Abstract
Targeted therapy (TT) has revolutionized cancer treatment, successfully applied in various settings. Adjuvant TT in resected early-stage gastrointestinal stromal tumors (GIST), melanoma, non-small cell lung cancer (NSCLC), and breast cancer has led to practice-changing achievements. In particular, standard treatments include BRAF inhibitors for melanoma, osimertinib for NSCLC, hormone therapy or HER2 TT for breast cancer, and imatinib for GIST. Despite the undeniable benefit derived from adjuvant TT, the optimal duration of TT and the appropriate managing of the relapse remain open questions. Furthermore, neoadjuvant TT is emerging as valuable, particularly in breast cancer, and ongoing studies evaluate TT in the perioperative setting for early-stage NSCLC. In this review, we aim to collect and describe the large amount of data available in the literature about adjuvant TT across different histologies, focusing on epidemiology, major advances, and future directions.
1. Introduction
In the last few decades, targeted therapy (TT) has completely revolutionized the cancer treatment landscape. Starting with the introduction of imatinib for advanced gastrointestinal stromal tumors (GIST) and hormone therapy for breast cancer, the identification of molecular targets for novel therapy has been the objective of the scientific community, in the light of precision oncology, which represents an established reality in the treatment of advanced cancers.
On the wave of the promising results obtained in the treatment of metastatic disease, an increasing number of studies are testing TT in the adjuvant setting, anticipating this approach to the early stages. Across some tumors, adjuvant TT is already a reality supported by solid data; in others, such as non-small cell lung cancer (NSCLC), substantial improvements have been recently achieved [1].
Imatinib was the first targeted therapy to be approved in the adjuvant setting. In the early 2000s, two trials, the Z9001 and Scandinavian Sarcoma Group trials, demonstrated the efficacy of adjuvant therapy with imatinib in resected high-risk GIST [2,3]. Based on these results, imatinib was approved by the European Medicines Agency (EMA) in the adjuvant setting for one year and three years of treatment. Based on the COMBI-AD trial [4], in 2018, dabrafenib and trametinib have been approved as adjuvant combination therapy for patients affected by stage III BRAFV600-mutant melanoma patients. In the landscape of breast cancer treatment, adjuvant TT stands as a cornerstone. However, there are several recent or ongoing studies aimed at further improving the outcome in this early disease setting.
Finally, resected lung cancer patients, in stage IB-IIIA NSCLC with EGFR exon 19 deletions or exon 21 L858R substitution mutations, can now benefit from adjuvant osimertinib after EMA approval in May 2021, based on the results of the ADAURA trial [5] (Figure 1).
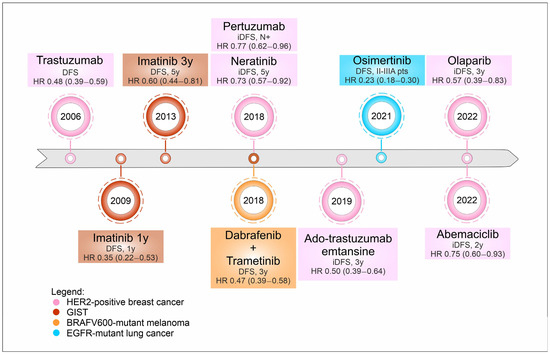
Figure 1.
Timeline of targeted therapies’ approval in the last decade across several histologies. Legend: DFS, disease free survival; HR, hazard ratio; y, years; iDFS, invasive disease-free survival; N+, nodal involvement; pts, patients.
In most trials leading to the approval of targeted therapies in the adjuvant setting, disease-free survival (DFS) has been the primary endpoint, while overall survival (OS) has been the secondary endpoint. While the first measures the time from the start of treatment until the recurrence of the disease, the second measures the time from the start of treatment until death from any cause.
OS has long been regarded as the most clinically significant outcome in clinical trials, offering a comprehensive evaluation of a treatment’s overall effectiveness. Nevertheless, in the context of early-stage cancer treatment trials, particularly when assessing perioperative or adjuvant therapies, OS can be influenced by the complexities of subsequent multiline therapies post-tumor recurrence. Consequently, alternative endpoints like DFS and other endpoints (such as pathological response, invasive disease-free survival, and event-free survival) are considered.
Here we discuss the past, the present, and the possible future directions of adjuvant targeted therapy in melanoma, GIST, NSCLC, and breast cancer.
2. Melanoma
2.1. Epidemiology and Prognosis
In the last few decades, the incidence of melanoma has grown steadily, with about 99,780 estimated new cases in 2022 in the United States, accounting for over 90% of skin cancer deaths due to its propensity to early systemic dissemination. Early stages are the most frequent clinical presentation with 78% of cases diagnosed in stages I and II (defined by the absence of nodal metastasis) and an excellent post-surgical excision 5-year survival rate that ranges from 98% (stage I) to 90% (stage II) [6]. However, the melanoma-specific 5-year survival rate ranges from 93% in stage IIIA disease (1–3 clinically occult, tumor-involved lymph nodes (N1a or N2a) and T1a, T1b, or T2a primaries) to 32% for those with stage IIID disease (patients with a thick and ulcerated primary (T4b), and either ≥4 tumor-involved regional nodes (N3a or N3b) or ≥2 tumor-involved nodes and evidence of microsatellite, satellite, or in-transit metastases (N3c)) [7]. Notably, approximately 40% of newly diagnosed melanomas harbor BRAF oncogenic mutation (V600E and V600K as the most frequent) [8].
Treatments targeting BRAF mutations have been translated from the metastatic into the adjuvant setting in stage III, achieving a significant benefit in terms of both relapse-free survival (RFS) and overall survival (OS) [4].
The main clinical trials exploring TT in the adjuvant setting are reported in Table 1, while selected ongoing trials are reported in Table 2.

Table 1.
Characteristics and results of the main clinical trials exploring targeted therapies in resected melanoma.

Table 2.
Ongoing clinical trial exploring targeted therapies in resected melanoma.
2.2. Major Advances in the Adjuvant Setting
Until 2012, interferon alfa (IFN-α) represented the only effective (and approved) adjuvant treatment available [12]. In 2015, the EORTC 18071 trial compared ipilimumab (an anti-CTLA4 antibody) and placebo in patients with stage III melanoma, demonstrating the benefit of ipilimumab in RFS, OS, and distant metastasis-free survival (DMFS) [13]. However, considering the concerning immune-related side effects reported with ipilimumab, the use of this agent in the adjuvant setting was not approved by the EMA, prioritizing anti-PD-1 or anti-BRAF/MEK treatment.
Different approaches targeting BRAF mutations have been explored. In the BRIM-8 trial, patients with radically resected melanoma and BRAF V600 mutation were randomized to receive double-blind vemurafenib (BRAF inhibitor) 960 mg ×2 daily or placebo for 12 months. The study enrolled patients in two different cohorts (cohort 1, stage IIC, IIIA, IIIB; cohort 2, stage IIIC). The primary endpoint was disease-free survival (DFS). The study showed no statistically significant difference in the hazard ratio (HR) for cohort 2 (HR = 0.80, 95% confidence interval (CI) 0.54–1.18) with a median DFS of 23.1 months in the vemurafenib group and 15.4 months in the placebo group. In cohort 1, the median DFS was not reached in the vemurafenib group, while it was 36.9 months in the placebo group (HR = 0.54, 95% CI 0.37–0.78, p = 0.0010) [9] (Table 1).
Currently, anti-PD-1 or BRAF-directed adjuvant treatments are indicated in melanoma patients with stage IIIA (with sentinel lymph node metastasis >1 mm), IIIB, IIIC, and IIID.
Regarding BRAF targeting, the efficacy of the combination of dabrafenib (BRAF inhibitor) and trametinib (MEK inhibitor) was demonstrated in the randomized, double-blind, phase III COMBI-AD study [4]. This study enrolled 870 stage IIIA, IIIB, or IIIC (according to AJCC 7) patients, harboring the BRAF V600E/K mutation. Patients were randomized to receive dabrafenib 150 mg ×2 day + trametinib 2 mg daily for 12 months vs. placebo, after radical surgery. The risk of disease recurrence was reduced by 53% in the TT arm compared to the control group (HR = 0.47, 95% CI 0.39–0.58, p < 0.001). The estimated 3-year OS was 86% vs. 77% in the two groups, with an HR = 0.57 (95% CI 0.42–0.79, p = 0.0006) in favor of the treatment arm, but this result cannot be considered significant considering the statistical limitations imposed by the interim analysis [4]. At ASCO 2020, the update with 5-year data from the COMBI-AD study was presented. At 5 years, RFS was 52% vs. 36% (HR = 0.51, 95% CI 0.42–0.61) in the dabrafenib plus trametinib vs. placebo arm, respectively [14]. The subgroup analysis and a subsequent post hoc analysis, conducted by re-staging patients enrolled according to the eighth version of the AJCC, showed no clinically significant differences [15]. Based on this evidence, the FDA and EMA approved the combination of dabrafenib–trametinib for resected stage III BRAFV600-mutant melanoma patients (Table 1).
2.3. Future Directions
The encouraging results obtained in the COMBI-AD trial led to the evaluation of novel approaches involving BRAF TT in the adjuvant setting. Ongoing studies include the Columbus-AD study (NCT05270044) evaluating the efficacy and safety of 12 months of BRAF (encorafenib) and MEK (binimetinib) inhibitors versus placebo in resected stage IIA/B/C melanoma patients. Furthermore, TT is also under evaluation in a combined neoadjuvant/adjuvant strategy (Table 2).
A perioperative combination of encorafenib plus binimetinib will be compared to standard adjuvant therapy in the phase II PREMIUM trial in BRAF V600E mutant patients in stage III (B/C/D) or oligometastatic resectable stage IV melanoma (NCT05097378). To date, a phase II study randomized 21 patients to receive the neoadjuvant/adjuvant treatment with dabrafenib and trametinib or the standard surgery ± adjuvant therapy. At the interim analysis, the perioperative strategy demonstrated longer event-free survival with 19.7 months, versus 2.9 months for standard care (HR = 0.016, 95% CI 0.00012–0.14, p < 0.0001) [10]. NeoCombi was a phase II study evaluating the efficacy of dabrafenib plus trametinib for 12 weeks before surgery, followed by 40 weeks of adjuvant therapy. The rate of pathological complete response (pCR) and the proportion of patients achieving a response at week 12 were the primary endpoints. At a median follow-up of 27 months, RECIST response was achieved by 86% of patients (46% complete responses and 40% partial response). After surgery, all patients achieved a pathologic response (49% pCR and 51% non-complete pCR) [11] (Table 1).
An interesting combination of immunotherapy and anti-BRAF/MEK TKIs will be evaluated in the NEO-TIM trial, a randomized phase II study, aiming to assess the perioperative approach of the combination of vemurafenib, cobimetinib (MEK inhibitor), and atezolizumab (anti-PD-L1) in stage IIIB/C/D and oligometastatic stage IV patients (BRAF wild-type and mutated) (NCT04722575).
3. Gastrointestinal Stromal Tumor (GIST)
3.1. Epidemiology and Prognosis
GIST accounts for less than 1% of all malignancies, but they are the most common mesenchymal tumors of the gastrointestinal tract [16].
The incidence is 1.5 cases/100,000/year. The most frequent sites of onset are in order: stomach (50%), small intestine (25%), esophagus (<5%), rectum (5%), and extra-intestinal localizations (<5%) [17]. The 5-year survival rates for GIST may vary from 95% for patients with localized disease to 52% for patients with advanced disease.
The histopathological diagnosis of GIST should include the mitotic index, tumor size, and consideration of the tumor’s anatomical location. Notably, the integrity of the tumor capsule represents a crucial prognostic factor in terms of risk of recurrence. Different approaches have been proposed to assess the risk of recurrence based on these prognostic factors, including nomograms and classifications incorporating mitotic index and tumor diameter.
About 85% of sporadic GISTs are characterized by the presence of mutations in tyrosine kinase receptors KIT and PDGFR-α genes, responsible for constitutive receptor activation and downstream proliferative signaling cascade [18]. Notably, KIT and PDGFR-α mutations are mutually exclusive. About 10% of adult GISTs and 85% of pediatric GISTs are associated with genetic syndromes, without KIT and PDGFR-α mutations [19]. The mutational analysis of the KIT and PDGFR genes is both a prognostic and predictive parameter of response to tyrosine kinase receptor inhibitors (TKIs) [16].
The main clinical trials exploring TT in the adjuvant setting are reported in Table 3, while selected ongoing trials are reported in Table 4.

Table 3.
Characteristics and results of the main clinical trials exploring targeted therapies in resected GIST.

Table 4.
Ongoing clinical trials exploring targeted therapies in resected GIST.
3.2. Major Advances in the Adjuvant Setting
Initially, conventional chemotherapy and radiation therapy have been of limited value as adjuvant treatments in radically resected GIST [21]. In 1998, Hirota et al., described for the first time, in five patients with GIST, the presence of an exon 11 mutation in the c-kit proto-oncogene [22]. Simultaneously, a molecule developed for the treatment of chronic myeloid leukemia, imatinib, demonstrated activity against aberrant gene products of KIT and PDGFR-α [21,23,24]. Based on the impressive efficacy in the first patient with metastatic GIST treated with imatinib [25], several trials, subsequently, confirmed the efficacy of imatinib in about 85% of patients with advanced GIST [26], becoming the first TKI approved in the treatment of solid tumors.
Surgical resection represents the primary choice in the treatment of localized GIST. After complete resection, adjuvant therapy with imatinib for 3 years is the standard of care in patients with a high risk of recurrence (risk assessed by mitotic index, neoplasm size, anatomical site, and rupture of the tumor at the time of surgery) and KIT or PDGFR-α sensitive mutation [27]. This type of approach was based on the results of three different trials.
The US American College of Surgeons Oncology Group (ACOSOG) Z9001 trial compared adjuvant treatment with imatinib 400 mg for one year vs. placebo after macroscopically complete surgical resection in patients with localized GIST with a diameter greater than 3 cm. The study demonstrated an advantage in high-risk patients treated with imatinib in terms of RFS, while no advantage was demonstrated in OS. The study design was characterized by the short follow-up time (median 19.7 months) and allowed crossover to imatinib upon recurrence [2].
The second study, the Scandinavian Sarcoma Group (SSG) XVIII/German (AIO) trial, showed improvement of adjuvant imatinib for 3 years in terms of both RFS and OS in high-risk patients, when compared to 1-year treatment, with an HR of 0.55 (95% CI 0.37–0.83) and of 0.46 (95% CI 0.32–0.65) in OS and DFS, respectively [3].
Finally, the EORTC/Inter-group trial compared 2-year imatinib adjuvant treatment versus observation alone in radically resected GIST with an intermediate or high risk of recurrence. No statistically significant differences were observed between the two groups in terms of imatinib failure-free survival (IFFS), considered as an overall survival surrogate. Imatinib did not improve either IFFS (HR = 0.87, 95.7% CI 0.65–1.15) or OS (HR = 0.88, 95% CI 0.65–1.21) [20] (Table 3).
Mutational analysis of KIT and PDGFR-α should be assessed for the definition of adjuvant therapy to identify patients harboring less-susceptible mutations. In particular, patients with the KIT exon 11 deletion mutation benefit most from the longer duration of adjuvant imatinib, while patients with the kit exon 9 mutation could benefit more from adjuvant imatinib with 800 mg/day dose (based on activity data in patients with advanced GIST in this specific genotype). At present, however, no clinical prospective study has been conducted that supports this indication [28,29]. On the other hand, the PDGFR-α D842V mutation appears to be insensitive to imatinib, as well as wild-type, SDH, and NF1 mutant GIST [30]. In this light, the evaluation of specific molecular subtypes is the cornerstone for the clinical management of GIST from localized to metastatic disease, given their relevance to predicting clinical behavior and the response to molecularly targeted agents.
3.3. Future Directions
Despite, to date, 3 years of imatinib remaining the standard of care in the adjuvant setting, the main open question is defining the optimal duration of the adjuvant therapy. Two studies are currently enrolling to evaluate whether prolonging imatinib therapy to 5 or 6 years is safe and effective (NCT02413736 and NCT02260505). In addition, a new molecule was evaluated. A highly selective TKI against c-Kit and PDGFR, masitinib, has been tested in completely resected GIST with a high risk of recurrence (NCT02009423) but the study has been discontinued due to a sponsor decision (Table 4).
4. Non-Small Cell Lung Cancer (NSCLC)
4.1. Epidemiology and Prognosis
Almost one-third of patients are diagnosed with resectable NSCLC. The 5-year survival rates for NSCLC may vary from 73% in stage IA disease to 13% in stage IV disease. The most crucial factor for predicting recurrence rates and prognosis is stage followed by tumor histology/molecular profile, age, and performance status. Currently, adjuvant chemotherapy is indicated in the presence of nodal metastasis or primary tumor with a maximum diameter bigger than 4 cm. However, adjuvant chemotherapy marginally improves DFS and OS (absolute benefits of 5.8% and 5.4% after 5 years, respectively), with a high recurrence rate (up to 50% after 5 years) [31].
Targeted therapies are already an established reality in the treatment of advanced NSCLC [32,33,34,35,36,37,38,39]. Targetable alterations include mutations in KRAS (20–30%), EGFR (10–15% of Caucasian patients and up to 40% of Asian patients), BRAF (2–4%), HER2 (1–2%), and MET (2–4%), and rearrangements in ALK (3–7%), ROS1 (1–2%), RET (1–2%), and NTRK (0.5–1%).
To date, only patients with EGFR mutant NSCLC can benefit from adjuvant TT, while several studies are currently ongoing to define the role of adjuvant TT in other molecular subgroups, as well as in the perioperative setting.
The main clinical trials exploring TT in the adjuvant setting are reported in Table 5, while selected ongoing trials are reported in Table 6.

Table 5.
Characteristics and results of the main clinical trials exploring targeted therapies in resected non-small cell lung cancer.

Table 6.
Ongoing clinical trials exploring targeted therapies in resected non-small cell lung cancer.
4.2. Major Advances in the Adjuvant Setting
In 2005, Tsuboi et al., in a phase III trial compared gefitinib (a first-generation EGFR TKI) to placebo in resected NSCLC [40]. Despite safety and feasibility being reported, no survival data were available due to an early trial closure due to a rising rate of interstitial lung disease (ILD) in patients treated with gefitinib [47].
Later, Goss et al., reported no benefit in terms of DFS (HR = 1.28, 95% CI 0.92–1.76, p = 0.14) or OS (HR = 1.24, 95% CI 0.90–1.71, p = 0.18) of 1-year adjuvant gefitinib in resected NSCLC (stage IB-IIIA) not selected for EGFR activating mutations [41].
In 2013, 6 months of consolidation treatment with gefitinib after platinum-based adjuvant chemotherapy demonstrated an improvement in DFS (HR = 0.37, 95% CI 0.16–0.85, p = 0.014), in the absence of OS benefit, in patients with resected stage IIIA NSCLC harboring EGFR mutations [48]. One phase II trial evaluating icotinib (a first-generation EGFR TKI), for 4–8 months, after platinum-based adjuvant chemotherapy, in resected NSCLC EGFR mutant stage IB-II-IIIA showed no significant increase in the proportion of disease-free patients (90.5% vs. 66.7%, p = 0.06) compared to observation alone [49]. Similarly, the RADIANT phase III trial, comparing 2-year erlotinib (a first-generation EGFR TKI) vs. placebo in an NSCLC adjuvant setting with EGFR-expressing tumors, but not necessarily EGFR-mutated tumors, demonstrated no benefit in terms of DFS (HR = 0.90, 95% CI 0.74–1.10, p = 0.32) or OS (HR = 1.13, 95% CI 0.88–1.44, p = 0.33) [43].
In 2018, the phase II EVAN trial reported a significant benefit in terms of DFS in favor of 2-year erlotinib compared to platinum-based chemotherapy in radically operated stage II-III NSCLC patients [50]. In the same year, 2-year gefitinib compared to platinum-based chemotherapy demonstrated, in 222 patients, improvement in RFS (HR = 0.60, CI 95% 0.42–0.87, p = 0.005) but not in OS outcomes (HR = 0.92, 95% CI 0.62–1.36, p = 0.67) [42,51].
Recently, the phase III IMPACT trial reported no significant benefit for adjuvant gefitinib when compared to platinum-based chemotherapy either in terms of RFS (HR = 0.92; 95% CI 0.67–1.28, p = 0.63) or in terms of OS (HR=1.03, 95% CI 0.65–1.65, p = 0.89) [45], while the EVIDENCE study comparing 2-year icotinib with platinum-based adjuvant chemotherapy demonstrated a significant benefit, for patients treated with icotinib, in terms of RFS (HR = 0.36, 95% CI 0.24–0.55, p < 0.0001) but not in OS (HR = 0.91, 95% CI 0.42–1.94, p = 0.80) [46].
The phase III ADAURA study randomized 682 radically operated NSCLC patients with stage IB-IIIA (VII edition of TNM staging) and classic EGFR activating mutations (exon 19 deletion or L588R exon 21 mutations) to receive either osimertinib 80 mg once daily (a third-generation EGFR TKI) or placebo for 3 years. The study achieved its primary endpoint by increasing RFS in patients with stage II-IIIA (HR = 0.23, 95% CI 0.18–0.30), with 70% of patients alive and disease-free at 48 months. The benefit was observed both in patients who had received prior adjuvant chemotherapy treatment (HR = 0.29, 95% CI 0.21–0.39) and in patients who had not received chemotherapy (HR = 0.36, 95% CI 0.24–0.55). Regarding the tolerability profile, grade 3 or 4 adverse events occurred in 23% of patients treated with osimertinib and 14% of patients treated with placebo [44]. The findings recently presented at ASCO 2023 suggest that the use of adjuvant osimertinib can also significantly improve OS, based on a median follow-up period of approximately 5 years. Specifically, among patients with stage II to IIIA, osimertinib was found to reduce the risk of death by 51% (HR = 0.49, 95% CI 0.33–0.73, p = 0.0004) [52].
Based on the positive results of the ADAURA study, osimertinib is currently indicated for adjuvant treatment after complete tumor resection in adult patients with stage IB-IIIA NSCLC whose tumors have EGFR exon 19 deletions or exon 21 L858R substitution mutations (Table 5).
4.3. Future Directions
4.3.1. EGFR Mutant NSCLC
Several trials are currently ongoing to better define the role of EGFR TKIs in resected NSCLC.
The ADAURA2 trial evaluates 3-year osimertinib vs. placebo in resected stage IA2 and IA3 EGFR mutant NSCLC (NCT05120349). The phase III ALCHEMIST trial compares 2-year erlotinib versus observation in patients with resected NSCLC stage IB-IIIA and EGFR mutation (NCT02193282). The efficacy of 6-month or 12-month icotinib following chemotherapy compared to chemotherapy alone in patients with resected stage IIA-IIIA NSCLC harboring an EGFR mutation is currently under evaluation in the ICTAN trial (NCT01996098).
Furmomertinib, a novel third-generation EGFR TKI, is currently under evaluation in the phase III study FORWARD in EGFR mutant patients with stage from IIA to IIIA NSCLC after complete resection (NCT04853342).
Finally, the APEX trial randomizes patients with EGFR mutant stage II-IIIA NSCLC following complete tumor resection, to evaluate the efficacy and safety of almonertinib, a third-generation EGFR TKI targeting both EGFR-sensitizing and T790M resistance mutations, combined with or without chemotherapy (NCT04762459).
Based on the positive results obtained from the ADAURA trial, the NEOADAURA trial is a phase III, randomized, controlled, three-arm, multi-center study evaluating the benefit and safety of neoadjuvant osimertinib alone or in combination with chemotherapy, versus standard-of-care chemotherapy alone, in patients with resectable EGFR mutant NSCLC (NCT04351555) (Table 6).
4.3.2. Other Oncogene-Addicted NSCLC
To date, no prospective data regarding the role of anti-ALK TKIs in the adjuvant/neoadjuvant setting have been published.
The ALCHEMIST screening trial is currently evaluating crizotinib (a first-generation ALK TKI) versus observation for up to 24 months after completion of standard-of-care chemotherapy and/or radiotherapy in ALK-positive patients (NCT02194738). In the ALINA trial adjuvant alectinib (a second-generation ALK TKI) for 24 months is compared with adjuvant platinum-based chemotherapy after surgical resection in patients with ALK-positive stage IB-IIIA NSCLC (NCT03456076) [53].
The activity of perioperative treatment with alectinib is being evaluated in the phase II ALNEO trial. Oral alectinib for 56 days before and 96 weeks after the surgery will be administered in potentially resectable stage II-III ALK-positive NSCLC (NCT05015010) (Table 6).
LIBRETTO-432 is a phase III study, evaluating 3 years of selpercatinib (a RET TKI) versus placebo after definitive locoregional treatment (surgery or radiotherapy) in participants with stage IB-IIIA RET fusion-positive NSCLC. Event-free survival is the primary endpoint and the estimated primary completion date is 2028 (NCT04819100) (Table 6).
NAUTIKA1 is a phase II study evaluating the efficacy and safety of 2 years of several targeted therapies in patients with resectable NSCLC in stages from IB to IIIB. In particular, the authors will evaluate neoadjuvant/adjuvant targeted therapies in patients harboring ALK, ROS1, NTRK, BRAF, and RET activating mutations with alectinib, entrectinib, vemurafenib, cobimetinib, and pralsetinib therapy, respectively. To date, only preliminary safety data of neoadjuvant alectinib for ALK+ NSCLC have been presented [54]. A perioperative approach with 8-week pre-operative capmatinib followed by 3-year adjuvant capmatinib is being evaluated in the phase II Geometry-N trial, enrolling patients with MET exon 14 mutations and/or high MET amplification stage I-IIIA NSCLC (NCT04926831).
5. Breast Cancer
5.1. Epidemiology and Prognosis
Breast cancer (BC) represents the first diagnosis of cancer in women and the second-leading cause of cancer death; in the last five years, the incidence rates increased by 0.5 annually, mainly due to early-stage and hormone receptor (HoR) positive disease.
The 5-years relative survival rate for localized, regional, and distant disease in American females is 99%, 86%, and 29%, respectively [6]. Thus, an unmet medical need is to prevent the development of a metastatic, and incurable, disease. Besides the stage at diagnosis, the tumor immunophenotype represents a further relevant factor associated with patient prognosis. Indeed, the survival rates range from 94% for HoR-positive to 85% and 77% for human epidermal growth factor receptor 2 (HER2) positive and triple-negative (TN) disease, respectively. Notably, the advances in personalized treatment for early/locally advanced disease recently introduced significantly improved (and will more deeply impact on) outcomes in featured tumor subtypes.
The main clinical trials exploring targeted therapies in the adjuvant setting are reported in Table 7; Table 8, while selected ongoing trials are reported in Table 9.

Table 7.
Characteristics and results of the main clinical trials exploring targeted therapies in resected HER2-positive breast cancer.

Table 8.
Characteristics and results of the main clinical trials exploring targeted therapies in resected hormone-positive and triple-negative breast cancer.

Table 9.
Ongoing clinical trials exploring targeted therapies in resected breast cancer.
5.2. Major Advances in the Adjuvant Setting
The therapeutic options for early breast cancer (eBC) have been improved in the last few years and the greater use of neoadjuvant therapy (NAT), in particular for HER2-positive and TN BC [77,78], led to consider residual disease as a risk factor, in addition to classic prognostic elements such as tumor histology, grade, stage, hormone receptors, and HER2 expression, in the choice of the best adjuvant treatment [79,80]. Indeed, the latest evidence in BC adjuvant therapies concerns escalation targeted strategies addressed to high-risk patients, defined by lymph node-positive disease, residual disease after neoadjuvant therapy, higher Ki67, and/or selected using a composite score, such as the clinical and pathological stage (CPS) and estrogen-receptor status and histologic grade (EG) (CPS + EG scoring system), and the analysis of pathogenic mutated genes [81].
Despite the survival improvements for HER2-positive disease with the introduction of anti-HER2 therapy, the rate of disease relapse after adjuvant trastuzumab is still estimated at 15 to 30%; therefore, the introduction of escalation targeted treatments, such as other monoclonal antibodies (mAbs), tyrosine kinase inhibitors, and antibody–drug conjugates (ADCs), was investigated to reduce the risk of relapse. Regarding HoR-positive HER2-negative disease, the cornerstone adjuvant treatment is represented by endocrine therapy (ET); recently, it was tested in addition to three targeted strategies against cyclin-dependent kinase 4 and 6, poly-adenosine-diphosphate–ribose-polymerase (PARP) and mTOR pathway, respectively. Conversely, triple-negative eBC is still lacking in adjuvant targeted therapies except for the BRCA-mutated subgroup.
5.2.1. HER2-Positive BC
Trastuzumab, a monoclonal antibody targeting the extracellular domain of the HER2 protein, represents a milestone in eBC targeted adjuvant therapy, reducing after one year of treatment the relative risk of recurrence by 40% and the risk of death by 30% in HER2-positive disease [55,56,80,82,83]. Phase III trials exploring adjuvant HER2-targeted agents are summarized in Table 7, Table 8 and Table 9. Although in the FinHER trial 9 weeks of trastuzumab plus chemotherapy versus chemotherapy alone showed a benefit [58,59], different trastuzumab durations (2 years, 9 weeks, or 6 months of trastuzumab) compared to 1 year of trastuzumab did not show superiority or non-inferiority efficacy [60,61,62,82,84], except for the PERSEPHONE trial (HR = 1.07, 90% CI 0.93–1.24, p = 0.011) [63]. Thus, one year is the standard duration of adjuvant trastuzumab for HER2-positive BC.
Concerning other therapeutic anti-HER2 mechanisms, lapatinib, an inhibitor of HER1 and HER2, failed to improve DFS both in place of trastuzumab for 1 year in the TEACH trial [64] and in a concurrent or subsequent addiction to trastuzumab in the ALTTO trial [65].
In July 2017, neratinib, a TKI against ErbB and HER2/HER4, was approved by the FDA for extended adjuvant treatment of HER2-positive eBC, based on the results of the ExteNET phase III randomized trial [66]. The 1-year administration of neratinib after trastuzumab-based therapy for stage II-IIIC disease showed a statistically significant 5-year invasive disease-free survival (iDFS) compared to placebo (HR = 0.73, 95% CI 0.57–0.92, p = 0.0083); the benefit was greater for HoR-positive disease (probably due to the crosstalk between estrogen and HER2 receptor signaling), for patients that started the treatment within 1 year of the end of trastuzumab, and for patients that had not achieved pCR after neoadjuvant therapy. Neratinib also improved the central nervous system (CNS) relapse of disease and demonstrated a numerical improvement in OS with a gain of 2.1% at 8 years (HR = 0.79, 95% CI, 0.55–1.13, p = 0.203) for the HoR-positive population who started treatment within one year of stopping trastuzumab [85]. In the ExteNET trial, 39% of patients reported G3-G4 diarrhea so a preemptive antidiarrheal strategy is recommended in patients who are candidates for neratinib [86]. In clinical practice, the role of neratinib remains not clarified given that new standards of care were subsequently approved.
In 2017 the therapeutic adjuvant landscape of HER2-positive eBC was enriched by the approval of pertuzumab, a monoclonal antibody against HER2 that prevents HER2/HER3 dimerization inhibiting cell survival and proliferation signaling, in combination with trastuzumab for 1 year and chemotherapy for high-risk patients (node-positive or tumor diameter greater than 1 cm), based on the primary analysis of the APHINITY trial [67]. The 6-year and 8.4-year follow-up analysis showed a statistical iDFS benefit (HR = 0.72, 95% CI 0.59–0.87) only for node-positive patients independently from HoR status, while OS superiority was not reached [87,88]. In conclusion, the addition of pertuzumab to adjuvant chemotherapy plus trastuzumab could be reserved only for node positive high-risk patients [80]. The use of pertuzumab after a pCR achieved with dual blockage remains an open question. The TRHYPAENA and BERENICE trials suggested continuing pertuzumab plus trastuzumab to complete 1 year of treatment in patients that reached pCR, even if trastuzumab alone remains the standard of care [89,90].
Trastuzumab emtansine (T-DM1), an antibody–drug conjugate (ADC) where the cytotoxic agent, emtansine, functions as a microtubule inhibitor and is covalently linked to trastuzumab, was the first ADC approved in 2019 as adjuvant treatment for HER2-positive residual breast or axilla invasive disease after taxane and trastuzumab-based neoadjuvant therapy. The phase III KATHERINE trial showed an improvement in 3-year iDFS (HR = 0.50, 95% CI 0.39–0.64, p < 0.001) with a relative 50% reduction in risk of relapse after 14 cycles of T-DM1 compared with trastuzumab [68]. The benefit was consistent in all subgroups, but no difference was shown in CNS relapse [91]. De-escalation chemotherapy strategy with the use of T-DM1 plus pertuzumab against taxane with pertuzumab and trastuzumab, after anthracycline-based chemotherapy, failed to meet the iDFS primary endpoint in the phase III KAITLIN study for HER2-positive eBC (node-positive or node-negative, HoR-negative and tumor size >2.0 cm) [69]. T-DM1 is now considered the new standard in the adjuvant context for residual invasive disease (Table 7).
5.2.2. Luminal (HER2-Negative) BC
Abemaciclib represents the first cyclin-dependent kinase 4 and 6 inhibitor (CDK4/6i) approved in combination with endocrine therapy (tamoxifen or an aromatase inhibitor) by the FDA in October 2021 as targeted adjuvant therapy for HoR-positive, node-positive eBC at high risk of recurrence and with a Ki67 ≥ 20% [92].
In the monarchE trial patients with four or more positive nodes, or one to three nodes and either a tumor size ≥ 5 cm, histologic grade 3, or central Ki-67 ≥ 20% who had completed the local and systemic treatment, were randomized to standard ET with or without abemaciclib (150 mg twice daily for 2 years). The iDFS primary endpoint was met at the first (HR = 0.75, 95% CI 0.60–0.93, p = 0.01) and second pre-planned analyses (HR = 0.664, 95% CI 0.578–0.762, p < 0.0001) in the intention-to-treat (ITT) population and pre-specified subgroups, and about 50% of patients reported grade ≥ 3 adverse events (AEs) [70,71].
International guidelines recommend abemaciclib for high-risk selected patients irrespective of the Ki-67 cut-off, even if the OS data are still immature [93].
The advantaged position of abemaciclib as a unique CDK4/6i approved in this scenario will probably soon be removed by ribociclib’s introduction in the early strategy treatment of BC.
Recently, the second interim analysis of the NATALEE trial was presented at the 2023 ASCO Annual Meeting, showing promising result for stage II-III BC adjuvant therapy [72].
Ribociclib for 3 years (400 mg, 3 weeks on/1 week off) plus ET was tested against ET alone in men and women with stage II-III BC at risk of recurrence. It is interesting to notice that node-negative patients were also included in the study; moreover, in the case of stage IIA (node negative), more additional high-risk inclusion criteria were required: grade 3 or grade 2 plus others including Ki67 ≥ 20% and, for the first time, genomic risk profiling and Oncotype DX recurrence score. The choice of 400 mg instead of 600 mg standard dose was justified by the authors for major tolerability, with an extended duration of 3 years for a prolonged cell cycle arrest. The addition of ribociclib to ET showed a statically significant absolute iDFS (primary endpoint) benefit of 3.3%, consistent between subgroups. However, at a median follow-up of 34 months, only 20.2% of participants in the experimental arm had completed 3 years of treatment, 56.8% had completed 2 years, and 74.7% of participants remained on the study treatment at data cutoff. We need a longer follow-up to confirm these results, even though they asked the important question of recognizing patients diagnosed with early stage BC that are really in need of an escalation treatment [72].
Likewise, palbociclib, another CDK4/6i, was tested in combination with ET in the adjuvant (2 years of treatment) and post-neoadjuvant (1 year) setting in the PALLAS [73] and PENELOPE-B [74] trials, respectively. PALLAS enrolled stage II-III HoR-positive HER2-negative patients with the same design as the monarchE, while the PENELOPE-B trial enrolled high-risk patients with residual disease (CPS + EG ≥ 3 score or ypN positive) after receiving taxane-based neoadjuvant therapy. Neither of them established the superiority of palbociclib over ET alone in terms of iDFS. Some hypotheses were generated to justify the difference between the two CDK4/6is tested, such as the higher rate of discontinuation and intermittent schedule of administration of palbociclib [94]; the idea of a lower-risk population enrolled in the PALLAS trial was rejected considering the same outcomes for the different subgroups [95].
The Olympia trial led to the PARP inhibitor olaparib indication for HoR-positive germinal BRCA1/2-mutated high-risk selected patients [76].
In this trial, a total of 325 HoR-positive patients (about 18% of each arm of the study population) were included after local and systemic standard treatment, selected based on residual invasive disease with a CPS + EG ≥ 3 or at least four nodes positive for surgery upfront, achieving an iDFS (HR = 0.68, 95% CI 0.40–1.13) and OS (HR = 0.897, 95% CI 0.449–1.784) improvement [96].
The abemaciclib and olaparib approvals opened an important question about the best adjuvant treatment choice for patients who were candidates for both drugs. The OS benefit and the power of targeting a specific gene alteration could justify the olaparib preference over abemaciclib, despite the absence of comparison data.
Finally, regarding mTOR inhibitors, the SWOG S1207 phase III trial results were recently presented [75]. Patients in four high-risk groups (including Oncotype DX recurrence score > 25 or MammaPrint high-risk category) after (neo)adjuvant chemotherapy were randomized to receive ET with or without everolimus: the primary iDFS (HR = 0.94, 95%CI 0.77–1.14, p = 0.52) and the secondary OS (HR = 0.97, 95 CI 0.75–1.26, p = 0.84) endpoints were not met (Table 8).
5.2.3. Triple-Negative BC
Triple-negative eBC, despite the approval of chemo-immunotherapy for neoadjuvant therapy [97], remains an orphan of new targeted therapies in the adjuvant setting except for the PARP inhibitor olaparib for BRCA-mutated disease [76]. The Olympia trial showed that the addition of 1-year olaparib after surgery and (neo)adjuvant chemotherapy for germinal BRCA1/2-mutated high-risk HER2-negative selected patients improved iDFS (HR = 0.58, 99.5% CI, 0.41–0.82, p < 0.001) and OS (HR = 0.68, 98.5% CI 0.47–0.97, p = 0.009) leading to its approval by the FDA in March 2022 [76,96]. A residual invasive disease after neoadjuvant therapy and a node-positive or tumor diameter ≥ 2 cm after initial surgery were selected as inclusion criteria for TNBC; the specific subgroup analysis confirmed a statistically significant benefit for TN disease (iDFS: HR = 0.62, 95% CI 0.487–0.787; OS: HR = 0.64, 95% CI 0.459–0.884). The characteristics of phase III trials with targeted therapy for HoR and triple-negative BC disease are summarized in Table 7, Table 8 and Table 9.
For patients that should continue pembrolizumab after neoadjuvant therapy, there are currently no data to help clinicians in the choice of the best adjuvant treatment after residual invasive disease. Future combination trials are needed and an adjuvant combination of olaparib plus pembrolizumab could be considered an option for the acknowledgment of good safety [98] (Table 8).
5.3. Future Perspectives
Over the last five years, many new drugs and new strategies focused on high-risk disease have been developed for eBC adjuvant treatment. The iDFS is commonly accepted as a surrogate endpoint for early-stage disease but the OS remains an important endpoint to confirm the first approval of new drugs. Future strategies are focusing on a combination of different drugs, the introduction of new intelligent therapies, and, above all, on recognizing high-risk patients by applying new technologies based on precision medicine. Ongoing phase III studies with adjuvant targeted therapy for BC are summarized in Table 7, Table 8 and Table 9.
Targeted therapies are being tested in HER2-positive patients not achieving pCR post-neoadjuvant therapy such as trastuzumab–deruxtecan (T-DXd), an ADC of trastuzumab linked to deruxtecan, a topoisomerase I inhibitor payload, against T-DM1 in the DESTINY-Breast05 trial (NCT04622319). The CompassHER2 RD trial (NCT04457596) is evaluating the combination of T-DM1 plus tucatinib, a HER2-specific TKI, for residual invasive disease to reduce CNS relapse considering the intracranial response rate demonstrated in the metastatic setting [99], while in the ASTEFANIA trial (NCT04873362) T-DM1 is evaluated in combination with atezolizumab, a mAb anti-PD-L1, in a potential combined effect in the cancer–immunity cycle [100].
The phase III SASCIA (NCT04595565) and ASCENT-5 (NCT05633654) ongoing clinical trials are testing sacituzumab govitecan, an ADC composed of a mAb against TROP-2 and SN38-topoisomerase I inhibitor, against physician’s choice treatment in patients with residual invasive disease post-neoadjuvant therapy in HER2-negative BC, as a single agent, and in TNBC, in combination with pembrolizumab.
Regarding HoR-positive disease, an ongoing clinical trial is still assessing the CDK4/6i potential benefit: the eMonarcHER trial (NCT04752332) is evaluating abemaciclib plus ET in patients with triple-positive BC who had completed adjuvant HER2-targeted therapy. Other studies are including the genomic assay platform Oncotype DX to select high-risk patients that could benefit from a CDK4/6i in addition to ET both as replacement of standard chemotherapy for ribociclib in the ADAPTcycle (NCT04055493) as (neo)adjuvant therapy and as escalation treatment after standard adjuvant chemotherapy for abemaciclib in the ADAPTlate trial (NCT04565054).
The COGNITION-GUIDE (NCT05332561) seven-arm umbrella phase II ongoing trial is a model of a precision medicine approach in a curative setting: patients with residual disease after neoadjuvant therapy and eventually after standard adjuvant therapy are allocated to a genomics-guided therapy determined by molecular alterations. Finally, futuristic approaches are evaluating the so-called “second-line” adjuvant treatments using circulating tumor DNA detection as a follow-up strategy to detect minimal residual disease (MRD) [101] and avoid a macroscopical relapse of disease: the DARE (NCT04567420) and the LEADER (NCT03285412) phase II trials are testing palbociclib and ribociclib in combination with ET for MRD in HoR-positive BC, respectively (Table 9).
6. Discussion
Targeted therapy is currently a consolidated reality in the treatment of advanced oncogene-addicted cancers, demonstrating significant improvement in survival and disease control outcomes. On the other hand, targeted therapy in the adjuvant setting is assuming an ever-increasing role and represents, to date, a treatment option in selected patients with potentially cured disease [3,4,45]. However, further research is still needed, and some questions remain open.
DFS and OS have been considered as primary and secondary endpoints, respectively, in almost all trials that led to the approval of TT in the adjuvant setting [3,4,45,95] (Figure 1).
OS has traditionally been considered the most clinically meaningful endpoint in clinical trials because it provides a comprehensive measure of the treatment overall effectiveness. However, in the context of early-stage cancer treatment, where perioperative or adjuvant therapies are evaluated, OS may be confounded by the effects of multiline therapy after tumor recurrence and may not fully capture the benefits of the treatments.
Therefore, alternative endpoints, such as DFS and other surrogate endpoints, are considered.
A meta-analysis conducted by Suciu et al., in 2018 aimed to evaluate whether DFS could be considered a valid surrogate endpoint for OS in resected stage II–III melanoma patients who were receiving adjuvant therapy with interferon or checkpoint inhibitors [102]. The study found that DFS was a valid surrogate endpoint for OS in this population and that improvements in DFS were associated with improvements in OS [102].
Another meta-analysis also evaluated the correlation between DFS and OS as endpoints in patients with resectable locally advanced NSCLC. The authors, analyzing a large sample of data for patients with resected NSCLC cancer, suggest DFS as a valid surrogate endpoint for OS in studies of adjuvant chemotherapy [103]. Notably, no data about adjuvant TT are collected in this study [103]. In addition, evaluation of OS data in early-stage cancer clinical trials can be challenging due to the lengthy follow-up duration required to observe enough events. These patients demonstrated a better prognosis than those with advanced disease, and, consequently, it may take significantly more time to gather an adequate number of occurrences for OS analysis.
Furthermore, no data about treatments used at the time of relapse have been reported (e.g., in ADAURA [44] and NCT00116935 [3]), thus leaving, at least, two open questions. How is OS influenced by the therapies at progression? What treatment can clinicians use at the time of disease relapse? While the first question remains still unanswered and more studies will be necessary, the second may find an answer according to cancer histology.
Currently, in some histologies, e.g., breast cancer, the duration after completing additional therapy can be taken into account when considering the possibility of reintroducing treatment or altering the treatment approach [104]. However, in other cancers, such as NSCLC, determining the most suitable therapy upon relapse can be more tangled. In this light, managing a patient with EGFR mutant NSCLC experiencing a relapse shortly after completing adjuvant osimertinib therapy can pose challenges, due to the insufficient evidence demonstrating the superiority of initiating first-line polychemotherapy instead of rechallenging. Borrowing the evidence from the advanced setting where molecular mechanisms of resistance to EGFR TKIs may include various genetic alterations (such as MET amplification, HER2 amplification, PIK3CA alterations, BRAF mutation, and KRAS mutation [105]), tissue or/and liquid rebiopsy could be useful to identify any molecular mechanisms responsible for resistance to TT. In the context of melanoma, evidence from studies on metastatic disease demonstrates that resistance mechanisms may manifest within the PI3K or NRAS signaling pathways. These data may potentially guide a future approach with targeted therapies (such as PI3K inhibitors) within the adjuvant setting or for effectively addressing recurrence [106]. On the other hand, concerning HER2-positive breast cancer, the emergence of recurrence during or following adjuvant therapy may possibly be attributed to a spectrum of molecular alterations, including the activation of the PI3K/AKT pathway, PTEN loss, or heightened ER activation. Each of these alterations could presents an intriguing opportunity for therapeutic intervention to be explored in dedicated clinical trials. PI3K inhibitors, mTOR inhibitors, and various endocrine therapies could emerge as valuable tools that can be harnessed to specific therapeutic targets [107].
TT is generally well-tolerated with a significantly better safety profile compared, in particular, to chemotherapy [108]. Nevertheless, these types of treatments are not exempt from potential side effects. Grade 3–4 adverse events are reported in percentages ranging from 23% to 41%, by studies evaluating TT in the adjuvant setting [4,45]. The exposure of potentially cured patients to treatments that can lead to non-negligible toxicity is justifiable to improve DFS and OS. However, in many cases (such as adjuvant imatinib or osimertinib) the duration of such therapies is not yet defined with certainty. Two studies are currently evaluating (and currently enrolling) whether prolonging imatinib therapy to 5 or 6 years is safe and effective (NCT02413736 and NCT02260505). On the other hand, the duration of adjuvant osimertinib, in the ADAURA study [44], was arbitrarily set at 3 years and probably influenced by the ADJUVANT trial [43,50]. Considering these premises, it is crucial to gain a deeper understanding of which patients are most likely to derive significant benefits from adjuvant TT.
The use/implementation of liquid biopsy, which involves the analysis of circulating tumor cells (CTCs) or circulating tumor DNA (ctDNA) in the blood to provide real-time monitoring of disease recurrence and to potentially guide treatment decisions, represents a promising research area, not only in advanced but also in early stages.
Abbosh et al., developed a patient-specific mutational panel assay using next-generation sequencing (NGS) technology on plasma samples, consisting of 12–30 single-nucleotide variants (SNVs). ctDNA was longitudinally evaluated in 24 patients with resected NSCLC, demonstrating that the detection of SNVs was associated with clinical evidence of lung cancer relapse [109]. Moreover, Chen et al., used the Oncomine Research Panel (134 cancer-related genes assay) to evaluate the potential of ctDNA as a biomarker of MRD in patients with TNBC who had residual disease after neoadjuvant chemotherapy. Despite the low detection rate of ctDNA mutations, all four patients with detectable ctDNA experienced disease relapse within 9 months [110].
Through the analysis of CTCs and ctDNA, physicians can gain insights into drug sensitivity or resistance and residual tumor cells, both during and after adjuvant therapy [111].
Finally, in recent years, neoadjuvant TT has emerged as a promising approach in early-stage cancer treatment. While for some histologies neoadjuvant TT is already a well-established reality (e.g., breast cancer), for some others, several studies are still ongoing. One of the main advantages is that neoadjuvant therapy allows the assessment of tumor response to treatment before surgery. This provides important information on the sensitivity of the tumor to the therapy, which can be used to guide subsequent treatment decisions.
In addition, in some histologies (e.g., HER2-positive breast cancer), neoadjuvant TT plus chemotherapy has been associated with a higher rate of pCR and better survival outcomes compared to neoadjuvant chemotherapy alone [112].
Regarding lung cancer, several studies, e.g., NeoADAURA [113] and ALNEO [114], are currently evaluating TT in a perioperative approach in resectable NSCLC.
7. Conclusions
In conclusion, adjuvant TT has emerged as a promising treatment option, with the potential for significant improvements in survival and disease control outcomes. In addition to historically proven effective therapies such as trastuzumab in breast cancer and imatinib in GIST, novel therapeutic approaches are progressively emerging, increasingly demonstrating promising outcomes. Examples include adjuvant BRAF inhibitors in melanoma, osimertinib in resected NSCLC, and abemaciclib in breast cancer, with even more recent advancements seen in perioperative strategies across diverse histologies. Ongoing research on the development of new therapies and understanding novel biomarkers will be crucial in realizing this potential and further enhancing the possibilities of early-stage cancer treatment.
Author Contributions
Conceptualization, M.S, L.B., L.C. and S.P.; writing—original draft preparation, M.S., L.B., L.P., D.T., I.T., J.I., A.A., M.M., E.B., L.C. and S.P.; writing—review and editing, M.S., L.B., L.C. and S.P.; supervision, M.M. and E.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
L.C. is supported by the Fondazione Associazione Italiana per la Ricerca sul Cancro (AIRC) under My First AIRC Grant (MFAG) No. MFAG25149. E.B. is supported by Institutional funds of the Università Cattolica del Sacro Cuore (UCSC-projects D1) and by the AIRC under Investigator Grant (IG) No. IG20583.
Conflicts of Interest
L.B. received speakers’ fees from Astra-Zeneca, MSD, and Roche, outside the submitted manuscript; and travel fees from Takeda. E.B. received speakers’ and travel fees from MSD, Astra-Zeneca, Celgene, Pfizer, Helsinn, Eli-Lilly, BMS, Novartis, and Roche. E.B. received institutional research grants from Astra-Zeneca and Roche. S.P. received honoraria or speakers’ fees from Astra-Zeneca, Eli-Lilly, BMS, MSD, Takeda, Amgen, Novartis, and Roche, outside the submitted manuscript. L.C. received speakers’ fee from Eli-Lilly, Novartis, and Astra-Zeneca. All remaining authors have declared no conflict of interest.
References
- Belluomini, L.; Riva, S.T.; Simbolo, M.; Nocini, R.; Trestini, I.; Avancini, A.; Tregnago, D.; Ferrara, M.G.; Caldart, A.; Dodi, A.; et al. Anticipating EGFR Targeting in Early Stages of Lung Cancer: Leave No Stone Unturned. Cells 2021, 10, 2685. [Google Scholar] [CrossRef]
- Dematteo, R.P.; Ballman, K.V.; Antonescu, C.R.; Maki, R.G.; Pisters, P.W.; Demetri, G.D.; Blackstein, M.E.; Blanke, C.D.; von Mehren, M.; Brennan, M.F.; et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: A randomised, double-blind, placebo-controlled trial. Lancet 2009, 373, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Joensuu, H.; Eriksson, M.; Sundby Hall, K.; Hartmann, J.T.; Pink, D.; Schutte, J.; Ramadori, G.; Hohenberger, P.; Duyster, J.; Al-Batran, S.E.; et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: A randomized trial. JAMA 2012, 307, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Hauschild, A.; Santinami, M.; Atkinson, V.; Mandala, M.; Chiarion-Sileni, V.; Larkin, J.; Nyakas, M.; Dutriaux, C.; Haydon, A.; et al. Adjuvant Dabrafenib plus Trametinib in Stage III BRAF-Mutated Melanoma. N. Engl. J. Med. 2017, 377, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Tsuboi, M.; He, J.; John, T.; Grohe, C.; Majem, M.; Goldman, J.W.; Laktionov, K.; Kim, S.W.; Kato, T.; et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 1711–1723. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Gershenwald, J.E.; Scolyer, R.A.; Hess, K.R.; Sondak, V.K.; Long, G.V.; Ross, M.I.; Lazar, A.J.; Faries, M.B.; Kirkwood, J.M.; McArthur, G.A.; et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 472–492. [Google Scholar] [CrossRef]
- Long, G.V.; Menzies, A.M.; Nagrial, A.M.; Haydu, L.E.; Hamilton, A.L.; Mann, G.J.; Hughes, T.M.; Thompson, J.F.; Scolyer, R.A.; Kefford, R.F. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J. Clin. Oncol. 2011, 29, 1239–1246. [Google Scholar] [CrossRef]
- Maio, M.; Lewis, K.; Demidov, L.; Mandala, M.; Bondarenko, I.; Ascierto, P.A.; Herbert, C.; Mackiewicz, A.; Rutkowski, P.; Guminski, A.; et al. Adjuvant vemurafenib in resected, BRAF(V600) mutation-positive melanoma (BRIM8): A randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 2018, 19, 510–520. [Google Scholar] [CrossRef]
- Amaria, R.N.; Prieto, P.A.; Tetzlaff, M.T.; Reuben, A.; Andrews, M.C.; Ross, M.I.; Glitza, I.C.; Cormier, J.; Hwu, W.J.; Tawbi, H.A.; et al. Neoadjuvant plus adjuvant dabrafenib and trametinib versus standard of care in patients with high-risk, surgically resectable melanoma: A single-centre, open-label, randomised, phase 2 trial. Lancet Oncol. 2018, 19, 181–193. [Google Scholar] [CrossRef]
- Long, G.V.; Saw, R.P.M.; Lo, S.; Nieweg, O.E.; Shannon, K.F.; Gonzalez, M.; Guminski, A.; Lee, J.H.; Lee, H.; Ferguson, P.M.; et al. Neoadjuvant dabrafenib combined with trametinib for resectable, stage IIIB-C, BRAF(V600) mutation-positive melanoma (NeoCombi): A single-arm, open-label, single-centre, phase 2 trial. Lancet Oncol. 2019, 20, 961–971. [Google Scholar] [CrossRef]
- Mocellin, S.; Pasquali, S.; Rossi, C.R.; Nitti, D. Interferon alpha adjuvant therapy in patients with high-risk melanoma: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2010, 102, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, A.M.; Chiarion-Sileni, V.; Grob, J.J.; Dummer, R.; Wolchok, J.D.; Schmidt, H.; Hamid, O.; Robert, C.; Ascierto, P.A.; Richards, J.M.; et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2015, 16, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Dummer, R.; Hauschild, A.; Santinami, M.; Atkinson, V.; Mandala, M.; Kirkwood, J.M.; Chiarion Sileni, V.; Larkin, J.; Nyakas, M.; Dutriaux, C.; et al. Five-Year Analysis of Adjuvant Dabrafenib plus Trametinib in Stage III Melanoma. N. Engl. J. Med. 2020, 383, 1139–1148. [Google Scholar] [CrossRef]
- Hauschild, A.; Dummer, R.; Schadendorf, D.; Santinami, M.; Atkinson, V.; Mandala, M.; Chiarion-Sileni, V.; Larkin, J.; Nyakas, M.; Dutriaux, C.; et al. Longer Follow-Up Confirms Relapse-Free Survival Benefit with Adjuvant Dabrafenib Plus Trametinib in Patients with Resected BRAF V600-Mutant Stage III Melanoma. J. Clin. Oncol. 2018, 36, 3441–3449. [Google Scholar] [CrossRef]
- Casali, P.G.; Blay, J.Y.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Bonvalot, S.; Boukovinas, I.; Bovee, J.; et al. Gastrointestinal stromal tumours: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 20–33. [Google Scholar] [CrossRef]
- DeMatteo, R.P.; Lewis, J.J.; Leung, D.; Mudan, S.S.; Woodruff, J.M.; Brennan, M.F. Two hundred gastrointestinal stromal tumors: Recurrence patterns and prognostic factors for survival. Ann. Surg. 2000, 231, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Corless, C.L.; Fletcher, J.A.; Heinrich, M.C. Biology of gastrointestinal stromal tumors. J. Clin. Oncol. 2004, 22, 3813–3825. [Google Scholar] [CrossRef]
- Boikos, S.A.; Pappo, A.S.; Killian, J.K.; Laquaglia, M.P.; Weldon, C.B.; George, S.; Trent, J.C.; Von Mehren, M.; Wright, J.A.; Schiffman, J.D.; et al. Molecular Subtypes of KIT/PDGFRA Wild-Type Gastrointestinal Stromal Tumors. JAMA Oncol. 2016, 2, 922. [Google Scholar] [CrossRef]
- Casali, P.G.; Le Cesne, A.; Poveda Velasco, A.; Kotasek, D.; Rutkowski, P.; Hohenberger, P.; Fumagalli, E.; Judson, I.R.; Italiano, A.; Gelderblom, H.; et al. Time to Definitive Failure to the First Tyrosine Kinase Inhibitor in Localized GI Stromal Tumors Treated with Imatinib As an Adjuvant: A European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Intergroup Randomized Trial in Collaboration with the Australasian Gastro-Intestinal Trials Group, UNICANCER, French Sarcoma Group, Italian Sarcoma Group, and Spanish Group for Research on Sarcomas. J. Clin. Oncol. 2015, 33, 4276–4283. [Google Scholar] [CrossRef]
- Dematteo, R.P.; Heinrich, M.C.; El-Rifai, W.M.; Demetri, G. Clinical management of gastrointestinal stromal tumors: Before and after STI-571. Hum. Pathol. 2002, 33, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Hirota, S.; Isozaki, K.; Moriyama, Y.; Hashimoto, K.; Nishida, T.; Ishiguro, S.; Kawano, K.; Hanada, M.; Kurata, A.; Takeda, M.; et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998, 279, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Buchdunger, E.; Zimmermann, J.; Mett, H.; Meyer, T.; Muller, M.; Druker, B.J.; Lydon, N.B. Inhibition of the Abl protein-tyrosine kinase in vitro and in vivo by a 2-phenylaminopyrimidine derivative. Cancer Res. 1996, 56, 100–104. [Google Scholar] [PubMed]
- Tuveson, D.A.; Willis, N.A.; Jacks, T.; Griffin, J.D.; Singer, S.; Fletcher, C.D.; Fletcher, J.A.; Demetri, G.D. STI571 inactivation of the gastrointestinal stromal tumor c-KIT oncoprotein: Biological and clinical implications. Oncogene 2001, 20, 5054–5058. [Google Scholar] [CrossRef]
- Joensuu, H.; Roberts, P.J.; Sarlomo-Rikala, M.; Andersson, L.C.; Tervahartiala, P.; Tuveson, D.; Silberman, S.L.; Capdeville, R.; Dimitrijevic, S.; Druker, B.; et al. Effect of the Tyrosine Kinase Inhibitor STI571 in a Patient with a Metastatic Gastrointestinal Stromal Tumor. N. Engl. J. Med. 2001, 344, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Casali, P.G.; Zalcberg, J.; Le Cesne, A.; Reichardt, P.; Blay, J.-Y.; Lindner, L.H.; Judson, I.R.; Schöffski, P.; Leyvraz, S.; Italiano, A.; et al. Ten-Year Progression-Free and Overall Survival in Patients with Unresectable or Metastatic GI Stromal Tumors: Long-Term Analysis of the European Organisation for Research and Treatment of Cancer, Italian Sarcoma Group, and Australasian Gastrointestinal Tr. J. Clin. Oncol. 2017, 35, 1713–1720. [Google Scholar] [CrossRef]
- Gold, J.S.; Gönen, M.; Gutiérrez, A.; Broto, J.M.; García-Del-Muro, X.; Smyrk, T.C.; Maki, R.G.; Singer, S.; Brennan, M.F.; Antonescu, C.R.; et al. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: A retrospective analysis. Lancet Oncol. 2009, 10, 1045–1052. [Google Scholar] [CrossRef]
- Joensuu, H.; Wardelmann, E.; Sihto, H.; Eriksson, M.; Sundby Hall, K.; Reichardt, A.; Hartmann, J.T.; Pink, D.; Cameron, S.; Hohenberger, P.; et al. Effect of KIT and PDGFRA Mutations on Survival in Patients with Gastrointestinal Stromal Tumors Treated with Adjuvant Imatinib: An Exploratory Analysis of a Randomized Clinical Trial. JAMA Oncol. 2017, 3, 602–609. [Google Scholar] [CrossRef]
- Debiec-Rychter, M.; Sciot, R.; Le Cesne, A.; Schlemmer, M.; Hohenberger, P.; van Oosterom, A.T.; Blay, J.Y.; Leyvraz, S.; Stul, M.; Casali, P.G.; et al. KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur. J. Cancer 2006, 42, 1093–1103. [Google Scholar] [CrossRef]
- Schaefer, I.-M.; Dematteo, R.P.; Serrano, C. The GIST of Advances in Treatment of Advanced Gastrointestinal Stromal Tumor. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 885–899. [Google Scholar] [CrossRef]
- Pignon, J.-P.; Tribodet, H.; Scagliotti, G.V.; Douillard, J.-Y.; Shepherd, F.A.; Stephens, R.J.; Dunant, A.; Torri, V.; Rosell, R.; Seymour, L.; et al. Lung Adjuvant Cisplatin Evaluation: A Pooled Analysis by the LACE Collaborative Group. J. Clin. Oncol. 2008, 26, 3552–3559. [Google Scholar] [CrossRef] [PubMed]
- Paez, J.G.; Janne, P.A.; Lee, J.C.; Tracy, S.; Greulich, H.; Gabriel, S.; Herman, P.; Kaye, F.J.; Lindeman, N.; Boggon, T.J.; et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science 2004, 304, 1497–1500. [Google Scholar] [CrossRef] [PubMed]
- Kwak, E.L.; Bang, Y.-J.; Camidge, D.R.; Shaw, A.T.; Solomon, B.; Maki, R.G.; Ou, S.-H.I.; Dezube, B.J.; Jänne, P.A.; Costa, D.B.; et al. Anaplastic Lymphoma Kinase Inhibition in Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2010, 363, 1693–1703. [Google Scholar] [CrossRef]
- Shaw, A.T.; Ou, S.-H.I.; Bang, Y.-J.; Camidge, D.R.; Solomon, B.J.; Salgia, R.; Riely, G.J.; Varella-Garcia, M.; Shapiro, G.I.; Costa, D.B.; et al. Crizotinib in ROS1-Rearranged Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2014, 371, 1963–1971. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Hu, H.; Pan, Y.; Li, Y.; Ye, T.; Li, C.; Luo, X.; Wang, L.; Li, H.; Zhang, Y.; et al. RET Fusions Define a Unique Molecular and Clinicopathologic Subtype of Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2012, 30, 4352–4359. [Google Scholar] [CrossRef] [PubMed]
- Farago, A.F.; Taylor, M.S.; Doebele, R.C.; Zhu, V.W.; Kummar, S.; Spira, A.I.; Boyle, T.A.; Haura, E.B.; Arcila, M.E.; Benayed, R.; et al. Clinicopathologic Features of Non–Small-Cell Lung Cancer Harboring an NTRK Gene Fusion. JCO Precis. Oncol. 2018, 1–12. [Google Scholar] [CrossRef]
- Planchard, D.; Besse, B.; Groen, H.J.M.; Souquet, P.-J.; Quoix, E.; Baik, C.S.; Barlesi, F.; Kim, T.M.; Mazieres, J.; Novello, S.; et al. Dabrafenib plus trametinib in patients with previously treated BRAFV600E-mutant metastatic non-small cell lung cancer: An open-label, multicentre phase 2 trial. Lancet Oncol. 2016, 17, 984–993. [Google Scholar] [CrossRef]
- Cappuzzo, F.; Bemis, L.; Varella-Garcia, M. HER2 Mutation and Response to Trastuzumab Therapy in Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2006, 354, 2619–2621. [Google Scholar] [CrossRef]
- Awad, M.M.; Oxnard, G.R.; Jackman, D.M.; Savukoski, D.O.; Hall, D.; Shivdasani, P.; Heng, J.C.; Dahlberg, S.E.; Jänne, P.A.; Verma, S.; et al. MET Exon 14 Mutations in Non–Small-Cell Lung Cancer Are Associated with Advanced Age and Stage-Dependent MET Genomic Amplification and c-Met Overexpression. J. Clin. Oncol. 2016, 34, 721–730. [Google Scholar] [CrossRef]
- Tsuboi, M.; Kato, H.; Nagai, K.; Tsuchiya, R.; Wada, H.; Tada, H.; Ichinose, Y.; Fukuoka, M.; Jiang, H. Gefitinib in the adjuvant setting: Safety results from a phase III study in patients with completely resected non-small cell lung cancer. Anticancer Drugs 2005, 16, 1123–1128. [Google Scholar] [CrossRef]
- Goss, G.D.; O’Callaghan, C.; Lorimer, I.; Tsao, M.-S.; Masters, G.A.; Jett, J.; Edelman, M.J.; Lilenbaum, R.; Choy, H.; Khuri, F.; et al. Gefitinib Versus Placebo in Completely Resected Non–Small-Cell Lung Cancer: Results of the NCIC CTG BR19 Study. J. Clin. Oncol. 2013, 31, 3320–3326. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.Z.; Wang, Q.; Mao, W.M.; Xu, S.T.; Wu, L.; Shen, Y.; Liu, Y.Y.; Chen, C.; Cheng, Y.; Xu, L.; et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II–IIIA (N1–N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): A randomised, open-label, phase 3 study. Lancet Oncol. 2018, 19, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.; Altorki, N.K.; Eberhardt, W.E.E.; O’Brien, M.E.R.; Spigel, D.R.; Crinò, L.; Tsai, C.-M.; Kim, J.-H.; Cho, E.K.; Hoffman, P.C.; et al. Adjuvant Erlotinib Versus Placebo in Patients with Stage IB–IIIA Non–Small-Cell Lung Cancer (RADIANT): A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2015, 33, 4007–4014. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Wu, Y.-L.; John, T.; Grohe, C.; Majem, M.; Wang, J.; Kato, T.; Goldman, J.W.; Laktionov, K.; Kim, S.-W.; et al. Adjuvant Osimertinib for Resected EGFR-Mutated Stage IB–IIIA Non–Small-Cell Lung Cancer: Updated Results from the Phase III Randomized ADAURA Trial. J. Clin. Oncol. 2023, 41, 1830–1840. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Mitsudomi, T.; Misumi, T.; Sugio, K.; Tsuboi, M.; Okamoto, I.; Iwamoto, Y.; Sakakura, N.; Sugawara, S.; Atagi, S.; et al. Randomized Phase III Study of Gefitinib Versus Cisplatin Plus Vinorelbine for Patients with Resected Stage II-IIIA Non–Small-Cell Lung Cancer with EGFR Mutation (IMPACT). J. Clin. Oncol. 2022, 40, 231–241. [Google Scholar] [CrossRef]
- He, J.; Su, C.; Liang, W.; Xu, S.; Wu, L.; Fu, X.; Zhang, X.; Ge, D.; Chen, Q.; Mao, W.; et al. Icotinib versus chemotherapy as adjuvant treatment for stage II–IIIA EGFR-mutant non-small-cell lung cancer (EVIDENCE): A randomised, open-label, phase 3 trial. Lancet Respir. Med. 2021, 9, 1021–1029. [Google Scholar] [CrossRef]
- Inoue, A.; Saijo, Y.; Maemondo, M.; Gomi, K.; Tokue, Y.; Kimura, Y.; Ebina, M.; Kikuchi, T.; Moriya, T.; Nukiwa, T. Severe acute interstitial pneumonia and gefitinib. Lancet 2003, 361, 137–139. [Google Scholar] [CrossRef]
- Li, N.; Ou, W.; Ye, X.; Sun, H.-B.; Zhang, L.; Fang, Q.; Zhang, S.-L.; Wang, B.-X.; Wang, S.-Y. Pemetrexed-Carboplatin Adjuvant Chemotherapy with or Without Gefitinib in Resected Stage IIIA-N2 Non-Small Cell Lung Cancer Harbouring EGFR Mutations: A Randomized, Phase II Study. Ann. Surg. Oncol. 2014, 21, 2091–2096. [Google Scholar] [CrossRef]
- Feng, S.; Wang, Y.; Cai, K.; Wu, H.; Xiong, G.; Wang, H.; Zhang, Z. Randomized Adjuvant Chemotherapy of EGFR-Mutated Non-Small Cell Lung Cancer Patients with or without Icotinib Consolidation Therapy. PLoS ONE 2015, 10, e0140794. [Google Scholar] [CrossRef]
- Yue, D.; Xu, S.; Wang, Q.; Li, X.; Shen, Y.; Zhao, H.; Chen, C.; Mao, W.; Liu, W.; Liu, J.; et al. Erlotinib versus vinorelbine plus cisplatin as adjuvant therapy in Chinese patients with stage IIIA EGFR mutation-positive non-small-cell lung cancer (EVAN): A randomised, open-label, phase 2 trial. Lancet Respir. Med. 2018, 6, 863–873. [Google Scholar] [CrossRef]
- Zhong, W.-Z.; Wang, Q.; Mao, W.-M.; Xu, S.-T.; Wu, L.; Wei, Y.-C.; Liu, Y.-Y.; Chen, C.; Cheng, Y.; Yin, R.; et al. Gefitinib Versus Vinorelbine Plus Cisplatin as Adjuvant Treatment for Stage II–IIIA (N1–N2) EGFR-Mutant NSCLC: Final Overall Survival Analysis of CTONG1104 Phase III Trial. J. Clin. Oncol. 2021, 39, 713–722. [Google Scholar] [CrossRef]
- Herbst, R.S.; Tsuboi, M.; John, T.; Kato, T.; Majem, M.; Grohé, C.; Wang, J.; Goldman, J.W.; Lu, S.; Su, W.-C.; et al. Overall survival analysis from the ADAURA trial of adjuvant osimertinib in patients with resected EGFR-mutated (EGFRm) stage IB–IIIA non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2023, 41, LBA3. [Google Scholar] [CrossRef]
- Solomon, B.J.; Ahn, J.S.; Barlesi, F.; Dziadziuszko, R.; Nishio, M.; Shaw, A.T.; Bordogna, W.; Meyenberg, C.; Wu, Y.-L. ALINA: A phase III study of alectinib versus chemotherapy as adjuvant therapy in patients with stage IB–IIIA anaplastic lymphoma kinase-positive (ALK+) non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2019, 37, TPS8569. [Google Scholar] [CrossRef]
- Lee, J.M.; Sepesi, B.; Toloza, E.M.; Lin, J.; Pass, H.I.; Johnson, B.E.; Heymach, J.V.; Johnson, M.L.; Ding, B.; Schulze, K.; et al. EP02.04-005 Phase II NAUTIKA1 Study of Targeted Therapies in Stage II–III NSCLC: Preliminary Data of Neoadjuvant Alectinib for ALK+ NSCLC. J. Thorac. Oncol. 2022, 17, S233–S234. [Google Scholar] [CrossRef]
- Piccart-Gebhart, M.J.; Procter, M.; Leyland-Jones, B.; Goldhirsch, A.; Untch, M.; Smith, I.; Gianni, L.; Baselga, J.; Bell, R.; Jackisch, C.; et al. Trastuzumab after Adjuvant Chemotherapy in HER2-Positive Breast Cancer. N. Engl. J. Med. 2005, 353, 1659–1672. [Google Scholar] [CrossRef]
- Romond, E.H.; Perez, E.A.; Bryant, J.; Suman, V.J.; Geyer, C.E.; Davidson, N.E.; Tan-Chiu, E.; Martino, S.; Paik, S.; Kaufman, P.A.; et al. Trastuzumab plus Adjuvant Chemotherapy for Operable HER2-Positive Breast Cancer. N. Engl. J. Med. 2005, 353, 1673–1684. [Google Scholar] [CrossRef]
- Slamon, D.; Eiermann, W.; Robert, N.; Pienkowski, T.; Martin, M.; Press, M.; Mackey, J.; Glaspy, J.; Chan, A.; Pawlicki, M.; et al. Breast Cancer International Research Group. Adjuvant trastuzumab in HER2-positive breast cancer. N. Engl. J. Med. 2011, 365, 1273–1283. [Google Scholar] [CrossRef]
- Joensuu, H.; Kellokumpu-Lehtinen, P.-L.; Bono, P.; Alanko, T.; Kataja, V.; Asola, R.; Utriainen, T.; Kokko, R.; Hemminki, A.; Tarkkanen, M.; et al. Adjuvant Docetaxel or Vinorelbine with or without Trastuzumab for Breast Cancer. N. Engl. J. Med. 2006, 354, 809–820. [Google Scholar] [CrossRef]
- Joensuu, H.; Bono, P.; Kataja, V.; Alanko, T.; Kokko, R.; Asola, R.; Utriainen, T.; Turpeenniemi-Hujanen, T.; Jyrkkiö, S.; Möykkynen, K.; et al. Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: Final results of the FinHer Trial. J. Clin. Oncol. 2009, 27, 5685–5692. [Google Scholar] [CrossRef]
- Pivot, X.; Romieu, G.; Debled, M.; Pierga, J.Y.; Kerbrat, P.; Bachelot, T.; Lortholary, A.; Espié, M.; Fumoleau, P.; Serin, D.; et al. 6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): A randomised phase 3 trial. Lancet Oncol. 2013, 14, 741–748. [Google Scholar] [CrossRef]
- Conte, P.; Frassoldati, A.; Bisagni, G.; Brandes, A.A.; Donadio, M.; Garrone, O.; Piacentini, F.; Cavanna, L.; Giotta, F.; Aieta, M.; et al. Nine weeks versus 1 year adjuvant trastuzumab in combination with chemotherapy: Final results of the phase III randomized Short-HER study. Ann. Oncol. 2018, 29, 2328–2333. [Google Scholar] [CrossRef] [PubMed]
- Mavroudis, D.; Saloustros, E.; Malamos, N.; Kakolyris, S.; Boukovinas, I.; Papakotoulas, P.; Kentepozidis, N.; Ziras, N.; Georgoulias, V. Six versus 12 months of adjuvant trastuzumab in combination with dose-dense chemotherapy for women with HER2-positive breast cancer: A multicenter randomized study by the Hellenic Oncology Research Group (HORG). Ann. Oncol. 2015, 26, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Earl, H.M.; Hiller, L.; Vallier, A.L.; Loi, S.; McAdam, K.; Hughes-Davies, L.; Harnett, A.N.; Ah-See, M.L.; Simcock, R.; Rea, D.; et al. 6 versus 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial. Lancet 2019, 393, 2599–2612. [Google Scholar] [CrossRef] [PubMed]
- Goss, P.E.; Smith, I.E.; O’Shaughnessy, J.; Ejlertsen, B.; Kaufmann, M.; Boyle, F.; Buzdar, A.U.; Fumoleau, P.; Gradishar, W.; Martin, M.; et al. Adjuvant lapatinib for women with early-stage HER2-positive breast cancer: A randomised, controlled, phase 3 trial. Lancet Oncol. 2013, 14, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Piccart-Gebhart, M.; Holmes, E.; Baselga, J.; de Azambuja, E.; Dueck, A.C.; Viale, G.; Zujewski, J.A.; Goldhirsch, A.; Armour, A.; Pritchard, K.I.; et al. Adjuvant Lapatinib and Trastuzumab for Early Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: Results from the Randomized Phase III Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization Trial. J. Clin. Oncol. 2016, 34, 1034–1042. [Google Scholar] [CrossRef]
- Martin, M.; Holmes, F.A.; Ejlertsen, B.; Delaloge, S.; Moy, B.; Iwata, H.; von Minckwitz, G.; Chia, S.K.L.; Mansi, J.; Barrios, C.H.; et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1688–1700. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Procter, M.; de Azambuja, E.; Zardavas, D.; Benyunes, M.; Viale, G.; Suter, T.; Arahmani, A.; Rouchet, N.; Clark, E.; et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N. Engl. J. Med. 2017, 377, 122–131. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Huang, C.S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef]
- Krop, I.E.; Im, S.A.; Barrios, C.; Bonnefoi, H.; Gralow, J.; Toi, M.; Ellis, P.A.; Gianni, L.; Swain, S.M.; Im, Y.H.; et al. Trastuzumab Emtansine Plus Pertuzumab Versus Taxane Plus Trastuzumab Plus Pertuzumab After Anthracycline for High-Risk Human Epidermal Growth Factor Receptor 2-Positive Early Breast Cancer: The Phase III KAITLIN Study. J. Clin. Oncol. 2022, 40, 438–448. [Google Scholar] [CrossRef]
- Johnston, S.R.D.; Harbeck, N.; Hegg, R.; Toi, M.; Martin, M.; Shao, Z.M.; Zhang, Q.Y.; Martinez Rodriguez, J.L.; Campone, M.; Hamilton, E.; et al. Abemaciclib Combined with Endocrine Therapy for the Adjuvant Treatment of HR+, HER2−, Node-Positive, High-Risk, Early Breast Cancer (monarchE). J. Clin. Oncol. 2020, 38, 3987–3998. [Google Scholar] [CrossRef]
- Johnston, S.R.D.; Toi, M.; O’Shaughnessy, J.; Rastogi, P.; Campone, M.; Neven, P.; Huang, C.S.; Huober, J.; Jaliffe, G.G.; Cicin, I.; et al. Abemaciclib plus endocrine therapy for hormone receptor-positive, HER2-negative, node-positive, high-risk early breast cancer (monarchE): Results from a preplanned interim analysis of a randomised, open-label, phase 3 trial. Lancet Oncol. 2023, 24, 77–90. [Google Scholar] [CrossRef]
- Slamon, D.J.; Stroyakovskiy, D.; Yardley, D.A.; Huang, C.-S.; Fasching, P.A.; Crown, J.; Bardia, A.; Chia, S.; Im, S.-A.; Martin, M.; et al. Ribociclib and endocrine therapy as adjuvant treatment in patients with HR+/HER2− early breast cancer: Primary results from the phase III NATALEE trial. J. Clin. Oncol. 2023, 41, LBA500. [Google Scholar] [CrossRef]
- Mayer, E.L.; Dueck, A.C.; Martin, M.; Rubovszky, G.; Burstein, H.J.; Bellet-Ezquerra, M.; Miller, K.D.; Zdenkowski, N.; Winer, E.P.; Pfeiler, G.; et al. Palbociclib with adjuvant endocrine therapy in early breast cancer (PALLAS): Interim analysis of a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2021, 22, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Marmé, F.; Martin, M.; Untch, M.; Bonnefoi, H.; Kim, S.B.; Bear, H.; McCarthy, N.; Melé Olivé, M.; Gelmon, K.; et al. Palbociclib for Residual High-Risk Invasive HR-Positive and HER2-Negative Early Breast Cancer-The Penelope-B Trial. J. Clin. Oncol. 2021, 39, 1518–1530. [Google Scholar] [CrossRef] [PubMed]
- Gregor, M.C.-M.; Barlow, W.E.; Pusztai, L.; Goetz, M.P.; Rastogi, P.; Ganz, P.A.; Mamounas, E.P.; Paik, S.; Bandos, H.; Gralow, J.; et al. Phase III randomized, placebo-controlled clinical trial evaluating the use of adjuvant endocrine therapy +/− one year of everolimus in patients with high-risk, hormone receptor (HR) positive and HER2-negative breast cancer (BC): SWOG/NRG/Alliance S1207 (NCT01674140). J. Clin. Oncol. 2015, 33, TPS637. [Google Scholar]
- Tutt, A.N.J.; Garber, J.E.; Kaufman, B.; Viale, G.; Fumagalli, D.; Rastogi, P.; Gelber, R.D.; de Azambuja, E.; Fielding, A.; Balmaña, J.; et al. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N. Engl. J. Med. 2021, 384, 2394–2405. [Google Scholar] [CrossRef] [PubMed]
- Spring, L.M.; Fell, G.; Arfe, A.; Sharma, C.; Greenup, R.; Reynolds, K.L.; Smith, B.L.; Alexander, B.; Moy, B.; Isakoff, S.J.; et al. Pathologic Complete Response after Neoadjuvant Chemotherapy and Impact on Breast Cancer Recurrence and Survival: A Comprehensive Meta-analysis. Clin. Cancer Res. 2020, 26, 2838–2848. [Google Scholar] [CrossRef] [PubMed]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef]
- Korde, L.A.; Somerfield, M.R.; Carey, L.A.; Crews, J.R.; Denduluri, N.; Hwang, E.S.; Khan, S.A.; Loibl, S.; Morris, E.A.; Perez, A.; et al. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J. Clin. Oncol. 2021, 39, 1485–1505. [Google Scholar] [CrossRef]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Jeruss, J.S.; Tucker, S.L.; Kolli, A.; Newman, L.A.; Gonzalez-Angulo, A.M.; Buchholz, T.A.; Sahin, A.A.; Cormier, J.N.; Buzdar, A.U.; et al. Validation of a Novel Staging System for Disease-Specific Survival in Patients with Breast Cancer Treated with Neoadjuvant Chemotherapy. J. Clin. Oncol. 2011, 29, 1956–1962. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.; Piccart-Gebhart, M.J.; Gelber, R.D.; Procter, M.; Goldhirsch, A.; de Azambuja, E.; Castro, G., Jr.; Untch, M.; Smith, I.; Gianni, L.; et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: Final analysis of the HERceptin Adjuvant (HERA) trial. Lancet 2017, 389, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.A.; Romond, E.H.; Suman, V.J.; Jeong, J.-H.; Sledge, G.; Geyer, C.E.; Martino, S.; Rastogi, P.; Gralow, J.; Swain, S.M.; et al. Trastuzumab Plus Adjuvant Chemotherapy for Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer: Planned Joint Analysis of Overall Survival From NSABP B-31 and NCCTG N9831. J. Clin. Oncol. 2014, 32, 3744–3752. [Google Scholar] [CrossRef] [PubMed]
- Joensuu, H.; Fraser, J.; Wildiers, H.; Huovinen, R.; Auvinen, P.; Utriainen, M.; Nyandoto, P.; Villman, K.K.; Halonen, P.; Granstam-Björneklett, H.; et al. Effect of Adjuvant Trastuzumab for a Duration of 9 Weeks vs 1 Year with Concomitant Chemotherapy for Early Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: The SOLD Randomized Clinical Trial. JAMA Oncol. 2018, 4, 1199–1206. [Google Scholar] [CrossRef]
- Chan, A.; Moy, B.; Mansi, J.; Ejlertsen, B.; Holmes, F.A.; Chia, S.; Iwata, H.; Gnant, M.; Loibl, S.; Barrios, C.H.; et al. Final Efficacy Results of Neratinib in HER2-Positive Hormone Receptor-positive Early-Stage Breast Cancer from the Phase III ExteNET Trial. Clin. Breast Cancer 2021, 21, 80–91.e87. [Google Scholar] [CrossRef] [PubMed]
- Barcenas, C.H.; Hurvitz, S.A.; Di Palma, J.A.; Bose, R.; Chien, A.J.; Iannotti, N.; Marx, G.; Brufsky, A.; Litvak, A.; Ibrahim, E.; et al. Improved tolerability of neratinib in patients with HER2-positive early-stage breast cancer: The CONTROL trial. Ann. Oncol. 2020, 31, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Piccart, M.; Procter, M.; Fumagalli, D.; de Azambuja, E.; Clark, E.; Ewer, M.S.; Restuccia, E.; Jerusalem, G.; Dent, S.; Reaby, L.; et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer in the APHINITY Trial: 6 Years’ Follow-Up. J. Clin. Oncol. 2021, 39, 1448–1457. [Google Scholar] [CrossRef]
- Loibl, S.; Jassem, J.; Sonnenblick, A.; Parlier, D.; Winer, E.; Bergh, J.; Gelber, R.D.; Restuccia, E.; Im, Y.H.; Huang, C.; et al. VP6-2022: Adjuvant pertuzumab and trastuzumab in patients with early HER-2 positive breast cancer in APHINITY: 8.4 years’ follow-up. Ann. Oncol. 2022, 33, 986–987. [Google Scholar] [CrossRef]
- Schneeweiss, A.; Chia, S.; Hickish, T.; Harvey, V.; Eniu, A.; Hegg, R.; Tausch, C.; Seo, J.H.; Tsai, Y.F.; Ratnayake, J.; et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: A randomized phase II cardiac safety study (TRYPHAENA). Ann. Oncol. 2013, 24, 2278–2284. [Google Scholar] [CrossRef]
- Swain, S.M.; Ewer, M.S.; Viale, G.; Delaloge, S.; Ferrero, J.M.; Verrill, M.; Colomer, R.; Vieira, C.; Werner, T.L.; Douthwaite, H.; et al. Pertuzumab, trastuzumab, and standard anthracycline- and taxane-based chemotherapy for the neoadjuvant treatment of patients with HER2-positive localized breast cancer (BERENICE): A phase II, open-label, multicenter, multinational cardiac safety study. Ann. Oncol. 2018, 29, 646–653. [Google Scholar] [CrossRef]
- Mamounas, E.P.; Untch, M.; Mano, M.S.; Huang, C.S.; Geyer, C.E., Jr.; von Minckwitz, G.; Wolmark, N.; Pivot, X.; Kuemmel, S.; DiGiovanna, M.P.; et al. Adjuvant T-DM1 versus trastuzumab in patients with residual invasive disease after neoadjuvant therapy for HER2-positive breast cancer: Subgroup analyses from KATHERINE. Ann. Oncol. 2021, 32, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Royce, M.; Osgood, C.; Mulkey, F.; Bloomquist, E.; Pierce, W.F.; Roy, A.; Kalavar, S.; Ghosh, S.; Philip, R.; Rizvi, F.; et al. FDA Approval Summary: Abemaciclib with Endocrine Therapy for High-Risk Early Breast Cancer. J. Clin. Oncol. 2022, 40, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Giordano, S.H.; Freedman, R.A.; Somerfield, M.R. Abemaciclib with Endocrine Therapy in the Treatment of High-Risk Early Breast Cancer: ASCO Optimal Adjuvant Chemotherapy and Targeted Therapy Guideline Rapid Recommendation Update. J. Clin. Oncol. 2022, 40, 307–309. [Google Scholar] [CrossRef]
- Mayer, E.L.; Fesl, C.; Hlauschek, D.; Garcia-Estevez, L.; Burstein, H.J.; Zdenkowski, N.; Wette, V.; Miller, K.D.; Balic, M.; Mayer, I.A.; et al. Treatment Exposure and Discontinuation in the PALbociclib CoLlaborative Adjuvant Study of Palbociclib with Adjuvant Endocrine Therapy for Hormone Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative Early Breast Cancer (PALLAS/AFT-05/ABCSG-42/BIG-14-03). J. Clin. Oncol. 2022, 40, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Gnant, M.; Dueck, A.C.; Frantal, S.; Martin, M.; Burstein, H.J.; Greil, R.; Fox, P.; Wolff, A.C.; Chan, A.; Winer, E.P.; et al. Adjuvant Palbociclib for Early Breast Cancer: The PALLAS Trial Results (ABCSG-42/AFT-05/BIG-14-03). J. Clin. Oncol. 2022, 40, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Geyer, C.E., Jr.; Garber, J.E.; Gelber, R.D.; Yothers, G.; Taboada, M.; Ross, L.; Rastogi, P.; Cui, K.; Arahmani, A.; Aktan, G.; et al. Overall survival in the OlympiA phase III trial of adjuvant olaparib in patients with germline pathogenic variants in BRCA1/2 and high-risk, early breast cancer. Ann. Oncol. 2022, 33, 1250–1268. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef]
- Tarantino, P.; Corti, C.; Schmid, P.; Cortes, J.; Mittendorf, E.A.; Rugo, H.; Tolaney, S.M.; Bianchini, G.; Andrè, F.; Curigliano, G. Immunotherapy for early triple negative breast cancer: Research agenda for the next decade. NPJ Breast Cancer 2022, 8, 23. [Google Scholar] [CrossRef]
- Murthy, R.K.; Loi, S.; Okines, A.; Paplomata, E.; Hamilton, E.; Hurvitz, S.A.; Lin, N.U.; Borges, V.; Abramson, V.; Anders, C.; et al. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 2020, 382, 597–609. [Google Scholar] [CrossRef]
- Hurvitz, S.A.; Bachelot, T.; Bianchini, G.; Harbeck, N.; Loi, S.; Park, Y.H.; Prat, A.; Gilham, L.; Boulet, T.; Gochitashvili, N.; et al. ASTEFANIA: Adjuvant ado-trastuzumab emtansine and atezolizumab for high-risk, HER2-positive breast cancer. Future Oncol. 2022, 18, 3563–3572. [Google Scholar] [CrossRef]
- Cescon, D.W.; Kalinsky, K.; Parsons, H.A.; Smith, K.L.; Spears, P.A.; Thomas, A.; Zhao, F.; DeMichele, A. Therapeutic Targeting of Minimal Residual Disease to Prevent Late Recurrence in Hormone-Receptor Positive Breast Cancer: Challenges and New Approaches. Front. Oncol. 2021, 11, 667397. [Google Scholar] [CrossRef] [PubMed]
- Suciu, S.; Eggermont, A.M.M.; Lorigan, P.; Kirkwood, J.M.; Markovic, S.N.; Garbe, C.; Cameron, D.; Kotapati, S.; Chen, T.T.; Wheatley, K.; et al. Relapse-Free Survival as a Surrogate for Overall Survival in the Evaluation of Stage II–III Melanoma Adjuvant Therapy. J. Natl. Cancer Inst. 2018, 110, 87–96. [Google Scholar] [CrossRef]
- Mauguen, A.; Pignon, J.-P.; Burdett, S.; Domerg, C.; Fisher, D.; Paulus, R.; Mandrekar, S.J.; Belani, C.P.; Shepherd, F.A.; Eisen, T.; et al. Surrogate endpoints for overall survival in chemotherapy and radiotherapy trials in operable and locally advanced lung cancer: A re-analysis of meta-analyses of individual patients’ data. Lancet Oncol. 2013, 14, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Caparica, R.; Lambertini, M.; Pondé, N.; Fumagalli, D.; de Azambuja, E.; Piccart, M. Post-neoadjuvant treatment and the management of residual disease in breast cancer: State of the art and perspectives. Ther. Adv. Med. Oncol. 2019, 11, 1758835919827714. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, L.E.; Kerr, K.M.; Menis, J.; Mok, T.S.; Nestle, U.; Passaro, A.; Peters, S.; Planchard, D.; Smit, E.F.; Solomon, B.J.; et al. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 339–357. [Google Scholar] [CrossRef]
- Tóvári, J.; Vári-Mező, D.; Surguta, S.E.; Ladányi, A.; Kigyós, A.; Cserepes, M. Evolving Acquired Vemurafenib Resistance in a BRAF V600E Mutant Melanoma PDTX Model to Reveal New Potential Targets. Cells 2023, 12, 1919. [Google Scholar] [CrossRef]
- Wu, X.; Huang, S.; He, W.; Song, M. Emerging insights into mechanisms of trastuzumab resistance in HER2-positive cancers. Int. Immunopharmacol. 2023, 122, 110602. [Google Scholar] [CrossRef]
- Mok, T.S.; Wu, Y.L.; Ahn, M.J.; Garassino, M.C.; Kim, H.R.; Ramalingam, S.S.; Shepherd, F.A.; He, Y.; Akamatsu, H.; Theelen, W.S.; et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N. Engl. J. Med. 2017, 376, 629–640. [Google Scholar] [CrossRef]
- Abbosh, C.; Birkbak, N.J.; Wilson, G.A.; Jamal-Hanjani, M.; Constantin, T.; Salari, R.; Le Quesne, J.; Moore, D.A.; Veeriah, S.; Rosenthal, R.; et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature 2017, 545, 446–451. [Google Scholar] [CrossRef]
- Chen, Y.H.; Hancock, B.A.; Solzak, J.P.; Brinza, D.; Scafe, C.; Miller, K.D.; Radovich, M. Next-generation sequencing of circulating tumor DNA to predict recurrence in triple-negative breast cancer patients with residual disease after neoadjuvant chemotherapy. NPJ Breast Cancer 2017, 3, 24. [Google Scholar] [CrossRef]
- Pantel, K.; Alix-Panabières, C. Liquid biopsy and minimal residual disease—latest advances and implications for cure. Nat. Rev. Clin. Oncol. 2019, 16, 409–424. [Google Scholar] [CrossRef]
- Buzdar, A.U.; Ibrahim, N.K.; Francis, D.; Booser, D.J.; Thomas, E.S.; Theriault, R.L.; Pusztai, L.; Green, M.C.; Arun, B.K.; Giordano, S.H.; et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: Results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J. Clin. Oncol. 2005, 23, 3676–3685. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, M.; Weder, W.; Escriu, C.; Blakely, C.; He, J.; Dacic, S.; Yatabe, Y.; Zeng, L.; Walding, A.; Chaft, J.E. Neoadjuvant osimertinib with/without chemotherapy versus chemotherapy alone for EGFR-mutated resectable non-small-cell lung cancer: NeoADAURA. Future Oncol. 2021, 17, 4045–4055. [Google Scholar] [CrossRef] [PubMed]
- Leonetti, A.; Minari, R.; Boni, L.; Gnetti, L.; Verzè, M.; Ventura, L.; Musini, L.; Tognetto, M.; Tiseo, M. Phase II, Open-label, Single-arm, Multicenter Study to Assess the Activity and Safety of Alectinib as Neoadjuvant Treatment in Surgically Resectable Stage III ALK-positive NSCLC: ALNEO Trial. Clin. Lung Cancer 2021, 22, 473–477. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).