591 | |
| Ca | 1 | 24.21 | 0.01 | 0.13 | 30.09 | 0.10 | 0.20 | −1.18 | 0.236 |
| 2 | 22.73 | 0.02 | 0.15 | 28.70 | 0.09 | 0.12 | −1.22 | 0.221 | |
| CRP | 1 | 23.44 | 0.55 | 3.23 | 17.82 | 0.20 | 34.30 | −1.28 | 0.200 |
| 2 | 19.77 | 0.40 | 7.40 | 18.29 | 0.70 | 37.30 | −0.32 | 0.749 | |
| Ferritin | 1 | 22.51 | 132.00 | 132.00 | 29.50 | 196.50 | 227.00 | −1.43 | 0.153 |
| 2 | 21.85 | 54.50 | 116.50 | 24.70 | 87.00 | 251.50 | −0.62 | 0.538 | |
| Folate acid | 1 | 16.75 | −0.10 | 5.08 | 18.40 | 0.00 | 3.10 | −0.35 | 0.725 |
| 2 | 16.58 | −0.55 | 10.85 | 14.00 | −1.00 | 3.30 | −0.66 | 0.508 | |
| Hb | 1 | 25.49 | 1.85 | 1.75 | 23.32 | 1.60 | 2.30 | −0.44 | 0.657 |
| 2 | 22.59 | 1.90 | 2.40 | 22.17 | 1.90 | 1.35 | −0.09 | 0.930 | |
| Hct | 1 | 24.64 | 3.90 | 4.33 | 26.23 | 4.80 | 4.90 | −0.32 | 0.746 |
| 2 | 22.69 | 4.50 | 6.00 | 21.78 | 3.90 | 2.70 | −0.19 | 0.850 | |
| MCV | 1 | 24.80 | 3.25 | 4.63 | 25.68 | 3.60 | 6.10 | −0.18 | 0.857 |
| 2 | 22.59 | 5.40 | 7.70 | 22.17 | 4.40 | 5.25 | −0.09 | 0.930 | |
| MCHC | 1 | 26.22 | 1.20 | 2.70 | 20.77 | 0.10 | 2.20 | −1.11 | 0.265 |
| 2 | 22.11 | 1.10 | 2.80 | 24.00 | 1.30 | 4.65 | −0.39 | 0.694 | |
| Osteocalcin | 1 | 21.22 | −1.40 | 9.80 | 22.40 | 0.15 | 18.0 | −0.27 | 0.790 |
| 2 | 21.71 | 3.00 | 13.68 | 20.63 | −0.10 | 25.90 | −0.22 | 0.823 | |
| Phosphate | 1 | 26.51 | −0.02 | 0.68 | 19.10 | −0.31 | 1.70 | −1.46 | 0.143 |
| 2 | 24.42 | −0.25 | 0.64 | 22.45 | −0.25 | 1.46 | −0.40 | 0.687 | |
| Phosphate in urine | 1 | 22.71 | −6.10 | 38.48 | 21.80 | −6.85 | 47.08 | −0.20 | 0.845 |
| 2 | 21.72 | −4.10 | 67.15 | 17.27 | −17.60 | 71.40 | −1.08 | 0.282 | |
| PTH | 1 | 24.07 | 0.40 | 14.35 | 25.95 | 11.40 | 32.50 | −0.39 | 0.695 |
| 2 | 22.67 | 0.40 | 17.95 | 17.22 | −5.30 | 35.15 | −1.18 | 0.238 | |
| Fe | 1 | 22.22 | 33.50 | 47.00 | 29.82 | 58.00 | 61.00 | −1.61 | 0.108 |
| 2 | 22.78 | 35.50 | 55.50 | 26.10 | 46.00 | 71.00 | −0.69 | 0.488 | |
| TIBIC | 1 | 21.59 | −82.00 | 107.00 | 27.95 | −43.00 | 86.00 | −1.35 | 0.176 |
| 2 | 21.89 | −77.00 | 90.00 | 26.90 | −39.50 | 56.50 | −1.06 | 0.287 | |
| Vitamin D3 | 1 | 21.34 | −0.15 | 7.95 | 19.78 | −1.40 | 5.60 | −0.35 | 0.729 |
| 2 | 21.05 | 2.90 | 16.70 | 18.85 | 4.20 | 7.20 | −0.52 | 0.606 | |
| iFGF23 | 1 | 25.10 | 0.97 | 11.04 | 24.60 | −1.84 | 58.10 | −0.10 | 0.921 |
| 2 | 24.71 | 2.18 | 11.72 | 19.64 | −0.94 | 26.93 | −1.09 | 0.274 | |
| P1NP | 1 | 23.43 | −3.00 | 35.69 | 23.78 | −3.78 | 51.46 | −0.07 | 0.945 |
| 2 | 23.23 | 0.34 | 23.79 | 22.20 | −0.70 | 73.53 | −0.22 | 0.827 | |
| MEASUREMENT “0” | |||||
|---|---|---|---|---|---|
| Phosphate | Phosphate in Urine | iFGF23 | |||
| MEASUREMENT “0” | Phosphate in urine | r | −0.09 | – | – |
| p | 0.531 | ||||
| iFGF23 | r | 0.23 | −0.13 | – | |
| p | 0.095 | 0.359 | |||
| MEASUREMENT AFTER 4 WEEKS | Phosphate | r | 0.50 | −0.17 | 0.14 |
| p | <0.001 | 0.249 | 0.351 | ||
| Phosphate in urine | r | 0.01 | 0.29 | −0.05 | |
| p | 0.933 | 0.056 | 0.768 | ||
| iFGF23 | r | 0.25 | −0.08 | 0.50 | |
| p | 0.081 | 0.592 | <0.001 | ||
| MEASUREMENT AFTER 12 WEEKS | Phosphate | r | 0.46 | 0.08 | −0.02 |
| p | 0.001 | 0.606 | 0.893 | ||
| Phosphate in urine | r | −0.16 | 0.31 | −0.08 | |
| p | 0.307 | 0.054 | 0.628 | ||
| iFGF23 | r | 0.17 | 0.02 | 0.57 | |
| p | 0.245 | 0.892 | <0.001 | ||
| MEASUREMENT “0” | ||||||
|---|---|---|---|---|---|---|
| Phosphate | Phosphate in Urine | IFGF23 | ||||
| Ferric derisomaltose | MEASUREMENT “0” | Phosphate in urine | R | −0.11 | - | - |
| p | 0.499 | |||||
| iFGF23 | R | 0.33 | −0.09 | - | ||
| p | 0.033 | 0.576 | ||||
| MEASUREMENT AFTER 4 WEEKS | Phosphate | R | 0.50 | −0.25 | 0.19 | |
| p | 0.001 | 0.134 | 0.259 | |||
| Phosphate in urine | r | −0.08 | 0.17 | 0.01 | ||
| p | 0.658 | 0.337 | 0.964 | |||
| iFGF23 | r | 0.28 | −0.01 | 0.63 | ||
| p | 0.082 | 0.946 | <0.001 | |||
| MEASUREMENT AFTER 12 WEEKS | Phosphate | r | 0.37 | −0.07 | 0.13 | |
| p | 0.026 | 0.718 | 0.438 | |||
| Phosphate in urine | r | −0.22 | 0.36 | −0.03 | ||
| p | 0.233 | 0.055 | 0.879 | |||
| IFGF23 | r | 0.29 | 0.02 | 0.68 | ||
| p | 0.085 | 0.899 | <0.001 | |||
| Ferric carboxymaltose | MEASUREMENT “0” | Phosphate in urine | r | −0.14 | - | - |
| p | 0.661 | |||||
| iFGF23 | r | −0.35 | −0.30 | - | ||
| p | 0.289 | 0.365 | ||||
| MEASUREMENT AFTER 4 WEEKS | Phosphate | r | 0.72 | −0.01 | −0.13 | |
| p | 0.019 | 0.978 | 0.733 | |||
| Phosphate in urine | r | 0.30 | 0.60 | −0.37 | ||
| p | 0.407 | 0.065 | 0.333 | |||
| iFGF23 | r | 0.17 | −0.22 | 0.01 | ||
| p | 0.648 | 0.536 | 0.989 | |||
| MEASUREMENT AFTER 12 WEEKS | Phosphate | r | 0.67 | 0.26 | −0.41 | |
| p | 0.035 | 0.474 | 0.240 | |||
| Phosphate in urine | r | 0.10 | 0.32 | −0.45 | ||
| p | 0.778 | 0.341 | 0.169 | |||
| iFGF23 | R | −0.01 | 0.13 | 0.16 | ||
| p | 0.975 | 0.707 | 0.641 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tulewicz-Marti, E.; Szwarc, P.; Więcek, M.; Lewandowski, K.; Korcz, T.; Cicha, M.; Rydzewska, G. Effect of Intravenous Iron Administration on Bone Mineral and Iron Homeostasis in Patients with Inflammatory Bowel Disease—Results of a Prospective Single-Centre Study. J. Pers. Med. 2023, 13, 458. https://doi.org/10.3390/jpm13030458
Tulewicz-Marti E, Szwarc P, Więcek M, Lewandowski K, Korcz T, Cicha M, Rydzewska G. Effect of Intravenous Iron Administration on Bone Mineral and Iron Homeostasis in Patients with Inflammatory Bowel Disease—Results of a Prospective Single-Centre Study. Journal of Personalized Medicine. 2023; 13(3):458. https://doi.org/10.3390/jpm13030458
Chicago/Turabian StyleTulewicz-Marti, Edyta, Paulina Szwarc, Martyna Więcek, Konrad Lewandowski, Tomasz Korcz, Malgorzata Cicha, and G. Rydzewska. 2023. "Effect of Intravenous Iron Administration on Bone Mineral and Iron Homeostasis in Patients with Inflammatory Bowel Disease—Results of a Prospective Single-Centre Study" Journal of Personalized Medicine 13, no. 3: 458. https://doi.org/10.3390/jpm13030458
APA StyleTulewicz-Marti, E., Szwarc, P., Więcek, M., Lewandowski, K., Korcz, T., Cicha, M., & Rydzewska, G. (2023). Effect of Intravenous Iron Administration on Bone Mineral and Iron Homeostasis in Patients with Inflammatory Bowel Disease—Results of a Prospective Single-Centre Study. Journal of Personalized Medicine, 13(3), 458. https://doi.org/10.3390/jpm13030458






