Combining Preoperative Clinical and Imaging Characteristics to Predict MVI in Hepatitis B Virus-Related Combined Hepatocellular Carcinoma and Cholangiocarcinoma
Abstract
1. Introduction
2. Materials and Methods
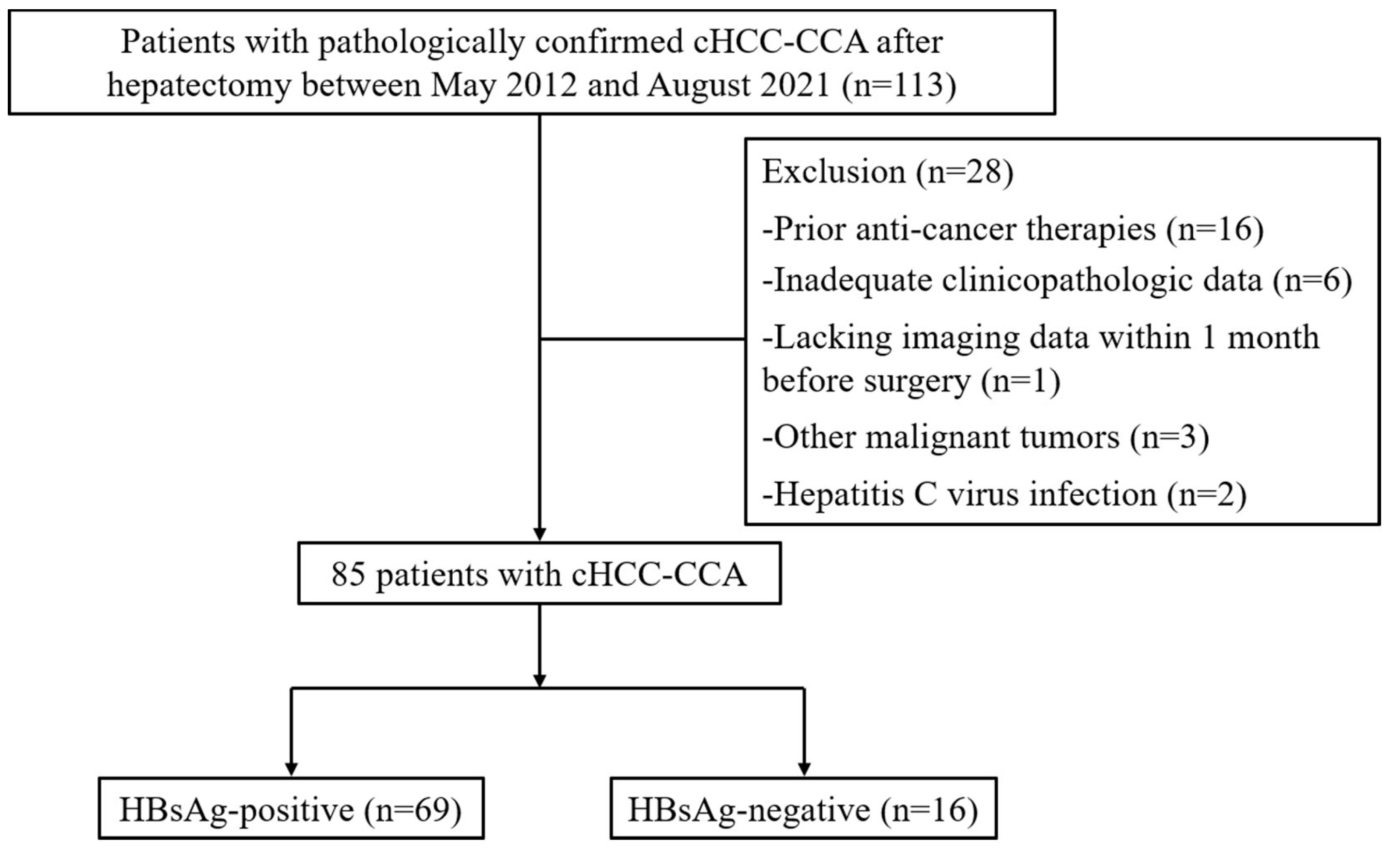
2.1. Study Population
2.2. Follow-Up
2.3. Image Analysis
2.4. Clinical Data and Pathological Evaluation
2.5. Statistical Analysis
3. Results
3.1. Clinical and Imaging Characteristics of Patients
3.2. Univariate and Multivariate Analyses
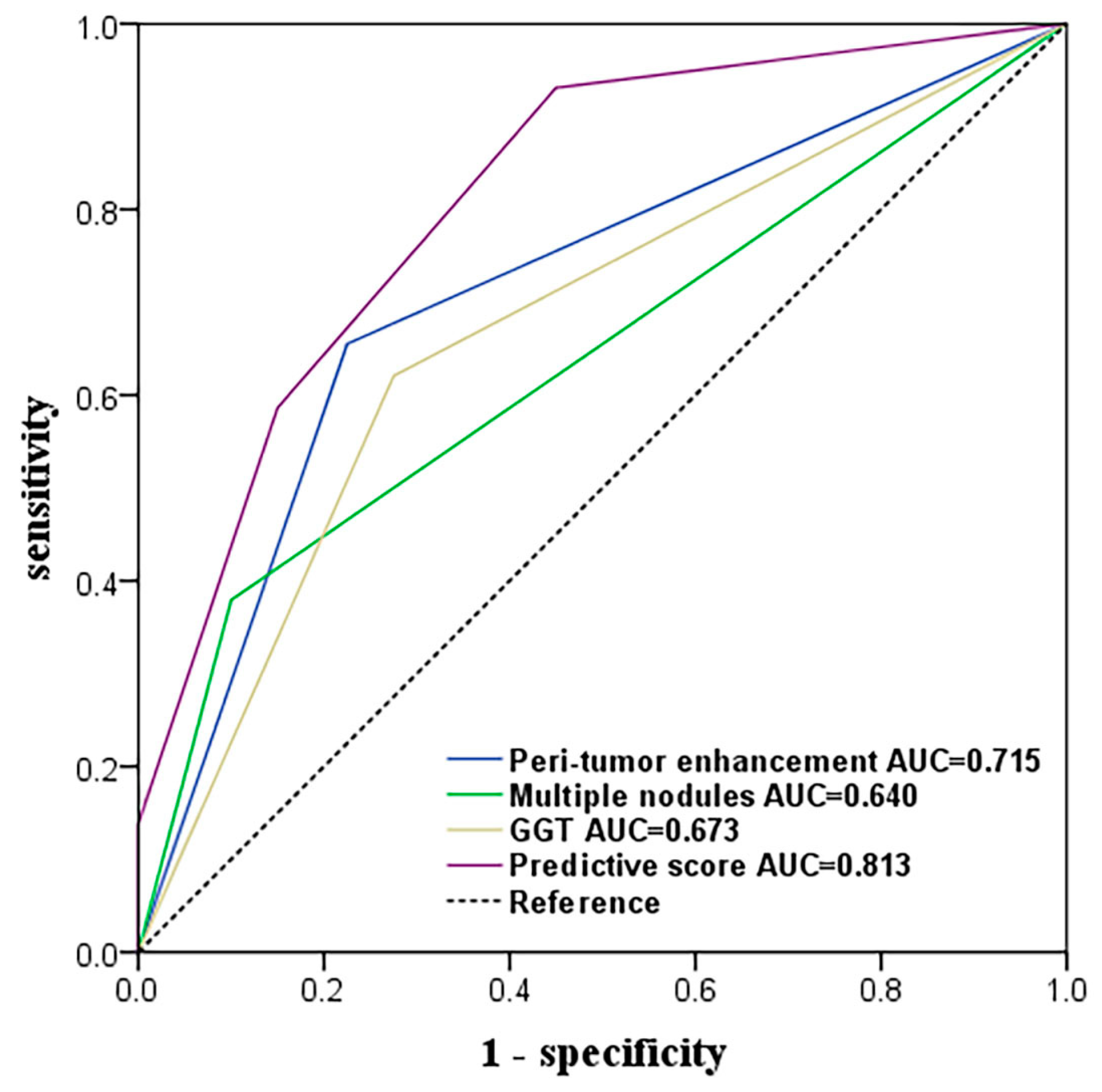
3.3. Ability to Predict MVI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramai, D.; Ofosu, A.; Lai, J.K.; Reddy, M.; Adler, D.G. Combined Hepatocellular Cholangiocarcinoma: A Population-Based Retrospective Study. Am. J. Gastroenterol. 2019, 114, 1496–1501. [Google Scholar] [CrossRef]
- Beaufrère, A.; Calderaro, J.; Paradis, V. Combined hepatocellular-cholangiocarcinoma: An update. J. Hepatol. 2021, 74, 1212–1224. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.J.; Lu, C.D.; Dong, H.; Fu, X.H.; Zhang, H.W.; Yao, X.P. Hepatitis B virus-related combined hepatocellular-cholangiocarcinoma: Clinicopathological and prognostic analysis of 390 cases. Eur. J. Gastroenterol. Hepatol. 2014, 26, 192–199. [Google Scholar] [CrossRef]
- Yin, X.; Zhang, B.H.; Qiu, S.J.; Ren, Z.G.; Zhou, J.; Chen, X.H.; Zhou, Y.; Fan, J. Combined hepatocellular carcinoma and cholangiocarcinoma: Clinical features, treatment modalities, and prognosis. Ann. Surg. Oncol. 2012, 19, 2869–2876. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Hsieh, S.Y.; Chang, C.J.; Lin, Y.J. Comparison of clinical characteristics of combined hepatocellular-cholangiocarcinoma and other primary liver cancers. J. Gastroenterol. Hepatol. 2013, 28, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Garancini, M.; Goffredo, P.; Pagni, F.; Romano, F.; Roman, S.; Sosa, J.A.; Giardini, V. Combined hepatocellular-cholangiocarcinoma: A population-level analysis of an uncommon primary liver tumor. Liver. Transpl. 2014, 20, 952–959. [Google Scholar] [CrossRef]
- Spolverato, G.; Bagante, F.; Tsilimigras, D.; Ejaz, A.; Cloyd, J.; Pawlik, T.M. Management and outcomes among patients with mixed hepatocholangiocellular carcinoma: A population-based analysis. J. Surg. Oncol. 2019, 119, 278–287. [Google Scholar] [CrossRef]
- Erstad, D.J.; Tanabe, K.K. Prognostic and Therapeutic Implications of Microvascular Invasion in Hepatocellular Carcinoma. Ann. Surg. Oncol. 2019, 26, 1474–1493. [Google Scholar] [CrossRef]
- Dhir, M.; Melin, A.A.; Douaiher, J.; Lin, C.; Zhen, W.K.; Hussain, S.M.; Geschwind, J.F.; Doyle, M.B.; Abou-Alfa, G.K.; Are, C. A Review and Update of Treatment Options and Controversies in the Management of Hepatocellular Carcinoma. Ann. Surg. 2016, 263, 1112–1125. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, S.G.; Park, E.H.; Hwang, S.; Ahn, C.S.; Moon, D.B.; Ha, T.Y.; Song, G.W.; Jung, D.H.; Kim, K.M.; et al. Surgical treatments and prognoses of patients with combined hepatocellular carcinoma and cholangiocarcinoma. Ann. Surg. Oncol. 2009, 16, 623–629. [Google Scholar] [CrossRef]
- Jiang, X.X.; Huang, X.T.; Huang, C.S.; Chen, L.H.; Liang, L.J.; Yin, X.Y. Long-term outcome and prognostic factors of combined hepatocellular carcinoma and cholangiocarcinoma after curative resection. Gastroenterol. Rep. 2020, 8, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wang, L.; Teng, F.; Zhang, T.; Zhao, Y.; Chen, Z. The clinical characteristics and prognostic factors of combined Hepatocellular Carcinoma and Cholangiocarcinoma, Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma after Surgical Resection: A propensity score matching analysis. Int. J. Med. Sci. 2021, 18, 187–198. [Google Scholar] [CrossRef]
- Pawlik, T.M.; Gleisner, A.L.; Anders, R.A.; Assumpcao, L.; Maley, W.; Choti, M.A. Preoperative assessment of hepatocellular carcinoma tumor grade using needle biopsy: Implications for transplant eligibility. Ann. Surg. 2007, 245, 435–442. [Google Scholar] [CrossRef]
- Zhao, W.C.; Fan, L.F.; Yang, N.; Zhang, H.B.; Chen, B.D.; Yang, G.S. Preoperative predictors of microvascular invasion in multinodular hepatocellular carcinoma. Eur. J. Surg. Oncol. 2013, 39, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Renzulli, M.; Brocchi, S.; Cucchetti, A.; Mazzotti, F.; Mosconi, C.; Sportoletti, C.; Brandi, G.; Pinna, A.D.; Golfieri, R. Can Current Preoperative Imaging Be Used to Detect Microvascular Invasion of Hepatocellular Carcinoma? Radiology 2016, 279, 432–442. [Google Scholar] [CrossRef]
- Yang, L.; Gu, D.; Wei, J.; Yang, C.; Rao, S.; Wang, W.; Chen, C.; Ding, Y.; Tian, J.; Zeng, M. A Radiomics Nomogram for Preoperative Prediction of Microvascular Invasion in Hepatocellular Carcinoma. Liver Cancer 2019, 8, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, S.K.; Zhou, J.; Sun, H.C.; Qiu, S.J.; Ye, Q.H.; Wang, L.; Fan, J. Positive HBcAb is associated with higher risk of early recurrence and poorer survival after curative resection of HBV-related HCC. Liver Int. 2016, 36, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Li, N.; Li, S.; Shi, J.; Guo, W.; Zheng, Y.; Cheng, S. Hepatitis B virus infection and active replication promote the formation of vascular invasion in hepatocellular carcinoma. BMC Cancer 2017, 17, 304. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Ma, X.; Lu, X.; Li, S.; Zeng, M.; Xu, K.; Yang, C. Combined hepatocellular-cholangiocarcinoma: Which preoperative clinical data and conventional MRI characteristics have value for the prediction of microvascular invasion and clinical significance? Eur. Radiol. 2020, 30, 5337–5347. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, C.W.; Zhu, G.Q.; Li, N.; Qian, X.L.; Chong, H.H.; Yang, C.; Zeng, M.S. A multidimensional nomogram combining imaging features and clinical factors to predict the invasiveness and metastasis of combined hepatocellular cholangiocarcinoma. Ann. Transl. Med. 2021, 9, 1518. [Google Scholar] [CrossRef]
- Sullivan, L.M.; Massaro, J.M.; D’Agostino, R.B., Sr. Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat. Med. 2004, 23, 1631–1660. [Google Scholar] [CrossRef]
- Cutolo, C.; Dell’Aversana, F.; Fusco, R.; Grazzini, G.; Chiti, G.; Simonetti, I.; Bruno, F.; Palumbo, P.; Pierpaoli, L.; Valeri, T.; et al. Combined Hepatocellular-Cholangiocarcinoma: What the Multidisciplinary Team Should Know. Diagnostics 2022, 12, 890. [Google Scholar] [CrossRef] [PubMed]
- Schizas, D.; Mastoraki, A.; Routsi, E.; Papapanou, M.; Tsapralis, D.; Vassiliu, P.; Toutouzas, K.; Felekouras, E. Combined hepatocellular-cholangiocarcinoma: An update on epidemiology, classification, diagnosis and management. Hepatobiliary Pancreat. Dis. Int. 2020, 19, 515–523. [Google Scholar] [CrossRef]
- Yang, P.; Si, A.; Yang, J.; Cheng, Z.; Wang, K.; Li, J.; Xia, Y.; Zhang, B.; Pawlik, T.M.; Lau, W.Y.; et al. A wide-margin liver resection improves long-term outcomes for patients with HBV-related hepatocellular carcinoma with microvascular invasion. Surgery 2019, 165, 721–730. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, H.; Chen, Y.; Zeng, J.; Huang, Q.; Zhang, J.; Zeng, Y.; Liu, J. Prognostic significance of three-tiered pathological classification for microvascular invasion in patients with combined hepatocellular-cholangiocarcinoma following hepatic resection. Cancer Med. 2022. [Google Scholar] [CrossRef]
- Wang, T.; Yang, X.; Tang, H.; Kong, J.; Shen, S.; Qiu, H.; Wang, W. Integrated nomograms to predict overall survival and recurrence-free survival in patients with combined hepatocellular cholangiocarcinoma (cHCC) after liver resection. Aging 2020, 12, 15334–15358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Hu, K.; Tang, B.; Tian, M.; Lu, S.; Yuan, J.; Li, M.; Chen, R.; Ren, Z.; Shi, Y.; et al. A New Scoring Method for Personalized Prognostic Prediction in Patients with Combined Hepatocellular and Cholangiocarcinoma After Surgery. J. Gastrointest. Surg. 2021, 25, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Wu, T.C.; Lin, H.Y.; Hung, C.M.; Hsieh, P.M.; Yeh, J.H.; Hsiao, P.; Huang, Y.L.; Li, Y.C.; Wang, Y.C.; et al. Clinical features and outcomes of combined hepatocellular carcinoma and cholangiocarcinoma versus hepatocellular carcinoma versus cholangiocarcinoma after surgical resection: A propensity score matching analysis. BMC Gastroenterol. 2021, 21, 20. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.; Yen, C.; Shan, Y.; Lin, Y.; Liu, I.; Huang, H.; Yeh, M.; Chan, S.; Tsai, H. Comparing the clinicopathological characteristics of combined hepatocellular-cholangiocarcinoma with those of other primary liver cancers by use of the updated World Health Organization classification. Histopathology 2021, 79, 556–572. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, Y.; Du, C.; Markowitz, G.J.; Fu, J.; Zhang, Z.; Liu, C.; Qin, W.; Wang, H.; Wang, F.; et al. Hepatitis B-Induced IL8 Promotes Hepatocellular Carcinoma Venous Metastasis and Intrahepatic Treg Accumulation. Cancer Res. 2021, 81, 2386–2398. [Google Scholar] [CrossRef]
- Lei, Z.; Li, J.; Wu, D.; Xia, Y.; Wang, Q.; Si, A.; Wang, K.; Wan, X.; Lau, W.Y.; Wu, M.; et al. Nomogram for Preoperative Estimation of Microvascular Invasion Risk in Hepatitis B Virus-Related Hepatocellular Carcinoma Within the Milan Criteria. JAMA Surg. 2016, 151, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.T.; Wang, Z.; Huang, X.W.; Chen, S.L.; Zheng, X.; Ruan, S.M.; Xie, X.Y.; Lu, M.D.; Yu, J.; Tian, J.; et al. Ultrasound-based radiomics score: A potential biomarker for the prediction of microvascular invasion in hepatocellular carcinoma. Eur. Radiol. 2019, 29, 2890–2901. [Google Scholar] [CrossRef] [PubMed]
- Sterling, R.K.; Wright, E.C.; Morgan, T.R.; Seeff, L.B.; Hoefs, J.C.; Di Bisceglie, A.M.; Dienstag, J.L.; Lok, A.S. Frequency of elevated hepatocellular carcinoma (HCC) biomarkers in patients with advanced hepatitis C. Am. J. Gastroenterol. 2012, 107, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhao, Y.; Jia, J.; Chen, H.; Bai, W.; Yang, M.; Yin, Z.; He, C.; Zhang, L.; Guo, W.; et al. The Prognostic Value of Alpha-Fetoprotein Response for Advanced-Stage Hepatocellular Carcinoma Treated with Sorafenib Combined with Transarterial Chemoembolization. Sci. Rep. 2016, 6, 19851. [Google Scholar] [CrossRef]
- Kim, H.; Park, M.S.; Choi, J.Y.; Park, Y.N.; Kim, M.J.; Kim, K.S.; Choi, J.S.; Han, K.H.; Kim, E.; Kim, K.W. Can microvessel invasion of hepatocellular carcinoma be predicted by pre-operative MRI? Eur. Radiol. 2009, 19, 1744–1751. [Google Scholar] [CrossRef] [PubMed]
- Lautt, W.W. Mechanism and role of intrinsic regulation of hepatic arterial blood flow: Hepatic arterial buffer response. Am. J. Physiol. 1985, 249 (Pt 1), G549–G556. [Google Scholar] [CrossRef]
- Nishie, A.; Yoshimitsu, K.; Asayama, Y.; Irie, H.; Tajima, T.; Hirakawa, M.; Ishigami, K.; Nakayama, T.; Kakihara, D.; Nishihara, Y.; et al. Radiologic detectability of minute portal venous invasion in hepatocellular carcinoma. AJR. Am. J. Roentgenol. 2008, 190, 81–87. [Google Scholar] [CrossRef]
- Pompella, A.; De Tata, V.; Paolicchi, A.; Zunino, F. Expression of gamma-glutamyltransferase in cancer cells and its significance in drug resistance. Biochem. Pharmacol. 2006, 71, 231–238. [Google Scholar] [CrossRef]
- Hanigan, M.H. Gamma-glutamyl transpeptidase: Redox regulation and drug resistance. Adv. Cancer Res. 2014, 122, 103–141. [Google Scholar]
- Hanigan, M.H.; Ricketts, W.A. Extracellular glutathione is a source of cysteine for cells that express gamma-glutamyl transpeptidase. Biochemistry 1993, 32, 6302–6306. [Google Scholar] [CrossRef] [PubMed]
- Corti, A.; Belcastro, E.; Dominici, S.; Maellaro, E.; Pompella, A. The dark side of gamma-glutamyltransferase (GGT): Pathogenic effects of an ’antioxidant’ enzyme. Free Radic. Biol. Med. 2020, 160, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; Huang, J.; Yang, L. The prognostic significance of pretreatment serum γ-glutamyltranspeptidase in primary liver cancer: A meta-analysis and systematic review. Biosci. Rep. 2018, 38, bsr20181058. [Google Scholar] [CrossRef] [PubMed]
- Ju, M.J.; Qiu, S.J.; Fan, J.; Zhou, J.; Gao, Q.; Cai, M.Y.; Li, Y.W.; Tang, Z.Y. Preoperative serum gamma-glutamyl transferase to alanine aminotransferase ratio is a convenient prognostic marker for Child-Pugh A hepatocellular carcinoma after operation. J. Gastroenterol. 2009, 44, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhao, R.; Chen, F.; Zhu, Y.; Chen, L. Preoperative prediction of microvascular invasion in non-metastatic hepatocellular carcinoma based on nomogram analysis. Transl. Oncol. 2021, 14, 100875. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, H.; Zhang, J.; Wu, Y.; Zhou, W.; Cheng, Z.; Lou, J.; Zheng, S.; Bi, X.; Wang, J.; et al. Prognostic value and predication model of microvascular invasion in patients with intrahepatic cholangiocarcinoma: A multicenter study from China. BMC Cancer 2021, 21, 1299. [Google Scholar] [CrossRef]
- Pawlik, T.M.; Delman, K.A.; Vauthey, J.N.; Nagorney, D.M.; Ng, I.O.; Ikai, I.; Yamaoka, Y.; Belghiti, J.; Lauwers, G.Y.; Poon, R.T.; et al. Tumor size predicts vascular invasion and histologic grade: Implications for selection of surgical treatment for hepatocellular carcinoma. Liver. Transpl. 2005, 11, 1086–1092. [Google Scholar] [CrossRef]
- Kim, B.K.; Han, K.H.; Park, Y.N.; Park, M.S.; Kim, K.S.; Choi, J.S.; Moon, B.S.; Chon, C.Y.; Moon, Y.M.; Ahn, S.H. Prediction of microvascular invasion before curative resection of hepatocellular carcinoma. J. Surg. Oncol. 2008, 97, 246–252. [Google Scholar] [CrossRef]
- Chandarana, H.; Robinson, E.; Hajdu, C.H.; Drozhinin, L.; Babb, J.S.; Taouli, B. Microvascular invasion in hepatocellular carcinoma: Is it predictable with pretransplant MRI? AJR. Am. J. Roentgenol. 2011, 196, 1083–1089. [Google Scholar] [CrossRef]
- Wang, W.T.; Yang, L.; Yang, Z.X.; Hu, X.X.; Ding, Y.; Yan, X.; Fu, C.X.; Grimm, R.; Zeng, M.S.; Rao, S.X. Assessment of Microvascular Invasion of Hepatocellular Carcinoma with Diffusion Kurtosis Imaging. Radiology 2018, 286, 571–580. [Google Scholar] [CrossRef]
- Leoni, S.; Sansone, V.; Lorenzo, S.; Ielasi, L.; Tovoli, F.; Renzulli, M.; Golfieri, R.; Spinelli, D.; Piscaglia, F. Treatment of Combined Hepatocellular and Cholangiocarcinoma. Cancers 2020, 12, 794. [Google Scholar] [CrossRef] [PubMed]
- Kassahun, W.T.; Hauss, J. Management of combined hepatocellular and cholangiocarcinoma. Int. J. Clin. Pract. 2008, 62, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Beppu, T.; Ishiko, T.; Mizumoto, T.; Masuda, T.; Okabe, K.; Baba, Y.; Okabe, H.; Takamori, H.; Kanemitsu, K.; et al. A 42-month disease free survival case of combined hepatocellular-cholangiocarcinoma with lymph node metastases treated with multimodal therapy. Gan Kagaku Ryoho 2006, 33, 1941–1943. [Google Scholar]
- Trikalinos, N.A.; Zhou, A.; Doyle, M.B.M.; Fowler, K.J.; Morton, A.; Vachharajani, N.; Amin, M.; Keller, J.W.; Chapman, W.C.; Brunt, E.M.; et al. Systemic Therapy for Combined Hepatocellular-Cholangiocarcinoma: A Single-Institution Experience. J. Natl. Compr. Canc. Netw. 2018, 16, 1193–1199. [Google Scholar] [CrossRef]
- Renzulli, M.; Ramai, D.; Singh, J.; Sinha, S.; Brandi, N.; Ierardi, A.M.; Albertini, E.; Sacco, R.; Facciorusso, A.; Golfieri, R. Locoregional Treatments in Cholangiocarcinoma and Combined Hepatocellular Cholangiocarcinoma. Cancers 2021, 13, 3336. [Google Scholar] [CrossRef] [PubMed]

| Variables | MVI-Negative (N = 40) | MVI-Positive (N = 29) | p Value |
|---|---|---|---|
| Age a | 51.1 ± 10.2 | 50.3 ± 8.0 | 0.702 |
| Gender (male) | 33 (82.5) | 23 (79.3) | 0.982 |
| HBcAb b | 38 (95.0) | 29 (100) | 0.506 |
| HBeAg | 7 (17.5) | 5 (17.2) | 1.000 |
| HBeAb | 30 (75.0) | 23 (79.3) | 0.897 |
| PLT < 100 × 109/L b | 2 (5.0) | 0 (0.0) | 0.506 |
| ALT > 50 (U/L) | 4 (10.0) | 6 (20.7) | 0.302 |
| GGT > 60 (U/L) | 11 (27.5) | 18 (62.1) | 0.009 |
| TBIL > 20 (umol/L) b | 3 (7.5) | 2 (6.9) | 1.000 |
| Alb < 40 (g/L) | 5 (12.5) | 5 (17.2) | 0.837 |
| AFP ≥ 400 (ng/mL) | 10 (25.0) | 5 (17.2) | 0.634 |
| CA 19-9 ≥ 35 (U/mL) | 11 (27.5) | 14 (48.3) | 0.129 |
| CEA ≥ 5 (ng/mL) | 8 (20.0) | 5 (17.2) | 1.000 |
| Tumor size (≥5 mm) | 14 (35.0) | 18 (62.1) | 0.048 |
| Multiple nodules | 4 (10.0) | 11 (37.9) | 0.013 |
| Tumor shape | 0.526 | ||
| Globular | 11 (27.5) | 5 (17.2) | |
| Lobulated | 19 (47.5) | 14 (48.3) | |
| Irregular | 10 (25.0) | 10 (34.5) | |
| Rim enhancement | 19 (47.5) | 19 (65.5) | 0.215 |
| Peri-tumor enhancement | 9 (22.5) | 19 (65.5) | 0.001 |
| Wash out | 22 (55.0) | 12 (41.4) | 0.383 |
| Delayed central enhancement | 7 (17.5) | 9 (31.0) | 0.305 |
| Peritumoral bile duct dilatation b | 2 (5.0) | 3 (10.3) | 0.643 |
| Enhancing capsule b | 5 (12.5) | 2 (6.9) | 0.690 |
| LR-M | 23 (57.5) | 21 (72.4) | 0.308 |
| Variables | Univariate Analyses | Multivariate Analyses | Score | |||||
|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | p Value | β | OR | 95%CI | p Value | ||
| GGT > 60 (U/L) Tumor size (≥5 mm) | 4.31 3.04 | 1.55–11.99 | 0.005 | 1.305 | 3.69 | 1.10–12.37 | 0.034 | 1 |
| 1.13–8.20 | 0.028 | |||||||
| Multiple nodules | 5.50 | 1.53–17.71 | 0.009 | 1.483 | 4.41 | 1.06–18.39 | 0.042 | 1 |
| Peri-tumor enhancement | 6.54 | 2.25–19.01 | <0.001 | 1.818 | 6.16 | 1.78–21.36 | 0.004 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.-S.; Zuo, M.-X.; Xie, C.-M. Combining Preoperative Clinical and Imaging Characteristics to Predict MVI in Hepatitis B Virus-Related Combined Hepatocellular Carcinoma and Cholangiocarcinoma. J. Pers. Med. 2023, 13, 246. https://doi.org/10.3390/jpm13020246
Huang S-S, Zuo M-X, Xie C-M. Combining Preoperative Clinical and Imaging Characteristics to Predict MVI in Hepatitis B Virus-Related Combined Hepatocellular Carcinoma and Cholangiocarcinoma. Journal of Personalized Medicine. 2023; 13(2):246. https://doi.org/10.3390/jpm13020246
Chicago/Turabian StyleHuang, Si-Si, Meng-Xuan Zuo, and Chuan-Miao Xie. 2023. "Combining Preoperative Clinical and Imaging Characteristics to Predict MVI in Hepatitis B Virus-Related Combined Hepatocellular Carcinoma and Cholangiocarcinoma" Journal of Personalized Medicine 13, no. 2: 246. https://doi.org/10.3390/jpm13020246
APA StyleHuang, S.-S., Zuo, M.-X., & Xie, C.-M. (2023). Combining Preoperative Clinical and Imaging Characteristics to Predict MVI in Hepatitis B Virus-Related Combined Hepatocellular Carcinoma and Cholangiocarcinoma. Journal of Personalized Medicine, 13(2), 246. https://doi.org/10.3390/jpm13020246





